Abstract
To identify the gene or genes on mouse Chromosome 9 that contribute to strain differences in fatness, we conducted an expanded mapping analysis to better define the region where suggestive linkage was found, using the F2 generation of an intercross between the C57BL/6ByJ and 129P3/J mouse strains. Six traits were studied: the summed weight of two adipose depots, the weight of each depot, analyzed individually (the gonadal and retroperitoneal depot), and the weight of each depot (summed and individual) relative to body size. We found significant linkage (LOD = 4.6) that accounted for the relative weight of the summed adipose depots, and another for the relative weight of the gonadal (LOD = 5.3) but not retroperitoneal (LOD = 0.9) adipose depot. This linkage is near marker rs30280752 (61.1 Mb, Build 34) and probably is equivalent to the quantitative trait locus (QTL) Adip5. Because the causal gene is unknown, we identified and evaluated several candidates within the confidence interval with functional significance to the body fatness phenotype (Il18, Acat1, Cyp19a1, Crabp1, Man2c1, Neil1, Mpi1, Csk, Lsm16, Adpgk, Bbs4, Hexa, Thsd4, Dpp8, Anxa2, and Lipc). We conclude that the Adip5 locus is specific to the gonadal adipose depot and that a gene or genes near the linkage peak may account for this QTL.
Introduction
Results of studies from our laboratory suggest that two strains of mice, C57BL/6ByJ (B6) and the 129P3/J (129), differ in adiposity (Bachmanov et al. 2001), and several quantitative trait loci (QTLs) account for the strain differences in overall fatness (Reed et al. 2003) and/or in the weight of individual adipose depots (Reed et al. 2006). The goal of subsequent studies is to find genes underlying these linkages that give rise to the observed variation in these traits. Chromosome 9 was selected for study here because several fatness QTLs identified from mouse crosses are clustered on it (Table 1) and also because the effect of this QTL is consistent among several experimental crosses. It is usually antagonistic, which means that the heavier and fatter strains (AKR/J, BKS, B6, LG/J, and NZB) contribute a fatness-lowering allele when interbred with leaner and lighter strains (129, A/J, SM/J, and SWR/J). We also chose to study this chromosome because it is not, to the best of our knowledge, the focus of other positional cloning efforts for obesity or related traits, and so its investigation is therefore likely to yield novel information. The goal of this analysis was to examine the effects of this locus on the retroperitoneal and gonadal depots individually, because other results (Reed et al. 2006) suggested that the strength of the genetic effects differed by depot. These two depots are both located in the abdominal cavity but have different characteristics in these mouse strains; the gonadal depot expands and fills quickly when mice are fed a high-fat diet, whereas the retroperitoneal depot expand more slowly (Bachmanov et al. 2001). After genotyping a large number of mice with a dense map, several approaches were used to nominate candidate genes within the linked region, which included bioinformatic analyses of the gene function and determining the gene sequence variants between the parental B6 and 129 strains.
Table 1.
Obesity QTLs on mouse Chromosome 9
| Name | Phenotype | Location (cM) | Strain 1 | Strain 2 | Minus allele | Mode | Ref |
|---|---|---|---|---|---|---|---|
| Carf2 | Percent fat | 10.0& | C57BL/6J-hg/hg | CAST/EiJ | C57BL/6J | Add | Corva et al. 2001 |
| Obq5 | Relative adiposity | 19.0 | C57BL/6J | KK/HlLt | C57BL/6J | Dom | Taylor et al. 1999 |
| No name | Relative gonadal fat | 27.0 | C57BL/6J | DBA/J | NAv | NAv | Lang et al. 2005 |
| No name | Gonadal fat | 27.0 | BKS.Cg-Leprdb+/+m | DBA/J | DBA/J | Add | Yaguchi et al. 2005 |
| Afw4 | Abdominal fat* | 29.0 | DUK | DU6 | DU6 | Add | Brockmann et al. 1998 |
| No name | Percent fat | 39.0 | SPRET/Ei | B6-Lipc−/− &129P2 | C57BL/6J | NAv | Farahani et al. 2004 |
| Adip5 | Percent fat (gonadal) | 42.0 | LG/J | SM/J | LG/J | Dom | Cheverud et al. 2001 |
| Adip5 | Relative adiposity | 42.0 | C57BL/6ByJ | 129P3/J | C57BL/6ByJ | Add | Reed et al. 2003 |
| Mob8 | Gonadal adipose depot# | 42.0 | C57BL/6J | CAST/Ei | C57BL/6J | Dom | Mehrabian et al. 1998 |
| Obq26 | Relative gonadal depot | 48.0 | NZB/BINJ | SM/J | NZB (male) SM (female) |
Add | Stylianou et al. 2006 |
| Dob2 | Adiposity, various | 48.0 | AKR/J | SWR/J | AKR/J | Add | West et al. 1994b |
| Adip5 | Percent fat (gonadal) | 65.0 | LGXSM | LGXSM | SM | NAv | Cheverud et al. 2004 |
| Obq18 ‡ | Percent fat | 65.0 | C57BL/6J | 129S1/SvImJ | Epistasis | NAp | Ishimori et al. 2004 |
NAv = not available; NAp = not applicable.
QTLs with confidence intervals that overlap the confidence interval of Adip5 are listed. Minus allele refers to the one that decreases the trait value. Mode refers to the mode of inheritance, Add = additive, Dom = dominant. In the case of Obq, Adip5, and Mob8, the KK, LG, and CAST alleles are dominant, respectively. Relative = weight of adipose tissue was adjusted for some aspect of body size, using either ratio methods or multiple regression.
Low marker density (n = 3) and low mapping precision.
Linkage is significant for the gonadal but not retroperitoneal depot.
Epistatic interaction with alleles on Chromosome 8.
For Afw4, there is no significant QTL when gonadal fat is expressed relative to body weight.
Method
Mice
C57BL/6ByJ (B6) and 129P3/J (129) inbred mice used for breeding and for phenotyping were obtained from the Jackson Laboratory (Bar Harbor, ME). The B6 × 129 F1 and F2 hybrids were bred at the Monell Chemical Senses Center as described in the companion article in this issue (Reed et al. 2006). F2 pups were weaned at 21–30 days of age and reared in same-sex groups. A total of 457 F2 mice (228 female and 229 male) were bred (Bachmanov et al. 2002). The mice were housed in a temperature-controlled vivarium at 23°C on a 12:12-h light:dark cycle and had free access to water and pelleted Teklad Rodent Diet 8604 (4.4% fat). All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center.
Adipose depot dissection
Mice were euthanized when they were 8–9 months old and then the retroperitoneal and gonadal depots were dissected and weighed. Dissection of the retroperitoneal depot included fat from the renal capsule and adipose tissue anchored to the wall of the body, dorsal to the kidney. The retroperitoneal depot is located between the first and second lumbar vertebrae and the fifth and sixth lumbar vertebrae. The appearance of the retroperitoneal depot is similar in male and female mice. For the gonadal depot, the adipose tissue near the epididymis and vesicular gland was dissected from male mice, and the fat near the ovary and uterus was dissected from female mice, with the landmarks near the seventh lumbar vertebrae, approximately at the iliac crest, and the first and second caudal vertebrae (Iwaki et al. 2001). Mesenteric and omental fat-containing tissue was not removed. Variables measured were the weight of the right and left retroperitoneal and gonadal adipose depot (four depots total, weighed individually to the nearest 0.01 g). Measures of body size were also made: body weight (to the nearest 0.1 g) and body length (base of the lower incisors to anus, distance to the nearest 0.1 cm).
Linkage analysis
The six phenotypes used in the linkage analysis were the absolute depot weight (adjusted for age and litter size) and the relative depot weight (adjusted for age, litter size, body weight, and body length) of the retroperitoneal depot, gonadal depot, and the sum of both depots. Adjustments were made using multiple regression methods (Statistica; StatSoft, Tulsa, OK). Female and male mice differed in the mean and variance for many traits, so the regression analysis was conducted separately within sex groups and the normalized values were combined before linkage analysis. The residuals obtained from the multiple regression analyses were standardized to a common scale so they could be pooled.
There are two common ways to adjust the fat weight to body size: multiple regression or ratio methods (such as dividing fat weight by body weight). We chose to adjust the data with regression methods for two reasons: First, more than one covariate can be used to normalize the data within groups (e.g., age and litter size). Second, regression methods are less likely to produce residual correlations among phenotypes. Previous QTL studies of body size and its relationship to muscle mass in mice suggest that multiple regression methods lead to more accurate assessment of QTL effects than ratio methods (Lang et al. 2005).
