Abstract
When pancreatic tissue is injured after duct obstruction, acinoductal metaplasia is observed. Similar metaplastic changes occur when exocrine pancreatic cells are isolated and cultured. We demonstrate that under these experimental conditions the exocrine acinar cells lose their differentiated characteristics: expression of the acinar transcription factors p48/Ptf1α and Mist1 is decreased or lost, whereas expression of the embryonic transcription factor Pdx1 is increased. The receptors Notch1 and Notch2, members of the DSL family of Notch ligands, and the target genes in the Notch-signaling pathway Hes1, Hey1, and Hey2 become strongly up-regulated. We noted also reduced expression of Sel1L, a Notch repressor that is normally highly expressed in exocrine pancreas. Stimulation of Notch by its ligand Jagged1 diminished the proliferation of cultured metaplastic exocrine cells. Chemical inhibition of Notch signaling resulted in increased proliferation and induction of the cell-cycle regulator p21Cip1. This effect seems to be Hes1-independent and mainly coincides with decreased Hey1 and Hey2 mRNA expression. In conclusion, we demonstrate that during acinoductal metaplasia the Notch-signaling pathway is activated concomitantly with changes in transcription factor expression of pancreatic acinar cells. In addition, we show that Notch signaling is implicated in the suppression of proliferation of these metaplastic exocrine cells. The latter may be important in protection from neoplastic transformation.
Damage to the pancreas typically causes exocrine cell metaplasia that can lead to different phenotypic conversions.1–3 It has been proposed that these metaplastic cells may represent precursor cells in tissue repair or regeneration, and that they can be exploited as targets for cell-based regenerative therapy of chronic disorders like diabetes.2 On the other hand, the metaplastic exocrine cells can also become a target for genetic damage, leading to pancreas cancer.4 It is therefore important to study what changes occur in gene expression during exocrine pancreas metaplasia, and to get insight into how these cells can become metaplastic without evolving into pancreatic neoplasia. One can take advantage of this knowledge to manipulate the exocrine cell population for regenerative medicine and to find ways for preventing cancer development.
We searched for the mechanisms that regulate the plasticity in proliferation and differentiation of pancreatic exocrine cells. We studied pancreatic duct ligation, an established model of tissue damage in which acini become metaplastic and transform into tubular duct-like structures.5 From these duct-like complexes new endocrine islet cells and eventually the normal exocrine acini are regenerated. We previously showed that the endocrine regeneration process could be considerably stimulated by exogenous gastrin administration.6,7 Gastrin however is also a strong mitogen for the metaplastic cells,6 and its role in development of pancreatic cancer has been shown.8 Nevertheless, in the duct ligation model in which this hormone also becomes overexpressed,9 the cells do not become neoplastic.
To complement the in vivo experimental model, we studied an in vitro model of exocrine cell metaplasia.10 In this primary culture model, we previously showed conversion of acinar cells to duct-like cells, similar to the duct ligation model. Depending on the culture conditions, hepatocyte-like cells and islet β-cells can also be obtained.11,12
In the present study, we further define the cellular changes at the level of transcription factor expression during acinoductal metaplasia in these experimental models. We focused on Notch signaling because it is known to represent a crucial regulator of cell fate decisions during embryonic development and in many different systems including the pancreas.13 In the embryonic pancreas, Notch1 and Notch2 receptors, as well as Hes1 are expressed in early Pdx1-positive precursor cells between E11 and E12.14 These precursor cells co-express p48. Cells expressing Notch1, Hes1, and p48 develop into exocrine cells with maintenance of p48 expression and loss of Notch1 and Hes1 expression. Developing endocrine cells show lower Hes1 expression, are p48-negative, and display Ngn3 expression. Notch3 and Notch4 are expressed by the mesenchyme and endothelial cells. The Notch ligand Dll1 is found scattered in the duct epithelium and the ligands Jagged1 and Jagged2 were demonstrated in the endothelial cells of the developing pancreas.13,14 Loss of function of Dll1 or its downstream targets RBPJκ or Hes1 leads to premature endocrine cell differentiation at the expense of precursor cells.13,15 Forced expression of Notch under the Pdx1 promotor causes cystic lesions, and suppresses acinar and endocrine cell differentiation.16,17 For each of the Notch pathway components, little or no expression is observed in the adult pancreas, with some Hes1 protein expressed by centro-acinar cells.18 In the adult pancreas, reactivation of Notch expression has been reported. The Notch receptors and ligands become frequently expressed in metaplastic duct lesions and PanIN epithelium.18 Notch1, Notch2, Hes1, Jagged2, and low levels of Dll1 become also expressed in the pancreatic exocrine epithelium during experimental pancreatitis.19 However, little is known about the role of the Notch signaling pathway when reactivated in adult pancreas and this has not been related with regenerative processes. We report that Notch re-expression is associated with modulation of the adult rat exocrine cell phenotype, and we show that Notch signaling is involved in the control of cell proliferation of metaplastic exocrine cells and possibly has a role in pancreas regeneration.
Materials and Methods
Chemicals and Antibodies
The γ-secretase inhibitors L685,458 and DFK-167 were purchased from, respectively, Calbiochem (La Jolla, CA) and MD Biosciences (St. Paul, MN). Recombinant Jagged1 was from R&D systems (Minneapolis, MN). Recombinant human epidermal growth factor (EGF) and BrdU were purchased from Sigma (St. Louis, MO). Primers, Taq polymerase, SuperScriptII Rtase, and reverse transcriptase-polymerase chain reaction (RT-PCR) buffers were purchased from Invitrogen (Paisley, UK). We used antibody Val 1744 from Cell Signaling Technology (Beverly, MA) to detect the Notch intracellular domain (NICD). Monoclonal anti-p21 was from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal anti-BrdU from MP Biomedicals (Aurora, OH), monoclonal anti-vimentin from Chemicon (distribution firm; Biognost, Heule, Belgium), and monoclonal anti-cytokeratin 20 from Novocastra (Newcastle, UK). Antibodies against Pdx1, Hes1, Mist1, and p48/Ptf1 were gifts from, respectively, Dr. O. Madsen, Dr. Tetsuo Sudo, Dr. S.F. Konieczny, and Dr. F.X. Real. Sheep anti-mouse and donkey anti-rabbit biotinylated were from Amersham (Buckinghamshire, UK), goat anti-guinea pig biotinylated from Vector Laboratories (Burlingame, CA). For Western blot we used mouse anti-p21 and goat anti-β-actin from Santa Cruz Biotechnology. Secondary horseradish peroxidase-labeled anti-goat (Jackson Laboratory, Bar Harbor, ME) and anti-mouse (Amersham) were used. For immunofluorescence fluorescein isothiocyanate-coupled anti-rabbit antibody (Jackson ImmunoResearch, West Grove, PA) was used.
