Abstract
The combined effects of myosin II and actin enable muscle and nonmuscle cells to generate forces required for muscle contraction, cell division, cell migration, cellular morphological changes, the maintenance of cellular tension and polarity, and so on. However, except for the case of muscle contraction, the details are poorly understood. We focus on nonmuscle myosin and actin in the formation and maintenance of hair and skin, which include highly active processes in mammalian life with respect to the cellular proliferation, differentiation, and movement. The localization of nonmuscle myosin II and actin in neonatal rat dorsal skin, mystacial pad, hair follicles, and vibrissal follicles was studied by immunohistochemical technique to provide the basis for the elucidation of the roles of these proteins. Specificities of the antibodies were verified by using samples from the relevant tissues and subjecting them to immunoblotting test prior to morphological analyses. The myosin and actin were abundant and colocalized in the spinous and granular layers but scarce in the basal layer of the dorsal and mystacial epidermis. In hair and vibrissal follicles, nonmuscle myosin and actin were colocalized in the outer root sheath and some hair matrix cells adjoining dermal papillae. In contrast, most areas of the inner root sheath and hair matrix appeared to comprise very small amounts of myosin and actin. Hair shaft may comprise significant myosin during the course of its keratinization. These results suggest that the actin-myosin system plays a part in cell movement, differentiation, protection and other key functions of skin and hair cells.
Keywords: myosin, actin, hair, skin, rat
I. Introduction
The skin of most mammals has hair that grows to a length of several millimeters and sometimes to more than 1 cm in the course of a month. This means that there are thousands of hair cells, whose diameter and height may be <10 µm, that must be generated in a hair bulb each day. The newly produced hair epidermal cells differentiate and form at least three inner root sheath layers, as well as the hair cuticle, hair cortex, and medulla in the follicle [18, 20]. This busy, active process requires the rapid replacement of basal cells by the next group of dividing cells so as to maintain a constant rate of hair growth as well as the voracious uptake not only of the necessary nutrients but also of hormone-like regulatory substances. In the skin epidermis, the spinous layer consists of amorphous cells with protrusive membrane structures [7, 16]. As the skin and hair cells that are undergoing differentiation are sandwiched between a highly proliferative cell mass and a keratinized hard wall, they require a means of resisting the resultant compression. This situation suggests that a force-generating mechanism, such as that provided by the combination of nonmuscle myosin and actin [15] to achieve cell movement, facilitate changes in cell shape, and reinforce the cytoskeleton, may be at work in the skin and hair follicles.
Recent advances in the study of the myosin superfamily have shown that it consists of at least 18 classes; one of these, the class II conventional myosin group in humans, contains the products of 16 genes, including 6 skeletal muscle, 2 cardiac muscle, 1 smooth muscle, and 3 nonmuscle myosin heavy chains [3, 9]. Herein, we focused on the nonmuscle conventional myosins, which have been suggested to be active in cytokinesis [8, 14], cell movement [1, 22], chemotaxis [2, 6], the postmitotic spreading of cells [4], cell shape [6, 15, 24], stabilization of the actin cytoskeleton [29], incorporation of sperm [23] and enucleation of erythroid cells [26]. Jazwinska et al. studied the distribution of skin and follicular antigens as they cross-reacted with antibodies raised against skeletal muscle myosin heavy chains [10]. In our investigation, we used antibodies against muscle and nonmuscle myosin heavy chains to study the localization of muscle and nonmuscle myosins in the skin and hair follicles based on the authentic antigen-antibody reactions.
II. Materials and Methods
Preparation of samples
Sprague-Dawley rats were obtained from Nippon SLC Inc. (Hamamatsu, Japan). Animals 5 to 7 days old were used for all experiments. The skin tissue used for immunoblotting was cut into small fragments in the presence of 100-fold diluted protease inhibitors (the undiluted mixture is a product of Wako Pure Chemical Industries Ltd., Osaka, Japan; code 160-19501) dissolved in phosphate-buffered saline, frozen quickly in liquid nitrogen, and stored at −80°C until use. The tissues for immunohistochemical staining were fixed in 10% formalin neutral buffer solution (Wako) for 24 hr at 4°C, dehydrated, and embedded in Shandon Histoplast paraffin (Thermo Electron Corp., Pittsburgh, PA). The blocks were sectioned at 4 µm thickness with a Leica RM2135 microtome (Leica Microsystems AG, Wetzler, Germany).
Primary antibodies
Mouse anti-chicken gizzard actin monoclonal antibody (clone C4) was purchased from MP Biochemicals Inc., Aurora, OH. Mouse anti-human skeletal muscle myosin heavy chain monoclonal antibody (clone A4) was purchased from Upstate Corp., Charlottesville, VA. Rabbit anti-human platelet myosin polyclonal antibody (BT-561) was purchase from Biomedical Technologies Inc., Stoughton, MA. C4 and A4, both consist of mouse ascites and additives, were diluted to 1:100 or 1:200 by 0.1%BSA in PBS, while BT-561 (a package of purified IgG solution) was diluted to 1:20 by the same buffer for histochemical study. For immunoblotting, C4, A4, and BT561 were diluted to 1:500, 1:500 and 1:100, respectively.