DNA extraction and genotyping
Genomic DNA was purified from mouse tails either by phenol/chloroform extraction and precipitation with ethanol (Hogan et al. 1986) or by a sodium hydroxide method (Truett et al. 2000). To select markers on Chromosome 9 that are polymorphic between the parental strains, we consulted several databases (see Website References and Table 2). We used both microsatellite (simple sequence repeats) and single nucleotide polymorphism (SNP) markers. Microsatellite markers were amplified, scored, and checked as described in the companion article (Reed et al. 2006) using either radioactive methods to visualize the band sizes, done in our laboratory, or using fluorescently labeled primers, conducted by the Australian Genome Research Facility (Melbourne, Australia). For some SNPs, we designed primers for the Orchid UHT system (Princeton, NJ), and the markers were typed by a commercial vendor (DNAprint; Coconut Grove, FL). For other SNPs, we used fluorescently labeled primers and probes and genotyped these markers through allelic discrimination in our laboratory using an ABI PRISM 7000 real-time PCR system (ABI Assay-by-Design, Applied Biosystems, Foster City, CA). Genotypes were checked for accuracy by comparison with the haplotypes of flanking markers, and those with unlikely recombination patterns were retyped as needed. In some cases, genotypes were confirmed by DNA sequencing.
Table 2.
Polymorphic markers on Chromosome 9
| # | Marker | n | Position (bp) | cM observed | JAX cM |
|---|---|---|---|---|---|
| 1 | D9Mit218 | 398 | 9,036,544 | 4.0 | 4.0 |
| 2 | rs13475347 | 439 | 26,879,442 | 15.3 | |
| 3 | D9Mit297 | 422 | 33,979,821 | 22.9 | 15.0 |
| 4 | rs30127992 | 440 | 35,276,833 | 25.6 | |
| 5 | D9Mit67 | 251 | 36,972,122 | 26.9 | |
| 6 | D9Mit91 | 159 | 37,183,740 | 27.1 | |
| 7 | D9Mit2 | 423 | 37,336,441 | 27.3 | 17.0 |
| 8 | D9Mit205 | 389 | 37,139,864 | 27.9 | 18.0 |
| 9 | rs33763745 | 421 | 38,122,554 | 28.5 | |
| 10 | rs33756006 | 441 | 42,289,186 | 31.4 | |
| 11 | D9Mit25 | 341 | 44,328,753 | 34.0 | 26.0 |
| 12 | rs30353028 | 407 | 50,600,947 | 37.7 | |
| 13 | rs30042362 | 439 | 57,854,008 | 41.3 | |
| 14 | rs29794257 | 425 | 59,831,074 | 42.7 | |
| 15 | rs30280752 | 438 | 61,187,869 | 44.5 | |
| 16 | rs33642294 | 275 | 61,173,765 | 45.6 | |
| 17 | D9Mit32 | 400 | 67,372,839 | 49.2 | 35.0 |
| 18 | rs30145295 | 435 | 72,541,925 | 52.5 | |
| 19 | D9Mit306 | 438 | 77,645,881 | 52.8 | 42.0 |
| 20 | rs4222028 | 419 | 79,975,159 | 55.5 | |
| 21 | D9Mit182 | 439 | 101,376,935 | 56.6 | 55.0 |
| 22 | rs29599615 | 422 | 105,843,664 | 66.8 | |
| 23 | rs3023231 | 441 | 106,954,769 | 70.0 | |
| 24 | D9Mit37 | 428 | 110,803,888 | 74.5 | 61.0 |
| 25 | rs29829423 | 436 | 115,237,603 | 84.0 | |
| 26 | D9Mit152 | 147 | 121,826,196 | 91.2 | 73.0 |
Marker position (bp) is from Build 34 of the mouse genome. The rs prefix indicates the SNP listing in the public NCBI database. Positions of the markers with a D9Mit prefix were estimated by using the primer sequence as input in a BLAT search, using the first base pair of the left-most primer as the bp location. For SNP markers, the location is given for the variant base pair. The observed linkage map was obtained through analysis of recombination events and computed with MAPMAKER/EXP. We assumed that the first marker (D9Mit218) was 4.0 cM from the centromere based on information available through the Mouse Genome Database (MGD) hosted by the Jackson Laboratory (JAX). MGD cM positions were used to anchor this marker. SNP markers (rs) have not been integrated into the MGD linkage map; therefore, no cM positions are listed. In two cases, underlined in the table, there was a disagreement between the physical and genetic maps for adjacent markers. In these instances, we assumed that the experimental genetic map was correct and we used this order in subsequent analyses. The region between 79 and 101 Mb has less recombination per unit physical distance compared with the other regions.
Mapping of Chromosome 9
Linkage analysis was conducted using 442 mice genotyped with 26 markers on Chromosome 9. Several of the original 457 mice died before the end of the experiment or were missing some phenotype information and were therefore not included in the subsequent analyses. The average DNA marker density was 3.5 cM, with no gap greater than 12 cM (Table 2).
Linkage analysis
Chromosome 9 linkage maps were created using MAPMAKER/EXP. Trait analysis was undertaken using MAPMAKER/QTL (Lander et al. 1987), and thresholds for suggestive and significant linkage were used as described previously (Lander and Kruglyak 1995). The confidence intervals were generated by MAPMAKER. LOD scores are reported at marker locations. The percentage of variance accounted for is also reported at the marker nearest the maximal LOD score, and the direction of dominance is expressed relative to the effect of the 129 allele. For each trait, three genome scans were conducted: using males-only, using females-only, and using a combined group of both sexes. Sex-dependent linkage was defined as described in the companion article (Reed et al. 2006). In supplemental analyses, QTLs were fixed at linkage peaks and evaluated using MAPMAKER/QTL or regression methods. A second analysis was done to refine linkage peaks. Genotypes from two other linked regions (Reed et al. 2003) (D2Mit12 and D16Mit6) were used as covariates in the analysis to control for background genotype effects. Finally, traits were grouped by genotype at the Chromosome 9 marker nearest the peak LOD score, and a one-way ANOVA was conducted to evaluate the marker genotype effect. Post hoc analysis of means was conducted using a least squares difference test (Statistica; StatSoft, Tulsa, OK).
Candidate gene analysis of Chromosome 9
We chose a region, defined by 1.0-LOD drops from the highest peak, ignoring lower peaks, to search for candidate genes (Fig. 1). This subregion was anchored at rs30280752 (61.1 Mb; Build 34), at the peak linkage for relative gonadal and combined fat depots, with flanking regions 10 Mb up- and downstream. Searching a smaller region for candidate genes seemed warranted because studies of other QTLs indicate that the location of the cloned gene is usually within 2 cM of the original peak LOD score (Price 2006).
Fig. 1.
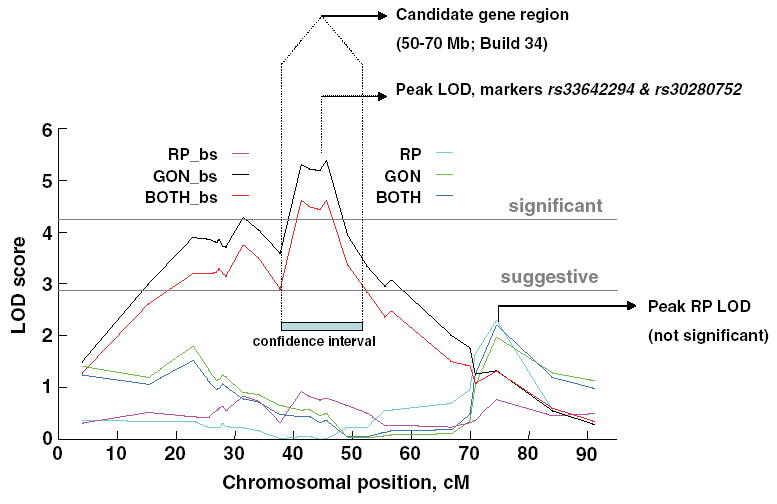
Mapping results for absolute and relative adipose depot weight.
RP = retroperitoneal adipose depot, GON = gonadal adipose depot, BOTH = sum of the RP and GON depot. RP, GON, and BOTH are expressed either as absolute adipose-depot weights or relative to body size (bs). LOD scores plotted are obtained using unconstrained (free) model. Peaks for the absolute weight of the depots near the telomere do not reach threshold for suggestive linkage (observed LOD = 2.3; criterion LOD = 2.8 for an unconstrained model).
