Abstract
Failures in fracture healing are mainly caused by a lack of vascularization. Adult human circulating CD34+ cells, an endothelial/hematopoietic progenitor-enriched cell population, have been reported to differentiate into osteoblasts in vitro; however, the therapeutic potential of CD34+ cells for fracture healing is still unclear. Therefore, we performed a series of experiments to test our hypothesis that functional fracture healing is supported by vasculogenesis and osteogenesis via regenerative plasticity of CD34+ cells. Peripheral blood CD34+ cells, isolated from total mononuclear cells of adult human volunteers, showed gene expression of osteocalcin in 4 of 20 freshly isolated cells by single cell reverse transcriptase-polymerase chain reaction analysis. Phosphate-buffered saline, mononuclear cells, or CD34+ cells were intravenously transplanted after producing nonhealing femoral fractures in nude rats. Reverse transcriptase-polymerase chain reaction and immunohistochemical staining at the peri-fracture site demonstrated molecular and histological expression of human-specific markers for endothelial cells and osteoblasts at week 2. Functional bone healing assessed by biomechanical as well as radiological and histological examinations was significantly enhanced by CD34+ cell transplantation compared with the other groups. Our data suggest circulating human CD34+ cells have therapeutic potential to promote an environment conducive to neovascularization and osteogenesis in damaged skeletal tissue, allowing the complete healing of fractures.
Whereas embryonic stem cells in the blastocyst stage have the ability to generate any differentiated cells in the body, most adult stem cells have limited potential for postnatal tissue/organ regeneration. Among phenotypically characterized adult stem/progenitor cells,1–3 the hematopoietic system has traditionally been considered as an organized, hierarchic system with multipotent, self-renewing stem cells at the top, lineage-committed progenitor cells in the middle, and lineage-restricted precursor cells, which give rise to terminally differentiated cells, at the bottom.4 Recently, adult human peripheral blood CD34+ cells have been reported to contain intensive endothelial progenitor cells (EPCs) as well as hematopoietic stem cells (HSCs).5 Tissue ischemia and cytokines mobilize EPCs from bone marrow (BM) into peripheral blood, and mobilized EPCs specifically home to sites of nascent neovascularization and differentiate into mature endothelial cells (ECs) (vasculogenesis).6,7 In the case of the immunodeficient rat model of acute myocardial infarction, transplanted human CD34+ cells or ex vivo expanded EPCs incorporate into the site of the myocardial neovascularization, differentiate into mature ECs, augment capillary density, inhibit myocardial fibrosis and apoptosis, and preserve the left ventricular function.8–10 In addition, intravenously transplanted CD34+ cells efficiently incorporate into ischemic tissue.9,11
In recent years, in an attempt to meet clinical demands, interest has turned to bone formation as an alternative category of regenerative medicine. It is anticipated that by optimizing the process of fracture repair, a biological approach results in the restoration of normal structure and function in the injured skeletal tissue. Although most fractures typically heal with callus formation that bridges the fracture gap, a significant proportion (5 to 10%) of fractures fail to heal and result in delayed union or persistent nonunion.12,13 Inappropriate neoangiogenesis is considered to be a crucial factor in failed bone formation and remodeling.14,15 Notably, appropriate vascularization is emerging as a prerequisite for bone development and regeneration, and indeed there appears to be a developmental reciprocity between ECs and osteoblasts (OBs).16 Under such recognition, human CD34+ cells, which are capable of generating ECs in an appropriate environment,6,7 have also been reported to differentiate into OBs in vitro.17–19 In addition, a recent report demonstrated that CD34+ osteoblastic cells line the cavities of the cartilage in the fracture site in a rabbit tibial osteotomy model.20 These observations provoked the hypothesis that human peripheral blood CD34+ cells play a key role in fracture healing via vasculogenesis and osteogenesis. Therefore, we first confirmed that mouse ScaI+ lineage marker− (Lin−) cells, quite similar to human CD34+ cells,7,21,22 were mobilized to peripheral blood in the natural course of the fracture healing process. Next, we investigated whether transplantation of circulating human CD34+ cells contributed to both vasculogenesis and osteogenesis for functional bone healing after fracture in an immunodeficient rat model.
In the present series of studies, we demonstrate that mouse ScaI+Lin− cells are mobilized into the peripheral blood in the natural course of fracture healing and that human peripheral blood CD34+ cells, containing osteo/endothelial progenitor cells already expressing osteocalcin (OC), which were recruited to the fracture site after systemic delivery, develop a favorable environment for fracture healing by enhancing vasculogenesis and osteogenesis and finally lead to functional recovery from fracture. The present findings have important clinical implications for cell-based therapy that will enhance bone repair after fracture.
Materials and Methods
Isolation of Mouse Lin− Cells and Assessment of ScaI+Lin− Cells
To confirm the kinetics of ScaI+Lin− cells in the natural course of fracture healing, we detected ScaI+Lin− cells at prefracture and 1, 4, 7, and 14 days after fracture by fluorescence-activated cell sorting (FACS) analysis (n = 3 in each). Peripheral blood cells were aspirated from the hearts of 10-week-old fractured mice 1, 4, 7, and 14 days after fracture and from those of unfractured mice and mixed with phosphate-buffered saline (PBS) containing 5% fetal calf serum (n = 3 in each). MNCs were obtained by a Histopaque-1083 (Sigma Co., St. Louis, MO) density gradient centrifugation at 400 × g for 20 minutes. The light-density MNCs were collected, washed twice with Dulbecco’s PBS supplemented with 2 mmol/L ethylenediaminetetraacetic acid, and counted manually. Separation of Lin− cells was performed to deplete mature hematopoietic cells7,21 such as T cells, B cells, natural killer (NK) cells, monocytes/macrophages, granulocytes, and erythrocytes by labeling MNCs with a Lin− separation kit (BD Pharmingen, San Diego, CA), containing biotin-conjugated Mac1, B220, CD3e, Ter119, Ly6G, and CD45R antibodies followed by streptavidin-conjugated magnetic beads and BD IMagnet separation. Then, Lin− MNCs were counted, and the number of ScaI+Lin− cells was calculated from the rate of ScaI+ cells in Lin− MNCs by FACS analysis and the number of Lin− MNCs.
Isolation of CD34+ Cells from Adult Human Volunteers
Human peripheral blood total MNCs were obtained from healthy male volunteers age 31.7 ± 1.2 years (n = 3). CD34+ cells were isolated from the MNCs by the AutoMACS system (Miltenyi Biotec, Auburn, CA) using anti-CD34 microbeads (Miltenyi Biotec). The CD34+ cell fraction had a purity of >97%, as determined by FACS analysis using a CD34-specific monoclonal antibody (Becton Dickinson, San Jose, CA). Institutional review board approval for the collection of peripheral blood MNCs from healthy human volunteers and informed consent regarding the experimental use of the cells from the volunteers were obtained.
Flow Cytometry Studies and Monoclonal Antibodies
Regular flow cytometric profiles were analyzed with a FACS Calibur analyzer and CELLQuest software (Becton Dickinson Immunocytometry Systems, Mountain View, CA). The instrument was aligned and calibrated daily using a four-color mixture of CaliBRITE beads (BD Biosciences) with FACSComp software (BD Bioscience). Dead cells were excluded from the plots’ beads on propidium iodide (PI) staining (Sigma Co.). Human CD34+ cells or mouse Lin− cells were washed twice with Hanks’ balanced salt solution containing 3.0% fetal calf serum, incubated with 10 μl of FcR blocking reagent to increase the specificity of monoclonal antibodies (Miltenyi Biotec) for 20 minutes at 4°C, and incubated with the monoclonal antibodies for 30 minutes at 4°C. The stained cells were washed three times with PBS containing 3.0% fetal calf serum, resuspended in 0.5 ml of Hanks’ balanced salt solution/3% fetal calf serum/propidium iodide, and analyzed by FACScan Caliber flow cytometer (Becton-Dickinson, Franklin Lakes, NJ). Cells (1 × 106) were processed through the cytometer, and 3 × 104 cells per sample were analyzed for human CD34+ cell or mouse Lin− cell fraction. The following monoclonal anti-human antibodies were used to characterize the CD34+ cell population: CD34-APC (BD Pharmingen), CD34-FITC (BD Pharmingen), CD45-FITC (BD Pharmingen), CD133-APC (BD Pharmingen), c-Kit-FITC (Nichirei), CD31-FITC (BD Pharmingen), CD105 (BD Pharmingen), VE cadherin (VE-cad)-FITC (BD Pharmingen), KDR-PE (BD Pharmingen), Tie2-PE (BD Pharmingen), IgG1-FITC isotype controls (BD Pharmingen), IgG1-APC isotype controls (BD Pharmingen), and propidium iodide (Sigma Co.). The following monoclonal anti-mouse antibodies were used to characterize the Lin− MNCs: cKit-APC (BD Pharmingen), ScaI-FITC (BD Pharmingen), IgG2a-PE isotype controls (BD Pharmingen), IgG2a-FITC isotype controls (BD Pharmingen), and propidium iodide (Sigma Co.).
Induction of Femoral Fracture with the Periosteum Cauterized and Cell Transplantation
Female athymic nude rats (F344/N Jcl rnu/rnu; CLEA Japan, Inc.), age 8 to 12 weeks and weighing 150 to 170 g, were used in this study. The rats were fed a standard maintenance diet and provided with water ad libitum. The institutional animal care and use committees of Riken Center for Developmental Biology approved all animal procedures including human cell transplantation.
