Abstract
Molecular mechanisms of prostate cancer progression are frequently studied in mice by orthotopic injection of aggressive cell lines, which yield primary tumors that spontaneously metastasize to lymph nodes. In this report, we characterized the human prostate carcinoma cell line 22Rv1 in an orthotopic system and evaluated the functional relevance of the hyaluronidase Hyal1, a correlate of invasive human prostate cancer, to progression in this model. To provide real-time insights into these processes, we first validated use of an epidermal growth factor-conjugated fluorophore to illuminate orthotopic prostate tumors and their metastases in whole animal imaging. Animals receiving intraprostatic injections were tracked throughout a 6-week period. Tumor sizes were correlated 92% with total fluorescence intensities of 22 prostate tumors. In contrast to the highly tumorigenic and metastatic PC3M-LN4 cells, the 22Rv1 line was orthotopically tumorigenic but not metastatic, despite larger tumor sizes. Lymph node metastasis was successfully imaged in animals with PC3M-LN4 tumors on endpoint dissection. Stable transfection of 22Rv1 cells with Hyal1 did not alter growth kinetics of primary orthotopic tumors, but all animals implanted with Hyal1 transfectants exhibited tumor-positive para-aortic lymph nodes. Hyal1 is implicated as an inducer of prostate cancer metastatic progression.
Prostate cancer is a heterogeneous disease without well-defined progression. Metastases often occur with no prior indication of tumor invasiveness.1 Early tumors may be responsive to androgen ablation therapy, but recurrence is hormone-refractory and/or metastatic.2,3 Changes in expression of the hyaluronidase Hyal1 within the prostate extracellular matrix have been shown to accompany invasive prostate cancer progression.4–8 Although a role for Hyal1 in tumor growth and angiogenesis has been established,5 its ability to induce metastatic progression has not been investigated.
Mechanistic study of prostate cancer progression at the molecular level is a challenge because clinically relevant aspects of human progression, such as bone metastasis, are difficult to reproduce in mice. Cell lines are useful for testing causality of candidate tumor genes in vitro, but they may not display features of human prostate cancer progression when placed in mice. Nonetheless, orthotopic (intraprostatic) injection of tumor cells is a well-characterized method in which the relevant tumor microenvironment is preserved, and aggressive cell lines will give rise to spontaneous lymph node metastases.9,10 Use of this model in conjunction with noninvasive methods for tracking the growth and metastasis of tumors during progression could advance the elucidation of molecular metastatic mechanisms, but the anatomical location of the tumors places challenges on the interpretation of tracking experiments.
Relatively few cell culture models exist for human prostate cancer. Highly aggressive derivative lines such as PC3M-LN4 have been selected from PC3, an androgen-independent line, for enhanced metastatic potential using orthotopic injection.10 PC3M-LN4 cells are a relevant model for cancer progression because they reproduce the aspects of rapid androgen-independent growth in the prostate of either an intact or castrated mouse and of spontaneous metastasis to lymph nodes. However, additional tumor cell models are necessary for the study of early tumorigenesis and metastatic initiation, which are not accessible with PC3M-LN4 cells. One candidate line is 22Rv1, derived originally from a human primary prostate tumor.11 The cells exhibit androgen sensitivity, androgen receptor gene expression, and PSA secretion12 and are easily transfected and handled in culture, but they have not been characterized in orthotopic models. A major objective of the current study was to perform a side-by-side comparison of the tumorigenic and metastatic potential of 22Rv1 and PC3M-LN4 cells in an orthotopic model. An additional objective was to characterize the effect of stable Hyal1 overexpression in these cells on orthotopic tumor growth and metastatic potential.
To facilitate the longitudinal comparisons in these experiments, we opted for a noninvasive imaging modality that was both sensitive and versatile without requiring specific reporter genes or cell lines. (Several excellent recent reviews describe existing noninvasive imaging modalities including magnetic resonance imaging, ultrasound, and optical techniques.13–19) Optical imaging has the potential to monitor growth and regression of tumors in a continuous and noninvasive manner if sites of malignancy are optically distinguishable from normal tissue. Because the absorbance spectra of bodily fluids and tissues exhibit local minima in the near-infrared (NIR) region,20 imaging in the NIR offers the advantages of relatively deep tissue penetration and low autofluorescence.21 A recent successful innovation for targeting of tumor cells in whole animals has been to couple ligands of tumor cell surface receptors to small molecule fluorophores.22–29 For example, epidermal growth factor (EGF) has been labeled with Cy5.5 to monitor EGF receptor-targeted therapy in breast cancer xenografts in vivo.23 Because the EGF receptor is elevated manyfold relative to normal cells, not only in breast tumors30 but also in tumors of the lung,31 ovary,32 brain,33 and prostate,34 we chose to interrogate it as a potential biomarker for our prostate cancer studies. With EGF as a targeting agent, we selected a fluorophore, IRDye 800CW, that exploits the high-sensitivity region of the NIR spectrum (780 to 850 nm) and has been used with previous success.29
We report the use of an orthotopic model to compare tumor growth kinetics and potential lymph node metastasis of two human prostate carcinoma cell lines, PC3M-LN4 and 22Rv1, the latter of which had not been previously tested. In addition, we provide the first evidence that Hyal1 expression may be sufficient to induce metastasis of prostate tumor cells thus implanted. To facilitate this and future studies of molecular metastatic mechanisms, we optimized and validated a straightforward approach for noninvasive monitoring of tumor growth. In particular, we have determined that the IRDye 800CW EGF conjugate is an effective and specific targeting agent for prostate tumor cells in mice and that progression of primary tumors and para-aortic node metastases can be detected and tracked longitudinally in a semiquantitative manner by optical imaging of this agent. 22Rv1 cells were found to be highly tumorigenic at the primary site, giving rise to ≈3.5-fold larger orthotopic tumors than PC3M-LN4 cells. 22Rv1-untransfected and control-transfected cells did not grow invasively or metastasize to nodes in a 5- to 7-week time course, whereas PC3M-LN4 cells and 22Rv1 Hyal1 transfectants were metastatic by 5 weeks. 22Rv1 cells orthotopically implanted may thus be an appropriate model for the study of molecular changes that accompany the progression of prostate cancer.
Materials and Methods
Cell Culture, Materials, and Reagents
PC3M-LN4 human prostate adenocarcinoma cells derived from PC3 were kindly provided by Dr. Isaiah J. Fidler (MD Anderson Cancer Center, Houston, TX) and maintained in minimal essential medium containing 10% fetal bovine serum, sodium pyruvate, and nonessential amino acids. 22Rv1 human prostate adenocarcinoma cells were purchased from American Type Culture Collection (Rockville, MD) and maintained in RPMI 1640 medium containing 10% fetal bovine serum. Selection, characterization, and maintenance of 22Rv1 stable transfectants in standard medium containing 1.5 mg/ml G418 has been previously described.35 Standard mouse chow and a purified maintenance diet (AIN-93M) were obtained from Harlan Teklad (Madison, WI). EGF (human recombinant) was obtained from Upstate Biotechnology (Charlottesville, VA). C225 (also known as Erbitux or Cetuximab; Imclone, New York, NY) was graciously provided by Dr. Chun Li (MD Anderson Cancer Center). IRDye 800CW free dye and NHS ester were supplied by LI-COR Biosciences (Lincoln, NE). The Odyssey infrared imaging system and Aerius automated infrared imaging system were also provided by LI-COR Biosciences. TO-PRO-3 was obtained from Molecular Probes, Inc. (Eugene, OR).