Manual and automated search strategies were used to select candidate genes, and the results were compared between these two approaches. For the manual search strategy, known genes (with RefSeq identifiers assigned) were extracted from the Build 34 genome assembly through the University of California at Santa Cruz browser, and the known function of each gene was examined one-by-one, and those involved in the regulation of body fat and its deposition (e.g., increased body fatness, depot-specific deposition, and adipogenesis) were identified, and chosen as candidates. Mice with targeted or spontaneous alleles of genes from this region were evaluated by examining the phenotype data cataloged in the Mouse Genome Database or by using gene-specific searches of NCBI Entrez Gene and subsequent PubMed links (see Website References). For the automated search, we used the algorithms implemented in Positional Medline Database, which filters candidate genes by location, function, gene expression, and function networks (see Website References).
Protein-coding (cSNP) and splicing sequence variants between the B6 and 129 strains were identified by a query of Mouse SNP Build 126 between 50 Mb and 70 Mb which corresponded respectively to the region between 40–50 cm. (UCSC Browser, Build 34; see Website References). Those genes with protein-coding and splicing variants were added to the list of candidate genes. This final list of genes was then ordered into three levels of priority—high, medium, and low—based on two criteria: gene function and the presence of cSNPs between the parental strains. Those candidates with known obesity-related functions and with known cSNPs were given high priority. Functional candidate genes within discordant haplotype blocks (which are more likely to differ in DNA sequence; see details below), but with no known cSNPs, were given medium priority. The lowest priority was assigned to either functional candidates that were in concordant blocks or to genes that were not candidates based on function but which contained cSNPs. The presence of SNPs in introns or immediately upstream or downstream of the coding regions was considered neutral information when rating priority because they are usually inconsequential, although they can sometimes change gene function, for example, if they are in specific promoter elements. Thus, because of the uncertainty about whether this type of variation would change gene function, this information did not alter the priority position of the gene.
To determine what was known about candidate genes and their role in human obesity, the location of each human gene ortholog was (1) determined by a search of the human NCBI Build 35, and matched to (2) the Obesity Gene Map and (3) the Online Mendelian Inheritance in Man (OMIM; see Website References). Information about the previous associations between human obesity and the candidate genes was considered neutral information when ranking mouse candidates, although in some cases it did contribute to the original identification of the candidate gene based on function.
Haplotype analysis
A prerequisite for a gene to be a candidate for a quantitative trait locus (QTL) is that it must vary in DNA sequence between the parental strains. Some genomic regions are less likely to contain between-strain variation because they are descended recently from the same ancestral chromosome. Using this concept, we attempted to assess whether genes in the confidence interval were contained in an identical haplotype block and therefore could be given a lower priority as candidates (Wade et al. 2002). To evaluate the haplotype blocks on Chromosome 9, a list of SNPs for the available B6 and 129 substrains was obtained (Mouse Genome Database; see Website References). The B6 and 129 substrains were treated, for the purposes of this analysis, as though they were identical to the strains used here, e.g., alleles of C57BL/6J = alleles of C57BL/6ByJ. Regions of haplotype concordance and discordance were identified, and the size and location of each block type were assessed. It was assumed that the transition from discordant haplotype to concordant haplotype occurred midway between adjacent markers. Candidate genes were mapped onto blocks by base pair location and genes that fell between or spanned discordant and concordant blocks were classified as discordant.
Results
Significant linkage peaks were found at the rs30280752 marker near the middle of Chromosome 9 for two phenotypes, relative gonadal depot weight and the relative weight of both depots combined (Fig. 1, Table 3). The peak LOD scores, mode of inheritance, and the percentage of variance that was accounted for are shown in Table 3. Compared with the original genome scan, more dense genotyping of a larger number of mice reduced the confidence interval (Reed et al. 2006). The central region of Chromosome 9 was significantly linked to the gonadal depot (LOD = 5.3) but was not linked to the retroperitoneal depot (LOD = 0.9). Although two peaks are observed, a primary and a secondary peak (Fig. 1), fixing the QTL at marker rs30280752 and rescanning the chromosome did not reveal statistical support for the existence of a second and independent QTL. Using markers on other chromosomes to control for residual genetic background did not change the location or shape of the linkage curves.
Table 3.
Significant linkages on Chromosome 9
| Locus | Trait | Markers near peak | Peak and CI (cM) | LOD score | Plus allele | Mode | % Variance |
|---|---|---|---|---|---|---|---|
| Adip5 | GON (bs) | rs30280752 | 45.6 (17.0–49.2) | 5.3 | 129 | Add | 5.5 |
| RP & GON (bs) | rs30280752 | 45.6 (15.3–51.2) | 4.6 | 129 | Add | 4.7 | |
| rs33642294 |
CI = confidence interval; GON (bs) = gonadal adipose depot relative to body size; RP & GON (bs) = combined weight of the retroperitoneal and gonadal depot relative to body size. The plus allele contributed by the 129 mouse strain increased the trait value. Mode refers to the mode of inheritance. % variance is the total amount of trait variance accounted for by the marker nearest the peak LOD score, as estimated by MAPMAKER/QTL using an additive model.
None of the linkages was sex-specific, defined as having a 1-LOD difference between male-only and female-only analysis. For the relative weight of the gonadal depot, the peak LOD score for males was 3.2 (n = 225; unconstrained model, LOD score reported at marker rs30042362) and the peak LOD score for this trait in females was 2.4 near the same location (n = 217). Likewise, for the relative weight of the combined depots, the peak LOD scores were 2.9 for males-only and 2.0 for females-only for the same model and approximate location, which did not reach the criterion of a 1-LOD difference for a sex-dependent effect.
In agreement with the International Committee on Standardized Genetic Nomenclature for Mice (see Website References), this QTL was named Adip5 because it substantially overlaps a previously discovered QTL of the same name (Cheverud et al. 2001), meets the criterion of significant linkage, and shares at least one parental strain in common (B6) with other significant QTLs in the region. There were other QTL symbols that would have also met these criteria equally well (e.g., Mob8, see Table 1) but Adip5 was chosen because we have used this symbol previously (Reed et al. 2003).
To illustrate the relationship between genotype and phenotype, traits were grouped by genotype at the marker nearest the peak LOD score (rs30280752) and the mean value for each trait was calculated. There was a significant effect of genotype on the gonadal depot relative to body size [F(2,435) = 12.231, p = 0.00001] and for both depots combined [F(2,435) = 10.378, p = 0.00004; Fig. 2]. The effect of the genotype is additive, with the heterozygous mice having an intermediate phenotype. There was no effect on absolute size for either the summed weight of both depots or for the retroperitoneal depot relative to body size (all p values >0.05). B6 alleles of the rs30280752 marker increased body length [effect of genotype, F(2,435) = 4.2372, p = 0.02] and tended to increase body weight [effect of genotype, F(2,435) = 2.3167, p = 0.10]. Thus, it appears that B6 alleles at the rs30280752 marker are associated with increases of body weight and body length but with a reduction of body fatness. The body weight and body length effects might also be a result of alleles of genes associated with nearby QTLs on Chromosome 9 (Bwq6, Bdln4, Bdln5; see Reed et al. 2003).
Fig. 2.
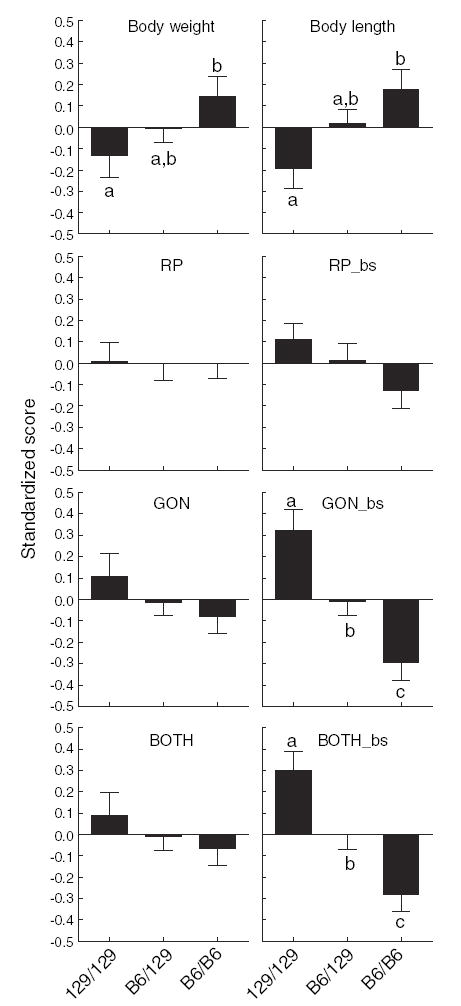
Means for each trait expressed as z scores with the standard error of the mean. “129” refers to the alleles of the 129P3/J strain and “B6” refers to alleles of the C57BL/6ByJ strain. Data are grouped by genotype at marker rs30280752. The means of groups with different subscripts differ by post hoc analysis. See text for significance tests and other details.
