Abstract
We investigated the expression of estrogen receptors (ERs), insulin-like growth factor 1 (IGF-1), and IGF-1R (receptor) in human cholangiocarcinoma and cholangiocarcinoma cell lines (HuH-28, TFK-1, Mz-ChA-1), evaluating the role of estrogens and IGF-1 in the modulation of neoplastic cell growth. ER-α, ER-β, IGF-1, and IGF-1R were expressed (immunohistochemistry) in all biopsies (18 of 18) of intrahepatic cholangiocarcinoma. ER-α was expressed (Western blot) only by the HuH-28 cell line (intrahepatic cholangiocarcinoma), whereas ER-β, IGF-1, and IGF-1R were expressed in the three cell lines examined. In serum-deprived HuH-28 cells, serum readmission induced stimulation of cell proliferation that was inhibited by ER and IGF-1R antagonists. 17β-Estradiol and IGF-1 stimulated proliferation of HuH-28 cells to a similar extent to that of MCF7 (breast cancer) but greater than that of TFK-1 and Mz-ChA-1, inhibiting apoptosis and exerting additive effects. These effects of 17β-estradiol and IGF-1 were associated with enhanced protein expression of ER-α, phosphorylated (p)-ERK1/2 and pAKT but with decreased expression of ER-β. Finally, transfection of IGF-1R anti-sense oligonucleotides in HuH-28 cells markedly decreased cell proliferation. In conclusion, human intrahepatic cholangiocarcinomas express receptors for estrogens and IGF-1, which cooperate in the modulation of cell growth and apoptosis. Modulation of ER and IGF-1R could represent a strategy for the management of cholangiocarcinoma.
Cholangiocarcinoma is a malignant tumor arising from the epithelial cells (cholangiocytes) lining the biliary tree and characterized by a poor prognosis and scarce response to current therapies.1,2 The incidence and mortality for cholangiocarcinoma are increasing worldwide.3 Estrogens are positive growth modulators for normal and neoplastic cells expressing estrogen receptors (ERs).4–7 They bind ER-α and/or ER-β subtypes and modulate cell growth by both direct genomic and nongenomic pathways, in which different intracellular transduction signals are involved but with a major role played by MAP kinases.4–7 The role played by estrogens and their receptors in the growth of ER-positive neoplasms represents the basis for the pharmacological treatment and/or prevention of different cancers (mainly breast cancer) with ER antagonists.8,9
We have recently shown that 1) human and rat cholangiocytes express both ER-α and/or ER-β subtypes, 2) estrogens positively modulate cholangiocyte proliferation, and 3) ERs are overexpressed during cholangiocyte proliferation associated with human cholangiopathies.10–13 Furthermore, studies in rat cholangiocytes indicate that estrogens interact with and potentiate the effect of growth factors on cholangiocyte proliferation.14,15 Specifically, by interacting at both receptor and postreceptor levels, 17β-estradiol markedly potentiates the proliferating effect of insulin-like growth factor 1 (IGF-1) on isolated rat cholangiocytes.15 Similar interactions between IGF-1 and estrogens modulate neoplastic cell growth of tumors expressing ERs, which may include breast, ovary, and endometrial cancers.16–18 Little information exists on the role of estrogens and IGF-1 in the modulation of growth and progression of cholangiocarcinoma. In the present study, we investigated the expression of ER and IGF-1R in human cholangiocarcinoma and human cholangiocarcinoma cell lines and evaluated the role of estrogens and IGF-1 in the modulation of neoplastic cell growth.
Materials and Methods
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise indicated. Media and serum for cell culture were obtained from Life Technologies, Inc. (Gaithersburg, MD). The IGF-1R blocking antibody αIR3 was obtained from Oncogene-DBA (DBA Italia, srl, Segrate, Milan, Italy).
Light Microscopy and Immunohistochemistry of Human Cholangiocarcinoma
We investigated 18 patients (nine females, age 60 to 75 years, and nine males, age 63 to 73 years) with intrahepatic cholangiocarcinoma presenting as a single mass lesion within the liver. In 10 of 18 patients, US-guided liver biopsies were investigated, whereas in 8 of 18 patients (four female, four male) specimens were obtained after surgical resection (four patients) or liver transplantation (four patients). As normal controls, we investigated 10 liver biopsies with a normal histology from patients (five females, age 58 to 69 years, and five males, age 60 to 72 years) submitted to laparotomy. Liver fragments (0.5 cm) were fixed in 10% buffered formalin for 2 to 4 hours and embedded in low-temperature fusion paraffin (55 to 57°C), and 3- to 4-μm sections were stained with hematoxylin and eosin and Masson’s trichrome. For immunohistochemistry, sections were mounted on glass slides coated with 0.1% poly-l-lysine. After deparaffination, endogenous peroxidase activity was blocked by a 30-minute incubation in methanolic hydrogen peroxide (2.5%). The endogen biotin was then blocked by Biotin Blocking System (DAKO, code X0590; DAKO, Copenhagen, Denmark) according to the instructions supplied by the vendor. Sections were hydrated in graded alcohol and rinsed in phosphate-buffered saline (PBS, pH 7.4) before applying the primary antibody. Sections were incubated overnight at 4°C with antibodies for cytokeratin 19 (monoclonal antibody CK-19; DAKO), proliferating cellular nuclear antigen (PCNA) (PC10; DAKO), ER-α [a cocktail of three monoclonal antibodies: SC-314, D12, F10 (33% of each); Santa Cruz Biotechnology. Inc., Santa Cruz, CA], ER-β (monoclonal antibody; GenTex, San Antonio, TX), IGF-1 (Santa Cruz), or IGF-1R (Santa Cruz). Samples were then rinsed with PBS for 5 minutes, incubated for 10 minutes at room temperature with secondary biotinylated antibody (DAKO LSAB Plus System; HRP, Milan, Italy), incubated with DAKO ABC (DAKO LSAB Plus System; HRP, Milan, Italy), and finally developed with 3-3′ diaminobenzidine. For all immunoreactions, negative controls were also included. Light microscopy and immunohistochemistry observation were taken by BX-5 1 light microscopy (Olympus, Tokyo, Japan) with a videocam (Spot Insight; Diagnostic Instrument, Inc., Sterling Heights, MI) and processed with an Image Analysis System (IAS; Delta Sistemi, Rome, Italy). Light microscopy and immunohistochemical observations were independently performed by three pathologists in a blind manner. ER-α and ER-β immunohistochemical expression was evaluated as previously described.11–13 Briefly, six slides were analyzed per each specimen of normal liver or cholangiocarcinoma. Neoplastic or normal cholangiocytes were counted in a random, blinded manner in six nonoverlapping fields (magnification ×20) of each slide and the data expressed as percentage of positive cells. The use of human material has been approved by the local institutional review board.
Cancer Cell Lines
Mz-ChA-1 cells (gallbladder origin)19 were a gift from Dr. J.G. Fitz (University of Texas, Southwest Medical Center, Dallas, TX). HuH-28 (intrahepatic bile duct)20 and TFK-1 (extrahepatic bile duct)21 cholangiocarcinoma cell lines were acquired from Cancer Cell Repository, Tohoku University, Tohoku, Japan. Mz-ChA-1, TFK-1, and HuH-28 cells were maintained in CRML 1066 medium containing 10% fetal bovine serum. The human HCC cell line Alex (PRF/PLC/5) was a gift from Prof. R. Mazzanti (University of Florence, Florence, Italy) and was maintained in Eagle’s minimum essential medium containing 10% fetal bovine serum. The human colon carcinoma SW 480 cell line (a gift from Dr. E. Porfiri, Polytechnic University of Ancona, Ancona, Italy) was cultured in Leibovitiz’s L15 medium containing 10% fetal bovine serum.
