Abstract
Febrile seizures are frequent during early childhood, and prolonged (complex) febrile seizures are associated with an increased susceptibility to temporal lobe epilepsy. The pathophysiological consequences of febrile seizures have been extensively studied in rat pups exposed to hyperthermia. The mechanisms that trigger these seizures are unknown, however. A rise in brain pH is known to enhance neuronal excitability. Here we show that hyperthermia causes respiratory alkalosis in the immature brain, with a threshold of 0.2–0.3 pH units for seizure induction. Suppressing alkalosis with 5% ambient CO2 abolished seizures within 20 s. CO2 also prevented two long-term effects of hyperthermic seizures in the hippocampus: the upregulation of the Ih current and the upregulation of CB1 receptor expression. The effects of hyperthermia were closely mimicked by intraperitoneal injection of bicarbonate. Our work indicates a mechanism for triggering hyperthermic seizures and suggests new strategies in the research and therapy of fever-related epileptic syndromes.
Febrile seizures are the most common type of convulsive events in humans between the ages of 6 months and 6 years, with a prevalence that varies from 3% to 14% between different populations worldwide1,2. Although most febrile seizures are apparently benign, one-third of them are ‘complex,’ with a prolonged duration (>10–20 min), and are associated with a risk of subsequent epilepsy3,4. Febrile seizures have been extensively studied in a well-established animal model, in which prolonged seizure activity is evoked by exposing rat pups to a hyperthermic environment that raises the body temperature to a level comparable to human fever5,6. Work on this model has mainly focused on the short- and long-term consequences of experimental febrile seizures, including effects on neuronal survival7, receptor and channel expression8, excitability9–11, plasticity12 and seizure susceptibility13. Notably, however, there is little information available on the mechanisms that are responsible for the triggering and maintenance of these hyperthermia-induced seizures.
It is known that an elevated body temperature results in an increase in the rate of breathing, especially in young children14–16, which reflects the general role of ventilation in the regulation of mammalian body temperature17–19. Although changes in breathing pattern do not necessarily have an effect on the partial pressure of CO2 (PCO2) in the blood, it has been reported that hyperventilation (by definition, a decrease in PCO2 resulting in an alkaline shift in pH20) can develop during ongoing hyperthermia18. This is interesting in the present context, as it is generally known that a rise in pH leads to an increase in neuronal excitability and often to epileptiform activity both in vitro and in vivo20–30.
The present work shows that, in the rat pup model of complex experimental febrile seizures, hyperthermia leads to thermal tachypnea and to a consequent, age-dependent respiratory alkalosis that triggers and sustains convulsions. A moderate elevation of ambient CO2 to 5% results in the abolishment of the hyperthermia-induced respiratory alkalosis and in a fast block of the seizures. Our results suggest new designs for therapies aimed at preventing or interrupting febrile seizures and related convulsive events. Our observations are also likely to be useful in devising strategies in the search for factors at the genetic and systems31–34 levels that enhance an individual’s susceptibility for fever-related epileptic syndromes.
RESULTS
Age dependence of hyperthermia-induced tachypnea and seizures
To examine whether hyperthermia-induced seizures are associated with changes in respiration, we made parallel measurements of body temperature and breathing frequency in rat pups at postnatal days (P)8–P11 and P22–P23. In the P8–P11 rats (n = 21), breathing frequency was 163 ± 14 breaths/min under control conditions, and an increase in body temperature was closely paralleled by an increase in the breathing rate. The hyperthermia-induced seizures occurred when the breathing rate increased by about 60%, to a value of 254 ± 44 breaths/min ( Fig. 1a). This rate was achieved when the body temperature increased from its control level of 33.4 ± 0.9 to 41.8 ± 0.7 °C. Both the accelerated breathing and the seizures were promptly terminated by reducing the body temperature. This was followed by a slow, transient undershoot to 140 ± 17 breaths/min in the breathing rate.
Figure 1.