Characterization of EGF-IRDye 800CW in Vitro
EGF was labeled with IRDye 800CW (NHS ester) by standard coupling methods. Unreacted dye was removed by dialysis. The specificity and activity of the IRDye 800CW EGF conjugate were evaluated on PC3M-LN4 and 22Rv1 cells by In-Cell Western, an in vitro cell-based assay.36 In brief, cells were grown to ∼90% confluency in a microtiter plate and starved for 2 hours in serum-free (22Rv1) or 1% serum-containing (PC3M-LN4) media. Starvation media were replaced with media containing either IRDye 800CW only (0.002 to 3.3 μmol/L), IRDye 800CW EGF (0.01 to 70 nmol/L), unlabeled EGF (0.01 to 2.14 μmol/L) plus IRDye 800CW EGF (70 nmol/L), or C225 (0.5 to 200 μg/ml) plus IRDye 800CW EGF (70 nmol/L). Cells were incubated at 37°C and 5% CO2 for 2 minutes and fixed with 4% formaldehyde solution for 20 minutes. Four washes in 1× phosphate-buffered saline plus 0.1% Triton X-100 removed unbound dye and permeabilized the cells. The plates were blocked in Odyssey blocking buffer (LI-COR Biosciences) for 1.5 hours and then incubated with TO-PRO-3 DNA stain (1:5000) for normalization of cell number using the 700-nm channel. Washing steps were repeated, and the plate was scanned with an Aerius NIR automated laser-scanning microplate imager. Quantifications were normalized by ratiometric analysis of the 700-nm channel applied to the 800-nm channel.
In Vivo Animal Imaging
NIR fluorescence imaging in vivo used a prototype LI-COR Biosciences small animal imager. The instrument is a light-tight chamber equipped with a cooled charge-coupled device camera, area illumination via diode lasers, and a selection of excitation and detection optical fluorescent filters tuned specifically for IRDye 800CW. Images were acquired and analyzed with Wasabi software from Hamamatsu Photonics (Hamamatsu City, Japan) or Adobe Photoshop (Adobe Systems Inc., San Jose, CA). For longitudinal tracking, immunocom-promised mice were imaged in a sealed, sterilized, portable compartment, through which HEPA-filtered air was circulated. All mice were shaved before image collection and anesthetized with 2% isoflurane throughout all procedures.
Male NOD/SCID mice (Jackson Laboratories, Bar Harbor, ME) used in the imaging experiments were maintained under the supervision and guidelines of the University of Nebraska–Lincoln Institutional Animal Care and Use Committee. To establish initial conditions, the IRDye 800CW EGF targeting agent (1 nmol) was injected via the tail vein into a tumor-negative mouse and evaluated for systemic clearance by NIR imaging at 1, 2, 4, 6, 8, 24, 48, and 72 hours. Mice bearing subcutaneous tumors (diameter, ≈0.5 cm) were then injected with IRDye 800CW EGF (1 nmol) and evaluated for tumor-specific retention of the fluorophore by NIR imaging at 24-hour intervals for up to 8 days. Signal-to-noise ratios (SNRs) calculated as described below were used to determine an optimal time point of 72 to 96 hours for imaging tumors. Therefore, all longitudinal images were collected at 4 days after injection of the targeting agent.
PC3M-LN4 or 22Rv1 human prostate carcinoma cells were trypsinized, washed, and resuspended in serum-free medium for subcutaneous injections in the flank (0.5 × 106 cells) of male NOD/SCID mice (four per cell line).37 Injections of the IRDye 800CW EGF targeting agent (1 nmol) via tail vein were begun 3 days after tumor cell implantation and continued weekly for 5 weeks. Images of all mice in the longitudinal study were taken 4 days after injection of the targeting agent. Four additional control mice received tumor cells but no targeting agent and were carried through the study for comparison purposes. These animals were used for the in vivo specificity experiment described below. It should be noted that the mean tumor size among animals repeatedly injected with IRDye 800CW EGF did not differ significantly from that of animals injected only at the study endpoint, indicating that the quantity of EGF-labeled fluorophore did not affect tumor growth in this time frame.
Orthotopic Injection of Prostate Tumor Cells
Six-week-old male NOD/SCID mice were anesthetized with 2% isoflurane. In a sterile field, a lower midline incision was made to access the prostate. The left lobe of the dorsal prostate was injected with 1 × 105 22Rv1 (n = 4) or PC3M-LN4 (n = 4) cells from a single cell suspension in serum-free medium, using a 27-gauge needle fitted on a 50-μl Hamilton syringe. Animals were sutured, and sutures were removed 10 days after surgery. Animals were longitudinally imaged as described above. Three additional animals were orthotopically injected, but they did not receive injections of the targeting agent until the endpoint of the study, at which time they were subjected to the specificity challenge described below. To examine the role of the hyaluronidase Hyal1 in orthotopic tumor growth and metastasis, animals were injected with 22Rv1 cells stably transfected with a control (n = 10) or Hyal1 (n = 10) expression vector. Growth of the tumors was tracked by optical imaging at 2, 4, and 6 weeks, each time after intravenous injection of 1 nmol of the targeting agent. All animals injected with 22Rv1 and its stable lines were used in the statistical calculations to validate imaging parameters as accurate measures of tumor size. Control animals carried through the study and injected only once at the endpoint confirmed that no differences in tumor size occurred within animal groups as a function of EGF fluorescent labeling.
In Vivo Specificity Challenge
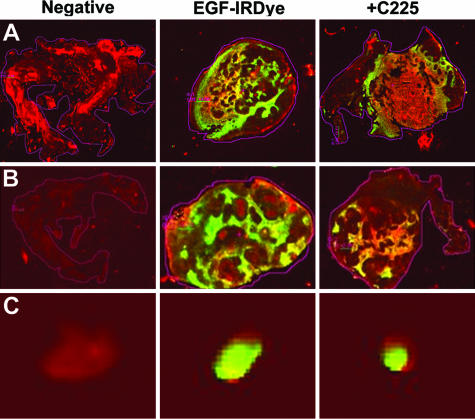
At week 5, three animals with subcutaneous tumors and three with orthotopic tumors were used for an in vivo dye specificity challenge experiment. One animal in each group was injected with IRDye 800CW EGF (1 nmol). A second animal received an injection of C225 (2 mg) via a tail catheter 1 hour before receiving an injection of IRDye 800CW EGF (1 nmol). The third mouse was injected with the 0.9% saline vehicle. After final imaging, animals were sacrificed and tumors, as well as organs suspected of background targeting agent accumulation (ie, kidney, liver, stomach), were removed for imaging to confirm signal content. Tumors were weighed, measured, and snap-frozen in OCT compound for cryosectioning. Sections (8-μm thickness) were scanned in both the 700-nm channel, for tissue autofluorescence, and the 800-nm channel, for the targeted IRDye 800CW EGF fluorescence signal, using the Odyssey NIR laser-scanning imager. The area-weighted fluorescence signal from the 800-nm channel was used to compare targeting agent specificity among experimental conditions. This experiment was repeated once with an additional three mice for each tumor site (subcutaneous and orthotopic) and yielded essentially identical results. Representative data are shown (Figure 2).
FIGURE 2.
In vivo specificity of EGF receptor targeting. Male NOD/SCID mice with subcutaneous or orthotopic tumors were injected intravenously with vehicle (negative), IRDye 800CW EGF, or with C225 blocking mAb (2 mg i.v.) followed in 1 hour by IRDye 800CW EGF. At 96 hours after injection, subcutaneous (A) and prostatic (B) tumors or lymph nodes (C) excised from the in vivo specificity challenge animals were snap-frozen and cryosectioned for NIR analysis on Odyssey. Tissue autofluorescence is detected in the 700-nm channel and shown in red. Fluorescence of the IRDye 800CW conjugate is detected in the 800-nm channel and shown in green. All images are merged. Differences in fluorescence intensity per unit area, normalized to the vehicle control, were quantified as given in Table 1 and discussed in the text. Fluorescence intensity per unit area was quantified to confirm tumor penetration by the targeting agent and degree of targeting specificity as shown in Table 1.
