Abstract
Experiments with amyloid-β (Aβ)-42-immunized transgenic mouse models of Alzheimer’s disease have revealed amyloid plaque disruption and apparent cognitive function recovery. Neuropathological examination of patients vaccinated against purified Aβ-42 (AN-1792) has demonstrated that senile plaque disruption occurred in immunized humans as well. Here, we examined tissue histology and quantified and biochemically characterized the remnant amyloid peptides in the gray and white matter and leptomeningeal/cortical vessels of two AN-1792-vaccinated patients, one of whom developed meningoencephalitis. Compact core and diffuse amyloid deposits in both vaccinated individuals were focally absent in some regions. Although parenchymal amyloid was focally disaggregated, vascular deposits were relatively preserved or even increased. Immunoassay revealed that total soluble amyloid levels were sharply elevated in vaccinated patient gray and white matter compared with Alzheimer’s disease cases. Our experiments suggest that although immunization disrupted amyloid deposits, vascular capture prevented large-scale egress of Aβ peptides. Trapped, solubilized amyloid peptides may ultimately have cascading toxic effects on cerebrovascular, gray and white matter tissues. Anti-amyloid immunization may be most effective not as therapeutic or mitigating measures but as a prophylactic measure when Aβ deposition is still minimal. This may allow Aβ mobilization under conditions in which drainage and degradation of these toxic peptides is efficient.
Sporadic Alzheimer’s disease (AD) affects the aged with a prevalence approaching 40 to 50% by age 80. At present, 4 million Americans are affected with AD at an estimated annual care cost of almost 100 billion dollars. Because the number of individuals 65 years or older is growing rapidly due to a general average life expectancy increase, it is estimated that the total incidence of AD will quadruple by the year 2050.1 Therefore, it is urgent to find a means of preventing, delaying the onset, or reversing the course of AD.
Alzheimer’s disease is characterized by the massive accumulation of extracellular amyloid fibrils in both the brain parenchyma and in the walls of cerebral blood vessels. The deposited fibrillar amyloid is mainly composed of amyloid-β (Aβ) peptides, 40/42 amino acid-residue molecules derived by proteolytic processing of larger amyloid precursor proteins (APPs) by the concerted actions of the β- and γ-secretases. The relevance of Aβ peptides to sporadic AD pathogenesis is strongly supported by the fact that mutations in the APP and presenilin genes both result in early-onset familial AD. Moreover, a formally similar suite of pathophysiological and cognitive changes is observed in multiple strains of transgenic (Tg) mice that overexpress APP and/or other APP processing genes. The fibrillar and soluble forms of Aβ interfere with the normal brain architecture and function, resulting in profound neuroinflammation, gliosis, severe neuronal injury, and vascular damage and in the induction of neurofibrillary tangle (NFT) and protracted dementia development. The clearly preeminent role of Aβ in AD provides strong experimental support to the amyloid cascade hypothesis as a mechanism central to AD pathogenesis.
Among the multiple remedial avenues so far explored, immunotherapy promises to be one of the most effective interventions. Several single (APP) and double Tg (APP/presenilin) mouse strains have been generated that produce amyloid structures similar to those observed in AD. Active and passive anti-Aβ immunization therapies were tested in Tg animals and resulted in amyloid deposit disaggregation and the reversal of cognitive deficits.2–4 Immunotherapy has also been successful in reducing amyloid levels in the brains of aging Caribbean Vervet monkeys.5
Encouraged by the impressive results observed in animal models, active Aβ vaccination clinical trials were initiated in humans. Three hundred individuals were vaccinated with the AN-1792/QS-21 antigen/adjuvant complex, and 72 subjects received placebo treatment. Of the 300 vaccinated subjects, 18 (6%) developed aseptic meningoencephalitis, whereas no placebo group subjects developed this condition during the same time frame. In the immunized cohort, a total of 59 individuals had desirable plasma antibody titers ≥1:2200. Thirteen patients from this vaccine-responsive subgroup developed meningoencephalitis (22%), whereas only 5 (2%) of a total pool of 241 “nonresponders” evidenced this adverse outcome. No significant differences were observed between vaccinated and placebo-treated subjects with respect to a battery of individual psychometric tests, although neuropsychological test battery z-scores demonstrated differences favoring the antibody responders. In addition, significant cerebrospinal fluid (CSF)-tau decreases were evident in the antibody-responsive patient group.6,7 Intriguingly, 45 of the high anti-amyloid antibody titer responding individuals had, as measured by magnetic resonance imaging, a greater brain volume reduction with an enhanced ventricular enlargement for which there is presently no certain explanation.8 It has been suggested that this reduction may be attributed to amyloid deposit removal. It is also possible that the reduction of brain volume is due to hydrodynamic changes in CSF and brain interstitial fluid. In addition, cognitive functions showed a slower decline in 20 AD patients who generated acceptable levels of antibodies after receiving primer and a booster of aggregated Aβ.9 There is also evidence that the administration of intravenous immunoglobulins, a complex mixture of IgG that contains antibodies against Aβ, results in an amelioration of dementia symptoms in AD patients, supporting the tenet that amyloid deposits are subject to immunological therapy.10,11 However, larger scale longitudinal studies with complete neuropathological analyses are required to rigorously confirm the results of the pilot studies.
The initial AD patient vaccination study was terminated because of an unanticipated adverse event: the emergence of vaccination-associated meningoencephalitis. So far, there are three published complete neuropathological reports concerning AN-1792/QS-21-immunized individuals.12–14 Two of these reports describe subjects who developed meningoencephalitis, whereas the third concerns a vaccinated subject who died without meningoencephalitis development. We isolated, characterized, and compared the amyloid species present in the gray matter (GM) and white matter (WM) as well as cortical and leptomeningeal vessels of two AN-1792/QS-21-vaccinated AD patients, one of whom developed meningoencephalitis. Our analyses reveal that parenchymal amyloid plaque deposits regressed to a remarkable extent in both patients, suggesting that simple differences in ultimate antibody titers or activity alone do not account for the adverse outcome of meningoencephalitis in some patients. A full understanding of the direct and emergent effects of amyloid vaccination on the AD patient brain necessitates a larger sample of the AN-1792 experimental cohort undergoing comprehensive biochemical assessments and comparisons.
Materials and Methods
Human Subjects
Case 1
Male, age 76, with mild to moderate cognitive impairment [mini mental state examination (MMSE) = 18], received two intramuscular doses of 225 μg of AN-1792. Nine months after the last immunization, the patient developed cerebral edema and neurological symptoms of meningoencephalitis. Magnetic resonance imaging revealed lesions in the WM. Two months later, the patient evidenced serious global cognitive deterioration and a greatly decreased MMSE score of 7. On neuropathological examination, the brain weight was 1130 g. There was softening of WM, signs of demyelination, enlarged ventricles, and multiple hemorrhages in the GM, uncommon in AD (the patient did not have a history of cardiovascular disease). Amyloid plaques were distributed nonuniformly and were mostly of the diffuse type with a lesser amount of compact amyloid core plaques presenting multinucleated cells loaded with dense aggregates of Aβ. Many apparently disaggregated senile plaques with amyloid deposits were observed in this patient. These apparent senile plaque debris structures, referred to as “collapsed plaques” by Ferrer et al,13 consisted of extracellular plaques depleted of amyloid. These structures probably represent the complex nonamyloid glycoproteins and glycolipids that account for 30% of the AD plaque composition. In addition, severe cerebrovascular amyloidosis, abundant NFT deposits, marked inflammatory T-lymphocyte infiltration and severe small vessel disease (lipohyalinosis) were present. The apolipoprotein E (Apo E) genotype for this patient was ε3/ε4. A comprehensive account of the neuropathological changes and detailed immunocytochemical profile of this patient are given by Ferrer et al.13
Case 2
Male, age 71, with moderate to severe cognitive impairment (MMSE = 12). This individual received three intramuscular doses of 225 μg each of AN-1792 and died 1 year after the first immunization. The cause of death was recorded as “failure to thrive.” The brain weighed 1100 g and exhibited a normal macroscopic appearance. White matter and ventricles looked normal, and frontal lobes were almost free of extracellular amyloid deposits and neuritic plaques. Aβ immunoassay, performed by Masliah et al,14 demonstrated low cortical Aβ levels. Aggregates of Aβ were observed within microglial/macrophage cells, and there was severe cerebrovascular amyloidosis in the frontal lobe but less apparent in the parietal and temporal lobes. In addition, there were abundant NFTs distributed in the cerebral cortex.14 The Apo E genotype of this individual was ε3/ε4.
Biochemical Analyses
The biochemical analyses of Aβ peptides were performed on GM from temporal lobe including the entorhinal cortex (case 1) and on parietal and temporal cortex (case 2). The postmortem delay time before freezing of the tissue was approximately 4 hours for case 1 and 12 hours for case 2. Brain sections were subjected to Campbell-Switzer silver stain15 and 1.0% aqueous thioflavine-S for 10 minutes. To assess the distribution and relative number of senile and diffuse plaques in the cerebral cortex, brain sections were immunocytochemically stained. This step also permitted the estimation of the amount of tissue required to perform subsequent biochemical experiments. Briefly, brain sections were deparaffinized and treated with 80% formic acid for 20 minutes. Sections were incubated overnight with anti-Aβ antibodies 6E10 and 4G8 diluted 1:1000 (Signet Laboratories, Dedham, MA). Anti-mouse IgG was used as a secondary antibody (1:10,000), and then developed with diaminobenzidine in 0.5% nickel ammonium sulfate. Hematoxylin was used as a counterstain.