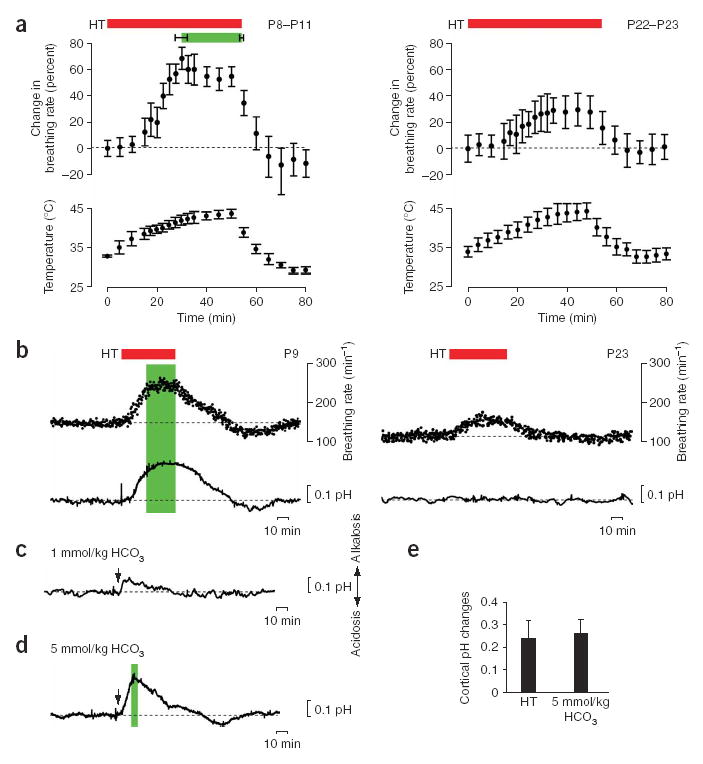
Hyperthermia-induced behavioral seizures are associated with brain alkalosis. (a) Hyperthermia (HT)-induced changes in body temperature (lower panels) and breathing rate (upper panels) in P8–P11 (n = 21; left panels) and P22–P23 (right panels) rat pups. The green bar marks the onset and end of the hyperthermia-induced seizures; the red bar indicates exposure to hyperthermia. Data here and below are mean ± (or +) s.d. Seizures did not occur in P22–P23 rats. (b) Simultaneous recording of hyperthermia-induced changes in breathing rate and intracortical pH in a P9 (left) and a P23 (right) rat. The green bar indicates hyperthermia-induced seizures. (c) Intraperitoneal application (arrow) of 1 mmol/kg bicarbonate induces a small (~0.1 pH units) alkaline shift in pH in the brain of a P9 rat, but no seizure activity. (d) Application of 5 mmol/kg bicarbonate in a P9 rat induces an intracortical alkalosis of 0.24 pH units. The green bar indicates seizure activity. (e) Summary of cortical pH changes in P8–P11 rat pups at threshold for seizure initiation during hyperthermia (n = 9) and upon injection of 5 mmol/kg bicarbonate (n = 7). The two sets of data are not statistically different (P = 0.424).
In the P22–P23 group (n = 14), exposure to hyperthermia resulted in an increase in body temperature to 44.2 ± 1.1 °C from its control level of 34.3 ± 0.7 °C, and in a 28% increase in breathing frequency from 112 ± 11 breaths/min to 144 ± 23 breaths/min ( Fig. 1a). In contrast to the younger rats, the 55 min of hyperthermia experienced by the older rats did not evoke seizures5.
Hyperthermia induces respiratory alkalosis in the immature brain
The above experiments show that changes in body temperature produce marked changes in breathing rate in rat pups19. But this thermal tachypnea does not, as such, necessarily imply that there would be a reduction in systemic PCO2 with a consequent increase in pH affecting the brain18,20. Hence, we carried out experiments on freely moving rat pups at P8–P11 and at P22–P23 with an implanted cortical pH electrode.
At P8–P11, the accelerated breathing during hyperthermia was closely paralleled by a rise in intracortical pH ( Fig. 1b). The onset of seizure activity took place when the intracortical pH was elevated by 0.23 ± 0.08 units from its control level of 7.22 ± 0.096 (n = 9). Thereafter, the pH shift showed a further slight increase to a maximum of 0.27 ± 0.04 units above control. After reduction of body temperature, the recovery of cortical pH was, in parallel with the respiration rate, followed by a slight but consistent rebound below the initial control level ( Fig. 1b). Hence, the changes in the rate of breathing and brain pH were tightly and monotonically linked to alterations in body temperature at P8–P11. In contrast to this linkage, hyperthermia produced a moderate increase in respiration rate but no detectable change in brain pH in the more mature, P22–P23 rats (pH, 7.26 ± 0.08 and 7.29 ± 0.09 in control and hyperthermia, respectively; n = 8, P = 0.52).