All surgical procedures were performed under anesthesia and normal sterile conditions. Anesthesia was performed with ketamine hydrochloride (60 mg/kg) and xylazine hydrochloride (10 mg/kg) administered intraperitoneally. A lateral parapatellar knee incision on the right limb was made to expose the distal femoral condyle. An animal model of femoral fracture was applied using a modification of the method described by Bonnarens and Einhorn.23 To avoid significant displacement of the fracture by obtaining the well-aligned stability of the fracture site, a 1.2-mm-diameter K-wire was inserted from the trochlear groove into the femoral canal in a retrograde manner using a motor-driven drill. The wire was advanced until its proximal end was positioned stably in the greater trochanter, and the distal end was cut close to the articular surface of the knee. A thin saw cut at a depth of a 3 mm was applied mid-shaft after minimal lateral exposure to weaken the bone and to avoid complex fractures. A transverse femoral shaft fracture was then produced in the right femur of each rat using a C-shaped instrument applying three-point bending. After this procedure, each rat received additional surgery to produce a nonunion in the fractured shaft according to the method of Kokubu and colleagues.24 The periosteum was cauterized (OPTEMP, variable low temperature cautery; Alcon Manufacturing, Ltd., Fort Worth, TX) circumferentially at a distance of 2 mm on each side of the fracture. The wound was then irrigated with 10 ml of sterile saline, and the muscle and skin were closed in layers with 5-0 nylon sutures. Postoperative pain was managed by administration of subcutaneous injection of buprenorphine hydrochloride after surgery. Unprotected weight bearing was allowed immediately after operation. The left unfractured femur served as a control. Thirty minutes after the production of the fracture, rats received an intravenous transplantation of 1 × 105 CD34+ cells or 1 × 105 total MNCs resuspended with 100 μl of PBS or the same volume of PBS without cells through their tail vein (n = 15 in each group).
Three rats were randomly selected from each group and sacrificed for the histological study after radiological evaluation of fracture healing at each time point: weeks 2, 4, and 8. The six remaining rats in each group were sacrificed at week 8 for biomechanical testing as described below. If the fracture was not a stable transverse fracture or if any evidence of deep infection was seen, the animals were excluded from the study and replaced with additional animals. Thus, eight rats with comminuted fractures and six rats with infection identified by radiographs were replaced during the experiment.
Targeting Cell Analysis with Qtracker Cell Labeling Kit
To target human CD34+ cells or MNCs after intravenous infusion and confirm their recruitment into fracture site, Qtracker 655 cell labeling kit (Quantum Dot Corp.) was applied for the human cells before transplantation in three additional rats in each group according to the manufacturer’s instructions. In brief, Qtracker cell labeling kits deliver fluorescent quantum dot (qdot) nanocrystals into the cytoplasm of live cells using a custom targeting peptide and the long-term stability and brightness of qdots make them ideal candidates for live cell targeting and imaging.25,26 The 1 × 105 CD34+ cells or MNCs were incubated for 60 minutes with the Qtracker labeling solution (1 μl of Qtracker regent A and B) and 0.2 ml of Dulbecco’s modified Eagle’s medium in eight-well Lab-Tek chambered coverglass system. The cells were washed twice with Dulbecco’s modified Eagle’s medium and cell labeling was confirmed under fluorescence microscopy. The labeled cells were intravenously transplanted into each animal 30 minutes after fracture. All animals were sacrificed at day 7 for targeting cell analysis with Qtracker.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis of RNA Isolated from CD34+ Cells and Peri-Fracture Site Tissue
Total RNA was obtained from the human CD34+ cells immediately after isolation and from the rat tissues in peri-fracture site at day 14 using Trizol (Life Technologies) according to the manufacturer’s instructions. The first-strand cDNA was synthesized using the RNA LA PCR kit version 1.1 (Takara), amplified by Taq DNA polymerase (Advantage-GC cDNA PCR kit; Clontech, Palo Alto, CA; and AmpliTaq Gold DNA polymerase; Applied Biosystems, Foster City, CA). PCR was performed using a PCR thermocycler (MJ Research PTC-225). Human CD31 (hCD31), human VE-cadherin (hVE-cad), human osteocalcin (hOC), human collagen1A1 (hCol1A1), human vascular endothelial growth factor (hVEGF), human fibroblast growth factor 2 (hFGF2), and human hepatocyte growth factor (hHGF) were amplified by TaqDNA polymerase (Advantage-GC cDNA PCR kit; Clontech) in the following conditions: 35 cycles of 30-second initial denaturation at 94°C, annealing at 56°C for 1 minute, and 30 seconds of extension at 72°C according to the manufacturer’s instructions. Subsequently, PCR products were visualized in 1.5% ethidium bromide-stained agarose gels. Human umbilical vein endothelial cells and hOBs (NHOst cells; Cambrex Bio Science, Walkersville, MD) were used for the positive control for human-specific endothelial and bone-related genes.
Primers
To avoid interspecies cross-reactivity of the primer pairs between human and rat genes, we designed the following human-specific primers using Oligo software (Takara). None of the primer pairs showed any cross-reactivity to rat genes (data not shown). hCD31 primer sequence (363 bp): sense 5′-ATCGATCAGTGGAACTTTGCCTATT-3′; anti-sense 5′-GTGGCATTTGAGATTTGATAGA-3′; hVE-cad primer sequence (461 bp): sense 5′-ACGCCTCTGTCATGTACCAAATCCT-3′; anti-sense 5′-GGCCTCGACGATGAAGCTGTATT-3′; hOC primer sequence (417 bp): sense 5′-AAGCAAGTAGCGCCAATCT-3′; anti-sense 5′-GGAAGTAGGGTGCCATAACAC-3′; hCol1A1 primer sequence (502 bp): sense 5′-CCTGGCCCCATTGGTAATGTT-3′; anti-sense 5′-CCCCCTCACGTCCAGATTCAC-3′; hVEGF primer sequence (186 bp): sense 5′-CAACATCACCATGCAGATTATGC-3′; anti-sense 5′-CCACAGGGACGGGATTTCTTG-3′; hFGF2 primer sequence (282 bp): sense 5′-AGCACAGTAACACTATCCTGCA-3′; anti-sense 5′-AAACGGAAACGCTCACCATAA-3′; hHGF primer sequence (282 bp): sense 5′-ACGAACACAGCTATCGGGGTA-3′; anti-sense 5′-CATCAAAGCCCTTGTCGGGAT-3′; hGAPDH primer sequence (596 bp): sense 5′-CTGATGCCCCCATGTTCGTC-3′; anti-sense 5′-CACCCTGTTGCTGTAGCCAAATTCG-3′; and rGAPDH primer sequence (320 bp): sense 5′-GTGCCAGCCTCGTCTCATAGA-3′; anti-sense 5′-CGCCAGTAGACTCCACGACAT-3′.
Single-Cell PCR
Single-CD34+ cells derived from peripheral blood using the MACS system were put individually into PCR tubes with 4.5 μl of lysis buffer containing 50 mmol/L Tris-HCl, 75 mmol/L KCl, 5 U of SuperRNaseIN (Ambion), 7.5 U of PrimeRNase inhibitor (Eppendorf), 0.5% Nonidet P-40, 1 mmol/L dithiothreitol, 50 μmol/L dNTP, and 15 nmol/L MO-dT30 primer (5′-AAGCAGTGGTATCAACGCAGAGTGGCCATTACGGCCGTACTT-(dT)30-3′). Tubes were then incubated at 65°C for 2 minutes and cooled to 45°C for 2 minutes. Reverse transcription was then performed by the addition of 100 U of SuperScript III (Invitrogen). After incubation at 45°C for 15 minutes, the reaction was terminated by heating at 65°C for 10 minutes. Next, 1.5 μl of RNA digestion mixture, 2 U of RNase H (Invitrogen), and 25 mmol/L MgCl2 was added into each tube, and RNA digestion was performed by incubating at 37°C for 15 minutes and then inactivating at 65°C for 10 minutes. After RNA digestion, 6.5 μl of reaction mixture [5× terminal transferase buffer (Roche), 3 mmol/L CoCl2, 1.5 mmol/L dATP, and 15 U of TdT (Promega)] was added, poly(A) tailing was performed by incubating at 37°C for 15 minutes, and inactivation was performed at 65°C for 10 minutes. cDNA amplification was performed using ExTaq polymerase (Takara Biochemicals, Japan). In brief, poly(A)-tailed cDNA (13 μl) was split 4 μl each into two tubes containing 16 μl of primary PCR reaction solution containing ExTaq PCR buffer (Takara Biochemicals), 2 mmol/L dNTP, 10 μmol/L MO-dT30 primer, and 1 U of ExTaq polymerase (Takara Biochemicals). PCR was performed with one cycle of 1 minute at 94°C, 2 minutes at 50°C, and 2 minutes at 72°C, followed by 35 cycles of 30 seconds at 94°C, 30 seconds at 60°C, and 2 minutes at 72°C. After combining split tubes into one tube, 2 μl of first-amplified cDNA was added to 18 μl of second PCR mixture, ExTaq PCR buffer (Takara Biochemicals), 2 mmol/L dNTP, 2 μmol/L MO-dT30 primer, and 1 U of ExTaq polymerase (Takara Biochemicals), and second PCR was performed for 35 cycles of 30 seconds at 94°C, 30 seconds at 60°C, and 2 minutes at 72°C. Finally, amplified cDNA purified with Qiagen PCR purification kit according to the manufacturer’s procedure, and then PCR analysis for specific gene expression was performed using each purified cDNA.