Immunoblotting
Stored frozen samples were struck with a stainless steel rod (SK200, Tokken Inc., Chiba, Japan) and crushed into powdery pieces. The temperature was kept below freezing during this operation. The powdery pieces were then heated at 95°C for 2 min in 125 mM Tris-HCl (pH 6.8), 4.3% sodium dodecyl sulfate, 10% 2-mercaptoethanol, 30% glycerol, and 0.01% bromophenol blue solution. Polyacrylamide gradient gels (4 to 20%) for electrophoresis were purchased from Daiichi Pure Chemicals Co., Ltd. (Tokyo, Japan). Immunoblotting ABC-POD kits for mice and rabbits were purchase from Wako; the experiments were performed according to the manufacturer’s manual with Wako supplies except for the blotting buffer (EzBlot), which was obtained from Atto Corp. (Tokyo, Japan). EzBlot is composed of three different buffers for anode, membrane gel, and cathode, respectively, to facilitate the transfer. We transferred the proteins to polyvinylidenefluoride (PVDF) membrane at 2 mA/cm2 for 60 min. Marker proteins were obtained from Daiichi Pure Chemicals.
Immunohistochemistry
For immunohistochemical experiments, we used Histofine Simple-Stain Rat MAX-PO (MULTI) as the secondary antibody, a product of Nichirei Co., Tokyo, Japan. The experiment was carried out according to the procedure described by the manufacturer. MAX-PO consists of a polymer conjugated with Fab secondary antibody and peroxidase. Localizations of the complex were visualized by 3-3'-diaminobenzidine (DAB). Incubation of sections with primary antibodies prepared as described before was carried out for 24 hr at 4°C and then with MAX-PO for 30 min at room temperature. Sections colored by DAB were poststained briefly with methylgreen-pyronine [17]. In addition to the immunoperoxidase staining, some sections were stained with hematoxylin and eosin for histological examinations. An Olympus BX-51 microscope equipped with a Camedia C-3040 digital camera system was used to take photographs.
Immunofluorescent staining was carried out using fluorescein-4-isothiocyanate (FITC)-conjugated donkey anti-mouse IgG antibody and Rhodamine Red-X-conjugated donkey anti-rabbit IgG antibody, which were obtained from Jackson Immunoresearch Laboratories, Inc. West Grove, PA. The former was subjected to 200- and the latter to 500-fold dilution. For nuclear counterstaining, they were then mixed with 2 µg/ml of 4',6-diamidino-2-phenylindole dihydrochloride (DAPI). The incubation with the secondary antibodies was performed for 30 min at room temperature.
III. Results
Immunoblotting
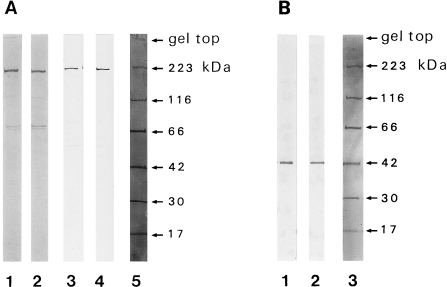
Both the anti-skeletal muscle myosin II heavy chain antibody (Fig. 1A: lane 1, 2) and anti-nonmuscle myosin II heavy chain antibody (Fig. 1A: lane 3, 4) were shown to react with proteins corresponding to the molecular weight of myosin heavy chain. Anti-actin antibody reacted with the protein corresponding to actin (Fig. 1B). Samples in Figure 1A1, A3, and B1 were obtained from the dorsal skin and those in Figure 1A2, A4, and B2 were from the mystacial pad. The reactions were shown to be largely specific, though minor bands around 70 kDa were detected in the reactions of the antibody against anti-skeletal muscle myosin heavy chain (Fig. 1A1 and A2). Significant differences in the patterns in dorsal skin versus mystacial pad samples were not observed.
Fig. 1.
Immunoblot of skin proteins reacted with antibodies against myosin and actin. A) Rat dorsal skin proteins (lanes 1, 3) or mystacial pad proteins (lanes 2, 4), obtained from the animals at postpartum day 7, were reacted with mouse monoclonal antibody (clone A4) against human skeletal muscle myosin heavy chain (lanes 1, 2) or were reacted with rabbit polyclonal antibody (BT561) against human platelet myosin heavy chain (lane 3, 4). B) Rat dorsal skin proteins (lane 1) or mystacial pad proteins (lane 2), obtained from the animals at postpartum day 7, were reacted with mouse monoclonal antibody (clone C4) against chicken gizzard actin. Lanes A5 and B3 represent the molecular weight markers (rabbit skeletal muscle myosin, 223 kDa; E. coli β-galactosidase, 116 kDa; bovine serum albumin, 66 kDa; rabbit skeletal muscle aldolase, 42 kDa; bovine erythrocyte carbonic anhydrase, 30 kDa; horse skeletal muscle myoglobin, 17 kDa).
