Abstract
In this study we performed comparisons of pulmonary responses between two different respiratory syncytial virus (RSV) antigenic subgroup A strains, A2 and Line 19. Line 19 strain induced significant dose-responsive airway hyperreactivity (AHR) in BALB/c mice at days 6 and 9 after infection, whereas the A2 strain induced no AHR at any dose. Histological examination indicated that A2 induced no goblet cell hyper/metaplasia, whereas the Line 19 induced goblet cell expansion and significant increases in gob5 and MUC5AC mRNA and protein levels in vivo. When examining cytokine responses, A2 strain induced significant interleukin (IL)-10 expression, whereas Line 19 strain induced significant IL-13 expression. When IL-13−/− mice were infected with Line 19 RSV, the AHR responses were abrogated along with gob5 gene expression. There was little difference in viral titer throughout the infection between the line 19- and A2-infected mice. However, the A2 strain grew to significantly higher titers than the Line 19 strain in HEp-2 cells in vitro. Thus, RSV Line 19-induced airway dysfunction does not correlate with viral load in vivo. These data demonstrate that different RSV strains of the same antigenic subgroup can elicit differential immune responses that impact the phenotypic expression of RSV-induced illness.
The initiation of respiratory syncytial virus (RSV)-mediated pathophysiological changes in the lungs of infants is likely a complex response that depends on both virus and host factors.1–5 Numerous laboratories have initiated studies of RSV infection in animals to outline the responses in the lung during primary and secondary responses to the virus, as well as long-term consequences of the RSV-induced response. Evidence suggests that the ability of mice to effectively respond to RSV infection is dependent on the strain of mice used, the quality of the CD8+ T-cell cytotoxic responses, as well as the level of specific cytokines produced within the lungs of mice.6,7 Thus, several variables contribute to the ability of the host to quickly and effectively clear the infectious virus and therefore minimize the progression of disease.
The yearly epidemic between November and April can overwhelm hospital emergency rooms and in-patient services.8–10 However, the severity of disease can fluctuate significantly from year to year. Although the reason for the year-to-year fluctuation in severity is not clear, it is possible that RSV genomic diversity contributes to fluctuations in disease severity. We decided to examine the phenotypic response to two different RSV strains to determine whether strain differences could impact illness. One of the two isolates used is available from the American Type Culture Collection (Manassas, VA) and has been maintained at Vanderbilt University (A2).11–15 The A2 strain has been used for a number of important studies including both in vitro and in vivo modeling of disease responses and establishing important vaccine parameters. An antigenic subgroup A isolate of RSV was derived from a clinically sick infant at the University of Michigan and originally identified as Line 19.16 Line 19 has been used to establish an animal model of severe RSV-induced disease with significant airway hyperreactivity (AHR) and mucus overproduction.17–19 In the present studies we have examined the differences in responses during a primary mouse infection with these two viruses in side-by-side studies to outline the phenotypic parameters. These data demonstrate that different subgroup A strains of RSV induce considerably different disease characteristics. RSV strain differences may be an important component of RSV disease severity observed in the clinic.
Materials and Methods
Mice
BALB/c J mice (6 weeks of age) were purchased from the Jackson Laboratories (Bar Harbor, ME). These mice were maintained in specific pathogen-free facilities. The interleukin (IL)-13−/− mice on a BALB/c background were originally graciously provided by Dr. Andrew McKenzie (Medical Research Council, Cambridge, UK) and maintained at the University of Michigan. Guidelines were followed for the care and use of animals as indicated by our animal review board.
RSV Propagation and Titer Determination
The A2 strain of RSV was originally provided by Dr. Robert Chanock, National Institutes of Health, Rockville, MD. Master stocks and working stocks of RSV were prepared as previously described.12 Line 19 RSV16 was propagated in HEp-2 cells (American Type Culture Collection) and was titered using Vero76 cells (American Type Culture Collection), as previously described.20 The viral titer for each virus was determined by RSV-specific antibodies to visualize the plaques in infected cultures and enumerate positive plaques in serially diluted Vero cell cultures. The titer for each virus was determined within a month of performing the studies, and the virus stocks were stored in liquid nitrogen until use.
Animal Infections and Treatments
Mice were anesthetized and infected with human RSV A2 or Line 19 at the indicated dose.17,21,22 The animals were then examined at various time points after challenge for AHR, cytokine expression, and levels of virus growth.
Real-Time Polymerase Chain Reaction (PCR)
Quantitative analysis of whole lung mRNA was performed using 0.5 μg of RNA reverse-transcribed in a total volume of 20 μl, as previously described.17 Subsequently, 1 μl of cDNA was then used to assess cytokine and chemokine production using predeveloped reagents that were purchased from Applied Biosystems (Foster City, CA). The primers and probes used for the detection of mRNA expression were determined using predeveloped primer/probe sets (PE Biosystems, Foster City, CA) for cytokine analysis. A predeveloped primer/probe set for murine GAPDH (PE Biosystems) was used as an internal control for quantification of the total amount of cDNA used in the reaction. Results were normalized to GAPDH expression and presented as fold increase in mRNA expression compared to the level detected at day 0 using the comparative CT method (ΔΔCT) methodology as outlined by Applied Biosystems (http://www.appliedbiosystems.com/support/tutorials/pdf/performing_rq_gene_exp_rtpcr.pdf). As outlined by the literature, this technique provides a standardized methodology that is quantitative based on a standard housekeeping gene expression for sample comparisons.
