Abstract
Insulin resistance induces nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (NASH). We used a high-fat, high-calorie solid diet (HFD) to create a model of insulin resistance and NASH in nongenetically modified rats and to study the relationship between visceral adipose tissue and liver. Obesity and insulin resistance occurred in HFD rats, accompanied by a progressive increase in visceral adipose tissue tumor necrosis factor (TNF)-α mRNA and in circulating free fatty acids. HFD also decreased adiponectin mRNA and peroxisome proliferator-activated receptor (PPAR)-α expression in the visceral adipose tissue and the liver, respectively, and induced hepatic insulin resistance through TNF-α-mediated c-Jun N-terminal kinase (JNK)-dependent insulin receptor substrate-1Ser307 phosphorylation. These modifications lead to hepatic steatosis accompanied by oxidative stress phenomena, necroinflammation, and hepatocyte apoptosis at 4 weeks and by pericentral fibrosis at 6 months. Supplementation of n-3 polyunsaturated fatty acid, a PPARα ligand, to HFD-treated animals restored hepatic adiponectin and PPARα expression, reduced TNF-α hepatic levels, and ameliorated fatty liver and the degree of liver injury. Thus, our model mimics the most common features of NASH in humans and provides an ideal tool to study the role of individual pathogenetic events (as for PPARα down-regulation) and to define any future experimental therapy, such as n-3 polyunsaturated fatty acid, which ameliorated the degree of liver injury.
Nonalcoholic fatty liver disease (NAFLD) is commonly associated with the clinical features of the metabolic syndrome such as obesity, type II diabetes, and dislipidemia.1 The clinical importance of NAFLD is due to its high prevalence (25% of the general population) and its wide spectrum of histological damage ranging from simple steatosis, which is generally nonprogressive, to nonalcoholic steatohepatitis (NASH), which can lead to cirrhosis, hepatocellular carcinoma, and liver failure.1,2
Insulin resistance is central to the pathogenesis of the metabolic syndrome, and recent data indicate that NAFLD should be considered the hepatic manifestation of the metabolic syndrome.3 It has been demonstrated that a series of molecular alterations in insulin signaling occurs in the setting of insulin resistance, finally resulting in triglyceride accumulation in the liver. The insulin receptor belongs to a subfamily of receptor tyrosine kinases that includes insulin-like growth factor-1 and insulin receptor-related receptor. After insulin binding, its receptor undergoes autophosphorylation and catalyzes the tyrosine phosphorylation of cellular proteins such as members of the insulin receptor substrate (IRS) family. In addition to tyrosine phosphorylation, IRS proteins undergo serine phosphorylation, which may attenuate signaling by decreasing insulin-stimulated tyrosine phosphorylation. Several kinases have been implicated in this process, including phosphatidylinositol 3-kinase (PI3K)/Akt, protein kinase C (PKC), and the mitogen-activated protein kinase (MAPK) pathway.4
The study of the pathogenetic or therapeutic factors involved in NASH has been hampered by the absence of a suitable experimental model because most studies use rodents with genetic defects or involve feeding rats a diet lacking in choline and methionine (MCD), creating a nutritional deficiency that is not common in patients with NASH.5 Recently a high-fat liquid diet, given to normal rats for a short period of time, was shown to cause steatosis, inflammation, oxidative stress, and increased collagen synthesis.6 However, the liquid diet represents a time-consuming system to be diffusely used as a model of NASH, and the relationship between the liver and other organs involved in the energy balance, such as adipose tissue, was not investigated.6 In this regard, the adipocyte is no longer regarded as a passive depot for storing excess energy in the form of triglyceride but as a cell that actively regulates the energy balance and that secretes proinflammatory cytokines, such as TNF-α, that are able to influence insulin sensitivity and metabolic processes in peripheral tissues, liver included.7 This occurs via specific transcription factors, such as the nuclear hormone receptor family of peroxisome proliferator-activated receptors (PPARs), which regulate both inflammation and lipid metabolism. The PPARs are nuclear receptors that bind to fatty acid-derived ligands and activate the transcription of genes that regulate lipid metabolism. Three PPAR isoforms have been described: α, δ (or β), and γ. PPARγ is found in adipocytes, macrophages, and muscle cells, where it regulates adipocyte differentiation, fatty acid uptake and storage, and glucose metabolism; PPARγ expression is low in tissues that express predominantly PPARα. The primary sites of action of PPARα are liver, heart, muscle, and kidney. PPARα activates a program of target gene expression involved in fatty acid uptake, β-oxidation, transport into peroxisomes, and ω-oxidation of unsaturated fatty acids. Several experimental studies suggest that PPARα might increase fatty acid catabolism and that, in the pathophysiologic context of the metabolic syndrome or high-fat diet, PPARα-induced fatty acid catabolism might prevent hepatic fat deposition.8–11 The hepatic expression and the finding that different compounds such as fibrates and n-3 polyunsaturated fatty acids (PUFA) act as its agonists make PPARα an attractive target in the pathophysiology and treatment of fatty liver.12
We thus took advantage of a high-fat, high-calorie solid diet13 to pursue the aims of our study 1) to create a model of insulin resistance and NASH in nongenetically modified animals, 2) to study the relationship between the visceral adipose tissue and the liver in this model, and 3) to evaluate the possible anti-steatogenic effects of n-3 PUFA as natural ligands of PPARα.
Materials and Methods
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise indicated. The following antibodies were used: anti-diphosphorylated extracellular signal-regulated kinase (ERK)1/2 (pERK) (1:5000 final dilution), anti-ERK2 (1:1000), anti-Akt (1:2000), anti-Ser-473 phosphorylated Akt (pAkt) (1:1000), anti-insulin receptor substrate-1 (IRS-1), anti-JNK2 (1:1000), anti-Thr-183/Tyr185 phosphorylated JNK (pJNK) (1:800), anti-PPARα (1:400) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-Ser-307 phosphorylated IRS-1 (IRS-1Ser307) (Cell Signaling Technology, Danvers, MA), and anti-β-actin (Sigma Chemical Co.).
Male Sprague-Dawyley CD rats (100 to 120 g body weight) (Charles River Laboratories, Como, Italy) were fed either a control pellet diet (5% of energy derived from fat, 18% from proteins, and 77% from carbohydrates; 3.3 kcal/g) (Harlan Italy, San Pietro al Natisone, UD, Italy) or a high-fat pellet diet (HFD) (58% of energy derived from fat, 18% from protein, and 24% from carbohydrates; 5.6 kcal/g) (Laboratorio Dottori Piccioni, Gessate, Milano, Italy).13 The amount of lipids was provided by saturated (0.9 and 30.4 g/100 g diet) and unsaturated (4.6 and 5.3 g/100 g diet) fat with n-6-to-n-3 PUFA ratios of 11.3 and 85.9 in the control and HFD diet, respectively. Body weight and food intake were measured weekly. Rats were sacrificed after 1, 3, and 6 months of treatment (n = 6 for each time point). Each experiment was performed following the guidelines of the local committee for care and use of laboratory animals.
Preparation of Tissue and Histological Evaluation
Portal blood was obtained before liver removal. After liver removal, the epididymal fat was removed to measure visceral adipose tissue, and its weight and volume were recorded.14 The degree of necroinflammatory liver injury was quantitated according to Brunt.1 The parenchymal extension of fibrotic tissue was determined by morphometry as previously described.15 Plasma insulin, glucose, ALT, tumor necrosis factor-α (TNF-α), adiponectin, and free fatty acid (FFA) levels were determined by commercially available kits.