Brain alkalosis and seizure activity evoked by bicarbonate
To examine whether the hyperthermia-induced brain alkalosis is sufficient to trigger the seizures observed at P8–P11, we used intraperitoneal injections of bicarbonate to evoke pH shifts in the brain. Bicarbonate at 1 mmol/kg induced a small alkalosis in the cortex (0.09 ± 0.03 units, n = 8), but no seizures were observed ( Fig. 1c). But increasing the dose to 5 mmol/kg produced a fast increase in cortical pH of 0.29 ± 0.05 units which was accompanied by seizures in seven of seven experiments ( Fig. 1d). Both the duration of the alkaline cortical pH shift after injection of bicarbonate and the associated behavioral seizure activity were more brief (~5 min) than those observed under hyperthermia. This is expected, considering that the bicarbonate injection produced only a transient alkaline load. Apart from their more brief duration, the behavioral characteristics of the bicarbonate-induced seizures were similar to those induced by hyperthermia. The threshold pH change at seizure onset after administration of bicarbonate was considerably similar (0.26 ± 0.06 units; n = 7) to what was seen under hyperthermia ( Fig. 1e).
Elevation of CO2 suppresses hyperthermia-induced ictal activity
The data described so far support the idea that the experimental febrile seizures are triggered by brain alkalosis. If this is so, a straightforward and testable prediction is that inhibition of respiratory alkalosis should suppress the hyperthermia-induced ictogenesis. We carried out simultaneous recordings with electrodes implanted into the hippocampus and into the adjacent temporal cortex in the nonanesthetized, freely moving P8–P11 pups. In 12 of 12 experiments in which movement artifacts did not preclude an accurate analysis of the exact onset times of the electrographic ictal events, a near-coincident seizure onset in the hippocampus and the adjacent temporal cortex was seen, with a mean delay of 1.3 ± 0.8 s in the cortical versus hippocampal activity ( Fig. 2a). The frequency of field potential discharges during the ictal activity was 3.0–3.5 Hz in both the hippocampus and cortex (n = 26).
Figure 2.
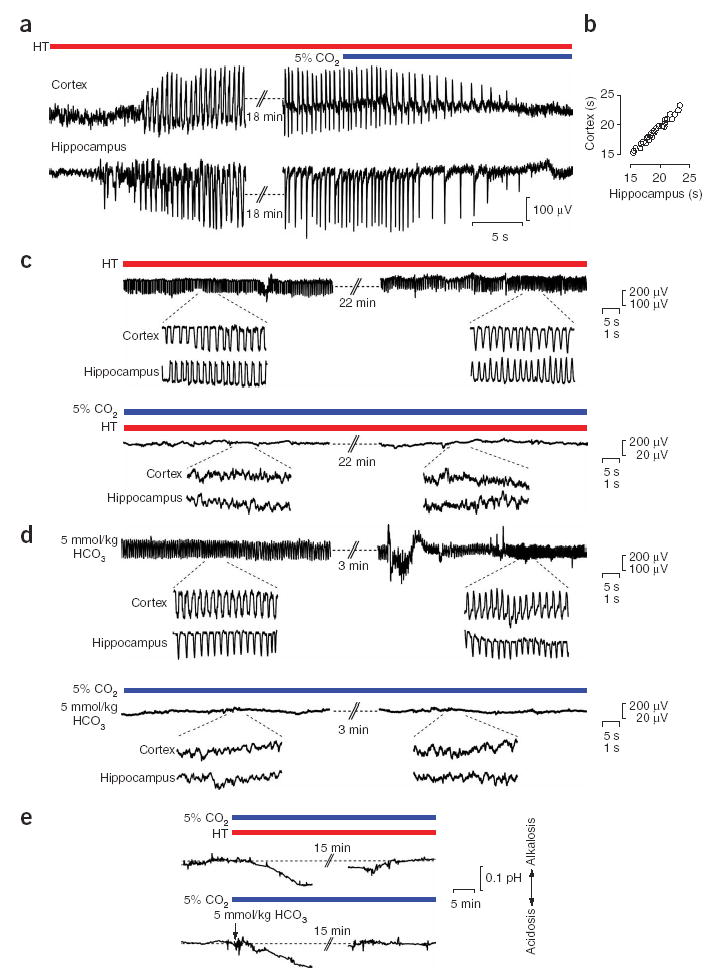
Exposure of rat pups to 5% ambient CO2 blocks hyperthermia- and bicarbonate-induced brain alkalosis and associated ictal activity. (a) Recording in a P9 rat showing simultaneous hyperthermia (HT)-induced ictal activity in the hippocampus and in the temporal cortex, and the fast antiepileptic action of ambient 5% CO2. (b) Time delay between exposure of the pups to 5% CO2 to the block of ongoing ictal activity in the cortex and hippocampus in the P8–P11 rats (n = 29). (c) Cortical recording during a continuous 25-min hyperthermia-induced seizure (upper panel) in a P9 rat. Under identical hyperthermic conditions, but in the presence of 5% CO2, no ictal activity is observed (lower panel). The insets show simultaneous cortical and hippocampal recordings at an expanded scale. (d) Cortical recording immediately after the onset and before the end of ictal activity evoked by intraperitoneal application of 5 mmol/kg bicarbonate (upper panel) in a P10 rat. The bicarbonate-induced ictal activity is completely suppressed by exposure to 5% CO2 (lower panel). The insets show simultaneous cortical and hippocampal recordings at an expanded scale. (e) The alkalosis induced by hyperthermia or intraperitoneal injection of 5 mmol/kg bicarbonate (P9 rats) is completely abolished in the presence of 5% CO2. During prolonged exposure to 5% CO2, a slow acid shift is seen.