Primers
hCD34 primer sequence (91 bp): sense 5′-TGCCTCTTCTGTGGGTGACC-3′; anti-sense 5′-TCCAACCGTCATTGAAACCAG-3′; hOC primer sequence (96 bp): sense 5′-GCTCAATCCGGACTGTGACG-3′; anti-sense 5′-CAGAGCGACACCCTAGACCG-3′; hGAPDH primer sequence (95 bp): sense 5′-GCATTGCCCTCAACGACC-3′; anti-sense 5′-CATGTGGGCCATGAGGTCC-3′.
Tissue Harvesting
Rats were euthanized with an overdose of ketamine and xylazine. Bilateral femurs were harvested and quickly embedded in optimal cutting temperature compound (Miles Scientific, Elkhart, IN), snap-frozen in liquid nitrogen, and stored at −80°C for histochemical and immunohistochemical staining as described below. Rat femurs in optimal cutting temperature blocks were sectioned, and 6-μm serial sections were mounted on silane-coated glass slides and air-dried for 1 hour before being fixed with 4.0% paraformaldehyde at 4°C for 5 minutes and stained immediately.
Morphometric Evaluation of Capillary Density and OB Density
Histochemical staining (n = 3 in each group) for isolectin B4 (Vector Laboratories, Burlingame, CA) as a rat EC marker or OC (Santa Cruz Biotechnology, Santa Cruz, CA) as a rat OB marker was visualized with diaminobenzidine (Vector Laboratories), and capillary or OB density was morphometrically evaluated as the average value in five randomly selected fields of soft tissue in the peri-fracture site (Figure 3M, zones a and b). To address the location of chondrocytes in the fractured sections, toluidine blue was used for counter staining. Capillaries were recognized as tubular structures positive for isolectin B4. OBs were recognized as lining or floating cells positive for OC on new bone surface. All morphometric studies were performed by two examiners blind to treatment.
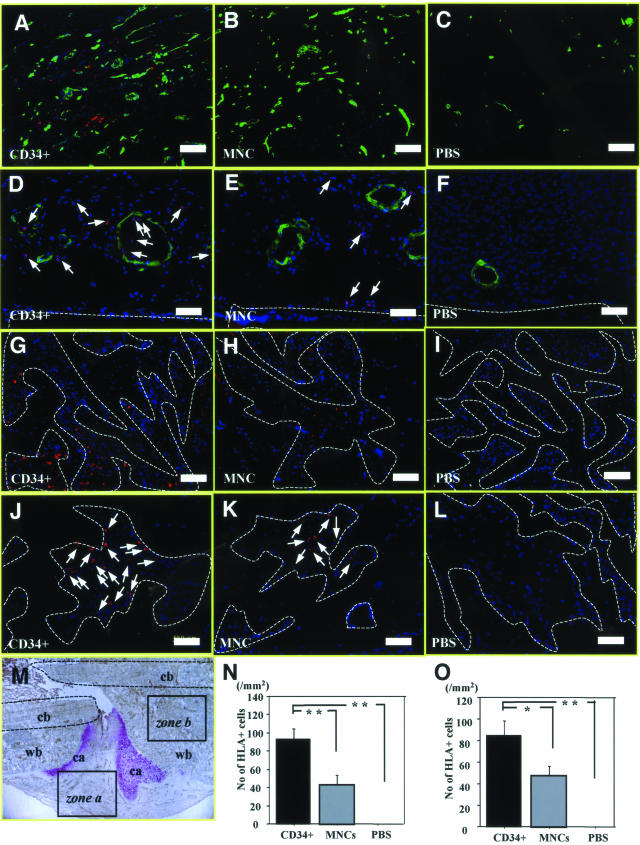
FIGURE 3.
Recruitment of fluorescent-labeled CD34+ cells into fracture site. A–C: Histochemical staining for isolectin B4 (green), a rat-specific EC marker, using tissue samples of fracture sites obtained from rats receiving Qtracker-labeled human cells (red) 1 week after fracture in the granulation area shown as zone a in M. More efficient recruitment of human cells with neovasculature-like structure was accompanied with enhancement of neovascularization by recipient rat cells in animals treated with CD34+ cells (A) compared with those receiving MNCs (B) or PBS alone (C). D–F: Double-fluorescent immunostaining for human leukocyte antigen (HLA)-ABC (red) and smooth muscle actin (green) using the tissue sample of granulation area. The massive recruitment of the human cells in the granulation area with relatively rare incorporation along the inner layer of SMA-positive smooth muscle cells was observed in CD34+ group. The human cells lining along the inner layer were morphologically compatible with ECs (D). In contrast, less recruitment of human cells in the granulation area and no human cells along the inner layer of arterioles were observed in animals receiving MNCs (E). Human cells were not found in the PBS group (F). G–I: Pursuit of Qtracker-labeled human cells 1 week after fracture in newly formed bone area shown as zone b in M. Human cell (red) recruitment into the newly formed bone area (surrounded by broken lines) was more abundant in animals treated with CD34+ cells (G) compared with those receiving MNCs (H) or PBS alone (I). J–L: Fluorescent immunohistochemistry for HLA-ABC demonstrated massive recruitment of human cells (red: arrows) in CD34+ group (J). In contrast, less recruitment of the HLA-ABC-positive cells was observed in animals receiving MNCs (K) and no human cells in PBS group (L). M: Representative photomicrograph of immunostaining for isolectin B4 (brown) and toluidine blue counter staining for anatomical indication of following areas: zone a, granulation zone; zone b, newly formed bone zone; cb, cortical bone; ca, cartilage; wb, woven bone. N: The number of HLA-ABC-positive cells observed in zone a was significantly greater in CD34+ group compared with other groups. **P < 0.01. O: The number of HLA-ABC-positive cells in zone b was significantly greater in CD34+ group compared with other groups. *P < 0.05; **P < 0.01. Blue fluorescence indicates DAPI for nuclear staining. Scale bars = 100 μm (A–C, G–F); 50 μm (D–F, J–L). Original magnifications: ×100 (A–C, G–I); ×200 (D–F, J–L); ×40 (M).
Physiological Assessment of Tissue Perfusion by Laser Doppler Perfusion Imaging
Laser Doppler perfusion imaging (LDPI) (n = 3 in each group) (Moor Instrument, Wilmington, DE)27,28 was used to measure serial blood flow in both legs throughout the course of 2 weeks after fracture. The ratio of fractured/intact (contralateral) blood flow was calculated to evaluate the serial blood flow recovery after fracture. The measurement was done under anesthesia with the animals supine and both limbs fully extended.
Immunofluorescent Staining
To detect transplanted human cells in the rat fracture site, immunohistochemistry (n = 3) was performed with the following human-specific antibodies: human leukocyte antigen (HLA)-ABC (BD Pharmingen) to detect various kind of human cells, hCD31 (DAKO, Glostrup, Denmark) for human ECs, and hOC (Biogenesis) for human OBs. Staining specificity for human cells without cross reaction to rat cells was confirmed by histochemical staining for HLA-ABC, hCD31, and hOC using human and rat heart or bone samples (data not shown). Double immunohistochemistry with HLA-ABC or hCD31 and smooth muscle actin (SMA) was performed to detect various human cells or human ECs in the arterioles. To estimate how many intravenously injected cells had recruited to the fracture site, the number of HLA-ABC-positive cells was morphometrically counted as the average value in five randomly selected soft tissue fields in the peri-fracture site. The secondary antibodies for each immunostaining were as follows: Alexa Flour 594-conjugated goat anti-mouse IgG1 (Molecular Probes) for HLA-ABC and hCD31 staining and Alexa Flour 488-conjugated goat anti-mouse IgG2a (Molecular Probes) for SMA. Immunohistochemistry with hOC was performed to detect human OBs along the newly formed bone surface. The secondary antibody for hOC is Cy3-conjugated AffiniPure goat anti-rabbit IgG (H+L) (Jackson ImmunoResearch, West Grove, PA). 4,6-Diamidino-2-phenylindole (DAPI) solution was applied for 5 minutes for nuclear staining.
Inhibition of Neovascularization
To investigate the hypothesis that neovascularization is essential for supporting endogenous bone regeneration, we used an anti-angiogenic agent, soluble (s) Flt1 (VEGF receptor 1), known to inhibit proliferation of ECs.29 First, we investigated whether transplanted human CD34+ cells released angiogenic factors (hVEGF, hFGF2, hHGF) at the fracture site compared with the MNCs. Next, rats subjected to fracture and CD34+ cell transplantation were divided into two groups; one group receiving sFlt1 (20 μg/kg, subcutaneous; R&D Systems, Minneapolis, MN) once daily for 14 days and the other receiving PBS only (n = 3 in each group). On day 14 after the cell transplantation, capillary and OB density, blood flow, and callus formation were assessed in each group.
Radiographical Assessment of the Fracture Healing
Radiographs of the fractured legs were serially taken at weeks 0, 2, 4, and 8 under anesthesia with the animal supine and both limbs fully extended. Fracture union was identified by the presence of bridging callus on two cortices. Radiographs of each animal were examined by three observers blinded to treatment. To evaluate the fracture healing process, callus formation was monitored radiographically, and relative callus areas detected by radiography were quantified with NIH Image (National Institutes of Health, Bethesda, MD) at week 2 in all groups.