Candidate genes
We confined the search to a 20-Mb segment that flanked the maximum LOD score (LOD = 5.3, 61.1 Mb), positioned between 50 and 70 Mb (Build 34). Within this interval there were 361 known genes. A manual search identified 16 candidate genes, and an automated search, using “obesity” as a keyword, identified 75 genes. Except for three genes (Thsd4, Neil1, and Lsm16), all genes found during the manual search were found during the automated search. The automated search missed these candidates for different reasons: In the case of Lsm16, the identification of the gene as a candidate came from data appearing in unpublished conference proceedings, and in the case of Thsd4, domains of this gene that suggested its role in adipogenesis were not identified in the automated algorithms. In the case of Neil1, the data linking it to obesity were too recent to be included in the automated search databases. All genes identified by both methods were reviewed (N = 77), but most candidates from the automated search were discarded because of search artifacts, e.g., weak or no evidence of their role in adipose depot weight or obesity. The list of 16 genes nominated as candidates based on their known function is shown in Table 4, listed by gene symbol, accession number, and a summary of their functional connection to obesity. Three genes are involved in the formation of new adipocytes (adipogenesis) (Crabp1, Csk, and Thsd4). Six genes have a role in metabolism: four in carbohydrate metabolism (Man2c1, Mpi1, Adpgk and Hexa) and two in lipid metabolism (Acat1 and Lipc). In addition, we examined three genes associated with diabetes, insulin sensitivity, and/or the ability to use glucose as a fuel (Il18, Dpp8, and Anxa2). Nine genes that contained cSNPs between the B6 and 129 strains were identified by searching dbSNP (Mouse SNP Build 126); of these genes, three were functional candidates (Csk, Adpdk, and Thsd4) and six were not (Table 5). No DNA sequence variants that would be predicted to influence splicing were found.
Table 4.
Mouse candidate genes selected based on function
| Gene symbol | Accession | Name | Function |
|---|---|---|---|
| Il18 | NM_008360 | Interleukin 18 | A proinflammatory cytokine involved in energy balance; knockout mice are hyperphagic, obese, and insulin-resistant (Netea et al. 2006) |
| Acat1 | NM_144784 | Acetyl-coenzyme A acetyltransferase 1 precursor | Enzyme involved in cholesterol metabolism; upregulated in adipose tissue of hormone-sensitive lipase knockout mice compared with controls (Harada et al. 2003) |
| Cyp19a1 | NM_007810 | Cytochrome P450, family 19, subfamily a, polypeptide | Encodes aromatase, an essential enzyme for the synthesis of endogenous estrogens, knockout mice have 50%–80% larger gonadal adipose depots compared with controls (Jones et al. 2001) |
| Crabp1 | NM_013496 | Cellular retinoic acid binding protein I | Adipogenesis; a retinoid-binding protein involved in adipocyte differentiation and downstream targets of PPARγ (Okuno et al. 2002) |
| Man2c1 | NM_028636 | Mannosidase alpha class 2C member 1 | Carbohydrate metabolism; increased mannosidase enzyme activity in obese subjects relative to controls (Corral et al. 1992) |
| Neil1 | NM_028347 | Nei endonuclease VIII-like | Knockout of this DNA repair enzyme leads to abdominal obesity in mice (Vartanian et al. 2006) |
| Mpi1 | XM_134931 | Mannose phosphate isomerase 1 | Carbohydrate metabolism (Okazaki et al. 2002) |
| Csk | NM_007783 | c-src tyrosine kinase | Adipogenesis; may mediate the inactivation of c-Src by IGF-1 during adipogenesis (Sekimoto and Boney 2003) |
| Lsm16 | NM_153799 | LSM16 homolog | Mice with a targeted mutation in this gene have less fat than controls (Vauti et al. 2003) |
| Adpgk | NM_028121 | ATP-dependent glucokinase | Carbohydrate metabolism; involved in glycolysis (Ronimus and Morgan 2004) |
| Bbs4 | NM_175325 | Bardet-Biedl syndrome 4 homolog | Knockout mice are fatter than controls (Mykytyn et al. 2004). Human patients with Bardet-Biedl syndrome have an obese phenotype |
| Hexa | NM_1010421 | Beta-hexosaminidase alpha chain precursor | Carbohydrate metabolism; glycoprotein catabolism; activity decreased in the livers of obese rats compared with controls (Garcia Pascual et al. 1992) |
| Thsd4 | NM_172444 | Thrombospondin, type I, domain containing 4 | Adipogenesis; shares domains with genes of the Adamts family that regulate adipogenesis through extracellular matrix remodeling (Lilla et al. 2002) |
| Dpp8 | NM_028906 | Dipeptidylpeptidase 8 | Aminopeptidase activity; a protein family member inactivates circulating incretins and affects glucose tolerance (Pederson et al. 1998) |
| Anxa2 | NM_007585 | Annexin A2 | Upregulated in liver of mice with no white adipose tissue; its human ortholog is a candidate gene for diabetes (Mir et al. 2003) |
| Lipc | NM_008280 | Lipase, hepatic | Alleles are associated with body fatness in genetic models of mouse obesity. Alleles of this gene are associated with abdominal adiposity in humans (Fabsitz et al. 1989) |
Gene symbol is the official locus designation for the mouse gene. Accession numbers are indexed at NCBI (see Website References). The Name column contains additional description of the gene identity. The Function column lists key features relevant to obesity in mice and humans.
Table 5.
Priority ranking of candidate genes
| Gene symbol | Build 34 (bp) | Haplotype block | cSNPs known | Allele | Function | Human ortholog location | Human phenotype |
|---|---|---|---|---|---|---|---|
| High priority | |||||||
| Csk | 57,740,571–57,759,105 | Discordant | rs33644792 | P312H | + | Chr15:72,861,767–72,882,557 | No known phenotype |
| Adpgk | 59,318,498–59,343,126 | Discordant | rs13480222 | A318T | + | Chr15:70,830,762–70,863,114 | No known phenotype |
| Thsd4 | 60,087,012–60,259,136 | Discordant | rs6224703a | V410M | + | Chr15:69,807,941–69,858,019 | No known phenotype |
| Medium priority | |||||||
| Il18 | 50,637,695–50,654,164 | Discordant | None known | NA | + | Chr11:111,519,186–111,540,050 | Obesity |
| Cyp19a1 | 54,269,958–54,297,852 | Discordant | None known | NA | + | Chr15:49,288,961–49,418,086 | Obesity |
| Crabp1 | 54,882,658–54,891,163 | Discordant | None known | NA | + | Chr15:76,419,749–76,427,622 | No known phenotype |
| Man2c1 | 57,244,266–57,255,699 | Discordant | None known | NA | + | Chr15:73,435,185–73,447,994 | No known phenotype |
| Neil1 | 57,256,746–57,260,524 | Discordant | None known | NA | + | Chr15:73,426,462–73,434,639 | No known phenotype |
| Mpi1 | 57,658,200–57,666,660 | Discordant | None known | NA | + | Chr15:72,969,914–72,977,130 | Hepatic-intestinal |
| Bbs4 | 59,438,725–59,470,220 | Discordant | None known | NA | + | Chr15:70,765,587–70,817,869 | Obesity |
| Hexa | 59,656,332–59,681,867 | Discordant | None known | NA | + | Chr15:70,422,832–70,455,393 | Tay-Sachs |
| Dpp8 | 65,154,659–65,204,852 | Discordant | None known | NA | + | Chr15:63,526,027–63,597,095 | No known phenotype |
| Anxa2 | 69,585,297–69,623,409 | Discordant | None known | NA | + | Chr15:58,426,642–58,477,477 | No known phenotype |
| Lipc | 70,939,916–71,079,289 | Discordant | None known | NA | + | Chr15:56,511,466–56,648,364 | Obesity |
| Low priority | |||||||
| Pts | 50,593,944–50,600,968 | Discordant | rs29794257 | G6D | − | Chr11:111,602,308–111,609,903 | Hyperphenylalaninemia |
| Acat1 | 53,644,099–53,673,629 | Concordant | None known | NA | + | Chr11:107,497,467–107,523,485 | Ketoacidosis |
| AK006055 | 57,281,276–57,290,213 | Discordant | rs13465819 | G230S | − | Chr15:73,285,398–73,290,513 | No known phenotype |
| Lsm16 | 57,822,503–57,864,085 | Concordant | None knownb | NA | + | Chr15:72,709,952–72,775,413 | No known phenotype |
| Gramd2 | 59,824,652–59,833,313 | Discordant | rs29794257 | R255C | − | Chr15:70,239,201–70,277,180 | No known phenotype |
| Uaca | 60,912,093–60,997,458 | Discordant | rs3667578 | Q425H | − | Chr15:68,733,947–68,842,904 | No known phenotype |
| BC039571 | 67,790,284–67,791,775 | Discordant | rs3721766 | M127L | − | Chr15:60,243,408–60,244,436 | No known phenotype |
| Vps13c | 67,871,263–67,936,662 | Discordant | rs3722443 | T1502I | − | Chr15:59,931,879–60,139,939 | No known phenotype |
NA = not applicable.
