Abstract
The involvement of genes controlling embryonic processes in the etiology of diseases often escapes attention because of the focus given to their inherent developmental role. Hoxa5 belongs to the Hox gene family encoding transcription factors known for their role in skeletal patterning. Hoxa5 is required for embryonic respiratory tract morphogenesis. We now show that the loss of Hoxa5 function has severe repercussions on postnatal lung development. Hoxa5−/− lungs present an emphysema-like morphology because of impaired alveogenesis. Chronic inflammation characteristics, including goblet cell hyperplasia, mucus hypersecretion, and recruitment of inflammatory cells, were also observed. Altered cell specification during lung morphogenesis triggered goblet cell anomalies. In addition, the defective motility of alveolar myofibroblast precursors in the embryonic lung led to the mispositioning of the alveolar myofibroblasts and to abnormal elastin deposition postnatally. Both goblet cell hyperplasia and elastic fiber abnormalities contributed to the chronic physiopathological features of Hoxa5−/− lungs. They constituted an attractive stimulus to recruit activated macrophages that in turn generated a positive feedback loop that perpetuated macrophage accumulation in the lung. The present work corroborates the notion that altered Hox gene expression may predispose to lung pathologies.
Lung morphogenesis relies on finely orchestrated processes that initiate from the outpocketing and the elongation of the foregut endoderm into the surrounding mesenchyme. The lung bud then branches extensively giving rise to saccules that expend and subdivide to ultimately produce alveoli. In the mouse, the main stem bronchi form at approximately embryonic day (E) 9.5, and ramification organized by signaling centers shapes the respiratory tree during the pseudoglandular period that extends from E14.0 to E16.5. At late gestational stages, saccules are found at the end of each terminus of the pulmonary tree. The last step of lung morphogenesis occurs between postnatal day (P) 5 and P30, and it aims at multiplying the respiratory surface for vital gas exchanges after birth by modifying the distal lung architecture through the process of alveogenesis. During each step of lung development, the concerted action of transcriptional regulators, signaling molecules, their associated receptors and downstream effectors governs branching morphogenesis and lung maturation by integrating cellular proliferation, differentiation, apoptosis, and migration.1–3 Proper expression of these factors is further required to maintain pulmonary homeostasis.
Impairment of any step of lung morphogenesis and maturation can cause pathologies and compromise viability. Beside premature death at birth, impaired lung development and maturation can lead to consequences similar to those observed in multiple pulmonary disorders resulting from inflicted alveolar damages, such as chronic obstructive pulmonary disease (COPD). This complex pathology stems from a chronic inflammatory process coupled to goblet cell hyperplasia and mucus hypersecretion.4,5 As well, emphysema, which is a major component of the morbidity and mortality of COPD, is defined as the enlargement of peripheral airspaces because of the destruction of alveolar septas.6 Mouse molecular genetics have provided important insights into the regulatory networks underlying lung morphogenesis and pathology. For instance, the loss of Pdgfa gene function perturbs alveolar septation, which results in lungs with an emphysema-like appearance.7 Pdgfa−/− surviving mice are missing alveolar smooth muscle cells, which contribute to elastin deposition during alveogenesis.8,9 Accordingly, the amount of lung elastin is reduced in these mutants. Elastin constitutes the most abundant extracellular matrix component in the lung, participating in the elastic fibers that allow the tissue to stretch and recoil without damage during respiration.10 Deposition of elastin by alveolar myofibroblasts is coupled to alveolar septation and critical for distal airway branching.11–13
Lung formation relies on several transcription factors including the proto-oncogene N-myc, forkhead domain proteins such as Foxa2, and homeobox-containing proteins.3,14,15 Expression of some key regulators of lung embryonic development persists through adulthood, suggesting that they may participate in postnatal alveogenesis. For instance, Foxa2 regulates several genes involved in lung morphogenesis and homeostasis, such as Nkx2.1,16 Sftpb (coding for surfactant protein B; SP-B),17 and Muc5ac (coding for mucin MUC5AC).18 Foxa2 also takes part in alveogenesis because its specific deletion in airway epithelial cells results in airspace enlargement. The loss of Foxa2 function further causes goblet cell hyperplasia and increased mucus production, pathological features commonly associated to chronic respiratory diseases in humans.18
Although much emphasis has initially been focused on their role in skeletal patterning, the Hox gene family also serves essential functions in the elaboration of organs as evidenced by the variety of defects encountered in Hox mutant mice.19 This family comprises 39 members clustered in four complexes, each located on a different chromosome. Approximately 20 Hox genes, predominantly from paralogous groups 2 to 6, are expressed in the lung, where they display a distinct spatio-temporal pattern of expression.14,20 Despite the fact that mutant mouse lines for several lung-expressed Hox genes have been generated, the consequences on respiratory tract development and function have been examined for a limited number of these. Although the Hoxa1 mutation does not affect lung development per se, it results in perinatal death caused by anoxia, a problem due to defective hindbrain patterning.21 The combined mutation of the Hoxa1 and Hoxb1 genes results in hypoplastic lungs with a reduced number of lobes but a normal histology, implying some functional redundancy between Hox1 paralogs during lung formation.22 The Hoxa3 gene participates to the patterning of the proximal airways, more specifically to the development of the larynx.23 Although no lung phenotype has been reported in Hoxb5−/− mice,24 abnormal Hoxb5 expression correlates with alterations in bronchiolar branching.25 Moreover, the HOXB5 gene was found aberrantly expressed in patients suffering from bronchopulmonary sequestration and congenital cystic adenomatoid malformation.26
The analysis of the Hoxa5 mutant mouse line has revealed the preponderant role of this gene in lung development and maturation.27,28 The loss of Hoxa5 function severely compromises viability at birth because of trachea and lung dysmorphogenesis. Moreover, the Hoxa5 mutation affects the embryonic expression of epithelial regulators of lung development and function, such as the Nkx2.1, Foxa2, and N-myc genes. Its absence also causes a decrease in surfactant protein production in newborns. Hoxa5 is still strongly expressed in postnatal lung, and surviving Hoxa5−/− adults present lung anomalies with deficient alveolar septation and atelectasis.28,29 These anatomical limitations impact on the breathing pattern of Hoxa5−/− mice that compensate for their decreased alveolar surface area available for gas exchange by an increase in breathing frequency and overall minute ventilation. In humans, aberrant HOXA5 expression has been shown to correlate with lung diseases.30 Altogether, these data unambiguously establish a critical role for Hoxa5 in lung ontogeny and point toward its implication in lung maturation and function. Here, we address the postnatal pulmonary function of the Hoxa5 gene, with the objective of deciphering the etiology of the impaired postnatal lung development in Hoxa5−/− mice.
Materials and Methods
Mouse Strains and Genotyping
The establishment of the Hoxa5 mutant mouse line in the MF1-129/SvEv-C57BL/6 mixed background has been previously described.29 Embryonic age was estimated by considering the morning of the day of the vaginal plug as E0.5. Animals were genotyped by Southern blot analysis. Hoxa5 mutant mice were intercrossed with PdgfαrGFP/+ mice (provided by Dr. Philippe Soriano, Fred Hutchinson Research Center, Seattle, WA). This mouse line carries a green fluorescent protein (GFP) allele fused to a histone 2B (H2B) moiety introduced into the Pdgfαr endogenous locus. The H2B portion allows the nuclear localization of GFP when expressed. The genotype of the PdgfαrGFP allele was determined by polymerase chain reaction.31
Tissue Collection and Histology
Wild-type and Hoxa5−/− mice were sacrificed at birth (P0), P5, P15, P31, and P60 (four wild-type and six mutant mice for each age). The body and wet lung weights were obtained by direct measurements for each specimen to determine the lung weight over the body weight ratio. Excised lungs were fixed overnight in ice-cold 4% paraformaldehyde prepared in phosphate-buffered saline. For P31 and P60 mice, lungs were fixed by tracheal instillation of ice-cold fixative until fully inflated under direct observation. Samples were processed, paraffin-embedded, and sectioned (4 μm) before staining according to standard procedures: hematoxylin and eosin (H&E) for alveolar morphology, periodic acid/Schiff for detection of mucus-producing goblet cells, Masson’s trichrome for detection of ciliated cells, and Weigert to visualize elastic fibers. All experiments were performed according to the guidelines of the Canadian Council on Animal Care and approved by the institutional animal care committee.
For GFP analysis of Hoxa5;PdgfαrGFP compound mutants, lungs from E16.5 embryos were harvested and fixed for 2 hours in ice-cold 4% paraformaldehyde. Samples were transferred in 30% sucrose and 0.2 mol/L phosphate buffer (40 mmol/L NaH2PO4, 150 mmol/L Na2HPO4) overnight at 4°C and then embedded in OCT. Cryostat sections (4 μm) were placed on poly-l-lysine-coated slides, counterstained with 10 μg/ml 4′6′-diamidino-2′-phenylindole (DAPI), and washed in phosphate-buffered saline (PBS) before mounting in aqueous media. For RNA preparation, lungs were snap-frozen in liquid nitrogen.
