Abstract
Mycobacteria have acquired an intracellular lifestyle within the macrophage, which is best exemplified by the enlarged infected histiocytes seen in lepromatous leprosy. To survive within the cell, mycobacteria must escape intracellular bactericidal mechanisms. In a study of Mycobacterium bovis Bacille Calmette-Guérin (M. bovis BCG) infection, it was shown that the host protein, CORO1A, also known as tryptophan aspartate-containing coat protein (TACO), accumulates on the phagosomal membrane, resulting in inhibition of phagosome-lysosome fusion, and thus augmenting intracellular survival. In this study, we show that CORO1A strongly localizes on the membrane of phagosomes that contain Mycobacterium leprae (M. leprae), where Toll-like receptor 2 was also visualized by immunostaining. When cultured macrophages were infected with M. leprae, CORO1A recruitment from the plasma membrane to the phagosomal membrane was observed. Moderate to strong CORO1A retention was observed in late lesions that contained foamy histiocytes, in which M. leprae were difficult to detect by acid-fast staining. These results suggest that components accumulating within the phagosome rather than viable bacilli are responsible for the retention of CORO1A, and that there is also a bactericidal mechanism in the macrophage that might counter the effects of CORO1A.
Keywords: Mycobacterium leprae, macrophage, phagosome, CORO1A, Toll-like receptor
I. Introduction
The adoption of an intracellular lifestyle confers several advantages on microbial pathogens: they are inaccessible to humoral and complement-mediated immune attack; they no longer require a specific adherence mechanism to maintain their site of infection; and they have accessibility to a range of nutrients. However, in order for mycobacteria to survive intracellularly, they also need to escape from intracellular bactericidal mechanisms.
Mycobacterium leprae (M. leprae) is one of the most successful intracellular pathogens, surviving within the phagosome of host macrophages. Such intracellular parasitization is most readily seen in the histiocytic lesions of lepromatous leprosy, which are characterized by an accumulation of histiocytes (termed leproma cells). They have enlarged phagosomes in which M. leprae lives and replicates. Because these phagosomes remain unfused with lysosomes, degradation of bacilli by bactericidal enzymes is prevented, resulting in their survival [8].
An in vitro study using Mycobacterium bovis Bacille Calmette-Guérin (M. bovis BCG) demonstrated that an important host protein, termed tryptophan aspartate-containing coat protein (TACO), plays an essential role in the inhibition of phagosome-lysosome fusion, as well as in the survival of bacilli within macrophages [7]. In the mouse macrophage cell line, J774.1, it was shown that TACO, which initially was found on the plasma membrane in association with tubulin, was recruited to the phagosomal membrane following infection by M. bovis BCG [7]. TACO represents a component of the phagosome coat, and retention of TACO prevents phagosomes from fusing with lysosomes, thereby contributing to the long-term survival of bacilli within the phagosome. The phagosomal localization of TACO was only transient in macrophages engulfing dead mycobacteria, whereas localization was quite stable when live bacilli were used. In addition, M. bovis BCG was completely digested in liver Kupffer cells, which lack TACO expression [7]. Mouse TACO is an ortholog of human CORO1A, also known as p57 [13], which encodes a 461-amino acid protein, that shares 40% amino acid identity with coronin, an actin-binding protein of the cellular slime mold Dictyostelium discoideum [6]. CORO1A actually binds to actin [13], and is thought to be important for cell mobility and endocytic activity, since it accumulates in cortical sites of moving cells and contributes to the dynamics of the intracellular actin system [6].
Macrophages and other immune cells have evolved several mechanisms to detect and combat pathogens. Among these mechanisms, toll like receptors (TLRs) are the pattern recognition receptors (PRRs) that sense and distinguish various components derived from pathogens, i.e. pathogen-associated molecular patterns (PAMPs) [1, 4]. TLR2 in combination with TLR1 or TLR6 are the most important for recognition of mycobacteria [10, 18], and bacterial lipopeptides, such as lipoarabinomannan (LAM), are well-known ligands for TLR2. Notably, several studies have demonstrated that TLR2 is recruited and localized on the phagosome membrane following exposure to its ligands, such as zymosan [17].