Histological Assessment of the Fracture Healing
Histological evaluation (n = 3 in each group) was performed with toluidine blue staining to address the process of endochondral ossification at weeks 2, 4, and 8. The degree of fracture healing was evaluated at weeks 2, 4, and 8 in each group using a five-point scale proposed by Allen and colleagues30 as follows: grade 4, complete bony union; grade 3, an incomplete bony union (presence of a small amount of cartilage in the callus); grade 2, a complete cartilaginous union (well-formed plate of hyaline cartilage uniting the fragments); grade 1, an incomplete cartilaginous union (retention of fibrous elements in the cartilaginous plate); and grade 0, the formation of a pseudoarthrosis (most severe form of arrest in fracture repair). All morphometric studies were performed by two orthopedic surgeons blind to treatment.
Biomechanical Assessment of the Fracture Union
At week 8, six rats in each treatment group were used for biomechanical evaluation.31–34 After euthanasia, the fractured femurs and the contralateral nonfractured intact femurs were dissected free from the surrounding muscle. After the intramedullary K-wires were removed, a standardized three-point bending test was performed on the fractured site and on the same portion of the intact contralateral femur using a load torsion and bending tester (Computer Control System Shimazu Autograft; Simazu Co., Kyoto, Japan). The bending force was applied with cross-head at a speed of 2 mm/minute until fracture occurred. The ultimate stress (N/mm2), the extrinsic stiffness (N/mm) and the failure energy (Nmm) were interpreted and calculated from the load deflection curve, which was recorded continuously in the computerized monitor linked to the tester. The percentage ratio of each parameter in the fractured (right) femur versus unfractured (left) femur was calculated in each animal.
Statistical Analysis
All values were expressed as mean ± SE. Paired Student’s t-tests were performed for comparison of data before and after treatment. The comparisons among three groups were made using the one-way analysis of variance. Post hoc analysis was performed by Fisher’s PLSD test. A probability value <0.05 was considered to denote statistical significance.
Results
Augmented Mobilization of Mouse ScaI+Lin− Cells by Fracture Stress
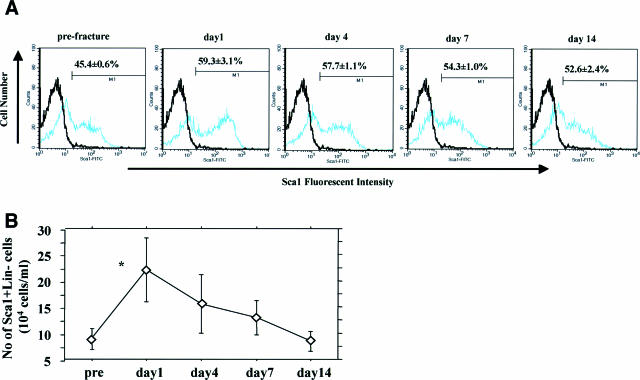
To confirm the kinetics of peripheral blood mouse ScaI+Lin− cells in the natural course of fracture healing, we serially performed FACS analysis to assess number of ScaI+Lin− cells in the peripheral blood after fracture. The MNCs obtained from the mice were 38.9 ± 4.8 × 104 cells, and the number of Lin− MNCs after lineage maker depletion by MACS system was 26.5 ± 3.0 × 104 cells. The percentage of peripheral blood ScaI+Lin− cells in relation to Lin− MNCs by FACS analysis had a tendency to peak at day 1 after fracture and gradually decrease thereafter; however, these changes were not statistically significant (prefracture, 45.4 ± 0.6; 1 day after fracture, 59.3 ± 3.1; 4 days after fracture, 57.7 ± 1.1; 7 days after fracture, 54.3 ± 1.0; 14 days after fracture, 52.6 ± 2.4%) (Figure 1A). The number of ScaI+Lin− cells, calculated from the number of Lin− MNCs and the percentage of ScaI+ cells in the number of Lin− MNCs, significantly increased at day 1 after fracture and then gradually decreased (prefracture, 8.3 ± 1.4; 1 day after fracture, 22.1 ± 6.1; 4 days after fracture, 15.6 ± 9.7; 7 days after fracture, 12.9 ± 5.7; 14 days after fracture, 8.4 ± 1.9 × 104 cells/ml; P < 0.05 for prefracture versus 1 day after fracture) (Figure 1B). These results indicate that mouse ScaI+Lin− cells, quite similar to human CD34+ cells, were mobilized into the peripheral blood at an early phase of the fracture healing process.
FIGURE 1.
Mobilization of mouse ScaI+Lin− cells by fracture stress. A: Percentage of peripheral blood ScaI+Lin− cells to Lin− MNCs shows an increase with the peak at day 1 after fracture. Black line represents isotype control (negative control), and blue line indicates sample data. x axis shows fluorescent intensity of cells, and y axis demonstrates the number of cells. B: Number of ScaI+Lin− cells significantly peaked at day 1 after fracture and then gradually decreased. *P < 0.05.
Phenotypic Characterization of CD34+ Cells Obtained from Adult Human Volunteers
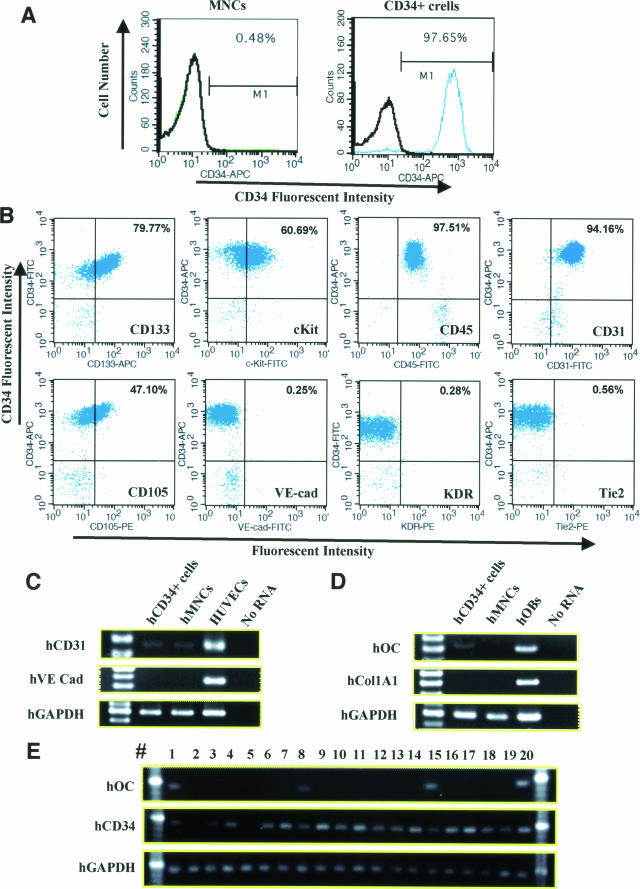
Peripheral blood total mononuclear cells (MNCs) were obtained from healthy male volunteers, age 31.7 ± 1.2 years (n = 3), and CD34+ cells were isolated from the MNCs by AutoMACS. The MNCs contained a CD34+ cell fraction at the rate of <0.5% and the CD34+ cell fraction had a purity of >97% as determined by FACS analysis (Figure 2A). The freshly isolated CD34+ cells were also characterized as positive for cell surface markers of CD45, CD133, c-Kit, CD31, and CD105 (Figure 2B). RT-PCR analysis of the CD34+ cells revealed weak expression of the human-specific genes, hCD31 and hOC, but not of another EC marker, hVE-cad, or another bone-related marker, hCol1A1 (Figure 2, C and D). RT-PCR analysis at the single cell level of the CD34+ cells revealed that 4 of 20 CD34+cells expressed hOC (Figure 2E). These results indicate that human peripheral blood CD34+ cells contain not only endothelial progenitor but also osteo-progenitor cells.19,35
FIGURE 2.
Phenotypic characterization of human peripheral blood MNCs and CD34+ cells by FACS and RT-PCR analysis. A: FACS analysis to evaluate positivity for CD34 in MNCs (left) and CD34+ cells (right). Numbers are percentages of the cells positive for CD34. B: FACS analysis to characterize CD34+ cells by positivity for various cell surface markers. Human CD34+ cells were positive for CD133, c-Kit, CD45, CD31, and CD105 and negative for VE-cad, KDR, and Tie2. Numbers are percentages of double-positive cells for CD34 (y axis) and each antibody (x axis). C and D: RT-PCR analysis for human-specific genes of endothelial (C) and osteoblastic (D) lineages was performed in freshly isolated CD34+ cells and MNCs. The analysis of CD34+ cells revealed weak expression of hCD31 and hOC but not of the other endothelial marker (hVE-cad) or bone-related marker (hCol1A1). Cultured human umbilical vein endothelial cells and OBs were used for positive control for human-specific endothelial and bone-related genes. E: RT-PCR analysis at the single cell level of the CD34+ cells. Four of 20 CD34+ cells expressed both CD34 and hOC.