Water-Soluble Amyloid
The water-soluble material represents soluble interstitial fluid Aβ, Aβ monomers and low molecular weight oligomers loosely attached to amyloid plaques, and soluble Aβ free in the cytosol.16 The leptomeninges covering the cerebral cortex were separated and stored at −80°C. Twenty grams of cerebral cortex was finely minced with a razor blade, suspended in 150 ml of ammonium bicarbonate buffer (0.1 mol/L NH4HCO3, 5 mmol/L ethylenediamine tetraacetic acid, and 0.01% sodium azide), with three tablets of complete enzyme inhibitors (Roche, Mannheim, Germany), and allowed to stand at 4°C for 48 hours. The brain cortex was homogenized with a 40-ml capacity Tenbroeck glass homogenizer (six manual gentle strokes) and centrifuged at 150,000 × g for 3 hours (Beckman SW28 rotor) in a Beckman Optima LE-80K ultracentrifuge (Beckman, Fullerton, CA). The water-soluble fraction was lyophilized and analyzed by fast protein liquid chromatography (FPLC) (Amersham Biosciences, Piscataway, NJ) and high performance liquid chromatography (HPLC) (Thermo Separation Products, Schaumburg, IL).
Sodium Dodecyl Sulfate (SDS)-Insoluble Amyloid
The water-insoluble pellets were dispersed and stirred for 12 hours in 50 ml of 0.1 mol/L NH4HCO3. Fifty milliliters of 30% SDS was added (final SDS concentration 15%) and stirred for an additional 14 hours at room temperature. To remove the SDS-insoluble tufts of parenchymal blood vessels, the suspension was filtered through a series of 300-, 45-, and 25-μm nylon meshes. The SDS-containing filtrate was centrifuged at 300,000 × g for 5 hours at 20°C in an SW41 Ti Beckman rotor. The resulting pellets were suspended in 60 ml of 0.1 mol/L ammonium bicarbonate, pH 10.0 (titrated with NH4OH), and after 24 hours of continuous stirring, centrifuged at 300,000 × g for 5 hours at 20°C. The pellets were stored, and the supernatant was lyophilized and analyzed by FPLC and HPLC.
Leptomeningeal Vascular Amyloid
The leptomeninges were washed 10 times with 500 ml of cold water to remove blood cells by hypotonic shock. Vessels larger than 600 μm in diameter were eliminated, and the smaller vessels were finely minced and washed three times (5 minutes at 1500 × g) with 0.1 mol/L Tris-HCl and 3 mmol/L CaCl2, pH 8.0. Vessels were suspended in the wash buffer amended with 0.3 mg/ml Clostridium histolyticum collagenase CL3 (Worthington Biochemicals Corp., Lakewood, NJ), and 10 μg/ml DNase I (Sigma-Aldrich, St. Louis, MO) was added to create a final suspension of 100 ml, which was incubated in a shaker at 37°C for 3 hours. The preparation was then filtered through a 150-μm nylon mesh, and the filtrate was centrifuged at 3000 × g for 10 minutes. The supernatant was eliminated, and the pellet was washed with water, suspended in water, and centrifuged at 3000 × g. The pellet, containing sheets of leptomeningeal vascular amyloid, was dissolved in 4 ml of glass distilled formic acid (GDFA) with the aid of a glass homogenizer, left at room temperature for 30 minutes, and centrifuged at 500,000 × g for 10 minutes in a TL 100 rotor (Beckman Optima TLX ultracentrifuge). The supernatant was lyophilized and analyzed by FPLC and HPLC.
Cortico-Vascular Amyloid
The 300-, 45-, and 25-μm nylon mesh-retained vessel tufts from the cortical SDS-insoluble amyloid preparation were immediately shaken in 500 ml of distilled water to suspend the retained vessels. The material was allowed to settle, and the clarified liquid was decanted with the remaining volume centrifuged at 1500 × g for 10 minutes. The pellets containing the cortical blood vessels were washed twice with distilled water to remove remaining SDS and were suspended in 0.1 mol/L Tris-HCl and 3 mmol/L CaCl2, pH 8.0. Next, 0.3 mg/ml collagenase CL3 (Worthington) and 10 μg/ml DNase I (Sigma) were added, and samples were incubated in a shaker at 37°C for 3 hours. The preparation was filtered through a 40-μm nylon mesh, and the filtrate fraction was centrifuged at 3000 × g. The pellet was treated with 4 ml of GDFA, spun at 500,000 × g as described above, and analyzed by FPLC and HPLC.
Diffuse Amyloid (P3)
This fraction represents the most insoluble of the amyloid aggregates and is composed primarily of Aβ 17–42 peptides recovered after high-speed centrifugation of the SDS/alkaline insoluble pellets. This material was dissolved in 80% GDFA with the aid of a glass homogenizer, allowed to stand at room temperature for 1 hour, parceled into 0.7-ml aliquots in thick-walled polyallomer tubes, and centrifuged at 500,000 × g for 15 minutes in a Beckman Optima TLA-ultracentrifuge using a 120.2 rotor. The dark brown pellets containing formic acid-insoluble material mostly composed of lipofuscin remnants were discarded, and the clear supernatant was submitted to FPLC-GDFA chromatography. Column eluate samples containing amyloid fraction P3 were pooled, reduced in volume by Speed-Vac centrifugation, and subjected to size-exclusion HPLC chromatography on a GPC-100 column, equilibrated, and developed with 80% GDFA. The GPC-100 column fraction containing the P3 was refined further by chromatography on a reverse-phase Zorbax SB-C8 column as described below.
White Matter Aβ
The WM was separated from the adjacent GM to avoid contamination from cortical amyloid. The WM (3 g) was finely minced and thoroughly homogenized in 18 ml of 80% GDFA. The homogenate was centrifuged at 250,000 × g for 1 hour using 11-ml polyallomer tubes in a Beckman SW41 rotor. The fatty material collected at the top of the tube and the minute bottom pellets were discarded. The transparent supernatant was brought to 20 ml by the addition of GDFA and centrifuged under the same conditions. The clear supernatant was submitted to FPLC, and further purification of Aβ peptides was achieved by reverse-phase chromatography on a C8 column Zorbax RX-C8 (Mac-Mod, Chadds Ford, PA) (see below).
Separation of Amyloid Peptides by FPLC
The lyophilized water-soluble fraction, the SDS-insoluble fraction, leptomeningeal-vascular amyloid and cortico-vascular Aβ, the P3 fraction, and the WM Aβ were all independently submitted to size-exclusion chromatography using a Superose 12 column (1 × 30 cm; General Electric, Uppsala, Sweden) equilibrated with 80% GDFA. In the case of the water-soluble and SDS-insoluble fractions, aliquots of 40 mg each of the lyophilized material were dissolved in 500 μl of 80% GDFA and chromatographed at 15 ml/h with column output monitored by UV absorbance at 280 nm. The 2- to 8-kd fraction was collected, acid was removed, and volume was reduced to 50 μl by Speed-Vac centrifugation and stored in a ultra-low freezer (−80°C). In some instances to further refine the separation of the Aβ peptides from higher and lower molecular mass flanking contaminants, the FPLC Superose 12 chromatographic step was repeated twice.
HPLC Purification of Aβ Peptides
The FPLC fractions containing 2- to 8-kd molecules were further purified by reverse-phase HPLC. Five FPLC runs were pooled and concentrated to 50 μl, 200 μl of 90% GDFA was added, and the final volume was adjusted to 500 μl with distilled water. The specimens were loaded onto a reverse-phase Zorbax SB-C8 column (4.6 × 250 mm; Mac-Mod) equilibrated with 20% acetonitrile/water and 0.1% trifluoroacetic acid (TFA). Separations were performed using a linear (20 to 40%) acetonitrile gradient developed for a period of 60 minutes at 80°C at 1 ml/minute flow rate. The column eluate was monitored at 214-nm UV absorbance. Alternatively, the Aβ peptides were further purified in a semipreparative reverse-phase column Zorbax RX-C8 (9.4 × 250 mm; Mac-Mod). The chromatography was developed with a linear gradient formed by 20% acetonitrile/water, 0.1% TFA and 60% acetonitrile/water, 0.1% TFA, at 1.5 ml/minute at room temperature. The chromatography was monitored at 214 nm. The FPLC-enriched diffuse plaque Aβ-related peptides were purified by size-exclusion chromatography on a silica-based Syncropack GPC-100 column (5-μm particle; 100-Å pore; 500 × 7.8 mm; Synchrom, Inc., Lafayette, IN), equilibrated, and developed at room temperature with 80% GDFA at a flow rate of 0.5 ml/minute. The chromatography was monitored at 280 nm. All HPLC columns were calibrated to establish the retention times for Aβ tetramer, Aβ trimer, Aβ dimer, Aβ monomer, and Aβ-P3 (residues 17–42 species). The Aβ monomers were synthesized by California Peptide Research, Inc. (Napa, CA) and further purified in our laboratory by size-exclusion FPLC (Superdex 75) and reverse-phase HPLC (C8). The Aβ oligomers were generated by dialyzing synthetic monomer Aβ 1–42, dissolved in 80% GDFA (3 mg/ml SpectraPor 6, 1000 d cut-off) against 4 L of 50 mmol/L Tris-HCl buffer, pH 7.5, followed by incubation at 37°C for a period of 8 weeks. Before the opening of the dialysis bag, the Aβ was dialyzed for 24 hours against 0.1 mol/L ammonium bicarbonate followed by lyophilization.17
Automatic Peptide Sequencing
The HPLC-purified Aβ peptides were dissolved in 50 μl of 50% (v/v) acetonitrile and 0.1% TFA. Approximately 30 μl of the sample was dried on a fiberglass disk (Beckman Coulter, Fullerton, CA). Sequencing was performed on a Porton 2090E gas-phase protein sequencer (Beckman Coulter) attached to an on-line Hewlett-Packard 1090L HPLC.