It is not known whether the observations regarding TACO activity that enhanced the survival of M. bovis BCG are universal for other mycobacteria, especially in vivo. In this study we investigate the expression and localization of CORO1A in macrophages infected with M. leprae both in vivo and in vitro.
II. Materials and Methods
Tissue samples and staining
Archived formalin-fixed, paraffin-embedded tissue sections from 42 cases of leprosy patients were subjected to immunohistochemical staining as described [12, 15]. Briefly, deparaffinized sections were incubated with rabbit anti-TACO antibody [7], which is specific for both mice and human CORO1A, was kindly provided by Dr. J. Pieters (Basel Institute for Immunology, Basel, Switzerland), rabbit anti-human Toll-like receptor 2 (TLR2) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or with anti-lipoarabinomannan (LAM) antibody (LRC, National Institute of Infectious Diseases, Japan), for 1 hr at room temperature. Slides were washed with Dulbecco’s phosphate buffered saline (DPBS) containing 0.01% Triton X-100. The peroxidase- or alkaline phosphatase-labeled streptavidin-biotin method using the LSAB2 kit (DAKO, Carpinteria, CA) and DAB (3,3-diaminobenzidine tetrahydrochloride) or BCIP/NBT (5'-bromo-4-chloro-3-indoxyl phosphate/nitro blue tetrazolium chloride) was employed according to the manufacturer’s protocol. Sections were then stained using carbol fuchsin to visualize acid-fast mycobacteria and counterstained with hematoxylin. For the Fite method of Ziehl-Neelsen staining, olive oil/xylene (1:2 ratio) was used to deparaffinize sections. Archived formalin-fixed, paraffin-embedded tissues were used according to the guidelines approved by the National Institute of Infectious Diseases (Tokyo, Japan).
Macrophage cell culture and M. leprae infection
Murine macrophage cell lines, RAW264.7, P388D1, and J774.1, were obtained from the American Type Culture Collection (ATCC; Manassas, VA). They were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 50 mg/ml penicillin/streptomycin at 37°C in 5% CO2. Peritoneal resident macrophages were harvested from Balb/c mice as previously described [9]. Briefly, peritoneal cells were recovered in Hanks’ balanced salt solution (HBSS), washed, suspended in DMEM supplemented with 15% FBS, and plated on glass coverslips in 24 well tissue culture plates. After 4 hr incubation, unadhered cells were removed by gentle rinsing with HBSS. M. leprae was prepared from footpads of nude mice as described [9] and added to the culture at a multiplicity of infection (MOI) of 20:1.
RNA preparation and Northern blot analysis
RNA from cultured macrophages was prepared using RNeasy Mini Kits (QIAGEN Inc., Valencia, CA) and minor modifications of the manufacturer’s protocol as described [14, 16]. Briefly, cells were washed with DPBS, resuspended in 600 µl of Lysis solution, and passed through a QIAshredder (QIAGEN). After 600 µl of 70% ethanol was added, the mixture was purified through a spin column, washed with 600 µl of RW1 wash solution, and washed twice with 500 µl of RPE wash solution. RNA was eluted with 30 µl of RNase-free water. RNA samples were electrophoresed on denaturing agarose gels and capillary blotted on a nylon membrane using a Turboblotter (Schleicher & Schuell, Keene, NH). After UV cross-linking, hybridization was performed as follows. cDNA probes were labeled with 33P-dCTP using a BcaBEST Labeling Kit (Takara Bio, Otsu, Japan). After the Nytran membranes (Schleicher & Schuell) were prehybridized with 10 ml of QuickHyb Hybridization Solution (Stratagene) for 1 hr at 68°C, 1×107 cpm radiolabeled probe was added after it had been premixed with 100 µl sonicated salmon sperm DNA (Stratagene), heated at 94°C for 5 min, and then chilled on ice. After hybridization for 3 hr, membranes were washed and analyzed using a BAS1000 BioImaging analyzer (Fujifilm, Tokyo, Japan). Reprobing was performed after incubating membranes in 50% formamide, 50 mM Tris-Cl (pH 8.0), and 10% SDS for 1 to 2 hr at 65°C. Mouse CORO1A cDNA was obtained by RT-PCR using primers 5'-ACCTCCTGCCGTGACAAGCG-3' and 5'-TCCTGGAACAGGTCCGACTTTC-3'.