An inhibition of hyperventilation-induced physiological effects can be effectively achieved by increasing the ambient CO2 level while keeping the oxygen level constant35. In agreement with this, application of 5% CO2 during fully developed hyperthermia-induced seizures completely blocked the electrographic ictal activity within approximately 15–25 s in both the hippocampus and the cortex ( Fig. 2a,b). The markedly fast block of seizure activity was seen in all 22 experiments of this kind. In addition, we found that the electrographic ictal activity and the associated behavioral manifestations were completely and invariably blocked when 5% CO2 was preapplied shortly before, and maintained throughout, the exposure to hyperthermia ( Fig. 2c). Exposure of the pups to 5% CO2 had no effect on body temperature during hyperthermia (41.8 ± 0.9 °C and 42.1 ± 1.1 °C in the absence versus presence of 5% CO2; n = 8, P = 0.78).
As expected on the basis of the behavioral observations, the bicarbonate-induced electrographic seizures at P8–P11 were characterized by a rapid onset and brief duration of about 4.4 ± 1.8 min. Exposure to 5% CO2 at the time of bicarbonate injection completely blocked the hippocampal and cortical ictal activity as well as their behavioral correlates (n = 11; Fig. 2d).
Finally, direct measurements of cortical pH in the P8–P11 pups during the exposure to 5% CO2 showed that the cortical alkalosis in response to hyperthermia was completely abolished. There was a slight, delayed acidosis that achieved its maximum value of 0.10 ± 0.02 units (n = 5) with a delay of 15–20 min ( Fig. 2e). The brain alkalosis seen in response to injection of 5 mmol/kg bicarbonate was also blocked by 5% CO2 and, again, a small and slowly developing acid shift took place (0.11 ± 0.03 units, n = 5; Fig. 2e).
CO2 blocks the long-term effects of hyperthermia
Previous work has shown a long-lasting upregulation of the hyperpolarization-activated cation current (Ih) in response to hyperthermia-induced seizures10. To test whether blocking seizure activity by exposure of the pups to 5% CO2 inhibits this effect of hyperthermia on Ih, we carried out whole-cell recordings from CA1 pyramidal cells in hippocampal slices 8–11 d after the experimental manipulations. Indeed, the hyperthermia-induced enhancement of the hyperpolarization-activated ‘sag’ potential and of the rebound depolarization10 was completely blocked in slices taken from pups that were exposed to hyperthermia in the presence of 5% CO2 ( Fig. 3a). Furthermore, the hyperthermia-induced increase in the average number of action potentials evoked by the rebound depolarization10 was also blocked in these slices ( Fig. 3a).
Figure 3.