Measurement of AHR
AHR was measured using a mouse plethysmograph using ventilation of anesthetized mice that is specifically designed for the low tidal volumes (Buxco, Troy, NY) as previously described.21–23 Briefly, the mouse to be tested was anesthetized with sodium pentobarbital and intubated via cannulation of the trachea with an 18-gauge metal tube. The mouse was subsequently ventilated with a Harvard pump ventilator (tidal volume, 0.4 ml; frequency, 120 breaths/minute; positive end-expiratory pressure, 2.5 to 3.0 cm H2O; Harvard Apparatus, Holliston, MA). The plethysmograph was sealed and readings monitored by computer. The Buxco software calculates resistance by dividing the change in pressure (Ptp) by the change in flow (F) (Ptp/F; units = cm H2O/ml/second). After determining a dose-response curve (0.001 to 0.5 mg), an optimal dose was chosen: 0.2 mg of methacholine. This dose was used throughout the rest of the experiments in this study. After the methacholine challenge, the response was monitored and the peak airway resistance recorded as a measure of AHR.
Histological Analysis
Lung tissue was preserved with 4% paraformaldehyde at various time points after challenge. The fixed lungs were embedded in paraffin and multiple 50-μm sections were differentially stained either with hematoxylin or eosin (H&E) or with periodic acid-Schiff (PAS) stain for mucus expression. MUC5AC (graciously provided by S.B.H.) and gob5 protein expression was evaluated using immunohistochemistry with specific antibodies as previously described.15
Virus Growth in Vitro
Eighty percent confluent HEp-2 cells grown in 10% Eagle’s minimal essential medium (EMEM) in six-well dishes were inoculated in triplicate with line 19 or A2 at a multiplicity of infection of 0.5 in a volume of 350 μl. The virus was allowed to adsorb for 1 hour at room temperature on a rocking platform. The inocula were removed, and the cells were washed three times with 2 ml of 10% EMEM before addition of 3 ml of fresh 10% EMEM. The cells were incubated at 37°C in 5% CO2. At the indicated times, 200-μl aliquots of supernatant were taken and replaced with an equal volume of fresh 10% EMEM. Aliquots were centrifuged 2000 rpm for 5 minutes to remove debris, frozen in a dry ice/ethanol bath, and stored at −80°C. All samples were titrated at the same time. Each sample was titrated in triplicate by standard plaque assay on HEp-2 cells.
Quantitation of Virus in Lung Tissue
Left lungs were aseptically removed, and infectious RSV was titrated by plaque assay on HEp-2 cells as described above, with the following modification. Lungs were individually ground with precooled mortar and pestle and sterile ground glass. Glass and tissue debris were removed from lung homogenates by centrifugation (15 minutes, 2000 rpm). Supernatants were immediately assayed in triplicate.
Statistics
Statistical significance was determined by analysis of variance with a Student Neuman-Keuhl posttest. Values of P < 0.05 were considered significant.
Results
Infection with Two Isolates of RSV Exhibits Differences in Pathophysiological Responses
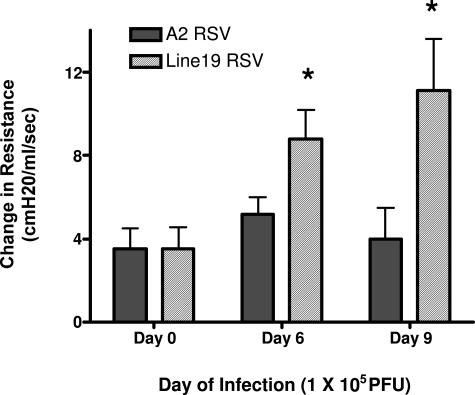
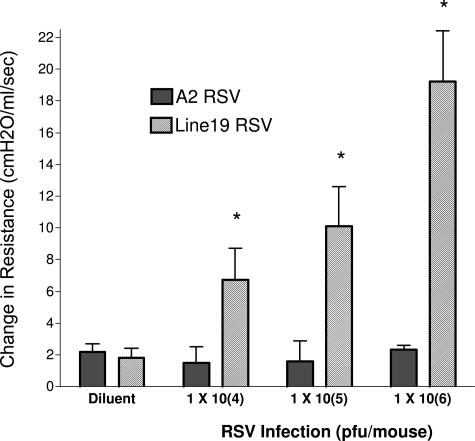
Previous studies with mouse models of RSV have identified divergent responses in AHR, mucus, and immune cytokine expression. In the present studies we have investigated two RSV strains that have, in the past, demonstrated substantial differences in responses in mice. The A2 strain and the Line 19 strain of virus were grown in HEp-2 cell lines, and infectivity was titrated by plaque assays using immunodetection of RSV-specific plaque formation (see Materials and Methods). The virus strains were adjusted to similar titers (2 × 106 PFU/ml) before use. To assess differences in the responses of these two strains of RSV, we infected mice by intratracheal administration using 1 × 105 PFU. After 6 or 9 days after infection the infected animals were anesthetized and assessed for development of AHR using methacholine-induced airway constriction. As depicted in Figure 1, the Line 19 strain induced significant AHR at both 6 and 9 days after infection, whereas the A2 strain did not induce AHR. Thus, these different isolates of RSV induced divergent responses that alter the severity and course of pulmonary disease. To further analyze these responses, we examined a dose-dependent infection because one difference in many studies is the dose of virus that is used to infect mice. These animals were analyzed at day 9 of infection. Assessing the induction of AHR in a dose-dependent manner, we found that there was a significant increase in AHR induction dependent on dose of the Line 19 virus (Figure 2). Even at the lowest dose examined, 1 × 104 PFU, the Line 19 strain induced significant increases in AHR at day 9 after infection. In contrast, examining AHR in the A2 virus at similar titers gave no significant increase in AHR. We have examined higher doses of A2 given into the airway and have found no significant increase in AHR at 107 PFU infection dose (data not shown).
Figure 1.
Differential induction of AHR in Line A2 and 19 A strain viruses. Six-week-old BALB/c mice were infected with 1 × 105 PFU of a specific RSV strain of virus and assessed for AHR at days 6 or 9 of infection by box plethysmography in anesthetized, ventilated animals. Peak airway resistance was recorded after an intravenous tail vein administration of methacholine (250 μg/kg). Data represent mean ± SE from five to six mice per group. *P < 0.05.