Animal Experiments
Duct-ligation was performed in adult male Wistar rats from Janvier (Le Genest-St-Isle, France), as described.5 In brief, exocrine ducts draining the splenic part of the rat pancreas are ligated with a silk thread obstructing the exocrine fluid in the splenic part. Seven days after the duct ligation has been applied, the ligated part of the pancreas has undergone metaplastic transformation and comparisons are made with the unligated part of the same pancreas, which serves as a control.5 In addition, we collected main ducts from normal adult rat pancreata by manual microdissection, eliminating the acinar and endocrine tissue as much as possible. Experiments were approved by the ethics committee of the Free University of Brussels. The principles of laboratory animal care (NIH publication no. 85-23, revised 1985) were followed and specific national laws on this issue were complied with. This study was performed in accordance with the Declaration of Helsinki as revised in 2000.
Exocrine Cell Culture Model
Exocrine acinar cells from adult rat pancreas were collected and cultured as previously described.10,12 Briefly, acinar cells, obtained by centrifugal elutriation from collagenase-digested rat pancreas, were cultured in suspension at an average density of 85.104 cells per ml. Culture medium consists of RPMI 1640 glutamax medium (Gibco BRL, Paisley, UK) with 10% fetal bovine serum (Gibco BRL), penicillin (75 μg/ml) (Continental Pharma, Brussels, Belgium), streptomycin (100 μg/ml), and geneticin sulfate (50 μg/ml) (both from Sigma). The cells were precultured (in suspension) for a total of 5 days, the period in which the acinoductal conversion takes place. Medium was replaced daily. Cells did not proliferate unless they were allowed to attach to plastic. For this purpose, cells were transferred on day 4 of culture into tissue culture treated wells (Falcon; BD Biosciences, Erembodegem, Belgium). After 1 day, nonadherent cells were washed off and serum concentration was reduced to 1%. Unless differently indicated, monolayers were studied after 3 days of monolayer culture, with or without EGF, Jagged1, or the γ-secretase inhibitors added at the indicated concentrations. In control cultures the solvent (dimethyl sulfoxide) was added at the same concentration. To test the effect of Jagged1 on cell growth, culture plates were precoated with Jagged1 (20 μg/ml) for 2 hours in the CO2 incubator.20 In all monolayer conditions, 10 μmol/L BrdU was added to the cultures 24 hours before harvesting to measure cell proliferation. BrdU-labeling was scored as the percentage of BrdU-labeled epithelial cells in monolayers, and in each experiment at least 1000 cells were counted independently by two persons.
RT-PCR
Total RNA (0.5 μg) was used after extraction from pancreas tissue or 1 μg from dissected exocrine ducts with TRIzol (Invitrogen) and from cultured exocrine cells with the Gene Elute Total RNA kit (Sigma). RNA quality was controlled with Agilent-technology (2100 Bioanalyzer; Agilent, Palo Alto, CA), an automated process that estimates the RNA integrity by the entire electrophoretic trace of the RNA sample, including the absence or presence of RNA degradation (www.agilent.com/chem/labonachip). Semiquantative RT-PCR was performed according to the manufacturer’s protocol. The primer pairs and number of cycles used were the following: β-actin (27 cycles) forward 5′-ACTATCGGCAATGAGCGGTTC-3′ and reverse 5′-AGAGCCACCAATCCACACAGA-3′; Mist1 (33 cycles) forward 5′-CCAGCCGCTTTGAACTCCTAA-3′ and reverse 5′ TTCCCGTAGCCGCACAATATG-3′; p48/Ptf1 (30 cycles) forward GCTCCTGGAGCATTTTCCCG-3′ and reverse 5′ CTGAGGAACTCTACCTCCGC-3′; Pdx1 (35 cycles) forward 5′-CTCGCTGGGAACGCTGGAACA-3′ and reverse 5′-GCTTTGGTGGATTTTCATCCACGG-3′; Notch1 (30 cycles) forward 5′-CTTGTGAAAATGACGCCCG-3′ and reverse 5′-CCTTATTGCCTGCATCCTCCT-3′; Notch2 (33 cycles) forward 5′-GCTCCACACCAACTTACGCAT-3′ and reverse 5′-GATAATGACCACAGCAACCGC-3′; Hes1 (28 cycles) forward 5′-GTCCCCGGTGGCTGCTAC-3′ and reverse 5′-AACACGCTCGGGTCTGTGCT-3′; Hey1 (35 cycles) forward 5′-AAAGACGGAGAGGCATCATCG-3′ and reverse 5′-GCAGTGTGCAGCATTTTCAGG-3′; Hey2 (35 cycles) forward 5′-ATTTGAAGATGCTCCAGGCAAC-3′ and reverse 5′-GGCCTTCCACAGAGCTTAGGTA-3′; Jagged1 (30 cycles) forward 5′-ATGGCCTCCAACGATACTCCT-3′ and reverse 5′-ACATGTACCCCCATAGTGGCA-3′; Jagged2 (30 cycles) forward 5′-GCGTTCTTTCACCCTCATCGT-3′ and reverse 5′-ACGGCTTCTTTGCACTCCTTG-3′; Dll1 (30 cycles) forward 5′-CGGCTTCTATGGCAAGGTCT-3′ and reverse 5′-TCCACATTGTCCTCGCAGTA-3′; p21Cip1 (28 cycles) forward 5′-TCTTGCACTCTGGTGTCTCACG-3′ and reverse 5′-TGAAGGCTAAGGCAGAAGATGG-3′; Sel1L (26 cycles) forward 5′-GATGAAGATCCTGAACGGCAG-3′ and reverse 5′-TATCAGGTTGCCTCCAAGAGC-3′. For each gene, PCR product accumulation was assessed at various cycle numbers to determine linear detection of the PCR product. Quantification of ethidium bromide-stained PCR products was done with the NIH Image 1.63 computer software: density is measured in a fixed area (able to incorporate each separate band), background density is substracted, and all measurements are divided by the value of the β-actin band of the corresponding sample. The value for control pancreas is set at 1(=100%). Negative controls consist of water instead of RNA but with all buffers and enzymes added.