Proliferation, Apoptosis, and Immunohistochemical Analyses
To assess the proliferation rate, lung sections were immunostained with a rabbit polyclonal antibody against the phosphorylated histone H3 (pH3, 1/200 dilution; Upstate Biotechnology, Lake Placid, NY).32 Apoptotic cells were detected by terminal transferase (TdT) DNA end labeling.33 Cell types were revealed with the following antibodies: Clara cells with a goat polyclonal antibody reacting with rodent Clara cell secretory protein 10 (CC10, 1/400 dilution; provided by Dr. Gurmukh Singh, Veteran’s Affairs Medical Center, Pittsburgh, PA); type II pneumocytes with a rabbit polyclonal antibody against SP-B (1/1000 dilution; Chemicon International, Temecula, CA), and a goat polyclonal antibody against Foxa2 (1/500 dilution, Santa Cruz Biotechnology, Santa Cruz, CA); alveolar myofibroblasts with a mouse monoclonal anti-α-smooth-muscle actin (α-SMA) antibody (1/10,000 dilution; Sigma Chemical Co., St. Louis, MO); and alveolar macrophages with a mouse monoclonal Mac-3 antibody (1/1000 dilution; Cedarlane, Hornby, ON, Canada). A chicken polyclonal antibody recognizing the mucin MUC5AC was obtained from Dr. Samuel Ho (1/15,000 dilution; University of Minnesota, Minneapolis, MN). A goat polyclonal antibody against mouse MMP-12 was also used (1/200 dilution; Santa Cruz). For Foxa2 immunostaining, ratio of positively stained cells on the total cell number was evaluated at P31 for a minimum of nine random areas with a total of at least 900 cells per specimen on average.
Bronchoalveolar Lavages, Cytokine, and MMP Activity Measurements
Bronchoalveolar lavages were performed on 3-month-old wild-type (n = 23) and Hoxa5−/− (n = 19) mice. For each animal, trachea was cannulated, and three 1-ml aliquots of saline were flushed into the lungs, withdrawn by syringe, pooled, and centrifuged.34 Differential cell counts (a minimum of 100 cells/specimen) of bronchoalveolar lavage fluids (BALF) were performed on cytospin preparations after staining with Diff-Quik (Dade Diagnostics, Newark, DE). The levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were measured in BALF of wild-type (n = 18) and Hoxa5−/− (n = 15) animals by enzyme-linked immunosorbent assay according to the manufacturer’s directions (R&D Systems, Minneapolis, MN).
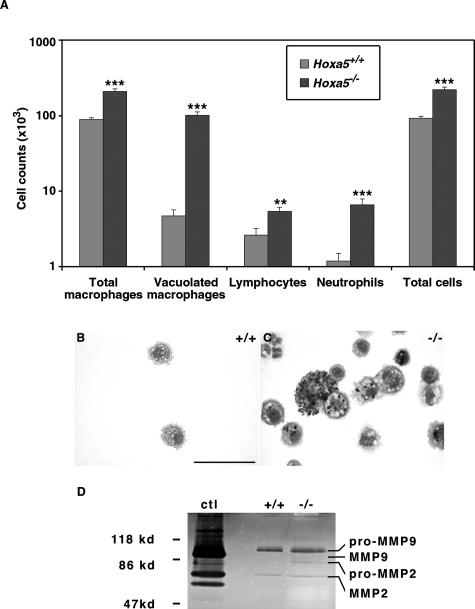
MMP activity was measured in macrophage-conditioned media isolated from adult wild-type and Hoxa5−/− mice (n = 5 per genotype) according to Wert and colleagues35 with the following modifications. After a 24-hour culture of the macrophages isolated from BALF, the conditioned media were harvested, centrifuged (100,000 × g, 1 hour), and concentrated using Amicon Ultra-4 filtration devices (Millipore, Bedford, MA). The concentrated samples were then electrophoresed under non-reducing conditions into a 10% zymogram gelatin gel. A 30-μl aliquot of conditioned medium from HT1080 cells containing MMP2 and MMP9 activities was run in parallel.36 Control gels were incubated in the presence of 20 mmol/L ethylenediaminetetraacetic acid before staining. These experiments were repeated three times, and Figure 7D is representative of the results obtained.
FIGURE 7.
Increased number of inflammatory cells and MMP activity in Hoxa5−/− BALF. A: Differential cell counts showed an increase of macrophages, lymphocytes, and neutrophils in Hoxa5−/− specimens when compared with controls. Statistically significant differences are denoted by asterisks (**P < 0.002, ***P < 0.0001). Diff-Quik staining also revealed that Hoxa5−/− BALF contained large, vacuolated macrophages with dark inclusions not seen in control samples (B, C). D: MMP activities in wild-type (+/+) and Hoxa5−/− (−/−) alveolar macrophage-conditioned media in gelatin gel zymogram. Data are presented as the reverse image of the original zymogram. Conditioned media from the HT1080 cell line served as a positive control (ctl). Slightly elevated MMP2 and MMP9 activities were observed in Hoxa5−/− alveolar macrophage-conditioned medium. Scale bar = 50 μm.
Tropoelastin Expression Analyses
Total RNA was isolated from P0, P5, P15, P31, P60, and adults (>4 months old) wild-type and Hoxa5−/− lungs (four wild-type and six mutant mice for each age) according to the TRIzol extraction protocol (Invitrogen, Carlsbad, CA). Northern blot analyses were performed using 32P-labeled cDNA tropoelastin (provided by Dr. Elaine Davis, McGill University, Montréal, QC, Canada) and GAPDH probes to evaluate and compare elastin production. Northern blots were quantified with the NIH ImageJ software (Bethesda, MD).
Wounding and Boyden Chamber Assays of Primary Embryonic Lung Mesenchymal Cells
Lungs from E15.5 embryos, obtained by timed matings of Hoxa5+/+ or Hoxa5−/− dams with Hoxa5+/−;PdgfαrGFP/+ males, were dissected and dissociated by trypsin digestion to obtain single cell suspensions (five Hoxa5+/+;PdgfαrGFP/+, four Hoxa5+/+;Pdgfαr+/+, 16 Hoxa5−/−;PdgfαrGFP/+, and four Hoxa5−/−;Pdgfαr+/+ specimens). Mesenchymal cells were separated from epithelial cells by differential adhesion37 and grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and penicillin/streptomycin until they reached confluency. Under these culture conditions, cells fully differentiated into smooth muscle cells.37,38 Cultures were then starved by replacing the medium with Dulbecco’s modified Eagle’s medium containing 0.5% fetal bovine serum for 36 hours before wounding assays. These were performed by applying a sharp razor blade to monolayer cultures.39 Plates were rinsed and low-serum medium was added. Invasion of the wounded area was followed under an inverted fluorescent microscope and pictures were taken at times 0, 12, and 24 hours after wounding. For Boyden chamber assays, primary E15.5 lung mesenchymal cells (six Hoxa5+/+;PdgfαrGFP/+, three Hoxa5+/+;Pdgfαr+/+, eight Hoxa5−/−;PdgfαrGFP/+, and four Hoxa5−/−;Pdgfαr+/+ specimens) were cultured as described above. Once confluent, cells were starved for 24 hours and then trypsinized and counted.40 A total of 1 × 105 cells were added to the upper compartment of Boyden chambers (8.0-μm pore size; Falcon; BD Biosciences Discovery Labware, Bedford, MA) that were previously coated with 0.1% gelatin. Each specimen was analyzed in triplicates. Cells were allowed to migrate for 24 hours in low-serum medium. Non-migratory cells were removed from the upper membrane surface with a cotton swab. The migratory cells were then fixed in 95% ethanol and 5% acetic acid for 10 minutes followed by Harris’s hematoxylin staining overnight. After several water washes, cells from five fields per triplicate were counted.
In parallel, primary lung mesenchymal cultures were tested for wounding assays with a 1000-μl pipette tip. After either the razor blade or the Boyden chamber assays, some cultures were subsequently plated in 10% fetal bovine serum-containing medium until they reached confluency (two Hoxa5+/+;PdgfαrGFP/+, nine Hoxa5+/+;Pdgfαr+/+, 15 Hoxa5−/−;PdgfαrGFP/+, and 13 Hoxa5−/−;Pdgfαr+/+ specimens). They were then starved for 48 hours before wounding with a 1000-μl pipette tip.41 After the scratch, plates were rinsed and low-serum medium was added. Pictures were taken at times 0, 12, and 24 hours to follow the invasion of the wounded area by the cells.
Immunofluorescence Analysis
Primary E15.5 lung mesenchymal cells were trypsinized and plated on poly-l-lysine-coated coverslips and cultured overnight in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Cells were fixed with 2% paraformaldehyde in PBS for 10 minutes, followed by one wash in PBS and 0.1% Triton and two washes in PBS. After a 10-minute incubation with blocking solution (PBS with 2% bovine serum albumin and 0.5% normal goat serum), cells were incubated with primary antibodies for 3 hours at room temperature. The Hoxa5 rabbit polyclonal and the α-smooth muscle actin mouse monoclonal antibodies were used at a dilution of 1/20042 and 1/1000, respectively. After three washes, cells were incubated with the appropriate Alexa Fluor 594 and Alexa Fluor 488 secondary antibodies and 10 μg/ml DAPI for 1 hour at room temperature. For some specimens, phalloidin-rhodamine (1/200) was added. Cells were washed and mounted in aqueous medium.