Aβ 40 and 42 Enzyme-Linked Immunosorbent Assays (ELISAs)
All preparations were performed at 4°C. One hundred milligrams of tissue was homogenized in 800 μl of 5 mol/L guanidine HCl and 50 mmol/L Tris-HCl, pH 8.0, with an electric grinder. The homogenates were shaken for 3 hours and then centrifuged in a 50.4-Ti rotor (Beckman) for 30 minutes at 60,000 × g. The supernatants were collected and stored at −80°C. Tissue extractions were also performed in 50 mmol/L Tris-HCl, pH 8.0, following the above protocol. The ELISA Aβ 40 (Immuno-Biological Laboratories, Minneapolis, MN) and Aβ 42 (Innogenetics, Belgium, Germany) kits were used according to the manufacturers’ instructions.
Mass Spectrometry of Aβ Peptides
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) was performed as described in detail by Esh et al.18 The complete mass spectrometry data are presented as Supplemental Tables 1 to 4 (http://ajp.amjpathol.org).
Western Blot Analysis
A detailed description of the methods used is provided by Esh et al.18 Briefly, formic acid or acetonitrile was removed by drying with vacuum centrifugation and replaced with 200 μl of distilled water. This procedure was repeated three times. The pellets were then suspended in NuPage LDS sample buffer (Invitrogen, Carlsbad, CA) containing 50 mmol/L dithiothreitol, heated for 10 minutes at 80°C, and loaded onto 4 to 12% Bis-Tris gels (Invitrogen) with NuPage MES running buffer. Proteins were transferred onto nitrocellulose membranes (Invitrogen), and the membranes were blocked in 5% nonfat dry milk in phosphate-buffered saline and 0.5% (v/v) Tween 20 (Fluka, St. Louis, MO). Aβ peptides were detected with anti-Aβ40 and anti-Aβ42 (Biosource, Camarillo, CA) as primary antibodies and goat anti-rabbit horseradish peroxidase conjugated as a secondary antibody (Pierce, Rockford, IL). The blots were developed with SuperSignal West Pico chemiluminescent substrate and CL-Xpose film (Pierce) and Kodak GBX developer (Sigma). The films were scanned with a GS-800 calibrated densitometer (Bio-Rad, Hercules, CA) and analyzed with Quantity One software (Bio-Rad).
Results
The histopathological studies, immunoassays, chemical extractions, and FPLC and HPLC purification procedures were conducted on the brain tissue of case 1 and case 2. However, because of limitations in time and resources and because of the remarkable similarities in chromatographic profiles, mass spectrometry analyses were performed on case 1 only.
Morphological Features
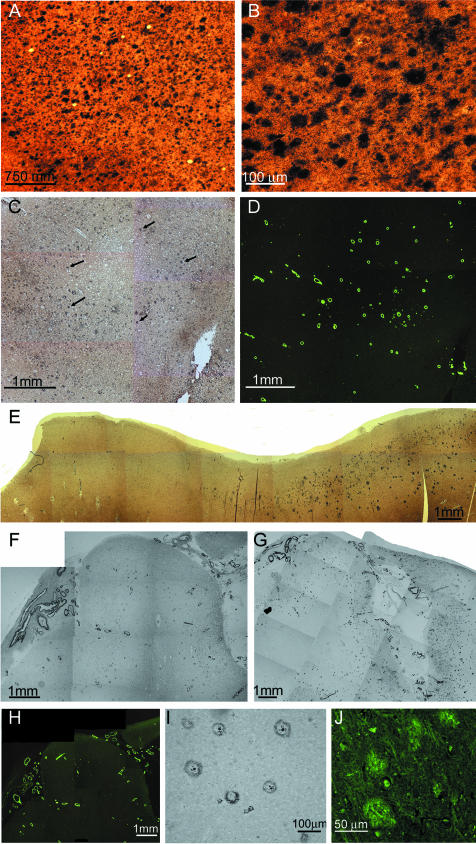
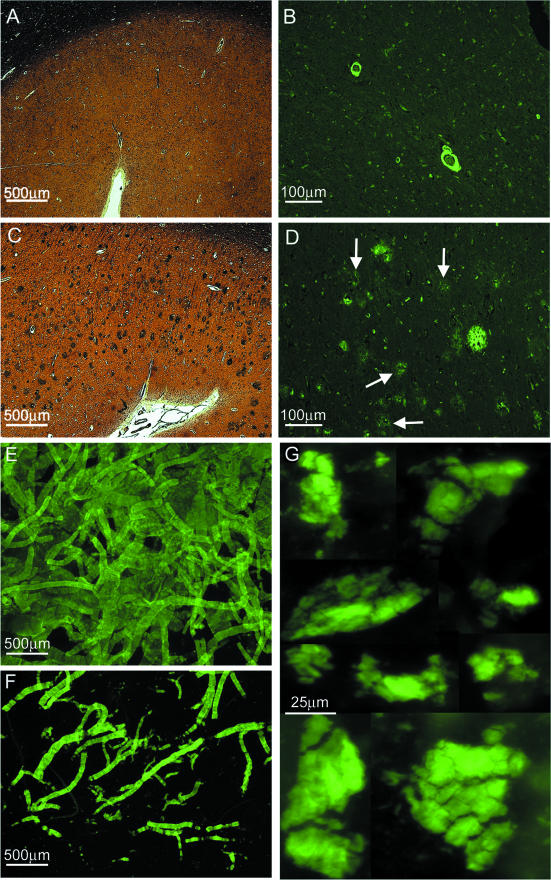
It is evident that the amyloid deposits in the cerebral cortex of AN-1792-vaccinated human cases focally disappeared, suggesting that polyclonal anti-Aβ antibodies crossed the blood-brain barrier. These antibodies were induced using synthetic fibrillar Aβ 1–42 as an antigen, and the resulting antibodies apparently disassembled fibrillar amyloid plaques. In case 1, there were patchy areas in which the diffuse and compact amyloid deposits were totally absent, although abundant accumulations of amyloid were present in the cortical vessels (Figure 1, C, D, E, and H). In this study, the term “diffuse amyloid deposits” describes plaques that are a conglomerate of nonfibrillar Aβ peptides organized into amorphous aggregates. They stain faintly with thioflavine-S (Figure 1J) or thioflavine-T, are negative for Congo red birefringence, and are very visible and stain intensely with silver dyes such as Campbell-Switzer (Figure 1, A and B). In other areas of the cortex, there were focally decreased densities of compact core plaques as revealed by histochemical stains and the 6E10 antibody probing, with remaining diffuse amyloid deposits clearly demonstrated by the 4G8 antibody (Figure 1, F and G), respectively. NFT density remained high in regions with focally depleted plaques. Earlier neuropathological reports of NFTs in AN-1792 vaccinated cases13,14 found no apparent changes in their abundance and distribution. Previous biochemical studies have revealed that the diffuse deposits of Aβ in sporadic AD are mainly composed of a mixture of Aβ 17–42 and Aβ 1–42.19 In some areas, the remaining amyloid plaques exhibited a peculiar morphology, consisting of a clearly defined halo of amyloid-positive material and diminutive centrally situated punctiform core. Between these two structures, there was a clear area devoid of Aβ immunoreactivity (Figure 1I). Concerning case 2, histological sections of the frontal lobe stained with Campbell-Switzer and thioflavine-S revealed a complete absence of amyloid core plaques and diffuse plaques (Figure 2, A and B) in agreement with previously published data.14 However, although the parietal and temporal cortex demonstrated sparse core amyloid plaques, diffuse plaques were abundant (Figure 2, C and D). Histological sections of case 1 showed an extensive deposition of Aβ in the cortical vessels and in the leptomeningeal vasculature (Figure 1, D and H; Figure 2, E and F). However, they were less pronounced in case 2.
Figure 1.
Brain tissue sections showing cortical areas with and without amyloid deposits. A: Section of frontal cortex from a nonvaccinated sporadic AD patient showing numerous plaques. Campbell-Switzer stain (×25). B: Campbell-Switzer silver-stained area of the frontal cortex of the same patient shown in A at higher magnification (×100). C: Section of the frontal cortex from case 1, stained by Campbell-Switzer stain demonstrating moderate number of diffuse amyloid deposits. Arrows point to examples of diffuse deposits (×25). D: The same area as in C stained by thioflavine-S showing an abundant number of vascular amyloid deposits (×25). E: A photomontage of the temporal cortex from case 1 demonstrating the transition between an area with no amyloid deposits (left) and an area with numerous diffuse plaques (right). Campbell-Switzer silver stain (×25). F: A photomontage of the superior frontal gyrus from case 1 stained by the 6E10 monoclonal antibody against Aβ residues 1 to 16. A very low number of amyloid core plaques are observed, whereas deposits of leptomeningeal- and cortico-vascular amyloid are abundant (×25). G: The same region as in F but stained with the monoclonal antibody 4G8 against Aβ residues 17 to 24, revealing numerous diffuse plaques, represented as fine powdery material, and vascular amyloid deposits (×25). H: The same area as in F and G, stained with thioflavine-S illustrating numerous vascular deposits of amyloid in both leptomeningeal and cortical vessels (×25). I: A temporal lobe section from case 1 stained with the 6E10 antibody showing a group of amyloid plaques. Four of these plaques contain a small centrally situated core of amyloid with a peripheral halo of immunoreactive material (×100). J: Diffuse deposits of Aβ in the parietal lobe from case 1 stained with thioflavine-S. These deposits were enhanced to delineate their boundaries and increase the contrast against the neuropil background (×200).
