Abstract
Cellular senescence of biliary epithelial cells with p16INK4a and p21WAF1/Cip expression in damaged small bile ducts may be critical for progressive bile duct loss in primary biliary cirrhosis. We investigated the involvement of bmi1, a polycomb group gene repressing p16INK4a expression, in the pathogenesis of biliary cellular senescence. Bmi1 expression was examined immunohistochemically in livers taken from the patients with primary biliary cirrhosis (n = 18) and other diseased (n = 19) and normal livers (n = 16). We examined the effect of oxidative stress and a short interference RNA (siRNA) targeting bmi1 on cellular senescence in cultured mouse biliary epithelial cells. Bmi1 was widely expressed in the nuclei of biliary epithelial cells in the control livers. In contrast, bmi1 expression was significantly decreased in damaged small bile ducts in 43% of livers with primary biliary cirrhosis of stage 1/2, coordinating with the increased p16INK4a expression. In cultured biliary epithelial cells, oxidative stress by H2O2 treatment significantly decreased bmi1 expression, followed by increased P16INK4a expression. A knockdown of bmi1 induced increased p16INK4a expression, decreased cell proliferation, and increased cellular senescence. In conclusion, the decreased bmi1 expression caused by oxidative stress may be involved in the pathogenesis of cellular senescence of biliary epithelial cells in primary biliary cirrhosis.
Primary biliary cirrhosis (PBC) is an organ-specific autoimmune disease and presents with chronic, progressive cholestasis and liver failure.1–3 PBC is characterized histologically as a cholangitis of small bile ducts (chronic nonsuppurative destructive cholangitis; CNSDC) eventually followed by the extensive loss of small bile ducts.2–4 Although there have been many studies on the immunopathological features,5–7 there have been few studies on the pathogenesis of bile duct loss in PBC. We have recently reported cellular senescence of biliary epithelial cells (BECs) with the augmented expression of p16INK4a and p21WAF1/Cip in damaged small bile ducts in PBC and suggested that the cellular senescence may be involved in the pathogenesis of progressive bile duct loss in PBC.8
Cellular senescence is defined as a condition in which a cell no longer has the ability to proliferate. Senescent cells are irreversibly arrested at the G1 phase of the cell cycle and do not respond to various external stimuli but remain metabolically active. Senescent cells display several characteristics, including histological changes in vitro and in vivo,9,10 shortened telomeres, increased expression of p16INK4 and p21WAF1/CIP, and increased activity of senescence-associated β-galactosidase (SA-β-gal).11 Although we have also shown a possible association of oxidative stress with cellular senescence in PBC,8 the mechanism inducing cellular senescence in PBC has not so far been fully clarified.
Recently, bmi1, a transcriptional receptor belonging to the polycomb group gene family,12 has been noted as a senescence regulator.13 A critical target of bmi1 is the INK4a locus, which encodes the p16INK4a and p19ARF (p14ARF in humans) tumor suppressor proteins.14 Overexpression of bmi1 extends the replicative life spans of mouse and human fibroblasts,14,15 possibly by repressing p16INK4a and p19/p14ARF. In the absence of bmi1, both the p16INK4a and p19 ARF genes from the Ink4a locus are expressed.16 Mouse embryonic fibroblasts (MEFs) from bmi1 knockout mice show a premature senescence feature with the increased expression of p16INK4a14.
Given the critical role of bmi1 in the regulation of cellular senescence and p16INK4a expression, we hypothesized that the decreased expression of bmi1 may be closely associated with the cellular senescence of BECs in PBC. Therefore, we examined bmi1 expression and its correlated expression with senescence-associated p16INK4a and p21WAF1/CIP in BECs in PBC and control human livers. Furthermore, we took advantage of cultured mouse BECs to address the issues whether or not oxidative stress could influence bmi1 expression and whether or not a knockdown of bmi1 expression could induce the up-regulation of p16INK4a and cellular senescence. We also examined the involvement of the other polycomb group protein EZH2 that is required for the recruitment of bmi1 to polycomb group bodies.17
Materials and Methods
Human Study
Classification of Intrahepatic Biliary Tree
The intrahepatic biliary tree is classified into the intrahepatic large and small bile ducts by their size and distribution in the portal tracts.4,18 In this study, septal and interlobular bile ducts are termed small bile ducts. Bile ductules were evaluated separately and not included in the small bile ducts.
Bile ducts were largely classified into inflamed and noninflamed bile ducts. Inflamed bile ducts included the bile ducts surrounded and occasionally infiltrated by inflammatory cells such as bile ducts involved in CNSDC in PBC and bile ducts embedded in infiltrating lymphoid cells in chronic viral hepatitis (CVH).
Liver Tissue Preparation
A total of 53 liver tissue specimens (all were wedge biopsied or surgically resected) were collected from the liver disease files of our laboratory and affiliated hospitals. The liver specimens consisted of 18 PBC, 14 CVH livers, five livers from extrahepatic biliary obstruction (EBO), and 16 histologically normal livers. All PBCs were from the patients fulfilling the clinical, serological, and histological characteristics consistent with the diagnosis of PBC.3 PBC livers were staged histologically.3,19 Twelve and six PBC were of stages 1/2 and of stages 3/4, respectively. Seven CVH were regarded as F0-2 and seven as F3/4, respectively.20 Two and twelve CVH cases were serologically positive for hepatitis B surface B antigen (HBsAg) and anti-hepatitis C viral antibody (HCVAb), respectively. Causes of EBO were obstruction or stenosis of the bile duct at the hepatic hilum or the extrahepatic bile duct, because of carcinoma or stone, and the duration of jaundice was less than 1 month. Histologically normal livers were obtained from surgically resected livers for traumatic hepatic rupture or metastatic liver tumor. The liver tissues used were taken from the part sufficiently away from the trauma and tumor. There are no significant differences between the liver samples we used as histologically normal livers, liver tissue from organ donor, and histologically normal wedge biopsied livers in the expression of bmi1 and Ki67 antigen in preliminary study.
Liver tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin. More than 20 serial sections, 4 μm thick, were cut from each block. Several were processed routinely for histopathological study, and the remainder was processed for the following immunohistochemistry.
Immunohistochemistry
Bmi1 was detected by using mouse monoclonal anti-bmi1 antibody (clone 229F6, dilution 1:400; Upstate Technology, Lake Placid, NY). This monoclonal antibody is characterized14,21 and has been reported to work on paraffin sections.22 As positive controls for immunohistochemistry, paraffin-embedded material from tonsil and lymph node was used. In addition, we used another monoclonal antibody recognizing bmi1 (clone 1.T.21, dilution 1:100; Abcam, Cambridge, UK) to confirm the immunostaining. The staining pattern by both antibodies was similar in preliminary study and the former antibody was mainly used. In selected serial sections, P16INK4a was also detected using mouse monoclonal anti-p16INK4a protein (clone JC2, dilution 1:100; Neomarkers, Freemont, CA). Dual immunostaining of bmi1 and p16INK4a was also performed in the selected sections as described previously.23 In brief, deparaffinized sections were pretreated in ethylenediaminetetraacetic acid buffer (pH 9) in a pressure-cooker at 100°C, 5 minutes for bmi1, or in a microwave oven in citrate buffer (pH 6) at 95°C, 20 minutes for P16INK4a. The sections were immersed in 0.3% H2O2 in methanol for 20 minutes to abolish endogenous peroxidase activities. The sections were then immersed in 5% normal goat serum for 60 minutes and incubated with the primary antibody at 4°C overnight. The Envision+ solution (DAKO, Glostrup, Denmark) was then applied for 30 minutes at room temperature. The reaction products were visualized using 3–3′-diaminobenzidine tetra hydrochloride (Sigma Chemical Co., St. Louis, MO) and H2O2. The sections were then lightly counterstained with methyl green or hematoxylin. A similar dilution of the control mouse or control rabbit IgG (DAKO) was applied instead of the primary antibody as negative control. Positive and negative controls were routinely included. At least five portal tracts per liver section were evaluated.