Statistical Analysis
Repeated measures for the linear mixed model were performed to assess the difference between genotypes at all stages studied when genotype is considered the fixed effect and area is the random effect. The procedure PROC MIXED from the SAS System (Cary, NC) was used.43 A Student’s t-test was performed for Foxa2 immunostaining positivity and for Boyden chamber assays.
Results
Abnormal Lung Morphology in Hoxa5−/− Mice
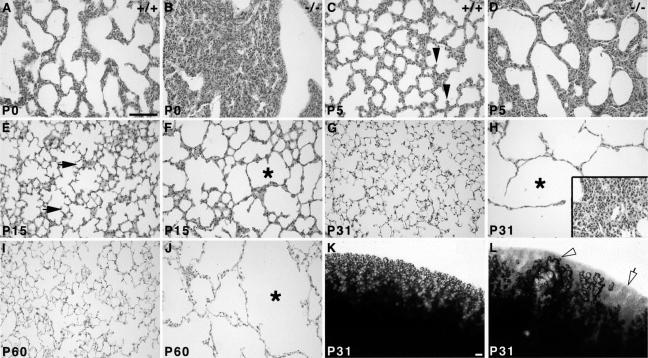
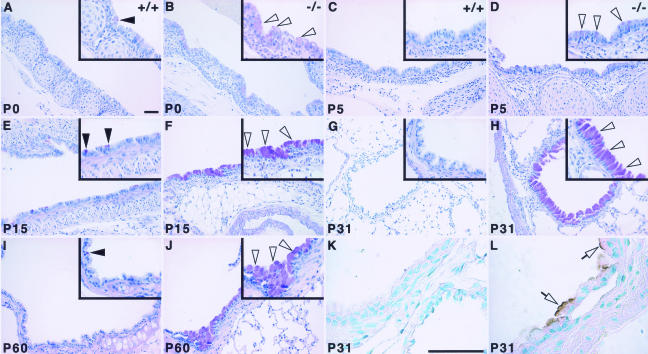
Embryonic lung development is perturbed in Hoxa5−/− mutants leading to a high rate of perinatal lethality.27 Surviving adult mutants present a severe emphysema-like phenotype characterized by enlarged airspaces and an overall decrease in septa number.28 On macroscopic examination, some areas of Hoxa5−/− lungs were completely depleted of alveoli, whereas others showed large tubular structures (shown for P31; Figure 1, K and L). To assess the origin of the morphological defects, lung histological analyses were performed at different ages after birth. At P0, wild-type lungs showed expanded respiratory saccules (Figure 1A). In contrast, Hoxa5−/− lungs were collapsed with a thickened parenchymal layer, consistent with the tragic outcome that accompanies the loss of Hoxa5 function (Figure 1B).27 At P5, growing septa were observed in wild-type lungs, whereas very few were seen in Hoxa5−/− specimens (Figure 1, C and D). At P15, only some alveoli were formed in Hoxa5−/− samples and airspace enlargement was already obvious compared with wild-type lungs (Figure 1, E and F). These alterations became more prominent with age (Figure 1, G–J). Areas of atelectasis were also detected (Figure 1H). These results suggest that the emphysema-like morphology observed in Hoxa5−/− animals originated from impaired septation, a key process in pulmonary alveogenesis. They also support the notion that Hoxa5 plays a critical role in postnatal lung development.
FIGURE 1.
Comparative histology (A–J) and macroscopic analysis (K, L) of postnatal wild-type and Hoxa5−/− lungs. At P0, mutant lungs were collapsed in contrast to wild-type specimens (A, B). Alveogenesis initiated normally at P5 in control samples (C, arrowheads), whereas in Hoxa5−/− lungs, the parenchyma was thickened and few septa were noticed (D). At P15, alveoli were readily observed in controls (E, arrows), but Hoxa5−/− lungs rather displayed enlarged airspaces (F, asterisk). At P31 (G, H, K, L) and P60 (I, J), Hoxa5−/− lungs presented an emphysema-like phenotype (H, J, L) with areas depleted of alveoli (open arrow and asterisk) or large tubular structures (open arrowhead), whereas other regions were atelectatic (inset in H) when compared with the normal lung architecture (G, I, K). Scale bars = 100 μm.
Consequences of the Hoxa5 Mutation on Pulmonary Weight, Proliferation, and Apoptosis
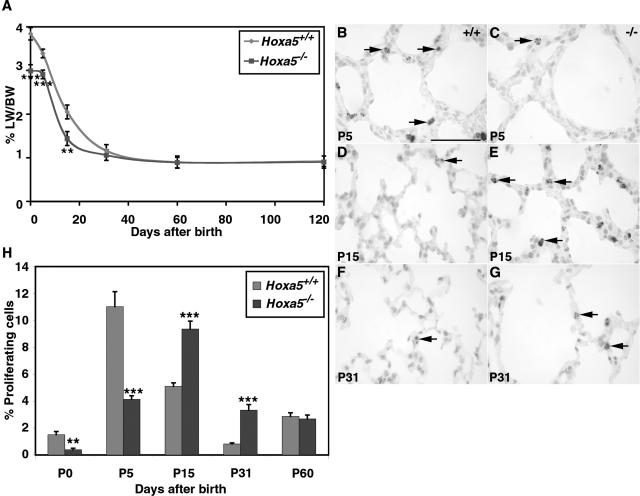
To explore the etiology of the septation defect, we first analyzed lung growth by measuring the lung weight/body weight ratio at different ages (Figure 2A). No significant difference was observed between the body weight of wild-type and Hoxa5−/− mice from P0 to adult age. At P0, P5, and P15, the ratio was statistically lower for Hoxa5−/− animals (P < 0.0075). However, the difference was resolved by P31 suggesting that recovery occurred.
FIGURE 2.
Lung weight/body weight ratio (A) and proliferation rate (B–H) of wild-type and Hoxa5−/− lungs. A: The lung weight/body weight (LW/BW) ratio of Hoxa5−/− mutants was statistically lower than that of controls at P0, P5, and P15. However, a compensatory effect was observed from P31 onwards. Detection of proliferating cells with an anti-phosphorylated histone H3 antibody (B–G, arrows) showed that proliferation was lower in Hoxa5−/− specimens compared with wild-type lungs at P0 (H) and P5 (B, C, H). At P15 (D, E, H) and P31 (F–H), more proliferating cells were detected in mutant lungs than in controls. H: No difference was observed at P60 between both groups. Statistically significant differences are denoted by asterisks (**P < 0.0075, ***P < 0.0006). Scale bar = 50 μm.
To decipher the underlying cellular process involved in the lung growth discrepancy, we examined lung proliferation and apoptosis. Compared with wild-type samples, decreased proliferation was first observed in P0 and P5 Hoxa5−/− lungs (Figure 2, B, C, and H). This was followed by increased proliferation at P15 and P31 (Figure 2, D–H). This increase could not be associated to a specific cell type. No significant difference was noted at P60 (Figure 2H). The changes in the temporal profile of proliferation in Hoxa5−/− lungs could explain the lower lung weight/body weight ratios observed at early stages and the compensatory effect seen later on.
We also investigated the contribution of apoptosis to the phenotype. Consistent with the low apoptotic rate normally observed in postnatal lungs,44 few positive cells were detected by terminal dUTP nick-end labeling (TUNEL) assay in the pulmonary tissue of both wild-type and Hoxa5−/− specimens at all stages tested (not shown). Thus, apoptosis did not contribute significantly to the delayed lung growth. These findings showed that the absence of Hoxa5 function impacts on lung proliferation during the critical period of alveogenesis without altering programmed cell death.