Lipid Content of the Liver
Hepatic lipids were extracted as previously described.16 The lipid extracts were resuspended in methanol and used for the evaluation of cholesterol and triglycerides levels by commercial kits from Sigma Chemical Co.
Measurement of Thiobarbituric Acid Reactive Substances (TBARS) and Lipid Hydroperoxides in the Liver
The levels of hepatic lipid peroxides were spectrophotometrically determined by the thiobarbituric acid method.17 A malondialdehyde solution freshly made by hydrolysis of 1,1,3,3-tetrametoxypropane was used as standard. The results were expressed as nanomoles of malondialdehyde per gram of liver. The levels of hepatic lipid hydroperoxides were evaluated using the xylenol orange assay as previously described.18 A solution of t-butylhydroperoxide solution (7.3 × 10−4 mol/L) was used as standard. The results were expressed as nanomoles of lipid hydroperoxides per gram of liver. In parallel experiments TBARS and hydroperoxides levels were evaluated either in the absence or in the presence of the antioxidant butyl-hydroxy-toluene (BHT; 1 mmol/L) to block spontaneous and additional generation of reactive oxygen species.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RNA isolation, reverse transcription, and PCR for TNF-α and adiponectin mRNA were performed as described previously.19,20 For every PCR assay, expression of β-actin was used as an internal control. Results were visualized by agarose electrophoresis.
Western Blotting
Western blot on total liver proteins or nuclear extracts was performed as previously described.21,22 After electrophoresis on 9% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transfer to a nitrocellulose membrane, immunoblotting was performed according to the manufacturer’s instructions for each primary antibody. After electrotransfer, equal loading of the proteins was checked by staining the blots with 0.2% Ponceau-S Red.22 Immunoreactive proteins were then detected by enhanced chemiluminescence system according to the manufacturer’s instructions (ECL; Amersham Biosciences Europe GmbH, Cologno Monzese, Italy). The intensity of the bands was then quantified by scanning video densitometry (Kodak Digital Sciences, Rochester, NY) and expressed as arbitrary units.
Immunoprecipitation
Tissue samples (5 mg) were lysed in radio-immunoprecipitation assay buffer. Immunoprecipitation was performed for 2 hours at 4°C with 2 μg of an antibody against total IRS-1 (final dilution 1:100) according to manufacturer’s instructions. Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and then immunoblotted with an antibody against total IRS-1 or IRS-1Ser307.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nicked End Labeling (TUNEL) Assay
For in situ detection of apoptosis at the single cell level, we used the TUNEL method (ApopTag kit; Oncor, Gaithersburg, MD). The specimens were examined using a computerized image analysis system (Cue 3; Galai Production Ltd., Migdal Haemek, Israel) connected to an Olympus microscope (Olympus Vanox AHBT3, Olympus Optical Co. Ltd., Tokyo, Japan), and data were expressed as the number of TUNEL-positive cells per field (×100).23
Measurement of n-6-to-n-3 PUFA Ratio
n-6 and n-3 PUFA were measured by means of a gas-chromatographic technique coupled with selected ion monitoring equipment (Agilent Technologies, Cernusco sul Naviglio, Milan, Italy) as elsewhere described, and data were expressed as nanograms per milliliter.24
JNK Phosphorylation in Vitro
Hepatocytes were isolated from normal CD Sprague-Dawley rats (200 to 250 g body weight) as previously described.25 Hepatocytes were then plated in six-well plates (0.5 × 106 cells/well) coated with rat type I collagen in Waymouth’s medium containing 10% fetal bovine serum, 0.1 mmol/L insulin, and 0.1 mmol/L dexamethasone in a humidified atmosphere at 37°C and 5% CO2. After 3 hours, the culture was washed with phosphate-buffered saline, and cells were incubated overnight with hormone-free medium containing 0.2 mmol/L l-glutamine, 5 mg/ml transferrin, 1 nmol/L selenium, and 10 nmol/L free fatty acids in RPMI. Cells were then incubated with TNF-α (30 ng/ml) or insulin (100 nmol/L) for 30 minutes and lysed in radio-immunoprecipitation assay buffer as previously described.26,21 Cell extracts (50 μg/lane) were then used for Western blotting as indicated above.
Statistical Analysis
Results are expressed as mean ± SD. Group means were compared by analysis of variance followed by the Student-Newman-Keuls test whether the former was significant. A P value of <0.05 was considered statistically significant.
Results
General Effects of Control and High-Fat Diets
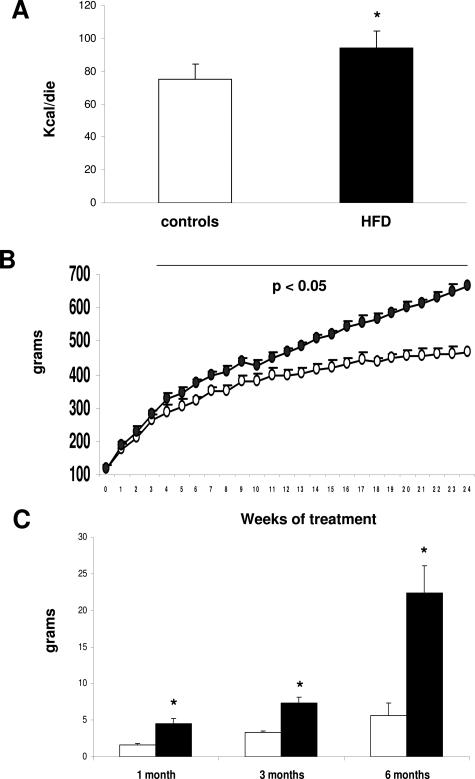
Rats fed HFD consumed significantly more calories on a “per day” basis than controls (Figure 1A), and this was associated with a progressive increase in rat body weight compared with control animals (Figure 1B). The increase in body weight was associated with the development of visceral obesity, as shown by the progressive increase in epididymal fat weight and volume (Figure 1C; data not shown) and by the development of insulin resistance, as shown by higher insulin and glucose values in the portal blood compared with controls (Table 1). Thus, these data indicate that the HFD diet is able to induce obesity and insulin resistance in nongenetically modified animals.
Figure 1.
Effect of HFD on the daily calories consumed (A) and on rat body weight (B) and epididymal fat weight (C). White bars, control animals; black bars, HFD-treated animals. Open circles, control animals; black circles, HFD-treated animals. Data are presented as mean ± SD. *P < 0.05 versus controls.
Table 1.
Effect of the HFD on Insulin, Glucose, and ALT Values in the Portal Blood
| Biochemical parameters | Controls | 1 month | 3 months | 6 months |
|---|---|---|---|---|
| Insulin (μg/L) | 0.2 ± 0.03 | 0.7 ± 0.08* | 0.7 ± 0.05* | 0.9 ± 0.01* |
| Glucose (mg/dl) | 111.8 ± 12.3 | 147.1 ± 18.9* | 151.0 ± 15.7* | 203.5 ± 18.2* |
| ALT (IU/ml) | 39.3 ± 4.2 | 97.6 ± 14.7 | 132.1 ± 12.7 | 148.4 ± 19.8 |
P < 0.05 versus controls.