Western Blot
Total cellular protein fraction was extracted in radioimmunoprecipitation buffer (RIPA: 150 mmol/L NaCl, 1% Nonidet P-40, 1% deoxycholate, 0.1% sodium dodecyl sulfate, 50 mmol/L Tris base, pH 7.5, 2 mmol/L ethylenediaminetetraacetic acid) with protease inhibitors (10 mmol/L Na3VO4, 50 mmol/L NaF, 1 mmol/L Na4P2O7.10 H2O, 10 mmol/L β-glycerophosphate, and 10 mmol/L p-nitrophenylphosphate) and complete protease inhibitor cocktail tablets (Roche, Brussels, Belgium). Concentrations were determined by the BCA method (Pierce, Rockford, IL). Proteins were separated on sodium dodecyl sulphate-polyacrylamide gels and electroblotted to nitrocellulose membranes. Loading of equal amount of proteins (50 μg per sample) was evaluated by Ponceau staining and by detection of β-actin in the same blot after stripping. Comparison for the same protein between two different samples is always shown on the same gel. Antibodies were used as follows: mouse anti-p21:1/100; goat anti-β-actin, 1/1500; donkey anti-goat-horseradish peroxidase, 1/8000; sheep anti-mouse horseradish peroxidase, 1/10000. Immunoreactivity was revealed with ECL detection (Amersham).
Immunohistochemistry
Cell pellets were fixed for 1 hour in buffered 4% paraformaldehyde, entrapped in 2% agarose gel (40°C) (Sigma), and processed for paraffin embedding. Tissues were fixed with the same fixative for 4 hours and processed for paraffin embedding. Paraffin sections were used for immunostaining as described.21 Antigen retrieval on paraffin sections was performed by microwave heating in antigen retrieval solution-citrate buffered (Prosan, Merelbeke, Belgium) for detection of p48/Ptf1, Pdx1, Hes1, and Mist1. To assess cell proliferation, to detect NICD, and to demonstrate p21CIP1 expression, monolayer-cultured cells were stained directly in the culture wells. Permeabilization of the cells in the monolayers was achieved by incubation in methanol at −20°C. For BrdU staining, additional incubation in 2 N HCl and borate buffer was done.
For NICD detection in suspension-cultured cells, cell aggregates were fixed using 4% formaldehyde in PBS, subsequently permeabilized with cold methanol (20 minutes at −20°C) and nonspecific background signals were eliminated using Image-IT FX signal enhancer (Invitrogen). Samples were incubated overnight at 4°C with rabbit anti-NICD. Secondary detection was performed with TRITC-conjugated donkey anti-rabbit IgG. DNA was visualized with SytoxGreen reagent (Invitrogen) and samples were mounted using Vectashield anti-fade (Vector Laboratories, Inc.). These samples were visualized using a Leica TCS SP confocal microscope and image processing was done with Volocity LE software (Improvision, Coventry, UK).
For all other immunohistochemistry and immunocytochemistry, the avidin-biotin complex method was applied. The primary antibodies were used at the following dilutions: anti-p48/Ptf1,22 10 ng/μl; anti-Mist1,23 1/10; anti-Pdx1,24 1/1000; anti-Hes1,25 1/60; anti-BrdU, 1/10; anti-vimentin, 1/200; anti-cytokeratin 20, 1/10; anti-p21Cip1, 1/30; and anti-NICD, 1/20. The secondary antibodies were the following: sheep anti-mouse biotinylated:1/300; goat anti-guinea pig biotinylated, 1/1000; and donkey anti-rabbit biotinylated, 1/300.
Statistics
Results are presented as means ± SEM. Data are analyzed by Student’s t-test. Statistical significance is considered when P values are <0.05.
Results
In Vivo Model of Acinoductal Metaplasia Induced by Duct Ligation
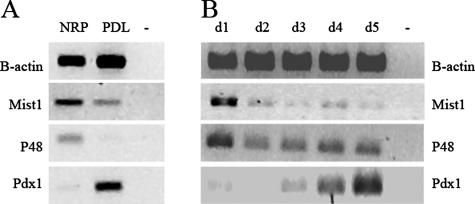
As reported previously, in the ligated part of the pancreas the acini lose amylase immunoreactivity and completely transform into tubular ductal complexes expressing cytokeratin 20 within 1 week.5,6 With RT-PCR we find that Pdx1 expression is 29.3 ± 9-fold increased over control pancreas (P = 0.02, n = 5), with p48/Ptf1 expression being decreased to 27 ± 19% of control pancreas (P < 0.01, n = 5) and Mist1 expression to 38 ± 17% of control pancreas (P = 0.02, n = 7) (Figure 1A). It should be noted that the increase of Pdx1 may be overestimated as the islet-mass (which is known to highly express Pdx1) is increased in ligated pancreas.5,7 These changes are confirmed at the protein level, in which the ductal complexes stain positive for Pdx1 protein and display diminished immunoreactivity for p48/Ptf1 and Mist1, whereas the nuclei of the original acini show high p48/Ptf1 and Mist1 expression and have no Pdx1 expression (Figure 2).
FIGURE 1.
A: RNA expression profile of pancreas transcription factors in normal rat pancreas (NRP) versus duct-ligated rat pancreas (PDL). In ligated pancreas Mist1 and p48/Ptf1α are down-regulated, whereas Pdx1 is strongly induced. B: RNA expression profile of rat exocrine cells during the 5-day culture period. Mist1 expression is already lost at the second day of culture, p48/Ptf1 expression is reduced and Pdx1 induced during the 5-day culture period. −, without template.
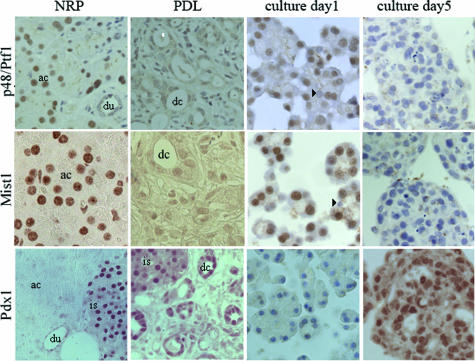
FIGURE 2.