In Vitro Study
Cell Culture and Treatments of Mouse Intrahepatic BECs
Mouse intrahepatic BECs (mBECs) were isolated from 8-week-old female BALB/c mice and were purified and cultured as described previously.24,25 The maintenance and passage of cultured BECs were made as described previously.24,25 The BECs were used during passage numbers 10 to 15 and cultured up to 8 days, as the longer culture time accelerated senescence in preliminary study. The confluency of the cells was kept less than 80% during experiments. In several experiments, mBECs were treated with H2O2, 112.5 μmol/L for 2 hours, washed throughout to remove H2O2, and cultured in fresh medium. UV irradiation (2 or 5 J/m2) was performed by a UV spectrolinker XL-1000 (Spectronics, Westbury, NY). BECs were incubated in phosphate-buffered saline during UV irradiation, then washed and cultured in fresh media. Serum-free media was applied for BECs to make the condition of cytokine/growth factor deprivation in some experiments.
Gene Silencing of bmi1 and EZH2 by Short Interfering RNA (siRNA)
The expression of bmi1 and EZH2 was silenced using an siRNA directed against the coding region of mouse bmi1 and EZH2 designed and synthesized by Qiagen (Hilden, Germany). In addition to nonlabeled siRNA, Alexa-488-labeled siRNA (Qiagen) was synthesized. A negative control siRNA labeled by Alexa-488 (Qiagen) was used as a control. The targeted sequence of mouse bmi1 was 5′-AAGCGGGTACTACCGTTTATT-3′. The targeted sequence of the negative control, which has no homology with mammalian genes, was 5′-AATTCTCCGAACGTGTCACGT-3′. One day before transfection, mBECs were plated in a type I collagen-coated 35-mm dishes (5 × 105 cells) or Lab-Tek chambers (5 × 104 cells/well), and then the cells were transiently transfected with 100 nmol/L bmi1 siRNA by using Dreamfect transfection reagents (OZ Biosciences, Marseilles, France) according to the manufacturer’s protocol.
RNA Extraction and Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from the cells with the Qiagen RNeasy mini kit (Qiagen) according to the manufacturer’s protocol. After cDNA was synthesized, real-time quantitative RT-PCR analysis for bmi1, EZH2, p16INK4a, and p21WAF1/Cip mRNAs were performed in duplicate for each sample using the ABI Prism 7700 sequence detection system instrument and software (PE Applied Biosystems, Inc., Foster City, CA). Sequence-specific primers and probes for mouse bmi1, EZH2, p16INK4a, p21WAF1/Cip, and GAPDH as the internal control were purchased from Applied Biosystems. Amplifications were performed under standard conditions.
Immunofluorescence Staining for Cultured Cells
The mBECs growing in a Lab-Tek chamber were fixed with 5% buffered formalin and ice-cold methanol for 20 minutes each. After blocking with 3% bovine serum albumin, the cells were incubated with either of the primary antibodies for bmi1 (described above) overnight. The cells were then incubated with Alexa-546-labeled goat anti-mouse IgG (Molecular Probes, Eugene, OR) for 30 minutes, counterstained with 10 μg/ml 4′ and 6-diamidino-2-phenylindole (DAPI), and evaluated under a conventional fluorescence microscope (Olympus, Tokyo, Japan).
Cell Growth and BrdU Incorporation
Cell proliferation activity was assessed on day 4 after treatment by using a 5-bromo-2′-deoxyuridine (BrdU) labeling and detection kit (Roche, Nonenwald, Germany), according to the manufacturer’s protocol. The nuclei were simultaneously stained with DAPI. At least 1 × 103 total cells were checked and counted to assess the BrdU-labeling index with a conventional fluorescence microscope (Olympus).
Assay for Cellular Senescence
SA-β-gal activity was detected by using the senescence detection kit (BioVision, Mountain View, CA) according to the manufacturer’s protocol.11 The proportion of senescent cells in each condition was assessed at day 6 by counting the percentage of SA-β-gal-positive cells in at least 1 × 103 total cells using light microscopy. In addition, we performed the colony-forming assay to confirm the cellular senescence. One day after H2O2 treatment or siRNA transfection, the cells were harvested and counted, and 200 viable cells were seeded in type I collagen-coated 60-mm dishes. The number of colonies (>1 mm in diameter) was counted at day 5 after the fixation with methanol and Giemsa staining.
Immunoblotting
The cell lysate samples (15 μl) were solubilized, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane as described previously.26 After transfer, the membranes were processed for immunoblotting as described previously.26 The primary antibodies for bmi1 and p21WAF1/Cip1 are described above. P16INK4a and α-tubulin were detected by using rabbit polyclonal antibody for p16INK4a (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse monoclonal anti-α-tubulin (clone TU-01; Zymed, South San Francisco, CA). The protein expression of bmi1, p16INK4a, and p21WAF1/Cip were quantified relative to α-tubulin by using NIH image.
Statistical Analysis
Statistical analysis for the difference in human study used the Wilcoxon rank sum test. Statistical analysis of the difference in the in vitro assays was performed with the Student’s t-test. A P value less than 0.01 was considered significant.
Results
Decreased Expression of bmi1 in Small Bile Duct Lesions in PBC
Bmi1 was expressed in the nuclei of most BECs lining small bile ducts in normal, CVH, and EBO livers (Figure 1, A and B). The expression of bmi1 was decreased in BECs in the small bile ducts involved in CNSDC in PBC stage 1/2 (Figure 1C). The decreased expression of bmi1 was observed in 10 of 12 PBC of stage 1/2 (83.3%) and 1 of 6 PBC stages 3/4 (16.7%), whereas bmi1 expression was not decreased in any of the other diseased (14 CVH and 5 EBO livers) and 16 normal livers. When small bile ducts were subdivided into inflamed and noninflamed, the decreased expression of bmi1 was detected in 40 of 51 (78.4%) and one of one (100%) inflamed small bile ducts in PBC stage 1/2 and PBC stage 3/4, respectively, whereas it was not seen in any of noninflamed small bile ducts in PBC and other livers (Table 1). Inflamed large bile ducts were not present in all liver tissue sections. The decreased expression of bmi1 was not detected in noninflamed large bile ducts at all even in PBC stage 1/2 in which bmi1 expression was decreased in the damaged small bile ducts (Table 1). The incidence of the decreased expression of bmi1 in BECs was significantly higher in PBC stage 1/2, when compared with those in other groups (P < 0.01).