Altered Cell Specification in Hoxa5−/− Respiratory Tract
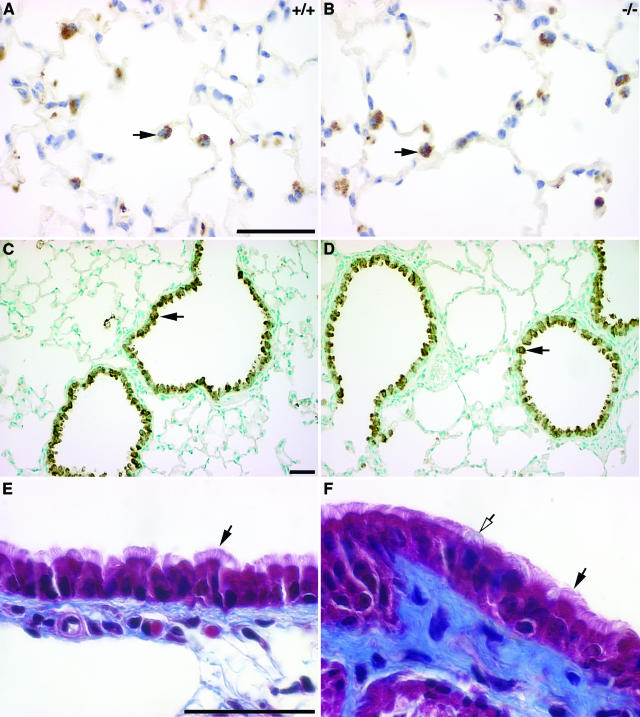
The mature respiratory tract epithelium is lined by a variety of cell types accomplishing specialized functions that guarantee proper lung homeostasis. The fact that surfactant protein production is reduced at birth in Hoxa5−/− pups,27 combined with our observations that the Hoxa5 mutation perturbs epithelial cell specification in the stomach32 prompted us to determine whether changes in cell specification occurred in the lung epithelium as well. Using SP-B as a marker, we examined type II pneumocytes, an epithelial cell type that maintains fluid homeostasis in the alveolar lumen and secretes surfactant. From P5 onwards, the number and the localization of SP-B-positive cells in the alveolar parenchyma were not altered in Hoxa5−/− lungs compared with wild-type samples (shown for P60; Figure 3, A and B). However, a secreted proteinaceous material reacting with the antibody was detected in the collapsed lungs of P0 mutants (not shown). Foxa2 immunostaining confirmed the normal distribution of type II pneumocytes in the distal lung but also revealed a small but significant increase in their number in P31 Hoxa5−/− lungs (22.25 ± 3.26% Foxa2-positive cells in wild-type versus 25.32 ± 4.91% in Hoxa5−/− specimens; P < 0.0002; not shown). This likely reflected the increased proliferation observed at this age (Figure 2H). Thus, the decreased surfactant protein production occurring in Hoxa5−/− newborns does not result from a change in cellularity but rather from reduced surfactant protein gene expression as already shown.27
FIGURE 3.
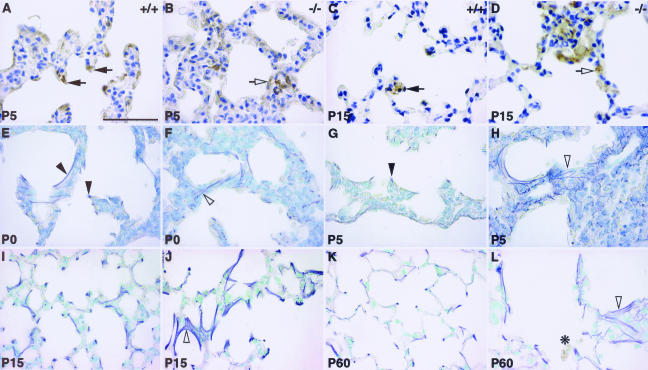
Correct specification of type II pneumocytes (A, B), Clara cells (C, D), and ciliated cells (E, F) in Hoxa5−/− lungs. No major difference was detectable between wild-type and Hoxa5−/− lungs regarding type II pneumocytes and Clara cells as shown by immunostaining with SP-B (A, B) and CC-10 (C, D) antibodies specific for these cell types, respectively (black arrows, shown for P60). Masson’s trichrome revealed that ciliated cells (black arrows) were similarly distributed in mutant (E) and wild-type lungs (F). However, mucus-producing goblet cells were more often visualized in mutant samples (F, open arrow). Scale bars = 50 μm.
Using a CC10-specific antibody, we also looked at Clara cells, a nonciliated secretory cell type normally localized along the bronchial tree and bronchioles. Clara cells were similarly detected along the respiratory bronchi and bronchioles for both genotypes from birth to P60 (Figure 3, C and D). The ciliated columnar cells and the mucus-secreting goblet cells are the major cell types lining the proximal respiratory epithelium, and they constitute the primary barrier against pathogenic agents. No major difference was noted for ciliated cells between wild-type and mutant specimens from birth to adulthood (Figure 3, E and F). However, we noticed a higher proportion of goblet cells in the epithelium of the trachea and the bronchi of Hoxa5−/− mutants, which prompted us to further examine this cell population. Although goblet cells were only sparsely found in the trachea and primary bronchi of wild-type specimens, they were dramatically increased along the trachea and the primary bronchi of mutant samples (Figure 4). Furthermore, they ectopically arose in more distal structures such as the secondary bronchi and bronchioles (Figure 4, B, D, F, H, and J). This augmentation in goblet cell number was already detected at E18.5 (not shown). From P15 onwards, the severity of the hyperplasia was salient (Figure 4, E–J). In experimentally induced mucus hypersecretion of the respiratory airways, Muc5ac gene expression is observed.45 When Hoxa5−/− samples were immunostained with the MUC5AC antibody, positive cells were readily visualized along the bronchi of the mutant lungs from P15 onwards, whereas control tissues remained negative (shown for P31; Figure 4, K and L). Thus, the loss of Hoxa5 function affects the specification of goblet cells and causes hyperplasia, metaplasia, and mucus hypersecretion.
FIGURE 4.
Goblet cell abnormal distribution in Hoxa5−/− lungs. Goblet cells were revealed by periodic acid/Schiff staining in postnatal lungs (A–J). At all ages tested, only a few positive cells were detected in wild-type samples (A, C, E, G, I; arrowheads), mostly scattered along the epithelium of the trachea (A, C) and the primary bronchi (E, I). No goblet cell was detected distally to the primary bronchi in controls (G). In Hoxa5−/− lungs, an increase in goblet cell number was systematically observed at every stage (B, D, F, H, J; open arrowheads), most obviously from P15 onwards (F, H, J). Furthermore, positive cells were detected in more distal structures such as the secondary bronchi (H) and bronchioles (not shown). Mucin MUC5AC, a marker of mucus hypersecretion, was detected by immunostaining (K, L). Although control samples remained negative (K), positive cells stained at their apical side were observed along the bronchi of Hoxa5−/− specimens from P15 onwards (shown for P31; L, open arrows). Scale bars = 50 μm.
Anomalies in Alveolar Myofibroblasts Localization and in Elastin Deposition in Hoxa5−/− Lungs
We also looked at alveolar myofibroblasts because they are a prominent player in alveoli formation. These cells are normally localized at the tip of growing septa where they synthesize and deposit elastic fibers.7 Elastogenesis is a highly regulated process that first peaks at birth and then at P10 to P14. Afterward, it declines through maturation to reach unappreciable levels in the adult mouse lung.46 The α-SMA marker was used to reveal alveolar myofibroblasts. As expected, these cells were found at the tip of the growing septa in wild-type specimens at P5 and P15 (Figure 5, A and C). However in Hoxa5−/− lungs, alveolar myofibroblasts appeared trapped in the parenchyma surrounding alveoli (Figure 5, B and D). Later on, α-SMA expression decreased in alveolar myofibroblasts rendering their presence undetectable for both genotypes (not shown).47
FIGURE 5.
Perturbed alveolar myofibroblasts localization (A–D) and elastin deposition (E–L) in Hoxa5−/− lungs. Alveolar myofibroblasts were located at the tip of the septa of control specimens at P5 and P15, as revealed by immunostaining with an α-SMA antibody (A, C; arrows). In Hoxa5−/− lungs, they were trapped within the parenchyma (B, D; open arrow). Weigert staining allowed visualization of elastic fibers (E–L). In P0 and P5 control specimens, elastic fibers were localized along the respiratory saccules and at the tip of the growing septa (E, G; arrowheads). In Hoxa5−/− lungs, elastin appeared tangled in the parenchyma (F, H; open arrowhead). At P15 onwards, Hoxa5−/− lungs displayed disorganized fibers (J, L; open arrowhead) compared with wild-type lungs (I, K). Moreover, inflammatory cells were detected in P60 mutant lungs (L, asterisk). Scale bar = 50 μm.
To determine whether the abnormal distribution of alveolar myofibroblasts observed in mutants correlated with anomalies in elastin deposition, Weigert staining was performed (Figure 5, E–L). Before birth, elastic fiber formation appeared normal in both groups (not shown). In P0 and P5 controls, elastin was deposited at the tip of the septa and along the respiratory saccules, as expected (Figure 5, E and G).8 In contrast, elastic fibers were disorganized and aberrantly distributed within the pulmonary tissue of P0 and P5 Hoxa5−/− lungs (Figure 5, F and H). From P15 onwards, the fibers appeared more abundant, disorganized, and fragmented (Figure 5, I–L). Northern analyses of tropoelastin expression failed to reveal differences in tropoelastin transcript levels between wild-type and Hoxa5−/− lungs at all ages examined that would correlate with the elastic fiber anomalies seen in mutants (not shown). Thus, Hoxa5 function is required for the correct positioning of alveolar myofibroblasts, which in turn warrants proper elastin deposition and septa formation, both being essential for normal lung architecture and function.