Statistical Analysis
Images analyzed in a longitudinal series for each particular mouse were normalized using the same look-up table with a common minimum and maximum value. SNR was calculated using the following formula: SNR = ((Maximum tumor intensity) − (Mean background intensity))/Standard deviation (SD) of mean background.
Regions of interest (ROIs) with identical areas were used for both tumor and background. ROIs were quantified for total pixel and mean pixel values. The SD of mean backgrounds was calculated using three to five ROIs. This calculation yields the number of SDs over background represented by the suspect tumor/signal. To validate the use of optical parameters as a correlate for tumor size, linear regression analysis was performed on the SNR, predicted tumor area, area-weighted fluorescence intensity, or total fluorescence relative to tumor wet weight or caliper volume. As a control, regression analysis between caliper volume and wet weight was also performed. P values <0.001 were considered significant. Linear regression analyses were performed with JMP statistical software (SAS Instruments, Inc., Cary, NC).
Results
In Vitro Efficacy of EGF for Targeting Prostate Tumor Cell Lines
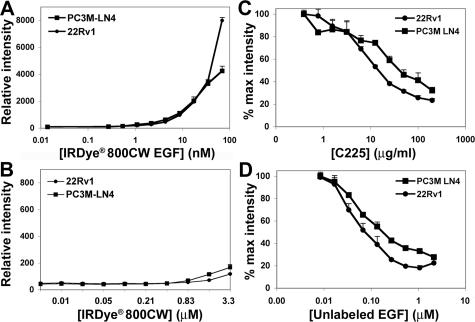
EGF has previously been used effectively as a tumor cell targeting agent in mice,23 but its efficacy for prostate tumor illumination and for long-term imaging are unknown. We coupled EGF to IRDye 800CW, an aqueous soluble, nontoxic fluorophore, and tested its specificity for prostate tumor cells in vitro before its use in vivo. Confluent monolayer cultures of human prostate tumor cell lines PC3M-LN4 and 22Rv1 were treated with increasing concentrations of IRDye 800CW EGF to establish a dose-response curve for binding of the targeting agent to EGF receptors expressed on the cell surface. Fluorescence increased in a concentration-dependent manner for both cell lines with saturation beginning at ≈70 nmol/L for PC3M-LN4 cells (Figure 1A). The same measurements with unconjugated dye showed negligible increases in fluorescence throughout an even higher concentration course (Figure 1B). Specificity of the conjugate for the EGF receptor was evaluated by competition with a monoclonal antibody, C225, that blocks ligand binding38 (Figure 1C) or with unlabeled EGF (Figure 1D). The antibody reduced fluorescence in a concentration-dependent manner with IC50 ≈ 5 μg/ml. Likewise, unlabeled EGF competition was dose-dependent with KI ≈ 70 nmol/L.
FIGURE 1.
In vitro specificity of IRDye 800CW EGF for tumor cell lines. PC3M-LN4 and 22Rv1 human prostate tumor cell lines were cultured to 90% confluence in 96-well plates. Cells were incubated with increasing concentrations of IRDye 800CW EGF (A) or unconjugated, nonreactive IRDye 800CW (B) for 2 minutes and then washed, fixed, permeabilized, and stained with TO-PRO-3. Plates were scanned on Aerius. The 800-nm signal, normalized to the 700-nm control, is plotted as the mean ± SD of three replicate wells. To demonstrate fluorescence specificity, cells were incubated with 70 nmol/L IRDye 800CW EGF in the presence of increasing concentrations of either C225 blocking antibody (C) or unlabeled EGF (D).
Kinetics of Clearance and Specificity of EGF Tumor Targeting in Vivo
Clearance of the targeting agent from normal mice was evaluated by injecting animals with 1 nmol of IRDye 800CW EGF via the tail vein and collecting NIR images throughout time from 15 minutes to 24 hours. Fluorescence intensity in ROIs encompassing the whole animal or limited to the abdominal area was quantified as a measure of clearance. The analysis confirmed that 75% of the signal cleared after 8 hours, and >90% cleared after 24 hours. A similar time course was monitored for clearance of the targeting agent from tumor-positive animals. Mice bearing subcutaneous PC3M-LN4 tumors of ≈0.5 cm in diameter were injected and repeatedly imaged at 24-hour intervals. As observed for the tumor-negative controls, whole animal signal had diminished by >90% within 24 hours (not shown). Analysis of SNR comparing fluorescence intensity in the tumor relative to background indicated that the time of maximal sensitivity for imaging was 72 to 96 hours, and subsequent longitudinal images were collected at 96 hours. The fluorescence signal from the targeting agent decreased after 4 days, so we opted to reinject animals weekly for longitudinal tracking.
To verify that EGF receptor was mediating retention of the targeting agent by prostate tumor cells in all relevant tissues in vivo, we performed a competition by preinjecting tumor-bearing mice with the C225 EGF receptor blocking antibody before IRDye 800CW EGF administration. Subcutaneous and intraprostatic tumors were harvested, volumetrically measured with digital calipers, and cryosectioned to quantify fluorescence per unit area (mm2). Images are shown in Figure 2, and the results of analysis are presented in Table 1. Signal intensities for the specificity controls are merged, with red representing tissue autofluorescence in the 700-nm channel and green for specific fluorescence in the 800-nm channel resulting from the targeting agent. Yellow indicates overlap of the signals. Signal intensity was evident throughout the tumors. C225 preinjection blocked ≈46 and ≈49% of the signal localization detected in subcutaneous and orthotopic tumors, respectively (Figure 2, A and B). Hematoxylin and eosin (H&E) staining verified tumor content and integrity (not shown). Lymph node fluorescence from spontaneously metastatic orthotopic PC3M-LN4 tumors (Figure 2C) was partially reduced to a similar extent (≈34%) as observed in the primary tumors, consistent with specific targeting in all tumor-bearing tissues. Thus, in addition to an effective tumor-imaging tool, the targeting agent also serves as a fluorescence reporter for EGF receptor function in the tissue sections.
TABLE 1.
Summary of Ratiometric Tissue Analysis of Tumors
| Treatment | Fluorescence signal*/mm2 by tissue
|
||
|---|---|---|---|
| Subcutaneous | Prostate | Nodes | |
| Vehicle | 0.01 | 0.14 | 0.01 |
| IRDye 800CW EGF | 0.69 | 1.00 | 1.15 |
| C225 + IRDye 800CW EGF | 0.38 | 0.51 | 0.76 |
| Percentage of signal blocked | 45.70 | 49.00 | 33.74 |
Integrated intensity in the 800 channel normalized to unit area of the section or tissue sample.