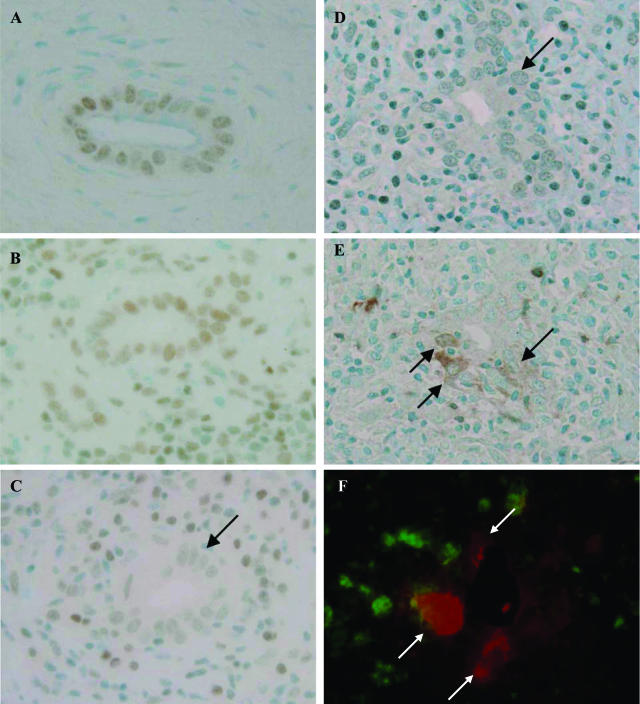
Figure 1.
Decreased expression of bmi1 and its relation with p16INK4a expression in BECs at the site of chronic nonsuppurative destructive cholangitis in PBC. A and B: bmi1 was expressed in the nuclei of BECs in normal livers (A) and CVH livers (B). C: The expression of bmi1 was decreased (arrows) in BECs at the site of chronic nonsuppurative destructive cholangitis. PBC, stage 2. D and E: Correlated expression of bmi1 (D) and p16INK4a (E) as a senescence-associated marker. The expression of bmi1 was decreased in BECs in the small bile ducts involved in chronic nonsuppurative destructive cholangitis (D, arrow), and the expression of p16INK4a was increased in BECs in the same damaged small bile duct (E, arrow). PBC, stage 2. Immunostaining for bmi1 (A–D) and p16INK4a (E). F: Dual immunostaining of bmi1 (green fluorescence) and p16INK4a (red fluorescence) demonstrated the absence of bmi1 expression in nuclei of BECs expressing p16INK4a (arrows). Original magnifications, ×400.
Table 1.
Frequency of the Decreased Expression of bmi1 in Small Bile Ducts in Primary Biliary Cirrhosis and Other Hepatobiliary Diseases
| Diseases | No. of patients | Type of BD | No. of BD | No. of BD showing decreased expression of bmi1 (%) |
|---|---|---|---|---|
| PBC, stage 1/2 | 12 | Large, noninflamed | 3 | 0 |
| Small, inflamed | 51 | 40 (78.4%)* | ||
| Small, noninflamed | 74 | 0 | ||
| PBC, stage 3/4 | 6 | Large, noninflamed | 3 | 0 |
| Small, inflamed | 1 | 1 (100%) | ||
| Small, noninflamed | 22 | 0 | ||
| CVH | 14 | Large, noninflamed | 9 | 0 |
| Small, inflamed | 50 | 0 | ||
| Small, noninflamed | 170 | 0 | ||
| EBO | 5 | Large, noninflamed | 2 | 0 |
| Small, inflamed | 3 | 0 | ||
| Small, noninflamed | 60 | 0 | ||
| Normal liver | 16 | Large, noninflamed | 12 | 0 |
| Small, inflamed | 3 | 0 | ||
| Small, noninflamed | 146 | 0 |
PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; CVH, chronic viral hepatitis; EBO, extrahepatic biliary obstruction; BD, bile ducts.
P < 0.01 versus PBC, stage 1/2, small, noninflamed and other groups.
Bmi1 was expressed in the nuclei of bile ductules as well as in bile ducts in the control livers. The decreased expression of bmi1 was detected in bile ductules, especially in those involved in chronic portal inflammation in 4 of 12 PBC stages 1/2 and one of six PBC stages 3/4. The decreased expression of bmi1 was not seen in the other diseased and normal livers. The expression of bmi1 was rather faint in hepatocytes, when compared with that in small bile ducts and bile ductules and decreased expression of bmi1 was not detectable in hepatocytes in PBC and other livers. The expression of bmi1 was detected in the nuclei of lymphocytes, but it was faint in endothelial and stromal cells in portal tracts.
When the co-related expression of bmi1 and p16INK4a as a senescence-related marker was evaluated using serial sections and dual immunostaining, the expression of bmi1 was decreased in the BECs in damaged small bile ducts involved in CNSDC (Figure 1D), and the expression of p16INK4a was increased in BECs at the same lesion (Figure 1E). The expression of p16INK4a was observed in the cytoplasm and the nuclei of BECs (Figure 1, E and F). Dual immunostaining demonstrated the absence of bmi1 expression in nuclei of BECs expressing p16INK4a (Figure 1F). In contrast, p16INK4a was not expressed in small bile ducts where the expression of bmi1 was not decreased.
Oxidative Stress Decreased the Expression of bmi1 and Increased p16INK4a, and p21WAF1/Cip Expression in Cultured mBECs
When examined by real-time PCR, bmi1 mRNA expression was significantly lower in BECs on days 2, 3, and day 4 after H2O2 (112.5 μmol/L, 2 hours) treatment (Figure 2A). p16INK4a mRNA expression was increased and significantly higher on days 2, 3, 4, and day 5 after H2O2 treatment. p21WAF1/Cip mRNA expression was significantly high at day 1, and then gradually decreased. According to immunoblot analysis (Figure 2, B and C), bmi1 protein expression was significantly decreased on days 3, 4, and 5 after H2O2 treatment. P16INK4a protein expression was significantly higher after H2O2 treatment. P21WAF1/Cip protein expression was transiently high on day 2 after H2O2 treatment and normalized thereafter.
Figure 2.
The effect of oxidative stress on bmi1, p16INK4a, and p21WAF1/Cip expression in BECs. A: bmi1 mRNA expression was significantly lower in BECs on days 2, 3, and 4 after H2O2 (112.5 μmol/L, 2 hours) treatment. P16INK4a mRNA expression was increased significantly on days 3, 4, and 5 after H2O2 treatment. P21WAF1/Cip mRNA expression was high on day 1, gradually decreased on days 2, 3, and 4 after H2O2 treatment. Expression of mRNA was quantified with real-time PCR. The expression was normalized as a ratio using GAPDH as a housekeeping gene. Data are expressed as the mean ± SD. *P < 0.01, **P < 0.05 compared to the control, n = 3 for each group. B: Immunoblot analysis for bmi1, p16INK4a, and p21WAF1/Cip protein expression in BECs treated with H2O2 (112.5 μmol/L, 2 hours) and control BECs. A pool of protein samples prepared in three independent experiments was used and analysis was performed twice. C: Intensity of each band in immunoblot analysis (B) was quantified by densitometry, normalized as a ratio using α-tubulin as an internal control, and statistically analyzed. Bmi1 protein expression was significantly lower in BECs on days 3, 4, and 5 after H2O2 treatment. P16INK4a protein expression was significantly high after H2O2 treatment. P21WAF1/Cip protein expression was high on day 2 after H2O2 treatment and normalized thereafter. *P < 0.01, **P < 0.05 compared to control.