Inflammatory Response in Hoxa5−/− Lungs
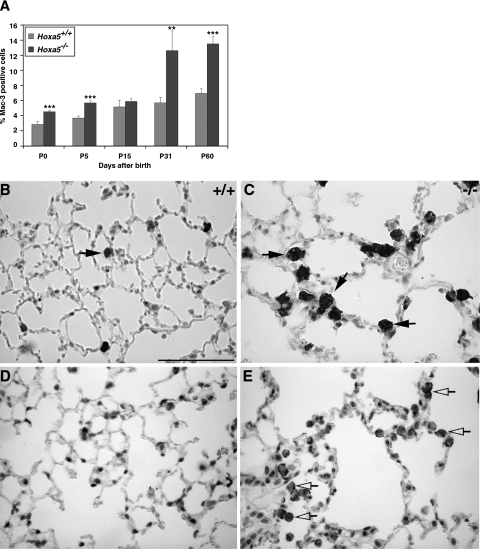
Goblet cell hyperplasia and mucus hypersecretion can be associated to the recruitment of inflammatory cells in the lung.4 Because inflammatory cells were detected in P60 Hoxa5−/− specimens (Figure 5L), we tested for the putative involvement of inflammation in Hoxa5−/− postnatal lung phenotype. Although immunostaining with a Mac-3 macrophage marker detected a few sparse positive cells in controls, a high percentage of macrophages was observed in mutant lungs (Figure 6, A–C). This increase was statistically significant from birth to adulthood, except for P15. Macrophages from Hoxa5−/− lungs also presented an atypical morphology indicative of an inflammatory response.
FIGURE 6.
Increased percentage of alveolar macrophages in Hoxa5−/− lungs. A Mac-3-specific antibody was used to detect macrophages in the lung (A–C, arrows) showing that the proportion of macrophages was statistically higher in Hoxa5−/− lungs than in controls. Asterisks denote statistical differences (**P < 0.01, ***P < 0.005). C: Moreover, at P31 (not shown) and P60, accumulation of large vacuolated macrophages was observed in Hoxa5−/− lungs. MMP-12 immunostaining revealed that the majority of macrophages in mutant samples produced this elastase (shown for P60; D and E, open arrows). Scale bar = 50 μm.
We performed bronchoalveolar lavages to further assess the importance of this inflammatory response in Hoxa5−/− lungs. Microscopic observations of recovered cells confirmed the hypertrophic and foamy morphology of the macrophages in mutant samples (Figure 7, B and C). Moreover, Hoxa5−/− BALF cell count was higher than that of controls and a statistically significant increase was observed for each inflammatory cell type identified (Figure 7A). Vacuolated macrophages constituted the predominant cell population in mutant specimens. These data confirmed that Hoxa5−/− lungs were under an inflammatory challenge.
Resident macrophages and recruited monocytes play a major role in amplifying the inflammatory response in the lower respiratory tract through the increased production of proinflammatory cytokines, such as TNF-α and IL-6.48 TNF-α is also a main participant in the activation of macrophages to produce MMPs involved in lung remodeling. The levels of TNF-α and IL-6 were measured by enzyme-linked immunosorbent assay assays in BALF. No statistically significant difference in cytokine levels was detected between both genotypes (not shown). Considering the augmentation in macrophages and the abnormal aspect of elastic fibers in Hoxa5−/− lungs, we immunostained lung sections with a specific antibody against MMP-12, a major elastolytic protease produced by activated alveolar macrophages.49 The majority of macrophages stained positively for MMP-12 in mutant samples, suggesting enhanced elastase production in Hoxa5−/− lungs (shown for P60; Figure 6, D and E). The proteinase activity present in wild-type and Hoxa5−/− alveolar macrophage-conditioned media was also directly tested by zymogram assays on gelatin gels. In the representative experiment shown in Figure 7D, 5.8 × 104 and 27.9 × 104 cells were obtained from pools of five wild-type and five Hoxa5−/− animals, corresponding to 81 and 282 μg of proteins, respectively. Because of the augmented macrophage number, the Hoxa5−/−-conditioned medium showed slightly elevated MMP9 and MMP2 activities (Figure 7D). Under these conditions, MMP12 activity was not detected. Thus, the absence of Hoxa5 function leads to an inflammatory response associated with augmented MMP production and activities.
Impaired Dispersal of Alveolar Myofibroblast Progenitors in Hoxa5−/− Lungs
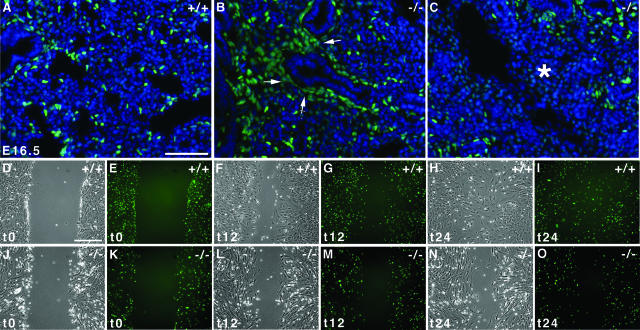
Pdgfαr-positive cells most likely constitute the progenitors of alveolar myofibroblasts responsible for elastin deposition.11 We took advantage of the PdgfαrGFP/+ mouse line carrying a GFP knocked-in allele at the Pdgfαr locus to follow the progenitor population and its behavior in Hoxa5−/− lungs.31 GFP staining faithfully reproduces the native wild-type Pdgfαr expression pattern in the PdgfαrGFP/+ mouse line and thus can serve as a lineage tracer. Before the canalicular stage and up until E15.5, clusters of Pdgfαr-positive cells occur at the distal epithelial branches. Between E16.5 and E17.5, these cells multiply and spread to acquire positions as solitary cells in the terminal sac walls. It was previously proposed that a prenatal block in the distal spreading of the alveolar myofibroblast precursors could result in alveolar septation failure.11,13 When we assessed the distribution of Pdgfαr/GFP-expressing cells in E16.5 Hoxa5+/+ lungs, they displayed the expected behavior being dispersed in the lung parenchyma (Figure 8A). In contrast, most Pdgfαr/GPF-positive cells remained clustered around the epithelium in Hoxa5−/− lungs, indicating a failure of the alveolar myofibroblast progenitors to distally spread (Figure 8B). Areas devoid of progenitors were also found, indicating their incapacity to reach certain regions of the lung (Figure 8C). These observations suggested that the Hoxa5−/− impaired alveogenesis may originate from the inability of alveolar myofibroblast progenitors to properly invade the developing lung.
FIGURE 8.
Defective motility of alveolar myofibroblast progenitors. A–C: Visualization of the GFP-positive alveolar myofibroblasts progenitors in E16.5 wild-type lungs showed that progenitors were dispersed in the lung parenchyma (A). In contrast, most Pdgfαr/GFP-positive cells remained clustered around the epithelium in Hoxa5−/− lungs, indicating a block in distal spreading of the alveolar myofibroblast progenitors (B, arrows). Progenitors also failed to invade some lung regions (C, asterisk). Sections were counterstained with DAPI in blue. D–O: Wounding assays of primary mesenchymal cells isolated from lungs of E15.5 Hoxa5+/+;PdgfαrGFP/+ (D–I) and Hoxa5−/−;PdgfαrGFP/+ (J–O) embryos after 0 (t0; D, E, J, K), 12 (t12; F, G, L, M), and 24 hours (t24; H, I, N, O). Wild-type cells invaded the wounded area within 12 hours of the assay (F, G), filling the gap by t24 (H, I). In contrast Hoxa5−/−;PdgfαrGFP/+ cells showed reduced migration at both time points (L–O). Scale bars = 50 μm.
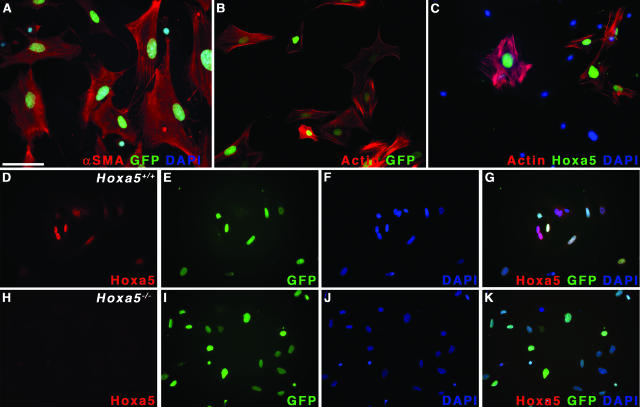
To address if a cell motility problem could underlie the defective dispersal of alveolar myofibroblast progenitors, we performed wounding assays on primary mesenchymal cells isolated from lungs of E15.5 Hoxa5+/+ and Hoxa5−/− embryos carrying or not the PdgfαrGFP allele. If the Hoxa5 mutation affects the motility of alveolar myofibroblast precursors, GFP-expressing cells should be impaired in their capacity to invade the denuded area in wounding assays. Under the culture condition applied, mesenchymal pulmonary cells differentiated into smooth muscle cells as demonstrated by the expression of α-SMA (Figure 9A) and that of GFP (Figure 8, E, G, I, K, M, and O; Figure 9, A and B).37,38 In razor blade wounding assays, four of five Hoxa5+/+;PdgfαrGFP/+ cultures displayed extensive invasion of the denuded area, whereas 11 of 16 Hoxa5−/−;PdgfαrGFP/+ cultures showed a reduced capacity to do so. This difference in behavior was also confirmed by scratch assays with a pipette tip (Figure 8, D–O). Within 24 hours, 10 of 11 wild-type samples filled the scratch (Figure 8, H and I). In contrast, 17 of 28 Hoxa5−/− cultures were impaired in wound closure (Figure 8, N and O). Migration in Boyden chambers was also tested but no difference in the migratory behavior in response to gelatin could be revealed between both genotypes (wild type: 55.9 ± 22.9 cells, n = 9; Hoxa5−/−: 61.0 ± 31.2 cells, n = 12; P = 0.07). Thus, these results established a role for Hoxa5 in mesenchymal cell motility and indicated a mechanism by which the lack of Hoxa5 function compromises the spreading of alveolar myofibroblast progenitors into the parenchyme during embryonic lung development.