Protein expression of the pancreas transcription factors p48/Ptf1, Mist1, and Pdx1 in sections of normal pancreas (NRP), duct-ligated pancreas (PDL), freshly isolated acini (culture day 1), and cultured metaplastic acinar cells (culture day 5): p48/Ptf1α and Mist1 expression are confined to the acinar tissue in normal pancreas and are lost in the ductal complexes that form after ligation of the pancreatic ducts. Pdx1 is highly expressed in islets of normal pancreas and to a lower extent in ducts and becomes up-regulated in ductal complexes after ligation. Freshly isolated acinar cells express p48/Ptf1 and Mist1 but not Pdx1. After 5 days of culture, the cells are negative for p48/Ptf1 and Mist1, but have gained Pdx1 expression. The nuclear staining for the transcription factors is revealed with diaminobenzidine resulting in a brown precipitate, and counterstained with hematoxylin except for Pdx1 and Mist1 staining in NRP, PDL, and culture day 5. ac, Acinus; du, duct; is, islet; dc, ductal complex; arrowhead, centro-acinar cell. Scale bar = 10 μm.
Acinoductal Metaplasia in a Primary Culture Model
We showed before that rat acinar cells cultured in suspension in the presence of serum factors lose their acinar phenotypic characteristics and gain duct-like characteristics.10 Within 4 days of suspension culture, the amylase-expressing acini convert into amylase-negative cytokeratin-20-positive duct-like cells. We now show that at the RNA level, Mist1 expression is decreased very rapidly and p48/Ptf1 expression declines also but to a lesser extent. Pdx1 expression strongly increases (Figure 1B).10 Immunostainings show highly reduced p48/Ptf1 and Mist1 protein expression in the nucleus whereas Pdx1 expression is up-regulated (Figure 2). Western blot analysis still detects some p48/Ptf1 and Mist1 protein (not shown).
The Notch Pathway Is Induced during Acinoductal Metaplasia
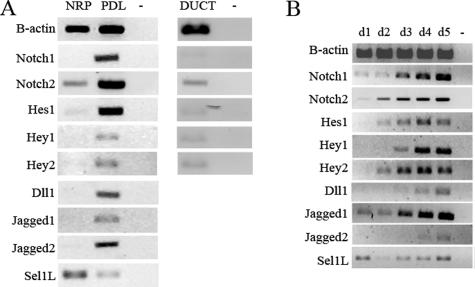
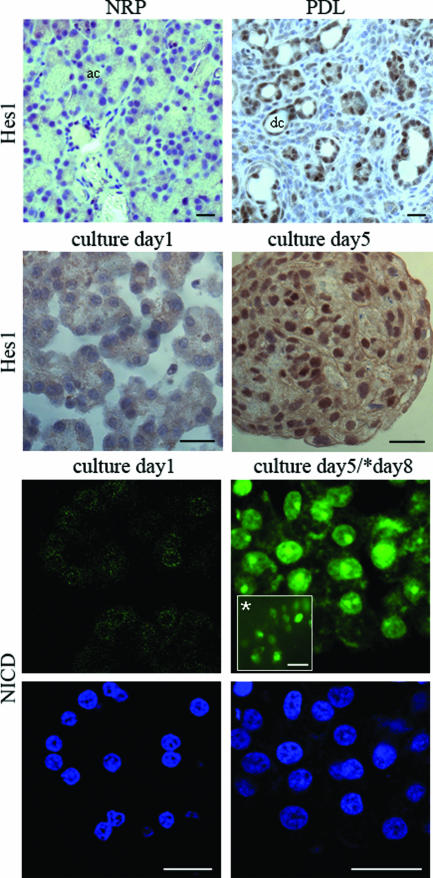
The expression of Notch receptors, Notch ligands, and downstream target genes Hes1, Hey1, and Hey2 was evaluated. Notch1 and Notch2 expression at the RNA level are strongly up-regulated together with Hes1 in duct-ligated pancreas (3.3 ± 0.4-fold increase for Notch1, P < 0.01, n = 5; 2.6 ± 0.6-fold increase for Notch2, P = 0.005, n = 5; 1.7 ± 0.2-fold increase for Hes1, P = 0.03, n = 5) (Figure 3A). These genes were not or very weakly expressed in samples of normal rat pancreas (Figure 3A). To exclude the possibility that these genes are expressed by the minority of ducts present in the tissue samples, we also analyzed samples of microdissected pancreatic duct. These samples lacked Notch1, Hes1, Hey1, and Hey2 expression and showed very weak Notch2 expression (Figure 3A) while the duct-specific cytokeratin 20 expression could be readily demonstrated (not shown). This indicates that Notch signaling is not active in normal ducts, unlike the metaplastic duct-like cells. With immunohistochemistry we demonstrate that Hes1 expression is highly induced in metaplastic duct tissue whereas analysis of normal rat pancreas shows no or a very low number of Hes1-expressing cells (Figure 4). Expression of Hey1 mRNA was 6.1 ± 1.5-fold (P < 0.01, n = 5) and of Hey2 4.7 ± 1.0-fold (P < 0.01, n = 5) up-regulated in duct-ligated pancreas (Figure 3A). In cultured exocrine cells, Notch1, Notch2, Hes1, Hey1, and Hey2 were also significantly induced at the RNA level (Figure 3B). Using the Val1744 antibody we could demonstrate NICD immunoreactivity in cells that were cultured, whereas the original acinar cells were negative (Figure 4). Also Hes1 immunoreactivity follows the same pattern (Figure 4).
FIGURE 3.
A: RNA expression profile of Notch and related genes in normal rat pancreas (NRP) versus ligated rat pancreas (PDL) and in normal rat pancreatic ducts (DUCT), Notch receptor, the target genes, and ligands are induced in ligated pancreas (PDL), concomitant with down-regulated Sel1L expression. Notch signaling is not active in pieces of normal pancreas tissue rich of ducts (DUCT). B: RNA expression profile of rat exocrine cells during a 5-day culture period. Notch receptor, the target genes, and ligands are induced during culture, concomitant with a more transient down-regulated Sel1L expression. −, without template.
FIGURE 4.
Protein expression of Hes1 and NICD in normal pancreas (NRP), duct-ligated pancreas (PDL), freshly isolated acini (culture day 1) and cultured metaplastic acinar cells [culture day 5 in suspension and culture day 8 as monolayer (*inset)]. The nuclear staining for Hes1 is revealed with diaminobenzidine resulting in a brown precipitate, and counterstained with hematoxylin. NICD was detected with TRITC and nuclei were visualized in the same samples with SytoxGreen reagent. Hes1 and NICD expression are highly induced in ductal complexes and cultured cells but nearly undetectable in normal pancreas and freshly isolated acini. ac, acinus; dc, ductal complex. Scale bar = 10 μm.