Massive Recruitment of CD34+ Cells into the Fracture Site
To simulate the clinical situation of the nonhealed fracture, a reproducible model of femoral fracture23 with cauterized periosteum, which led to nonunion 8 weeks after fracture,12,24 was applied in nude rats. Human CD34+ cells isolated from peripheral blood were labeled with the Qtracker cell labeling kit (Qtracker; Quantum Dot Corp.) and intravenously administered via the tail vein 30 minutes after fracture production. MNCs labeled with Qtracker or PBS alone were similarly infused into control animals. Rats were sacrificed 1 week after the cell infusion and then histochemical staining for isolectin B4, a rat-specific EC marker, was performed. Fluorescent microscopy demonstrated a massive recruitment of human cells with tubular structure, which were morphologically compatible with vascular cells, and the enhancement of neovascularization by rat ECs around the granulation area (Figure 3M, zone a) in animals treated with CD34+ cells (CD34+ group) (Figure 3A). In contrast, recruitment of human cells into this area was rarely observed in rats receiving MNCs, and augmentation of recipient vascularity was not obvious in animals receiving MNCs or PBS (Figure 3, B and C). To further confirm the human cell incorporation into the fracture site, especially into the arterioles, tissue samples were stained with HLA-ABC and SMA. The double immunostaining demonstrated the massive recruitment of the human cells in the granulation area with relatively rare incorporation along the inner layer of SMA-positive smooth muscle cells in CD34+ group. The human cells lining along the inner layer were morphologically compatible with ECs (Figure 3D). In contrast, less recruitment of human cells in the granulation area and no human cells along the inner layer of arterioles were observed in animals receiving MNCs (Figure 3E). No human cells were found in the area of PBS group (Figure 3F). The number of human cells in the granulation area was significantly higher in the CD34+ group compared with the other groups (CD34+, 93.1 ± 11.3/mm2; MNC, 43.1 ± 10.4/mm2; PBS, 0.0 ± 0.0/mm2, respectively; P < 0.01 for CD34+ versus MNC or PBS group) (Figure 3N).
Fluorescent microscopy 1 week after the cell infusion also demonstrated abundant recruitment and distribution of human cells in the newly bone formed area (zone b in Figure 3M) as well as the granulation area in CD34+ group (Figure 3G). In contrast, recruitment of human cells into zone b was rarely observed in other groups (Figure 3, H and I). To quantitatively assess the number of recruited cells, tissue samples were stained with HLA-ABC, and the number of HLA-ABC-positive cells was morphometrically counted in the newly formed bone area. Fluorescent microscopy demonstrated a massive recruitment of human cells in CD34+ group (Figure 3J). In contrast, there was less recruitment of HLA-positive cells in animals receiving MNCs (Figure 3K) and no human cells were seen in the PBS group (Figure 3L). The number of HLA-ABC-positive cells in the newly formed bone area was significantly greater in the CD34+ group compared with the other groups (CD34+, 84.7 ± 13.5/mm2; MNC, 47.2 ± 9.0/mm2; PBS, 0.0 ± 0.0/mm2 respectively; P < 0.05 for CD34+ versus MNC, P < 0.01 for CD34+ versus PBS group) (Figure 3O). These morphological findings indicate that human peripheral blood CD34+ cells infused intravenously are recruited into not only the granulation zone but also the newly formed bone zone in the fracture healing process.
Human CD34+ Cell-Derived Vasculogenesis and Osteogenesis
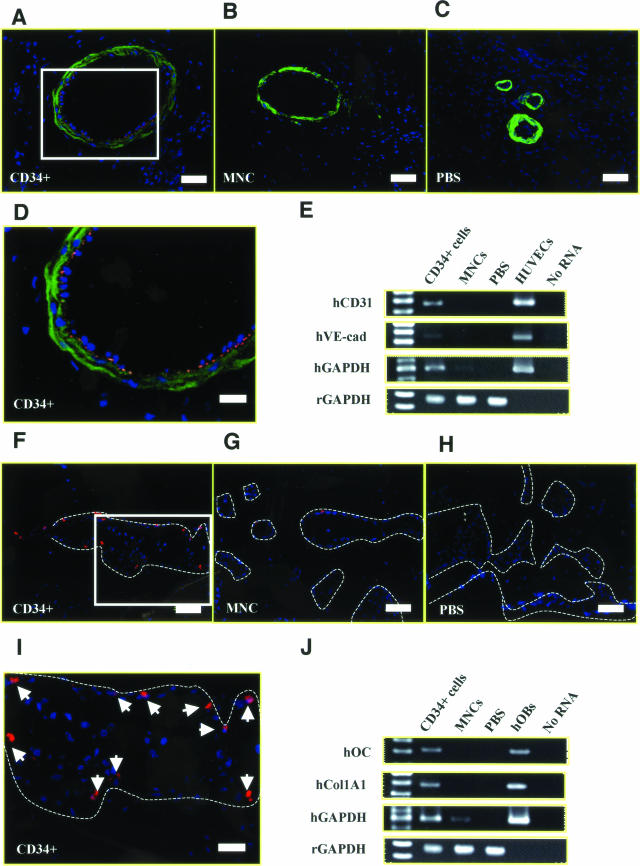
Next, we performed experiments to characterize the human cells recruited into the fracture sites. To histologically demonstrate the phenomenon of human cell-derived vasculogenesis, immunohistochemical staining for hCD31, a human-specific EC marker, was performed using tissue samples obtained 2 weeks after cell infusion. Differentiated human ECs derived from the transplanted CD34+ cells were detected as hCD31+ cells in the vasculature in the peri-fracture area (Figure 4, A and D), whereas hCD31+ cells were not identified in the MNC (Figure 4B) or PBS group (Figure 4C). To further confirm this phenomenon in terms of transcription, RT-PCR analysis of tissue RNA isolated from the peri-fracture site was performed. The molecular approach revealed the gene expression of human-specific EC markers (hCD31 and hVE-cad) in the CD34+ group, but not in the other groups (Figure 4E).
FIGURE 4.
Human CD34+ cell-derived vasculogenesis and osteogenesis. Immunohistochemical staining and RT-PCR for human-specific EC or OB markers was performed using tissue samples harvested at week 2. A–D: Differentiated human ECs were identified in zone a as hCD31-positive cells (red) in animals receiving CD34+ cells compared with MNC (B) or PBS group (C). E: RT-PCR analysis of tissue RNA isolated from the peri-fracture site demonstrated the expression of human-specific EC markers (hCD31, hVE-cad) in animals treated with CD34+ cells but not in control animals. Cultured human umbilical vein endothelial cells were used for positive control, and no RNA served as negative control. F–I: Differentiated human OBs were identified in zone b as hOC-positive cells (red) in animals receiving CD34+ cells compared with MNC (G) or PBS group (H). J: RT-PCR analysis of RNA isolated from the peri-fracture site demonstrates the expression of human-specific bone-related markers (hOC, hCol1A1) in animals treated with CD34+ cells, but not in control animals. Cultured hOBs were used for positive control. Blue fluorescence represents DAPI. Broken lines, newly formed bone surface. Scale bars = 50 μm (A–C, F–H); 20 μm (D, I). Original magnifications: ×200 (A–C, F–H); ×400 (D, I).
To identify human cell-derived osteogenesis, immunohistochemical staining for hOC, a human-specific OB marker was performed using tissue samples obtained 2 weeks after cell infusion. Human OBs derived from the transplanted CD34+ cells were observed as lining cells along the newly formed bone surface (Figure 4, F and I), whereas only a few human OBs were identified in the MNC group (Figure 4G) and no human OBs were identified in the PBS group (Figure 4H). RT-PCR analysis of tissue RNA isolated from the peri-fracture site demonstrated the expression of human-specific bone-related markers (hOC and hCol1A1) in the CD34+ group, but not in other groups (Figure 4J). These results indicate that human peripheral blood CD34+ cells differentiate into both ECs and OBs in a fracture-induced environment.
Enhancement of Intrinsic Angiogenesis and Osteogenesis in Animals Receiving CD34+ Cells
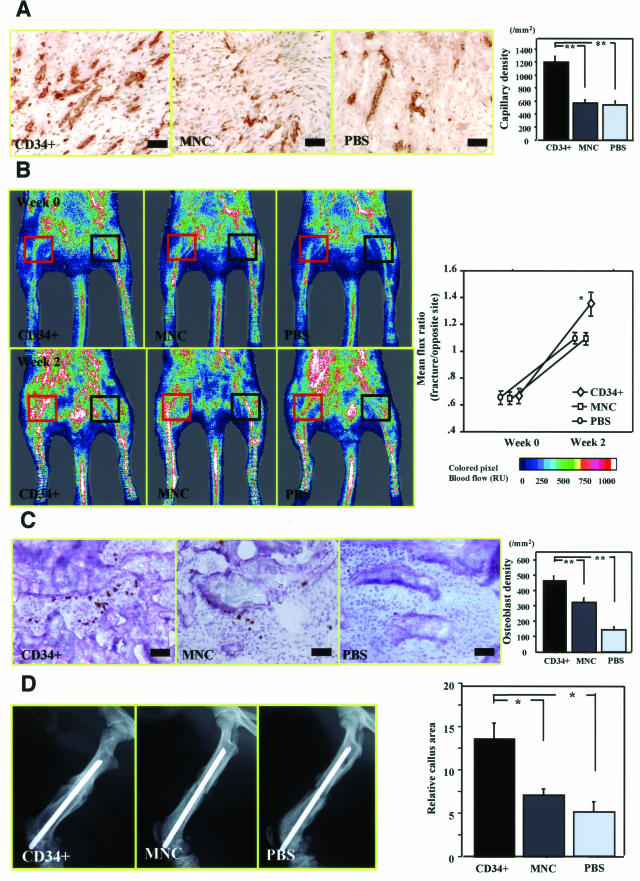
Enhanced angiogenesis and osteogenesis by recipient’s cells after cell transplantation were further confirmed by immunostaining for rat-specific markers. Vascular staining with isolectin B4 (marker for rat EC, but not human) using tissue samples at week 2 after fracture demonstrated enhanced neovascularization around the granulation area (Figure 3M, zone a) in CD34+ group compared with MNC or PBS group (Figure 5A). Neovascularization assessed by capillary density was significantly enhanced in CD34+ group compared with the other groups (CD34+, 1187.5 ± 79.3/mm2; MNC, 562.5 ± 36.6/mm2; PBS, 532.5 ± 49.3/mm2, respectively; P < 0.01 for CD34+ versus MNC or PBS group) (Figure 5A).