FIGURE 9.
Hoxa5 protein detection in primary embryonic lung myofibroblasts. A: Mesenchymal cells isolated from E15.5 lungs adopted a myofibroblast phenotype as shown by the co-expression of Pdgf-α receptor/GFP and α-SMA. B: The actin staining observed with phalloidin was identical to that of α-SMA in GFP-positive cells. C and D: The Hoxa5 protein was readily detected in the nucleus of wild-type myofibroblasts. D, E, and G: Furthermore, GFP and Hoxa5 protein fluorescence extensively overlapped in this cell population. H–K: The Hoxa5 protein was not detected in Hoxa5−/− cell cultures. Nuclei were counterstained with DAPI (A, C, F, G, J, K). Scale bar = 100 μm.
Hoxa5 Expression in the Lung Mesenchyme
To assess whether Hoxa5 exerts a cell-autonomous effect on alveolar myofibroblast cell specification, Hoxa5 immunofluorescence analyses were performed on primary embryonic lung mesenchymal cells. The Hoxa5 protein was detected in the nuclei of embryonic lung myofibroblasts (Figure 9C), in concordance with our previous observations that Hoxa5 transcripts were restricted to the lung mesenchyme during embryonic development.27 These myofibroblasts also produced α-SMA and the Pdgfα receptor as evidenced by the GFP labeling (Figure 9, A and B). Hoxa5 immunostaining in wild-type primary myofibroblasts substantially overlapped with Pdgfα receptor expression (Figure 9, D–G). As expected, Hoxa5 was not detected in Hoxa5−/− cells (Figure 9, H–K). No expression of Hoxa5 was observed in the embryonic and postnatal lung epithelia, precluding a direct role of Hoxa5 in goblet cell specification (not shown).27 Thus, Hoxa5 could regulate the specification and the behavior of alveolar myofibroblast progenitors in a cell-autonomous manner.
Discussion
Hoxa5 Carries a Broad Spectrum of Activities during Lung Development and Maturation
Hoxa5 function is instrumental throughout lung ontogeny and maturation. During embryogenesis, Hoxa5 participates in the development of the respiratory tract where it exerts its action through the regulation of mesenchymal-epithelial interactions (J.A. and L.J., unpublished).27 After birth, Hoxa5 plays a prominent role in alveogenesis initiation and lung homeostasis because the Hoxa5 mutation causes impaired septa formation, goblet cell hyperplasia, and accumulation of inflammatory cells. The involvement of Hoxa5 in postnatal processes is also exemplified by its requirement for proper gut maturation and mammary gland development.50,51 The panoply of postnatal phenotypes displayed by Hox mutants indicates that aside from the crucial role of Hox genes in embryo patterning, they fulfill a variety of functions in adult life.19
Our study indicates that the postnatal lung defects of Hoxa5−/− mutants have an embryonic origin, pointing to altered cell specification and behavior of goblet cells and alveolar myofibroblast progenitors. Because Hoxa5 remains expressed in the adult lung,29 a definitive approach to establish the Hoxa5 postnatal role would be to conditionally ablate the gene function in the lung after birth.
Hoxa5 Impacts on Proliferation but Not Apoptosis during Alveogenesis
In Hoxa5−/− postnatal lungs, proliferation is transiently perturbed while the apoptotic rate remains unchanged. The loss of Hoxa5 function has been associated with changes in proliferation and/or apoptosis in a number of situations. As for the postnatal lung, in the mammary gland, the pectoral girdle, and the thyroid gland, proliferation but not apoptosis is affected in Hoxa5 mutants.51–53 Both processes are diminished in the adult Hoxa5−/− stomach.32 In contrast, ectopic Hoxa5 expression in the developing spinal cord increases apoptosis without changing proliferation.54 Finally, HOXA5 was proposed to control programmed cell death in certain human breast tumor cell lines.55 Because apoptosis is not influenced by the loss of Hoxa5 function in the postnatal lung, it is unlikely to contribute to the defective alveogenesis. As for proliferation, the transitory changes observed can explain the lung growth recovery, but it remains to be determined how these variations impact on lung alveogenesis. However, it cannot be excluded that inflammation may influence the proliferative response during this period. Nonetheless, Hoxa5 involvement in the regulation of proliferation and apoptosis most likely depends on the tissue-specific context and will require further examination to dissect its participation in these basic cellular processes.
Contribution of Altered Cell Specification to Impaired Alveogenesis in Hoxa5−/− Mutants
Altered cellular specification is consistently observed in tissues affected by the Hoxa5 mutation.32,51,53 In the lung, goblet cells and alveolar myofibroblasts are affected by the loss of Hoxa5 function. The overabundance of mucus-producing cells is a common feature of the Hoxa5 mutant lung, stomach, and colon phenotypes, suggesting that Hoxa5 may serve a particular function in their differentiation.32 The specific ablation of Foxa2 in the lung epithelium also results in goblet cell hyperplasia and defective alveogenesis without affecting lung morphology before birth.18 In E12.5 Hoxa5−/− lungs, Foxa2 expression is diminished, whereas in postnatal mutant lungs, the Foxa2 protein is normally detected.27 The latter observation suggests that abnormal alveogenesis seen in Hoxa5−/− lungs is a Foxa2-independent process. However, we cannot rule out the possibility that early perturbations in Foxa2 expression in the embryonic lung may have late repercussions and contribute to the Hoxa5−/− postnatal lung phenotype.
Goblet cell hyperplasia could result from lung inflammation.4 However, the presence of ectopic goblet cells secondary to an inflammatory stimulus is unlikely because the number of goblet cells is already increased in Hoxa5−/− fetal lung specimens before any macrophage recruitment (not shown). The prenatal accumulation of goblet cells rather supports the view that altered proximodistal patterning occurs in the Hoxa5−/− respiratory tract. Indeed goblet cells, which are normally restricted to the trachea and primary bronchi, are now ectopically found in more distal structures, like secondary bronchi and bronchioles. In support of this, Hoxa5 participates in the regionalization of the gut and its mutation causes the stomach to adopt intestinal-like characteristics.32 Other experimental data, such as the presence of ectopic cartilage in distal Hoxa5−/− lungs and the reduced pulmonary branching, also strengthen the hypothesis of perturbed proximodistal patterning of the lung (J.A. and L.J., unpublished).27 Altogether, these observations are in accordance with the expected role of Hox genes in providing essential cues to pattern the respiratory tract.
Alveolar myofibroblasts are also affected by the loss of Hoxa5 function. The postnatal lung phenotype of Hoxa5−/− mutants bears some similarities with that of Pdgfa−/− mutants including emphysema morphology and atelectasis.7,11,28 However, the etiology of impaired alveogenesis in Hoxa5 mutants differs from that of Pdgfa−/− mice. Alveolar myofibroblasts are lacking in Pdgfa−/− lungs, whereas they are mispositioned in Hoxa5−/− postnatal lungs. Their disappearance in the Pdgfa−/− strain, attributable to a block in proliferation and distal spreading, results in reduced elastic fiber deposition and progression to an emphysematous morphology. In Hoxa5−/− lungs, alveolar myofibroblasts are rather trapped in the parenchyma where they produce abnormal elastic fibers. Nonetheless, Pdgfa−/− and Hoxa5−/− lungs share a common defect: the subset of mesenchymal cells from which alveolar myofibroblasts originate and that expresses the Pdgfα receptor fails to spread into the distal fetal lung. Because Hoxa5 expression in myofibroblasts extensively overlaps with that of the Pdgf α receptor, this supports the notion that Hoxa5 acts cell autonomously to participate in the specification of this cell population and to influence its motility.
According to the wounding assays, Hoxa5 affects the capacity of alveolar myofibroblast progenitors to correctly progress to their appropriate position in the lung parenchyma. In Boyden chamber assays, the absence of Hoxa5 function does not impact on the migratory behavior suggesting that Hoxa5−/− lung primary mesenchymal cells can properly respond to gelatin. Hox gene involvement in migration has been previously described for endothelial cells and keratinocytes in wound healing in vivo.41,56 The Pdgfa-Pdgfαr signaling network is known to provide appropriate guiding cues that ascertain septa formation.11,13 It is tempting to speculate that Hoxa5 regulates Pdgfαr gene expression because both genes are co-expressed in the lung myofibroblasts. On the other hand, Hoxa5 may influence the outcome of Pdgf signaling. Both alternatives are not mutually exclusive and further characterization of the Hoxa5−/− lung phenotype should help to decipher this question. Altogether, these results strengthen the notion that proper specification and positioning of the alveolar myofibroblast cell population during embryonic life is critical to ensure proper alveogenesis after birth. They also identify a role for Hoxa5 in cell motility.