III. Results
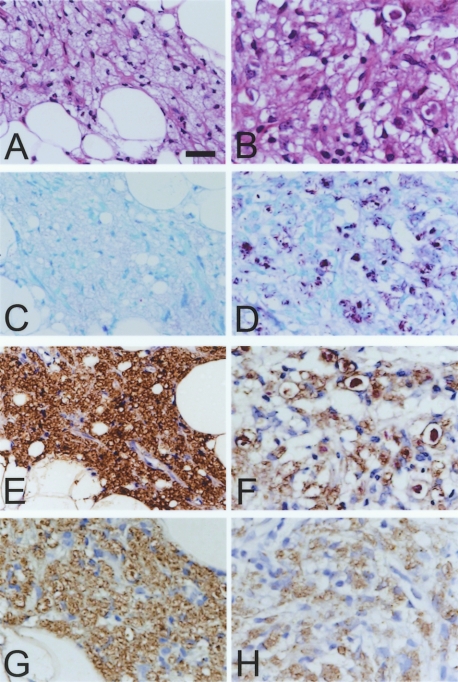
Intracellular parasitization by mycobacteria is best exemplified in lepromatous leprosy. The most characteristic feature of lepromatous leprosy is the dermal lesion, containing an accumulation of foamy histiocytes within enlarged phagosomes that contain acid-fast bacilli (M. leprae) (Fig. 1A and B). In order to study subcellular localization of CORO1A in such macrophages, skin biopsy specimens from patients with lepromatous leprosy were immunostained for CORO1A. As shown in Figure 1C and D, CORO1A localized to the phagocytic vacuoles containing M. leprae. Of note, there did not appear to be a direct association between bacilli and CORO1A, which were separated by lipids accumulated within the phagocytic vacuole (Fig. 1C and D). Phagosomal localization of CORO1A was confirmed by immunostaining serial sections of skin biopsy specimens for TLR2, which is known to localize on phagosomal membranes that contain mycobacteria, and which is responsible for initiation of innate immune responses [17, 18]. In the skin lesions of lepromatous leprosy, CORO1A localized to the same histiocytic lesion where TLR2 was also stained as shown in low magnification (Fig. 1E and F, respectively). In higher magnification, both were localized to the phagocytic vacuoles containing M. leprae (Fig. 1G and H).
Fig. 1.
Histopathological characterization of the skin lesions of leprosy. Hematoxylin and eosin staining of a tissue section from lepromatous leprosy shows accumulation of histiocytes in the dermis (A). Fite staining reveals numerous bacilli stained with pink coloration within the histiocytes (B). Immunostaining for CORO1A, shown as brown coloration, followed by acid-fast staining and hematoxylin counterstaining (C and D). Immunostaining for CORO1A (E and G), and for TLR2 (F and H), followed by acid-fast staining and hematoxylin counterstaining (both E and F, and G and H, are serial sections). Phagosomal membrane was visualized by light brown coloration in these figures and pink bacilli can be recognized in each phagocytic vacuole. Sections from a skin biopsy from tuberculoid leprosy were subjected to hematoxylin and eosin staining (I), Fite staining (J), LAM immunostaining (K) and CORO1A immunostaining (L). Both Fite and LAM staining were negative, and CORO1A staining was weakly visualized to the plasma membrane of histiocytes. Original magnification: ×100 (A, B, E and F; Bars=100 µm); ×200 (G, H, I, J, K and L; Bar=20 µm); ×400 (C and D; Bar=20 µm).
Tuberculoid leprosy is characterized by strong cellular immune responses against M. leprae, accompanied by the appearance of epithelioid cells and giant cells (Fig. 1I). In biopsies of tuberculoid leprosy lesions, M. leprae was infrequently detected either by Fite staining or immunostaining of LAM, a component of the mycobacterial cell wall (negative staining shown in Fig. 1J and K, respectively). CORO1A expression in tissue macrophages was only weakly detected on the plasma membrane and cytoplasm in tuberculoid leprosy (Fig. 1L).