Effect of HFD on Visceral Adipose Tissue Synthesis of TNF-α and on Secretion of TNF-α and FFAs
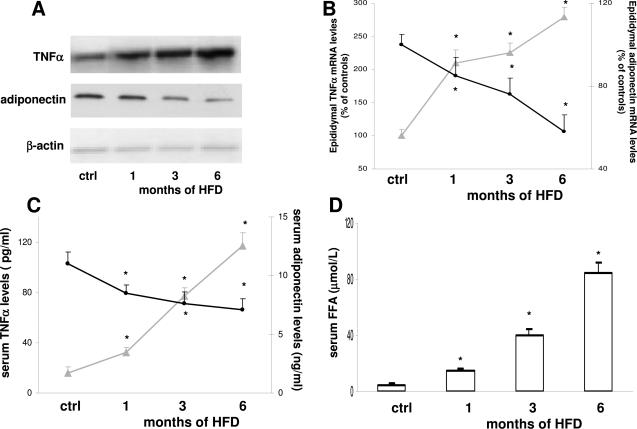
It has been hypothesized that several factors released from the hypertrophic visceral adipose tissue could contribute to the development of hepatic insulin resistance.7 To study this in our model, we measured TNF-α and adiponectin synthesis in the visceral adipose tissue as well as TNF-α, adiponectin, and FFA levels in the portal blood. By RT-PCR, single bands of 705 and 108 bp for TNF-α and adiponectin mRNA, respectively, were observed in the epididymal adipose tissue of control rats. The level of TNF-α mRNA expression was strikingly elevated in adipose tissue obtained from HFD animals compared with controls. When normalized to the β-actin mRNA bands, a twofold increase was evident at 1 month, followed by a progressive increase up to 6 months (Figure 2, A and B). This increased synthesis was associated with higher TNF-α levels in the portal blood (Figure 2C). In parallel, the intensity of the mRNA adiponectin band decreased as early as 1 month. This reduction peaked at 6 months (60% of control value) and led to a constant decrease in adiponectin serum levels (Figure 2, B and C). Finally, enhanced levels of FFA were observed in the portal blood after 1 month of HFD, again progressively increasing and peaking at 6 months of treatment (Figure 2D).
Figure 2.
Effect of HFD on TNF-α and adiponectin mRNA levels (A and B), levels of TNF-α and adiponectin in serum blood (C), and levels of FFA in serum blood (D). No differences were observed between controls at the different time points, which were thus considered as a single control group. A representative agarose gel of RT-PCR for TNF-α and adiponectin mRNA is shown in A. Gray lines, TNF-α; black lines, adiponectin; white bars, FFA. Data are presented as mean ± SD. *P < 0.05 versus controls.
Effect of HFD on PPARα Expression
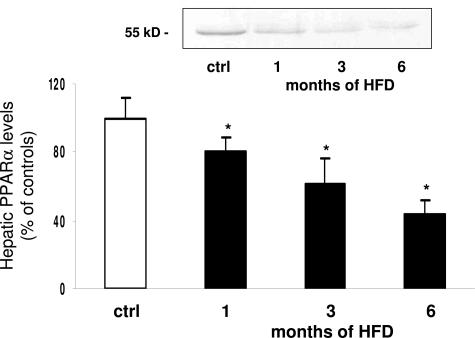
In the PPAR family, the α-isoform is involved in hepatic lipid metabolism and is regulated by adipokines such as TNF-α and adiponectin.8,27,28 We thus measured PPARα protein expression in liver nuclear extracts from control and HFD animals. PPARα was readily detected in the liver of control rats, and its level was significantly reduced (by 20%) at 1 month of HFD, reaching 40% of control values at 6 months (Figure 3).
Figure 3.
Effect of HFD on PPARα expression in whole-liver homogenates. No differences were observed between controls at the different time points, which were thus considered as a single control group. A representative Western blot is shown. White bar, controls; black bars, HFD-treated animals. Data are presented as mean ± SD. *P < 0.05 versus controls.
Effect of HFD on Deposition of Lipid and Production of TBARS and Lipid Hydroperoxides
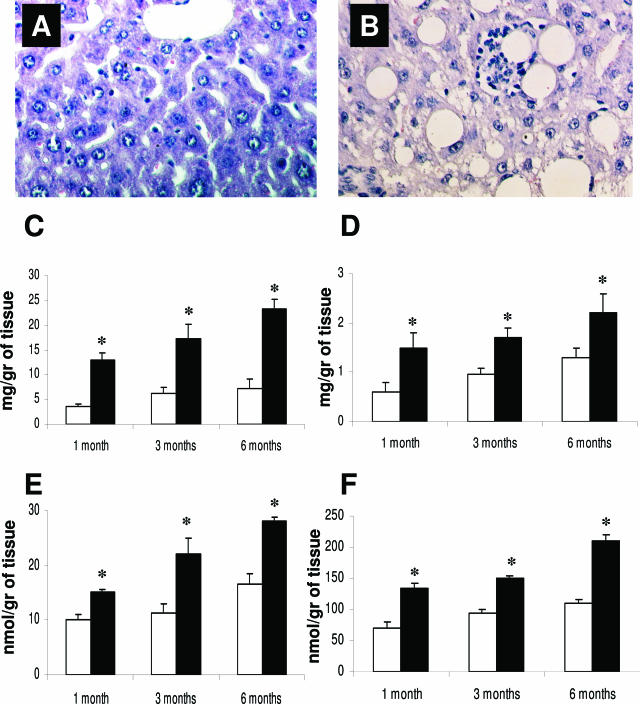
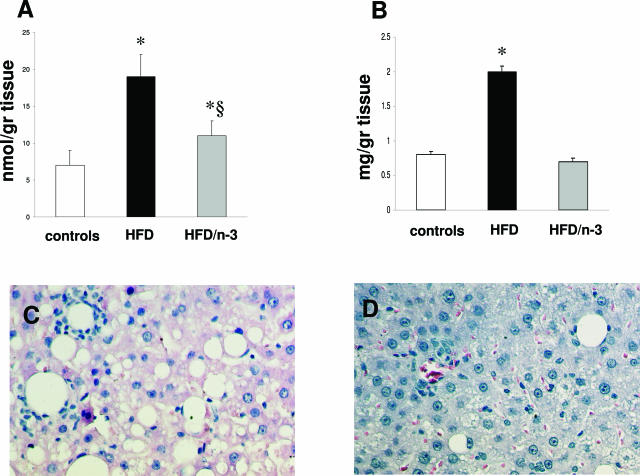
Insulin resistance and decreased PPARα expression were associated with liver steatosis (as shown in Figure 4, A and B). Fatty liver was quantified by measuring triglyceride and cholesterol content in the liver, both of which were increased 1 month after HFD (Figure 4, C and D). This was associated with increased hepatic production of TBARS and lipid hydroperoxides, measured as expression of lipid peroxidation (Figure 4, E and F). Interestingly, hepatic TBARS and hydroperoxide production showed a highly significant linear correlation with both cholesterol and triglyceride liver content (r = 0.78 and 0.63 for triglyceride and r = 0.67 and 0,.84 for cholesterol content versus TBARS and lipid hydroperoxides production, respectively). To evaluate the role of spontaneous and additional generation of reactive oxygen species, TBARS and hydroperoxide levels were evaluated in parallel experiments in both the absence and the presence of the antioxidant BHT (1 mmol/L). The addition of BHT did not modify the differences between controls and HFD-treated animals in TBARS and hydroperoxide levels (data not shown).
Figure 4.