Contribution of Inflammatory Stimuli to Alveolar Defects in Hoxa5−/− Mutants
The increased presence of inflammatory cells in Hoxa5−/− lungs raises questions about the cause of their accumulation and their contribution to the pathological features of the lung architecture. Although in genetic mouse models of emphysema, alveolar remodeling is progressive and related to the activation of alveolar macrophages and inflammation rather than developmental abnormalities,57 the lack of alveoli formation in Hoxa5−/− lungs favors the view that the inflammatory response is secondary to developmental defects. In Hoxa5 mutants, activated macrophages are abundantly found in the lung as early as birth and most of them express MMP12. This recruitment coincides with the abnormal elastin deposition by the mispositioned alveolar myofibroblasts. Elastin fragments are chemotactic for monocytes,49 thus supporting the notion that macrophages accumulate in response to this cue. The presence of abundant activated macrophages in Hoxa5−/− lungs also correlates with a modest increase in MMP activity. In turn, this activity may generate a positive feedback loop that can perpetuate macrophage accumulation, thereby contributing to a chronic inflammatory condition.
Likewise, whereas goblet cell hyperplasia are generally observed under inflammatory conditions,4,45 the accumulation of goblet cells arises before birth in Hoxa5−/− lungs, and thus precedes any sign of inflammation. This favors the view that misspecification of goblet cells may contribute to inflammation. The onset of mucin MUC5AC production at P15, a marker specific for inflammatory conditions in mouse and human chronic lung diseases endorses the chronic inflammation affecting the Hoxa5−/− lungs.18,45
In summary, the altered cell specification resulting from the Hoxa5 mutation causes ectopic and hyperplastic goblet cells, and abnormal positioning of myofibroblasts in the postnatal lung, both factors contributing to chronic physiopathological features of the mutant lungs. The lack of formation of alveolar walls precludes the examination of the contribution of the chronic inflammation to the remodeling of the Hoxa5−/− lungs. Nonetheless, these results exemplify the importance of genetic contributions to pulmonary diseases. Understanding how Hoxa5 warrants proper normal lung development should therefore shed light on the etiology of chronic lung pathologies.
Relevance of Hox Genes to Pulmonary Diseases
The respiratory dynamics rely on the viscous and elastic properties within lung parenchyma. Any alteration of surfactant or elastic fibers can jeopardize normal pulmonary function. Hoxa5−/− mutants adapt their res-piratory behavior and present a modified breathing pattern to compensate for the emphysematous morphology.28 When parameters of respiratory mechanics are measured in Hoxa5−/− mutants, they reveal a decreased lung hysterisivity, a species-independent constant corresponding to the ratio between resistance and elastance of alveolar parenchyma (not shown).58 The change in hysterisivity in Hoxa5−/− mutants reflects the structural lung anomalies.
The Hoxa5 mutant phenotype shares similarities, without fulfilling all characteristics, of COPD, the sixth most common cause of death worldwide in 1990 that is predicted to become third by 2020.5 Yet, COPD has been relatively neglected in research on its cellular and molecular mechanisms. Environmental factors such as cigarette smoking are known to affect the susceptibility to develop COPD but genetic factors also contribute, including the inherited deficiency in α1-antitrypsin, the primary inhibitor of neutrophil elastase, and MMPs that have been identified as key players in lung remodeling in both humans and mice.49,59–63 In addition, most patients with COPD exhibit airway mucus hypersecretion and goblet cell hyperplasia that may, in a certain subsets of these patients, contribute to morbidity and mortality.4 Although the emphysema-like architecture of the Hoxa5−/− lung does not result from a structural remodeling and destruction of the alveoli with a postnatal onset, understanding the process of normal lung development is crucial for devising strategies to restore normal lung architecture and function in emphysema and lung homeostasis.64
Until recently, indirect evidence pinpoints to a potential role of Hox genes in alveogenesis as downstream effectors of the therapeutic action of retinoic acid for premature infants.65 Hoxa5 might represent one of these important effectors. Moreover, patients suffering from primary pulmonary hypertension and emphysema have augmented and decreased pulmonary HOXA5 expression, respectively, demonstrating the necessity of proper levels of Hoxa5 expression in the adult lung.30 The present work corroborates the notion that altered Hox gene expression may predispose to lung pathologies. The study of the cellular and molecular mechanisms underlying the pathological characteristics observed in Hoxa5−/− mice may provide significant insights that can translate into better therapeutic strategies.
Acknowledgments
We thank M. Lemieux for careful care of the mouse colony; F. Harel for the statistical analyses; Drs. G. Singh and S. Ho for providing the CC10 and MUC5AC antibodies, respectively; Dr. E. Petitclerc for the HT1080 conditioned media and advice; Dr. P. Soriano for the Pdgfαr mouse line; Dr. E. Davis for the mouse tropoelastin probe; and Dr. J. Hogg for insightful comments at the initial stages of this project.
Footnotes
Address reprint requests to Lucie Jeannotte, Centre de Recherche de L’Hôtel-Dieu de Québec, 9, rue McMahon, Québec, QC, Canada, G1R 2J6. E-mail: lucie.jeannotte@crhdq.ulaval.ca.
Supported by the Canadian Institutes of Health Research (grant MOP-15139 to L.J.) and the Fonds de la Recherche en Santé du Québec (Chercheur National Award to L.J.).
I.M., J.A., and M.L. contributed equally to this work.
References
- Perl A-K, Whitsett JA. Molecular mechanisms controlling lung morphogenesis. Clin Genet. 1999;56:14–27. doi: 10.1034/j.1399-0004.1999.560103.x. [DOI] [PubMed] [Google Scholar]
- Cardoso WV. Lung morphogenesis revisited: old facts, current ideas. Dev Dyn. 2000;219:121–130. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1053>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;15:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Mucus hypersecretion in chronic obstructive pulmonary disease. Chronic obstructive pulmonary disease: pathogenesis to treatment. Novartis Symposium. 2001;234:65–83. doi: 10.1002/0470868678.ch5. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Small airways in COPD. N Engl J Med. 2004;350:2635–2637. doi: 10.1056/NEJMp048102. [DOI] [PubMed] [Google Scholar]
- Snider GL. Emphysema: the first two centuries—and beyond. A historical overview, with suggestions for future. Am Rev Respir Dis Res. 1992;146:1334–1344. doi: 10.1164/ajrccm/146.5_Pt_1.1334. [DOI] [PubMed] [Google Scholar]
- Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Noguchi A, Reddy R, Kursar JD, Parks WC, Mecham RP. Smooth muscle isoactin and elastin in fetal bovine lung. Exp Lung Res. 1989;4:537–552. doi: 10.3109/01902148909069617. [DOI] [PubMed] [Google Scholar]
- Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Schwarzbauer JE. Elastic fibers; building bridges between cells and their matrix. Curr Biol. 2002;12:279–281. doi: 10.1016/s0960-9822(02)00800-x. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Karlsson L, Hellström M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- Prodhan P, Kinane TB. Developmental paradigms in terminal lung development. Bioessays. 2002;24:1052–1059. doi: 10.1002/bies.10177. [DOI] [PubMed] [Google Scholar]
- Cardoso WV. Transcription factors and pattern formation in the developing lung. Am J Physiol. 1995;13:429–442. doi: 10.1152/ajplung.1995.269.4.L429. [DOI] [PubMed] [Google Scholar]
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Shaw-White JR, Wert SE, Whitsett JA. Hepatocyte nuclear factor 3 activates transcription of thyroid transcription factor 1 in respiratory epithelial cells. Mol Cell Biol. 1996;16:3626–3636. doi: 10.1128/mcb.16.7.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Kaestner KH, Ang S-L, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveogenesis and goblet cell hyperplasia. Development. 2004;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- Aubin J, Jeannotte L. Implication des gènes Hox dans les processus d’organogenèse chez les mammifères. French. Médecine/Sciences. 2001;17:54–62. [Google Scholar]
- Takahashi Y, Hamada J, Murakawa K, Takada M, Tada M, Nogami I, Hayashi N, Nakamori S, Monden M, Miyamoto M, Katoh H, Moriuchi T. Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Exp Cell Res. 2004;293:144–153. doi: 10.1016/j.yexcr.2003.09.024. [DOI] [PubMed] [Google Scholar]