Foamy histiocytes in late lesions from lepromatous leprosy sometimes have a xanthomatous appearance, as demonstrated in Figure 2A, and acid-fast bacilli were not observed because of degenerative changes (Fig. 2C). However, CORO1A was rather strongly immunostained in such lesions (Fig. 2E). Positive immunostaining of LAM, which persists for a long period of time within infected macrophages, confirmed the preexistence of M. leprae in these foamy macrophages (Fig. 2G). In a different lesion within the same tissue section (Fig. 2B), both CORO1A staining and LAM staining were relatively weak (Fig. 2F and H, respectively), in spite of the existence of numerous acid-fast bacilli (Fig. 2D). These results, together with the observation that there was no direct association between bacilli and CORO1A, as shown in Figure 1G and H, suggest that products from M. leprae accumulate within the phagosome, and that these components, and not necessarily the direct interaction of the phagosomal membrane with live bacilli, might be important for the retention of CORO1A on the phagosomal membrane.
Fig. 2.
Accumulation of CORO1A on phagosomal membranes is independent of the existence of live bacilli. Serial sections of a skin biopsy from lepromatous leprosy were subjected to hematoxylin and eosin staining (A and B), Fite staining (C and D), CORO1A immunostaining (E and F) and LAM immunostaining (G and H). Photomicrographs of A, C, E and G are taken from the same area, and B, D, F and H are from a different area within the same specimen. Original magnification: ×200. Bar=20 µm.
To confirm the localization of CORO1A in vitro, murine peritoneal macrophages were infected with M. leprae, and the infected cells were fixed with formalin, immunostained for CORO1A, and then stained for acid-fast bacilli. CORO1A was initially distributed on the plasma membrane of the macrophage (Fig. 3A). However, 4 hr after infection it relocalized to phagosomal membranes containing M. leprae (Fig. 3B, C and D). Of note, some engulfed bacilli were observed in which there was no recruitment of CORO1A to the phagosomal membrane (Fig. 3C and D, arrows), which has been reported previously [11].
Fig. 3.
CORO1A localization in the mouse peritoneal macrophage in vitro. Macrophages grown on cover slips were infected with M. leprae and subjected to CORO1A immunostaining, shown as dark coloration of BCIP/NBT, followed by acid-fast staining. Macrophages before infection of M. leprae (A) and those from 4 hr after infection (B, C and D). Arrows indicate M. leprae without a CORO1A coat around phagosomes. Original magnification: ×400. Bar=10 µm.
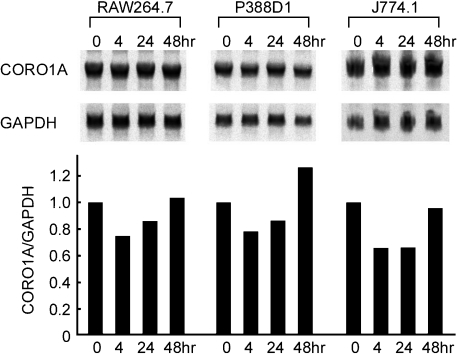
In addition to changes in subcellular localization, the pattern of CORO1A immunostaining suggested possible changes in its expression level following M. leprae infection. We therefore examined changes in CORO1A RNA levels following M. leprae infection, using three independent macrophage cell lines. Northern blot analysis revealed that mRNA levels were decreased 4 hr after M. leprae infection, and then gradually recovered by 48 hr to levels that were similar or slightly increased over the levels seen before infection (Fig. 4). Such a tendency was observed similarly in the three different macrophage cell lines tested.
Fig. 4.
Changes in CORO1A mRNA expression following M. leprae infection. Murine macrophage cell lines RAW264.7, P388D1, and J774.1 were utilized. Cells (5×105) were cultured in 10 cm dishes and infected with 5×106 organisms of M. leprae. After incubation for the indicated time, total RNA was obtained, and Northern blot analysis was performed as described in Materials and Methods. The density of each band was evaluated using Fuji Image Gauge software, and normalized to the mRNA levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Ratios relative to the original levels are shown in bar graphs below each Northern blot. CORO1A mRNA levels relative to GAPDH decreased in 4 hr following infection and recovered to the original levels in 48 hr. The same experiments were repeated three times using different batches of cells, and the typical results for each were presented.