Position in bp is given relative to Mouse Build 34. See text for details about haplotype blocks. Alleles are denoted by the amino acid substitution (using single letter codes) at the numbered position of the protein sequence, e.g., proline (P) is substituted for histidine (H) as position 312, P312H. Genes chosen for their functional relevance to obesity are denoted by a ‘‘+,’’ whereas those chosen only because they have a cSNP are denoted by ‘‘−.’’
The allele was confirmed by direct sequencing of the parental strains (Accession Nos. DQ424862 and DQ517441).
The protein-coding regions of this gene were sequenced to detect variants but none were found (Accession Nos. DQ240818 and DQ240819).
High priority is given to genes with a function related to obesity and known protein-coding variants. Medium priority is given to functional candidates within discordant haplotype blocks. Lower priority is given to functional candidates in concordant blocks or genes with protein coding variants but without an obvious functional relationship to obesity or gonadal depot weight. Within priority category, genes are ordered by location. Genes in transition areas between discordant and concordant blocks were categorized as discordant because the crossover points are not known. Human orthologs and their chromosome location in base pairs are relative to Build 35 of the human genome. When known, the human phenotype is listed in the right column (OMIM; see Website References).
Query of the homologous genes in humans suggested that alleles of some of these candidate genes are associated with human obesity, notably BBS4, alleles of which lead to a rare form of genetic disorder with obesity as a feature. Association with human obesity of three other genes, LIPC, IL18, and CYP19A1, was found based on differences between obese and lean human populations either in frequency of alleles in the non-coding regions or in gene or protein function or concentration (Table 5). For some candidate genes (e.g., HEXA), the effects of alleles in the human population are described, but obesity is not part of the clinical description. For the remaining genes (e.g., DPP8), no human disease-causing alleles have been reported.
Haplotype block analysis
On Chromosome 9 there were 6778 SNPs, 3830 (57%) of which were discordant and 2948 (43%) of which were concordant between the B6 and 129 strains. There were 736 haplotype blocks, the average size of the blocks was approximately 162 kb, and about 66% of the entire chromosome was discordant. The largest block was a discordant stretch 3.1 Mb long, from 17.3 to 20.4 Mb. For the region of linkage, from 50 to 70 Mb, there were 1280 SNPs in 119 blocks. The average block size in the candidate region was 183 kb, and the largest block (1.8 Mb) was discordant (Fig. 3). The percentage of discordant genome within the interval from 50 to 70 Mb was slightly higher compared with the whole chromosome (75% vs. 66%).
Fig. 3.
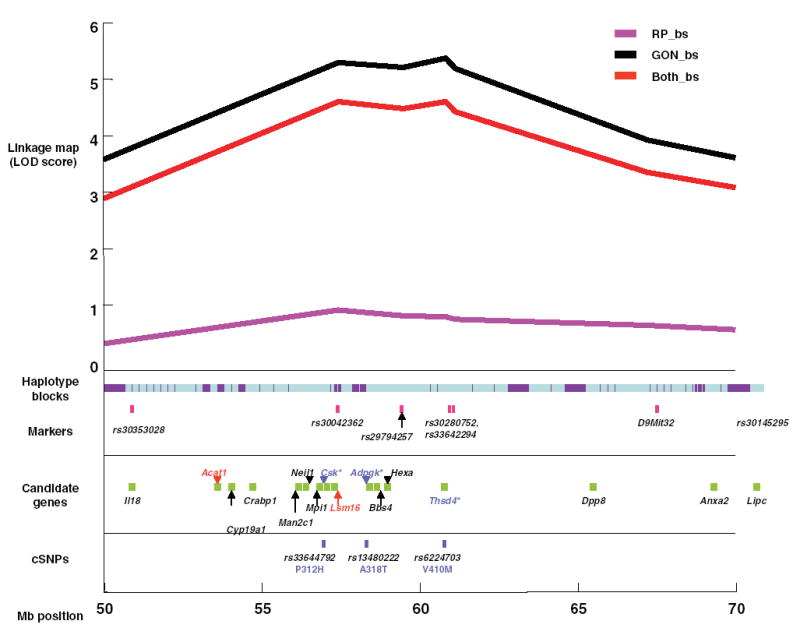
Detailed map of linkage peak region (50–70 Mb). The linkage peak was anchored to markers within 10 Mb from the peak. The haplotype blocks are displayed under the linkage map with light blue demonstrating discordant regions and purple demonstrating regions of concordance. Markers used in mapping the chromosome are displayed in pink immediately under the haplotype blocks. Beneath the markers, candidate genes that were chosen for their functional significance (Table 4) are displayed as follows: gene names in red are lower-priority candidates, gene names in black are medium-priority candidates, and gene names in blue are the highest-priority candidates. Directly beneath the candidate genes are the known cSNPs located within the sequence of the highest priority candidates. The amino acid residue changes and their positions within the polypeptide sequence of the resulting protein are also in blue under the SNP identifier. The approximate position of the map is shown in 5-Mb increments (bottom).
Genes within the discordant blocks are more likely to contain cSNPs than those within concordant blocks. Therefore, three candidate genes that were within discordant blocks and contained known cSNPs were given a higher priority (Table 5). Other genes within discordant blocks were given medium priority rating because of the higher likelihood that a possible cSNP or other functional variant may exist, although none are known based on currently available data. Candidates within the concordant blocks have a lower likelihood of containing cSNPs and thus were given a lower-priority rating. For one gene within a concordant block (Lsm16), we sequenced its open reading frame to determine whether there might be cSNPs that were missed by assuming that the gene was in a concordant block, but none were found.
Discussion
This genetic mapping study supports the existence of a QTL for relative gonadal fat weight on mouse Chromosome 9 with a peak at 45.6 cM and a confidence interval between 17 and 49 cM. Compared with the original genome scan (Reed et al. 2006), these data more precisely define the linked region and provide better statistical support for depot specificity. There are genotype-by-adipose depot interactions in other mouse crosses (Cheverud et al. 2004), and the results reported here are consistent with those from a previous study suggesting that a locus at or near Adip5 has a stronger effect on some depots than others (Stylianou et al. 2006; West et al. 1994a). Our results agree with other studies, summarized in Table 1, showing that usually alleles of this locus from the B6 and other fatter or larger strains are antagonistic and reduce adiposity, and the “plus” alleles are found in the 129 strain and other leaner or smaller strains. We therefore gather that this locus is likely to be involved in the expansion of adipose depot weight specifically, rather than in generalized growth factors leading to a larger or smaller mouse. Furthermore, because B6 body weight- and body length-increasing alleles (Bwq6, Bdln4, Bdln5 QTLs) are nearby and may act to dilute the effects of the 129 body fatness alleles, we may have an explanation as to why this linkage is apparent only when adipose depot weight is adjusted for body size (i.e., body weight and body length) in our study.
The depot specificity of this linkage suggests that effects of allelic variation of the Adip5 locus are stronger in the gonadal depot than in the retroperitoneal depot, which leads to an association with gonadal but not with retroperitoneal depot weight. Therefore, it would be reasonable to search for genes whose expression levels differ by adipose depot and/or that result in depot-specific expansion of adipose tissue. Many of the factors that influence the rate at which new adipose cells (adipogenesis) are generated are expressed at levels that are depot-dependent (Voros et al. 2003). These genes may include those responsible for providing new vasculature (angiogenesis). One study revealed that angiogenesis and adipogenesis may be under dual control by components common to both processes, including vascular endothelial growth factor, its receptors, angiopoietins, ephrins, matrix metalloproteinases, and the plasminogen enzymatic system (Hausman and Richardson 2004).