Figure 2.
Development of AHR on day 9 after infection is dose-dependent in Line 19 but not A2 RSV. To verify that the effect observed in Figure 1 was not dose-specific, increasing doses of virus were administered to 6-week-old BALB/c mice and AHR was examined at 9 days after challenge with intravenous methacholine administration. Lower doses of neither Line 19 nor A2 elicited significant increases in AHR (data not shown). Data represent mean ± SE from five to six mice per group. *P < 0.05.
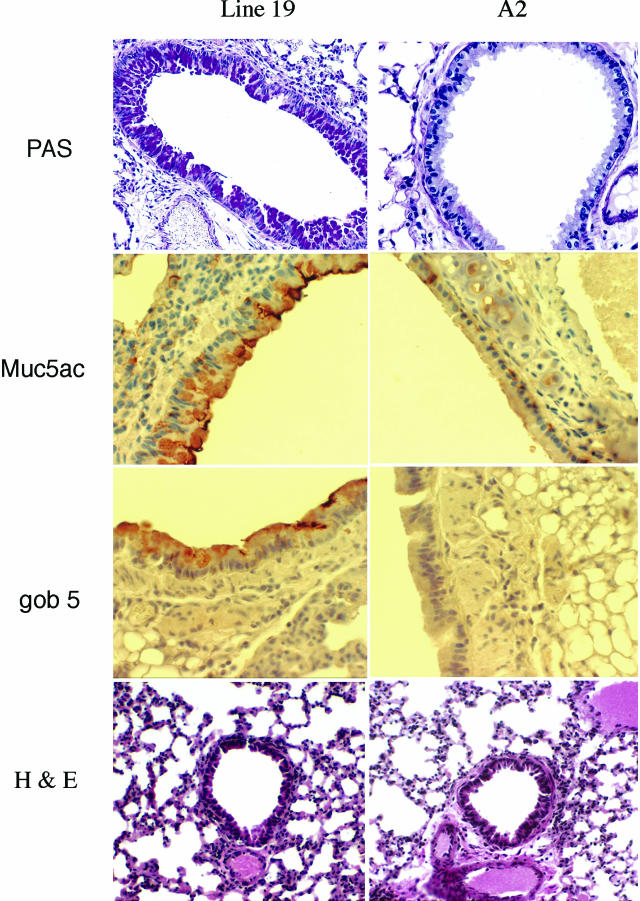
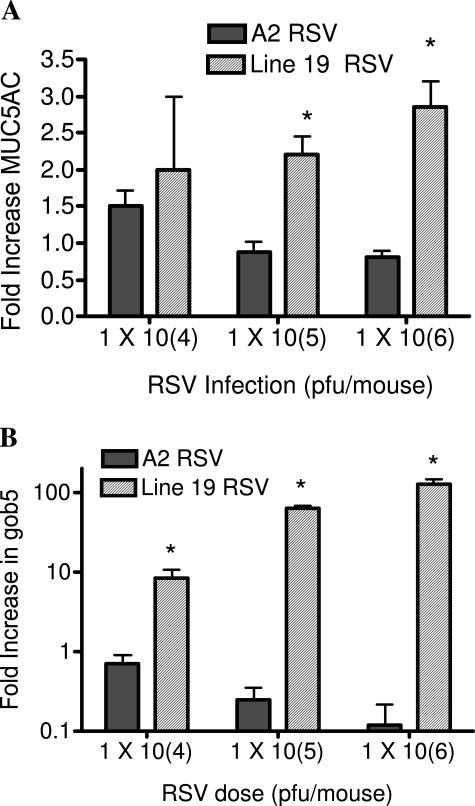
Possibly one of the most devastating aspects of severe RSV infection in infants is the overproduction of mucus. When comparing these two strains of virus, the A2 strain induced no histologically detectable goblet cell expansion and no PAS staining, whereas the Line 19 strain induced significant goblet cell hyper/metaplasia as indicated by the strong PAS staining in the airways of the Line 19-infected mice (Figure 3). Furthermore, immunohistochemistry for two proteins that have been closely associated with mucus overproduction in the airway, gob5 and MUC5AC, indicated similar differences as the PAS staining. Specifically, the immunohistochemical staining pattern for these two proteins clearly stained the airways of mice infected with Line 19 but not those infected with A2 strain RSV (Figure 3). The examination of the inflammatory response indicates no difference in the type of inflammation in the histological sections because both strains induced a primarily lymphocytic infiltration (Figure 3). To further assess and quantitate the mucus-associated differences, pulmonary mRNA was assessed for MUC5AC and gob5 (Figure 4). The data indicates that although the Line 19 strain induced significant increases in MUC5AC at higher concentrations on 9 days after infection, the A2 strain significantly decreased the expression of MUC5AC compared to naive animals, directly correlating with the presence of goblet cells in the airways stained by PAS and by the specific antibodies. The gob5 mRNA levels were increased in a dose-dependent manner only by Line 19. Thus, these two virus strains demonstrate significant differences in pathophysiological responses in airways of BALB/c/J mice.
Figure 3.
PAS staining of histological sections demonstrate goblet cell development in BALB/c mice infected with Line 19 but not with A2. Lungs from experiments in Figure 1 were processed for histology and stained with PAS reagent to identify the mucus-positive cell populations in the lungs of infected mice. No staining was found in uninfected mice. Photomicrographs are representative of all of the animals in the group, demonstrating significant mucus cell staining at 9 days after RSV challenge. Using specific antibodies against MUC5AC and gob5 proteins, lung sections were specifically stained by immunohistochemistry and representative staining is shown for Line 19 and A2. The H&E staining of histological sections demonstrated that both Line 19 and A2 viruses induced significant and similar inflammation within the lungs of the mice.
Figure 4.