Cell Proliferation and Apoptosis
Cell lines, cultured in the appropriate medium containing 10% fetal bovine serum, were deprived of serum for 48 hours. Then, cells were maintained in serum-deprived conditions for an additional 48 hours (controls = C) or exposed to serum, 17β-estradiol, IGF-1, and/or receptor antagonists for an additional 48 hours. Specifically, cell medium was replaced with fresh serum-free or serum-containing medium to which the tested agent was added. 17β-Estradiol and ICI 182,780 were dissolved in dimethyl sulfoxide whereas IGF-1 and αIR3 were dissolved in saline as a stock solution that was then added (dilution, 1:100,000) to serum-free culture medium. In these experimental conditions, proliferation, apoptosis, and immunoblots were evaluated as described below.
Cell proliferation was assessed by a commercially available colorimetric cell proliferation assay (CellTiter 96 aqueous nonradioactive cell proliferation assay, MTS kit; Promega, Madison, WI), by following the manufacturer’s instructions. Proliferation index was calculated as the ratio (multiplied × 100) between cell numbers in unstimulated and stimulated cultures as described.22 In selected experiments, proliferation was also evaluated by PCNA protein expression (Western blot) or by [3H]thymidine incorporation as previously described.23 In these latter experiments, [3H]thymidine was added into the culture medium (1 μCi/ml) for the last 2 hours of each treatment.
Apoptosis was evaluated by a caspase 3 colorimetric assay kit (Sigma Chemical Co.), based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp p-nitro-anilide (Ac-DEVD-pNA) by caspase 3, resulting in the release of the p-nitroaniline (pNA). For this assay, cells were lysed in the appropriate lysis buffer provided by the vendor (50 mmol/L HEPES, pH 7.4, 5 mmol/L CHAPS, and 5 mmol/L dithiothreitol). The concentration of the pNA released from the substrate was calculated from the absorbance values at 405 nm. Caspase 3 activity resulting from the measured concentration of pNA [controls (48 hours plus 48 hours serum-free) = 1.66 ± 0.11 μmol pNA/minute/ml] was expressed as percent changes with respect to controls.
Western Blot Analysis
For Western blot analysis, cells were solubilized in lysis buffer containing 15 mmol/L Tris-HCl (pH 7.4), 5 mmol/L ethylenediaminetetraacetic acid, 100 mmol/L NaCl, 1% Igepal, 2 mmol/L phenylmethyl sulfonyl fluoride, 2 mmol/L benzamidine, and 1% aprotinin on ice for 30 minutes. After centrifugation at 10,000 × g for 20 seconds at 4°C, the supernatant was recovered, and protein concentration was determined with the protein assay-dye reagent (Bio-Rad Laboratories GmbH, Segrate, Milan, Italy). Cell extracts (10 μg) were diluted in 6× LSB (Laemmli sample buffer) containing 0.3 mol/L 2-mercaptoethanol and resolved by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Western blotting was performed as described12–15 by using the following primary antibodies from Santa Cruz: anti-PCNA, specific mouse monoclonal antibody (1:300 dilution); anti-IGF-1, goat polyclonal antibody (1:100 dilution); anti-IGF-1Rb, rabbit polyclonal antibody (1:400 dilution); anti-ER-α rabbit polyclonal antibody (1:200 dilution); anti-ER-β rabbit polyclonal antibody (1:200 dilution); anti-tERK, rabbit polyclonal antibody (1:1000 dilution); anti-pERK, mouse monoclonal antibody (1:800 dilution); anti-AKT, mouse monoclonal antibody (1:200 dilution); and anti-pAKT, rabbit polyclonal antibody (1:300 dilution). As secondary antibodies, anti-mouse IgG peroxidase-conjugated (1:2000; Sigma), anti-rabbit IgG peroxidase-conjugated (1:10,000; Sigma), or anti-goat IgG peroxidase-conjugated (1:10,000; Sigma) antibody was used. The intensity of the bands was determined by scanning video densitometry (Ultra Violet Products, Cambridge, UK) and expressed as arbitrary densitometric units normalized to β-actin expression (ie, tested protein/β-actin × 100).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis of ER-α in Cell Lines
Total cellular RNA was extracted by the Micro-Fast Track II kit (Invitrogen, San Diego, CA) according to the instructions of the vendor. Total RNA (1 μg) was used for first strand cDNA synthesis by AMV reverse transcriptase (Roche Diagnostics, Mannheim, Germany). PCR primers for ER-α, 5′-AAGGAGACTCGCTACTGT-3′ (sense) and 5′-TCAAAGATCTCCACCATGCC-3′ (anti-sense), were based on the published sequence.24 GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as housekeeping gene.25 Primers were synthesized by Invitrogen. PCR conditions used were as follows: 30 cycles of 1 minute at 94°C, 1 minute at 57°C, and 2 minutes at 72°C.
Transfection of HuH-28 Cells with IGF-1 Receptor Anti-Sense Oligonucleotides
An 18-mer anti-sense phosphorothioate oligonucleotide (S-ODN) targeted against codons 2 to 7 of the prepropeptide IGF-I receptor sequence,26,27 sense, and mismatch control oligonucleotides were obtained from Invitrogen (Invitrogen S.R.L San Giuliano Milanese, Milan, Italy). The sequences were 5′-TCCTCCGGAGCCAGACTT-3′ (anti-sense), 5′-AAGTCTGGCTCCGGAGGA-3′ (sense), 5′-TGAGCCCTCCTCCGTAGA-3′ (mismatch primer 1) and 5′-CTCTGAGCCAGACGTCTC-3′ (mis-match primer 2). HuH-28 cells were kept in CMRL 1066 medium with penicillin-streptomycin-glutamine 1% + 10% fetal bovine serum (Invitrogen). Per protocol instruc-tions, 1 day before transfection, HuH-28 cells were plated in growth medium (10% fetal bovine serum + 0.5% antibiotics) to obtain 50% confluency at the time of transfection. Phosphorothioate oligonucleotides were transfected into cells using oligofectamine reagent (Invitrogen). Per protocol instructions, the cells were washed two times with serum-free medium and then incubated with S-ODN-oligofectamine solution at 37°C in a CO2 incubator for 4 hours (serum-free medium). Then, a medium containing 30% serum and 1% antibiotics was added to the cells without removing the transfection mixture, and after 2 days (30% serum) the protein expression of IGF-1R and PCNA (proliferation marker) was analyzed.
Statistical Analysis
Data are presented as arithmetic mean ± SEs. Statistical analysis was conducted by using one-way analysis of the variance with pair-wise comparison by the Fisher’s protected least-significant difference test. In all cases, P < 0.05 was considered significant.
Results
Immunohistochemistry for ER, IGF-1, and IGF-1R in Human Liver Biopsies
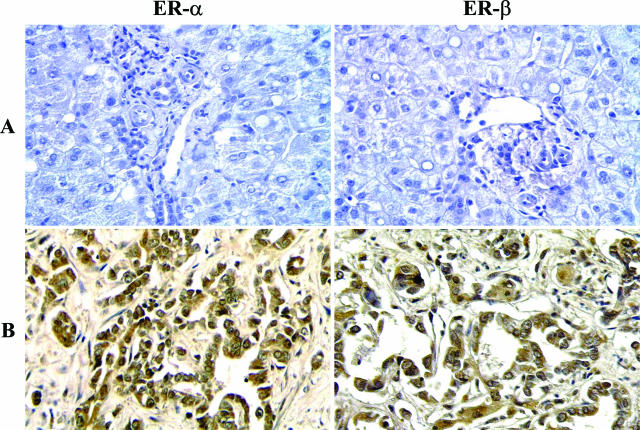
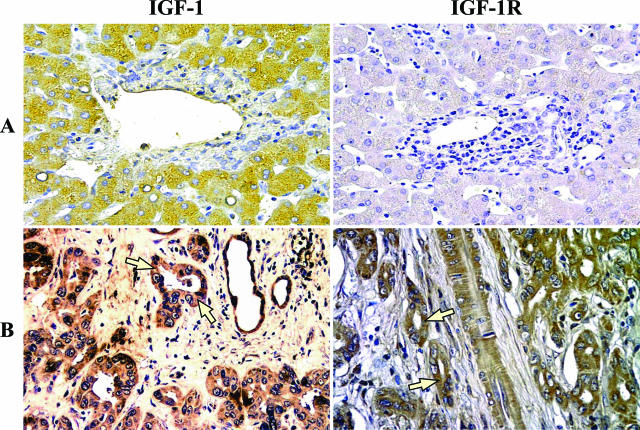
Cholangiocytes of intrahepatic bile ducts of normal human liver (n = 10 biopsies) were all negative at the immunohistochemical analysis for ER-α, ER-β, IGF-1, and IGF-1R (Figure 1A and Figure 2A). In contrast, all 18 human intrahepatic cholangiocarcinomas showed a marked positivity for both ER-α and ER-β, which involves 81.0 ± 3.5% and 82.5 ± 3.7% cells, respectively, with a staining located at both the cytoplasmic and nuclear level (Figure 1B). The 18 biopsies of human cholangiocarcinoma showed an intense positivity for both IGF-1 and IGF-1R, which involved 60.8 ± 2.8% and 64.4 ± 3.2% cells, respectively, with staining predominantly located at the cytoplasmic level (Figure 2B).