Early Detection and Tracking of Orthotopic Prostate Tumor Growth
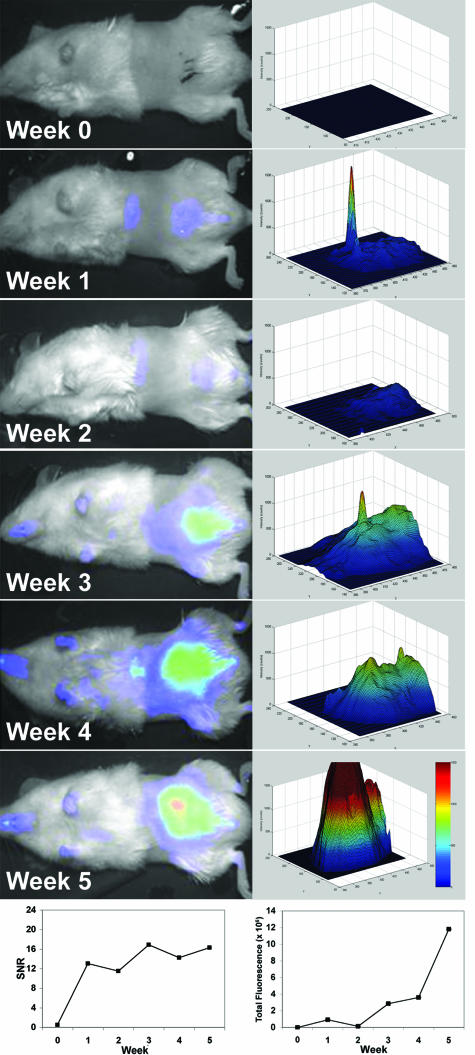
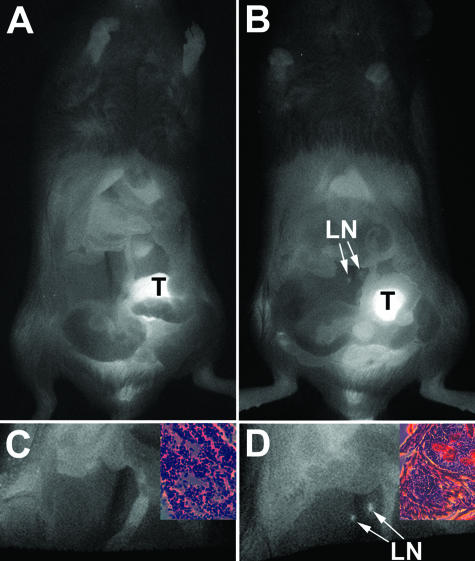
PC3M-LN4 cells have been well characterized in the orthotopic implantation model of prostate cancer progression by our laboratory and others, so the conditions for development of both large primary tumors and spontaneous lymph node metastases are established. Our first objective was to compare the growth kinetics and metastatic phenotype of these cells directly with those of untransfected 22Rv1 cells, previously uncharacterized in this model. Three days after surgical implant of tumor cells in the prostate, all animals were imaged (week 0) and subsequently injected with the targeting agent. Four days (96 hours) later, the week 1 images were collected. To track the growth of tumors in each cohort of mice, animals were reinjected weekly and all images obtained after 96 hours. A representative series of images collected on a single mouse implanted with 22Rv1 cells is shown in Figure 3 (left column). The graph at the lower left depicts the time-dependent change in SNR, which is strictly an indicator of tumor presence, for the animal shown. SNR of 3 SDs above the mean background was considered to be our limit of detection because ROIs with a lower signal failed to develop into tumors throughout time. The earliest time point at which we detected SNR >3 SD above background in orthotopic tumors was at 1 week, even though tumors were not palpable until 3 weeks. A topology plot of fluorescence intensity within the tumor area was generated for each of the weekly images (right column). The total fluorescence within an ROI encompassing the tumor was calculated for each mouse. Total fluorescence was plotted for the representative animal (bottom right) to illustrate the orthotopic growth curve. As expected, SNR did not correlate with tumor growth, because it is calculated using absolute signal intensity, which has a maximum value. However, total fluorescence above the imposed SNR limit of detection continued to indicate exponential growth.
FIGURE 3.
Longitudinal growth kinetics and statistical identification of intraprostatic tumors. Male NOD/SCID mice were injected orthotopically with PC3M-LN4 or 22Rv1 tumor cells. At day 3 after implantation of cells, animals were imaged (week 0), injected intravenously with IRDye 800CW EGF (1 nmol), and imaged again 96 hours later (week 1). To track the progress of the tumors longitudinally, mice were reinjected weekly with 1 nmol of probe and imaged 96 hours later. The left column shows the progression of a representative 22Rv1 tumor in color-enhanced fluorescence images superimposed on the white light images. SNR plotted for this animal throughout the time course of the study are shown in the bottom left. In the column on the right, total fluorescence in an ROI encompassing the tumor was quantified for each weekly image by plotting the signal intensity relative to unit area as determined using the SNR cutoff. Fluorescence intensity is color enhanced to assist visualization of differences in signal. Total fluorescence in the ROI is plotted in the lower right as a semiquantitative measure of tumor size.
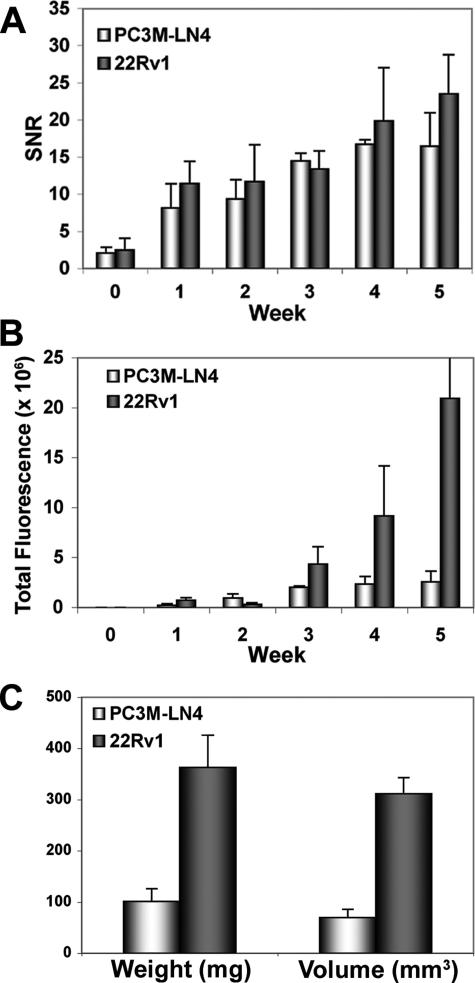
To compare the two cell lines for limits of tumor detection and predicted tumor size, SNR and total fluorescence, respectively, were calculated for all animals tracked in the study (n = 4 per cell line). The mean SNR values for each group plotted ± SEM illustrate comparable detection of tumors in each group of animals (Figure 4A). However, starting at week 3, results from fluorescence growth curves for the two cell lines (Figure 4B) predicted larger tumors in the 22Rv1-injected mice relative to PC3M-LN4 mice. By week 4, the difference in size was approximately threefold, magnified to approximately fivefold at week 5. Because normalization of EGF binding to cell number in vitro indicated only slightly higher functional expression of EGF receptor by 22Rv1 relative to PC3M-LN4 cells, we attributed the increased signal intensity to tumor size. On endpoint analysis of harvested tumors, the actual difference measured by wet weight and caliper dimensions was 3.6-fold (Figure 4C), in good agreement with prediction by total fluorescence. The strength of this correlation was subsequently pursued by similar analysis of a larger number of animals.
FIGURE 4.
Imaging and endpoint parameter comparison of PC3M-LN4 and 22Rv1 tumors. Male NOD/SCID mice bearing orthotopic PC3M-LN4 (n = 4) or 22Rv1 (n = 4) tumors were imaged weekly, and images were analyzed as described for the representative animal in Figure 3. A: SNR ± SEM for all tumors in the study is plotted by tumor type. B: Using the SNR to determine areas of the tumors by signal cutoff, total fluorescence ± SEM was plotted for all eight tumors. C: At the study endpoint, tumors were harvested, weighed, and measured in three dimensions with digital calipers. Mean weight or volume ± SEM is plotted for each group. In all graphs, white bars represent results from PC3M-LN4 tumors and dark bars are 22Rv1.