Localization of myosin by peroxidase method
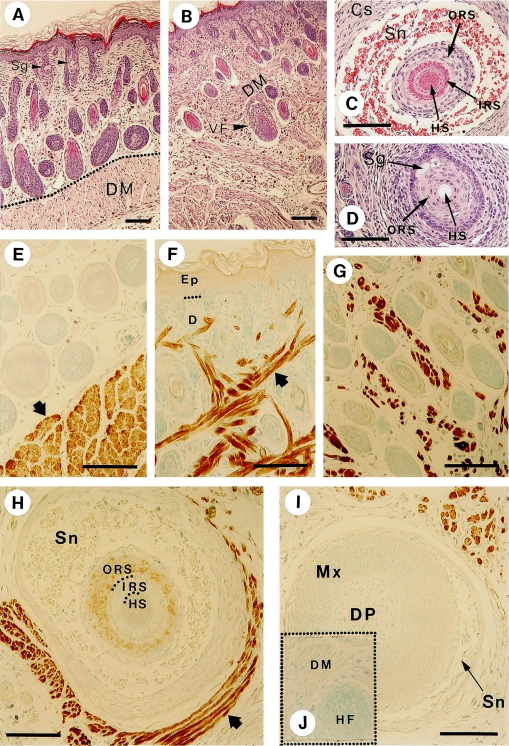
Hematoxylin-eosin staining and immunohistochemical visualization of the myosin were carried out for the sections obtained from dorsal and mystacial skin tissues. Although the epidermal structures of the ordinary hair follicle and that of the vibrissal follicle are essentially the same, the dermal tissues surrounding both types of follicles are very different [5]. The vibrissal follicle is surrounded by a highly developed sinus and capsule in the intermediate height area (Fig. 2C). These dermal tissues are reduced at the upper height area of the follicle (Fig. 2D). In the upper area, the hair shaft was not stained by hematoxylin-eosin (Fig. 2D).
Fig. 2.
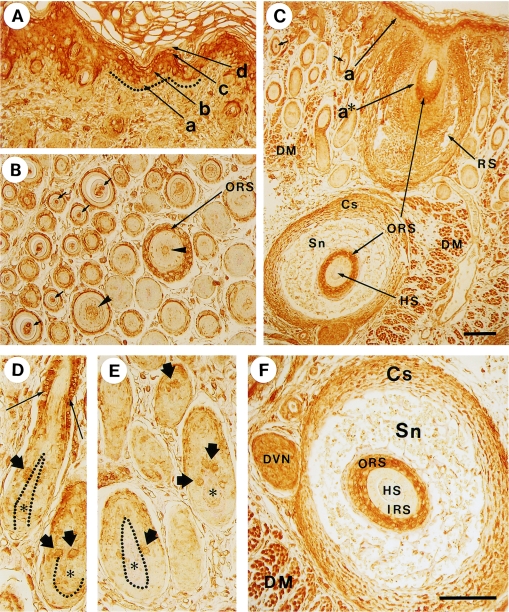
Histological views and localization of myosin in the skin and hair follicles detected by anti-skeletal muscle myosin antibody. Anti-myosin antibody clone A4 was used as the primary antibody in the immunohistochemical experiments. The results of hematoxylin and eosin staining are shown in A–D; A: dorsal skin, B: mystacial pad, C: a cross section of vibrissal follicle in the intermediate area and D: that in the upper (near-skin surface) area. The panels E–J represent the results of immunohistochemical staining; E: dorsal skin (the arrow denotes the dermal muscle arranged as a layer in the dermis). F, G: cheek skin (the arrow denotes the dermal muscle interwoven in the dermis of the mystacial vibrissal pad). H: a cross section of vibrissal follicle at the intermediate level. I: a cross section of vibrissal follicle at the level of the hair bulb. The arrow denotes the stained skeletal muscles surrounding the vibrissa follicle. J (inserted in I): a control sample of a section of vibrissal follicle processed without the primary antibody. In place of the antibody, the sample was treated with 1% bovine serum albumin. Sg, sebaceous gland; DM, dermal muscle; VF, vibrissal follicle; HS, hair shaft; IRS, inner root sheath; ORS, outer root sheath; Sn, sinus; Cs, capsule; Mx, hair matrix; DP, dermal papilla. Bars=100 µm.