Figure 1.
Immunohistochemistry for ER-α and ER-β in human normal liver and cholangiocarcinoma. A: Intrahepatic bile ducts of the normal liver were negative at immunohistochemical analysis for ER-α (left) and ER-β (right). B: Biopsies of human cholangiocarcinoma showing an intense positivity for both ER-α (left) and ER-β (right) involving both the cytoplasm and nucleus. Light microscopy. Original magnifications, ×20.
Figure 2.
Immunohistochemistry for IGF-1 and IGF-1R in human normal liver and cholangiocarcinoma. A: Intrahepatic bile ducts of the normal liver were negative at immunohistochemical analysis for IGF-1 (left) and IGF-1R (right). B: Biopsies of human cholangiocarcinoma showing an intense positivity for both IGF-1 and IGF-1R at cytoplasmatic level (arrows). Light microscopy. Original magnifications, ×20.
Western Blot Analysis of ER, IGF-1, and IGF-1R in Human Cholangiocarcinoma Cell Lines
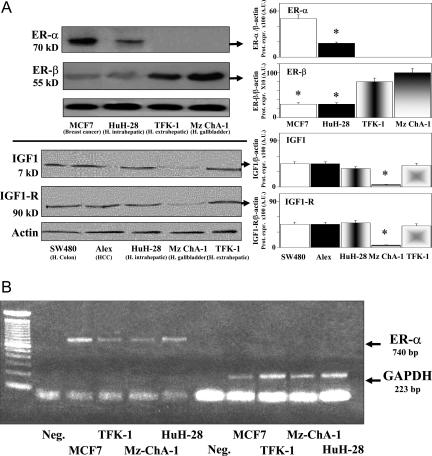
Western blot analysis shows (Figure 3A) that ER-α was expressed by the HuH-28 (human intrahepatic) cell line but not by the TFK-1 (human extrahepatic) or Mz-ChA-1 (human gallbladder) cell lines (five independent experiments). In contrast, by qualitative RT-PCR the message for ER-α was also detected in TFK-1 and Mz-ChA-1 cells (Figure 3B). Therefore, Western blot data should be indicative for a very low level of ER-α protein expression in these two cell lines. In comparison with the MCF7 breast cancer cell line used as control, the expression (Western blot) of ER-α was significantly lower (P < 0.05, five independent experiments; Figure 3A) in HuH-28 cells. ER-β was similarly expressed in HuH-28 (intrahepatic) and in MCF7 breast cancer (positive control) cell lines but highly expressed in both TFK-1 (human extrahepatic) and Mz-ChA-1 (human gallbladder) cell line (P < 0.05 versus MCF7 or HuH-28, five independent experiments; Figure 3A).
Figure 3.
A: Western blot analysis of ER, IGF-1, and IGF-1R in human cholangiocarcinoma cell lines. Cell lines, maintained in the appropriate culture medium (see Materials and Methods) with 10% fetal bovine serum, were solubilized in lysis buffer, and then the cell extract was resolved by 10% SDS-PAGE. The protein mass was determined by evaluating the intensity of the bands by scanning video densitometry and expressed (Prot. Expr.) as arbitrary densitometric units (A.U.) normalized to β-actin expression (ie, tested protein/β-actin × 100). Top: ER-α was expressed by the HuH-28 (intrahepatic) cholangiocarcinoma cell line and by the MCF7 breast cancer cell line (positive control) but not by the TFK-1 (human extrahepatic) and Mz-ChA-1 (human gallbladder) cell lines. ER-β was similarly expressed in HuH-28 (intrahepatic) and in MCF7 breast cancer (positive control) cell lines but markedly higher expressed in both TFK-1 (human extrahepatic) and Mz-ChA-1 (human gallbladder) cell lines. *P < 0.05 versus the other cell lines; five independent experiments. Bottom: IGF-1 and IGF-1R protein expression was similar in HuH-28 (intrahepatic) and TFK-1 (human extrahepatic) cholangiocarcinoma cell lines without significant differences with respect to cell lines derived from human hepatocellular carcinoma (Alex) or human colon carcinoma (SW480) used as positive controls. In contrast, the MZ-ChA-1 cell line showed a protein mass of IGF-1 and IGF-1R significantly lower than all of the other cell lines investigated. *P < 0.01 versus the other cell lines; five independent experiments. B: RT-PCR analysis of ER-α in different cell lines. Total cellular RNA was extracted from different cells lines and used (1 μg) for first strand cDNA synthesis by AMV reverse transcriptase. PCR primers for ER-α were based on the published sequence.24 GAPDH was used as a housekeeping gene.25 Neg., negative control. The figure is representative of three independent experiments with similar findings.
The protein mass of IGF-1 and IGF-1R (five independent experiments; Figure 3A) was similar in HuH-28 (intrahepatic) and TFK-1 (human extrahepatic) cholangiocarcinoma cell lines without significant differences with respect to cell lines derived from human hepatocellular carcinoma (Alex) or human colon carcinoma (SW480) used as positive controls. In contrast, the Mz-ChA-1 (gallbladder) cell line showed a protein mass of IGF-1 and IGF-1R significantly lower (P < 0.01, five independent experiments; Figure 3A) than all of the other cell lines investigated.
Role of Estrogens, IGF-1, and Their Receptors in the Modulation of Cell Line Growth
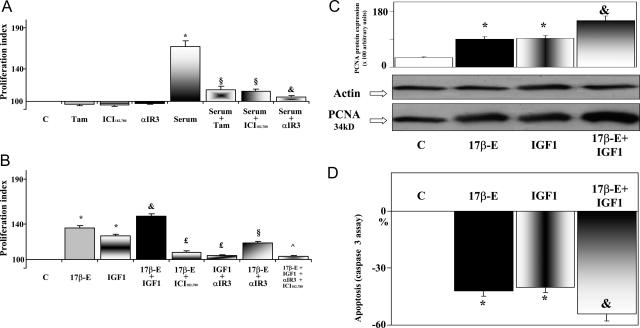
Because the HuH-28 (intrahepatic) cell line, similar to human intrahepatic cholangiocarcinoma, expresses the protein for both ER-α and ER-β, we mainly focused on this cell line to evaluate the role and mechanism by which estrogens and IGF-1 modulate cell proliferation and apoptosis. For this purpose, HuH-28 cells were deprived of serum for 48 hours, a maneuver that elicited a 65.6 ± 8% decrease of proliferation index (10 independent experiments) and a 45 ± 5% (10 independent experiments) increase of apoptosis. Serum-deprived HuH-28 cells were left without serum (controls) or exposed to serum, 17β-estradiol, IGF-1, and/or receptor antagonists for an additional 48 hours. In controls, 48 + 48 hours serum-free starvation elicited a 75.4 ± 6% (10 independent experiments) decrease of proliferation index and a 54 ± 4% (10 independent experiments) increase of apoptosis with respect to HuH-28 cells cultured in serum-containing media. [3H]Thymidine incorporation decreased from 7560 ± 586 dpm/mg protein to 3050 ± 209 dpm/mg protein after 48 hours (P < 0.01) and to 2035 ± 197 dpm/mg protein after 48 + 48 hours (P < 0.05 versus 48 hours) serum-free starvation (10 independent experiments for each protocol). The significant [3H]thymidine incorporation demonstrates that a certain rate of proliferation still persists in HuH-28 cells starved without serum for 48 or 48 + 48 hours. We also performed flow cytometry analysis of propidium iodide-stained HuH-28 cells (not shown), indicating that after 48 hours of serum-free starvation HuH-28 cells do not completely arrest in G1 phase, but at least 24% of cells are still under proliferation (S phase). When serum-deprived HuH-28 cells were exposed for 48 hours to the ER antagonists tamoxifen (1 μmol/L, 10 independent experiments) and ICI 182,780 (1 μmol/L, 10 independent experiments) or to the IGF-1R blocking antibody αIR3 (1 μg/ml, 10 independent experiments), no significant changes in proliferation index were seen (Figure 4A). Readmission of serum for 48 hours induced a marked increase of proliferation (+67 ± 7%, 10 independent experiments) which was inhibited (P < 0.01) for ∼80% (10 independent experiments) by the two ER antagonists, tamoxifen and ICI 182,780, and to a higher extent (93% inhibition) by the IGF-1R blocking antibody αIR3 (10 independent experiments; Figure 4A). This indicates that serum-induced proliferation of HuH-28 cells primarily depends on activation of ER and/or IGF-1R.