Validation of Fluorescence Imaging Parameters for Estimating Tumor Size
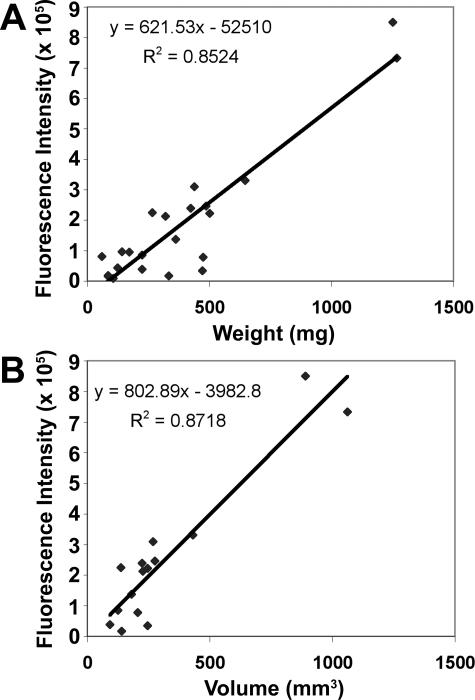
To validate the use of NIR optical imaging for the purpose of tracking orthotopic tumor growth, we analyzed images of a larger cohort of mice (18 animals in addition to the four used in the longitudinal study above) bearing 22Rv1 tumors of varied size, only some of which were palpable. These mice were sacrificed after imaging, and tumors were excised for caliper volume and weight determination. Statistical analysis of variance and linear regression were then performed to evaluate potential correlations between SNR, predicted area of tumors, area-weighted fluorescence, or total fluorescence as dependent variables and wet weight or caliper volume of tumors as independent variables (Figure 5). No correlation was found between SNR and tumor volume or weight (data not shown). Area-weighted fluorescence correlated somewhat with volume (r2 = 0.58) and wet weight (r2 = 0.66). Predicted tumor area also partially indicated both volume and weight (r2 = 0.57 for both). However, results showed strong statistical correlation between total fluorescence and either tumor wet weight (r = 0.92, r2 = 0.85, P < 0.001; Figure 5A) or caliper volume (r = 0.93, r2 = 0.87, P < 0.001; Figure 5B). This relationship is significant enough not only to predict the presence of a tumor but to track changes in its size (progression or regression) that are at least 15% of its starting size.
FIGURE 5.
Correlation of optical imaging parameters with endpoint tumor size. Excised orthotopic prostate tumors of varying sizes from a total of 22 animals were weighed and measured with standard digital calipers to determine volume. Mean fluorescence intensity was determined for the total tumor region above the incision line. Linear regression analysis was performed using total fluorescence relative to wet weight (A) or caliper volume (B).
Optical Imaging of Lymph Node Metastasis: 22Rv1 Cells Are Highly Tumorigenic but Nonmetastatic
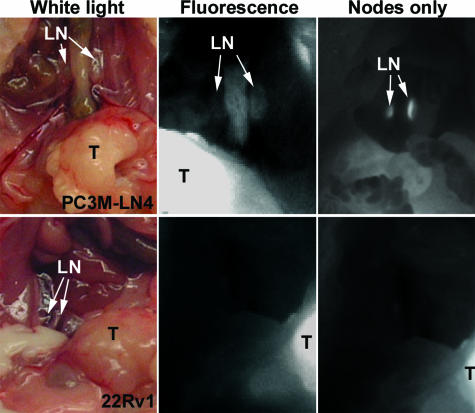
To visualize lymph nodes, mice were sacrificed and the abdominal cavity was opened to expose the prostate (Figure 6, white light images). From these images and the longitudinal analyses presented, it was evident that 22Rv1 cells were highly tumorigenic in an orthotopic model using NOD/SCID mice. Tumors were not apparently invasive, and animals inspected by whole-body necropsy were found to be macroscopically devoid of metastasis. Animals were imaged after sacrifice, with prostates intact (Figure 6, center panels), which showed clearly that the swollen lymph nodes of the PC3M-LN4-injected mice were fluorescent, although the 22Rv1-injected animals’ nodes were not. Greater sensitivity in the images and improved visualization of fluorescence in the lymph nodes was afforded by covering the primary tumors before imaging (right panels). Similar images were collected and analyzed for each mouse in the study and histological confirmation of macroscopic metastasis was performed (not shown but see Figure 8). Consistent with the representative images presented, all animals with PC3M-LN4 tumors were node-positive, and all tumor-bearing 22Rv1 animals were node-negative.
FIGURE 6.
Positive lymph node identification. Abdominal cavities of mice bearing orthotopic PC3M-LN4 (top) or 22Rv1 (bottom) tumors were opened to reveal the prostate tumors (T) and the para-aortic lymph nodes (LN). White light images (left) of positive and negative nodes were confirmed by fluorescence (center), in which primary tumors are clearly visible in both, but only the PC3M-LN4 nodes fluoresce. After excising or covering the primary tumor, sensitivity of node detection increased (right).
FIGURE 8.
Hyal1 overexpression induces metastasis to para-aortic lymph nodes. Abdominal cavities of mice bearing orthotopic 22Rv1 control (A and C) or Hyal1-overexpressing (B and D) tumors were opened and reimaged to reveal the prostate tumors (T) and the para-aortic lymph nodes (LN). Primary tumors are clearly visible in both sets of whole animal images (A and B), but node fluorescence was only detected in the animals with tumors overexpressing Hyal1. On covering the primary tumor (C and D), sensitivity of node detection increased, but control nodes remained negative (C), whereas positive nodes in the Hyal1 animals became more defined (D). Insets illustrate H&E-stained frozen sections of the respective excised lymph nodes.
Hyal1 Does Not Change Primary Tumor Growth Rate but Induces Spontaneous Lymph Node Metastasis
The ultimate goal of validating a versatile method for the detection and tracking of tumor tissue in mice was to have a way of monitoring phenotypic effects in whole animals resulting from manipulations in tumor cell gene expression. The hyaluronidase Hyal1 has been correlated with aggressive progression of human prostate cancer, and its expression in subcutaneous tumors yields a dose-responsive effect in which it promotes growth at low levels and suppresses growth at higher levels.5 Overexpression of Hyal1 in aggressive PC3M cells, another PC3-derived line, was found to modestly increase incidence of node metastasis.39 In this study, having determined that 22Rv1 cells were nonmetastatic 6 weeks after orthotopic injection, we used stable transfectants of these cells to determine whether Hyal1 expression would be sufficient to induce metastasis.