Generally, rat skin consists of epidermis, dermal connective tissue, and dermal muscle, arranged from outside to inside, as shown in Figure 2A and E. This order is disrupted in the mystacial pad, where the dermal muscle invades the dermal connective tissue area up to just beneath the epidermis, as detected by the antibody against skeletal muscle myosin (Fig. 2F and G) in addition to the histological view (Fig. 2B). The vibrissal follicle is surrounded by a skeletal muscle (not arrector pili), which is reserved for the voluntary movement of the whiskers (Fig. 2H). Although cells of the outer root sheath in Figure 2H are faintly stained, we have not determined whether or not this is due to the cross-reactivity of the antibody. Generally other vibrissal follicle cells were not stained for skeletal myosin (Fig. 2H and I). In the inserted Figure 2J in Figure 2I as well as Figure 3A, B represents control experiments without the addition of primary antibodies. Non-specific staining was not observed except that at the bottom end of the epidermal cornified layer (Fig. 3A).
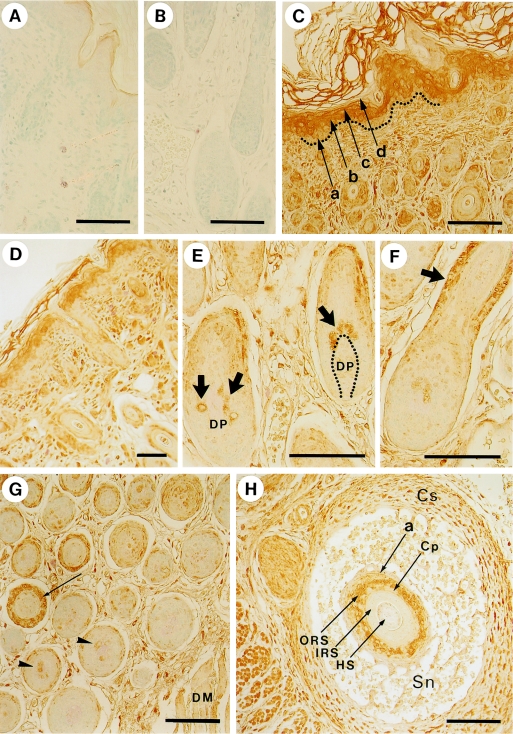
Fig. 3.
Localization of myosin in the skin and hair follicles detected by an anti-non-muscle myosin antibody. A, B) Control experiments in which the primary antibody was replaced by 1% bovine serum albumin. Samples were obtained from dorsal skin. C) Dorsal skin. In the epidermis of dorsal skin, a, b, c, and d represent the basal, spinous, granular, and innermost cornified layer, respectively. Dotted line indicates the basement membrane. D) Face skin. E) Hair bulbs in dorsal skin. The arrows show positive hair matrix cells adjacent to the dermal papilla (DP). F) Hair follicle in dorsal skin. The arrow denotes the positive staining of the outer root sheath. G) Middle (left side) and inner (right side) areas of dermis in dorsal skin. Each cross section of hair follicle in the middle area has a ring of positive staining in the outer root sheath (arrow), whereas that in inner area, which is adjacent to the dermal muscle (DM), is negative. Some of the hair matrix cells are stained (arrowheads). H) An oblique section of vibrissal follicle. Cs, capsule; Sn, sinus; a, border between epidermis and dermis, equivalent to the basement membrane; ORS, outer root sheath; Cp, companion layer; IRS, inner root sheath; HS, hair shaft. Bars=100 µm.
Figure 3C shows that the nonmuscle myosin II is located in the spinous (arrow b) and granular (arrow c) layers of the epidermis, while it appears to be scarce in the proliferative basal layer (arrow a). These results suggest that myosin II may participate in forming a stratified structure of the epidermis as a component of the cellular membrane cortex and/or cytoskeleton, which probably provides sealing and strength to the skin. The signal of myosin II appears to be eliminated when the cells enter into the keratinization stage (Fig. 3C and D).
Compared with the hair follicle cells, both interfollicular dermal cells and epidermal cells give a strong signal of myosin II (Fig. 3D). Nevertheless, some cells in the hair follicle were stained. One group of these stained cells was observed in the hair bulb, as indicated by the thick arrows in Fig. 3E and the arrowheads in Fig. 3G. These cells were generally adjacent to the dermal papillae. The other group of cells is located around the middle height of the outer root sheath (Fig. 3F, thick arrow; 3G, thin arrow; and 3H, thin arrow ORS). In addition to these groups, the peripheral area (corresponding to the hair cuticle) of the hair shaft may be stained specifically (Fig. 3H, arrow HS). This is further investigated by immunofluorescence staining and the result was confirmed as shown in Figure 7; the details are mentioned in a latter part of this paper. The companion layer was not necessarily stained as shown by the arrow Cp in Figure 3H. Nonmuscle myosin II was distributed widely in both epidermal and dermal tissues, including skeletal muscle [15, 21, 27] (Fig. 3). It is interesting to note that most of the basal layer of skin epidermis, hair matrix, dermal papillae, hair cortex, and inner root sheath exhibited a very low signal level for nonmuscle myosin II.