Oxidative Stress by H2O2 Treatment Decreased Cell Proliferation and Increased Cellular Senescence in Cultured mBECs
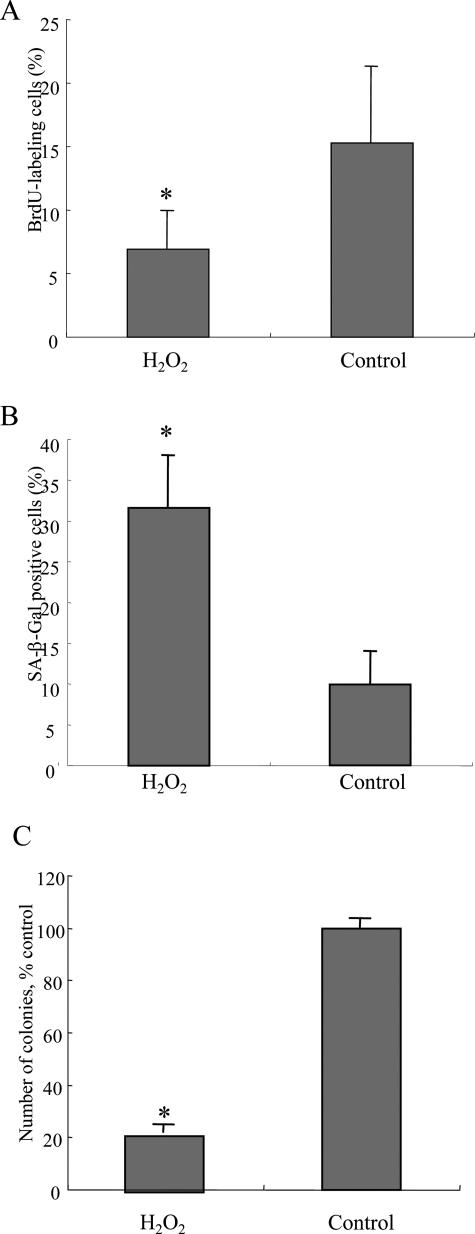
The BrdU labeling index on day 4 after H2O2 treatment was significantly lower in BECs with H2O2 treatment (6.9 ± 3.0%), when compared with control mBECs (15.3 ± 8.1%; P < 0.01) (Figure 3A). These findings suggest that oxidative stress decreased cell proliferation of mBECs. Cellular senescence of mBECs with and without H2O2 treatment was assessed by the detection of SA-β-gal. As shown in Figure 3B, the SA-β-gal-labeling index on day 6 after H2O2 treatment was significantly higher in BECs with H2O2 treatment (31.6 ± 6.38%), when compared with control BECs (9.9 ± 4.0%) (P < 0.01) (Figure 3B). The BECs with H2O2 treatment showed lower colony-forming activity (21.3 ± 2.4% control) after passaging (P < 0.01) (Figure 3C). These findings confirmed our previous report8 showing the increased cellular senescence in mBECs by oxidative stress with H2O2 treatment.
Figure 3.
Oxidative stress inhibits cell proliferation and induces cellular senescence. A: Cell proliferation was assessed by 5-bromo-2′-deoxyuridine (BrdU) assay on day 4 after H2O2 treatment. The labeling index of BrdU was counted for at least 1000 cells in each group. The labeling index of BrdU was significantly low in BECs treated with H2O2 (112.5 μmol/L, 2 hours), when compared with control BECs. Data are expressed as the mean ± SD. *P < 0.01 compared to control. B: Cellular senescence was assessed by senescence-associated β-galactosidase activity (SA-β-gal) on day 6 after H2O2 treatment. Percentage of cells positive for SA-β-Gal activity was significantly higher in BECs treated with H2O2 (112.5 μmol/L, 2 hours), when compared with the control BECs. Data are expressed as the mean ± SD. *P < 0.01 compared to the control. C: Cellular senescence was assessed by colony-forming assay on day 6 after H2O2 treatment. Number of colonies was significantly lower in BECs treated with H2O2 (112.5 μmol/L, 2 hours), when compared with the control BECs. Data are expressed as the mean ± SD. *P < 0.01 compared to the control.
The Effect of UV Irradiation and Serum Deprivation on bmi1 Expression
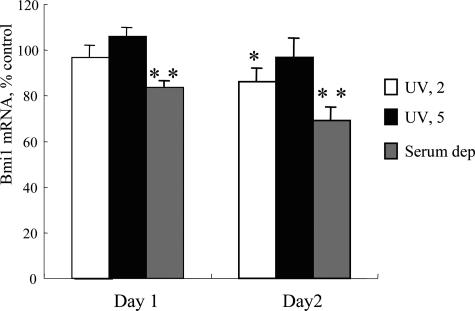
Apoptotic cells recognized as cells with fragmented and/or condensed nuclei by nuclear staining with DAPI were significantly increased on day 1 after UV irradiation (5 J/m2) (4.48 ± 1.38%), when compared with control (2.29 ± 1.56%) (P < 0.05). Apoptotic cells were not significantly increased in UV irradiation (2 J/m2) (2.61 ± 0.63%) and serum deprivation (3.96 ± 1.75%). When examined by real-time PCR, bmi1 mRNA expression was significantly lower in BECs on days 1 and 2 after serum deprivation (P < 0.01) (Figure 4). bmi1 mRNA expression was slightly lower in BECs on day 2 after UV irradiation (2 J/m2) (P < 0.05) (Figure 4).
Figure 4.
The effect of UV irradiation and serum deprivation on bmi1 expression in BECs. bmi1 mRNA expression was significantly lower in BECs on days 1 and 2 after serum deprivation and on day 1 after UV irradiation (2 J/m2). bmi1 mRNA was quantified with real-time PCR and the expression was normalized as a ratio using GAPDH as a housekeeping gene. Data are expressed as the mean ± SD. *P < 0.01, **P < 0.05 compared to the control, n = 3 for each group. Serum dep., serum deprivation.
Knockdown of bmi1 Induced the Up-Regulation of p16INK4a, Decreased Cell Proliferation, and Increased Cellular Senescence in Cultured mBECs
Validation of siRNA Targeting bmi1
To verify the significance of decreased bmi1 in BECs for the induction of cellular senescence, we took advantage of siRNA targeting bmi1 to inhibit bmi1 expression in mBECs. bmi1 mRNA expression was significantly lower in BECs transfected with siRNA targeting bmi1 on days 2, 3, and 4 (Figure 5A). Immunoblot analysis for bmi1 confirmed the decreased expression of bmi1 in BECs transfected with siRNA targeting bmi1 at the protein level (Figure 5B). Bmi1 protein expression was suppressed in the cells transfected with bmi1 siRNA, but not those with control siRNA (Figure 5C). These findings indicate that the siRNA targeting bmi1 designed in this study successfully knocked down bmi1 expression in mBECs.
Figure 5.
Validation of siRNA targeting bmi1. A: bmi1 mRNA expression was significantly lower in BECs transfected with siRNA targeting bmi1 on days 2, 3, and 4. bmi1 mRNA was quantified with real-time PCR. The expression was normalized as a ratio using GAPDH as a housekeeping gene. Data are expressed as the mean ± SD. *P < 0.01, **P < 0.05 compared to the control, n = 3 for each group. B: Immunoblot analysis for bmi1 protein expression in BECs transfected with siRNA targeting bmi1 and a control siRNA. Day 3 after transfection. Three independent experiments showed similar results. C: bmi1 expression labeled by red fluorescence was suppressed in the cells transfected with bmi1 siRNA showing green fluorescence in the cytoplasm (arrows) (left). In contrast, bmi1 expression was not decreased in the cells transfected with control siRNA (right). Day 3 after transfection.