Figure 4.
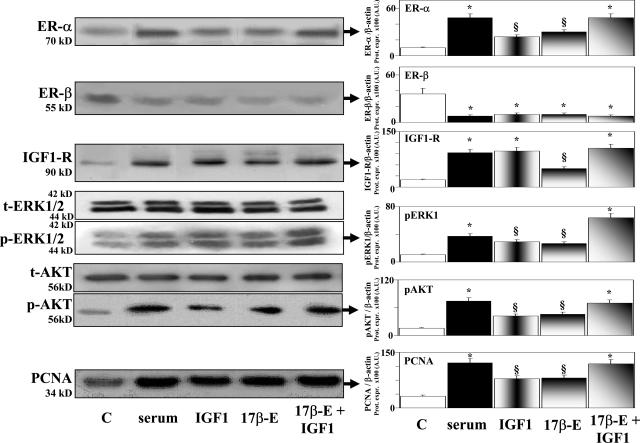
Effects of serum, 17β-estradiol, IGF-1, and receptor antagonists on proliferation index, PCNA protein expression, and apoptosis of HuH-28 cell line. HuH-28 cells cultured in CRML 1066 medium containing 10% fetal bovine serum were deprived of serum for 48 hours. Then the cells were maintained in serum-deprived conditions for further 48 hours (controls, C) or exposed to serum, 17β-estradiol, IGF-1, and/or receptor antagonists for a further 48 hours. Proliferation index, protein expression of PCNA, and apoptosis were determined. A: Effect of serum readmission on proliferation index of HuH-28 cell line in the presence or absence of ER and IGF-1R antagonists. HuH-28 cells deprived of serum for 48 hours were maintained under serum-deprived conditions for a further 48 hours (controls, C) or exposed (48 hours) to serum in the presence or absence of the ER antagonists, tamoxifen (Tam, 1 μmol/L) and ICI 182,780 (1 μmol/L), or the IGF-1R blocking antibody αIR3 (1 μg/ml), which were added into the culture medium. Proliferation index was calculated as the ratio (multiplied × 100) between cell number (MTS assay) in stimulated and unstimulated (control) cultures. *P < 0.01 versus controls; §P < 0.01 versus serum; &P < 0.05 versus serum plus Tam or serum plus ICI 182,780. Ten independent experiments for each protocol. B: Effect of 17β-estradiol, IGF-1 and antagonists of ER and IGF-1R on proliferation index of HuH-28 cell line. HuH-28 cells deprived of serum for 48 hours were maintained under serum-deprived conditions for further 48 hours (controls, C) or exposed (48 hours) to 17β-estradiol (17β-E, 10 nmol/L), IGF-1 (10 ng/ml, 1.3 nmol/L), the ER antagonist, ICI 182,780 (1 μmol/L), or the IGF-1R blocking antibody, αIR3 (1 μg/ml) that were added into the culture medium. *P < 0.01 versus C; &P < 0.05 versus 17β-E or IGF-1; £P < 0.01 versus 17β-E and IGF-1, respectively; §P < 0.01 versus 17β-E; ^ P < 0.01 versus 17β-E and IGF-1. Ten independent experiments for each protocol. C: Western blot analysis of PCNA (proliferation marker) in HuH-28 cell lines exposed to 17β-estradiol and IGF-1. HuH-28 cells deprived of serum for 48 hours were maintained under serum-deprived conditions for an additional 48 hours (controls, C) or exposed (48 hours) to 17β-estradiol (17β-E, 10 nmol/L), IGF-1 10 ng/ml (1.3 nmol/L), or 17β-estradiol and IGF-1, which were added into culture medium. For Western blot analysis, cells were solubilized in lysis buffer and then resolved by 10% SDS-PAGE. The protein mass was determined by evaluating the intensity of the bands by scanning video densitometry and expressed (Prot. Expr.) as arbitrary densitometric units (A.U.) normalized to β-actin expression (ie, tested protein/β-actin × 100). *P < 0.01 versus controls (C); &P < 0.05 versus 17β-estradiol or IGF-1 alone. Six independent experiments for each protocol. D: Effect of 17β-estradiol and IGF-1 on apoptosis (caspase 3 assay) of HuH-28 cell lines. HuH-28 cells deprived of serum for 48 hours, were maintained under serum-deprived conditions for an additional 48 hours (controls, C) or exposed (48 hours) to 17β-estradiol (17β-E, 10 nmol/L), IGF-1 (10 ng/ml, 1.3 nmol/L), or 17β-estradiol and IGF-1 added into the culture medium. Apoptosis was evaluated by measuring caspase 3 activity from the hydrolysis of Ac-DEVD-pNA with release of the pNA. Caspase 3 activity resulting from the measured concentration of pNA was expressed as percent changes with respect to controls. *P < 0.01 versus controls (C); &P < 0.05 versus 17β-estradiol or IGF-1 alone. Eight independent experiments were performed for each protocol.
17β-Estradiol (10 nmol/L) or IGF-1 (10 ng/ml, 1.3 nmol/L) added for 48 hours to HuH-28 cells cultured in serum-containing media failed to induce a significant increase of proliferation index (1.2 ± 0.9%, five independent experiments, and 0.5 ± 0.4%, five independent experiments, respectively). When 17β-estradiol (10 nmol/L) or IGF-1 (10 ng/ml, 1.3 nmol/L) were added for 48 hours in the medium of serum-deprived (48 hours) HuH-28 cells, they induced a significant (P < 0.01, 10 independent experiments) increase of proliferation index (35.5 ± 2.3% and 26.1 ± 2.3%, respectively), and when the two agents were added together, an additional increase (48.5 ± 2.7%) of proliferation index was observed, which was significantly (P < 0.05, 10 independent experiments) higher than that induced by 17β-estradiol or IGF-1 alone (ie, 17β-estradiol + IGF-1 versus 17β-estradiol or IGF-1 alone = P < 0.05; Figure 4B). In the same experimental conditions, the minimal concentration capable to significantly increase the proliferation index was 1 nmol/L for 17β-estradiol (7.5 ± 1.1% increase, eight independent experiments) and 1 ng/ml for IGF-1 (8.4 ± 0.9% increase, eight independent experiments). High concentrations of 17β-estradiol (100 nmol/L, five independent experiments) or IGF-1 (100 ng/ml, five independent experiments) failed to induce further increase of the proliferation index with respect to the concentration used in the study (not shown).