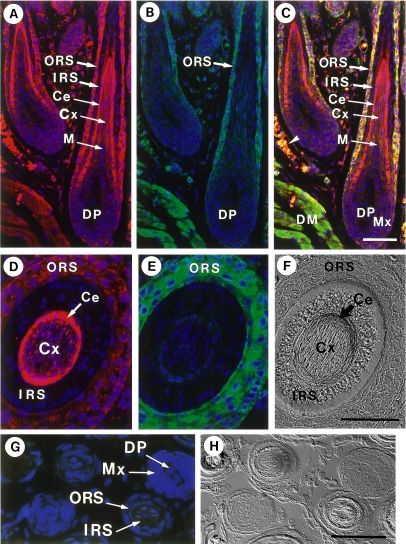
Fig. 7.
Immunofluorescence localization of non-muscle myosin in hair cuticle. Samples were stained for non-muscle myosin (red) (A, E) and actin (green) (B, F). Merged images are shown in C. Nuclei were stained with DAPI (blue). Corresponding Nomarski images are also shown (F). ORS, outer root sheath; IRS, inner root sheath; Ce, hair cuticle; Cx, hair cortex; M, medulla; Mx, hair matrix; DP, dermal papilla; DM, dermal muscle. A–C) A section of hair follicles prepared from the dorsal skin. An arrowhead in C designates autofluorescence of erythrocytes. D–F) A section of vibrissa follicle. G) Immunohistochemical control. A section was processed in the absence of primary antibodies. Merged images are shown in G). H) is a Nomarski image corresponding to G). Bars=50 µm.
Localization of actin by immunoperoxidase method
The localization of actin in the skin, hair follicles, and dermal tissues was found to be essentially the same as that of nonmuscle myosin II (Fig. 4). Namely, the following areas were stained: spinous and granular layers of the epidermis (Fig. 4A and C), some undetermined cells of the hair matrix (Fig. 4D and E, thick arrows), outer root sheath of the hair follicle (Fig. 4B thin, long arrow ORS; 4C, thin, long arrows ORS; and 4D, thin arrows), interfollicular dermal cells, and dermal muscle (for example, Fig. 4C and F). As shown in Figure 4F, the distribution of actin is not uniform in capsule cells. The outermost cells exhibited dense staining, though its significance is not known. The staining of the basal layer of epidermis (Fig. 4A, arrow a) was faint, whereas the spinous and granular layers were stained distinctly, as in the reaction with the anti-nonmuscle myosin antibody (Fig. 3C). Small arrows and arrowheads in Fig. 4B designate the staining of the hair shaft.
Fig 4.
Localization of actin in skin and hair follicles. Results of control experiments were the same with those shown in Figure 4. The magnification of A, B, D, E and F are the same. Bars=100 µm. A) In the epidermis, a, b, c, and d represent the basal, spinous, granular, and innermost keratinized layer, respectively. B) Middle (left side) and inner (right side) areas of dermis in dorsal skin. ORS, outer root sheath. Small arrows and arrowheads denote the hair shafts including positively-stained medulla. C) Facial skin and vibrissal follicle. The basal layer of mystacial skin (a), and its extension surrounding the vibrissa (a*) are shown. RS, ring sinus; DM, dermal muscle; ORS, outer root sheath; Cs, capsule; Sn, Sinus; HS, hair shaft. D, E) The positive cells of hair matrix (thick arrows) and middle height outer root sheath (thin arrows) are seen. The border between hair matrix and dermal papilla (*) is indicated by dotted line. F) An oblique section of mystacial vibrissal follicle sectioned at the intermediate height. Cs, capsule; Sn, sinus; ORS, outer root sheath; IRS, inner root sheath; HS, hair shaft; DVN, deep vibrissal nerve; DM, dermal muscle.
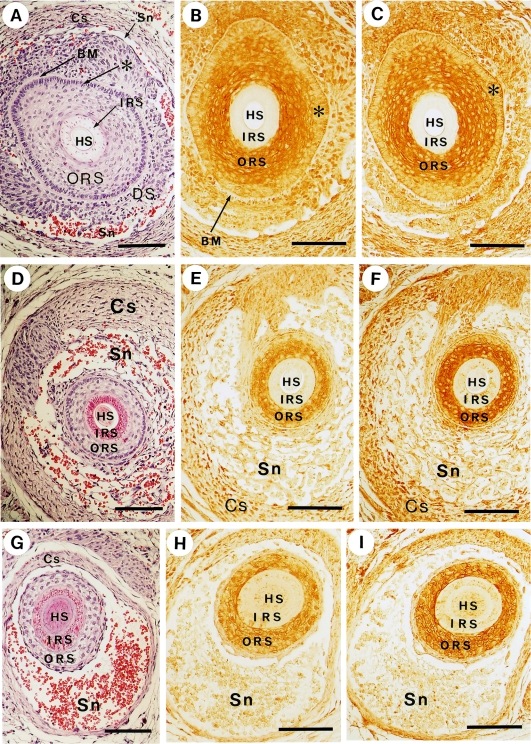
The infundibular area of the hair follicle is surrounded by a sheath connected to the basal layer of the epidermis. This monolayer sheath (or extended basal layer, Fig. 5A, asterisk) was clearly distinguishable from the outer root sheath, because the former is highly sensitive to hematoxylin staining. The peroxidase staining of this area by both anti-myosin and anti-actin antibodies was indistinct as compared with that of the outer root sheath (Fig. 5B and C, compare the areas of * and ORS) and was surrounded by an intercellular space corresponding to the basement membrane (Fig. 5A and B, BM). For both antibodies, the hair shaft and inner root sheath were not stained (Fig. 5B and C), though they were slightly reactive in lower areas (Fig. 5H and I). In the lower areas, endothelial cells, sinus, and capsule envelop the follicle (Fig. 5D and G) and the extended basal layer becomes indistinguishable in the lowest section (Fig. 5G). The capsule appears to be stained significantly (Fig. 5E and F).