IV. Discussion
Despite the fact that the macrophage is one of the most important immune cells protecting the body from infection, pathogenic mycobacteria such as M. tuberculosis and M. leprae parasitize the macrophage by evading its intracellular killing mechanisms. In this regard, the lepromatous leprosy lesion is the most classic example demonstrating intracellular parasitization of mycobacteria within the macrophage. It has been shown in the murine model of mycobacterial infection that TACO, the mouse CORO1A ortholog, accumulates in phagosomes containing mycobacteria and inhibits their fusion with lysosomes. Therefore, CORO1A has been considered as an essential host protein that permits the intracellular survival of mycobacteria [7], although the molecular mechanisms underlying such a function of CORO1A is still unknown.
In this study we demonstrated for the first time that CORO1A localizes on phagosomal membranes containing M. leprae in tissue specimens from lepromatous leprosy patients. Because M. leprae cannot be cultivated in vitro, it is not possible to directly determine the contribution of CORO1A to M. leprae survival. In the study of M. bovis BCG infection, however, it was clearly shown that the existence of CORO1A enhances intracellular survival of bacilli [7]. Therefore, the observed accumulation of CORO1A around phagocytic vacuoles that contained M. leprae strongly indicates that CORO1A is a factor in the pathogenesis of leprosy. After translocation to the phagocytic vacuole, stable retention of CORO1A occurred only when viable, but not inactivated M. bovis BCG (killed by either heat or antibiotics) was engulfed [7]. It was shown that phagosomes containing bacterial clumps, rather than individual or low numbers of M. bovis BCG, are required for CORO1A retention [11]. M. leprae infection as shown in Figure 4 in this study supports that observation. Thus, it may be possible that individual isolated bacilli, are processed by a different phagocytic pathway. Another possibility for the lack of CORO1A accumulation on some phagosomes is the low viability of M. leprae prepared from nude mice, because CORO1A is not maintained on phagosomes that engulf dead bacilli [7]. Using the freeze-substitution method in electron microscopy, Amako et al. performed ultrastructural studies of M. leprae freshly prepared from nude mice footpads, and reported that most bacilli were degraded [2].
Although the mycobacterial factor that contributes to the anchoring of CORO1A has not yet been determined, it has been observed in Helicobacter pylori strains that the vacuolating cytotoxin, VacA, plays an important role in the retention of CORO1A, as well as in the interruption of lysosomal fusion [19]. In leproma cells, bacilli appeared to be floating in lipid-filled phagosomes, and therefore direct association between M. leprae and CORO1A was not evident. Moreover, CORO1A accumulation on phagosomal membranes seemed to be stronger in late lesions where viable bacilli were not present. These results suggest that, rather than the existence of live bacilli, some component accumulating in the phagosome contributes to the retention of CORO1A. It may also suggest that for these late lesions the bactericidal mechanism was effective despite the CORO1A coat on the phagosomal membrane.
An interesting observation is that both CORO1A and TLR2 localize on the phagosomal membrane containing M. leprae. TLR2 activates the innate immune response for protecting cells against pathogens [18], while CORO1A inhibits fusion of the lysosome to the phagosome, allowing mycobacterial parasitization [7]. The Arg677Trp polymorphism of TLR2, which is unable to initiate downstream signaling cascades, has been reported to be associated with lepromatous leprosy [5], as well as tuberculosis [3]. Within the infected macrophage both mycobacteria-killing and mycobacteria-tolerant mechanisms, mediated at least in part by TLR2 and CORO1A, respectively, may be activated. The balance between such competing mechanisms might be one factor in determining whether the pathogen is killed or instead parasitizes the host macrophage.
V. Acknowledgments
The authors thank Dr. J. Pieters (Basel Institute for Immunology, Basel, Switzerland), for the rabbit anti-TACO antibody. We also thank Dr. M. Matsuoka for providing nude mice infected with M. leprae, and Dr. S. Toratani for peritoneal macrophages. We are also grateful to C. Sakamoto, K. Kawazu, S. Wattanopakayakit, P. D. Bang, and J. Rudeeaneksin for their technical assistance and valuable discussion.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (C) from the Ministry of Education, Culture, Sport, Science and Technology of Japan (No. 13226132 to K.S.), and by a Grant-in-Aid for Research on Emerging and Reemerging Infectious Diseases from The Ministry of Health, Labor, and Welfare of Japan (to M.M.).