Results of proliferation index were confirmed by protein expression of PCNA (Western blot), which was significantly (P < 0.01) enhanced by 17β-estradiol and IGF-1 (six independent experiments; Figure 4C), and when the two agents were added together in the culture medium, the protein mass of PCNA further increased (P < 0.05) with respect to 17β-estradiol or IGF-1 alone (six independent experiments; Figure 4C). In contrast, apoptosis (caspase-3 assay) was inhibited by 17β-estradiol or IGF-1 (P < 0.01 versus controls, eight independent experiments) and an additional inhibitory effect was observed when serum-deprived HuH-28 cells were exposed to 17β-estradiol + IGF-1 (P < 0.05 versus 17β-estradiol or IGF-1 alone, eight independent experiments; Figure 4D).
Figure 4B also shows how the stimulatory effect of 17β-estradiol or IGF-1 on proliferation index was inhibited (P < 0.01) by ∼80% by the highly specific ER antagonist ICI 182,780 (10 independent experiments) and the IGF-1R blocking antibody αIR3 (10 independent experiments). Furthermore, the additive effect of 17β-estradiol + IGF-1 was almost completely inhibited (94%, P < 0.01) by ICI 182,780 + αIR3 (10 independent experiments). However, we observed that αIR3 inhibited ∼50% of the stimulatory effect of 17β-estradiol on the proliferation index (10 independent experiments, P < 0.01; Figure 4B), suggesting that an intact, functioning IGF-1R is also required for the stimulatory effect of estrogens on HuH-28 cell growth.
Changes in the protein expression of receptors and signaling proteins induced by serum readmission, IGF-1, or 17β-estradiol in serum-deprived HuH-28 cells are shown in Figure 5. In association with enhanced PCNA protein expression, the protein expression of IGF-1R and ER-α was increased whereas, in contrast, the protein mass of ER-β was decreased in serum-deprived HuH-28 cells stimulated to proliferate by the addition of serum, IGF-1, 17β-estradiol, or IGF-1 + 17β-estradiol (P < 0.01 versus serum-deprived controls, eight independent experiments; Figure 5). In association with higher PCNA, 17β-estradiol and IGF-1 induced, in serum-deprived HuH-28 cells, an enhanced protein expression of phosphorylated (p)-AKT and p-ERK1/2 (P < 0.01 versus controls, eight independent experiments; Figure 5). When cells were exposed to 17β-estradiol + IGF-1, an additional increase of p-AKT and p-ERK1/2 was seen (17β-estradiol + IGF-1 versus 17β-estradiol or IGF-1 alone = P < 0.05, eight independent experiments). The protein expression of total (t)-AKT and t-ERK were similar in the different experiments. Finally, Figure 5 demonstrates that serum readmission also induced an enhanced protein expression of p-AKT and p-ERK (P < 0.01 versus controls, eight independent experiments).
Figure 5.
Effect of serum, IGF-1, 17β-estradiol, and 17β-estradiol and IGF-1 on the protein expression (Western blot) of ER-α, ER-β, IGF-1R, ERK1/2, AKT, and PCNA in HuH-28 cell lines. HuH-28 cells deprived of serum for 48 hours were maintained under serum-deprived conditions for an additional 48 hours (controls, C) or exposed (48 hours) to serum, 17β-estradiol (17β-E, 10 nmol/L), IGF-1 (10 ng/ml, 1.3 nmol/L), or 17β-estradiol and IGF-1, which were added into the culture medium. For Western blot analysis, cells were solubilized in lysis buffer, and then the cell extract was resolved by 10% SDS-PAGE. The protein mass was determined by evaluating the intensity of the bands by scanning video densitometry and expressed (Prot. Expr.) as arbitrary densitometric units (A.U.) normalized to β-actin expression (ie, tested protein/β-actin × 100). *P < 0.01 versus controls (C); §P < 0.01 versus controls, P < 0.05 versus serum or 17β-estradiol and IGF-1. Ten independent experiments were performed for each protocol.
Transfection of HuH-28 Cells with IGF-1R Anti-Sense Oligonucleotides
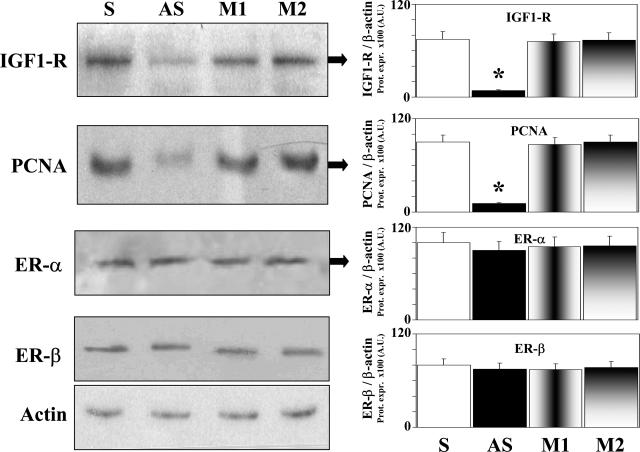
An 18-mer anti-sense S-ODN targeted against a sequence near the translation initiation site of the human IGF-I receptor mRNA reduced by ∼90% (P < 0.01, four independent experiments) the protein expression (Western blot) of PCNA (marker of proliferation) in the HUH-28 cell lines and this effect was associated with a similar decrease (P < 0.01, four independent experiments) of IGF-1R protein expression (Figure 6). The sense and both mismatch control ODNs did not have any effect on the protein expression of both PCNA and IGF-1R. The protein expression of ER-α and ER-β was unchanged by transfection of HuH-28 cells with IGF-1R anti-sense oligonucleotides (Figure 6).
Figure 6.
Effect of transfection of HuH-28 cells with IGF-1-R anti-sense phosphorothioate oligonucleotides on IGF-1, ER, and PCNA protein expression. One day before transfection, HuH-28 cells were plated in growth medium (10% fetal bovine serum and 0.5% antibiotics) to obtain 50% confluency at the time of transfection. Phosphorothioate oligonucleotides were transfected into cells using oligofectamine reagent (Invitrogen). Cells were washed two times with serum-free medium and then incubated with S-ODN-oligofectamine solution at 37°C in a CO2 incubator for 4 hours (serum-free medium). Then, a medium containing 30% serum and 1% antibiotics was added to the cells without removing the transfection mixture and after 2 days (30% serum) the protein expression of IGF-1R, ER, and PCNA (proliferation marker) was analyzed. For Western blot analysis, cells were solubilized in lysis buffer, and then the cell extract was resolved by 10% SDS-PAGE. The protein mass was determined by evaluating the intensity of the bands by scanning video densitometry and expressed (Prot. Expr.) as arbitrary densitometric units (A.U.) normalized to β-actin expression (ie, tested protein/β-actin × 100). *P < 0.01 versus other columns; four independent experiments. S, sense; AS, anti-sense; M1, mismatch 1; M2, mismatch 2.