Figure 2.
Brain tissue sections from case 2 and whole montage preparations of isolated blood vessels and of purified P3 pellets from case 1. A: A gyrus of the frontal cortex stained by Campbell-Switzer showing a total absence of amyloid plaques (×25). B: The same area as in A, revealing vascular amyloid deposits stained by thioflavine-S as well as numerous neurofibrillary tangle-bearing neurons (×100). C: A section of the parietal cortex demonstrating amyloid cores and diffuse plaques stained by Campbell-Switzer (×25). D: The same section as in C, showing amyloid core and diffuse plaques stained by thioflavine-S. This fluorochrome stains the diffuse amyloid deposits (arrows) very faintly (×100). E: Whole mounted isolated leptomeningeal blood vessels stained with thioflavine-S (×25). F: Whole mounted isolated cortical vessels showing a heavy burden of vascular amyloid (×25). On a semiquantitative basis, there was a severe vascular amyloid load (3) in both cases under study, being more severe in case 1. G: Whole mount preparation depicting the amorphous flocks of water/SDS insoluble nonfibrillar P3 recovered by high-speed ultracentrifugation. The high g forces compacted the otherwise Aβ diffuse aggregates into a mass of thioflavine-S-positive material (×400).
Immunoassay Quantification of Aβ Peptides
Before the general analytical preparations to separate the soluble and insoluble fractions, the amount of Aβ peptides present in the GM and WM of case 1 and case 2 and those in sporadic AD and age-matched non-demented (ND) subjects were quantified by ELISA (Table 1). Homogenization of the brain tissue with 5 mol/L guanidine hydrochloride (GHCl) yielded 868 and 186 μg/g total Aβ for cases 1 and 2, respectively. In comparison, the average amount of Aβ for neuropathologically confirmed AD cases (n = 31) was 406 μg/g tissue and for age-matched ND controls (n = 22) was 221 μg/g tissue. Interestingly, the water-soluble Aβ obtained after a gentle homogenization of finely minced cerebral cortex in aqueous buffer was 61 μg/g for case 1 and 13.5 μg/g for case 2. These values were in sharp contrast with those obtained from AD (n = 7) and ND (n = 4), which both amounted to 3.9 μg/g tissue. In both vaccinated cases, the levels of Aβ in the WM, extracted by 5 mol/L GHCl, were 96.8 μg/g for case 1 and 16.9 μg/g for case 2. In the GM and WM of case 1, Aβ n-40 and n-42 were equally represented in the GHCl extract. In case 2, the Aβ n-40-to-n-42 ratio differed, being 2.8:1 in the GM and 1.8:1 in the WM. Most of the water-soluble Aβ corresponded to the n-40 isoform in both of the cases.
Table 1.
Aβ Quantification by ELISA
| Aβ 40 (μg/g wet weight) | Aβ 42 (μg/g wet weight) | Total Aβ | |
|---|---|---|---|
| Case 1 GM (GHCl) | 428.7 | 439.6 | 868.3 |
| Case 1 GM (Tris) | 58.4 | 2.7 | 61.1 |
| Case 1 WM (GHCl) | 44.3 | 52.5 | 96.8 |
| Case 2 GM (GHCl) | 137.0 | 48.8 | 185.8 |
| Case 2 GM (Tris) | 13.5 | 0.00 | 13.5 |
| Case 2 WM (GHCl) | 11.0 | 5.9 | 16.9 |
| 31 AD GM (GHCl) | 144.0 (32.3) | 262.1 (18.1) | 406.1 (41.4) |
| 7 AD GM (Tris) | 3.3 (0.8) | 0.6 (0.18) | 3.9 (0.9) |
| 22 ND GM (GHCl) | 16.3 (3.9) | 204.3 (34.9) | 220.6 (37.1) |
| 4 ND* GM (Tris) | 1.5 | 2.4 | 3.9 |
GHCl, homogenized in 5 mol/L guanidine HCl buffer; Tris, homogenized in Tris buffer.
Values represent a blend of four samples; SEM given in parentheses.
Leptomeningeal and Cortico-Vascular Aβ Peptides
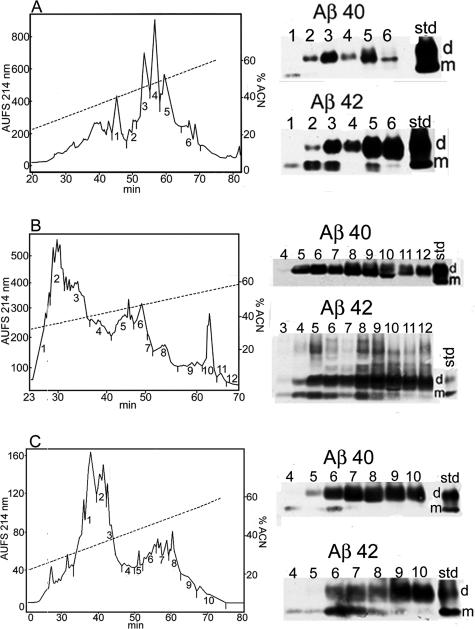
We developed techniques that permitted the independent separation and purification of the Aβ peptides present in the walls of leptomeningeal vessels and the cortico-vascular vessels. This result was achieved by the combined use of collagenase digestion of the extracellular matrix and GDFA extraction/solubilization of Aβ. Both tissue fractions were independently submitted to FPLC for Aβ enrichment (Figure 3, A and B). On subsequent HPLC, the leptomeningeal amyloid produced three amyloid-containing fractions, whereas the parenchymo-vascular amyloid produced seven fractions as depicted in Figure 4, A and B. Because we obtained a limited amount of purified vascular Aβ, a decision was made to investigate these fractions by mass spectrometry to maximize the amount of information at the peptide structural level. A large number of peptides were detected by MALDI-TOF. In the case of the leptomeningeal vessels, 21 Aβ potential peptides were observed that started at positions 1 through 5 and 7 through 10 and ending at positions 37 through 42 (Supplemental Table 1). In the case of the cortico-vascular vessels, a total of 26 likely Aβ-related peptides were identified with N termini at positions 1 through 5 and 7 to 9 and 11 and with C termini at positions 32 and 37 through 42 (Supplemental Table 1). In some instances, these peptides were modified by formylation at hydroxyl groups or by modification of Met to Met-sulfone or Met-sulfoxide. Peptides with N-terminal pyroglutamyl were also observed at positions Glu3 or Glu11 of the Aβ sequence. In sporadic AD, the leptomeningeal amyloid is mainly composed of Aβ 1–40 with a lesser, variable amount of Aβ 1–42. Characteristically in sporadic AD, the leptomeningeal amyloid exhibits very limited N-terminal degradations and posttranslational modifications that, in contrast, are commonly observed in the GM amyloid plaques.20–22 These limited modifications are probably due to the constraints imposed by the biochemical constitution of the vascular environment, in which endothelial cells, pericytes, and myocytes predominate. However, in the vaccinated individuals, the composition of the leptomeningeal and cortico-vascular Aβ is more reminiscent of that of the typical AD amyloid plaques with more N-terminally degraded Aβ peptides possessing shorter C termini. These observations suggest a mobilization from the soluble cortical pool to the vascular deposits.12,13 In support of this contention are the recent observations of Boche et al,23 who reported a larger number of cortical vessels containing Aβ n-40 and Aβ n-42 in AN-1792-immunized individuals. The elevated Aβ n-42 probably originated from the senile plaques, where it represents the major Aβ component. The apparent increase in amyloid angiopathy was also accompanied by a larger number of microhemorrhages than those observed in nonimmunized AD controls.23 The composition of the Aβ present in the walls of the cortico-vascular amyloid in sporadic AD is intermediate between the plaque core and leptomeningeal amyloid, with a lesser degree of N-terminal degradations and posttranslational modifications than those observed in the plaque core amyloid.20
Figure 3.
Chromatographic patterns of crude brain and cerebrovascular proteins separated by Superose 12 FPLC from immunized individuals with the AN-1792 antigen. The collected proteins, spanning the molecular masses from 2 to 8 kd, are indicated by the horizontal bar. The Superose 12 columns were calibrated using monomeric and dimeric Aβ. A: Leptomeningeal vessels. The vascular amyloid is very abundant in the walls of leptomeningeal arteries (Figure 2E), where it represents the most abundant protein. B: A pure preparation of cortical vessels extracted with GDFA. The Aβ peptide-containing fraction is highly enriched by the FPLC step. C: Cerebral cortex water-soluble proteins obtained after gentle homogenization of the brain tissue and high-speed ultracentrifugation. The lyophilized supernatant was dissolved in GDFA and submitted to FPLC. To increase the yield and eliminate contaminating flanking proteins, with higher and lower molecular masses, the fractions eluted within the 2- to 8-kd portion (trace a) were concentrated and re-chromatographed (trace b). D: The vascular depleted cerebral cortex SDS-insoluble/formic acid-soluble Aβ peptides were separated by FPLC (trace a). To further enrich the Aβ peptides, the specimens were re-chromatographed under the same conditions (trace b). E: The FPLC profile of the WM GDFA-soluble proteins. To enhance the purification of the Aβ peptides (trace a), the 2- to 8-kd fractions from four FPLC runs were pooled, concentrated, and re-chromatographed (trace b).