H&E staining in control (A) and HFD-treated (B) rat for 3 months. The effect of HFD on triglyceride and cholesterol content in the liver (C and D) and on TBARS and hydroperoxide production (E and F). No alterations were observed in the liver of the rats fed the control diet (A), whereas HFD induced pronounced hepatic steatosis and inflammatory cells infiltrate surrounding steatotic hepatocytes (lipogranuloma). White bars, controls; black bars, HFD-treated animals. Data are presented as mean ± SD. *P < 0.05 versus controls. Final magnification, ×100.
Effect of HFD on Intracellular Signaling Pathways
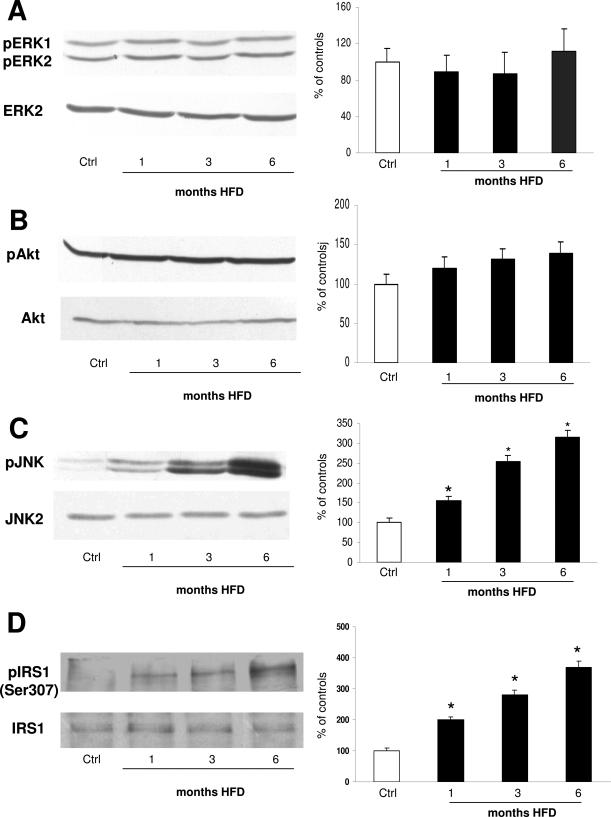
It has been reported that insulin resistance can be associated with specific alterations in the intracellular signaling pathways, such as Ser307 phosphorylation of IRS-1, and that this process can be mediated in vitro by specific kinases such as PI3K/Akt, PKC, and the MAPK system.4 To elucidate this in the liver in vivo, we took advantage of using antibodies specifically recognizing protein phosphorylation, which parallels enzyme activation21 (Figure 5, A–D). In rats fed the control diet, no differences were observed in ERK, Akt, and JNK phosphorylation at 1, 3, and 6 months. Animals were thus considered as a single control group. In HFD animals, despite the increase in portal insulin levels, no increase in pERK was observed, whereas pAkt slightly increased without reaching the statistical significance (Figure 5, A and B). In striking contrast, increases in JNK1/2 Thr183/Tyr185 phosphorylation were observed at 1 month of HFD, progressively increasing and then reaching a 3.5-fold elevation at 6 months (Figure 5C).
Figure 5.
Effect of HFD on phosphorylation of ERK (A), Akt (B), JNK (C), and IRS-1Ser307 (D). On the left, representative Western blots are shown. On the right, the densitometric analysis of phosphorylation level is shown. No differences were observed between controls at the different time points, which were thus considered as a single control group. Data are presented as mean ± SD. *P < 0.05 versus controls.
IRS-1 is an adaptor protein that mediates the activation of downstream effectors of insulin signaling. In several in vitro models of insulin resistance, it has been shown that under the effect of TNF-α, phosphorylated JNK associates with the principal insulin receptor substrate IRS-1 and induces insulin resistance by Ser307 phosphorylation.29 To verify this in the liver in vivo, total IRS-1 was immunoprecipitated from whole-liver lysates. Immunopurified proteins were then immunoblotted with antibodies against total IRS-1 or IRS-1Ser307. No differences were observed in control and HFD-treated animals concerning total IRS-1 levels. IRS-1Ser307 was barely detectable in immunoprecipitates from control rat livers. However, IRS-1Ser307 levels progressively increased during HFD treatment, as shown by the IRS-1-to-IRS-1Ser307 ratio in Figure 5D.
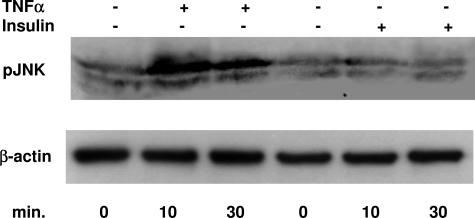
To confirm the role of TNF-α in mediating JNK phosphorylation, primary cultures of rat hepatocytes were incubated with TNF-α (30 ng/ml) or insulin (100 nmol/L). At 10 minutes TNF-α induced a striking increase in JNK phosphorylation that was maintained at 30 minutes, whereas this effect was not observed after insulin incubation (Figure 6).
Figure 6.
Effect of TNF-α (30 ng/ml) and insulin (100 nmol/L) on JNK phosphorylation. Hepatocytes were isolated and cultured as described in Materials and Methods and then incubated with TNF-α (30 ng/ml) or insulin (100 nmol/L) for the indicated period of time. Cell lysates (50 μg/lane) were separated by electrophoresis, transferred to nitrocellulose, and then incubated with the specific antibody. β-Actin was used to show equal loading.