Fig. 5.
Co-localization of non-muscle myosin and actin in the outer root sheath. Bars=100 µm. Panels A, D and G show sections of vibrissal follicles stained with hematoxylin-eosin. Panels B, E, H and panels C, F, I represent immunostaining using anti-myosin antibody (BT561) and anti-actin antibody (C4) respectively. Each set of panels, (A, B, C), (D, E, F) and (G, H, I), consists of adjacent sections. HS, hair shaft; IRS, inner root sheath; ORS, outer root sheath; BM, basement membrane; DS, dermal sheath; Sn, sinus; Cs, capsule; *, the extension of the epidermal basal layer.
Colocalization of myosin and actin by immunofluorescence method
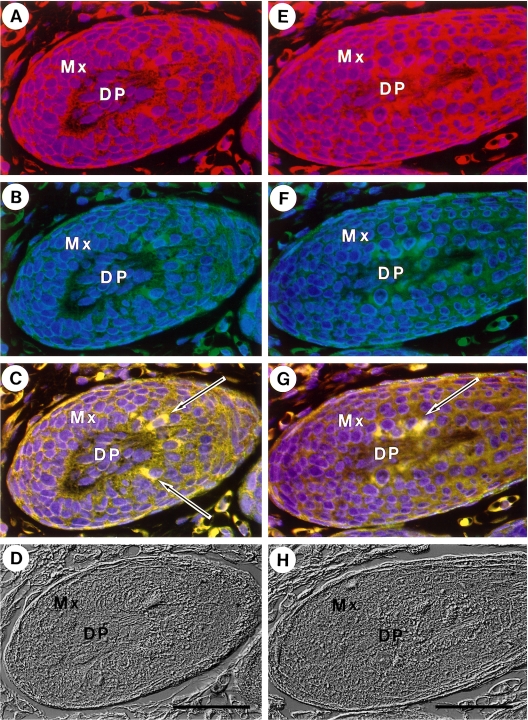
The cells in the hair matrix stained with anti-nonmuscle myosin antibody (red) were shown to be identical to those stained with anti-actin antibody (green) as shown in Figure 6. Arrows in C and G denote the special cells rich in both nonmuscle myosin and actin (yellow) by overlapping Figure 6A, B, and Figure 6E, F, respectively. At present, the function of these cells is unknown. Most of the other cells in the hair matrix were not stained distinctly by either antibody. By Nomarski observation, the outer root sheath in the hair bulb was distinguished from the matrix cells (Fig. 6D and H).
Fig. 6.
Co-localization of non-muscle myosin and actin in special hair matrix cells. Immunofluorescence staining. Panel A–D and panel E–H are the results obtained from a single section. Samples were stained for non-muscle myosin (red) (A, E) and actin (green) (B, F). Merged images are shown in C and G. Nuclei were stained with DAPI (blue). Corresponding Nomarski images are also shown (D, H). Bars=50 µm.
Figures 7A and D show the staining of hair cuticle (Ce) by anti-myosin antibody. Interestingly this tissue is not stained with anti-actin antibody (Fig. 7B and E). Double-labeling for myosin and actin showed that the hair cuticle was rich in myosin but scarce in actin (Fig. 7C). This observation makes for a marked contrast to the outer root sheath and dermal muscle (Fig. 7C), where both myosin and actin were abundant. The staining for actin in the hair cortex was weak (Fig. 7A). This may suggest possible participation of myosin in the keratinization of the hair shaft without the aid of actin. Nomarski microscopy could distinguish between hair cortex, hair cuticle, inner root sheath (three layers) and outer root sheath (Fig. 7F).
IV. Discussion
The anagen hair follicle is a minute organ that contains many proliferating and differentiating cells. Hair follicles of rats start to grow from several days before birth, and essentially all of them are in the anagen phase at postpartum days 5 to 7. The anagen hair follicle is thus an excellent model for the observation of the morphological processes of cellular differentiation because most cells accumulate in an orderly fashion according to their particular stage of maturation [7, 18]. The cells of the keratinized hair shaft and inner root sheath are apparently elevated passively by the pressure of cell division from underneath. On the other hand, the cells of the hair bulb, outer root sheath, and dermal papillae are thought to move on their own initiative. Our main object was to study the distributions of nonmuscle myosin and its partner, actin, in the hair follicle. For comparative study, skin epidermis and vibrissal follicles were investigated in parallel. The distribution of skeletal muscle myosin was also studied as a reference.