Figure 4.
Chromatographic profiles of vascular Aβ and P3 peptides. A: This trace corresponds to the leptomeningeal vascular Aβ peptides purified on a Zorbax AB-C8 reverse-phase column. The Aβ peptides with C-terminal at residue 40 are mostly eluted in peak 2, and those ending at position 42 concentrate in peak 3. The final overall purity of the leptomeningeal vascular Aβ is about 98%. The dotted line indicates the slope of the acetonitrile gradient. B: The cortico-vascular amyloid demonstrates a more complex elution pattern than that shown in A when purified under the same conditions. This is mainly due to a higher level of posttranslational modifications and to a higher amount of peptides ending at position 42/43. C: Chromatographic pattern of P3 on a GPC-100 silica-based size-exclusion column eluted with 80% GDFA. Most of this material corresponds to the sequence of Aβ residues 17 to 42, the chief component of the diffuse plaques. D: The peptides purified in C were resolved by reverse-phase chromatography on a Zorbax SB-C8 column. The six Aβ-related peptides, analyzed by automatic amino acid sequence and MALDI-TOF MS, corresponded to isoforms of Aβ 17–42. However, MALDI-TOF also suggested the minor presence of Aβ 1–42 represented by the 1–10 and 11–42 fragments. The dotted line indicates the slope of the acetonitrile linear gradient.
Water-Soluble Cortical Aβ Peptides
The 2- to 8-kd water-soluble fractions separated by FPLC/GDFA (Figure 3C) submitted to reverse-phase chromatography (Zorbax RX-C8 column) yielded six fractions containing Aβ peptides (Figure 5A). Western blots demonstrated the presence of more dimers than monomers. Scanning densitometry showed a total ratio of Aβ 40 monomers to Aβ 40 dimers of 2:98, whereas the ratio of Aβ 42 monomers to Aβ 42 dimers was 17:83 (Figure 5A). MALDI-TOF suggested the presence of 87 Aβ-related peptides. Because our analyses account for peptides present in the soluble brain homogenate, they are also likely to detect peptides derived from Aβ degradation by amino-, carboxy-, and endo-peptidases, including those carrying posttranslationally modified amino acids by isomerization and racemization as well. Amino acid modifications due to naturally occurring or artifactual formylation, oxidation, and cyclization were also detected in some Aβ peptides. We identified Aβ-related peptides with N termini commencing from residues 1 through 27 (with the exclusion of those starting at residues 10 and 25). Most of these peptides (59%) had their N termini at residues 1 through 9 and 11, indicating β-secretase digestion and probably amino-peptidase degradation. The C termini, on the other hand, included peptides ending at residues 14 through 43 (with the exception of those ending at positions 16, 19, and 32). However, the majority of these peptides (53%) had their C termini at positions 36 through 43, suggesting processing by the γ-secretase. Three Aβ-related peptides: 17–36, 17–41, and 17–43, may have resulted from α- and γ-secretase hydrolysis (Supplemental Table 2).
Figure 5.
Elution patterns of GM and WM Aβ-related peptides separated by reverse-phase RX-C8 HPLC. Trial and error coupled with Western blot analysis established that the Aβ peptides eluted between 40 to 60% acetonitrile concentration as indicated by the slope of the dashed line. Other unrelated peptides eluted at lower and higher acetonitrile concentration. The antibodies used were against Aβ 40 and Aβ 42 (Biosource) with a 0.5 μg/ml dilution. A: The GM water-soluble fraction generated six peaks containing Aβ peptides. B: The SDS-insoluble fraction produced 10 Aβ-containing fractions. C: The WM yielded seven Aβ-containing fractions. In all three cases, there was a preponderance of dimeric Aβ and more Aβ n-42 over Aβ n-40. Because of the hydrophobic moiety of the transmembrane C-terminal Aβ peptide, monomers associate into dimers to shield their thermodynamically unfavorable domains creating a very stable structure.16,17,73
SDS-Insoluble Cortical Aβ Peptides
In both Aβ-vaccinated cases under study, there was a remarkable reduction in the number of SDS-insoluble amyloid cores, which are abundant in the SDS-insoluble fraction obtained from the cerebral cortex in the vast majority of neuropathologically severe sporadic AD cases.24 However, in addition to the scarce amyloid cores, this fraction must have contained a substantial quantity of fibrillar water-insoluble Aβ, which originated from disassembled cortical amyloid cores that were solubilized by the alkaline treatment and remained in the supernatant after ultracentrifugation. After FPLC separation (Figure 3D), these Aβ peptides were submitted to reverse-phase HPLC chromatography (Figure 5B). Western blots indicated the presence of Aβ in 9 of the 12 fractions recovered with a preponderance of dimers over monomers. The ratio of Aβ 40 monomers to Aβ 40 dimers was 0:100 and that of Aβ 42 monomers to Aβ 42 dimers was 13:87 (Figure 5B). However, the more insoluble Aβ n-42 fraction revealed on the Western blot a small amount of trimers, tetramers, and larger oligomers up to ∼30 kd (Figure 5B). The ratios of Aβ monomers to Aβ dimers in the water-soluble fraction and SDS-insoluble fraction were very similar probably because they are, to some extent, derived from the same pool of insoluble plaque fibrillar amyloid. Mass spectrometry (MALDI-TOF) analysis of 13 chromatographic fractions suggested the presence of 100 Aβ-related peptides that could originate from Aβ itself, APP, and its secretase by-products (Supplemental Table 3). The chromatographic fractions contained Aβ peptides with N termini from position 1 through 23 (except for those starting at positions 9 and 21). The C termini encompassed peptides ending from positions 17 through 43 (excluding those ending at positions 18, 20, and 24). These peptides were present in their pristine condition and in their modified forms by formylation and oxidation. N-terminal pyroglutamyl was detected at positions 3 and 11. As in the case of the SDS-insoluble (fibrillar Aβ) of sporadic AD,21 the SDS-insoluble fraction of the vaccinated cases under study also contained abundant Aβ peptides with posttranslational modifications (isomerization and racemization) and extensively degraded N termini.
Diffuse Plaque (P3) Aβ Peptides
This material represents the most insoluble of all Aβ brain parenchymal peptides and is recovered by high-speed centrifugation as the insoluble pellet that remains after water, SDS, and alkaline extractions. Staining by thioflavine-S revealed homogeneous amorphous conglomerates that resemble a flock of cotton wool (Figure 2G). The GDFA-solubilized P3 fraction Aβ peptides were submitted to size-exclusion chromatography on a GPC-100 column, which yielded a single large peak and with lesser minor peaks (Figure 4C). The larger peak was subsequently resolved by reverse-phase chromatography (Zorbax SB-C8) into six well-separated peaks (Figure 4D). On automatic amino acid sequencing, all fractions, with the exception of peak 4, yielded the peptide Leu-Val-Phe corresponding to residues 17-18-19 of Aβ. Further automatic Edman degradation analyses performed on peak 1 revealed the amino acid sequence of Leu-Val-Phe-Phe-Ala-Glu-Asp-Val-Gly (Aβ residues 17 through 25). The molecular weight of 2409.4 d on the mass spectrometry (MS) reflectron mode confirmed the identity of these peptides as Aβ 17–42, with an oxidized Met 35. Interestingly, peaks 4 and 6 also contained peptides with a molecular mass of 991.73, which may correspond to the Aβ peptide residues 3 to 10 with an N-terminal pyroglutamyl residue. In addition, peak 5 also showed a peptide with a mass of 3437.68 d, which may correspond to the Aβ peptide amino acid residues 11 to 43. The different retention times may represent isoforms containing iso-Asp at position 23 and possibly 27 that do not change the molecular mass because they are the results of β-shifts and/or also due to racemization of Asp and Ser residues as previously observed on reverse-phase chromatography.21,25,26
White Matter Aβ Peptides
Overall, Aβ40 and Aβ42 were equally represented in the WM with a ratio of 46:54 for case 1. Case 2 had almost two times more Aβ 40 than Aβ42 in the WM (Table 1). To increase the Aβ yield, four of the initial FPLC/GDFA runs were pooled and re-chromatographed under the same conditions (Figure 3E). Western blots of the C8 reverse-phase HPLC (Figure 5C) demonstrated the presence of monomeric and dimeric Aβ peptides in seven fractions, with Aβ dimers more abundant than monomers. The proportion of Aβ40 monomers to dimers was 4:96 and of Aβ42 was 26:74. On mass spectrometry, there were 107 peptides with N termini from residues 1 through 27 (except those starting at residue 20) and C termini from residues 14 through 43 (excluding those ending at residues 15 and 18). In qualitative terms, the peptides ending at residue 36 through 43 represented 53% of the total Aβ peptides identified by MALDI-TOF (Supplemental Table 4). Overall, many peptides were posttranslationally modified as described for the cortical Aβ.