In addition, although the process of adipogenesis is interrelated with the process of lipid accumulation or lipogenesis, the adipogenic process itself is committed to providing an increase in the number of mature adipose cells for lipid deposition and storage, not for increasing the total amount of lipid available for storage. Therefore, if adipogenesis is increased in a particular depot, we might expect available lipids to be preferentially stored in that depot, increasing the size of that depot without a subsequent increase in the total body fat or total body weight of the mouse. This depot-specific increase in fat deposition, which may be the result of an increase in adipogenesis, meshes with the observed phenotype and, therefore, genes related to this process were considered prime candidates.
One of the highest-priority candidate genes fits this profile quite well. The Thsd4 gene shares some conserved domains with a family of proteins (Adam-ts-1) that are secreted, bind to the extracellular matrix, and control its breakdown, which is a key component to allow the growth and expansion of newly formed adipocytes during adipogenesis (Lilla et al. 2002). Thsd4 is most similar in structure to the Adamts-l subfamily of genes and contains the spacer and TSP1 repeat domains. These domains are those required to bind and cleave substrates and may regulate the function of the ADAMTS proteins (Hirohata et al. 2002). Moreover, the Tsp1 repeat domain has an inhibitory role in angiogenesis, and thus also possibly adipogenesis, by interfering with the function of vascular endothelial growth factor (Fukumura et al. 2003; Iruela-Arispe et al. 1999a, 1999b). Consequently, the Thsd4 gene has features consistent with the role of Adip5 and contains a cSNP. Another high-priority gene that also contains a cSNP variant and is involved in adipogenesis is Csk. Csk interacts with insulin-like growth factor 1 receptors in preadipocytes, causing them to become permissive for differentiation into mature adipocytes (Sekimoto and Boney 2003). A QTL that influences the plasma concentration of insulin-like growth factor binding protein-5 is near Adip5 (Mohan et al. 2003); therefore, it is interesting to note that the CSK and IGFBP5 proteins are both involved in the IGF-1 pathway (Arbet-Engels et al. 1999; Ferry et al. 1999; Salih et al. 2004). A third medium-priority candidate gene Crabp1 may also play a role in adipogenesis as a downstream target of PPARG (Okuno et al. 2002) or by binding to retinoic acid, a known inhibitor of adipocyte differentiation. However, to date there are no known cSNPs in the Crabp1 gene. A more detailed description of the remaining candidate genes selected for their functional relevance is included in Table 4. Although we searched for candidate genes within a narrow region, multiple smaller nearby peaks for fatness and other traits may signal the presence of multiple influential QTL.
Because the origins of mouse strains used in genetic mapping experiments are well described and inbred strains are becoming more densely genotyped, new methods that exploit the recent ancestral history of the strains are being used to map QTLs (Grupe et al. 2001; Mehrabian et al. 2005; Pletcher et al. 2004). This approach is similar to haplotype-sharing methods that identify disease-causing alleles in genetically isolated human populations and, when used in mice, has already met with some success (Liao et al. 2004). There is concern that this method may be useful only when there is a one-to-one correspondence between genotype and phenotype, the range of phenotype among inbred strains is wide, and the effect of genotype is large. Another limitation is that no single standard criterion has emerged to assign haplotype blocks or strain distribution patterns (Ideraabdullah et al. 2004; Pletcher et al. 2004; Wiltshire et al. 2003; Yalcin et al. 2004; Zhang et al. 2005). Given these issues, we took a conservative strategy: We assumed that a single discordant SNP (sometimes called an orphan SNP) broke a concordant block, and we did not exclude candidate genes or regions based on the haplotype block results, although genes within concordant blocks were assigned a lower priority.
The use of mice as a model system for the genetic influences on obesity is based on the premise that mice and humans share regulatory systems of body weight and fatness. To date, this has proved to be the case and there are many examples of correspondence between alleles in particular genes and phenotypes in mice and humans. For instance, in the mouse, mutations in the leptin gene cause early-onset, extreme obesity (Zhang et al. 1994), and the same is true for humans (Montague et al. 1997). The linkage on mouse Chromosome 9 reported here has genomic regions of conserved synteny on two human chromosomes, 11 and 15 (Perusse et al. 2005). Trying to screen mouse candidate genes using information from humans is probably premature, but there are three specific points worth considering: First, one of the candidate genes in the mouse (Bbs4) was identified because the corresponding human gene has alleles that lead to one form of Bardet-Biedl syndrome (Mykytyn et al. 2001), a disorder that has obesity among its cardinal features. Milder alleles of BBS4 genes might contribute to ordinary obesity (Croft et al. 1995), although linkage results in otherwise normal populations suggest that this is rare (Perusse et al. 2005; Reed et al. 1995). Furthermore, mice with targeted alleles of this gene are obese (Mykytyn et al. 2004). Another candidate gene in the mouse, Lipc, was an early human candidate gene in the study of obesity (Hasstedt et al. 1997), and some studies have shown associations between an allele in the LIPC promoter (−514C>T) and visceral obesity in humans (St-Pierre et al. 2003). Another gene was identified as a candidate because of its association with obesity in humans (IL18). Obese women were observed to have higher levels of the proinflammatory cytokine IL18 protein in their blood, and its levels were subsequently reduced following weight loss (Esposito et al. 2002, 2003). In line with the observed phenotype in humans, Il18 knockout mice are hyperphagic, obese, and insulin-resistant (Netea et al. 2006). Considered together, these studies suggest that Il18 protein may be secreted from adipose tissue and provide regulatory information to the brain or other areas about body fatness. A fourth mouse candidate gene, Cyp19a1, was identified because when knocked out it produces a fat phenotype in mice, and also because alleles of this gene are associated with human visceral obesity (Baghaei et al. 2003; Remes et al. 2003; Tworoger et al. 2004). Thus, four genes from this small genomic region are implicated in both mouse and human obesity.
To detect the specific genes that might account for linkage results in mice reported here, we focused our mapping efforts on Chromosome 9, a region linked to adipose depot weight identified from the genome scan and from the related work of other researchers. Candidate gene assessment was conducted by searching databases for genes and their functions and considering the pattern of shared and unshared ancestral chromosomal segments. Several genes emerged that had functions consistent with the effects of Adip5, four of which have alleles of the human ortholog that are associated with obesity and fat patterning (BBS4, LIPC, IL18, and CYP19A1). Other genes discussed earlier (Csk, Thsd4, and Crabp1) may be regulated in a depot-specific manner, influencing the patterning of fat. Our ongoing positional cloning efforts that involve the construction of consomic and congenic mouse strains harboring Adip5, along with gene expression studies that include both the examination of expression of our individual candidate genes and whole-genome microarrays in the depots of interest, should assist and further the gene-identification process.
Obesity is a complex disorder that is a result of the small effects of many genes and the interaction of these genes with the environment. The chromosomal locations of many areas that show linkage to obesity-related traits are now known in mice and their corresponding locations are known in humans. We have identified one such region along mouse Chromosome 9 that accounts for fatness that is specific to the gonadal depot. This locus differs from others in that smaller and/or leaner strains carry alleles that increase trait values. This is an important finding because the genes that underlie this locus may be more influential in predicting fat patterning as opposed to contributing to overall body fatness and, thus, may be affected and have consequences that differ from that of genes that have more generalized effects on obesity and body size. Identifying the genes that contribute uniquely to body fatness will hopefully give us a better understanding of the specific types of obesity, including fat patterning and its development, and will assist with identifying the genes and their variants underlying the individual differences in the predisposition to obesity. This information will also help in developing individually tailored treatment of this disease.
Website References
http://genome.ucsc.edu/: University of California at Santa Cruz Genome (UCSC) Bioinformatics
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=gene: National Center Biotechnology Information (NCBI) Entrez Gene
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi: NCBI PubMed
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=snp: NCBI Entrez SNP with Mouse SNP Build 126
http://www.informatics.jax.org/: Mouse Genome Database
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM: NCBI Online Mendelian Inheritance in Man (OMIM)
http://aretha.jax.org/pub-cgi/phenome/mpdcgi?rtn=docs/home: Mouse Phenome Database, hosted by The Jackson Laboratory
http://www.informatics.jax.org/mgihome/nomen: International Committee on Standardized Genetic Nomenclature for Mice, hosted by The Jackson Laboratory
http://omicspace.riken.jp/index.html: Positional Medline Database
http://obesitygene.pbrc.edu/: Obesity Gene Map
Acknowledgments
Grants from the National Institutes of Health funded this research (R01DK058797 to DRR, R01AA011028 and R01DC00882 to AAB, and R01DK046791 and R01AA12715 to MGT). The authors acknowledge Matthew Thomas, Senior Scientist at DNAPrint, for his assistance with genotyping services, and Dr. Kelly Ewen-White and Paige Stevenson, the Genotyping Section at the Australian Genome Research Facility, for additional genotyping. Maria Theodorides and Fujiko Duke provided excellent technical assistance. Early data collection for this experiment was conducted in the laboratory of R. Arlen Price, and his support is gratefully acknowledged. Patricia Watson provided helpful editorial advice. Discussions with Hong Ji, Mark I. Friedman, and Caroline M. Pond enhanced the quality of this work.