Dose-dependent increases in mucus-associated genes, MUC5AC and gob5, in animals infected by Line 19 but not by A2. Animals from a separate RSV dose study were assessed for pulmonary levels of MUC5AC and gob5 at 6 days after infection with the two A strain viruses using quantitative PCR analysis. The data represent the mean ± SE from five to six mice per group. *P < 0.05.
The Differential Induction of Cytokines/Chemokines
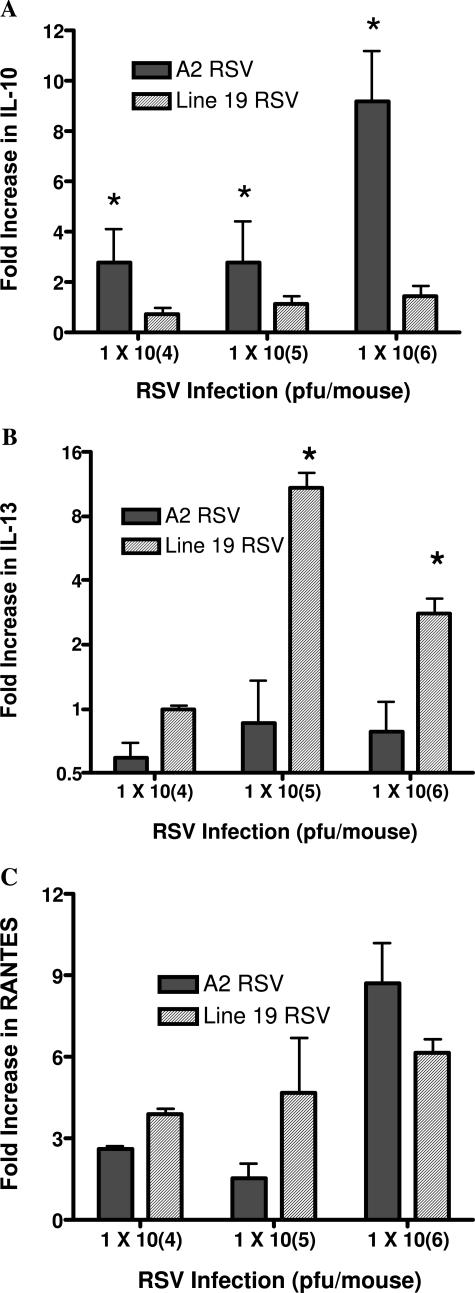
The cytokine environment in the lung during a viral infection likely dictates the severity of the pathophysiology. A number of cytokines have previously been outlined to be important in both the clearance of the virus as well as induction of AHR and mucus. To determine whether there were any differences in specific cytokine expression, lung mRNA was isolated and the expression patterns of specific cytokines determined in infections of each of the viruses (Figure 5). The associated production of IL-10 with the A2 virus, but not Line 19 virus, specifically related to the differential pathophysiological responses observed (Figure 5A). IL-13 has been closely associated with mucus overproduction. When we examined the expression of IL-13 mRNA in the two viral infections, we found that although the Line 19 strain induced significant increases in IL-13, the A2 strain did not increase the IL-13 expression (Figure 5B). Interestingly, when interferon-γ expression was assessed there was no difference in the level of expression between the A2 and Line 19 A strain viruses (data not shown). In addition to immune cytokines examined above, chemokine levels have also been correlated with severe disease. When comparisons were made between the two isolates of RSV for the expression of CCL5 (RANTES) mRNA, a similar profile could be observed. The data in Figure 5C illustrates that CCL5 is similarly up-regulated by the two viruses. Thus, no clear difference could be observed with these viruses in CCL5 expression.
Figure 5.
Differential regulation of cytokines in two RSV A strain lines. Isolated pulmonary mRNA from lungs of 6-day infected mice was assessed for cytokine expression levels using specific primers and probes for quantitative PCR analyses. The dose-dependent increase of IL-10 in A2 (A) and increase of IL-13 in Line 19 (B) correlated with the differences observed in AHR and mucus overexpression. C: No difference in expression of CCL5 (RANTES) was observed in the studies. Data represent mean ± SE from five to six mice per group. *P < 0.05.
Attenuation of Line 19 Pathogenesis in IL-13−/− Mice
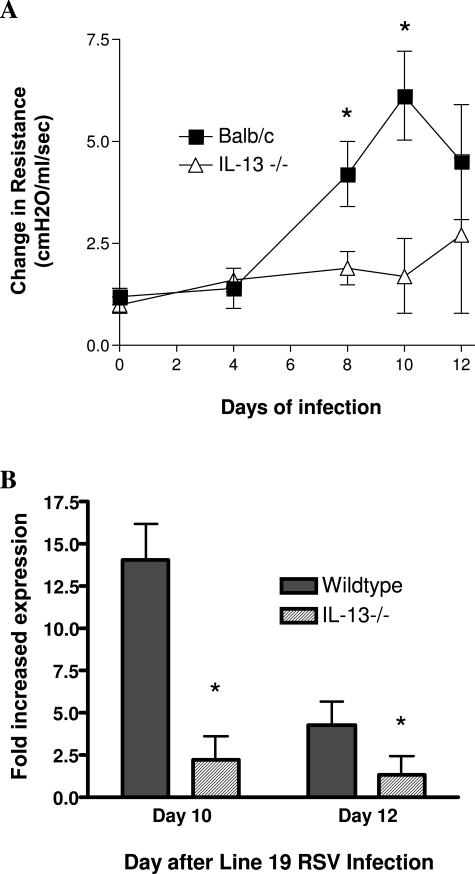
To better define whether IL-13 played a significant role in the development of the airway pathogenesis associated with Line 19 RSV infection, we infected mice deficient in IL-13. The IL-13−/− mice on a BALB/c background did not respond to Line 19 infection with AHR (Figure 6A) as compared to wild-type animals. As indicated in multiple publications, IL-13 is also often associated with development of mucus overproduction and increased gene expression. In these studies we compared the up-regulation of gob5 gene expression and found that the IL-13−/− animals demonstrated significantly lower expression than the wild-type BALB/c mice infected with Line 19 (Figure 6B). Together, these data demonstrate that one of the major pathogenic mediators induced by Line 19 was IL-13.