The Effect of bmi1 Knockdown on p16INK4a and p21WAF1/Cip Expression
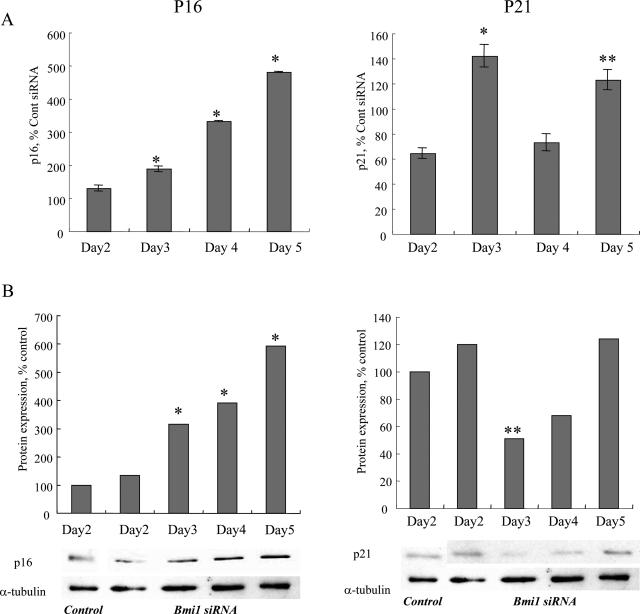
It is conceivable that knockdown of bmi1 increases p16INK4a expression, which is repressed by bmi1 in the basal state. As expected, p16INK4a mRNA expression was significantly higher in BECs transfected with siRNA targeting bmi1 on days 3, 4, and 5 (Figure 6A). P16INK4a protein expression was significantly higher in mBECs on days 3, 4, and 5 after bmi1 siRNA transfection (Figure 6B). p21WAF1/Cip mRNA was significantly higher on days 3 and 5 (Figure 5A). p21WAF1/Cip protein expression was rather low on day 3 after siRNA transfection.
Figure 6.
The effect of bmi1 knockdown on p16INK4a and p21WAF1/Cip expression. A: P16INK4a mRNA expression was significantly higher in BECs transfected with siRNA targeting bmi1 on days 3, 4, and 5 (left). P21WAF1/Cip mRNA was significantly higher on days 3 and 5 after siRNA transfection. P16INK4a and p21WAF1/Cip mRNA was quantified with real-time PCR. The expression was normalized as a ratio using GAPDH as a housekeeping gene and was shown as percentage of control. Data are expressed as the mean ± SD. *P < 0.01, **P < 0.05 compared to the control, n = 3 for each group. B: Immunoblot for p16INK4a and p21WAF1/Cip protein expression in BECs transfected with siRNA targeting bmi1 and a control siRNA. The intensity of each band in immunoblot analysis was quantified by densitometry, normalized as a ratio using α-tubulin as an internal control and statistically analyzed. P16INK4a protein expression was significantly higher in BECs on days 3, 4, and 5 after bmi1 siRNA transfection (left). P21WAF1/Cip protein expression was low on day 3 after siRNA transfection. *P < 0.01, **P < 0.05 compared to the control. Three independent experiments showed similar results.
Bmi1 Knockdown Inhibits Cell Proliferation
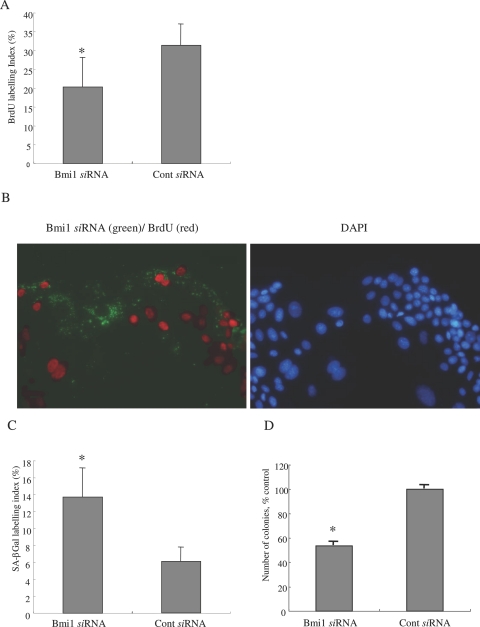
Knockdown of bmi1 should decrease cell proliferation by inducing p16INK4a expression as a CDK inhibitor. As expected, the labeling index of BrdU was significantly low in BECs transfected with bmi1 siRNA (20.3 ± 7.8%), when compared with BECs transfected with the control siRNA (31.4 ± 5.6%; P < 0.01) (Figure 7A). Most BECs transfected with bmi1 siRNA were negative for BrdU labeling, as shown in Figure 7B. These findings clearly suggest that the knockdown of bmi1 inhibits cell proliferation.
Figure 7.
The knockdown of bmi1 inhibits cell proliferation and induces cellular senescence. A: Cell proliferation was assessed by 5-bromo-2′-deoxyuridine (BrdU) assay. The labeling index of BrdU was counted for at least 1000 cells in each group. The labeling index of BrdU was significantly low in BECs transfected with bmi1 siRNA, when compared with BECs transfected with control siRNA. Data are expressed as the mean ± SD. *P < 0.01. Day 4 after transfection. B: Most BECs transfected with bmi1 siRNA showing green fluorescence in cytoplasm were negative for BrdU-labeling (red fluorescence). C: Cellular senescence was assessed by the senescence-associated β-galactosidase activity (SA-β-gal). Percentage of cells for positive for SA-β-Gal activity was significantly higher in BECs transfected with bmi1 siRNA, when compared with BECs transfected with control siRNA. Data are expressed as the mean ± SD. *P < 0.01. Day 6 after transfection. D: Number of colonies was significantly lower in BECs transfected with bmi1 siRNA, when compared with BECs transfected with control siRNA. Data are expressed as the mean ± SD. *P < 0.01. Day 6 after transfection.
Bmi1 Knockdown Induces Cellular Senescence
Bmi1 knockdown should increase cellular senescence via the increased expression of p16INK4a in BECs. As shown in Figure 7C, the SA-β-gal-labeling index was significantly higher in BECs transfected with bmi1 siRNA (13.7 ± 3.4%), when compared with BECs transfected with control siRNA (6.1 ± 1.7%) (P < 0.01). The BECs transfected with bmi1 siRNA showed lower colony-forming activity (53.4 ± 3.3% control) after passaging (P < 0.01) (Figure 7D). These findings indicate that the knockdown of bmi1 increases cellular senescence in mBECs.
The Knockdown of EZH2 Decreases the Nuclear Expression of bmi1 but Did Not Affect Cell Proliferation and Cellular Senescence
Since recent studies show that the other polycomb protein, EZH2, is essential for bmi1 recruitment to the polycomb body,17 we examined the association of EZH2 with bmi1 expression and the involvement in the induction of cellular senescence.
The Effect of Oxidative Stress, UV Irradiation, and Serum Deprivation on EZH2 Expression in BECs
When examined by real-time PCR, EZH2 mRNA expression was significantly lower (P < 0.01) in BECs on day 4 after H2O2 (112.5 μmol/L, 2 hours) treatment (Figure 8A). EZH2 mRNA expression was significantly lower (P < 0.01) in BECs on days 1 and 2 after serum deprivation and on day 2 after UV irradiation (5 J/m2) (Figure 8B).
Figure 8.