Nonmuscle myosin II is known to be widely distributed in species and tissues [3, 9, 11, 13] and seems to function in several types of cells [2–4, 6–8, 22, 24, 25, 28]; however, useful information about myosin and actin in skin/hair is not available. Cross reactions between anti-skeletal muscle myosin antibodies and follicular proteins were reported by Jazwinska et al., with varying results depending on the antibodies used [10]. Since their experimental strategy would not be appropriate to obtain definitive results, it is difficult to make a direct comparison between their results and ours.
We explain our experimental results as follows: 1) The colocalization of myosin and actin in the spinous layer, as found in the present study, is consistent with the reported results suggesting that protrusion and retraction of lamellipodia/filopodia are correlated with the dynamism of the myosin and actin molecules [24, 25]. Myosin and actin in the granular layer may be the residues which are slated to disappear in the innermost part of the cornified layer. 2) In contrast to the cells of inner root sheath and hair shaft, those of the outer root sheath undergo cell division at a variety of heights. When the follicle grows downward, the cells of the outer root sheath must move toward the subcutaneous area. The outer root sheath has also been suggested to be the path of stem cells that are known to move upward and downward in the hair follicle [19], while the outer root sheath itself grows in both upward and downward directions depending on its height [12]. Since outer root sheath cells do not undergo terminal keratinization, they are expected to move voluntarily. It is therefore natural that myosin and actin, which seem to be required for cell migration [1, 22], are found to be localized in the outer root sheath. 3) Since myosin- and actin-rich hair matrix cells are usually adjacent to the dermal papillae, they may play a role in the input and output of nutritional substances and/or hormone-like factors. It is also possible that they are on the verge of moving toward a more distal niche of cell division. Another possibility is that they are melanocytes that have invaded the follicle. Further investigations are needed to verify these views. 4) It is of interest to note that hair cuticle was stained for myosin but not for actin. This suggests that myosin may participate in the distinctive keratinization of the hair cuticle [18]. For example, it may be correlated with the assembly of keratin molecules. 5) The dermal papilla cells are suggested to serve a special cytoskeletal function when they enter into the hair bulb, because they undergo drastic morphological change at that point [18]. But once they have succeeded in invagination, they appear to be relaxed. Therefore, we speculate that this might be the reason why the accumulation of myosin and actin was not observed in most cells of the dermal papillae. 6) Actively dividing cells in the basal layer of skin epidermis as well as those in the hair matrix seem to have meager amounts of actin and myosin. Although these proteins are components of the contractile ring, their quantities may be insignificant in comparison to those associated with the cytoskeleton and cortical skeletal structures.
V. Acknowledgment
We thank Dr. T. Matsuzaki (Shimane University), Dr. M. Morioka (University of Tokyo) and Dr. H. Takano-Ohmuro (Musashino University) for their helpful discussions.
VI. References
- 1.Bastian P., Lang K., Niggemann B., Zaenker K. S., Entschladen F. Myosin regulation in the migration of tumor cells and leukocytes within a three-dimensional collagen matrix. Cell. Mol. Life Sci. 2005;62:65–76. doi: 10.1007/s00018-004-4391-6. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ya’acov A., Ravid S. Epidermal growth factor-mediated transient phosphorylation and membrane localization of myosin II-B are required for efficient chemotaxis. J. Biol. Chem. 2003;278:40032–40040. doi: 10.1074/jbc.M306948200. [DOI] [PubMed] [Google Scholar]
- 3.Berg J. S., Powell B. C., Cheney R. E. A millennial myosin census. Mol. Biol. Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer L. P., Mitchison T. J. Myosin is involved in postmitotic cell spreading. J. Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebara S., Kuramoto K., Matsuura T., Mazurkiewicz J. E., Rice F. L. Similarities and differences in the innervation of mystacial vibrissal follicle-sinus complexes in the rat and cat: A confocal microscopic study. J. Comp. Neurol. 2002;449:103–119. doi: 10.1002/cne.10277. [DOI] [PubMed] [Google Scholar]
- 6.Even-Faitelson L., Rosenberg M., Ravid S. PAK1 regulates myosin II-B phosphorylation, filament assembly, localization and cell chemotaxis. Cell. Signal. 2005;17:1137–1148. doi: 10.1016/j.cellsig.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E. Beauty is skin deep: the fascinating biology of the epidermis and its appendages. Harvey Lect. 1998;94:47–77. [PubMed] [Google Scholar]