Figure 6.
Attenuation of Line 19-induced AHR (A) and gob5 expression (B) in IL-13−/− mice. Wild-type and IL-13−/− BALB/c mice were infected with 1 × 105 PFU of Line 19 RSV into the airway. A: After various time points the animals were examined for development of AHR using box plethysmography in anesthetized animals. Peak airway resistance was recorded after an intravenous tail vein administration of methacholine (250 μg/kg). B: After 10 or 12 days of infection, mRNA was isolated from lungs of infected animals, and gob5 gene expression was assessed using qPCR and the fold increase calculated in comparison to pulmonary gob5 mRNA from uninfected animals. Data represent mean ± SE from five to six mice per group. *P < 0.05.
Differential Viral Replication in Vivo and in Vitro
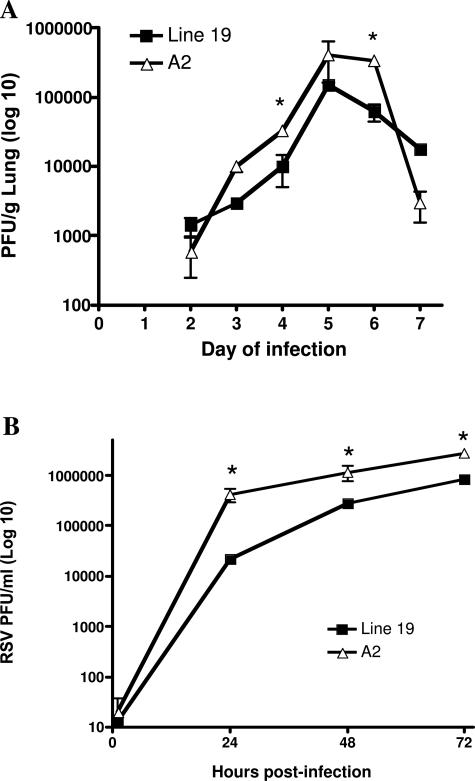
One of the considerations of the different RSV strains that needed to be examined was whether A2 and Line 19 differ in viral growth characteristics. The examination of growth kinetics of the two strains of RSV was examined using in vivo and in vitro assays. Plaque assays were performed to assess viral titers in infected mice. Although the viruses demonstrated similar growth curves, levels of infectious A2 were higher than Line 19 in the lung at 4 and 6 days after infection (Figure 7A). By day 9 of infection there were no detectable viral titers in either strain (data not shown). We also compared A2 and Line 19 growth kinetics in vitro by generating growth curves of infectious yield from HEp-2 cells infected and an multiplicity of infection of 0.5. These studies demonstrated that the A2 strain replicates to significantly higher titers in HEp-2 cells than Line 19 (Figure 7B). This was not surprising because titers of viral stocks of Line 19 are generally lower than titers of A2 stocks (data not shown). Because Line 19 grew to lower titers in the lungs of mice than A2, the increased AHR and mucus production associated with Line 19 infection does not correlate with higher viral load. Thus, virus load does not appear to be a significant factor in the physiological differences observed between these two RSV strains in vivo.
Figure 7.
Differential growth of A2 and Line 19 strains of RSV. A: Mice infected with increasing doses of Line 19 or A2 were assessed for pulmonary viral titers at various days after infection in mice given either Line 19 or A2 A strain RSV infection (1 × 105). Data represent mean ± SE from three mice per group. B: In vitro growth of the two strains of virus demonstrated a significant difference (P < 0.05) in growth as assessed by viral plaque assays in HEp-2 cells. *P < 0.05.
Discussion
The ability to conduct mechanistic studies in animal models of disease is paramount for understanding the progression of complex disease phenotypes. In the present studies the comparison of two RSV antigenic subgroup A strains, A2 and Line 19, has been examined. The clinical manifestations of illness during RSV infections are diverse and can depend on the host responses but may also depend on the specific RSV strain present. In the most severe cases of disease in infants the manifestation of illness can be severe airway reactivity, mucus overexpression, and a broad profile of leukocyte infiltration including neutrophils, lymphocytes, and eosinophils.24 Using animal models, many of these same effects have been observed at differing levels. Interestingly, using the most commonly available strain of RSV available to researchers, A2, the recapitulation of these parameters in primary mouse infection models has been centered on lymphocyte-associated inflammation and airway damage, along with weight loss.14,25–28 In other studies that have used other clinical isolates a number of other parameters have been reported, including AHR, mucus overexpression, and Th2-type cytokine production.18,20,23,29,30 Together, these data begin to question whether the differences in the responses are centered on laboratory methodology or more likely differences in RSV isolates that are used by the different researchers. This latter issue has been previously addressed by clinical studies,31–34 but little has been done to examine a mechanistic reason why different RSV isolates might display varying clinical attributes. More central to the focus of these studies may be whether specific clinical attributes can be modeled using one virus strain or another.