The effect of oxidative stress, UV irradiation, and serum deprivation on EZH2 expression in BECs. A: EZH2 mRNA expression was significantly lower in BECs on day 4 after H2O2 (112.5 μmol/L, 2 hours) treatment. B: EZH2 mRNA expression was significantly lower in BECs on days 1 and 2 after serum deprivation and on day 2 after UV irradiation (5 J/m2). A and B: EZH2 mRNA was quantified with real-time PCR and the expression was normalized as a ratio using GAPDH as a housekeeping gene. Data are expressed as the mean ± SD. *P < 0.01 compared to the control, n = 3 for each group. Serum dep, serum deprivation.
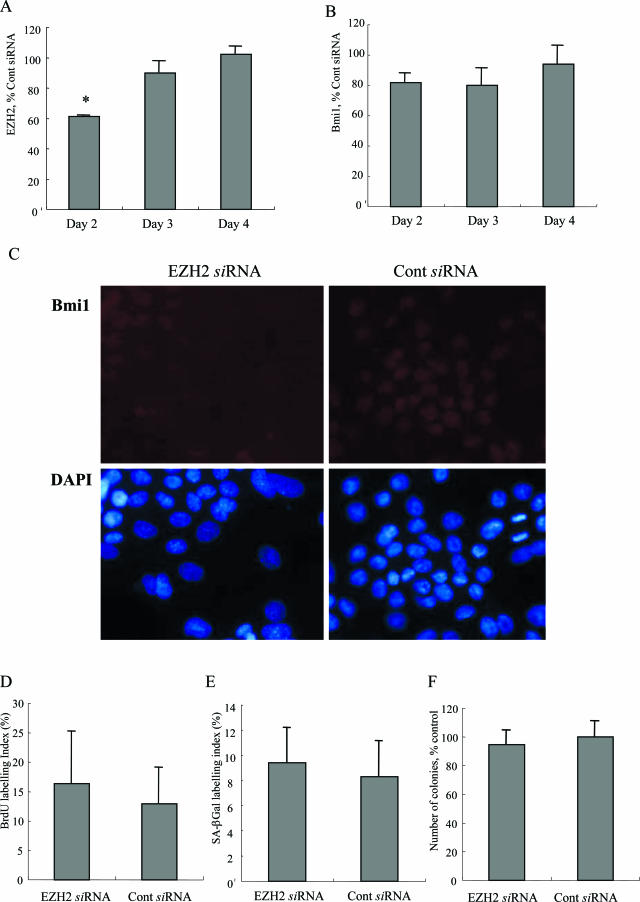
The Knockdown of EZH2 Slightly Decreases the Nuclear Expression of bmi1
EZH2 mRNA expression was significantly lower in BECs transfected with siRNA targeting EZH2 on day 2 (Figure 9A). bmi1 mRNA expression was slightly lower in BECs transfected with siRNA targeting EZH2 on days 2, 3, and 4, but there was no statistical difference (Figure 9B). When the expression of bmi1 was detected by immunocytochemistry, the nuclear expression of bmi1 was slightly decreased in the cells transfected with EZH2 siRNA, when compared with the cells transfected with control siRNA on day 4 after transfection (Figure 9C).
Figure 9.
The knockdown of EZH2 slightly decreases the nuclear expression of bmi1 but does not affect cell proliferation and cellular senescence. A: EZH2 mRNA expression was significantly lower in BECs transfected with siRNA targeting EZH2 on day 2. EZH2 mRNA was quantified with real-time PCR. The expression was normalized as a ratio using GAPDH as a housekeeping gene. Data are expressed as the mean ± SD. *P < 0.01 compared to the control, n = 3 for each group. B: bmi1 mRNA expression was slightly lower in BECs transfected with siRNA targeting EZH2 on days 2, 3, and 4, but there was no statistical difference. bmi1 mRNA was quantified with real-time PCR. The expression was normalized as a ratio using GAPDH as a housekeeping gene. Data are expressed as the mean ± SD. C: The nuclear expression of bmi1 labeled by red fluorescence was decreased in the cells transfected with EZH2 siRNA (left), when compared with the cells transfected with control siRNA (right). Day 4 after transfection. D: The labeling index of BrdU was not significantly different in BECs transfected with EZH2 siRNA (16.3 ± 8.8%), when compared with BECs transfected with control siRNA (12.9 ± 6.0%). Data are expressed as the mean ± SD. The labeling index of BrdU was counted for at least 1000 cells in each group. Day 4 after transfection. E: Percentage of cells positive for SA-β-Gal activity was not significantly different in BECs transfected with EZH2 siRNA, when compared with BECs transfected with control siRNA. Data are expressed as the mean ± SD. Day 6 after transfection. F: Number of colonies was not significantly different in BECs transfected with EZH2 siRNA, when compared with BECs transfected with control siRNA. Data are expressed as the mean ± SD. Day 6 after transfection.
The Knockdown of EZH2 Does Not Affect Cell Proliferation and Cellular Senescence
The labeling index of BrdU on day 4 was not significantly different in BECs transfected with EZH2 siRNA (16.3 ± 8.8%), when compared with BECs transfected with control siRNA (12.9 ± 6.0%; P > 0.01) (Figure 9D). When the cellular senescence was assessed by the SA-β-Gal labeling index (Figure 9E) and colony-forming assays (Figure 9F), there was no significant difference between BECs transfected with EZH2 siRNA and BECs transfected with control siRNA in both indexes of cellular senescence.
Discussion
Cellular senescence is a state in which a cell no longer has the ability to proliferate. We have reported previously that the BECs involved in bile duct damage in PBC frequently show markers of cellular senescence and that oxidative stress may be a candidate cause of cellular senescence in BECs in PBC.8 Cellular senescence of BECs may be critical for progressive bile duct loss in PBC, because cellular senescence can compromise the regenerative capacity of tissues.11 Recently, accumulating data suggest that bmi1, a polycomb group repressor, is critical for the inhibition of p16INK4a expression and the inhibition of cellular senescence. We hypothesized that the expression of bmi1 may be decreased at the site of bile duct damage by oxidative stress, resulting in cellular senescence with p16INK4a up-regulation. Therefore, we investigated the involvement of bmi1 and its relation to oxidative stress in the pathogenesis of cellular senescence in PBC.
A major conclusion of this study is that the expression of bmi1 is decreased in BECs involved in bile duct damage in PBC, whereas BECs in normal small bile ducts express bmi1 in other diseased liver and normal livers. In the late stage of PBC, the number of the remaining small bile ducts was limited and the decreased expression of bmi1 was detected in only one small bile duct involved in CNSDC. Therefore, it may be one possibility that the bmi1-expressing bile ducts were spared and survive in the late stage of PBC. It is of interest that the decreased expression of bmi1 co-localized the increased expression of p16INK4a in damaged bile ducts in PBC. Taken together, it is conceivable that the decreased expression of bmi1 may be responsible for the cellular senescence of BECs with the increased expression of p16INK4a in damaged bile ducts in PBC. Hepatic abnormality has not been reported so far in bmi1 knockout mice in which hematological impairment and neurological abnormality are major features. However, one possibility is that additional environmental factors might be required to trigger the development of PBC in bmi1 knockout mice, because both genetic and environmental factors are thought to be involved in the pathogenesis of PBC. Another possibility is that other compensatory mechanism(s) may relieve the influence of bmi1 defect in several organs in bmi1 knockout mice because the impact of bmi1 defect is various in each organ. When bmi1 is disrupted by siRNA, such compensatory mechanism(s) may not be induced promptly.