Effect of 17β-Estradiol and IGF-1 on the Proliferation of Cell Lines Showing Different Expression of ER and IGF-1R
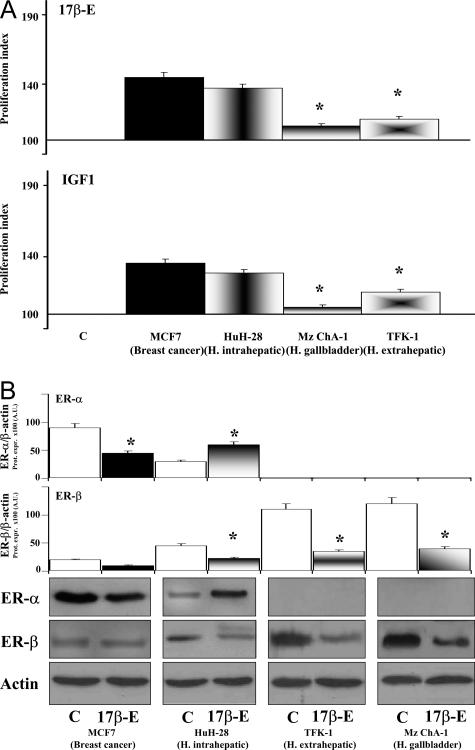
Figure 7A shows the effect of 17β-estradiol and IGF-1 on the proliferation of the HuH-28 cell line in comparison with the breast cancer cell line MCF7, which represents the prototype of estrogen-responsive cells, and the two other cholangiocarcinoma cell lines, TFK-1 (human extrahepatic) and Mz-ChA-1 (human gallbladder). When added into medium of 48-hour serum-deprived cells, 17β-estradiol (10 nmol/L) induced, after 48 hours of exposure, a similar increase of proliferation index in MCF7 (45.2 ± 3.6%, 10 independent experiments) and HuH-28 cells (37.4 ± 2.7%, 10 independent experiments; Figure 7A), both being significantly (P < 0.01) higher than TFK-1 (15.2 ± 1.7%, 10 independent experiments) and Mz-ChA-1 (10.4 ± 1.4%, 10 independent experiments). In MCF7 cells, the proliferative effects of 17β-estradiol were associated with a decreased protein expression of ER-α (P < 0.05 versus controls, eight independent experiments) and unchanged ER-β (Figure 7B). This is different from what was observed in HuH-28 cell lines in which the protein expression of ER-α increased (P < 0.05 versus controls, eight independent experiments) whereas that of ER-β decreased (P < 0.05 versus controls, eight independent experiments) after 17β-estradiol stimulation (Figure 7B). The protein expression of ER-β was also decreased (P < 0.05 versus controls, eight independent experiments) in TFK-1 and Mz-ChA-1 cells, which failed to express the ER-α protein even after stimulation with 17β-estradiol. IGF-1 (10 ng/ml) induced a similar increase of proliferation index in MCF7 (35.5 ± 3.1%, 10 independent experiments) and HuH-28 cells (28.8 ± 2.6%, 10 independent experiments), both being significantly (P < 0.01) higher than TFK-1 (15.5 ± 1.9%, 10 independent experiments) or Mz-ChA-1 (4.8 ± 1.6%, 10 independent experiments) cell line (Figure 7A).
Figure 7.
Effect of 17β-estradiol and IGF-1 on the proliferation index and protein expression of ER-α and -β in different cell lines. A: Cell lines cultured in medium containing 10% fetal bovine serum were deprived of serum for 48 hours. Then cells were maintained in serum-deprived conditions for a further 48 hours (controls, C) or exposed to 17β-estradiol (10 nmol/L) or IGF-1 (10 ng/ml), which were added into the culture medium for a further 48 hours. Proliferation index was calculated as the ratio (multiplied × 100) between cell number (MTS assay) in stimulated and unstimulated (control) cultures. *P < 0.01 versus MCF7 or HuH-28. Ten independent experiments for each protocol. B: For Western blot analysis, in the same experimental conditions described for A, cells were solubilized in lysis buffer and then the cell extract was resolved by 10% SDS-PAGE. The protein mass was determined by evaluating the intensity of the bands by scanning video densitometry and expressed (Prot. Expr.) as arbitrary densitometric units (A.U.) normalized to β-actin expression (ie, tested protein/β-actin × 100). *P < 0.05 versus controls (C, empty columns). Eight independent experiments were performed for each protocol.
Discussion
The main findings of this study indicate that: 1) ER-α, ER-β, IGF-1, and IGF-1R are expressed in human intrahepatic cholangiocarcinoma and the human intrahepatic cholangiocarcinoma cell line, HuH-28; 2) inhibitors of ER and IGF-1R markedly impair serum-induced growth of the HuH-28 cell line; 3) 17β-estradiol and IGF-1 stimulate proliferation and inhibit apoptosis of HuH-28 cell lines and exert additive effects; 4) the proliferative response of HuH-28 cells to 17β-estradiol and IGF-1 was similar to MCF7 (breast cancer) but higher than the other two cholangiocarcinoma cell lines (TFK-1 and Mz-ChA-1) lacking of ER-α expression at the Western blot analysis; 5) proliferation of HuH-28 cells induced by 17β-estradiol and IGF-1 is associated with enhanced protein expression of ER-α, p-ERK1/2, and pAKT but with decreased protein expression of ER-β; and 6) transfection of the HuH-28 cells with IGF-1-R anti-sense oligonucleotides markedly inhibits the proliferation of HuH-28 cell lines.
We first investigated by immunohistochemistry the expression of ER, IGF-1, and IGF-1R in biopsies of patients with intrahepatic cholangiocarcinoma, presenting as a single mass lesion within the liver. Although cholangiocytes of normal liver were negative, more than 80% of the cholangiocarcinoma cells were intensely positive for ER-α and ER-β in all 18 patients examined, with a staining involving both the cytoplasm and nucleus, the latter being indicative of activated receptors. IGF-1 and IGF-1R were also intensely positive in human intrahepatic cholangiocarcinoma with a staining predominantly located at the cytoplasmic level. Biopsies of cholangiocarcinoma were obtained from postmenopausal females or males and all had normal bilirubin serum levels. This should exclude that serum estrogen levels have influenced the immunohistochemical expression of ER. In addition, the cholangiocarcinomas examined display different degrees of differentiation. However, despite differences in sex, age, and degree of differentiation the immunohistochemistry showed very homogeneous features suggesting that the intense positive staining for ER and IGF-1 represents typical characteristics of cholangiocarcinoma. In many different tissues investigated, ER-α was always linked with a positive modulatory effect of estrogens on proliferation and growth.6,28–31 For ER-β in contrast, definitive conclusions are still lacking, also because recent data indicate a high heterogeneity of this receptor which could be constituted by different splice variants.28–31 Recently, a decreased expression of ER-β (mRNA and proteins) or an increased ratio ER-α/ER-β has been described in tumor versus normal tissues, including ovary, prostate, colon, and breast cancers.29,31–33 In these tissues, neoplastic transformation and progression have been associated with up-regulation ER-α and down-regulation of ER-β, indicating an opposite role of the two receptor subtypes in modulating estrogen-dependent neoplastic cell growth.29,31–33 In human intrahepatic cholangiocarcinoma, although the immunohistochemical expression of ER-β was similar with respect to benign cholangiocyte proliferation occurring during primary biliary cirrhosis, primitive sclerosing cholangitis, and alcoholic cirrhosis,10 the expression of ER-α was fourfold higher, suggesting a role in cancer progression. However, further studies aimed at investigating the isoforms of ER-β preferentially expressed during neoplastic and nonneoplastic cholangiocyte proliferation should be performed.
Next we investigated the expression of ER, IGF-1, and IGF-1R in different cholangiocarcinoma cell lines and found that only the cell line derived from human intrahepatic cholangiocarcinoma, HuH-28, expressed the protein (Western blot) of ER-α and ER-β as did the human cholangiocarcinoma biopsies. In previous studies, ERs were detected by immunoprecipitation in cholangiocarcinoma cell lines34 (OZ and SK-ChA-1), but immunohistochemical analysis was negative in both cell lines34 (OZ and SK-ChA-1) and biopsies of human cholangiocarcinoma.35 However, at variance with the present study, the antibodies used were not specific for ER subtype.34,35 HuH-28 cells also express IGF-1 and IGF-1R with a level of protein expression (Western blot) similar to cell lines derived from colon or hepatocellular carcinoma in which IGF-1 is thought to play a key role in modulating neoplastic growth.36,37 Therefore, we used HuH-28 cells to evaluate the role of estrogens and IGF-1 in modulating neoplastic cell growth. We showed that the serum-induced proliferation of HuH-28 cells is markedly inhibited by two different ER antagonists, tamoxifen and ICI 182,780,38 and also by the IGF-1R blocking antibody αIR3.39 This effect is specific on serum-induced proliferation, because in the absence of serum, ER and IGF-1R antagonists showed no effect. Therefore, most of the proliferative effect of serum is linked to activation of ER and or IGF-1R. When HuH-28 cells were exposed to IGF-1 or 17β-estradiol, proliferation was activated and apoptosis (caspase 3) was inhibited, and when the two agents were simultaneously present, additive effects were observed. Interestingly, the IGF-1R blocking antibody αIR3 partially inhibited the effects of 17β-estradiol suggesting the involvement of IGF-1R in the estrogen-stimulation of neoplastic cell growth. Because the specificity of αIR3 for IGF-1 is absolute39 our findings indicate a sort of interplay between estrogens and IGF-1 in modulating cholangiocarcinoma cell growth, in which IGF-1R plays a major role. This was further confirmed by our experiments showing that transfection of HuH-28 cell lines with IGF-1R anti-sense oligonucleotides induces, in association with depression of IGF-1R protein expression, a 90% inhibition of PCNA expression, indicating a marked impairment of cell proliferation.