Discussion
AN-1792 immunization dramatically altered several facets of Aβ distribution and deposition considered characteristic of AD. Despite remarkable senile plaque regression in some regions, the total ELISA-detectable Aβ levels present in brain tissue did not decrease commensurately. In addition to a greatly increased soluble amyloid level in the GM, the Aβ levels present in vaccinated patient WM were elevated to amounts far higher than any observed previously in AD patients. Although senile plaques were disaggregated, both diffuse and vascular amyloid deposits remained unaffected by vaccination. The implications of each of these findings will be considered in turn.
Senile Plaque Disruption: Amyloid Deposit Mobilization without Clearance
Near complete disaggregation of senile plaques was evident in some regions of the vaccinated patient brains. Although promising, this result must be balanced with the possibility that amyloid plaques rich in fibrillar Aβ may represent a defense mechanism that removes potentially neurotoxic soluble amyloid from the neuropil and insulates it with a layer of glial cells.17,27,28 This suggests that plaque disruption in the absence of removal of the solubilized amyloid or in situations in which removal is inefficient may inadvertently produce a destructive side effect. Dissolution of fibrillar amyloid plaque cores by immunoglobulins or complement is followed by microglia/macrophage receptor-mediated uptake, limited degradation of Aβ and eventual discharge of soluble Aβ into the neuropil.29,30 The Aβ HHQK domain, amino acid residues 13 to 16, is capable of unleashing neuroinflammation via activation of microglia,31,32 and low molecular weight Aβ oligomers are recognized to kill neurons and impair cognitive function.17,33 The oligomerization of Aβ-protein begins intracellularly in cells derived from human brain,16 and intraneuronal Aβ accumulation causes early cognitive impairment before the extracellular Aβ deposition in triple Tg mice.34 Whether the antibody-solubilized amyloid species constrained within the cortex and WM are toxic and can eventually be eliminated remains to be established. In addition to Aβ n-40 and n-42 residue length species and their multimers, AD patients have large quantities of soluble Aβ molecules present that are posttranslationally modified and difficult for proteolytic enzymes to degrade.35
In both vaccinated patients, the amount of soluble amyloid was substantially increased compared with sporadic AD patients (Table 1). Compared with the levels observed in the GM of nonimmunized sporadic AD patient pools, case 1 demonstrated a 2.1-fold increase in total Aβ (extracted by 5 mol/L guanidine HCl buffer), whereas case 2 showed a reduction (2.2-fold less) (Table 1). Case 1 developed meningoencephalitis, and it is possible that the added pathology associated with this adverse event increased Aβ levels above the typical mean for uncomplicated AD. In addition, AD pathology is known to exhibit great variation among individuals, and perhaps case 2 simply harbored a comparatively low amount of senile plaques, vascular amyloid deposits, and soluble Aβ before vaccination. However, it is remarkable that the total amounts of GM soluble amyloid (extracted with Tris buffer) are substantially increased in both vaccinated cases. Cases 1 and 2 demonstrated 15- and 3.5-fold higher levels of total soluble Aβ compared with AD and ND individuals, respectively (Table 1).
Despite a marked focal reduction in the number of amyloid plaques, there was no commensurate decrease in total brain tissue Aβ levels (measured by ELISA), similar to previous results obtained with immunized APP Tg animals. Although plaques were disrupted in vaccinated Tg mice, formic acid-extractable Aβ levels representing the combined pools of soluble and insoluble plaque and vascular-associated fibrillar Aβ fractions did not differ between Aβ immunized TgCRND8, PDAPP, and tg2576 Tg mice and nonimmunized rodents.36–39 These observations suggest that the removal of Aβ from the brain by immunization is not complete or occurs at substantially slower rates than senile plaque disruption.
Comprehending the ultimate effects of plaque amyloid dispersal on brain vascular function is vital. Soluble intra- and extracellular Aβ may exert deleterious effects on vascular endothelial and smooth muscle cell metabolism. Soluble Aβ is a potent vasoconstrictor capable of altering regional cerebral blood flow,40,41 and Aβ peptides are also powerful angiogenesis inhibitors.42,43 These activities may impact blood-brain barrier selectivity and vascular repair. In addition, Aβ interferes with the production of vascular relaxing factors and disturbs the balance between cerebral energy requirements and cerebral blood flow.44 The soluble and insoluble Aβ effects on vascular homeostasis may be the most aggressive pathology in AD, and it is possible that increased levels of soluble Aβ peptides could have important vascular pathophysiological consequences in immunized individuals.
Vascular and Diffuse Amyloid Deposits Resist AN-1792 Vaccine-Induced Disruption
A feature shared by both Tg mice and human AD patients is the firm recalcitrance of vascular amyloid deposits to disruption by immunization.4,13,14,36–38,45,46 Also common to both is that focal senile plaque density decreases were accompanied by increased vascular amyloid deposition suggesting an active, antibody-induced remodeling of amyloid accumulation patterns. It is possible that some antibody-solublilized senile plaque amyloid was re-deposited in the microvasculature during egress from the brain through the periarterial spaces. In support of this contention is the observation that the peptide composition of the water-soluble Aβ peptides (Supplemental Table 2) resembled that isolated from the vascular compartments (Supplemental Table 1). Moreover, of the water-soluble fraction extracted from the cerebral cortex, 96 and 100% of the Aβ peptides measured by ELISA were mostly composed of the more soluble Aβ n-40 for cases 1 and 2, respectively (Table 1). However, extractions with water-based buffers do not efficiently isolate the more insoluble Aβ n-42. Therefore, a more stringent analysis using formic acid extraction was used for the water-insoluble pellets. Subsequent MALDI-TOF MS analyses revealed a substantial amount of Aβ peptides ending at residues 42 and 43. Pre-existing impaired brain interstitial fluid drainage or an emerging periarterial occlusion could act to retain a permanent, antibody-dispersed Aβ pool in the cerebral cortex.47–50 In this scenario, the reported cognitive enhancement in Tg mice,4 rats,51 and immunized individuals may be due to the removal of a toxic soluble amyloid pool. Recent investigations have demonstrated that passive immunotherapy against Aβ in aged tg2576 Tg mice reversed cognitive deficits and reduced parenchymal plaque burden but resulted in a concomitant increase in vascular amyloid load and associated microhemorrhages.52 Moreover, in the 21-month-old APP23 Tg mice, passive vaccination with IgG against residues 3 to 6 of human Aβ resulted in a twofold increase in cerebral amyloid angiopathy-associated microhemorrhages.45 The same phenomenon occurred in aged PDAPP Tg mice in which vaccination at high doses of the 3D6 antibody, directed against the N-terminal region of Aβ, resulted in an increased incidence and severity of microhemorrhages.46 Intriguingly, all three human vaccinated cases, neuropathologically characterized,12–14 showed the highest score of 3 (severe) for vascular amyloid deposition in those areas in which the apparent removal of amyloid attained its maximal expression. Additional studies with APP23 mice,53 a Tg mouse model in which there is a remarkable load of vascular amyloid, have confirmed that elevated quantities of soluble amyloid (>50 μg/g) are ultimately accompanied by a prompt and overwhelming global leptomeningeal and cortico-vascular amyloid deposition, a phenomenon that has not been observed in other Tg mice studied.54
Immunization was apparently unable to remove diffuse deposits of Aβ. In case 1, there were large areas in the frontal parietal and temporal regions in which there were numerous diffuse plaques. In case 2, however, there were abundant diffuse plaques present in the temporal and parietal lobes, although they were absent from the frontal lobe, a finding consistent with the results of Masliah et al.14 We were able to isolate from both AN-1792-treated cases substantial quantities of the extremely insoluble nonamyloidogenic P3 peptide, the major peptide in the diffuse plaques,19 mainly composed of the Aβ sequence of residues 17 to 42. Because P3 deposits are not fibrillar and are not associated with activated proinflammatory microglia, it has been concluded that P3 is a “desirable” APP by-product of the α- and γ-secretases, the generation of which precludes amyloid formation. However, this peptide has also been observed in dystrophic neurites associated with amyloid plaques and in pathological cortical vesicular structures.55 Furthermore, the Aβ 17–42 induces apoptosis by activation of c-jun N-terminal kinase and caspases 8 and 3.56 The Tg mice transfected with APP751 cDNA, under the control of the rat neuron-specific enolase promoter, only develop diffuse Aβ deposits that correlate with age-dependent severe learning deficits.57 The C-terminal domain of Aβ has powerful membrane destabilizing properties that alter the permeability of cellular membranes, thus contributing to cytotoxicity.58–60 It has been recently suggested that the retention and accumulation of Aβ in lipid bilayers could induce severe disturbances in cellular metabolism and play a major role in AD pathology.61 These findings suggest that diffuse P3 deposits are not benign. Passive or active immunizations directed exclusively to N-terminal Aβ-42 sequences, such as AN-1792,62 will not remove potentially neurotoxic Aβ P3 peptides. However, a new generation of antibodies specifically directed against the C-terminal domain of Aβ and designed to remove both compact and diffuse plaques are undergoing experimental evaluation.63
Gross Enlargement of the White Matter Amyloid Pool
White matter lesions are present in two-thirds of patients with AD. They represent severe alterations in protein, lipid, and cholesterol metabolism; dysmyelination; demyelination; loss of axons and astrogliosis compounded by a reduced number of microvessels; concurrent ischemia/hypoxia; and interstitial fluid retention with dilation of periarterial spaces.49,64 In elderly ND individuals, the WM contains a small pool of soluble Aβ that probably originates in the GM and gradually diffuses into the WM.64 In the absence of visible WM Aβ deposits, such as those observed in the cerebral cortex of AD patients, it can be assumed that the WM Aβ peptides are either soluble oligomers or associated with structural molecules such as myelin sheets that may inhibit Aβ fibrillization. In addition, an amplified Aβ-mediated WM neuroinflammatory response would increase the complement-mediated production of membrane attack complexes that will further destroy myelin and axonal integrity.65 Experimentally, Aβ peptides induce severe oligodendrocyte damage by interfering with the sphingomyelinase-ceramide pathway66 as well as alterations in axonal cytoskeleton manifested by loss of neurofilament immunoreactivity.67 Both guanidine and formic acid extractions recovered a substantial amount of WM Aβ n-40 and Aβ n-42. In sporadic AD WM, the total average soluble Aβ is 2.2 μg/g tissue64 whereas the WM of immunized cases 1 and 2 contained 97 and 17 μg/g tissue of soluble total Aβ, representing 44 and 8 times more Aβ peptide load, respectively. The higher amount of total WM Aβ (∼ 5.8-fold) of case 1 over case 2 may be due to the postvaccination aseptic encephalitis that gravely affected the WM or to differences in prevaccination Aβ levels. The substantial increase in WM amyloid burden suggests that this compartment acted as the ultimate “sink” for Aβ released from disrupted senile plaques that could not escape through the vasculature. The ultimate consequences of a shifted amyloid tissue burden are unknown.