We also studied the mRNA expression of ligands for the Notch receptor. The Notch ligand Dll1 is 3.8 ± 0.2-fold increased after duct ligation versus unligated rat pancreas (P < 0.001, n = 5) (Figure 3A). Jagged1 and Jagged2 expression are, respectively, 4.0 ± 0.8-fold (P < 0.01, n = 5) and 3.8 ± 0.4-fold increased in ligated rat pancreas (P < 0.001, n = 5) (Figure 3A). In vitro induction of Jagged1 expression seems to be more prominent than Jagged2 expression (Figure 3A).
We find that the increase in Notch-expression is paralleled by down-regulation of Sel1L, a known negative regulator of Notch. Sel1L expression in ligated pancreas is 22 ± 9% of unligated pancreas (P = 0.02, n = 5) (Figure 3A). During exocrine cell culture Sel1L expression levels are decreased at day 3 (26 ± 4% of expression at day 1, P < 0.001, n = 4) with some recovery of expression at day 5 but still lower compared to freshly isolated exocrine cells (54 ± 7%, P = 0.013, n = 4) (Figure 3B).
Notch Signaling Regulates Proliferation of Metaplastic Exocrine Cells and Leads to Increased Expression of p21Cip1
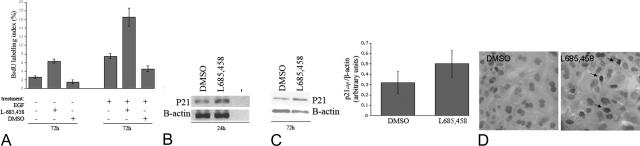
L685,458 (γ-secretase inhibitor X) is an established potent and selective pharmacological inhibitor that blocks intramembranous γ-secretase activity, by which production of the Notch intracellular domain (NICD) on ligand binding is inhibited. This results in decreased Notch signaling.26,27 Metaplastic exocrine cells were obtained after 4 days of suspension culture, and were further cultured as a monolayer (known to allow proliferation of these cells, unpublished observations), with or without L685,458 at 0.1 μmol/L. On 24 hours of treatment with L685,458, the expression of Hes1 at RNA and protein levels was unaffected (not shown) but the expression of Hey1 mRNA was 2.8-fold decreased by the treatment (P = 0.03, n = 4) and Hey2 mRNA was 5.2-fold decreased (P = 0.03, n = 4). The treatment did not affect the low p48 expression (P = 0.37, n = 4) and Mist1 expression was absent in both conditions. During this period, the BrdU-labeling index was increased 2.3-fold over control conditions with L685,458 (P = 0.005, n = 3). Without any other growth factors supplemented, the BrdU-labeling index remained 2.4-fold increased during 3 days in the presence of L685,458 (P < 0.01, n = 4) (Figure 5A). EGF (50 ng/ml) is a known mitogenic factor and gives 2.8-fold increase in proliferation (P < 0.001, n = 16). When EGF is combined with the γ-secretase inhibitor, an approximate sixfold increase is obtained (P = 0.02 versus EGF-alone, n = 4) (Figure 5A). Effects were confirmed using another chemical γ-secretase inhibitor (DFK-167) (data not shown). These data demonstrate that inhibition of Notch signaling stimulates cell proliferation. Metaplastic exocrine cells cultured on plates coated with Jagged-1 (20 μg/ml), which is a known stimulatory ligand of the Notch receptor, showed ∼1.6-fold reduction in cell proliferation, both in the absence and presence of the mitogen EGF in the medium (respectively, P < 0.01 and P = 0.03, n = 10) (Figure 5A).
FIGURE 5.
A: BrdU-labeling index (%) in exocrine cell monolayer culture treated for 72 hours with or without L685,458, EGF, and Jagged-protein. L685,458 enhanced proliferation, whereas Jagged1 reduced proliferation of cells that were treated or not with the mitogen EGF. B: RT-PCR for p21CIP1 expression in exocrine cell monolayer culture 24 hours with L685,458 or with the solvent alone (dimethyl sulfoxide). −, Without template. C: Western blot analysis of p21CIP1 in these culture conditions after 72 hours of exposure to L685,458 versus control. The relative density of the protein bands (p21Cip1/β-actin) is represented in the graph. p21CIP1 is up-regulated in L685,458 conditions at RNA and protein level. D: Nuclear p21Cip1 expression detected in monolayer cultured cells (with and without L685,458) by immunohistochemistry. The nuclear staining is revealed with diaminobenzidine resulting in a brown precipitate. In the L685,458-exposed cells, some nuclei stain more intensely compared to the control condition.
The expression of the cell cycle regulator p21Cip1 was up-regulated in L685,458-exposed monolayers of metaplastic exocrine cells. This effect was seen at RNA level (1.2-fold, P = 0.04, n = 3) (Figure 5B). Also protein levels of p21Cip1 were 1.5-fold higher in total protein extracts from L685,458-treated monolayers compared to controls (P = 0.005, n = 5) (Figure 5C). p21Cip1 expression was detected in the nucleus of monolayer cultured cells, with some nuclei having a more intense staining in the L685,458 condition than in the condition without L685,458 (Figure 5D).