- del Toro ED, Borday V, Davenne M, Neun R, Rijli FM, Champagnat J. Generation of a novel functional neuronal circuit in Hoxa1 mutant mice. J Neurosci. 2001;21:5637–5642. doi: 10.1523/JNEUROSCI.21-15-05637.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossel M, Capecchi MR. Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development. 1999;126:5027–5040. doi: 10.1242/dev.126.22.5027. [DOI] [PubMed] [Google Scholar]
- Chisaka O, Capecchi MR. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991;350:473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Rancourt DE, Tsuzuki T, Capecchi MR. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 1995;9:108–122. doi: 10.1101/gad.9.1.108. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Vosatka RJ, Nielsen HC. Hoxb-5 control of early airway formation during branching morphogenesis in the developing mouse lung. Biochim Biophys Acta. 2000;1475:337–345. doi: 10.1016/s0304-4165(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Pham L, Lessin M, Ralston SJ, Bhan I, Cutz E, Nielsen HC. Expression of Hoxb-5 during human lung development and in congenital lung malformations. Birth Defects Res. 2003;67:550–556. doi: 10.1002/bdra.10086. [DOI] [PubMed] [Google Scholar]
- Aubin J, Lemieux M, Tremblay M, Bérard J, Jeannotte L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol. 1997;192:432–445. doi: 10.1006/dbio.1997.8746. [DOI] [PubMed] [Google Scholar]
- Kinkead R, LeBlanc M, Gulemetova R, Lalancette-Hebert M, Lemieux M, Mandeville I, Jeannotte L. Respiratory adaptations to lung morphological defects in adult mice lacking Hoxa5 gene function. Pediatr Res. 2004;56:553–562. doi: 10.1203/01.PDR.0000139427.26083.3D. [DOI] [PubMed] [Google Scholar]
- Jeannotte L, Lemieux M, Charron J, Poirier F, Robertson EJ. Specification of axial identity in the mouse: role of the Hoxa-5 (Hox1.3) gene. Genes Dev. 1993;7:2085–2096. doi: 10.1101/gad.7.11.2085. [DOI] [PubMed] [Google Scholar]
- Golpon HA, Geraci MW, Moore MD, Miller HL, Miller GJ, Tuder RM, Voelkel NF. HOX genes in human lung. Altered expression in primary pulmonary hypertension and emphysema. Am J Pathol. 2001;158:955–966. doi: 10.1016/S0002-9440(10)64042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003;11:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J, Déry U, Lemieux M, Chailler P, Jeannotte L. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development. 2002;129:4075–4087. doi: 10.1242/dev.129.17.4075. [DOI] [PubMed] [Google Scholar]
- Giroux S, Charron J. Defective development of the embryonic liver in N-myc-deficient mice. Dev Biol. 1998;195:16–28. doi: 10.1006/dbio.1997.8823. [DOI] [PubMed] [Google Scholar]
- Vachon E, Bourbonnais Y, Bingle CD, Rowe SJ, Janelle M-F, Tremblay GM. Anti-inflammatory effect of pre-elafin in lipopolysaccharide-induced acute lung inflammation. Biol Chem. 2002;383:1249–1256. doi: 10.1515/BC.2002.138. [DOI] [PubMed] [Google Scholar]
- Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA. 2000;97:5972–5977. doi: 10.1073/pnas.100448997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Koike L, Miyata Y, Hirata M, Mimaki Y, Sashida Y, Yano M, Ito A. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases-1 production and suppression of production of matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080 cells. Cancer Res. 2002;62:1025–1029. [PubMed] [Google Scholar]
- Yang Y, Palmer KC, Relan N, Diglio C, Schuger L. Role of laminin polymerization at the epithelial mesenchymal interface in bronchial myogenesis. Development. 1998;125:2621–2629. doi: 10.1242/dev.125.14.2621. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132:2157–2166. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- Tétreault M-P, Chailler P, Rivard N, Ménard D. Differential growth factor induction and modulation of human gastric epithelial regeneration. Exp Cell Res. 2005;306:285–297. doi: 10.1016/j.yexcr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Le Boeuf F, Houle F, Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Scr kinase activities. J Biol Chem. 2004;279:39175–39185. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Myers CA, Charboneau A, Young DM, Boudreau N. HoxD3 accelerates wound healing in diabetic mice. Am J Pathol. 2003;163:2421–2431. doi: 10.1016/S0002-9440(10)63597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic M, Jeannotte L, Tuggle CK. Dynamic expression of murine HOXA5 protein in the central nervous system. Gene Express Patt. 2005;5:792–800. doi: 10.1016/j.modgep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- Kresch MJ, Christian C, Wu F, Hussain N. Ontogeny of apoptosis during lung development. Pediatr Res. 1998;43:426–431. doi: 10.1203/00006450-199803000-00020. [DOI] [PubMed] [Google Scholar]
- Borchers MT, Wert SE, Leikauf GD. Acrolein-induced MUC5ac expression in rat airways. Am J Physiol. 1998;274:573–581. doi: 10.1152/ajplung.1998.274.4.L573. [DOI] [PubMed] [Google Scholar]
- Mariani TJ, Reed JJ, Shapiro SD. Expression profiling of the developing mouse lung. Insights into the establishment of the extracellular matrix. Am J Respir Cell Mol Biol. 2002;26:541–548. doi: 10.1165/ajrcmb.26.5.2001-00080c. [DOI] [PubMed] [Google Scholar]
- Jostarndt-Fögen K, Djonov V, Draeger A. Expression of smooth muscle markers in the developing murine lung: potential contractile properties and lineal descent. Histochem Cell Biol. 1998;110:273–284. doi: 10.1007/s004180050289. [DOI] [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J. 2001;18:50–59. [PubMed] [Google Scholar]
- Parks WC, Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res. 2001;2:10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J, Chailler P, Ménard D, Jeannotte L. Loss of Hoxa5 gene function in mice perturbs intestinal maturation. Am J Physiol. 1999;277:C965–C973. doi: 10.1152/ajpcell.1999.277.5.C965. [DOI] [PubMed] [Google Scholar]
- Garin É, Lemieux M, Coulombe Y, Robinson GW, Jeannotte L. Stromal Hoxa5 function controls the growth and differentiation of mammary alveolar epithelium. Dev Dyn. 2006;235:1858–1871. doi: 10.1002/dvdy.20822. [DOI] [PubMed] [Google Scholar]
- Aubin J, Lemieux M, Moreau J, Lapointe J, Jeannotte L. Cooperation of Hoxa5 and Pax1 genes during formation of the pectoral girdle. Dev Biol. 2002;244:96–133. doi: 10.1006/dbio.2002.0596. [DOI] [PubMed] [Google Scholar]
- Meunier D, Aubin J, Jeannotte L. Perturbed thyroid morphology and transient hypothyroidism symptoms in Hoxa5 mutant mice. Dev Dyn. 2003;227:367–378. doi: 10.1002/dvdy.10325. [DOI] [PubMed] [Google Scholar]
- Abbott MA, Joksimovic M, Tuggle CK. Ectopic HOXA5 expression results in abnormal differentiation, migration and p53-independent cell death of superficial dorsal horn neurons. Dev Brain Res. 2005;159:87–97. doi: 10.1016/j.devbrainres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Chen H, Chung S, Sukumar S. HOXA5-induced apoptosis in breast cancer cells is mediated by caspases 2 and 8. Mol Cell Biol. 2004;24:924–935. doi: 10.1128/MCB.24.2.924-935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace KA, Hansen SL, Myers C, Young DM, Boudreau N. HOXA3 induces cell migration in endothelial and epithelial cells promoting angiogenesis and wound repair. J Cell Sci. 2005;118:2567–2577. doi: 10.1242/jcs.02399. [DOI] [PubMed] [Google Scholar]
- Brusselle GG, Bracke KR, Maes T, D’hulst AI, Moerloose KB, Joos GF, Pauwels RA. Murine models of COPD. Pulm Pharmacol Ther. 2006;19:155–165. doi: 10.1016/j.pupt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol. 1989;67:2408–2419. doi: 10.1152/jappl.1989.67.6.2408. [DOI] [PubMed] [Google Scholar]
- Laurel C-B, Ericsson S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. Scand J Clin Lab Invest. 1963;15:132–140. [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Russell RE, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, Fitzgerald M, Barnes PJ. Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol. 2002;283:867–873. doi: 10.1152/ajplung.00020.2002. [DOI] [PubMed] [Google Scholar]
- DeMeo DL, Silverman EK. Alpha1-antitrypsin deficiency. 2: Genetic aspects of alpha(1) antitrypsin deficiency: phenotypes and genetic modifiers of emphysema risk. Thorax. 2004;59:259–264. doi: 10.1136/thx.2003.006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy RF, Okada Y, Cole R, D’Armiento J. Progressive adult-onset emphysema in transgenic mice expressing human MMP-1 in the lung. Am J Physiol. 2003;284:727–737. doi: 10.1152/ajplung.00349.2002. [DOI] [PubMed] [Google Scholar]
- Mahadeva R, Shapiro SD. Chronic obstructive pulmonary disease #3: experimental animal models of pulmonary emphysema. Thorax. 2002;57:908–914. doi: 10.1136/thorax.57.10.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinoy MR, Nielsen HC, Volpe MV. Mesenchymal nuclear transcription factors in nitrogen-induced hypoplastic lung. J Surg Res. 2002;108:203–211. doi: 10.1006/jsre.2002.6550. [DOI] [PubMed] [Google Scholar]