Footnotes
The following sequences have been submitted to the GenBank database: Thsd4: Accession Nos. DQ424862 and DQ517441 and Lsm16: Accession Nos. DQ240818-DQ240819.
References
- 1.Arbet–Engels C, Tartare–Deckert S, Eckhart W. C-terminal Src kinase associates with ligand-stimulated insulin-like growth factor-I receptor. J Biol Chem. 1999;274:5422–5428. doi: 10.1074/jbc.274.9.5422. [DOI] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 2001;72:603–613. doi: 10.1016/s0031-9384(01)00412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Reed DR, Li X, Li S, Beauchamp GK, et al. Voluntary ethanol consumption by mice: genome-wide analysis of quantitative trait loci and their interactions in a C57BL/6ByJ x 129P3/J F2 intercross. Genome Res. 2002;12:1257–1268. doi: 10.1101/gr.129702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baghaei F, Rosmond R, Westberg L, Hellstrand M, Eriksson E, et al. The CYP19 gene and associations with androgens and abdominal obesity in pre-menopausal women. Obes Res. 2003;11:578–585. doi: 10.1038/oby.2003.81. [DOI] [PubMed] [Google Scholar]
- 5.Brockmann GA, Haley CS, Renne U, Knott SA, Schwerin M. Quantitative trait loci affecting body weight and fatness from a mouse line selected for extreme high growth. Genetics. 1998;150:369–381. doi: 10.1093/genetics/150.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheverud JM, Vaughn TT, Pletscher LS, Peripato AC, Adams ES, et al. Genetic architecture of adiposity in the cross of LG/J and SM/J inbred mice. Mamm Genome. 2001;12:3–12. doi: 10.1007/s003350010218. [DOI] [PubMed] [Google Scholar]
- 7.Cheverud JM, Ehrich TH, Hrbek T, Kenney JP, Pletscher LS, et al. Quantitative trait loci for obesity- and diabetes-related traits and their dietary responses to high-fat feeding in LGXSM recombinant inbred mouse strains. Diabetes. 2004;53:3328–3336. doi: 10.2337/diabetes.53.12.3328. [DOI] [PubMed] [Google Scholar]
- 8.Corral J, Miralles JM, Garcia-Pascual IJ, Corrales JJ, Garcia-Sastre A, et al. Increased serum N-acetyl-beta-D-glucosaminidase and alpha-D-mannosidase activities in obese subjects. Clin Invest. 1992;70:880–884. doi: 10.1007/BF00180432. [DOI] [PubMed] [Google Scholar]
- 9.Corva PM, Horvat S, Medrano JF. Quantitative trait loci affecting growth in high growth (hg) mice. Mamm Genome. 2001;12:284–290. doi: 10.1007/s003350010275. [DOI] [PubMed] [Google Scholar]
- 10.Croft JB, Morrell D, Chase CL, Swift M. Obesity in heterozygous carriers of the gene for the Bardet–Biedl syndrome. Am J Med Genet. 1995;55:12–15. doi: 10.1002/ajmg.1320550105. [DOI] [PubMed] [Google Scholar]
- 11.Edwards AL. Statistical Methods. New York: Holt, Rinehart and Winston; 1973. [Google Scholar]
- 12.Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, et al. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 13.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 14.Fabsitz RR, Nam JM, Gart J, Stunkard A, Price RA, et al. HLA associations with obesity. Hum Hered. 1989;39:156–164. doi: 10.1159/000153852. [DOI] [PubMed] [Google Scholar]
- 15.Farahani P, Fisler JS, Wong H, Diament AL, Yi N, et al. Reciprocal hemizygosity analysis of mouse hepatic lipase reveals influence on obesity. Obes Res. 2004;12:292–305. doi: 10.1038/oby.2004.37. [DOI] [PubMed] [Google Scholar]
- 16.Ferry RJ, Jr, Cerri RW, Cohen P. Insulin-like growth factor binding proteins: new proteins, new functions. Horm Res. 1999;51:53–67. doi: 10.1159/000023315. [DOI] [PubMed] [Google Scholar]
- 17.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia Pascual JJ, Villar E, Corrales JJ, Garcia-Sastre A, Garcia-Diez LC, et al. Enzymatic glycosidase activities in experimental obesity. Horm Metab Res. 1992;24:412–415. doi: 10.1055/s-2007-1003348. [DOI] [PubMed] [Google Scholar]
- 19.Grupe A, Germer S, Usuka J, Aud D, Belknap JK, et al. In silico mapping of complex disease-related traits in mice. Science. 2001;292:1915–1918. doi: 10.1126/science.1058889. [DOI] [PubMed] [Google Scholar]
- 20.Harada K, Shen WJ, Patel S, Natu V, Wang J, et al. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. Am J Physiol Endocrinol Metab. 2003;285:E1182–E1195. doi: 10.1152/ajpendo.00259.2003. [DOI] [PubMed] [Google Scholar]
- 21.Hasstedt SJ, Hoffman M, Leppert MF, Elbein SC. Recessive inheritance of obesity in familial non-insulin-dependent diabetes mellitus, and lack of linkage to nine candidate genes. Am J Hum Genet. 1997;61:668–677. doi: 10.1086/515509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 23.Hirohata S, Wang LW, Miyagi M, Yan L, Seldin MF, et al. Punctin, a novel ADAMTS-like molecule, ADAMTSL-1, in extracellular matrix. J Biol Chem. 2002;277:12182–12189. doi: 10.1074/jbc.M109665200. [DOI] [PubMed] [Google Scholar]
- 24.Hogan B, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1986. [Google Scholar]
- 25.Ideraabdullah FY, de la Casa-Esperon E, Bell TA, Detwiler DA, Magnuson T, et al. Genetic and haplotype diversity among wild-derived mouse inbred strains. Genome Res. 2004;14:1880–1887. doi: 10.1101/gr.2519704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iruela–Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999a;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- 27.Iruela–Arispe ML, Rodriguez–Manzaneque JC, Abu–Jawdeh G. Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation. 1999b;6:127–140. [PubMed] [Google Scholar]
- 28.Ishimori N, Li R, Kelmenson PM, Korstanje R, Walsh KA, et al. Quantitative trait loci that determine plasma lipids and obesity in C57BL/6J and 129S1/SvImJ inbred mice. J Lipid Res. 2004;45:1624–1632. doi: 10.1194/jlr.M400098-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Iwaki T, Yamashita H, Hayakawa T. A color atlas of sectional anatomy of the mouse. Tokyo: Adthree Publishing Co., Ltd; 2001. [Google Scholar]
- 30.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Misso ML, et al. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol. 2001;79:3–9. doi: 10.1016/s0960-0760(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 31.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 32.Lander E, Green P, Abrahamson J, Barlow A, Daley M, et al. MAPMAKER: An interactive complex package for constructing primary linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- 33.Lang DH, Sharkey NA, Mack HA, Vogler GP, Vandenbergh DJ, et al. Quantitative trait loci analysis of structural and material skeletal phenotypes in C57BL/6J and DBA/2 second-generation and recombinant inbred mice. J Bone Miner Res. 2005;20:88–99. doi: 10.1359/JBMR.041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao G, Wang J, Guo J, Allard J, Cheng J, et al. In silico genetics: identification of a functional element regulating H2-Ealpha gene expression. Science. 2004;306:690–695. doi: 10.1126/science.1100636. [DOI] [PubMed] [Google Scholar]
- 35.Lilla J, Stickens D, Werb Z. Metalloproteases and adipogenesis: a weighty subject. Am J Pathol. 2002;160:1551–1554. doi: 10.1016/S0002-9440(10)61100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehrabian M, Wen PZ, Fisler J, Davis RC, Lusis AJ. Genetic loci controlling body fat, lipoprotein metabolism, and insulin levels in a multifactorial mouse model. J Clin Invest. 1998;101:2485–2496. doi: 10.1172/JCI1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehrabian M, Allayee H, Stockton J, Lum PY, Drake TA, et al. Integrating genotypic and expression data in a segregating mouse population to identify 5-lipoxygenase as a susceptibility gene for obesity and bone traits. Nat Genet. 2005;37:1224–1233. doi: 10.1038/ng1619. [DOI] [PubMed] [Google Scholar]