FIGURE 5.
Enhanced intrinsic vascularization and osteogenesis by recipient’s cells and serial improvement of blood flow and enhancement of callus formation after CD34+ cell transplantation. A: Rat-specific vascular staining with isolectin B4 (brown) at week 2 demonstrated enhanced neovascularization in zone a in animals treated with CD34+ cells compared with those receiving MNCs or PBS alone. Intrinsic angiogenesis assessed by capillary density at week 2 was significantly enhanced after CD34+ cell transplantation compared with other treatments. **P < 0.01. B: Representative LDPI at week 0 (1 hour after fracture) and week 2 in each group. In these digital color-coded images, maximum perfusion values are indicated in white, medium values in green to yellow, and lowest values in dark blue. The skin blood flow within fracture site (red square) and intact contralateral site (black square) were evaluated as mean flux, and ratio of the mean flux in the fractured site with that in the contralateral site (mean flux ratio) was calculated. Severe reduction of the blood flow was similarly observed 1 hour after fracture with the periosteum cauterized in all groups, whereas the mean flux ratio at week 2 was significantly greater in animals treated with CD34+ cells compared with those receiving MNCs or PBS alone. *P < 0.05. C: Rat-specific OC staining (brown) to detect intrinsic OBs at week 2 revealed augmentation of osteogenesis in zone b in animals treated with CD34+ cells compared with those receiving MNCs or PBS alone. Intrinsic osteogenesis assessed by the OB density at week 2 was significantly enhanced after CD34+ cell transplantation compared with other groups. **P < 0.01. D: Relative callus area assessed by radiograph at week 2 was significantly larger in CD34+ group compared with other groups. *P < 0.05. Scale bars = 50 μm. Original magnifications, ×200.
OB staining for rat-specific OC (marker for rat OB but not human) using tissue samples at week 2 after fracture revealed augmentation of osteogenesis in the area of new bone formation (Figure 3M, zone b) in animals treated with CD34+ cells compared with those receiving MNCs or PBS alone (Figure 5C). Osteogenesis assessed by the OB density was significantly enhanced in CD34+ group compared with other groups (CD34+, 468.0 ± 25.3/mm2; MNC, 321.6 ± 28.8/mm2; PBS, 141.6 ± 14.0/mm2, respectively; P < 0.01 for CD34+ versus MNC or PBS group) (Figure 5C). These results indicate that administration of human CD34+ cells enhances both intrinsic angiogenesis and osteogenesis by recipient cells.
Improvement of Blood Flow and Enhancement of Callus Formation in Animals Receiving CD34+ Cells after Fracture
To evaluate blood flow recovery at the fracture site by physiological approach, LDPI was serially performed after fracture. LDPI analysis demonstrated severely low blood flow at the fracture sites 1 hour after fracture production and its recovery at week 2 in all groups (Figure 5B). The ratio of fractured/intact (contralateral) blood flow was significantly increased by week 2 in all groups (Figure 5B). There was no significant difference in the blood flow ratio 1 hour after fracture production among the groups, whereas the ratio at day 14 was significantly higher in CD34+ group compared with the other groups (CD34+, 1.344 ± 0.079; MNC, 1.096 ± 0.037; PBS, 1.085 ± 0.024, respectively; P < 0.05 for CD34+ versus MNC or PBS group) (Figure 5B).
To evaluate the fracture healing process, callus formation was monitored radiographically and the relative callus area detected by radiography was quantified with NIH Image at week 2 in all groups (Figure 5D). The relative callus area was significantly larger in the CD34+ group compared with the other groups (CD34+, 13.67 ± 1.76; MNC, 7.00 ± 0.57; PBS, 5.00 ± 1.15, respectively; P < 0.05 for CD34+ versus MNC or PBS group) (Figure 5D). These results indicate that administration of human peripheral blood CD34+ cells contribute to improvement of tissue perfusion and enhancement of bone formation after fracture.
Inhibition of Intrinsic Angiogenesis and Osteogenesis by Anti-Angiogenic Agent in Animals Receiving CD34+ Cell Transplantation
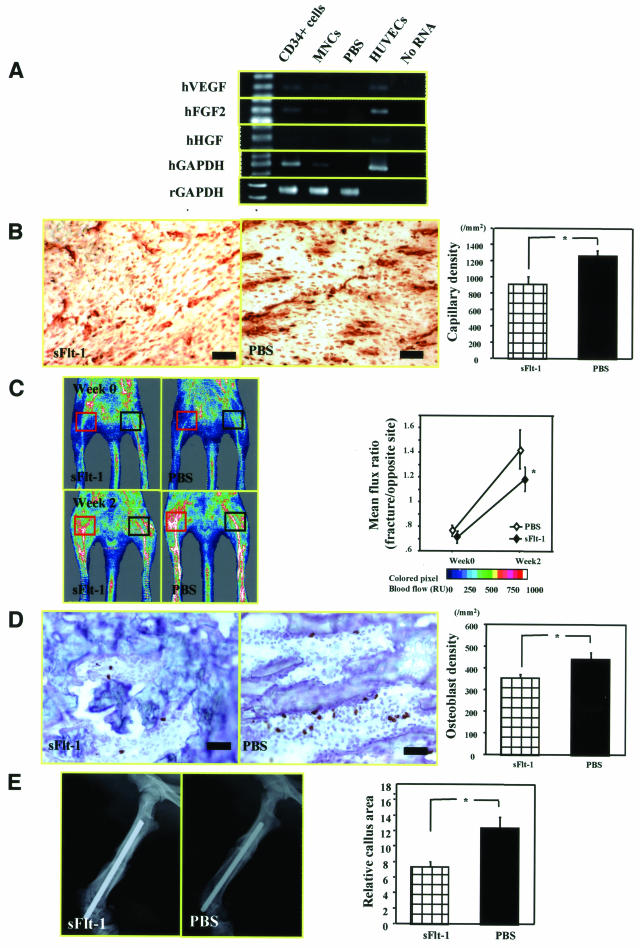
At first, we confirmed that transplanted human CD34+ cells released a greater amount of angiogenic factors (hVEGF, hFGF2, hHGF) than the MNCs at the fracture site (Figure 6A). Next, to investigate the hypothesis that neovascularization is essential to support endogenous bone regeneration after CD34+ cell infusion, we used an anti-angiogenic agent, sFlt1. Rat-specific vascular staining with isolectin B4 at week 2 demonstrated reduced capillary density in zone a in animals treated with CD34+ cells and sFlt1 compared with those receiving CD34+ cells and PBS (sFlt1, 908.3 ± 90.6/mm2; PBS, 1258.3 ± 69.1/mm2, respectively; P < 0.05 for sFlt1 versus PBS group) (Figure 6B). The skin blood flow within the fractured site and the intact contralateral site were calculated by LDPI. By week 2, the ratio of fractured/intact contralateral blood flow was significantly reduced in the sFlt1-treated group compared with the PBS group (sFlt1, 1.180 ± 0.096; PBS, 1.428 ± 0.159, respectively; P < 0.05 for sFlt1 versus PBS group) (Figure 6C).
FIGURE 6.
Inhibition of intrinsic angiogenesis and osteogenesis by anti-angiogenic agent in animals receiving CD34+ cell transplantation. A: RT-PCR revealed that fractured tissue sample contained higher amount of angiogenic factors (hVEGF, hFGF2, and hHGF) in human CD34+ group compared with MNC group. B: Rat-specific vascular staining with isolectin B4 (brown) at week 2 demonstrated reduced neovascularization in zone a in animals treated with CD34+ cells and sFlt1 compared with those receiving CD34+ cells and PBS. Intrinsic angiogenesis assessed by capillary density at week 2 is significantly reduced after sFlt1 treatment compared with PBS. *P < 0.05. C: Representative LDPI at weeks 0 and 2 in each group (red square, fracture site; black square, intact contralateral site). Mean flux ratio at week 2 was significantly less in animals treated with sFlt1 compared with rats receiving PBS. *P < 0.05. D: Representative rat-specific OC staining (brown) to detect intrinsic OBs at week 2 in zone b in animals treated with CD34+ cells and sFlt1 and those receiving CD34+ cells and PBS. Intrinsic osteogenesis assessed by the OB density at week 2 was significantly inhibited after sFlt1 treatment compared with PBS. *P < 0.05. E: Relative callus area assessed by radiograph at week 2 was significantly smaller in sFlt1-treated group compared with PBS group. *P < 0.05. Original magnifications, ×200.
Rat-specific OC staining at week 2 revealed a reduction of OB density, a parameter of intrinsic osteogenesis, in zone b in animals treated with CD34+ cells and sFlt1 compared with those receiving CD34+ cells and PBS (sFlt1, 352.0 ± 17.3/mm2; PBS, 440.7 ± 30.0/mm2, respectively; P < 0.05 for sFlt1 versus PBS group) (Figure 6D). The relative callus area assessed by radiograph at week 2 was significantly smaller in sFlt1-treated group compared with PBS group (sFlt1, 7.33 ± 0.67; PBS, 12.33 ± 1.53, respectively; P < 0.05 for sFlt1 versus PBS group) (Figure 6E). These findings indicate that the administration of human CD34+ cells contributed in an autocrine/paracrine manner to fracture healing by releasing angiogenic factors.