- 8.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 9.Golomb E., Ma X., Jana S. S., Preston Y. A., Kawamoto S., Shoham N. G., Goldin E., Conti M. A., Sellers J. R., Adelstein R. S. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J. Biol. Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- 10.Jazwinska A., Ehler E., Hughes S. M. Intermediate filament-co-localized molecules with myosin heavy chain epitopes define distinct cellular domains in hair follicles and epidermis. BMC Cell Biol. 2003;4:10. doi: 10.1186/1471-2121-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsuragawa Y., Yanagisawa M., Inoue A., Masaki T. Two distinct nonmuscle myosin-heavy-chain mRNAs are differentially expressed in various chicken tissues. Eur. J. Biochem. 1989;184:611–616. doi: 10.1111/j.1432-1033.1989.tb15057.x. [DOI] [PubMed] [Google Scholar]
- 12.Kayama H., Iida M., Ihara S., Matsuzaki T. Origin of the outer layers of the mouse vibrissal hair bulb. Abstract of the 28th Annual Meeting of the Molecular Biology Society of Japan. 2005. [Google Scholar]
- 13.Korn E. D., Hammer J. A. Myosins of nonmuscle cells. Ann. Rev. Biophys. Biophys. Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- 14.Mabuchi I. Biochemical aspects of cytokinesis. Int. Rev. Cytol. 1986;101:175–213. doi: 10.1016/s0074-7696(08)60249-1. [DOI] [PubMed] [Google Scholar]
- 15.Maciver S. K. Myosin II function in non-muscle cells. Bioessays. 1996;18:179–182. doi: 10.1002/bies.950180304. [DOI] [PubMed] [Google Scholar]
- 16.Morioka K., Takano-Ohmuro H., Sameshima M., Ueno T., Kominami E., Sakuraba H., Ihara S. Extinction of organelles in differentiating epidermis. Acta Histochem. Cytochem. 1999;32:465–476. [Google Scholar]
- 17.Morioka K., Sato-Kusubata K., Kawashima S., Ueno T., Kominami E., Sakuraba H., Ihara S. Localization of cathepsin B, D, L, LAMP-1 and µ-calpain in developing hair follicles. Acta Histochem. Cytochem. 2001;34:337–347. [Google Scholar]
- 18.Morioka K. Hair Follicle: Differentiation under Electron Microscope. Springer Verlag; Tokyo: 2005. [Google Scholar]
- 19.Oshima H., Rochat A., Kedzia C., Kobayashi K., Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 20.Paus R., Cotsarelis G. The biology of hair follicles. N. Engl. J. Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 21.Pompili E., De Luca A., Nori S. L., Maras B., De Renzis G., Ortolani F., Fumagalli L. Biochemical and immunohistochemical evidence for a non-muscle myosin at the neuromuscular junction in bovine skeletal muscle. J. Histochem. Cytochem. 2003;51:471–478. doi: 10.1177/002215540305100408. [DOI] [PubMed] [Google Scholar]
- 22.Schaar B. T., McConnell S. K. Cytoskeletal coordination during neuronal migration. Proc. Natl. Acad. Sci. U S A. 2005;102:13652–13657. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simerly C., Nowak G., de Lanerolle P., Schatten G. Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos. Mol. Biol. Cell. 1998;9:2509–2525. doi: 10.1091/mbc.9.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slager H. G., Good M. J., Schaart G., Groenewoud J. S., Mummery C. L. Organization of non-muscle myosin during early murine embryonic differentiation. Differentiation. 1992;50:47–56. doi: 10.1111/j.1432-0436.1992.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 25.Small J. V., Resch G. P. The comings and goings of actin: coupling protrusion and retraction in cell motility. Curr. Opin. Cell Biol. 2005;17:517–523. doi: 10.1016/j.ceb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Takano-Ohmuro H., Mukaida M., Morioka K. Distribution of actin, myosin, and spectrin during enucleation in erythroid cells of hamster embryo. Cell Motil. Cytoskeleton. 1996;34:95–107. doi: 10.1002/(SICI)1097-0169(1996)34:2<95::AID-CM2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Takeda K., Yu Z. X., Qian S., Chin T. K., Adelstein R. S., Ferrans V. J. Nonmuscle myosin II localizes to the Z-lines and intercalated discs of cardiac muscle and to the Z-lines of skeletal muscle. Cell Motil. Cytoskeleton. 2000;46:59–68. doi: 10.1002/(SICI)1097-0169(200005)46:1<59::AID-CM6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Tserentsoodol N., Shin B. C., Suzuki T., Takata K. Colocalization of tight junction proteins, occludin and ZO-1, and glucose transporter GLUT1 in the cells of the blood-ocular barrier in the mouse eye. Histochem. Cell Biol. 1998;110:543–551. doi: 10.1007/s004180050316. [DOI] [PubMed] [Google Scholar]
- 29.Vitale M. L., Carbajal M. E. Involvement of myosin II in dopamine-induced reorganization of the lactotroph cell’s actin cytoskeleton. J. Histochem. Cytochem. 2004;52:517–527. doi: 10.1177/002215540405200410. [DOI] [PubMed] [Google Scholar]