Effect of HFD on Liver Injury
ALT values showed a progressive increase in HFD animals compared with controls (Table 1). No histological signs of liver injury were observed in rats fed the chow diet, and thus these animals were considered as a single control group. In contrast, livers from HFD rats showed steatosis (predominantly macrovesicular) involving up to 66% of specimens, distributed in perivenular and periportal region by month 1, associated with occasional ballooned zone 3 hepatocytes and scattered and mild intraacinar inflammation (polymorphs). By 3 and 6 months, steatosis affected most of the hepatocytes, ballooning was diffusely present, and foci of mixed inflammatory cell infiltration and hepatocyte necrosis or apoptosis appeared throughout the lobule (Figure 4B). To quantify this, the degree of steatosis (1.24 ± 0.43 at 1 month, 2.40 ± 0.48 at 3 months, and 2.57 ± 0.49 at 6 months) and necroinflammation (1.75 ± 0.25 at 1 month, 2.2 ± 0.4 at 3 months, and 2.6 ± 0.3 at 6 months) were measured according to Brunt.1
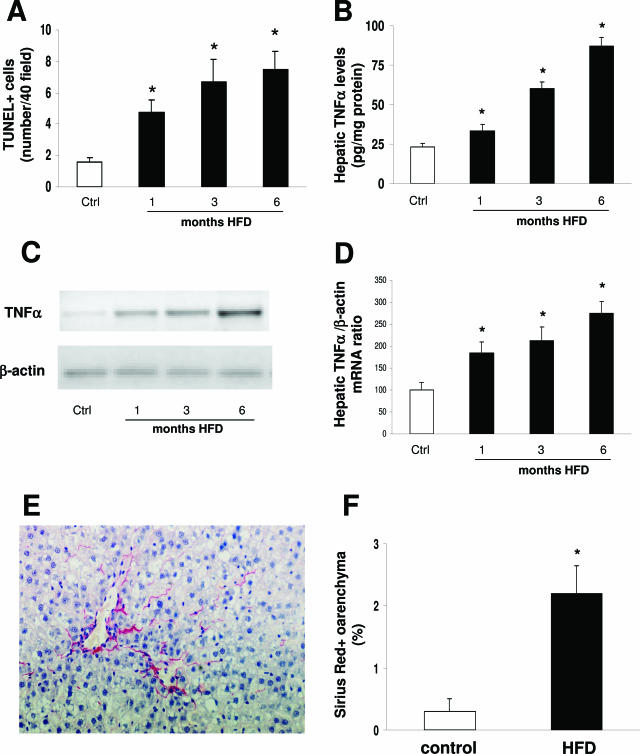
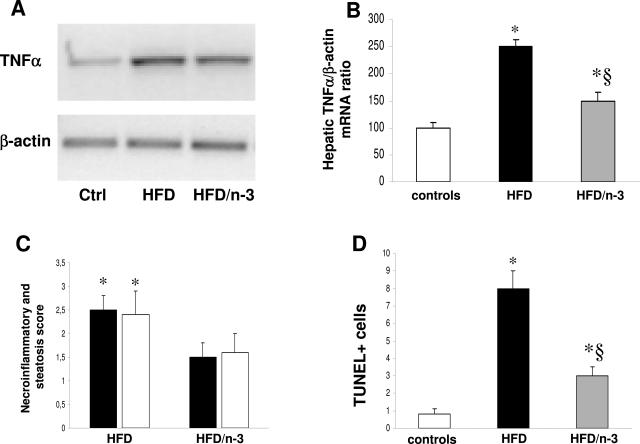
The number of apoptotic hepatocytes, as determined by TUNEL staining, increased in HFD-treated rats compared with controls already at 1 month (Figure 7A). It is known that both FFA and TNF-α can lead to hepatocyte apoptosis in vitro or in knockout models26,30 FFA hepatic levels progressively increased from 38.8 ± 4.4 μmol/mg protein at 1 month of HFD to 85.6 ± 9.4 and 210.3 ± 18.7 at 3 and 6 months, respectively (P < 0.05 versus controls, 8.5 ± 1.3). By enzyme-linked immunosorbent assay, TNF-α levels in hepatic lysates increased at 1 month in HFD-treated animals (Figure 7B). To determine whether the increased TNF-α levels were due to hepatic production or reflected adipose tissue release only, we examined hepatic mRNA levels by RT-PCR. When normalized to the β-actin mRNA bands, a 1.8-, 2.1-, and 2.5-fold increase was evident at 1, 3, and 6 months, respectively, in rats receiving HFD (Figure 7, C and D).
Figure 7.
Effect of HFD on hepatocyte apoptosis (A), TNF-α protein content in the liver (B), TNF-α mRNA levels in the liver (C and D), and collagen deposition (E and F). A representative agarose gel of RT-PCR for TNF-α mRNA is shown in C. Representative Sirius Red staining is shown in E. No differences were observed between controls at the different time points (A, B, and D), which were thus considered as a single control group. Data are presented as mean ± SD. *P < 0.05 versus controls.
We finally determined whether the different forms of liver injury (ie, steatosis, necroinflammation, and apoptosis) were associated with collagen deposition. By Sirius Red staining, no excessive matrix deposition was observed after 1 and 3 months of HFD compared with control rats. On the other hand, after 6 months of HFD, zone 3 pericellular and perisinusoidal matrix deposition was observed (Figure 7E), which was significantly increased compared with control animals, as determined by morphometry (Figure 7F).
Effect of n-3 PUFA Supplementation
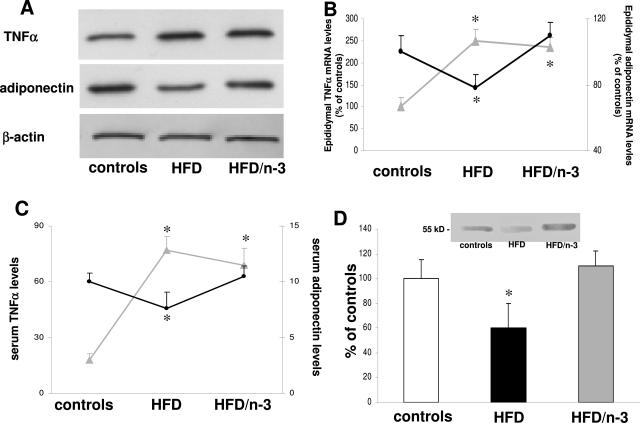
Because fatty liver deposition induced by HFD was associated with PPARα down-regulation, we next treated animals with a natural PPARα ligands, namely n-3 PUFA. Animals were treated with control diet or HFD for 1 month and then randomly divided into four groups receiving control diet or HFD for an additional 2 months supplemented or not with fish oil (3.3% [w/w]) containing eicosapentaenoic acid (146 mg/g diet) and docosahexaenoic acid (353 mg/g) (controls, controls/n-3 PUFA, HFD, and HFD/n-3 PUFA). Absorption and bioavailability of n-3 PUFA was documented by the reduction in the n-6-to-n-3 PUFA ratio both in the portal blood (7.2 ± 2.5, 4.5 ± 1.8, 16.3 ± 3.1, and 10.5 ± 2.3 in controls, controls/n-3 PUFA, HFD, and HFD/n-3 PUFA, respectively; P < 0.05 for HFD and HFD/n-3 PUFA versus their respective controls) and in the liver (11.4 ± 2.9, 6.6 ± 1.3, 21.5 ± 4.2, and 13.5 ± 2.1, respectively; P < 0.05 for HFD and HFD/n-3 PUFA versus their respective controls). In preliminary experiments, n-3 PUFA treatment did not determine any effect in terms of ALT values, histological appearance, PPARα expression, hepatic triglyceride, and cholesterol levels between controls and controls/n-3 PUFA (data not shown), and these animals were thus considered as a single control group for the further experiments of this study. However, in the epididymal adipose tissue of HFD/n-3 PUFA animals, n-3 PUFA administration restored adiponectin mRNA levels to control values, whereas no effects were observed on TNF-α synthesis (Figure 8, A and B). Compared with HFD animals, this was associated with an increase of adiponectin serum levels when HFD-treated rats received n-3 PUFA (Figure 8C). Administration of n-3 PUFA to HFD-treated animals induced an increased hepatic nuclear expression of PPARα that did not differ from control levels (Figure 8D).
Figure 8.
Effect of n-3 PUFA administration on TNF-α and adiponectin mRNA in visceral adipose tissue (A and B), TNF-α and adiponectin levels in portal blood (C), and PPARα expression in nuclear liver extracts (D). Rats were treated for 1 month with HFD and then divided into HFD or HFD/n-3 PUFA group for an additional 2 months. Gray lines, TNF-α; black lines, adiponectin. Data are presented as mean ± SD. *P < 0.05 versus controls.
These modifications in adiponectin synthesis and PPARα expression were associated with reduced cholesterol and triglyceride deposition in the liver (Figure 9, A and B). In HFD/n-3 PUFA, the progression of liver damage was not observed, and after 3 months, only small deposits of micromacrovesicular steatosis were observed (Figure 9, C and D). This was accompanied by a significant decrease in the mRNA levels of hepatic TNF-α (Figure 10, A and B), in the ALT values (141.3 ± 21.3 and 78.4 ± 14.7 UI/ml in HFD and HFD/n-3 PUFA animals, respectively; P < 0.05), in the steatosis and necroinflammatory score, and in the number of the apoptotic bodies (Figure 10, C and D).