Morphological and Functional Recovery of Fractured Bone in Animals Receiving CD34+ Cell Transplantation
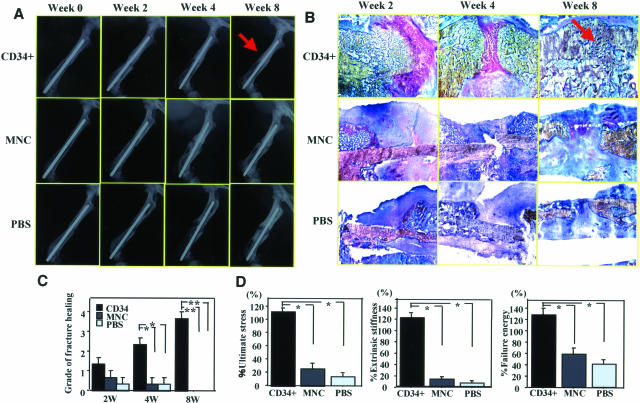
Morphological recovery of fractured bone in each group was evaluated by radiographical and histological examinations. In 66% of the rats (9 of 12) at week 4 and all rats at week 8 (nine of nine) in the CD34+ group, the fracture radiographically healed with bridging callus formation, whereas the fracture site in the other groups showed no bridging callus formation and resulted in nonunions, consistent with a previous report showing the natural course of this animal model (Figure 7A).20 Histological evaluation with toluidine blue staining demonstrated enhanced endochondral ossification consisting of numerous numbers of chondrocytes and newly formed trabecular bone at week 2, bridging callus formation at week 4, and complete union at week 8 in the CD34+ group (Figure 7B). In contrast, although a thick callus formation was observed at week 2, the healing process had stopped by week 4 and finally the callus was absorbed at week 8 in the other groups (Figure 6B). The degree of fracture healing assessed by Allen’s classification25 was significantly higher in CD34+ group compared with other groups at weeks 4 and 8 (2 weeks: CD34+, 1.3 ± 0.3; MNC, 0.7 ± 0.3; PBS, 0.3 ± 0.3, not significant for CD34+ versus other groups; 4 weeks: CD34+, 2.3 ± 0.3; MNC, 0.3 ± 0.3; PBS, 0.3 ± 0.3, P < 0.05 for CD34+ versus other groups; 8 weeks: CD34+, 3.7 ± 0.3; MNC, 0.0 ± 0.0; PBS, 0.0 ± 0.0, P < 0.01 for CD34+ versus other groups, respectively.) (Figure 7C).
FIGURE 7.
Radiographical, histological, and biomechanical evidence of fracture healing after CD34+ cell transplantation. A: Fracture healing was serially assessed by radiographs. By week 8, fracture was healed with bridging callus in all animals receiving CD34+ cells (arrow), but in no rats treated with MNCs or PBS alone. B: Serial assessment of fracture healing by histological examination with toluidine blue staining. Histological evaluation demonstrated the enhanced endochondral ossification consisting of numerous chondrocytes and newly formed trabecular bone at week 2, bridging callus formation at week 4, and complete union at week 8 (arrow) in animals receiving CD34+ cell transplantation. Although thick callus formation at week 2 was observed, the healing process had stopped by week 4, and the callus was finally absorbed at week 8 in animals receiving MNCs or PBS. C: The degree of fracture healing was assessed by Allen’s classification. The degree of fracture healing at weeks 4 and 8 was significantly higher after CD34+ cell transplantation compared with other treatments. *P < 0.05; **P < 0.01. D: Functional recovery after fracture is assessed by biomechanical three-point bending test at week 8. The percentage of all parameters (percent ultimate stress, percent extrinsic stiffness, percent failure energy) showing the ratio of each value in fractured side with contralateral side in animals receiving CD34+ cells was significantly superior to those in animals receiving MNCs or PBS. *P < 0.05.
Furthermore, to confirm the functional recovery of the fractured bone, biomechanical evaluation by a three-point bending test was performed at week 8 in all groups. Specimen length was similar in CD34+ cell group (15.2 ± 1.9 mm), MNC group (15.5 ± 2.1 mm), and PBS group (14.8 ± 1.3 mm). The percent ratios of all parameters in the fractured femur versus contralateral intact femur in CD34+ group were significantly superior to those in the other groups (percent ultimate stress: CD34+, 112.3 ± 5.3; MNC, 25.5 ± 8.3; PBS, 13.1 ± 4.3%, P < 0.01 for CD34+ versus other groups; percent extrinsic stiffness: CD34+, 121.1 ± 8.1; MNC, 12.1 ± 3.8; PBS, 6.8 ± 2.6%, P < 0.01 for CD34+ versus other groups; percent failure energy: CD34+, 127.3 ± 12.1; MNC, 57.3 ± 10.7; PBS, 40.3 ± 8.2%, P < 0.05 for CD34+ versus other groups) (Figure 7D). These results indicate that the femoral fractures with cauterized periosteum in the immunodeficient rats were morphologically and functionally healed by the administration of human peripheral blood CD34+ cells.
Discussion
Severe skeletal injury accompanied by fracture and loss of blood supply results in delayed union or established nonunion. Recovery of blood flow at the injury site is considered to be essential for adequate fracture healing.15,16 Therefore, appropriate neovascularization is emerging as a prerequisite for bone development and regeneration through developmental reciprocity between ECs and OBs.16 One of the most promising approaches for overcoming this clinical issue is by grafting the vascularized bone; however, this process requires painstaking microvascular surgical skill.12 Thus, the development of a more practical strategy for fracture healing is required, and many surgeons have begun looking at therapeutic neovascularization to answer this need. Recent advances in stem cell biology have suggested the feasibility and effectiveness of cell-based therapy. Human circulating CD34+ cells, EPC- and HSC-enriched fraction, have been shown to have therapeutic potential for ischemic diseases through vasculogenesis mechanism in our previous studies.5–7 As we anticipated, mouse ScaI+Lin− cells, quite similar to human CD34+ cells, were mobilized into peripheral blood in the natural course of fracture healing and human CD34+ transplantation induced significant vasculogenesis in regenerating tissues and enhanced functional recovery from nonhealing fracture in small animal models.
However, human CD34+ cells are reported to be not only hematopoietic and vasculogenic but also capable of differentiating into OBs in vitro.17–19 Quite recent reports demonstrated that the murine BM side population (SP) cells, which contain hematopoietic repopulating cells,36 can engraft in bone after intravenous transplantation37 and that a nonadhesive population of cultured BM cells contains primitive cells capable of generating into both hematopoietic and osteogenic lineages in vivo.38 In addition, Ford and colleagues20 recently reported that CD34+ osteoblastic cells line the cavities of the cartilage in the fracture site of rabbit tibial osteotomy model. Although several reports suggest the commitment of HSC- or EPC-rich cell population into osteogenic lineage cells, the morphological and physiological incorporation of these cells for medical application has never been proved.
In the present study, we used a reproducible animal model of nonhealing femur fracture with severe decrease in local blood flow, physiologically proven by LDPI examination. The natural history of this model is clearly relevant to the clinical situation of delayed union or nonunion. We identified in vivo multilineage plasticity of human peripheral blood CD34+ cells into OBs as well as ECs by not only immunohistochemistry but also by RT-PCR for human-specific cell markers. Recent reports demonstrated in vitro or in vivo trans-lineage differentiation of CD34+ cells into cardiomyocytes,39–42 and peripheral blood CD34+ cells were also shown to contain a cell fraction expressing not only hematopoietic and endothelial but also cardiac, skeletal, hepatic, and neural lineage markers after G-CSF administration or myocardial ischemia.43,44 However, the exact mechanism of the phenomena is not yet fully understood. Considering that CD34+ cells are EPC and HSC enriched but still a heterogeneous cell population, there may be several possible mechanisms underlying the multilineage differentiation: 1) differentiation of original multipotent stem cells into multiple lineages, 2) differentiation of multiple kinds of lineage-committed progenitor cells into each lineage, 3) transdifferentiation of hematopoietic/endothelial lineage cells into mesenchymal lineage, and 4) cell fusion between transplanted cells and recipient cells. The present RT-PCR results revealed a weak gene expression of human-specific OC in freshly isolated human CD34+ cells, strongly supported by single-cell PCR analysis (before transplantation) and enhancement of the gene expression 14 days after transplantation. These findings indicate that CD34+ cell fraction contains a few multipotent stem and/or committing osteogenic progenitor cells, subsequently representing the osteogenic activity under the microenvironment of fracture. Enhbali-Fatourechi and colleagues45 quite recently demonstrated that sorted OC-positive cells in human peripheral blood formed mineralized nodules in vitro and bone in an in vivo transplantation assay. Their novel report may support the hypothesis that CD34+ cell fraction contains a few osteo/endothelial progenitor cells, contributing to an enhanced functional recovery of the fracture.