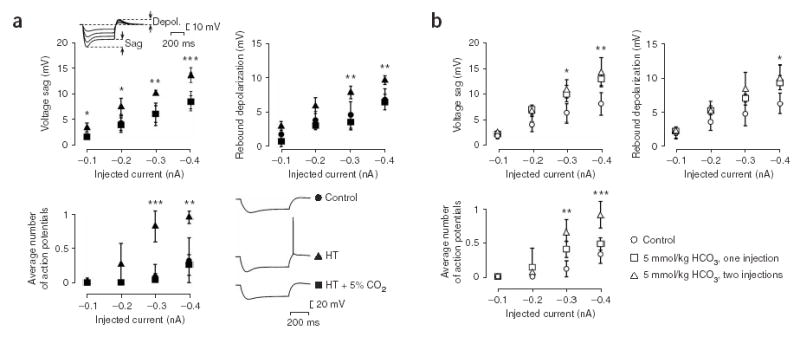
Ambient 5% CO2 applied during hyperthermia blocks the long-term upregulation of the Ih current. (a) The amplitudes of the Ih-generated voltage sag and rebound depolarization evoked by hyperpolarizing current pulses in CA1 pyramidal neurons in hippocampal slices are enhanced in slices from pups previously exposed to hyperthermia compared to control slices10, but there are no significant differences between neurons from control and hyperthermia + 5% CO2 rats (control, n (slices) = 6; hyperthermia, n = 7; hyperthermia + 5% CO2, n = 5). The inset shows recordings performed 10 d after the induction of hyperthermia-induced seizures at P9. The number of action potentials evoked by the rebound depolarization after hyperpolarizing currents ≥ 0.3 nA is increased in slices from pups previously exposed to hyperthermia10. No significant differences were found between neurons from hyperthermia + 5% CO2 and control rats (bottom left panel; control, n (slices) = 5; hyperthermia, n = 6; hyperthermia + 5% CO2, n = 4). Five to ten current pulses of each amplitude shown on the x-axis were applied. Specimen traces are shown on the bottom right. (b) After seizures caused by one or two bicarbonate injections, the Ih-generated sag (top left panel), the rebound depolarization (top right panel) and the number of action potentials evoked by the rebound depolarization after hyperpolarizing currents ≥ 0.3 nA (bottom panel) are enhanced (control, n (slices) = 8; one bicarbonate injection, n = 8; two bicarbonate injections, n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 for test versus control (ANOVA).
The effects of the bicarbonate-induced seizures on Ih were similar to those evoked by hyperthermia ( Fig. 3b). But two consecutive injections of bicarbonate were needed for effects that were quantitatively comparable to those induced by hyperthermia. This is probably attributable to the brief duration of the seizures induced by bicarbonate.
In addition to the upregulation of Ih, another salient molecular effect characteristic of complex experimental febrile seizures is a long-term change in cannabinoid signaling12. Therefore, we carried out CB1 receptor western blot analyses from hippocampi at 1–120 d after causing the hyperthermia-induced seizures at P10 ( Fig. 4a). We observed a rise in CB1 receptor protein that peaked 5 d after the hyperthermia-induced seizures to a level of about 170% versus control ( Fig. 4b). This was followed by a decline at 10 d, and a full recovery to the control level was evident at 120 d. In contrast to the hippocampi from pups that had undergone hyperthermia-induced seizures, no statistically significant changes in the expression of the CB1 protein were seen in hippocampi from pups exposed to hyperthermia in the presence of 5% CO2.
Figure 4.
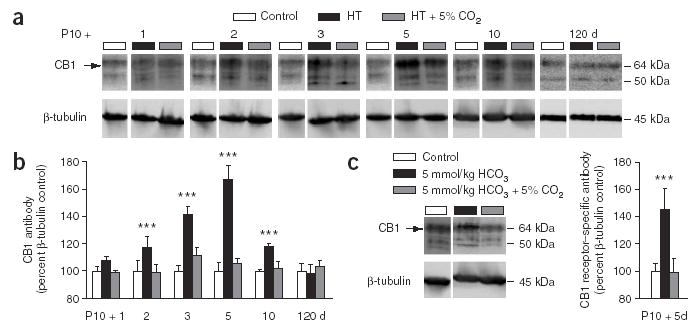
Ambient 5% CO2 applied during hyperthermia blocks the long-term upregulation of the CB1 receptors. (a) Western blots of CB1 receptor protein from hippocampi 1, 2, 3, 5, 10 and 120 d after exposing P10 rats to hyperthermia (red) or to the hyperthermia + 5% CO2 treatment (blue), and from control littermates (white). β-tubulin was used as a reference protein for the quantification shown in b. (b) Quantitative analysis of western blots. An upregulation of the CB1 receptor protein is evident after 2 d following hyperthermia, and a maximum is seen after 5 d. No significant change in CB1 receptor protein expression was found in the hyperthermia + 5% CO2 rats compared to controls (all values represent percentage changes normalized to β-tubulin levels). The value at each time point is based on data from 12 hippocampi. (c) After bicarbonate injection, an upregulation in CB1 receptor is seen at 5 d after seizures (n = 6). This effect is fully blocked in rats in which the seizures were suppressed by 5% CO2. ***P < 0.001, control versus hyperthermia, or control versus bicarbonate injection.