When we compared cell lines with different expression of IGF-1R and ER subtypes, we found that the two cell lines expressing ER-α protein (MCF7 and HuH-28) showed a higher proliferative response to estradiol with respect to cell lines (Mz-ChA-1, TFK-1) that lack, at the Western blot analysis, of ER-α and that the rate of proliferation induced by 17β-estradiol in HuH-28 was similar than MCF7, which should be considered the prototype of estrogen responsive cell.40,41 This further supports the role of ER-α in the estrogen-dependent modulation of neoplastic cell growth. A number of different studies showed that proliferation of MCF7 cells induced by 17β-estradiol or IGF-1 is associated with a decreased protein expression of ER-α but unchanged ER-β protein mass.40–43 This is the opposite of what was observed in HuH-28 cell lines exposed to 17β-estradiol, which showed enhanced ER-α and decreased ER-β protein expression. A decreased protein mass of ER-β has been also observed in the two other cholangiocarcinoma cell lines that lack of ER-α protein expression. Interestingly, proliferation of normal rat cholangiocytes induced by experimental cholestasis11 or 17β-estradiol12 administration in ovariectomized rats was associated with an increased expression of both ER-α and ER-β and this is different with respect to the neoplastic proliferation investigated in the present study. These differences, as well as the different behavior of the ER subtypes in MCF7 and HuH-28 cells stimulated by 17β-estradiol or IGF-1, could be related to a different expression of ER isoforms, because several ER isoforms have been described with different agonist interaction and different role in modulation of cell proliferation.44
As far as IGF-1 is concerned, MCF7 and HuH-28 cells showed a similar proliferative response that was much higher than the response of TFK-1 and Mz-ChA-1 cells. Although the lower response of Mz-ChA-1 cells is justified by the very low IGF-1R expression (see Figure 3), that of TFK-1 cell could be related to the absence of ER-α because of the cooperative action of the two receptors observed in our experiments and in previous studies.29,31–33 There is increasing scientific interest in the relationship between IGF-1, IGF-1R, and cancer because high serum concentrations of IGF-1 are associated with an increased risk of breast, prostate, colorectal, pancreas, and lung cancers.36,45–48 IGF-1 has a strong influence on cell proliferation and differentiation and is a potent inhibitor of apoptosis.49 The action of IGF-1 is predominantly mediated through IGF-1R, which is involved in several oncogenic processes and is overexpressed in many tumor cell lines and in some human tumors, in which it seems to play a critical role in transformation, carcinogenesis, and metastasis.45–49 As far as estrogen-sensitive cells are concerned, IGF-1 has been considered as one of the candidate growth factors for cross-talk with estrogens in the modulation of cell proliferation.50–55 Estrogens appear to act in several critical points of the IGF signal transduction pathway. Estrogens may regulate the expression of IGF-1R, IGF-1-binding proteins, and of crucial signaling proteins including IRS1, the principal substrate for the tyrosine kinase of IGF-1R.50 In addition, ER-α and IGF-1R, once activated by specific agonists, have been shown to co-precipitate, and their state of activation (phosphorylation) as well as the related signaling pathways have been shown to be potentiated by their coupling.50 Finally, signaling activated by estrogens and IGF-1 may converge at different common transduction pathways modulating proliferation, including ERK and phosphatidylinositol-3 kinase/Akt pathways.50–56 We found that the protein expression of phosphorylated (p)-ERK1/2 and pAKT was increased in HuH-28 cells induced to proliferate by IGF-1 and 17β-estradiol and a further increase was observed when IGF-1 and 17β-estradiol were added together, suggesting convergence of the two agents into similar signaling pathways. As far as receptors are concerned, although IGF-1R and ER-α were increased, we found that proliferation of HuH-28 cell lines induced by serum, IGF-1, or 17β-estradiol was associated with decreased protein mass of ER-β, in agreement with studies indicating, in other neoplasms, an inverse correlation between cell growth and ER-β and an anti-proliferative effect of this ER subtype.31–33
In conclusion, our study highlights the role of estrogens and IGF-1 in regulating the growth of human cholangiocarcinoma and suggests that modulation of ER and IGF-1R could represent a future therapeutic strategy for the management of cholangiocarcinoma.
Acknowledgments
We thank F. Lucarelli and Cinzia Tesse for technical assistance in immunoblotting and Tracie Dornbusch for English editing.
Footnotes
Address reprint requests to Domenico Alvaro, M.D., Department of Clinical Medicine, Division of Gastroenterology, University of Rome, via R. Rossellini 51, 00137 Rome, Italy. E-mail: domenico.alvaro@uniroma1.
Supported by the Scott and White Hospital and The Texas A&M University System (grant award to G.A.), the Veteran’s Administration (merit and research scholar awards to G.A.), the National Institutes of Health (grant DK 58411 to G.A.), Ministero dell’Istruzione, dell’Università e della Ricerca (grants PRIN 2003, 2005: 2003060137_002, 2003060498_002, 2005069739_003, and 2005067975_002 to D.A. and A.F.A.), Biomedicina (cluster C04, progetto n.ro 5, Cofin 2003 and ex 60% to E.G. and P.O. and grant Cofin 2004 2004068113_03 to S.G.C.).
D.A. and B.B. contributed equally to this article.