In addition to these sensitive assessments of donor cell differentiation at the incorporated site, quantitative histochemical analysis for rat ECs and OBs revealed the enhancement of intrinsic angiogenesis and osteogenesis by recipient cells after the administration of human CD34+ cells. Human CD34+ cells were reported to secrete numerous angiogenic factors in vitro, including VEGF, HGF, FGF2, and IGF1.46,47 Our in vivo data also demonstrated that gene expression levels of human angiogenic factors including VEGF, FGF2, and HGF at the fracture site were greater in the CD34+ cell group compared with the MNC group. Moreover, we demonstrated that the inhibition of angiogenesis by sFlt1 suppressed not only angiogenesis/vasculogenesis but also intrinsic osteogenesis, indicating that angiogenic factors released by the transplanted CD34+ cells contribute, at least in part, to fracture healing in a paracrine manner. Although the detailed mechanism of the paracrine effect of human CD34+ cells is still unclear, a cooperative signal between HSCs/EPCs and OBs may need to be further considered as a mechanism of multilineage regeneration by CD34+ cell transplantation. The niche regulating birth and differentiation of HSCs is known to consist of OBs, which line the inner surface of BM.48,49 Zhang and colleagues50 reported that depleting the receptor of bone morphogenic protein in OBs caused a doubling in both OB and HSC populations, offering some insight into the native of HSCs. Calvi and colleagues51 found the parallel expansion of HSCs when the number of OBs increased by parathyroid hormone infusion. In addition, Ponomaryov and colleagues52 reported that immature OBs and ECs control homing, retention, and repopulation of SRC (severe combined immunodeficiency repopulating cells) by secreting SDF-1 as a host defense in response to DNA damage. These findings indicate that osteogenesis and hematopoiesis/vasculogenesis closely regulate each other in terms of microenvironmental interaction for regenerative activity in BM. Microenvironmental interaction between osteogenic and vasculogenic lineage cells may involve not only paracrine regulatory factors but also direct cellular communications in developing CD34+ cells. Enhanced angiogenesis/vasculogenesis signals could exert a cellular commitment and promote development of CD34+ cells into the osteogenic lineage in response to a rigorous demand for skeletal tissue repair as a cooperative organogenesis mechanism.
The present results, showing the potential of CD34+ cells for multilineage plasticity, suggest that CD34+ cell transplantation contributes to forming an ideal local environment for fracture healing by supplying adequate blood flow and stimulating osteogenesis. The environmental contribution of CD34+ cells resulted in morphological and physiological healing of the fracture. Radiographical examination and histological toluidine blue staining demonstrated enhancement of endochondral ossification leading to bridging callus formation only after CD34+ cell transplantation, but this did not occur in the MNC and PBS groups. Finally, the biomechanical three-point bending test confirmed significant functional recovery from fracture after administration of CD34+ cells. These findings strongly suggest that peripheral blood CD34+ cells have significant potential for therapeutic application to the damaged skeletal tissue.
On the basis of preclinical achievements of cell-based therapy for injured bone53 or osteogenesis imperfecta,54 transplantation of whole BM cells or BM mesenchymal stem cells has been clinically applied for bone/cartilage regeneration.55,56 It remains to be clarified whether circulating CD34+ cell transplantation is superior to the BM cell therapy in terms of efficacy and safety for fracture healing. However, cell harvest from peripheral blood dose provides positives in that it is less invasive and safer than BM aspiration under general anesthesia, and magnetic sorting of CD34+ cells has been clinically applied in the hematology field for many years.57,58 The technical feasibility in a clinical situation and the present preclinical findings demonstrating morphological and functional fracture healing through concurrent vasculogenesis and osteogenesis strongly suggest promising results for the future clinical application of circulating CD34+ cells for nonhealing fracture.
In conclusion, human circulating CD34+ cells have potent vasculogenic and osteogenic plasticity in the fracture-induced environment, enabling them to make a remarkable contribution to morphological and functional bone healing.
Acknowledgments
We thank Drs. M. Osawa and S. Nishikawa (Riken CDB, Kobe, Japan) for technical support with single-cell PCR, Y. Masukawa and T. Itoh for secretarial assistance, and Ms. Janina Tubby for her editing assistance in preparing for this manuscript.
Footnotes
Address reprint requests to Takayuki Asahara, M.D., Stem Cell Translational Research, Kobe Institute of Biomedical Research and Innovation/Riken Center for Developmental Biology, 2-2 Minatojima-Minamimachi, Chuo-ku, Kobe 650-0047. E-mail: asa777@aol.com.
References
- Slack JM. Stem cells in epithelial tissues. Science. 2000;287:1431–1433. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- Korbling M, Estrov Z. Adult stem cells for tissue repair—a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Merchan EC, Forriol F. Nonunion: general principles and experimental data. Clin Orthop Relat Res. 2004:4–12. [PubMed] [Google Scholar]
- Marsh D. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res. 1998:S22–S30. doi: 10.1097/00003086-199810001-00004. [DOI] [PubMed] [Google Scholar]
- Colnot CI, Helms JA. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev. 2001;100:245–250. doi: 10.1016/s0925-4773(00)00532-3. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Long MW, Williams JL, Mann KG. Expression of human bone-related proteins in the hematopoietic microenvironment. J Clin Invest. 1990;86:1387–1395. doi: 10.1172/JCI114852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Hunt P, McElvain M, Black T, Kaufman S, Choi ES. Osteoblast precursor cells are found in CD34+ cells from human bone marrow. Stem Cells. 1997;15:368–377. doi: 10.1002/stem.150368. [DOI] [PubMed] [Google Scholar]
- Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derive from CD133 positive cells in mobilized peripheral blood and cord blood: proliferation, Oct-4 expression and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- Ford JL, Robinson DE, Scammell BE. Endochondral ossification in fracture callus during long bone repair: the localisation of ‘cavity-lining cells’ within the cartilage. J Orthop Res. 2004;22:368–375. doi: 10.1016/j.orthres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- Kokubu T, Hak DJ, Hazelwood SJ, Reddi AH. Development of an atrophic nonunion model and comparison to a closed healing fracture in rat femur. J Orthop Res. 2003;21:503–510. doi: 10.1016/S0736-0266(02)00209-7. [DOI] [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden M, Sirsjo A, Lindbom L, Nilsson G, Gidlof A. Laser-Doppler perfusion imaging of microvascular blood flow in rabbit tenuissimus muscle. Am J Physiol. 1995;269:H1496–H1500. doi: 10.1152/ajpheart.1995.269.4.H1496. [DOI] [PubMed] [Google Scholar]
- Wardell K, Jakobsson A, Nilsson GE. Laser Doppler perfusion imaging by dynamic light scattering. IEEE T Bio-Med Eng. 1993;40:309–316. doi: 10.1109/10.222322. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen HL, Wase A, Bear WT. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand. 1980;51:595–600. doi: 10.3109/17453678008990848. [DOI] [PubMed] [Google Scholar]
- Hak DJ, Makino T, Niikura T, Hazelwood SJ, Curtiss S, Reddi AH. Recombinant human BMP-7 effectively prevents non-union in both young and old rats. J Orthop Res. 2006;24:11–20. doi: 10.1002/jor.20022. [DOI] [PubMed] [Google Scholar]
- Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- Peng Z, Tuukkanen J, Zhang H, Jamsa T, Vaananen HK. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone. 1994;15:523–532. doi: 10.1016/8756-3282(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Makino T, Hak DJ, Hazelwood SJ, Curtiss S, Reddi AH. Prevention of atrophic nonunion development by recombinant human bone morphogenetic protein-7. J Orthop Res. 2005;23:632–638. doi: 10.1016/j.orthres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi G, Khosla S. Purification and characterization of circulating osteoblast lineage cells: expression of type1 collagen mRNA is a key difference between these and adherent bone marrow stromal cells. J Bone Miner Res. 2005;20:S12. [Google Scholar]
- Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- Dominici M, Pritchard C, Garlits JE, Hofmann TJ, Persons DA, Horwitz EM. Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. Proc Natl Acad Sci USA. 2004;101:11761–11766. doi: 10.1073/pnas.0404626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted-Davis EA, Gugala Z, Camargo F, Gannon FH, Jackson K, Kienstra KA, Shine HD, Lindsey RW, Hirschi KK, Goodell MA, Brenner MK, Davis AR. Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc Natl Acad Sci USA. 2003;100:15877–15882. doi: 10.1073/pnas.2632959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110:3803–3807. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells ‘hide out’ in the bone marrow. Leukemia. 2004;18:29–40. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, Gewirtz AM, Emerson SG, Ratajczak MZ. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Majka M, Ratajczak J, Ratajczak MZ. Autocrine/paracrine mechanisms in human hematopoiesis. Stem Cells. 2001;19:99–107. doi: 10.1634/stemcells.19-2-99. [DOI] [PubMed] [Google Scholar]
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- Zhu J, Emerson SG. A new bone to pick: osteoblasts and the haematopoietic stem-cell niche. Bioessays. 2004;26:595–599. doi: 10.1002/bies.20052. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D, Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Petite H, Viateau V, Bensaid W, Meunier A, de Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–963. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- Brugger W, Heimfeld S, Berenson RJ, Mertelsmann R, Kanz L. Reconstitution of hematopoiesis after high-dose chemotherapy by autologous progenitor cells generated ex vivo.[see comment] [retraction in Kanz L, Brugger W: N Engl J Med 2001 Jul 5;345(1):64; PMID: 11439953]. N Engl J Med. 1995;333:283–287. [Google Scholar]
- Kessinger A, Armitage JO. The evolving role of autologous peripheral stem cell transplantation following high-dose therapy for malignancies. Blood. 1991;77:211–213. [PubMed] [Google Scholar]