Again, the bicarbonate seizures mimicked the effects of hyperthermia-induced seizures, and a single injection was sufficient for a pronounced upregulation (144 ± 16%; n = 6) of CB1 receptor expression ( Fig. 4c). This suggests a lower threshold for the seizure effects on CB1 than on Ih. The bicarbonate-induced effect on expression of CB1 was completely suppressed when seizure activity was blocked by 5% CO2.
DISCUSSION
Here, we show that an increase in the body temperature of rat pups leads to a pronounced increase in the rate of respiration, which is followed by an increase in brain pH that triggers ictal activity. That the ictogenesis in the present model of febrile seizures5,6 is causally connected to the respiratory alkalosis is supported by several lines of evidence. First, there is an extensive amount of work showing that alkalosis of brain tissue leads to enhanced neuronal excitability and to epileptiform activity20–30. We found that in the P8–P11 pups (i) seizure activity induced by hyperthermia had a well-defined pH threshold of about 0.25 units; (ii) seizures were provoked by brain alkalosis of a similar magnitude after injection of bicarbonate; (iii) an increase in the ambient CO2 to 5% blocked the hyperthermia-associated brain alkalosis as well as the experimental febrile seizures, without affecting body temperature; and (iv) the bicarbonate-induced pH changes and seizures were also blocked by 5% ambient CO2. Finally, although hyperthermia led to a moderate increase in respiratory frequency, there was no respiratory alkalosis and, consistent with this, no seizures in rats at P22–P23. The dependence on age of experimental febrile seizures has been well characterized5,6 but, so far, no clear explanation has been provided for the high propensity of rats for hyperthermia-induced seizures at around P10. This topic will be discussed in detail below.
Prevention of brain alkalosis and the consequent experimental febrile seizures by 5% CO2 blocked two types of hyperthermia-induced changes at the level of neuronal communication and long-term plasticity: the increase in the Ih current in hippocampal pyramidal neurons10 and the increase in the expression of the CB1 cannabinoid receptor12. Our data indicate that the upregulation of both Ih and CB1 are caused by seizure activity and not by hyperthermia as such. Moreover, we found that with regard to the long-term modulation of Ih and CB1, the effects of the hyperthermia-induced seizures were closely mimicked by those caused by bicarbonate injection. This provides additional support for the key role of brain alkalosis. We also show for the first time that robust, hyperthermia-induced electrographic seizure activity can be recorded not only in the hippocampus6 but also in the temporal cortex. A major conclusion that can be drawn from the present work, however, is that the basic mechanisms underlying experimental febrile seizures cannot be elucidated by focusing solely on hippocampal and cortical functions. It is obvious that the temperature-sensitive mechanisms controlling respiratory activity and acid-base homeostasis have a key role in the generation of experimental febrile seizures.
Despite the abundance of data on the age dependence of experimental febrile seizures5, previous work has shed little light on the question of why the immature rat brain has a particularly high susceptibility for the generation of hyperthermia-induced seizures at the end of the second week of life. It should be noted that the experimental febrile seizures are evoked in normal, healthy rat pups that do not have any specific pathological susceptibility for hyperthermia-induced seizures. Our observations provide a new view into this problem.
In mammals, the neuronal mechanisms that control respiration have the dual task of playing an important role in thermoregulation and maintaining the partial pressures of blood gases (both oxygen and CO2) constant18,36,37. But the immature neuronal mechanisms that control respiration in the neonatal rat19,38 are apparently not able to cope with the above dual homeostatic functions when challenged by an abnormally high increase in ambient temperature. The overall result is a thermal tachypnea that strives to cool the body, but obviously does so at the expense of a net loss in CO2 homeostasis, which thereby becomes manifest as a substantial respiratory alkalosis in brain tissue. It is of interest to note that the CO2 chemosensitivity of rat pups reaches a minimum at around P10 (ref. 39). Our observation that hyperthermia leads to respiratory alkalosis in P8–P11 but not in P22–P23 rats are in agreement with the fact that the ‘nadir’ in CO2 chemosensitivity39 coincides with the developmental time window in which the hyperthermia-induced seizures have their lowest threshold5,6.