Figure 9.
Effect of n-3 PUFA administration on hepatic deposition of triglyceride (A) and cholesterol (B) and on the histological appearance of liver injury induced by HFD (C and D). Rats were treated for 1 month with HFD and then divided into HFD or HFD/n-3 PUFA group for an additional 2 months. Compared with the histological injury observed with HFD (C), the HFD/n-3 PUFA diet (D) elicited a striking decrease in fat accumulation and inflammatory infiltrate. Data are presented as mean ± SD. *P < 0.05 versus controls. §P < 0.05 versus HFD.
Figure 10.
Effect of n-3 PUFA administration on hepatic TNF-α mRNA levels (A and B), necroinflammatory and steatosis score (C), and number of apoptotic bodies (D). Rats were treated for 1 month with HFD and then divided into HFD or HFD/n-3 PUFA group for an additional 2 months. In C, black bars represent steatosis and white bars represent necroinflammation. Data are presented as mean ± SD. *P < 0.05 versus controls. §P < 0.05 versus HFD.
Discussion
Progress in the understanding of the pathophysiology of NASH has been hampered by the lack of experimental models adequately reproducing features of the human disease. Most of the data gained so far have been obtained using rodent models based either on genetic leptin defects, which are resistant to the development of fibrosis during chronic liver injury, or on the MCD diet.5 In the MCD model, steatosis, necroinflammation, and little fibrosis develops in zone 3 as a result of a nutritional deficiency to which rodents are selectively sensitive but that is uncommon in patients with NASH, because animals lose weight and are cachectic.5 Recently a high-fat, high-calorie liquid diet was able to reproduce some features of NASH in rats.6 However, the liquid diet represents a time-consuming method that was used for only 3 weeks and did not induce body or liver weight gain. Moreover no information on adipose tissue or insulin resistance was provided in the study. To overcome this and to further approximate the human disease, we developed a model based on a commercially available high-fat, high-calorie solid diet.13 Using such a model, we have been able to demonstrate in the present study that visceral obesity (increase in body weight and epididymal fat) in animals without genetic modifications is associated with insulin resistance (increased glucose and insulin levels), decreased PPARα expression, and alterations in insulin signaling and hepatic steatosis; that these alterations lead to oxidative stress, necroinflammatory liver injury, cell apoptosis, and collagen deposition; and that the specific treatment of one of these alterations by using a selective PPARα ligand, ie, n-3 PUFA, is able to reduce the degree of liver injury.
One may argue that it is unclear whether the effects observed in the HFD group are the result of increased caloric or fat intake. We chose HFD because it was shown to induce obesity and insulin resistance, being thus suitable for the aim of our study.13 A recent investigation by the same group31 showed that reducing the number of kilocalories consumed from a high-fat diet attenuates but does not prevent the development of type 2 diabetes and obesity in rodents, thus indicating a role of high-fat diet independent of the caloric intake. On the other hand, different diet manipulations have been shown to induce obesity and fatty liver in a number of different strains and species of rodents, suggesting that “over nutrition” with either carbohydrates or fats or both might play a role in the genesis of obesity-related NAFLD.5 In addition, when adult male C57BL6 became obese by feeding on high amounts of either fat or sucrose or combined high-fat high-sucrose, they developed the same alterations of the immune system independent from the source of the calories.32 The HFD we used is useful in reproducing the human condition because it contains predominantly saturated fat (30.4 g/100-g diet versus 5.3 g/100-g polyunsaturated), of which the diet of obese and NASH patients is rich.33,34 In addition, saturated fat intake positively correlates with insulin sensitivity index and alanine aminotransferase levels in patients with NASH and with the amount of hepatic steatosis in obese women.33,34 Thus, our model successfully reproduces most of the pathogenetic findings known to be responsible in humans for the occurrence of hepatic steatosis associated with obesity and insulin resistance.7
The most hypothesized pathogenetic mechanism to explain the development of NASH from NAFLD is the “two-hit” theory in which the first event is the accumulation of lipids in the liver mediated by insulin resistance.7,35 The conventional explanation for hepatic triglyceride accumulation is that visceral obesity and insulin resistance, mostly mediated by adipokines such as TNF-α, result in increased FFA release from adipocytes with the consequent enhancement of lipid delivery to the liver.7 By stimulating TNF-α expression in hepatocytes and in adipocytes, FFAs are also implicated in the etiology of insulin resistance, which has been shown to be associated with increased activation of PKCε in the liver.30,36,37 Therefore our model, in which visceral obesity and insulin resistance are associated with increased TNF-α synthesis and secretion from the visceral adipose tissue and to higher FFA levels in the portal blood, reproduces the key features of the metabolic syndrome, of which NAFLD represents the hepatic manifestation.3
Peripheral insulin resistance can induce hepatic steatosis, which in turn determines the occurrence of insulin resistance in the liver, characterized by reduced insulin-suppressing effect in hepatic glucose production that aggravates peripheral insulin resistance and contributes to hepatic lipogenesis.38 In the liver in vivo, we have shown that HFD treatment increased the levels of IRS-1Ser307, which inhibits the insulin receptor signaling.29 IRS-1Ser307 can be mediated in vitro by different kinases such PI3K; mitogen-activated, ERK-activating kinase; and JNK.39 We have observed no increase in the phosphorylation of Akt, an immediate downstream of PI3K, that mediates insulin- and insulin-like growth factor-1-stimulated IRS-1Ser307.39 It has been shown that TNF-α-mediated IRS-1Ser307 can be either MEK or JNK dependent,29,39 but in our model, no increase in ERK phosphorylation was observed, whereas activated JNK was strikingly increased in vivo already at 1 month. The role of JNK in the induction of hepatic insulin resistance has been recently stressed by Samuel et al36 in an acute model of insulin resistance induced by 3-day HFD. In this study, the short-term feeding induced insulin resistance and was associated with PKCε and JNK activity. It has been shown that FFAs induce insulin resistance by activating PKCθ in the muscle and in the adipose tissue in vivo and that a PKCθ/JNK pathway is responsible for IRS-1Ser307.37,40 Conflicting results exist on the role of the different JNK isoforms in inhibiting insulin signaling and in the pathogenesis of NASH. Schattenberg et al41,42 first described a role of JNK, mostly JNK2, in impairing IRS-2 but not IRS-1 phosphorylation in hepatocytes overexpressing the cytochrome P402E1 but then showed that jnk1−/− mice had significantly reduced levels of hepatic triglyceride accumulation, inflammation, lipid peroxidation, liver injury, and apoptosis compared with jnk2−/− mice in the MCD model. In addition, increased IRS-1Ser307 phosphorylation was found in the liver of obese wild-type mice compared with obese JNK1−/− animals, demonstrating that Ser307 of IRS-1 is a target for JNK action.29 Increased IRS-1Ser307 phosphorylation was also found in a cellular model of insulin resistance in liver cells treated with TNF-α, which was completely prevented by a JNK inhibitor.29 All of these data are in agreement with ours showing that insulin resistance in the livers occurs in the HFD model mimicking so closely the human condition of NASH, and it is not induced by insulin but mediated by TNF-α and FFA through a JNK-dependent IRS-1Ser307 phosphorylation. This was confirmed by the in vitro experiments showing that TNF-α but not insulin was able to stimulate JNK phosphorylation in primary cultured hepatocytes (Figure 6).