Discussion
We previously reported that acinar exocrine cells of the adult rodent pancreas retain differentiation plasticity and can undergo various phenotypic switches including transdifferentiation to duct-like,10 hepatocyte-like,11 and endocrine islet12 cells. These transdifferentiations are preceded by a dedifferentiation event wherein the cells lose amylase expression and gain CK20 expression.10 It remains to be discovered which are the key transcription factors and signaling pathway(s) involved in the initial dedifferentiation that leads to plasticity. Others have also reported that acinar cells can transdifferentiate into endocrine insulin cells,28 without evaluating in detail the preceding steps. CK19, the only duct cell-specific marker that was evaluated was found to increase during their culture procedure. In the present study, we demonstrate that after experimental injury to the pancreas or after cell isolation (another type of injury), there is a highly reduced expression of the exocrine acinar cell transcription factors Mist1 and p48/Ptf1α. A correlation between acinoductal phenotypic conversion and suppression of Mist1 expression has been reported before in transgenic mice.29 Also p48/Ptf1 was nearly completely suppressed in acinar-derived ductal complexes in chronic pancreatitis and in transforming growth factor-α-overexpressing cells in the pancreas.30 In a previous study,10 we noticed that p48/Ptf1 remained expressed to some extent during acinar cell culture but differences in expression level during the culture period were at that time not studied in depth. In addition, we report induced Pdx1 expression and reactivation of the Notch-signaling pathway, which are both features of protodifferentiated embryonic pancreas epithelium. Up-regulation of Pdx1 in exocrine cells was also reported in other pancreas regeneration models.31,32 Concomitant with the observed changes in pancreas-specific transcription factors, Notch1 and Notch2 expression, and ligands for this receptor were highly induced both in vivo and in vitro. Notch regulates precursor cell maintenance, proliferation, and differentiation in many cell types during embryonic development. Besides the role of pancreatic Notch in the lateral inhibition process to suppress endocrine differentiation,13,15 Notch has been found to maintain cells in the precursor state. It was reported that Notch1 and Notch2 expression starts to decline in embryonic mouse pancreas from E14.5 on.14 Others have reported that at E18.5 duct cells still express Notch1 and Hes1.33 We detected no or very low expression of Notch1 and Notch2 and its downstream targets in adult pancreas or in duct cells. Our observations indicate that re-expression of Notch occurs in metaplastic or dedifferentiated acinar cells. These cells are involved in vivo in the regeneration of pancreas tissue.2,3 Likewise, we previously demonstrated that these metaplastic exocrine cells also express gastrin/CCK-B receptors, whereas normal acinar or duct cells do not.6,7 In embryonic pancreas it has been shown before that Notch expression blocks terminal acinar cell differentiation, despite ongoing p48 expression.34 Our data also support the observations of a recent study, in which it was shown that Notch receptors are induced in the exocrine cells during pancreas regeneration in the course of caerulein-induced pancreatitis.19 In that experimental approach, similar dedifferentiation of the exocrine acinar cells was found. The extent of tissue damage and consequent cellular alterations after caerulein exposure seem to be more transient, probably explaining why the definitive turning-on of the ductal phenotype was not achieved. Moreover duct cell identification solely relied on DBA-lectin staining whereas in our experiments we have analyzed more duct cell markers.2,6,10–12 It should also be stressed that double-positive cells expressing acinar and ductal cell markers at the same time are rare in general, and that only in conditions in which the process can be slowed down, such transitional types of cells can be identified.35
After binding of ligands from the DSL family, Notch receptors undergo proteolytic steps and the NICD translocates to the nucleus where target genes are activated, among them Hes1.36 Hes1 is present in undifferentiated pancreatic precursor cells, repressing endocrine and exocrine differentiation.15,16 Besides Hes1, Hes-related proteins Hey1 and Hey2 are targets in the Notch pathway.36 We show that Notch1, Hes1, Hey1, and Hey2 are highly induced in our experimental models. Because of the availability of a reliable anti-Hes1 antibody,25 we further analyzed the expression of this target gene at the protein level in our experimental models, confirming that Hes1 protein is highly expressed in metaplastic duct cells and is not abundantly present in acini. Our analysis also reveals that different ligands (Dll1, Jagged1, or Jagged2) are induced that can trans-activate the Notch receptors. It should be noted that each of these Notch ligands could have different effects.37 Because of the lack of reliable antibodies, we did not perform a detailed study of which cells express these ligands. Different cell types besides the exocrine cells may be involved, eg, mesenchymal cells, inflammatory cells, or endothelial cells. Jagged1 and Jegged2 expression has been documented in some intralobular duct cells of human pancreas,18 and in mesenchymal cells of embryonic pancreas.33
An accumulation of Notch receptor can result from down-regulation of negative regulators or induction of stimulators. Sel1L is a gene that is highly expressed in normal exocrine pancreas and it is known in other species to function as a negative regulator of Notch signaling.38 We are the first to show that expression of Sel1L is significantly reduced with concomitant increase of Notch1 and Notch2 in the rat pancreas. Potential upstream signaling molecules that may switch on the Notch pathway include ligands for the EGF receptor (EGFR) and/or gp130.18,39 EGFR was reported before to act upstream of Notch in mouse exocrine cell culture.18,40 Both EGFR and gp130 are expressed in pancreatic exocrine cells.28,41
To uncover the function of Notch we used γ-secretase inhibitors that are known to suppress Notch signaling.26,27 In none of our conditions the addition of inhibitor influenced the Hes1 expression at RNA or protein levels. On the other hand, expression of the other known target genes Hey1 and Hey2 mRNA were significantly reduced by the use of this compound. Although it has been repeatedly reported that signaling via Notch can result in Hes1-independent effects,36 we see an increased Hes1 expression during culture of acinar cells and after ligation of exocrine ducts in vivo. We cannot exclude that during acinoductal metaplasia a parallel Notch/RBP-Jk-independent pathway is operating that results in the increased Hes1 expression.40 This might also explain why L685,458 addition does not prevent acinoductal conversion in our culture conditions (unpublished observations). We found that γ-secretase-inhibitors stimulated the proliferation of metaplastic exocrine cells and that this effect is coinciding with decreased Hey1 and Hey2 levels. We found this stimulatory effect on proliferation also in metaplastic exocrine cells derived from human pancreas (not shown). Addition of the Notch ligand Jagged1 to the cultures diminished cell growth. Notch has been reported to stimulate as well as to inhibit cell growth, depending on the cell type studied. Activated Notch causes growth suppression in several other cell types,42–46 but the relation of Notch expression and its role in controlling the growth of metaplastic exocrine cells from pancreas has not previously been described. After duct ligation, Notch expression is high, although proliferation of the metaplastic cells is highly increased when compared to the basal growth rate of exocrine acinar and duct cells.6 It is likely that the growth stimulus coming from local production of factors such as transforming growth factor-α and gastrin9 stimulates the proliferation in the ductal complexes but that Notch is a limiting factor preventing aberrant cell growth. Notch may thus represent an important factor in preventing neoplasia. When duct-ligated rat pancreas was examined 1 month after ligation, the exocrine compartment was regenerated and no cancerous development could be detected (unpublished data). In contrast, Elas/CCK2 mice which undergo continuous mitogenic stimulation by gastrin do develop tumors in cells that underwent acinoductal conversion.8 In the caerulein-treated mouse model, a similar transient increase in proliferation was noted as early as after duct ligation but the expression of Notch declined together with a decline in proliferation and together with restoration of the differentiated exocrine state within 1 week. In the duct ligation model it takes more than 14 days to restore normal tissue structure. So in the caerulein model Notch expression does not need to be sustained as long to control cell growth.