It is widely assumed that experimental febrile seizures as studied in the present rat pup model have, at least to a certain degree, a mechanistic basis similar to simple and complex febrile seizures in children. The fact that hyperthermia induces respiratory alkalosis in the immature brain leads to a number of consequences that are likely to be relevant for the understanding of the basic mechanisms underlying febrile seizures and related disorders, such as febrile seizures plus (FS+) and generalized epilepsy with febrile seizures plus (GEFS+)40,41. In addition to abnormalities in hippocampal and cortical functions, the disease mechanisms underlying FS, FS+ and GEFS+ may involve neuronal mechanisms that control respiration and acid-base homeostasis. Obviously, homeostatic malfunctions of these types are not mutually exclusive, and various kinds of susceptibility factors may coexist in an individual. There is notably little information available on the effects of fever on respiratory functions in children14. Hence, data from blood gas analyses (especially of CO2) during fever-related epileptic episodes will be important in order to further examine the relevance of the present observations for the human condition.
In future studies, it will be interesting to study whether some of the ion channel gene mutations underlying febrile seizures, FS+ and GEFS+ have an influence on the pH sensitivity of the corresponding mutated channels31,32,42. Furthermore, in view of the polygenic heterogeneity underlying epilepsy32, genes coding for proteins involved in the control of respiration36,37 provide a novel, functionally attractive class of candidate modifier or susceptibility genes for epilepsy. This, of course, does not exclude genuinely temperature-sensitive mechanisms in FS+, GEFS+ and other kinds of fever-related epileptic syndromes43.
The blocking effect of ambient 5% CO2 on the experimental febrile seizures in the rat pups was markedly fast and potent, with a delay of only ~ 20 s to a complete suppression of electrographic ictal activity. The prompt action of CO2 raises the possibility that, also in children, ongoing ictal activity associated with fever could be quickly suppressed by a rise in ambient CO2. Notably, CO2 also prevented the conspicuous effects on long-term neuronal plasticity that are known to take place after hyperthermia-induced seizures9–12. In conclusion, our work indicates a mechanism for triggering hyperthermia-induced seizures and suggests new strategies in the research and therapy of fever-related epileptic syndromes.
METHODS
Rats
We used pups from timed-pregnant Wistar rats which were kept together with their littermates except during experiments. The results are mainly from P8–P11 pups in which hyperthermic seizures were reliably induced, with comparisons to pups at P22–P23. All experiments were approved by the Committee for Animal Care and Use at the University of Helsinki and by the Ethic Committee of the Charité, University Medicine, Berlin.
Induction of seizures
We raised the body temperature of the pups, monitoring rectal temperature with a thermocouple (K101; Voltcraft) in a chamber with an ambient temperature of 48 ± 2 °C. The exposure to hyperthermia lasted 55 min and, at P8–P11, the latency to onset of hyperthermia-induced seizures (31.2 ± 3.7 min, n = 32 pups) showed little variation. The seizures had a broad spectrum of behavioral characteristics5,34 with a duration of ~ 25 min (24.6 ± 1.7 min). Initially, there was a sudden interruption of movements followed by oral automatisms. Thereafter, clonic movements of the limbs and the head, chewing of an extremity and tonic flexion of the body took place, often associated with a loss of postural control.
We used a thin tube connected to a 26-gauge polyethylene cannula (Becton Dickinson) placed intraperitoneally for application of bicarbonate (100 mM NaHCO3 and 154 mM NaCl). The seizures evoked by a single bicarbonate injection (5 mmol/kg) were much more brief, but markedly similar to those induced by hyperthermia. After a brief delay following injection (~ 2 min), an interruption of movements was seen, followed by hyperthermia-induced seizure–like oral automatisms and tonic-clonic movements of the limbs and the head. In some experiments on Ih, we applied two consecutive bicarbonate injections of 5 mmol/kg with an interval of 15 min.
We monitored the breathing patterns of the pups using a piezo crystal sensor (movement sensor 230; Siemens) and applied CO2 at constant oxygen partial pressure using a prewarmed gas mixture containing 5% CO2, 19% O2, 76% N2 (AGA). A 95% change in the CO2 concentration in the chamber took place in 8.7 ± 4.2 s (n = 25; CO2 analyzer CD-101; Datex).