Obesity and the metabolic syndrome represent chronic inflammatory states indicating that in addition to ROS, several immunomodulatory factors can contribute to insulin resistance and liver injury.38 Recently, several reports have at least partially elucidated the cellular and molecular mechanisms underlying this inflammatory response. Nuclear factor-κB (NF-κB) mediates induction of proinflammatory cytokines implicated in insulin resistance such as interleukin-1, interleukin-6, and TNF-α, and IKK-β is required for NF-κB activation.43 By selectively expressing constitutively active IKK-β in hepatocytes, mice exhibit a type 2 diabetes phenotype, profound hepatic insulin resistance, and increased production of proinflammatory cytokines such as TNF-α, as observed after HFD administration.44 A role of NF-κB in mediating hepatic and systemic insulin resistance was confirmed by the selective loss of IKK-β in hepatocytes (IKKβΔhep) or in myeloid cells (IKKβΔmye). IKKβΔhep mice retained insulin sensitivity and glucose tolerance in the liver in response to HFD and obesity but continued to develop peripheral insulin resistance, whereas IKKβΔmye mice on HFD showed global insulin sensitivity.45 These findings indicate that NF-κB-dependent inflammatory mediators produced in hepatocytes, such as TNF-α, are most likely to act in a paracrine manner to downmodulate insulin sensitivity in the liver and to favor liver injury. Hepatic TNF-α in animal models of NAFLD can also derive from liver lymphocyte modifications. When otherwise normal mice are fed with high-fat or high-sucrose diet, they become obese, develop fatty livers, and acquire hepatic innate immune system abnormalities, including increased NKT cell apoptosis. The latter reduces liver NKT cell populations and promotes excessive hepatic production of Th-1 cytokines (such as TNF-α) that could promote hepatic inflammation.32 Those studies did not evaluate the degree of liver injury. In our model, steatosis and ROS production were associated with increased hepatic TNF-α gene expression and proteins levels, necroinflammatory liver injury, hepatocyte apoptosis, and collagen deposition. In addition to its well-known role in inducing apoptosis, TNF-α can exert a fibrogenetic effect being released by hepatocytes and Kupffer cells and inducing hepatic stellate cells activation in a paracrine manner.30,46 Thus, it is reasonable to speculate that the association of visceral obesity and hepatic steatosis is needed for the progression from NAFLD to NASH, and this data confirms that our model reproduces most of the pathogenetic mechanisms leading to liver injury associated with the metabolic syndrome.
In addition to increased lipid afflux to the liver, steatosis can derive from reduced hepatic lipid oxidation and decreased VLDL excretion. Fatty acid metabolism in the liver is tightly regulated. Two classes of transcription factors, namely sterol response element binding proteins and PPARs play a major role in regulating lipid metabolism and energy homeostasis.7 We thus chose to evaluate the modifications of PPARα in our model of hepatic steatosis due to its central role in lipid oxidation and to its regulation by adipokines such as TNF-α and adiponectin.8,27,28 Adiponectin is a protein exclusively secreted from adipose tissue able to increase insulin sensitivity and FFA oxidation in the liver and to exert anti-inflammatory effects.38 Its importance in patients with NASH has been recently underlined by Musso et al,47,48 who found reduced adiponectin levels that correlated with the intake of PUFA and with the degree of necroinflammation and fibrosis. Although the three-dimensional structure of adiponectin closely resembles that of TNF-α, these two proteins have completely opposite effects, and both in vivo and in vitro experiments demonstrate that adiponectin and TNF-α antagonize each other’s action in their target tissues.49 More recently, adiponectin has been shown to directly activate PPARα receptors with consequent increase of fatty acid oxidation in cultured hepatocytes.28 In our model, the increased TNF-α expression associated with obesity in HFD-treated rats was accompanied by a reduction of both adiponectin synthesis in the visceral adipose tissue and PPARα nuclear protein levels in the liver. PPARα down-regulation could thus represent the link between the abnormalities observed in the visceral adipose tissue and the hepatic features of NASH, indicating that reduced lipid breakdown occurs in our model in addition to increased fatty acids afflux to the liver. In agreement with this, reduced expression of PPARα and fatty acid oxidizing enzymes, such as acyl-CoA oxidase, was observed in the MCD and alcohol model of liver steatosis and precedes the development of steatosis in Otsuka Long-Evans Tokushima Fatty rat, a model of human type 2 diabetes mellitus.11,50,51 The central role of PPARα in the development of NAFLD and NASH was confirmed in our model by treating animals with a natural PPARα ligand, namely n-3 PUFA. n-3 PUFA elicit their effects by coordinately suppressing lipid synthesis in the liver and up-regulating fatty acid oxidation in liver and skeletal muscle.12 Interaction of PPARα with its DNA recognition site is enhanced by PUFA, leading to the induction of genes encoding proteins involved in lipid transport, oxidation, and thermogenesis, with the n-3 PUFA being more potent than n-6 PUFA as in vivo activators of PPARα.12 After 1 month of HFD, rats were fed with HFD supplemented with an adequate amount of n-3 PUFA to evaluate their effect on an already established steatosis. n-3 PUFA administration reduced the increased n-6-to-n-3 PUFA ratio in the portal blood and in the liver observed in our model as well as in patients with NASH.24 Compared with HFD, n-3 PUFA treatment increased both adiponectin synthesis in the adipose tissue and plasma levels and restored hepatic PPARα nuclear expression finally leading to reduced cholesterol and triglyceride deposition. n-3 PUFA treatment was also associated with reduction in hepatic TNF-α mRNA levels, in necroinflammatory score and in the number of apoptotic bodies. It could thus be hypothesized that lipid overload reaches a threshold for progression to steatohepatitis in the absence of other aggravating factors, so that n-3 PUFA-induced PPARα-dependent intrahepatic fatty acid combustion reduces the susceptibility to steatohepatitis. An alternative explanation for the prevention of dietary steatohepatitis is that PPARα activation induced by n-3 PUFA has direct effects on inflammation or apoptosis, thus reducing liver injury as observed in the MCD and alcohol model.11,52,53 The protective effect of n-3 PUFA administration in our model, beyond their ability to up-regulate hepatic PPARα expression, could also result by the induction of adiponectin synthesis by adipose tissue as recently shown by Flachs et al.54 Adiponectin, in addition to having beneficial effects on lipid metabolism by increasing fatty acid β-oxidation, has also direct anti-inflammatory effects.55 In this regard, the protective role of adiponectin against liver injury may be partly due to its antagonistic effect against TNF-α. In our model, n-3 PUFA treatment restored adiponectin levels, and this was associated with decreased hepatic TNF-α and liver injury. In agreement with our findings, administration of adiponectin is able to suppress hepatic TNF-α and ameliorates both alcoholic and nonalcoholic fatty liver disease in mice,56 whereas previous reports showed that TNF-α production was markedly increased in adiponectin knockout mice.49,57 In this scenario, we may hypothesize a double protective effect of n-3 PUFA in NASH by a direct up-regulation of PPARα expression in the liver and by modifying the altered adiponectin-to-TNF-α ratio toward an anti-steatogenic and anti-inflammatory profile and parallels a similar effect observed when fish oil was given to patients with NAFLD, ameliorating the degree of steatosis and reducing ALT values.58
In conclusion, our study recapitulates the different pathogenetic and morphological events (hepatic steatosis, inflammation, and fibrosis) leading to NASH from visceral obesity and insulin resistance that have been observed in humans and underlines the role of n-3 PUFA as PPARα ligands in the experimental therapy of NASH. This model may thus represent an ideal tool to further study the pathogenesis of NASH and to modify each pathogenetic event (as for PPARα down-regulation in this study) to define any further future experimental therapy.