There have so far been few reports regarding factors that down-regulate bmi1 expression.27 A proliferation associated SNF-2-like gene (PASG) in mutant mice demonstrates a markedly increased expression of p16INK4a that is associated with the down-regulation of bmi1,27 although the mechanism remains to be determined.27 Because the involvement of oxidative stress has been suggested in the pathogenesis of cellular senescence in PBC,8,28,29 we examined the influence of oxidative stress in the expression of bmi1 and cellular senescence using cultured mBECs. We took advantage of mBECs, because nonneoplastic human BECs are not always conveniently available and the features of senescent mBECs are reportedly similar to those of senescent human BECs.30 Immortalized human BECs31 and cholangiocarcinoma cell lines32 appear to be inappropriate for the present study, because genetic and/or epigenetic alterations of p16INK4a gene and SV40-T-antigen-transformation affects directly the pathway of cellular senescence.
In the present study, we have reported firstly that oxidative stress decreased bmi1 expression. There have been no reports describing the down-regulation of bmi1 by oxidative stress to our knowledge. After H2O2 treatment, bmi1 mRNA expression was decreased, being followed by the decreased bmi1 protein expression in mBECs. P16INK4a expression was gradually increased in mBECs after H2O2 treatment, corresponding to the decreased bmi1 expression. The early increase of p16INK4a expression 1 day after H2O2 treatment may occur in a bmi1-independent manner (as discussed below). The present study also demonstrated the decreased cell proliferation activity and increased rate of cellular senescence after H2O2 treatment. These findings suggest that oxidative stress down-regulates bmi1 expression and causes the following increased expression of p16INK4a, the decreased cell proliferation activity, and increased rate of cellular senescence. In addition, we demonstrated that bmi1 mRNA expression was decreased by serum deprivation and UV irradiation (2 J/m2). This finding suggests that decreased bmi1 expression may be involved also in senescence induced by other factors.
To clarify the role of bmi1 in the regulation of p16INK4a expression and cellular senescence in BECs, we took advantage of siRNA targeting bmi1. The designed siRNA targeting bmi1 successfully inhibited the expression of bmi1 in the present study. As a result, the knockdown of bmi1 using siRNA induced the up-regulation of p16INK4a, decreased cell proliferation, and increased cellular senescence in BECs. The induction of cellular senescence in BECs by bmi1 inhibition is similar to the earlier premature senescence in cells from bmi1 knockout mice accompanied by the increased expression of p16INK4a 14. These results also support our hypothesis that decreased bmi1 expression is responsible for the induction of p16INK4a and cellular senescence in BECs.
The findings in the present study suggest that at least a part of cellular senescence by oxidative stress is induced via a bmi1-dependent pathway. In addition to the bmi1-dependent pathway, the immediate increase of p21WAF1/Cip and p16INK4a after H2O2 treatment observed in the present study indicated that oxidative stress influenced p21WAF1/Cip and p16INK4a expression via another pathway. Cells subjected to DNA damage induced by ultraviolet or X-radiation, to oxidative stress (induced by H2O2 or hyperoxia)33 induces p21WAF1/Cip expression rapidly via a p53-dependent pathway33,34 and enter stress-induced premature senescence. Therefore, it is likely that the immediate increase of p21WAF1/Cip expression in mBECs after H2O2 treatment may occur in a p53-dependent manner. In fact, the increased expression of p21WAF1/Cip as well as p16INK4a is also seen in damaged small bile ducts in PBC.8 Although several studies suggest that oxidative stress acts mainly through the p53-p21WAF1/Cip-Rb arm to induce senescence,34 other studies indicate that p16INK4a can be activated in response to oxidative stress, possibly through the action of the p38-MAPK protein.35 In the present study, an immediate increase of p16INK4a protein expression was seen on day 2 after H2O2 treatment without preceding bmi1 inhibition. This finding agreed with the latter studies. A transient response before day 1 might be responsible for the increased p16INK4a protein expression because p16INK4a mRNA expression on day 1 was rather low. Reportedly, an increased expression of p21WAF1/Cip1 is involved in the induction of senescence, whereas the p16INK4a accumulated in senescent cells is involved in the maintenance of senescence.36–38 Taken together, the immediate increase of p21WAF1/Cip as well as p16INK4a by oxidative stress seems to contribute to the induction of cellular senescence of mBECs in coordination with the bmi1-dependent increase of p16INK4a expression.
There have so far been no studies showing the effect of bmi1 inhibition on p21WAF1/Cip expression. However, it is suspected that p21WAF1/Cip is up-regulated by bmi1-inhibition through the pathway involving p19Arf, mouse double minute 2 (MDM2), and p53, resulting in cell-cycle arrest.13,39 In the present study, p21WAF1/Cip mRNA expression increased on days 3 and 5 because of bmi1 inhibition, although the up-regulation of p21WAF1/Cip protein is obscure. The increased p21WAF1/Cip mRNA expression on days 3 and 5 might be induced via a p19Arf-p53-dependent pathway. However, the reason why p21WAF1/Cip expression fluctuated due to bmi1 inhibition remains unclear and it may suggest the presence of a complex regulatory system between bmi1 and p21WAF1/Cip expression.
Recently, Hernandez-Munoz and colleagues17 reported that the other polycomb group protein EZH2 is required for the recruitment of bmi1 to polycomb group bodies. Therefore, we also examined the involvement of EZH2 in the regulation of bmi1 expression and the induction of cellular senescence. We have obtained several interesting results regarding this issue. Decreased EZH2 expression on day 4 after oxidative stress appears to have less impact on the induction of cellular senescence, when compared with the prompt decrease of bmi1. It is of interest that the knockdown of EZH2 by siRNA slightly decreased the nuclear expression of bmi1. This result agreed with the recent report by Hernandez-Munoz and colleagues.17 However, the knockdown of EZH2 does not affect cell proliferation and cellular senescence. Because the efficiency of EZH2 knockdown by siRNA was not complete in the present study, the decreased expression of EZH2 may not be enough to induce cellular senescence. Further studies are needed to clarify the significance of EZH2 and its association with bmi1 in the stress-induced cellular senescence.
Accumulating data suggest the importance of cellular senescence in the pathogenesis of several types of chronic liver diseases. We have reported cellular senescence in small bile ducts in PBC.8 Lunz and colleagues30 have previously implicated the stress-induced expression of p21WAF1/Cip in BECs as an important mediator of cellular senescence that precedes bile duct loss in chronic liver allograft rejection. Marshall and colleagues40 have recently reported the relation between hepatocyte G1 arrest, impaired regeneration, and fibrosis in chronic HCV infection. P21WAF1/Cip-mediated cell-cycle arrest may be a direct viral effect or may be induced by interferon-γ and transforming growth factor-β under conditions of oxidative stress and DNA damage.40 Hepatocytes showing SA-β-gal have been detected in chronic hepatitis due to HCV.41 It is conceivable that the oxidative stress-induced, bmi1-mediated up-regulation of p16INK4a may also impair the hepatocellular and biliary function, limit regeneration of BECs and hepatocytes, and contribute to the progression of chronic liver diseases, as well as PBC.