We also noted that γ-secretase exposure in monolayers of acinar-derived cells resulted in up-regulated p21Cip1 expression. Nuclear p21Cip1 expression was demonstrated with immunocytochemistry and the differences in expression between the culture conditions with and without L685,458 were quantified by RT-PCR and Western blotting. Increasing p21CIP1 higher than a certain threshold may be needed for cell-cycle entry of the metaplastic exocrine cells. P21CIP1 is a known target that can be repressed by activated Notch.47 P21CIP1 is sometimes described as a growth inhibitory protein but it also acts as an assembly factor for cyclinD1/cdk4 complexes, of which the enzymatic activity is necessary for phosphorylation of retinoblastoma protein and cell-cycle progression. This may depend on the stoichiometry of p21CIP1 relative to the cyclin.47 P21CIP1 overexpression is also a very early event in pancreatic neoplastic PanIN lesions, even before the aberrant expression of p53, cyclin D1, and DPC/Smad4 starts.48 In pancreatic acinar cells it has been shown that p48/Ptf1 is directly responsible for growth suppression via transcriptional activation of p21CIP1 30. In that situation, p21CIP1 thus functions as a cell-cycle inhibitory protein.
Notch receptors have been described before in metaplastic pancreas tissue in vivo.18,19 We here provide evidence that Notch is associated with the phenotypic conversion and is implicated in limiting the growth of the metaplastic cells as shown in our in vitro experiments. Restraining the proliferation of metaplastic cells can prevent them from developing pancreatic neoplasia and ultimately invasive cancer.18 Notch reactivation in the adult pancreas may also serve as a key in regeneration, as it does in liver49 and muscle50 regeneration. Our previous observations of cellular transdifferentiation10–12 suggest that the abundant population of acinar cells can serve as a source for replenishing lost or incapacitated cells after pancreatic injury. It remains to be studied whether Notch activation by different ligands can play a major role in phenotypic (re)-specification of the dedifferentiated acinar cells. This information might be exploited for regenerative medicine of, for example, diabetes or pancreatitis.
Acknowledgments
We thank E. De Blay, M. Baekeland, and W. Rabiot for their skillful technical assistance; Dr. S. Bonné and J. Mfopou Kunjom for helping and discussion of the results; Dr. D. Pipeleers for his general and logistic support; and Dr. O. Madsen, Dr. Tetsuo Sudo, Dr. S.F. Konieczny, and Dr. F.X. Real for providing, respectively, antibodies for Pdx1, Hes1, Mist1, and p48/Ptf1.
Footnotes
Address reprint requests to Ilse Rooman, Ph.D., Cell Differentiation Unit–Diabetes Research Center, Vrije Universiteit Brussel, Laarbeeklaan 103, B-1090 Brussels, Belgium. E-mail: irooman@vub.ac.be.
Supported by the Fund for Scientific Research–Flanders (FWO-Vlaanderen).
I.R. and N.D. contributed equally to this study.
I.R. is a postdoctoral research fellow from the Fund for Scientific Research-Flanders (FWO-Vlaanderen). L.B. is a research fellow of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
References
- Bockman DE. Toward understanding pancreatic disease: from architecture to cell signaling. Pancreas. 1995;11:324–329. doi: 10.1097/00006676-199511000-00002. [DOI] [PubMed] [Google Scholar]
- Bouwens L. Transdifferentiation versus stem cell hypothesis for the regeneration of islet beta-cells in the pancreas. Microsc Res Tech. 1998;43:332–336. doi: 10.1002/(SICI)1097-0029(19981115)43:4<332::AID-JEMT7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Lardon J, Bouwens L. Metaplasia in the pancreas. Differentiation. 2005;73:278–286. doi: 10.1111/j.1432-0436.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- Schmid RM. Acinar-to-ductal metaplasia in pancreatic cancer development. J Clin Invest. 2002;109:1403–1404. doi: 10.1172/JCI15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RN, Klöppel G, Bouwens L. Duct to islet cell differentiation and islet growth in the pancreas of duct ligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- Rooman I, Lardon J, Flamez D, Schuit F, Bouwens L. Mitogenic effect of gastrin and expression of gastrin receptors in duct-like cells of rat pancreas. Gastroenterology. 2001;121:940–949. doi: 10.1053/gast.2001.27998. [DOI] [PubMed] [Google Scholar]
- Rooman I, Lardon J, Bouwens L. Gastrin stimulates β-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes. 2002;51:686–690. doi: 10.2337/diabetes.51.3.686. [DOI] [PubMed] [Google Scholar]
- Mathieu A, Clerc P, Portolan G, Bierkamp C, Lulka H, Pradayrol L, Seva C, Fourmy D, Dufresne M. Transgenic expression of CCK2 receptors sensitizes murine pancreatic acinar cells to carcinogen-induced preneoplastic lesions formation. Int J Cancer. 2005;115:46–54. doi: 10.1002/ijc.20861. [DOI] [PubMed] [Google Scholar]
- Wang RN, Rehfeld JF, Nielsen FC, Kloppel G. Expression of gastrin and transforming growth factor-alpha during duct to islet cell differentiation in the pancreas of duct-ligated adult rats. Diabetologia. 1997;40:887–893. doi: 10.1007/s001250050764. [DOI] [PubMed] [Google Scholar]
- Rooman I, Heremans Y, Heimberg H, Bouwens L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia. 2000;43:907–914. doi: 10.1007/s001250051468. [DOI] [PubMed] [Google Scholar]
- Lardon J, De Breuck S, Rooman I, Van Lommel L, Kruhoffer M, Orntoft T, Schuit F, Bouwens L. Plasticity in the adult rat pancreas: transdifferentiation of exocrine to hepatocyte-like cells in primary culture. Hepatology. 2004;39:1499–1507. doi: 10.1002/hep.20213. [DOI] [PubMed] [Google Scholar]
- Baeyens L, De Breuck S, Lardon J, Kunjom JM, Rooman I, Bouwens L. In vitro generation of insulin-producing beta-cells from adult exocrine pancreatic cells. Diabetologia. 2005;48:49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signaling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Lammert E, Brown J, Melton DA. Notch gene expression during pancreatic organogenesis. Mech Dev. 2000;94:199–203. doi: 10.1016/s0925-4773(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Hald J, Hjorth P, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGFa-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Jensen JN, Cameron E, Garay MVR, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Murata-Oshawa M, Tohda S, Sakano S, Nara N. The Notch ligand, delta-1, partially inhibits GM-CSF-induced differentiation and apoptosis along with reducing the cleavage of PARP in U937 cells. Int J Mol Med. 2004;13:419–423. [PubMed] [Google Scholar]