The present studies have used consistent endpoint methodology to examine the viral responses in side-by-side studies to examine the overall responses of two different RSV A strain isolates. The A2 strain has had a long history in modeling responses both in vitro and in vivo, whereas the Line 19 strain has not been examined as extensively. There are clearly differences in physiological responses generated with the two strains. The AHR and mucus production were the most obvious. The fact that these two responses were altered corresponded primarily with differences in the ability to induce IL-10 and IL-13 during the viral infection and not in the level of virus present in the lungs. The A2 strain has been shown either not to express IL-13 and mucus or to down-regulate those responses11,13 while generating significant levels of IL-10 and interferon-γ.35–37 Previous studies have examined the consequences of IL-10 depletion in knockout animals and in animals overexpressing IL-10 but not neutralization during infection in a normal animal during primary infection.38,39 The overexpression of IL-13 in lungs of mice has been clearly linked to pathophysiological changes in not only allergic responses but in several other instances, including RSV infection.23,40–42 In the present studies we also demonstrated the relevance of IL-13 in the development of pathophysiology using IL-13−/− mice and indicated a significant attenuation of the developing AHR responses as well as gob5 gene expression during Line 19 RSV infection. Although both IL-10 and IL-13 have been previously associated with Th2 responses, they clearly have different functions. In particular, IL-10 can regulate a broad range of cytokine production and cellular responses whereas IL-13 can be closely associated with mucus overexpression. Together these results raise several questions about the nature of RSV infections and whether different clinical isolates separated by region or time induce different degrees of severity that is partially dependent on the virus itself along with the specific immune response generated.
There are several possible explanations that relate to the differences observed between the two strains. The differential expression of a particular RSV protein may help to skew the response. Studies have demonstrated that RSV G protein induced a much different immune response compared to RSV F protein on rechallenge with RSV.26,43–49 Thus, the preferential expression and presentation of a particular RSV protein during the infectious process by one isolate versus another may be sufficient to skew the response. It may be that through transcriptional or translational control, different RSV isolates could preferentially express specific proteins. Alternatively, proteins from various RSV strains may be differentially immunogenic. Given the fact that we observed no real difference in G protein mRNA expression, transcriptional control may not be a critical factor (data not shown). However, multiple investigations have identified that RSV strains differ greatly in their ability to promote a successful infection and/or promote inflammatory responses dependent on the nature and soluble versus membrane form of G protein.50–54 The alteration of RSV proteins may lend to the alteration of the immune response.
The initiation of the immune response by these two virus isolates has not been compared. However, the role of dendritic cells for the induction of the immune response is a primary mechanism for determining the outcome and the immune environment.55 The ability of RSV to alter the capacity of dendritic cells to elicit an effective CTL response is striking.7,56,57 Several studies have linked IL-10 expression in whole animals or in isolated dendritic cell/macrophage populations to RSV infection.36–38,58 The fact that the two RSV antigenic subgroup A strains that were examined differentially induced IL-10 expression in vivo in whole lungs may dictate one specific mechanism that alters the severity of the infectious process. It is tempting to speculate that it is at this early immune cell activation level that the different isolates may control the outcome of the immune response. One aspect that has remained relatively uncharacterized within RSV responses is the function of individual RSV proteins on the function of host cells. Is it possible that one or more of the 11 RSV proteins could have immune altering effects on the host system? Successful infectious organisms have developed one or more ways to evade or alter the immune response.59 Differences in the expression of specific RSV proteins by one of the isolates might account for these changes. Studies examining NS protein from RSV have focused on immunomodulatory aspects and suppression of type I interferon production.60
There is a wide spectrum of illness caused by RSV infection, attributable in large part to host-related factors such as age of the patient and degree of host immunocompetency. However, the results from this study strongly suggest that RSV strain-specific immunopathology may be an important contributor to the disparity observed in clinical illness. We are currently undertaking studies to sequence these two viral strains to determine the specific genome alterations that are responsible for the significant differences in RSV-induced airway dysfunction observed. The results of these studies may be important to further our understanding of RSV pathogenesis as well as provide valuable clues for vaccine development to control this clinically important virus.
Acknowledgments
We thank Takeda Chemical Industries for the kind gift of anti-gob-5 antibody.
Footnotes
Address reprint requests to Nicholas W. Lukacs, Ph.D., Associate Professor of Pathology, University of Michigan, 1301 Catherine St., Ann Arbor, MI 48109-0602. E-mail: nlukacs@umich.edu.
Supported in part by the National Institutes of Health (grants AI036302 to N.W.L. and AI054660 to R.S.P.).
References
- Openshaw PJ, Dean GS, Culley FJ. Links between respiratory syncytial virus bronchiolitis and childhood asthma: clinical and research approaches. Pediatr Infect Dis J. 2003;22:S55–S64. doi: 10.1097/01.inf.0000053887.26571.eb. [DOI] [PubMed] [Google Scholar]