In conclusion, the decreased expression of bmi1 is closely associated with the cellular senescence of damaged small bile ducts in PBC. The in vitro study demonstrated that oxidative stress induced the decreased expression of bmi1 and the subsequent increased p16INK4a expression, decreased cell proliferation activity, and cellular senescence. Taken together, the decreased expression of bmi1 caused by oxidative stress may be critical for the induction of cellular senescence of BECs in PBC. Given that the decreased expression of bmi1 may be responsible for the increased expression of p16INK4a and cellular senescence, therapeutic agents to maintain bmi1 expression may be effective for the prevention of cellular senescence of BECs and for the prevention of bile duct loss in PBC.
Footnotes
Address reprint requests to Yasuni Nakanuma, M.D., Department of Human Pathology, Kanazawa University Graduate School of Medicine, Kanazawa 920-8640, Japan. E-mail: pbcpsc@kenroku.kanazawa-u.ac.jp.
Supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports Science and Technology, Japan.
References
- Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- Kaplan M. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–1580. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- Portmann B, Nakanuma Y. MacSween R, Burt A, Portmann B, Ishak K, Scheuer P, Anthony P, editors. London: Churchill Livingstone,; Diseases of the bile ducts. Pathology of the Liver, (ed 4) 2001:pp 435–506. [Google Scholar]
- Nakanuma Y, Ohta G. Histometric and serial section observations of the intrahepatic bile ducts in primary biliary cirrhosis. Gastroenterology. 1979;76:1326–1332. [PubMed] [Google Scholar]
- Fussey S, Guest J, James O, Bassendine M, Yeaman S. Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci USA. 1988;85:8654–8658. doi: 10.1073/pnas.85.22.8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe EB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Ikeda H, Haga H, Manabe T, Nakanuma Y. Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol. 2005;205:451–459. doi: 10.1002/path.1729. [DOI] [PubMed] [Google Scholar]
- Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DR, Jr, Reid LM, Gupta S. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol. 1999;276:G1260–G1272. doi: 10.1152/ajpgi.1999.276.5.G1260. [DOI] [PubMed] [Google Scholar]
- Brodsky WY, Uryvaeva IV. Cell polyploidy: its relation to tissue growth and function. Int Rev Cytol. 1977;50:275–332. doi: 10.1016/s0074-7696(08)60100-x. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M, Berns A. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J, Dimri GP. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Hernandez-Munoz I, Taghavi P, Kuijl C, Neefjes J, van Lohuizen M. Association of BMI1 with polycomb bodies is dynamic and requires PRC2/EZH2 and the maintenance DNA methyltransferase DNMT1. Mol Cell Biol. 2005;25:11047–11058. doi: 10.1128/MCB.25.24.11047-11058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanuma Y, Sasaki M. Expression of blood-group-related antigens in the intrahepatic biliary tree and hepatocytes in normal livers and various hepatobiliary diseases. Hepatology. 1989;10:174–178. doi: 10.1002/hep.1840100209. [DOI] [PubMed] [Google Scholar]
- Ludwig J. Small-duct primary sclerosing cholangitis. Semin Liver Dis. 1991;11:11–17. doi: 10.1055/s-2008-1040417. [DOI] [PubMed] [Google Scholar]
- Desmet V, Gerber M, Hoofnagle J, Manns M, Scheuer P. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- Alkema MJ, Bronk M, Verhoeven E, Otte A, van ’t Veer LJ, Berns A, van Lohuizen M. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian polycomb complex. Genes Dev. 1997;11:226–240. doi: 10.1101/gad.11.2.226. [DOI] [PubMed] [Google Scholar]
- Vonlanthen S, Heighway J, Altermatt HJ, Gugger M, Kappeler A, Borner MM, van Lohuizen M, Betticher DC. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer. 2001;84:1372–1376. doi: 10.1054/bjoc.2001.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Sasaki M, Ohira S, Ohta T, Oda K, Nimura Y, Chen MF, Jan YY, Yeh TS, Nakanuma Y. Aberrant expression of CDX2 is closely related to the intestinal metaplasia and MUC2 expression in intraductal papillary neoplasm of the liver in hepatolithiasis. Lab Invest. 2004;84:629–638. doi: 10.1038/labinvest.3700087. [DOI] [PubMed] [Google Scholar]
- Katayanagi K, Kono N, Nakanuma Y. Isolation, culture and characterization of biliary epithelial cells from different anatomical levels of the intrahepatic and extrahepatic biliary tree from a mouse. Liver. 1998;18:90–98. doi: 10.1111/j.1600-0676.1998.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Zen Y, Harada K, Sasaki M, Tsuneyama K, Katayanagi K, Yamamoto Y, Nakanuma Y. Lipopolysaccharide induces overexpression of MUC2 and MUC5AC in cultured biliary epithelial cells: possible key phenomenon of hepatolithiasis. Am J Pathol. 2002;161:1475–1484. doi: 10.1016/S0002-9440(10)64423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Van De Water J, Kenny TP, Gallo ML, Leung PS, Nakanuma Y, Ansari AA, Coppel RL, Neuberger J, Gershwin ME. Immunoglobulin gene usage and immunohistochemical characteristics of human monoclonal antibodies to the mitochondrial autoantigens of primary biliary cirrhosis induced in the XenoMouse. Hepatology. 2001;34:631–637. doi: 10.1053/jhep.2001.27544. [DOI] [PubMed] [Google Scholar]
- Sun LQ, Lee DW, Zhang Q, Xiao W, Raabe EH, Meeker A, Miao D, Huso DL, Arceci RJ. Growth retardation and premature aging phenotypes in mice with disruption of the SNF2-like gene, PASG. Genes Dev. 2004;18:1035–1046. doi: 10.1101/gad.1176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneyama K, Harada K, Kono N, Sasaki M, Saito T, Gershwin ME, Ikemoto M, Arai H, Nakanuma Y. Damaged interlobular bile ducts in primary biliary cirrhosis show reduced expression of glutathione-S-transferase-pi and aberrant expression of 4-hydroxynonenal. J Hepatol. 2002;37:176–183. doi: 10.1016/s0168-8278(02)00105-8. [DOI] [PubMed] [Google Scholar]
- Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon RF, Jain SK, Warnes TW. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta. 2003;1637:142–150. doi: 10.1016/s0925-4439(02)00225-9. [DOI] [PubMed] [Google Scholar]
- Lunz JG, III, Contrucci S, Ruppert K, Murase N, Fung JJ, Starzl TE, Demetris AJ. Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21(WAF1/Cip1) as a disease marker and the influence of immunosuppressive drugs. Am J Pathol. 2001;158:1379–1390. doi: 10.1016/S0002-9440(10)64089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120:190–199. doi: 10.1053/gast.2001.20875. [DOI] [PubMed] [Google Scholar]
- Jhandier MN, Kruglov EA, Lavoie EG, Sevigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280:22986–22992. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- Chen QM. Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann NY Acad Sci. 2000;908:111–125. doi: 10.1111/j.1749-6632.2000.tb06640.x. [DOI] [PubMed] [Google Scholar]
- Chen JH, Stoeber K, Kingsbury S, Ozanne SE, Williams GH, Hales CN. Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J Biol Chem. 2004;279:49439–49446. doi: 10.1074/jbc.M409153200. [DOI] [PubMed] [Google Scholar]
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A, Rushbrook S, Davies SE, Morris LS, Scott IS, Vowler SL, Coleman N, Alexander G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology. 2005;128:33–42. doi: 10.1053/j.gastro.2004.09.076. [DOI] [PubMed] [Google Scholar]
- Paradis V, Youssef N, Dargere D, Ba N, Bonvoust F, Deschatrette J, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]