Footnotes
Address reprint requests to Dr. Gianluca Svegliati-Baroni, M.D., Università Politecnica delle Marche, Clinica di Gastroenterologia, Via Tronto, 60020 Ancona, Italy. E-mail: g.svegliati@univpm.it.
Supported by grants from Dottorato di ricerca “Alimenti e Salute” (to A.O., S.D.M., and C.C.), Borsa di Studio AISF “Mario Coppo” (to C.C.), Ricerca Scientifica di Ateneo (ex 60%) (to A.B.), Ricerca Finalizzata MIUR 2003 (to G.S.B., S.D.M., C.C., and S.S.), MIUR 2003060137_004 (to G.S.B., S.D.M., and S.S.), MIUR 2004068113_001 (to A.B., C.C., and S.D.M.), Ministero della Salute “Alimentazione per la Salute e la Prevenzione di Malattia” and Ente Cassa di Risparmio di Firenze (to A.C. and R.S.), and Fondazione Cariverona 2002 grant “Ambiente e sviluppo sostenibile.”
References
- Brunt E. Nonalcoholic steatohepatitis. Semin Liver Dis. 2004;24:3–20. doi: 10.1055/s-2004-823098. [DOI] [PubMed] [Google Scholar]
- Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- Saltiel A, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Koteish A, Diehl A. Animal models of steatosis. Semin Liver Dis. 2001;21:89–104. doi: 10.1055/s-2001-12932. [DOI] [PubMed] [Google Scholar]
- Lieber C, Leo M, Mak K, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli L. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- Browning J, Horton J. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman AI, Mangelsdorf D. Retinoid X receptor heterodimers in the metabolic syndrome. N Engl J Med. 2005;353:604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–132. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- Clarke S. Nonalcoholic steatosis and steatohepatitis: I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. Am J Physiol Gastrointest Liver Physiol. 2001;281:G865–G869. doi: 10.1152/ajpgi.2001.281.4.G865. [DOI] [PubMed] [Google Scholar]
- Surwit R, Feinglos M, Rodin J, Sutherland A, Petro A, Opara E, Kuhn C, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Barzilai N, She L, Liu B, Vuguin P, Cohen P, Wang J, Rossetti L. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–98. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Di Sario A, Casini A, Ridolfi F, Bendia E, Pigini P, Tonnini C, D’Ambrosio L, Feliciangeli G, Macarri G, Svegliati-Baroni G. Inhibition of the NA+/H+ exchanger reduces rat hepatic stellate cell activity and liver fibrosis: an in vitro and in vivo study. Gastroenterology. 2001;120:545–556. doi: 10.1053/gast.2001.21203. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Willmore W, Storey K. Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radic Biol Med. 1995;19:271–280. doi: 10.1016/0891-5849(95)00020-x. [DOI] [PubMed] [Google Scholar]
- Schoemaker MH, Gommans WM, de la Rosa LC, Homan M, Klok P, Trautwein C, van Goor H, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39:153–161. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655–1669. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svegliati-Baroni G, Ridolfi F, Caradonna Z, Alvaro D, Marzioni M, Saccomanno S, Candelaresi C, Trozzi L, Macarri G, Benedetti A, Folli F. Regulation and crosstalk of ERK/JNK/P70s6k in two rat models of liver injury and fibrosis: an in vivo and in vitro study. J Hepatol. 2003;39:528–537. doi: 10.1016/s0168-8278(03)00291-5. [DOI] [PubMed] [Google Scholar]
- Lemberger T, Saladin R, Vazquez M, Assimacopoulos F, Staels B, Desvergnet B, Wahli W, Auwerz J. Expression of the peroxisome proliferator-activated receptor a gene is stimulated by stress and follows a diurnal rhythm. J Biol Chem. 1996;271:1764–1769. doi: 10.1074/jbc.271.3.1764. [DOI] [PubMed] [Google Scholar]
- Feldstein A, Canbay A, Angulo P, Taniai M, Burgart L, Lindor K, Gores G. Hepatocyte apoptosis and Fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, Poniachik J. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- Svegliati Baroni G, D’Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel A, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720–726. doi: 10.1002/hep.510270313. [DOI] [PubMed] [Google Scholar]
- Schwabe R, Uchinami H, Qian T, Bennett B, Lemasters J, Brenner D. Differential requirement for c-Jun NH2-terminal kinase in TNFalpha- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- Beier K, Volkl A, Fahimi DH. TNF-alpha downregulates the peroxisome proliferator activated receptor-alpha and the mRNAs encoding peroxisomal proteins in rat liver. FEBS Lett. 1997;412:385–387. doi: 10.1016/s0014-5793(97)00805-3. [DOI] [PubMed] [Google Scholar]
- You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53:454–457. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- Tiikkainen M, Bergholm R, Vehkavaara S, Rissanen A, Hakkinen AM, Tamminen M, Teramo K, Yki-Jarvinen H. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–707. doi: 10.2337/diabetes.52.3.701. [DOI] [PubMed] [Google Scholar]
- Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Faga E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- Day C, James O. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu Z, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3–L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3–L1 adipocytes. Mol Endocrinol. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Friedman S, Arthur M. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium: direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Gambino R, Durazzo M, Biroli G, Carello M, Faga E, Pacini G, De Michieli F, Rabbione L, Premoli A, Cassader M, Pagano G. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology. 2005;42:1175–1183. doi: 10.1002/hep.20896. [DOI] [PubMed] [Google Scholar]
- Musso G, Gambino R, Biroli G, Carello M, Faga E, Pacini G, De Michieli F, Cassader M, Durazzo M, Rizzetto M, Pagano G. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438–2446. doi: 10.1111/j.1572-0241.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Yeon JE, Choi KM, Baik SH, Kim KO, Lim HJ, Park KH, Kim JY, Park JJ, Kim JS, Bak YT, Byun KS, Lee CH. Reduced expression of peroxisome proliferator-activated receptor-alpha may have an important role in the development of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2004;19:799–804. doi: 10.1111/j.1440-1746.2004.03349.x. [DOI] [PubMed] [Google Scholar]
- Wan YJ, Morimoto M, Thurman RG, Bojes HK, French SW. Expression of the peroxisome proliferator-activated receptor gene is decreased in experimental alcoholic liver disease. Life Sci. 1995;56:307–317. doi: 10.1016/0024-3205(94)00953-8. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart J, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Rao M, Reddy J. PPARalpha in the pathogenesis of fatty liver disease. Hepatology. 2004;40:783–786. doi: 10.1002/hep.20453. [DOI] [PubMed] [Google Scholar]
- Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, Ruzickova J, Kopecky J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol. 2003;171:5091–5099. doi: 10.4049/jimmunol.171.10.5091. [DOI] [PubMed] [Google Scholar]
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capanni M, Calella F, Centenaro G, Biagini M, Svegliati-Baroni G, Milani S, Raimondi L, Mugelli A, Surrenti C, Casini A. Prolonged n-3 PUFA dietary supplementation improves fatty liver in patients with NAFLD. Aliment Pharmacol Ther. 2006;23:1143–1151. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]