- Welliver RC. Respiratory syncytial virus and other respiratory viruses. Pediatr Infect Dis J. 2003;22:S6–S12. doi: 10.1097/01.inf.0000053880.92496.db. [DOI] [PubMed] [Google Scholar]
- Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. 2003;22:S76–S82. doi: 10.1097/01.inf.0000053889.39392.a7. [DOI] [PubMed] [Google Scholar]
- Gentile DA, Skoner DP. Effect of respiratory syncytial virus infection during early infancy on the ontogeny of cytokine immune responses. Allergy Asthma Proc. 2002;23:399–405. [PubMed] [Google Scholar]
- Panitch HB. Bronchiolitis in infants. Curr Opin Pediatr. 2001;13:256–260. doi: 10.1097/00008480-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Peebles RS, Jr, Hashimoto K, Graham BS. The complex relationship between respiratory syncytial virus and allergy in lung disease. Viral Immunol. 2003;16:25–34. doi: 10.1089/088282403763635429. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Durbin RK. Respiratory syncytial virus-induced immunoprotection and immunopathology. Viral Immunol. 2004;17:370–380. doi: 10.1089/vim.2004.17.370. [DOI] [PubMed] [Google Scholar]
- Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–S117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22:S21–S32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Graham BS, Ho SB, Adler KB, Collins RD, Olson SJ, Zhou W, Suzutani T, Jones PW, Goleniewska K, O’Neal JF, Peebles RS., Jr Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am J Respir Crit Care Med. 2004;170:306–312. doi: 10.1164/rccm.200301-030OC. [DOI] [PubMed] [Google Scholar]
- Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- Peebles RS, Jr, Sheller JR, Johnson JE, Mitchell DB, Graham BS. Respiratory syncytial virus infection prolongs methacholine-induced airway hyperresponsiveness in ovalbumin-sensitized mice. J Med Virol. 1999;57:186–192. doi: 10.1002/(sici)1096-9071(199902)57:2<186::aid-jmv17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol. 2002;168:2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Graham BS, Geraci MW, FitzGerald GA, Egan K, Zhou W, Goleniewska K, O’Neal JF, Morrow JD, Durbin RK, Wright PF, Collins RD, Suzutani T, Peebles RS., Jr Signaling through the prostaglandin I2 receptor IP protects against respiratory syncytial virus-induced illness. J Virol. 2004;78:10303–10309. doi: 10.1128/JVI.78.19.10303-10309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlocher ML, Ewasyshyn M, Sambhara S, Gharaee-Kermani M, Cho D, Lai J, Klein M, Maassab HF. Immunological properties of plaque purified strains of live attenuated respiratory syncytial virus (RSV) for human vaccine. Vaccine. 1999;17:172–181. doi: 10.1016/s0264-410x(98)00155-8. [DOI] [PubMed] [Google Scholar]
- Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis. 2004;189:1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- John AE, Gerard CJ, Schaller M, Miller AL, Berlin AA, Humbles AA, Lukacs NW. Respiratory syncytial virus-induced exaggeration of allergic airway disease is dependent upon CCR1-associated immune responses. Eur J Immunol. 2005;35:108–116. doi: 10.1002/eji.200425439. [DOI] [PubMed] [Google Scholar]
- Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Strieter RM, Gruber AD, Ho SB, Lukacs NW. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol. 2003;170:3348–3356. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- Tekkanat KK, Maassab H, Miller A, Berlin AA, Kunkel SL, Lukacs NW. RANTES (CCL5) production during primary respiratory syncytial virus infection exacerbates airway disease. Eur J Immunol. 2002;32:3276–3284. doi: 10.1002/1521-4141(200211)32:11<3276::AID-IMMU3276>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Tekkanat KK, Berlin A, Hogaboam CM, Miller A, Evanoff H, Lincoln P, Maassab H. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol. 2001;167:1060–1065. doi: 10.4049/jimmunol.167.2.1060. [DOI] [PubMed] [Google Scholar]
- Tekkanat KK, Maassab HF, Cho DS, Lai JJ, John A, Berlin A, Kaplan MH, Lukacs NW. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J Immunol. 2001;166:3542–3548. doi: 10.4049/jimmunol.166.5.3542. [DOI] [PubMed] [Google Scholar]
- Hoffman SJ, Laham FR, Polack FP. Mechanisms of illness during respiratory syncytial virus infection: the lungs, the virus and the immune response. Microbes Infect. 2004;6:767–772. doi: 10.1016/j.micinf.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Chang J, Choi SY, Jin HT, Sung YC, Braciale TJ. Improved effector activity and memory CD8 T cell development by IL-2 expression during experimental respiratory syncytial virus infection. J Immunol. 2004;172:503–508. doi: 10.4049/jimmunol.172.1.503. [DOI] [PubMed] [Google Scholar]
- Varga SM, Wissinger EL, Braciale TJ. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J Immunol. 2000;165:6487–6495. doi: 10.4049/jimmunol.165.11.6487. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Chargelegue D, Steward MW. Reduction of respiratory syncytial virus titer in the lungs of mice after intranasal immunization with a chimeric peptide consisting of a single CTL epitope linked to a fusion peptide. Virology. 1998;240:376–381. doi: 10.1006/viro.1997.8923. [DOI] [PubMed] [Google Scholar]
- Tang YW, Graham BS. Interleukin-12 treatment during immunization elicits a T helper cell type 1-like immune response in mice challenged with respiratory syncytial virus and improves vaccine immunogenicity. J Infect Dis. 1995;172:734–738. doi: 10.1093/infdis/172.3.734. [DOI] [PubMed] [Google Scholar]
- Tekkanat KK, Maassab H, Berlin AA, Lincoln PM, Evanoff HL, Kaplan MH, Lukacs NW. Role of interleukin-12 and stat-4 in the regulation of airway inflammation and hyperreactivity in respiratory syncytial virus infection. Am J Pathol. 2001;159:631–638. doi: 10.1016/S0002-9440(10)61734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171:1051–1060. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- Johansen J, Christensen LS, Hornsleth A, Klug B, Hansen KS, Nir M. Restriction pattern variability of respiratory syncytial virus during three consecutive epidemics in Denmark. APMIS. 1997;105:303–308. doi: 10.1111/j.1699-0463.1997.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Kneyber MC, Brandenburg AH, Rothbarth PH, de Groot R, Ott A, van Steensel-Moll HA. Relationship between clinical severity of respiratory syncytial virus infection and subtype. Arch Dis Child. 1996;75:137–140. doi: 10.1136/adc.75.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz MC, Kew OM, Anderson LJ. Genetic heterogeneity of the attachment glycoprotein G among group A respiratory syncytial viruses. Virus Res. 1994;33:203–217. doi: 10.1016/0168-1702(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Hendry RM, Talis AL, Godfrey E, Anderson LJ, Fernie BF, McIntosh K. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J Infect Dis. 1986;153:291–297. doi: 10.1093/infdis/153.2.291. [DOI] [PubMed] [Google Scholar]