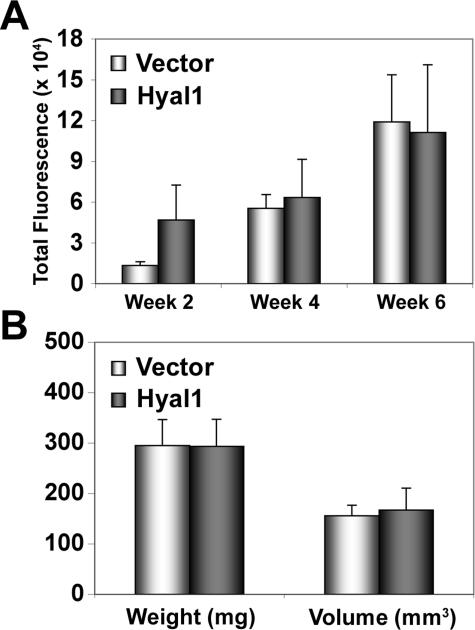
We previously selected and characterized stable 22Rv1 transfectants expressing the full-length coding sequence for Hyal1 or its expression vector control.35 Hyal1 transfectants secrete active Hyal1 into the culture media (34 ± 3.9 mU/ml for Hyal1 relative to 8.2 ± 2.4 mU/ml for control transfectants as measured by competitive binding assay) and into the extracellular space in tumors. Although the intrinsic growth rate of the cells was ∼30% faster than the controls, subcutaneous tumor size was unchanged. However, there was a significant increase in vascular density within Hyal1 tumors. To characterize the role of Hyal1 in the prostate microenvironment, we implanted the stable transfectants orthotopically. Changes in tumor size were monitored as above by optically imaging every 2 weeks after targeting agent injection. Images were analyzed in the manner described for the 22Rv1 parental line, and total fluorescence, the validated predictor of tumor size, was plotted to estimate tumor growth kinetics (Figure 7A). Although it appeared that Hyal1 tumors were approximately threefold larger than controls at week 2, by weeks 4 and 6, no differences were detectable. On sacrificing the animals, tumor weight and volume measurements confirmed virtually identical average tumor size between cohorts (Figure 7B).
FIGURE 7.
Hyal1 overexpression does not alter primary orthotopic tumor growth. Male NOD/SCID mice were injected orthotopically with 22Rv1 vector control or full-length Hyal1-transfected tumor cells. A: Animals were monitored longitudinally for tumor growth as described above, but only injected with the targeting agent, and imaged every 2 weeks as indicated. SNR cutoff was used to determine tumor area and total fluorescence, which is plotted for each cohort ± SEM as a measure of tumor growth. B: At the study endpoint, primary prostate tumors were excised to determine weight and caliper volume. Mean values ± SEM were compared and found to be nearly identical. Light bars represent 22Rv1 control tumor parameters and dark bars are 22Rv1 Hyal1 data.
Because lymph node positivity could not be confirmed noninvasively, animals were imaged again after dissection to expose the primary tumors and para-aortic nodes. Whole-animal images of control and Hyal1 animals revealed brightly fluorescent primary tumors of equivalent size (Figure 8, A and B). Some nonspecific fluorescence was also visible in the bladder and preputial gland, as verified by similar imaging of nontumor-bearing animals, but the seminal vesicles remained dark, suggesting absence of tumor invasion from the prostate. Nonetheless, the para-aortic nodes of the Hyal1 animals developed an opaque appearance relative to the transparent normal and control tumor-bearing mouse nodes. The tumor burden is most easily visualized by comparing Figure 8, C and D, in which the tumors have been covered to increase optical imaging sensitivity and the nodes appear brightly fluorescent (Figure 8D). Histological appearance of normal (Figure 8C) and tumor-infiltrated (Figure 8D) nodes are shown in the inset panels. No control tumor-bearing animals had fluorescent lymph nodes (eight of eight including the animals injected with 22Rv1 untransfected cells), whereas nine of nine animals with 22Rv1 Hyal1-overexpressing tumors were node-positive. These results, in conjunction with our previous observation that Hyal1 overexpression increased the angiogenic potential of the cells, are consistent with a role for Hyal1 in aggressive, metastatic progression of prostate cancer, despite its nominal effects on tumor growth.
Discussion
Two human prostate carcinoma cell lines, PC3M-LN4 and 22Rv1, were compared for tumorigenic and metastatic potential in orthotopic injections. 22Rv1 cells were highly tumorigenic, with statistically larger tumors forming throughout the same growth period as PC3M-LN4, previously deemed aggressive in this model. Unlike PC3M-LN4, however, 22Rv1 cells were not metastatic to retroperitoneal lymph nodes in a 5- to 7-week time period. Thus, these cells are a potential model for investigating mechanisms of metastasis initiation. Hyal1 is an enzyme previously implicated in aggressive progression of human prostate cancer. We used stable transfectants of 22Rv1 cells to investigate the role of Hyal1 in lymph node metastasis of prostate cancer. Semiquantitative volumetric estimates of orthotopic tumor growth, as well as visual identification of tumor-bearing lymph nodes, were performed and validated with receptor-targeted NIR fluorescence imaging. Longitudinal tracking and endpoint analysis of control and Hyal1-overexpressing 22Rv1 tumors revealed no differences in primary tumor growth kinetics. However, in contrast to the untransfected and control tranfected cells, Hyal1 transfected 22Rv1 cells gave rise to spontaneous retroperitoneal lymph node metastasis. These results implicate Hyal1 for the first time in the initiation of prostate tumor metastasis.
Hyaluronidases are a family of enzymes involved in extracellular matrix remodeling during normal processes such as developmental cell migration and mammalian fertilization.40–44 However, dysregulated expression of specific hyaluronidases is associated with pathological cellular motility and inappropriate vascular deposition.6,42,44,45 Histochemical analysis of human prostate cancer specimens has shown specifically that expression of Hyal1 correlates with poor patient outcome, particularly when detected concurrently with excess accumulation of its substrate, hyaluronan, in the tumors.8 Overexpression of Hyal1 in subcutaneously injected tumor cells has resolved this conundrum by demonstrating that elevated Hyal1 promotes tumor growth only to a point, beyond which high levels become deleterious to tumor growth.5 Hyal1 processes hyaluronan, which is synthesized and deposited in the extracellular space as a polymer. The processed fragments have differential effects on cells depending on oligosaccharide size. Larger oligosaccharides stimulate angiogenesis and may be more prevalent at tumorigenic concentrations of Hyal1, whereas smaller, more completely processed pieces generated by high Hyal1 levels, induce apoptosis.46,47
PC3M-LN4 cells may be metastatic in part because of increased cellular hyaluronan metabolism.37 Inhibition of hyaluronan synthesis by these cells significantly diminished the size of primary orthotopic tumors and reduced lymph node metastatic frequency. These cells also secrete detectable levels of Hyal1, whereas 22Rv1 cells synthesize very little hyaluronan and possess undetectable Hyal1 activity.35 Metastasis is a complex multigene phenomenon, but Hyal1 appears to be one factor that can influence the process if the conditions are otherwise favorable within the microenvironment. Tumors arising from 22Rv1 orthotopic injection grew very rapidly but did not metastasize unless Hyal1 was up-regulated. The Hyal1 polypeptide, independently of its enzymatic activity, could potentially influence metastasis. However, the cells expressing Hyal1 have activity at neutral pH in vitro, and tumors arising from these cells exhibit increased angiogenesis.35 Therefore, this result may indicate that Hyal1 remodeling of the extracellular matrix in the prostate provides physical conduits through which tumor cells migrate. Because very little hyaluronan is normally present in the prostate gland, Hyal1 activity would inflict little damage through production of apoptotic oligosaccharides, whose potent function could thereby favorably impact cell motility and facilitate metastasis.
In addition to the biological outcomes, a key component of this study was the development of an effective method to localize fluorescence signal in deep tumor tissue during whole animal imaging. This is particularly significant because it allows this imaging technique to be applied to preclinical models that preserve tumor microenvironments. Receptor targeting methodologies are context-sensitive, so the series of control experiments we described were essential for interpretation of outcomes, which could vary with fluorophore, biomarker, or tumor type. Here, in vivo fluorescence of the IRDye 800CW EGF targeting agent after systemic clearance was dramatically enhanced in tumors relative to the whole animals and was specific as shown by antibody blocking in subcutaneous, prostatic, and node-localized tumor tissue. By direct comparison with unlabeled EGF, we also verified that covalent conjugation to IRDye 800CW had no deleterious effect on EGF biological function, consistent with results from other investigators showing both fluorescein isothiocyanate-EGF48 and fluorescent quantum dot-EGF49 conjugates are effectively internalized by ligand-induced endocytosis of the bound EGF receptor complex. Furthermore, tumor size did not differ significantly among control and IRDye 800CW EGF-injected mouse cohorts, suggesting the concentrations of EGF used in the longitudinal experiments had negligible effects on longer term growth potentiation of tumor cells.