Amyloid Accumulation Dynamics and Vaccination
The cognitive improvement exhibited by vaccinated patients suggests that amyloid plaques play key roles in AD pathophysiology and the development of dementia. In addition, the results suggest that a patient with preclinical stages of AD may have incompletely evolved plaques that have not yet undergone extensive chemical modification, provoked inflammation, or managed to irreversibly damage surrounding neurons. Thus, there may be only a brief window of opportunity to reverse these plaques. The problem is that vascular Aβ deposits in all likelihood are forming concomitantly with senile plaques and may interfere with the complete removal of disrupted amyloid. Even with an antibody specific to Aβ 1–40, the dominant peptide in vascular amyloid, once preclinical AD has progressed to dementia, it may be too late to preserve the architecture and function of the vessels.49,68 These tenets suggest two quite interesting hypotheses: 1) AD plaques might initially be virtually absent but emerge rapidly once mild cognitive impairment appears. 2) Plaques from the early clinical stages of AD are substantially less modified chemically than those of full-blown AD, as in the case of the APP Tg mice where the amyloid does not have time to accumulate chemical modifications and thereby becomes more resistant to disruption. In support of these contentions is the early prophylactic immunization of several strains of Tg mice that results in minimal amyloid deposits.4,69 As the Tg mice age, the efficient removal of plaques by vaccination decreases.69–71 Arresting Aβ production in the Tg APP mice carrying the Swedish/Indiana mutations by doxycycline-controlled expression demonstrated that pre-existing amyloid deposits become very stable and difficult to clear as the animals age.72 Therefore, although “young” and comparatively unmodified amyloid in Tg mice and possibly in preclinical AD is more amenable to disruption, such efforts will be substantially less effective in fully developed AD, where modified Aβ is too abundant and difficult to eliminate. In addition, immunotherapy cannot remove the vascular amyloid because it is enmeshed with the extracellular matrix to too great an extent. We also hypothesize that impaired regional brain perfusion due to damaged microvasculature might explain the patchy plaque removal patterns observed in immunized individuals. These assumptions suggest that early intervention, well before vascular amyloid deposition is extensive and circulatory compromise is severe, may be a key feature of successful mitigation approaches.
In summary, examination of two AN-1792 immunized individuals revealed areas of the cerebral cortex in which the compact amyloid plaques were removed in some regions, leaving behind diffuse Aβ deposits. A remarkable increase in the soluble Aβ pool was evident in both cases and may have contributed to a more severe amyloid angiopathy and sharply elevated WM-soluble Aβ levels. In addition, the increasing deposits of amyloid in the brain vasculature may intensify blood-brain barrier distress and hamper brain perfusion. The elevated pool of soluble amyloid in GM and WM may further impair neuronal function and neural transmission and additionally promote neuroinflammation. Despite these potential pitfalls, the morphological and biochemical evidence supports immunization programs in humans by providing direct and substantial evidence that amyloid plaque deposits of mild cognitive impairment patients are amenable to disassembly by antibody therapies. However, more cases will need to be studied to verify these preliminary conclusions and to determine whether immunization may have deleterious consequences for AD patients or patients with non-AD dementias. Earlier anti-Aβ immunizations, combined with treatment strategies that are more preventative than therapeutic, may be needed to facilitate drainage and efficient degradation of the brain’s Aβ peptides and prevent the accumulation of toxic soluble and insoluble Aβ and subsequent deleterious neuronal and vascular damage.
Supplementary Material
Acknowledgments
We express our gratitude to Dr. Douglas Walker (Sun Health Research Institute) for performing Apo E genotyping.
Footnotes
Address reprint requests to Alex E. Roher, M.D. Ph.D., Sun Health Research Institute, 10515 W. Santa Fe Dr., Sun City, AZ 85351. E-mail: alex.roher@sunhealth.org.
Supported by National Institute on Aging grant RO1 AG-19795, by Arizona Alzheimer’s Disease Core Center grant P30 AG-19610, and by grants from the State of Arizona to the Arizona Alzheimer’s Research Consortium. Brain tissue from case 2 was obtained from University of California at San Diego Alzheimer’s Disease Research Center grant NIA AG-05131 and grant RO1 AG-18440.
Supplemental material for this article appears on http://ajp.amjpathol.org.
Related Commentary on page 738
References
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Curr Opin Immunol. 2004;16:599–606. doi: 10.1016/j.coi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Solomon B. Alzheimer’s disease and immunotherapy. Curr Alzheimer Res. 2004;1:149–163. doi: 10.2174/1567205043332126. [DOI] [PubMed] [Google Scholar]
- Gelinas DS, Dasilva K, Fenili D, George-Hyslop P, McLaurin J. Immunotherapy for Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14657–14662. doi: 10.1073/pnas.0404866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, deq Uervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Weksler ME, Gouras G, Relkin NR, Szabo P. The immune system, amyloid-beta peptide, and Alzheimer’s disease. Immunol Rev. 2005;205:244–256. doi: 10.1111/j.0105-2896.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- Dodel RC, Du Y, Depboylu C, Hampel H, Frolich L, Haag A, Hemmeter U, Paulsen S, Teipel SJ, Brettschneider S, Spottke A, Nolker C, Moller HJ, Wei X, Farlow M, Sommer N, Oertel WH. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1472–1474. doi: 10.1136/jnnp.2003.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1:213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid β-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR. Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem. 1996;271:20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- Esh C, Patton L, Kalback W, Kokjohn TA, Lopez J, Brune D, Newell AJ, Beach T, Schenk D, Games D, Paul S, Bales K, Ghetti B, Castano EM, Roher AE. Altered APP processing in PDAPP (Val717→Phe) transgenic mice yields extended-length Abeta peptides. Biochemistry. 2005;44:13807–13819. doi: 10.1021/bi051213+. [DOI] [PubMed] [Google Scholar]
- Gowing E, Roher AE, Woods AS, Cotter RJ, Chaney M, Little SP, Ball MJ. Chemical characterization of A beta 17–42 peptide, a component of diffuse amyloid deposits of Alzheimer disease. J Biol Chem. 1994;269:10987–10990. [PubMed] [Google Scholar]
- Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ. Beta-amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Lowenson JD, Clarke S, Wolkow C, Wang R, Cotter RJ, Reardon IM, Zurcher-Neely HA, Heinrikson RL, Ball MJ. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J Biol Chem. 1993;268:3072–3083. [PubMed] [Google Scholar]
- Kuo YM, Emmerling MR, Woods AS, Cotter RJ, Roher AE. Isolation, chemical characterization, and quantitation of Aβ 3-pyroglutamyl peptides from neuritic plaques and vascular amyloid deposits. Biochem Biophys Res Commun. 1997;237:188–191. doi: 10.1006/bbrc.1997.7083. [DOI] [PubMed] [Google Scholar]
- Boche D, Barton E, Neal J, Ferrer I, Wilkinson D, Bayer A, Holmes C, Weller RO, Nicoll JAR. Effects of Abeta immunotherapy on cerebral amyloid angiopathy in human Alzheimer’s disease. Abstracts of the 107th meeting of the British Neuropathological Society. Neuropathol Appl Neurobiol. 2006;32(Suppl 1):3–4. [Google Scholar]
- Roher AE, Palmer KC, Chau V, Ball MJ. Isolation and chemical characterization of Alzheimer’s disease paired helical filament cytoskeletons: differentiation from amyloid plaque core protein. J Cell Biol. 1988;107:2703–2716. doi: 10.1083/jcb.107.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenson JD, Clarke S, Roher AE. Chemical modification of deposited amyloid-β peptides. Methods Enzymol. 1999;309:89–105. doi: 10.1016/s0076-6879(99)09009-6. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Watanabe A, Ogawara M, Mori H, Shirasawa T. Isoaspartate formation and neurodegeneration in Alzheimer’s disease. Arch Biochem Biophys. 2000;381:225–234. doi: 10.1006/abbi.2000.1955. [DOI] [PubMed] [Google Scholar]
- Watson D, Castano E, Kokjohn TA, Kuo YM, Lyubchenko Y, Pinsky D, Connolly ES, Jr, Esh C, Luehrs DC, Stine WB, Rowse LM, Emmerling MR, Roher AE. Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer’s disease. Neurol Res. 2005;27:869–881. doi: 10.1179/016164105X49436. [DOI] [PubMed] [Google Scholar]