- Chung HL, Kim WT, Kim JK, Choi EJ, Lee JH, Lee GH, Kim SG. Relationship between atopic status and nasal interleukin 10 and 11 levels in infants with respiratory syncytial virus bronchiolitis. Ann Allergy Asthma Immunol. 2005;94:267–272. doi: 10.1016/S1081-1206(10)61307-5. [DOI] [PubMed] [Google Scholar]
- Bartz H, Buning-Pfaue F, Turkel O, Schauer U. Respiratory syncytial virus induces prostaglandin E2, IL-10 and IL-11 generation in antigen presenting cells. Clin Exp Immunol. 2002;129:438–445. doi: 10.1046/j.1365-2249.2002.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuska JR, Merolla R, Rebert NA, Hoffmann SP, Tsivitse P, Cirino NM, Silverman RH, Rankin JA. Respiratory syncytial virus induces interleukin-10 by human alveolar macrophages. Suppression of early cytokine production and implications for incomplete immunity. J Clin Invest. 1995;96:2445–2453. doi: 10.1172/JCI118302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela MJ, Kanehiro A, Dakhama A, Borish L, Joetham A, Tripp R, Anderson L, Gelfand EW. The failure of interleukin-10-deficient mice to develop airway hyperresponsiveness is overcome by respiratory syncytial virus infection in allergen-sensitized/challenged mice. Am J Respir Crit Care Med. 2002;165:824–831. doi: 10.1164/ajrccm.165.6.2105062. [DOI] [PubMed] [Google Scholar]
- Ruan Y, Okamoto Y, Matsuzaki Z, Endo S, Matsuoka T, Kohno T, Chazono H, Eiko I, Tsubota K, Saito I. Suppressive effect of locally produced interleukin-10 on respiratory syncytial virus infection. Immunology. 2001;104:355–360. doi: 10.1046/j.1365-2567.2001.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JJ, Dabbagh K, Ueki IF, Dao-Pick T, Burgel PR, Takeyama K, Tam DC, Nadel JA. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol. 2001;280:L134–L140. doi: 10.1152/ajplung.2001.280.1.L134. [DOI] [PubMed] [Google Scholar]
- Yang G, Volk A, Petley T, Emmell E, Giles-Komar J, Shang X, Li J, Das AM, Shealy D, Griswold DE, Li L. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28:224–232. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Alwan WH, Record FM, Openshaw PJ. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211–5218. [PubMed] [Google Scholar]
- Spender LC, Hussell T, Openshaw PJ. Abundant IFN-gamma production by local T cells in respiratory syncytial virus-induced eosinophilic lung disease. J Gen Virol. 1998;79:1751–1758. doi: 10.1099/0022-1317-79-7-1751. [DOI] [PubMed] [Google Scholar]
- Sparer TE, Matthews S, Hussell T, Rae AJ, Garcia-Barreno B, Melero JA, Openshaw PJ. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T, Baldwin CJ, O’Garra A, Openshaw PJ. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- Openshaw PJ, Anderson K, Wertz GW, Askonas BA. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990;64:1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham CR, Openshaw PJ, Ball LA, King AM, Wertz GW, Askonas BA. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986;137:3973–3977. [PubMed] [Google Scholar]
- Simmons CP, Hussell T, Sparer T, Walzl G, Openshaw P, Dougan G. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T cell responses. J Immunol. 2001;166:1106–1113. doi: 10.4049/jimmunol.166.2.1106. [DOI] [PubMed] [Google Scholar]
- Teng MN, Whitehead SS, Collins PL. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology. 2001;289:283–296. doi: 10.1006/viro.2001.1138. [DOI] [PubMed] [Google Scholar]
- Arnold R, Konig B, Werchau H, Konig W. Respiratory syncytial virus deficient in soluble G protein induced an increased proinflammatory response in human lung epithelial cells. Virology. 2004;330:384–397. doi: 10.1016/j.virol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bembridge GP, Lopez JA, Bustos R, Melero JA, Cook R, Mason H, Taylor G. Priming with a secreted form of the fusion protein of respiratory syncytial virus (RSV) promotes interleukin-4 (IL-4) and IL-5 production but not pulmonary eosinophilia following RSV challenge. J Virol. 1999;73:10086–10094. doi: 10.1128/jvi.73.12.10086-10094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes O, Martinez I, Valdivia A, Cancio R, Savon C, Goyenechea A, Melero JA. Unusual antigenic and genetic characteristics of human respiratory syncytial viruses isolated in Cuba. J Virol. 1998;72:7589–7592. doi: 10.1128/jvi.72.9.7589-7592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MA, Palucka AK, Barton T, Ghaffar F, Jafri H, Banchereau J, Ramilo O. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis. 2005;191:1105–1115. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Matsuse H, Machida I, Kawano T, Saeki S, Tomari S, Obase Y, Fukushima C, Kohno S. Regulation of mite allergen-pulsed murine dendritic cells by respiratory syncytial virus. Am J Respir Crit Care Med. 2004;169:494–498. doi: 10.1164/rccm.200305-663OC. [DOI] [PubMed] [Google Scholar]
- Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology. 2003;109:49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bont L, Heijnen CJ, Kavelaars A, van Aalderen WM, Brus F, Draaisma JT, Geelen SM, Kimpen JL. Monocyte IL-10 production during respiratory syncytial virus bronchiolitis is associated with recurrent wheezing in a one-year follow-up study. Am J Respir Crit Care Med. 2000;161:1518–1523. doi: 10.1164/ajrccm.161.5.9904078. [DOI] [PubMed] [Google Scholar]
- Piguet V. Receptor modulation in viral replication: HIV, HSV, HHV-8 and HPV: same goal, different techniques to interfere with MHC-I antigen presentation. Curr Top Microbiol Immunol. 2005;285:199–217. doi: 10.1007/3-540-26764-6_7. [DOI] [PubMed] [Google Scholar]
- Gotoh B, Komatsu T, Takeuchi K, Yokoo J. Paramyxovirus accessory proteins as interferon antagonists. Microbiol Immunol. 2001;45:787–800. doi: 10.1111/j.1348-0421.2001.tb01315.x. [DOI] [PubMed] [Google Scholar]