An additional consideration was that ligand-induced internalization of the EGF receptor could result in decreased cell surface receptor density,48 which might be expected to decrease sensitivity of the tumor fluorescence signal throughout the course of the study. However, this did not appear to interfere with efficacy of the tracking agent in vivo. Our pharmacokinetics suggest that internalization of the fluorophore likely contributes to its effective pooling in the tumor and facilitates the 72- to 96-hour retention that leads to highly sensitive SNR measurements. Clearance of the signal within a week and continued sensitivity to reinjection shows that there is ample time for the receptor to be redistributed to the membrane. Equivalent signal intensities at the endpoint of the study among animals with tumors of comparable size that received a single injection versus those that had been longitudinally injected confirmed sustained binding and uptake by tumor cells repeatedly exposed to the targeting agent.
We tracked tumor growth using total tumor fluorescence and found that signal intensity increased with tumor size. This is not simply attributable to repeat injections, because comparable signal intensity was measured in similarly sized tumors after a single injection. Furthermore, analysis of tissue sections showed uniform signal throughout the tumor. The tumor images are in two dimensions, so the increase in signal throughout time likely corresponds to an amplification of the targeting agent in three dimensions as the tumor enlarges within the abdominal cavity, and may be quantitative within a range. For example, based on indications that an SNR >3 SD above background is optimal for specific signal determination, we used this signal cutoff to calculate the area of the tumor and obtain its total fluorescence intensity, which shows good correlation to caliper measurements and wet weight of excised tumor tissue. This is probably a reasonable estimate for consistently placed intraprostatic tumors of ≤0.5 cm in diameter, and measurement of regression should be feasible by this method. However, considerable caution must be exercised in these interpretations because the planar imaging method used is less reliable at increasing tissue depths.14,16 For relatively small, superficial tumors, detected fluorescence signal increases are approximately linear with depth, as we have assumed in our calculations and validated with empirical measurement. As the size of the tumor increases, fluorescence intensity from deeper regions of the tumor is diminished in a nonlinear manner by tissue-dependent scattering of emitted photons.14,16,50 Thus, the data analysis method discussed here is applicable for tumor growth and regression quantification only within the specified parameters.
Prostate tumor growth in orthotopic models is not accessible to measurement by standard calipers, and palpation is not adequately sensitive, so we developed the optical imaging methodology described for use as a molecular caliper to monitor prostate cancer progression. Studying the impact of molecular changes within the prostate microenvironment that lead to enhanced or inhibited growth potential, or to initiation or prevention of metastasis, requires large cohorts of mice to establish significance. The use of imaging to monitor these effects longitudinally reduces the animal numbers dramatically, and animals and experimental outcomes both benefit from rapid image acquisition and minimal time under anesthesia. The level of resolution needed for reasonable monitoring of changes in tumor size throughout time is often not high enough to warrant intensive imaging by techniques such as magnetic resonance imaging or positron emission tomography/computed tomography. We have shown, in this direct comparison of prostate tumor cell lines that are differentially tumorigenic in an orthotopic model, that size differences can be reasonably approximated with fluorescence intensities from receptor-targeted fluorophore imaging. Our imaging results were validated by endpoint caliper and mass measurements of excised tumors that confirmed predicted tumor sizes.
An additional benefit of the technique is the ability to visualize and semiquantitatively analyze tumor burden in regional lymph nodes. At present, lymph node fluorescent signals are not sufficiently intense to penetrate tissue, so quantifications could only be performed in endpoint analysis. However, fluorescence imaging on dissection readily assists detection of tumor-containing tissue. Subsequent fluorescence intensity measurements can then be used to support traditional histomorphometric determinations of tumor burden, potentially in any tissue site. Because this quantification can be performed with intact tissue, the measurement represents total tumor burden, rather than an estimate extrapolated from two-dimensional evaluation of histological sections.
In conclusion, we have characterized an alternative androgen-responsive tumor cell line, 22Rv1, in an orthotopic model in which it was highly tumorigenic but did not metastasize. This observation addresses a mechanistic question of whether larger tumors more frequently metastasize in the orthotopic model. Clearly, tumorigenicity does not inherently correlate to metastatic potential, because the 22Rv1 cells grew more rapidly in the prostate than the highly metastatic PC3M-LN4 cells but failed to metastasize despite large tumor sizes. Furthermore, growth of primary tumors was identical between control and Hyal1-transfected 22Rv1 cells, but only the latter developed node metastases, demonstrating that specific molecular changes are relevant to metastatic initiation. In evaluating progression in this model, we also characterized an effective targeting agent for noninvasive optical detection and tracking of prostate tumors in mice and described computational methods that yield optical parameters indicative of tumor size, thus validating use of planar optical imaging to track orthotopic tumors and visually confirm lymph node positivity. The fluorophore used, IRDye 800CW, is sensitive and versatile and can be easily coupled to other ligands or targeting molecules of interest. Thus, this methodology for NIR monitoring of tumors in mice may be similarly applicable to surface expression of any molecular target or to longitudinal tracking of a variety of tumors.
Acknowledgments
We thank Patrick Humphreys for software consultation, and Joe Barycki, Amy Geschwender, and Mike Olive for critical evaluation of the manuscript.
Footnotes
Address reprint requests to Dr. Melanie A. Simpson, Assistant Professor, Department of Biochemistry, University of Nebraska–Lincoln, N241 Beadle Center, Lincoln, NE 68588-0664. E-mail: msimpson2@unl.edu.
Supported by the National Institutes of Health (National Cancer Institute grant R01 CA106584 and National Center for Research Resources grant P20 RR018759 to M.A.S.) and the US Army Medical Research and Materiel Command Prostate Cancer Research Program (PC030271 to M.A.S.).