- 38.Mir AA, Myakishev MV, Polesskaya OO, Moitra J, Petersen D, et al. A search for candidate genes for lipodystrophy, obesity and diabetes via gene expression analysis of A-ZIP/F-1 mice. Genomics. 2003;81:378–390. doi: 10.1016/s0888-7543(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 39.Mohan S, Masinde G, Li X, Baylink DJ. Mapping quantitative trait loci that influence serum insulin-like growth factor binding protein-5 levels in F2 mice (MRL/MpJ x SJL/J) Endocrinology. 2003;144:3491–3496. doi: 10.1210/en.2003-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montague CT, Farooqi S, Whitehead JP, Soos MA, Rau H, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 41.Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, et al. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet. 2001;28:188–191. doi: 10.1038/88925. [DOI] [PubMed] [Google Scholar]
- 42.Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, et al. Bardet–Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci USA. 2004;101:8664–8669. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 44.Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 45.Okuno M, Arimoto E, Nishizuka M, Nishihara T, Imagawa M. Isolation of up- or down-regulated genes in PPARgamma-expressing NIH-3T3 cells during differentiation into adipocytes. FEBS Lett. 2002;519:108–112. doi: 10.1016/s0014-5793(02)02720-5. [DOI] [PubMed] [Google Scholar]
- 46.Pederson RA, White HA, Schlenzig D, Pauly RP, McIntosh CH, et al. Improved glucose tolerance in Zucker fatty rats by oral administration of the dipeptidyl peptidase IV inhibitor isoleucine thiazolidide. Diabetes. 1998;47:1253–1258. doi: 10.2337/diab.47.8.1253. [DOI] [PubMed] [Google Scholar]
- 47.Perusse L, Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, et al. The human obesity gene map: the 2004 update. Obes Res. 2005;13:381–490. doi: 10.1038/oby.2005.50. [DOI] [PubMed] [Google Scholar]
- 48.Pletcher MT, McClurg P, Batalov S, Su AI, Barnes SW, et al. Use of a dense single nucleotide polymorphism map for in silico mapping in the mouse. PLoS Biol. 2004;2:e393. doi: 10.1371/journal.pbio.0020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price AH. Believe it or not, QTLs are accurate! Trends Plant Sci. 2006;11:213–216. doi: 10.1016/j.tplants.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Reed DR, Ding Y, Xu W, Cather C, Price R. Human obesity does not segregate with the chromosomal regions of Prader–Willi, Bardet–Biedl, Borjeson or Wilson–Turner syndromes. Int J Obes Relat Metab Disord. 1995;19:599–603. [PubMed] [Google Scholar]
- 51.Reed DR, Li X, McDaniel AH, Lu K, Li S, et al. Loci on chromosomes 2, 4, 9 and 16 for body weight, body length and adiposity identified in a genome scan of an F2 intercross between the 129P3/J and C57BL/6ByJ mouse strains. Mamm Genome. 2003;14:302–313. doi: 10.1007/s00335-002-2170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reed DR, McDaniel AH, Li X, Tordoff MG, Bachmanov AA. Quantitative trait loci for individual adipose depots weight in C57BL/6ByJ x 129P3/J F2 mice. Mamm Genome. 2006;17:xxx–xxx. doi: 10.1007/s00335-006-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Remes T, Vaisanen SB, Mahonen A, Huuskonen J, Kroger H, et al. Aerobic exercise and bone mineral density in middle-aged finnish men: a controlled randomized trial with reference to androgen receptor, aromatase, and estrogen receptor alpha gene polymorphisms small star, filled. Bone. 2003;32:412–420. doi: 10.1016/s8756-3282(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 54.Ronimus RS, Morgan HW. Cloning and biochemical characterization of a novel mouse ADP-dependent glucokinase. Biochem Biophys Res Commun. 2004;315:652–658. doi: 10.1016/j.bbrc.2004.01.103. [DOI] [PubMed] [Google Scholar]
- 55.Salih DA, Tripathi G, Holding C, Szestak TA, Gonzalez MI, et al. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc Natl Acad Sci USA. 2004;101:4314–4319. doi: 10.1073/pnas.0400230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sekimoto H, Boney CM. C-terminal Src kinase (CSK) modulates insulin-like growth factor-I signaling through Src in 3T3-L1 differentiation. Endocrinology. 2003;144:2546–2552. doi: 10.1210/en.2003-0187. [DOI] [PubMed] [Google Scholar]
- 57.St-Pierre J, Miller–Felix I, Paradis ME, Bergeron J, Lamarche B, et al. Visceral obesity attenuates the effect of the hepatic lipase −514C>T polymorphism on plasma HDL-cholesterol levels in French-Canadian men. Mol Genet Metab. 2003;78:31–36. doi: 10.1016/s1096-7192(02)00223-8. [DOI] [PubMed] [Google Scholar]
- 58.Stylianou IM, Korstanje R, Li R, Sheehan S, Paigen B, et al. Quantitative trait locus analysis for obesity reveals multiple networks of interacting loci. Mamm Genome. 2006;17:22–36. doi: 10.1007/s00335-005-0091-2. [DOI] [PubMed] [Google Scholar]
- 59.Taylor BA, Tarantino LM, Phillips SJ. Gender-influenced obesity QTLs identified in a cross involving the KK type II diabetes-prone mouse strain. Mamm Genome. 1999;10:963–968. doi: 10.1007/s003359901141. [DOI] [PubMed] [Google Scholar]
- 60.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, et al. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 61.Tworoger SS, Chubak J, Aiello EJ, Yasui Y, Ulrich CM, et al. The effect of CYP19 and COMT polymorphisms on exercise-induced fat loss in postmeno-pausal women. Obes Res. 2004;12:972–981. doi: 10.1038/oby.2004.119. [DOI] [PubMed] [Google Scholar]
- 62.Vartanian V, Lowell B, Minko IG, Wood TG, Ceci JD, et al. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase. Proc Natl Acad Sci USA. 2006;103:1864–1869. doi: 10.1073/pnas.0507444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vauti F, Meyer N, Ruiz P, Kumar S, Arnold H. Mutation of a novel gene in the mouse results in reduction of body weight and fat tissue; 17th International Mouse Genome Conference; Braunschweig, Germany. 2003. [Google Scholar]
- 64.Voros G, Maquoi E, Collen D, Lijnen HR. Differential expression of plasminogen activator inhibitor-1, tumor necrosis factor-alpha, TNF-alpha converting enzyme and ADAMTS family members in murine fat territories. Biochim Biophys Acta. 2003;1625:36–42. doi: 10.1016/s0167-4781(02)00589-4. [DOI] [PubMed] [Google Scholar]
- 65.Wade CM, Kulbokas EJ, Kirby AW, Zody MC, Mullikin JC, et al. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- 66.West DB, Goudey–Lefevre J, York B, Truett GE. Dietary obesity linked to genetic loci on chromosome 9 and 15 in a polygenic mouse model. J Clin Invest. 1994a;94:1410–1416. doi: 10.1172/JCI117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West DB, Waguespack J, York B, Goudey–Lefevre J, Price RA. Genetics of dietary obesity in AKR/J X SWR/J mice: segregation of the trait and identification of a linked locus on Chromosome 4. Mamm Genome. 1994b;5:546–552. doi: 10.1007/BF00354928. [DOI] [PubMed] [Google Scholar]
- 68.Wiltshire T, Pletcher MT, Batalov S, Barnes SW, Tarantino LM, et al. Genome-wide single-nucleotide polymorphism analysis defines haplotype patterns in mouse. Proc Natl Acad Sci USA. 2003;100:3380–3385. doi: 10.1073/pnas.0130101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yaguchi H, Togawa K, Moritani M, Itakura M. Identification of candidate genes in the type 2 diabetes modifier locus using expression QTL. Genomics. 2005;85:591–599. doi: 10.1016/j.ygeno.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Yalcin B, Fullerton J, Miller S, Keays DA, Brady S, et al. Unexpected complexity in the haplotypes of commonly used inbred strains of laboratory mice. Proc Natl Acad Sci USA. 2004;101:9734–9739. doi: 10.1073/pnas.0401189101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Hunter KW, Gandolph M, Rowe WL, Finney RP, et al. A high-resolution multistrain haplo-type analysis of laboratory mouse genome reveals three distinctive genetic variation patterns. Genome Res. 2005;15:241–249. doi: 10.1101/gr.2901705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]