VI. References
- 1.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Amako K., Takade A., Umeda A., Matsuoka M., Yoshida S., Nakamura M. Degradation process of Mycobacterium leprae cells in infected tissue examined by the freeze-substitution method in electron microscopy. Microbiol. Immunol. 2003;47:387–394. doi: 10.1111/j.1348-0421.2003.tb03375.x. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ali M., Barbouche M. R., Bousnina S., Chabbou A., Dellagi K. Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin. Diagn. Lab. Immunol. 2004;11:625–626. doi: 10.1128/CDLI.11.3.625-626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blander J. M., Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 5.Bochud P. Y., Hawn T. R., Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 2003;170:3451–3454. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 6.de Hostos E. L., Bradtke B., Lottspeich F., Guggenheim R., Gerisch G. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 1991;10:4097–4104. doi: 10.1002/j.1460-2075.1991.tb04986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari G., Langen H., Naito M., Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–447. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 8.Frehel C., Rastogi N. Mycobacterium leprae surface components intervene in the early phagosome-lysosome fusion inhibition event. Infect. Immun. 1987;55:2916–2921. doi: 10.1128/iai.55.12.2916-2921.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukutomi Y., Matsuoka M., Minagawa F., Toratani S., McCormick G., Krahenbuhl J. IL-10 treatment of macrophages bolsters intracellular survival of Mycobacterium leprae. Int. J. Lepr. Other Mycobact. Dis. 2004;72:16–26. doi: 10.1489/1544-581X(2004)072<0016:ITOMBI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Ozinsky A., Underhill D. M., Fontenot J. D., Hajjar A. M., Smith K. D., Wilson C. B., Schroeder L., Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. U S A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuller S., Neefjes J., Ottenhoff T., Thole J., Young D. Coronin is involved in uptake of Mycobacterium bovis BCG in human macrophages but not in phagosome maintenance. Cell. Microbiol. 2001;3:785–793. doi: 10.1046/j.1462-5822.2001.00155.x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K., Kobayashi M., Kawaoi A. Immunohistochemical demonstration of proliferating cell nuclear antigen in growing cells on formalin-fixed, paraffin-embedded tissue sections. Acta Histochem. Cytochem. 1992;25:13–18. [Google Scholar]
- 13.Suzuki K., Nishihata J., Arai Y., Honma N., Yamamoto K., Irimura T., Toyoshima S. Molecular cloning of a novel actin-binding protein, p57, with a WD repeat and a leucine zipper motif. FEBS Lett. 1995;364:283–288. doi: 10.1016/0014-5793(95)00393-n. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K., Lavaroni S., Mori A., Ohta M., Saito J., Pietrarelli M., Singer D. S., Katoh R., Kimura S., Kawaoi A., Kohn L. D. Autoregulation of thyroid-specific gene transcription by thyroglobulin. Proc. Natl. Acad. Sci. U S A. 1998;95:8251–8256. doi: 10.1073/pnas.95.14.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K., Lavaroni S., Mori A., Okajima F., Kimura S., Katoh R., Kawaoi A., Kohn L. D. Thyroid transcription factor 1 is calcium modulated and coordinately regulates genes involved in calcium homeostasis in C cells. Mol. Cell. Biol. 1998;18:7410–7422. doi: 10.1128/mcb.18.12.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K., Mori A., Ishii K. J., Saito J., Singer D. S., Klinman D. M., Krause P. R., Kohn L. D. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc. Natl. Acad. Sci. U S A. 1999;96:2285–2290. doi: 10.1073/pnas.96.5.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Underhill D. M., Ozinsky A., Hajjar A. M., Stevens A., Wilson C. B., Bassetti M., Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 18.Underhill D. M., Ozinsky A., Smith K. D., Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. U S A. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng P. Y., Jones N. L. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell. Microbiol. 2003;5:25–40. doi: 10.1046/j.1462-5822.2003.00250.x. [DOI] [PubMed] [Google Scholar]