References
- Hughes C, Murphy A, Martin C, Sheils O, O’Leary J. Molecular pathology of prostate cancer. J Clin Pathol. 2005;58:673–684. doi: 10.1136/jcp.2002.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin. 2005;55:300–323. doi: 10.3322/canjclin.55.5.300. [DOI] [PubMed] [Google Scholar]
- Cher ML. Mechanisms governing bone metastasis in prostate cancer. Curr Opin Urol. 2001;11:483–488. doi: 10.1097/00042307-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Ekici S, Cerwinka WH, Duncan R, Gomez P, Civantos F, Soloway MS, Lokeshwar VB. Comparison of the prognostic potential of hyaluronic acid, hyaluronidase (HYAL-1), CD44v6 and microvessel density for prostate cancer. Int J Cancer. 2004;112:121–129. doi: 10.1002/ijc.20368. [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Cerwinka WH, Isoyama T, Lokeshwar BL. HYAL1 hyaluronidase in prostate cancer: a tumor promoter and suppressor. Cancer Res. 2005;65:7782–7789. doi: 10.1158/0008-5472.CAN-05-1022. [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Lokeshwar BL, Pham HT, Block NL. Association of elevated levels of hyaluronidase, a matrix-degrading enzyme, with prostate cancer progression. Cancer Res. 1996;56:651–657. [PubMed] [Google Scholar]
- Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, Nadji M, Lokeshwar BL. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 2001;276:11922–11932. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- Posey JT, Soloway MS, Ekici S, Sofer M, Civantos F, Duncan RC, Lokeshwar VB. Evaluation of the prognostic potential of hyaluronic acid and hyaluronidase (HYAL1) for prostate cancer. Cancer Res. 2003;63:2638–2644. [PubMed] [Google Scholar]
- Rembrink K, Romijn JC, van der Kwast TH, Rubben H, Schroder FH. Orthotopic implantation of human prostate cancer cell lines: a clinically relevant animal model for metastatic prostate cancer. Prostate. 1997;31:168–174. doi: 10.1002/(sici)1097-0045(19970515)31:3<168::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, Fidler IJ. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. [PubMed] [Google Scholar]
- Sramkoski RM, Pretlow TG, II, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–409. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Gurfinkel M, Ke S, Wen X, Li C, Sevick-Muraca EM. Near-infrared fluorescence optical imaging and tomography. Dis Markers. 2003;19:107–121. doi: 10.1155/2004/474818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- Weissleder R. A clearer vision for in vivo imaging. Nature Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- Weissleder R. Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer. 2002;2:11–18. doi: 10.1038/nrc701. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Chance B. Optical method. Annu Rev Biophys Biophys Chem. 1991;20:1–28. doi: 10.1146/annurev.bb.20.060191.000245. [DOI] [PubMed] [Google Scholar]
- Citrin D, Lee AK, Scott T, Sproull M, Menard C, Tofilon PJ, Camphausen K. In vivo tumor imaging in mice with near-infrared labeled endostatin. Mol Cancer Ther. 2004;3:481–488. [PubMed] [Google Scholar]
- Ke S, Wen X, Gurfinkel M, Charnsangavej C, Wallace S, Sevick-Muraca EM, Li C. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003;63:7870–7875. [PubMed] [Google Scholar]
- Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grotzinger C. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat Biotechnol. 2001;19:327–331. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- Aina OH, Marik J, Gandour-Edwards R, Lam KS. Near-infrared optical imaging of ovarian cancer xenografts with novel alpha3-integrin binding peptide “OA02.”. Mol Imaging. 2005;4:439–447. doi: 10.2310/7290.2005.05169. [DOI] [PubMed] [Google Scholar]
- Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenografts. Cancer Res. 2004;64:8009–8014. doi: 10.1158/0008-5472.CAN-04-1956. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Wu Y, Xiong Z, Gambhir SS, Chen X. Near-infrared fluorescent RGD peptides for optical imaging of integrin alphavbeta3 expression in living mice. Bioconjug Chem. 2005;16:1433–1441. doi: 10.1021/bc0501698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston JP, Ke S, Wang W, Li C, Sevick-Muraca EM. Quality analysis of in vivo near-infrared fluorescence and conventional gamma images acquired using a dual-labeled tumor-targeting probe. J Biomed Opt. 2005;10:054010. doi: 10.1117/1.2114748. [DOI] [PubMed] [Google Scholar]
- Wang W, Ke S, Wu Q, Charnsangavej C, Gurfinkel M, Gelovani JG, Abbruzzese JL, Sevick-Muraca EM, Li C. Near-infrared optical imaging of integrin alphavbeta3 in human tumor xenografts. Mol Imaging. 2004;3:343–351. doi: 10.1162/15353500200404148. [DOI] [PubMed] [Google Scholar]
- Kraus MH, Popescu NC, Amsbaugh SC, King CR. Overexpression of the EGF receptor-related proto-oncogene erbB-2 in human mammary tumor cell lines by different molecular mechanisms. EMBO J. 1987;6:605–610. doi: 10.1002/j.1460-2075.1987.tb04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. EGF receptor as a therapeutic target. Lung Cancer. 2003;41(Suppl 1):S9–S14. doi: 10.1016/s0169-5002(03)00134-x. [DOI] [PubMed] [Google Scholar]
- Maihle NJ, Baron AT, Barrette BA, Boardman CH, Christensen TA, Cora EM, Faupel-Badger JM, Greenwood T, Juneja SC, Lafky JM, Lee H, Reiter JL, Podratz KC. EGF/ErbB receptor family in ovarian cancer. Cancer Treat Res. 2002;107:247–258. doi: 10.1007/978-1-4757-3587-1_11. [DOI] [PubMed] [Google Scholar]
- Kyritsis AP, Saya H. Epidemiology, cytogenetics, and molecular biology of brain tumors. Curr Opin Oncol. 1993;5:474–480. doi: 10.1097/00001622-199305000-00006. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo G, Tortora G, D’Armiento FP, De Rosa G, Staibano S, Autorino R, D’Armiento M, De Laurentiis M, De Placido S, Catalano G, Bianco AR, Ciardiello F. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res. 2002;8:3438–3444. [PubMed] [Google Scholar]
- Simpson MA. Concurrent expression of hyaluronan biosynthetic and processing enzymes promotes growth and vascularization of prostate tumors in mice. Am J Pathol. 2006;169:247–257. doi: 10.2353/ajpath.2006.060032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kovar J, Sissons S, Cox K, Matter W, Chadwell F, Luan P, Vlahos CJ, Schutz-Geschwender A, Olive DM. A cell-based immunocytochemical assay for monitoring kinase signaling pathways and drug efficacy. Anal Biochem. 2005;338:136–142. doi: 10.1016/j.ab.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Wilson CM, McCarthy JB. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. Am J Pathol. 2002;161:849–857. doi: 10.1016/S0002-9440(10)64245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J, Burtness B. Cetuximab: an epidermal growth factor receptor chimeric human-murine monoclonal antibody. Drugs Today (Barc) 2005;41:107–127. doi: 10.1358/dot.2005.41.2.882662. [DOI] [PubMed] [Google Scholar]
- Patel S, Turner PR, Stubberfield C, Barry E, Rohlff CR, Stamps A, McKenzie E, Young K, Tyson K, Terrett J, Box G, Eccles S, Page MJ. Hyaluronidase gene profiling and role of hyal-1 overexpression in an orthotopic model of prostate cancer. Int J Cancer. 2002;97:416–424. doi: 10.1002/ijc.1638. [DOI] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- Lin G, Stern R. Plasma hyaluronidase (Hyal-1) promotes tumor cell cycling. Cancer Lett. 2001;163:95–101. doi: 10.1016/s0304-3835(00)00669-8. [DOI] [PubMed] [Google Scholar]
- Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, Markowitz S, Willson J, Sy MS. Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1996;93:7832–7837. doi: 10.1073/pnas.93.15.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317–325. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- Mullegger J, Lepperdinger G. Degradation of hyaluronan by a Hyal2-type hyaluronidase affects pattern formation of vitelline vessels during embryogenesis of Xenopus laevis. Mech Dev. 2002;111:25–35. doi: 10.1016/s0925-4773(01)00593-7. [DOI] [PubMed] [Google Scholar]
- Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–38020. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Carraway KL, III, Cerione RA. Fluorescent-labeled growth factor molecules serve as probes for receptor binding and endocytosis. Biochemistry. 1993;32:12039–12045. doi: 10.1021/bi00096a014. [DOI] [PubMed] [Google Scholar]
- Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE, Jares-Erijman EA, Jovin TM. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol. 2004;22:198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- Zacharakis G, Kambara H, Shih H, Ripoll J, Grimm J, Saeki Y, Weissleder R, Ntziachristos V. Volumetric tomography of fluorescent proteins through small animals in vivo. Proc Natl Acad Sci USA. 2005;102:18252–18257. doi: 10.1073/pnas.0504628102. [DOI] [PMC free article] [PubMed] [Google Scholar]