References
- Gores GJ. A spotlight on cholangiocarcinoma. Gastroenterology. 2003;125:1536–1538. doi: 10.1016/j.gastro.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- Blendis L, Halpern Z. An increasing incidence of cholangio-carcinoma: why? Gastroenterology. 2004;127:1008–1009. doi: 10.1053/j.gastro.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Bottero D, Varricchio L, Nanayakkara M, Rotondi A, Auricchio F. Sex steroid hormones act as growth factors. J Steroid Biochem Mol Biol. 2002;83:31–35. doi: 10.1016/s0960-0760(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Koike S, Sakai M, Muramatsu M. Molecular cloning and characterization of rat estrogen receptor. Nucleic Acids Res. 1987;15:2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ERβ: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Thomas T, Gallo MA, Thomas TJ. Estrogen receptors as targets for drug development for breast cancer, osteoporosis and cardiovascular diseases. Curr Cancer Drug Targets. 2004;4:483–499. doi: 10.2174/1568009043332880. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Invernizzi P, Onori P, Franchitto A, De Santis A, Crosignani A, Sferra R, Ginanni-Corradini S, Mancino MG, Maggioni M, Attili AF, Podda M, Gaudio E. Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J Hepatol. 2004;41:905–912. doi: 10.1016/j.jhep.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Alpini G, Onori P, Perego L, Svegliati Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F, Gaudio E. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. 2000;119:1681–1691. doi: 10.1053/gast.2000.20184. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Alpini G, Onori P, Franchitto A, Glaser SS, Le Sage G, Gigliozzi A, Attili AF, Gaudio E. Effect of ovariectomy on the proliferative capacity of intrahepatic biliary epithelium. Gastroenterology. 2002;123:336–344. doi: 10.1053/gast.2002.34169. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Onori P, Drudi Metalli V, Svegliati-Baroni G, Folli F, Franchitto A, Alpini G, Mancino MG, Attili AF, Gaudio E. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology. 2002;36:297–304. doi: 10.1053/jhep.2002.34741. [DOI] [PubMed] [Google Scholar]
- Gigliozzi A, Alpini G, Baroni GS, Marucci L, Metalli VD, Glaser SS, Francis H, Mancino MG, Ueno Y, Barbaro B, Benedetti A, Attili AF, Alvaro D. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198–1209. doi: 10.1053/j.gastro.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, Glaser SS, Francis H, Cantafora A, Blotta I, Attili AF, Gaudio E. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 43:875–883. doi: 10.1016/j.jhep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Helle SI. The insulin-like growth factor system in advanced breast cancer. Best Pract Res Clin Endocrinol Metab. 2004;18:67–79. doi: 10.1016/s1521-690x(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Wimalasena J, Meehan D, Dostal R, Foster JS, Cameron M, Smith M. Growth factors interact with estradiol and gonadotropins in the regulation of ovarian cancer cell growth and growth factor receptors. Oncol Res. 1993;5:325–337. [PubMed] [Google Scholar]
- Hata H, Hamano M, Watanabe J, Kuramoto H. Role of estrogen and estrogen-related growth factor in the mechanism of hormone dependency of endometrial carcinoma cells. Oncology. 1998;55(Suppl 2):35–44. doi: 10.1159/000055257. [DOI] [PubMed] [Google Scholar]
- Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Buschenfelde KH. Biliary adenocarcinoma. Characterization of three new human tumor cell lines. J Hepatol. 1985;1:579–596. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- Kusaka Y, Tokiwa T, Sato J. Establishment and characterization of a cell line from a human cholangiocellular carcinoma. Res Exp Med (Berl) 1988;188:367–375. doi: 10.1007/BF01851205. [DOI] [PubMed] [Google Scholar]
- Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T, Fukuhara K, Suzuki T, Matsuno S. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30:1128–1133. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- Kanno N, LeSage G, Phinizy JL, Glaser SS, Francis H, Alpini G. Stimulation of α2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology. 2002;35:1329–1340. doi: 10.1053/jhep.2002.33330. [DOI] [PubMed] [Google Scholar]
- Inoue S, Hoshino S, Miyoshi H, Akishita M, Hosoi T, Orimo H, Ouchi Y. Identification of a novel isoform of estrogen receptor, a potential inhibitor of estrogen action, in vascular smooth muscle cells. Biochem Biophys Res Com. 1996;219:766–772. doi: 10.1006/bbrc.1996.0308. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser S, Robertson W, Phinizy JL, Rodgers R, Caligiuri A, LeSage G. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol. 1997;273:G518–G529. doi: 10.1152/ajpgi.1997.273.2.G518. [DOI] [PubMed] [Google Scholar]
- Muller M, Dietel M, Turzynski A, Wiechen K. Antisense phosphorothioate oligodeoxynucleotide down-regulation of the insulin-like growth factor 1 receptor in ovarian cancer cells. Int J Cancer. 1998;77:567–571. doi: 10.1002/(sici)1097-0215(19980812)77:4<567::aid-ijc16>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Inoue S. Estrogen receptors and their downstream targets in cancer. Arch Histol Cytol. 2004;67:435–442. doi: 10.1679/aohc.67.435. [DOI] [PubMed] [Google Scholar]
- Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537–551. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel P. Tissue-specific estrogenic response and molecular mechanisms. Toxicol Lett. 2002;28:217–224. doi: 10.1016/s0378-4274(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–3182. [PubMed] [Google Scholar]
- Bardin A, Hoffmann P, Boulle N, Katsaros D, Vignon F, Pujol P, Lazennec G. Involvement of estrogen receptor beta in ovarian carcinogenesis. Cancer Res. 2004;64:5861–5869. doi: 10.1158/0008-5472.CAN-04-0552. [DOI] [PubMed] [Google Scholar]
- Girault I, Andrieu C, Tozlu S, Spyratos F, Bieche I, Lidereau R. Altered expression pattern of alternatively spliced estrogen receptor beta transcripts in breast carcinoma. Cancer Lett. 2004;215:101–112. doi: 10.1016/j.canlet.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sampson LK, Vickers SM, Ying W, Phillips JO. Tamoxifen-mediated growth inhibition of human cholangiocarcinoma. Cancer Res. 1997;57:1743–1749. [PubMed] [Google Scholar]
- Nash JW, Morrison C, Frankel WL. The utility of estrogen receptor and progesterone receptor immunohistochemistry in the distinction of metastatic breast carcinoma from other tumors in the liver. Arch Pathol Lab Med. 2003;127:1591–1595. doi: 10.5858/2003-127-1591-TUOERA. [DOI] [PubMed] [Google Scholar]
- Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- Scharf JG, Braulke T. The role of the IGF axis in hepatocarcinogenesis. Horm Metab Res. 2003;35:685–693. doi: 10.1055/s-2004-814151. [DOI] [PubMed] [Google Scholar]
- Diel P, Smolnikar K, Michna H. The pure antiestrogen ICI 182780 is more effective in the induction of apoptosis and down regulation of BCL-2 than tamoxifen in MCF-7 cells. Breast Cancer Res Treat. 1999;58:87–97. doi: 10.1023/a:1006338123126. [DOI] [PubMed] [Google Scholar]
- Surmacz E. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin-like growth factor I receptor. Oncogene. 2003;2:6589–6597. doi: 10.1038/sj.onc.1206772. [DOI] [PubMed] [Google Scholar]
- del Rio B, Garcia Pedrero JM, Martinez-Campa C, Zuazua P, Lazo PS, Ramos S. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. J Biol Chem. 2004;279:38294–38302. doi: 10.1074/jbc.M403140200. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Statti G, Vivacqua A, Gabriele S, Rago V, Loizzo M, Menichini F, Amdo S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J Steroid Biochem Mol Biol. 2002;82:315–322. doi: 10.1016/s0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- Stoica A, Saceda M, Fakhro A, Joyner M, Martin MB. Role of insulin-like growth factor-I in regulation estrogen receptor-α gene expression. J Cell Biochem. 2000;76:605–614. doi: 10.1002/(sici)1097-4644(20000315)76:4<605::aid-jcb9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- Shi R, Yu H, McLarty J, Glass J. IGF-I and breast cancer: a meta-analysis. Int J Cancer. 2004;111:418–423. doi: 10.1002/ijc.20233. [DOI] [PubMed] [Google Scholar]
- Lin Y, Tamakoshi A, Kikuchi S, Yagyu K, Obata Y, Ishibashi T, Kawamura T, Inaba Y, Kurosawa M, Motohashi Y, Ohno Y. Serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and the risk of pancreatic cancer death. Int J Cancer. 2004;110:584–588. doi: 10.1002/ijc.20147. [DOI] [PubMed] [Google Scholar]
- Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R. Estrogen receptor α rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;75:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- Adesanya OO, Zhou J, Samathanam C, Powell-Braxton L, Bondy CA. Insulin-like growth factor 1 is required for G2 progression in the estradiol-induced mitotic cycle. Proc Natl Acad Sci USA. 1999;96:3287–3291. doi: 10.1073/pnas.96.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen RL, Shaw LE, Larsen C, Corcoran D, Darbre PD. Insulin-like growth factor receptor levels are regulated by cell density and by long term estrogen deprivation in MCF7 human breast cancer cells. J Biol Chem. 2001;276:40080–40086. doi: 10.1074/jbc.M105892200. [DOI] [PubMed] [Google Scholar]
- Zhou J, Anderson K, Bievre M, Ng S, Bondy CA. Primate mammary gland insulin-like growth factor system: cellular localization and regulation by sex steroids. J Invest Med. 2001;49:47–55. doi: 10.2310/6650.2001.34090. [DOI] [PubMed] [Google Scholar]
- Kassem M, Okazaki R, Harris SA, Spelsberg TC, Conover CA, Riggs BL. Estrogen effects on insulin-like growth factor gene expression in a human osteoblastic cell line with high levels of estrogen receptor. Calcif Tissue Int. 1998;62:60–66. doi: 10.1007/s002239900395. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Rev. 2001;37:320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- Shelton JG, Steelman LS, White ER, McCubrey JA. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle. 2004;3:372–379. [PubMed] [Google Scholar]