- Bouwens L, Wang RN, De Blay E, Pipeleers DG, Klöppel G. Cytokeratins as markers of ductal cell differentiation and islet neogenesis in the neonatal rat pancreas. Diabetes. 1994;43:1279–1283. doi: 10.2337/diab.43.11.1279. [DOI] [PubMed] [Google Scholar]
- Adell T, Gómez-Cuadrado A, Skoudy A, Pettengill OS, Longnecker DS, Real FX. Role of the helix-loop-helix transcription factor p48 in the differentiation phenotype of exocrine pancreas cancer cells. Cell Growth Differ. 2000;11:137–147. [PubMed] [Google Scholar]
- Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec. 2000;259:157–167. doi: 10.1002/(SICI)1097-0185(20000601)259:2<157::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Oster A, Jensen J, Serup P, Galante P, Madsen OD, Larsson LI. Rat endocrine pancreatic development in relation to two homeobox gene products (Pdx-1 and Nkx 6.1). J Histochem Cytochem. 1998;46:707–715. doi: 10.1177/002215549804600602. [DOI] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- Martys-Zage JL, Kim SH, Berechid B, Bingham SJ, Chu S, Sklar J, Nye J, Sisodia SS. Requirement for presenilin 1 in facilitating lagged 2-mediated endoproteolysis and signaling of notch 1. J Mol Neurosci. 2000;15:189–204. doi: 10.1385/jmn:15:3:189. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Eggert S, Reinhard FB, Vogel M, Paliga K, Baier G, Masters CL, Beyreuther K, Evin G. A novel epsilon-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry. 2002;41:2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA. 2005;102:15116–15121. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Tran T, Rukstalis M, Sun P, Damsz B, Konieczny SF. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Mol Cell Biol. 2004;24:2673–2681. doi: 10.1128/MCB.24.7.2673-2681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolosse A, Chalaux E, Adell T, Hagège H, Skoudy A, Real FX. PTF1a/p48 and cell proliferation. Gastroenterology. 2004;127:937–949. doi: 10.1053/j.gastro.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- Kritzik MR, Krahl T, Good A, Krakowski M, St-Onge L, Gruss P, Wright C, Sarvetnick N. Transcription factor expression during pancreatic islet regeneration. Mol Cell Endocrinol. 2000;164:99–107. doi: 10.1016/s0303-7207(00)00234-3. [DOI] [PubMed] [Google Scholar]
- Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of notch in organ development. Dev Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Lardon J, Huyens N, Rooman I, Bouwens L. Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Arch. 2004;444:61–65. doi: 10.1007/s00428-003-0930-z. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. Hes and Herp families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, Takeda Y, Chia W, Sankar N, Ng YK, Ling EA, Maciag T, Small D, Trifonova R, Kopan R, Okano H, Nakafuku M, Chiba S, Hirai H, Aster JC, Schachner M, Pallen CJ, Watanabe K, Xiao ZC. F3/contactin acts as a functional ligand for notch during oligodendrocyte maturation. Cell. 2003;115:163–175. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Orlandi R, Ronchini C, Granelli P, Malferrari G, Menard S, Biunno I. The expression of SEL1L and TAN-1 in normal and neoplastic cells. Int J Biol Markers. 2000;15:26–32. doi: 10.1177/172460080001500105. [DOI] [PubMed] [Google Scholar]
- Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23:1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Breuck S, Baeyens L, Bouwens L. Expression and function of leukemia inhibitory factor and its receptor in normal and regenerating rat pancreas. Diabetologia. 2006;49:108–116. doi: 10.1007/s00125-005-0079-1. [DOI] [PubMed] [Google Scholar]
- Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, Ball DW. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61:3200–3205. [PubMed] [Google Scholar]
- Aguilera C, Hoya-Arias R, Haegeman G, Espinosa L, Bigas A. Recruitment of IkappaBalpha to the hes1 promoter is associated with transcriptional repression. Proc Natl Acad Sci USA. 2004;101:16537–16542. doi: 10.1073/pnas.0404429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L, Lynch M, Silverman S, Fraser J, Boulter J, Weinmaster G, Gasson JC. The notch/jagged pathway inhibits proliferation of human hematopoetic progenitors in vitro. Stem cells. 1999;17:162–171. doi: 10.1002/stem.170162. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Tezuka Y, Matsuno K, Miyatani S, Morimura N, Yasuda M, Fujimaki R, Kuroda K, Hiraki Y, Hozumi N, Tezuka K. Suppression of differentiation and proliferation of early chondrogenic cells by Notch. J Bone Miner Metab. 2003;21:344–352. doi: 10.1007/s00774-003-0428-4. [DOI] [PubMed] [Google Scholar]
- Taylor KL, Henderson HM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc Res. 2002;64:372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- Castella P, Sawai S, Nakao K, Wagner JA, Caudy M. HES-1 repression of differentiation and proliferation in PC12 cells: role for the helix 3-helix 4 domain in transcription repression. Mol Cell Biol. 2000;20:6170–6183. doi: 10.1128/mcb.20.16.6170-6183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda M, Chang L, McLean G, Grim JE, Clurman BE, Smith LL, Karsan A. Notch activation induces endothelial cell cycle arrest and participates in contact inhibition: role of p21CIP1 repression. Mol Cell Biol. 2004;24:8813–8822. doi: 10.1128/MCB.24.20.8813-8822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biankin AV, Kench JG, Morey AL, Lee CS, Biankin SA, Head DR, Hugh TB, Henshall SM, Sutherland RL. Overexpression of p21WAF1/CIP1 is an early event in the development of pancreatic intraepithelial neoplasia. Cancer Res. 2001;61:8830–8837. [PubMed] [Google Scholar]
- Köhler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–1065. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]