- Lee HG, Casadesus G, Zhu X, Takeda A, Perry G, Smith MA. Challenging the amyloid cascade hypothesis: senile plaques and amyloid-beta as protective adaptations to Alzheimer disease. Ann NY Acad Sci. 2004;1019:1–4. doi: 10.1196/annals.1297.001. [DOI] [PubMed] [Google Scholar]
- Brazil MI, Chung H, Maxfield FR. Effects of incorporation of immunoglobulin G and complement component C1q on uptake and degradation of Alzheimer’s disease amyloid fibrils by microglia. J Biol Chem. 2000;275:16941–16947. doi: 10.1074/jbc.M000937200. [DOI] [PubMed] [Google Scholar]
- Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer’s amyloid β-peptide by microglial cells. J Biol Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Yu J, Karshin W, Tom D, Li J, Kazanskaia A, Kirkpatrick J, Roher AE. The HHQK domain of beta-amyloid provides a structural basis for the immunopathology of Alzheimer’s disease. J Biol Chem. 1998;273:29719–29726. doi: 10.1074/jbc.273.45.29719. [DOI] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Yu JH, Karshin W, Tom D, Li J, Kirkpatrick J, Kuo LM, Roher AE. Specific domains of beta-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J Neurosci. 1996;16:6021–6037. doi: 10.1523/JNEUROSCI.16-19-06021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Webster S, Emmerling MR, De Lima N, Roher AE. Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of A beta peptides of Alzheimer’s disease. Biochim Biophys Acta. 1998;1406:291–298. doi: 10.1016/s0925-4439(98)00014-3. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Knudsen E, Asuni A, Fitzer-Attas C, Sage D, Quartermain D, Goni F, Frangione B, Wisniewski T. An attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloid-beta derivatives. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. Beta-amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- Crawford F, Suo Z, Fang C, Sawar A, Su G, Arendash G, Mullan M. The vasoactivity of Aβ peptides. Ann NY Acad Sci. 1997;826:35–46. doi: 10.1111/j.1749-6632.1997.tb48459.x. [DOI] [PubMed] [Google Scholar]
- Paris D, Townsend K, Quadros A, Humphrey J, Sun J, Brem S, Wotoczek-Obadia M, DelleDonne A, Patel N, Obregon DF, Crescentini R, Abdullah L, Coppola D, Rojiani AM, Crawford F, Sebti S, Mullan M. Inhibition of angiogenesis by Aβ peptides. Angiogenesis. 2004;7:75–85. doi: 10.1023/B:AGEN.0000037335.17717.bf. [DOI] [PubMed] [Google Scholar]
- Paris D, Town T, Parker T, Humphrey J, Mullan M. Aβ vasoactivity: an inflammatory reaction. Ann NY Acad Sci. 2000;903:97–109. doi: 10.1111/j.1749-6632.2000.tb06355.x. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Cerebrovascular effects of amyloid-β peptides: mechanisms and implications for Alzheimer’s dementia. Cell Mol Neurobiol. 2003;23:681–689. doi: 10.1023/A:1025092617651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid-β accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol. 1998;153:725–733. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, Luehrs DC, Childress JL, Beach TG, Weller RO, Kokjohn TA. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med. 2003;9:112–122. [PMC free article] [PubMed] [Google Scholar]
- Weller RO, Cohen NR, Nicoll JA. Cerebrovascular disease and the pathophysiology of Alzheimer’s disease: implications for therapy. Panminerva Med. 2004;46:239–251. [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Beach TG, Sue LI, Scott S, Layne KJ, Kokjohn TA, Kalback WM, Luehrs DC, Vishnivetskaya TA, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Weller RO, Roher AE. The evolution of A beta peptide burden in the APP23 transgenic mice: implications for A beta deposition in Alzheimer disease. Mol Med. 2001;7:609–618. [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Crawford F, Mullan M, Kokjohn TA, Emmerling MR, Weller RO, Roher AE. Elevated Aβ and apolipoprotein E in AβPP transgenic mice and its relationship to amyloid accumulation in Alzheimer’s disease. Mol Med. 2000;6:430–439. [PMC free article] [PubMed] [Google Scholar]
- Higgins LS, Murphy GM, Jr, Forno LS, Catalano R, Cordell B. P3 beta-amyloid peptide has a unique and potentially pathogenic immunohistochemical profile in Alzheimer’s disease brain. Am J Pathol. 1996;149:585–596. [PMC free article] [PubMed] [Google Scholar]
- Wei W, Norton DD, Wang X, Kusiak JW. Abeta 17–42 in Alzheimer’s disease activates JNK and caspase-8 leading to neuronal apoptosis. Brain. 2002;125:2036–2043. doi: 10.1093/brain/awf205. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Ort M, Cimadevilla JM, Vondrous R, Cordell B, Koistinaho J, Bures J, Higgins LS. Specific spatial learning deficits become severe with age in β-amyloid precursor protein transgenic mice that harbor diffuse β-amyloid deposits but do not form plaques. Proc Nat Acad Sci USA. 2001;98:14675–14680. doi: 10.1073/pnas.261562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillot T, Goethals M, Vanloo B, Talussot C, Brasseur R, Vandekerckhove J, Rosseneu M, Lins L. Fusogenic properties of the C-terminal domain of the Alzheimer β-amyloid peptide. J Biol Chem. 1996;271:28757–28765. doi: 10.1074/jbc.271.46.28757. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides: implications for neurotoxicity. J Biol Chem. 1996;271:26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Marchesi VT. An alternative interpretation of the amyloid Abeta hypothesis with regard to the pathogenesis of Alzheimer’s disease. Proc Natl Acad Sci USA. 2005;102:9093–9098. doi: 10.1073/pnas.0503181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Bard F, Johnson-Wood K, Lee C, Hu K, Griffith SG, Black RS, Schenk D, Seubert P. Abeta42 immunization in Alzheimer’s disease generates Abeta N-terminal antibodies. Ann Neurol. 2005;58:430–435. doi: 10.1002/ana.20592. [DOI] [PubMed] [Google Scholar]
- Carty NC, Wilcock DM, Rosenthal A, Grimm J, Pons J, Ronan V, Gottschall PE, Gordon MN, Morgan D. Intracranial administration of deglycosylated modified C-terminal-specific anti-A antibody efficiently clears amyloid plaques without activating microglia in amyloid depositing transgenic mice. J Neuroinflammation. 2006;3:11. doi: 10.1186/1742-2094-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Weiss N, Kokjohn TA, Kuo Y: M, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, Emmerling M, Goux W, Beach T. Increased Aβ peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer’s disease. Biochemistry. 2002;41:11080–11090. doi: 10.1021/bi026173d. [DOI] [PubMed] [Google Scholar]
- Webster S, Lue LF, Brachova L, Tenner AJ, McGeer PL, Terai K, Walker DG, Bradt B, Cooper NR, Rogers J. Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer’s disease. Neurobiol Aging. 1997;18:415–421. doi: 10.1016/s0197-4580(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-β peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantaratnotai N, Ryu JK, Kim SU, McLarnon JG. Amyloid beta peptide-induced corpus callosum damage and glial activation in vivo. Neuroreport. 2003;14:1429–1433. doi: 10.1097/00001756-200308060-00005. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Yamada M, Frackowiak J, Mazur-Kolecka B, Weller RO. Cerebral amyloid angiopathy plays a direct role in the pathogenesis of Alzheimer’s disease: pro-CAA position statement. Neurobiol Aging. 2004;25:589–597. doi: 10.1016/j.neurobiolaging.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Levites Y, Das P, Price RW, Rochette MJ, Kostura LA, McGowan EM, Murphy MP, Golde TE. Anti-Abeta(42)- and anti-Abeta(40)-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Murphy MP, Younkin LH, Younkin SG, Golde TE. Reduced effectiveness of Aβ 1–42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging. 2001;22:721–727. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- Zhou J, Fonseca MI, Kayed R, Hernandez I, Webster SD, Yazan O, Cribbs DH, Glabe CG, Tenner AJ. Novel Abeta peptide immunogens modulate plaque pathology and inflammation in a murine model of Alzheimer’s disease. J Neuroinflammation. 2005;2:28. doi: 10.1186/1742-2094-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Gonzales V, Savonenko AV, Wen JC, Jenkins NA, Copeland NG, Younkin LH, Lester HA, Younkin SG, Borchelt DR. Persistent amyloidosis following suppression of Abeta production in a transgenic model of Alzheimer disease. PLoS Med. 2005;2:e355. doi: 10.1371/journal.pmed.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney MO, Webster SD, Kuo YM, Roher AE. Molecular modeling of the Aβ-42 peptide from Alzheimer’s disease. Protein Eng. 1998;11:761–767. doi: 10.1093/protein/11.9.761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.