Anesthesia
We used hypothermia44 for chronic implantation of the Ag-AgCl electrodes in P5–P6 pups and for implantation of cortical pH electrodes 24–48 h before the experiments at P8–P11, and ketamine-xylazine anesthesia45 for all pups older than P20.
Electrophysiology in vivo
We made direct-current recordings35,46 of cortical and hippocampal activity at P8–P11 using Teflon-coated silver wire (uncoated diameter, 0.125–0.25 mm; Advent Research Materials Ltd, with chlorided tips) implanted at P5 or P6. We sampled the signals at 0.5–3 kHz using a 12-bit data acquisition board (National Instruments).
We performed craniectomies without damaging the underlying dura using a standard miniature drill equipped with a 0.7-mm diameter carbide dental burr, and placed the electrodes at the following coordinates: 1.6–2.0 mm posterior from bregma, 1.6–2.0 mm lateral from midline, 1.8–2.2 mm below dura for hippocampal CA3 recordings and 0.3–0.7 mm below dura for recordings in the temporal cortex, with a subdural reference electrode above the cerebellum. We fixed the implanted electrodes using microconnectors (GM-4; Microtech) and dental acryl. We sutured the incision for electrode implantation using 6-0 monofilament nylon. After recovery from anesthesia, we returned pups into their original litter. To verify the electrode positions, we injected dye into the hippocampus and cortex, dissected the brain into coronal sections and subsequently examined the sections using light microscopy.
The threshold for detecting the onset of the electrographic seizure activity evoked by hyperthermia or bicarbonate was defined as the time point at which the ictal activity reached an amplitude greater than three standard deviations of the baseline activity.
Monitoring of cortical pH in vivo
We made H+-sensitive electrodes using plastic tubing with a tip outer diameter of 0.1–0.2 mm and a PVC-gelled membrane solution47. This enabled the construction of short and stable membrane columns with a signal that is not sensitive to changes in temperature48. We back-filled electrodes with a solution containing 150 mM NaCl, 20 mM HEPES and 10 mM NaOH.
We placed the H+-sensitive microelectrodes 0.5–1 mm into the cortex using a guiding plastic tubing (conical; tip outer diameter, 0.9 mm), which was implanted above the dura (2.0 mm posterior from bregma, 2.0 mm lateral from midline) and fixed with dental acryl. As the reference, we implanted an Ag-AgCl electrode close to the H+ microelectrode.
Electrophysiology in vitro.
We performed whole-cell recordings in hippocampal slices in a submerged-type chamber that was perfused with physiological solution at 21–22 °C10,49 (Supplementary Methods online).
Western blotting
We performed western blot analyses from hippocampi using a CB1-specific rabbit antibody (1:5,000) raised against the C terminus of the rat CB1 protein50. Optical densities of the bands were analyzed with the AIDA imaging software (Raytest; Supplementary Methods).
Statistical analysis
All data are presented as mean ± s.d. One-way analysis of variance (ANOVA) was followed by the Bonferroni-Dunn post hoc test.
Supplementary Material
Acknowledgments
We thank M. Heikura and M. Palviainen for technical assistance. This work was supported by grants from the Academy of Finland (to K.K., C.R., J.V.), the Sigrid Jusélius Foundation (to S.S., K.K., J.V., C.R.), the Biocentrum Helsinki Organization (to K.K., C.R.), and the Deutsche Forschungsgemeinschaft (SCHM1383/4-1, SFB665, SFB618, to D.S.). K.K. is a member of the Nordic Center of Excellence, WIRED.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(Suppl 2):S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsuboi T. Epidemiology of febrile and afebrile convulsions in children in Japan. Neurology. 1984;34:175–181. doi: 10.1212/wnl.34.2.175. [DOI] [PubMed] [Google Scholar]
- 3.Sagar HJ, Oxbury JM. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsions. Ann Neurol. 1987;22:334–340. doi: 10.1002/ana.410220309. [DOI] [PubMed] [Google Scholar]
- 4.French JA, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 5.Holtzman D, Obana K, Olson J. Hyperthermia-induced seizures in the rat pup: a model for febrile convulsions in children. Science. 1981;213:1034–1036. doi: 10.1126/science.7268407. [DOI] [PubMed] [Google Scholar]
- 6.Bender RA, Dube C, Baram TZ. Febrile seizures and mechanisms of epileptogenesis: insights from an animal model. Adv Exp Med Biol. 2004;548:213–225. doi: 10.1007/978-1-4757-6376-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toth Z, Yan XX, Haftoglou S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewster A, et al. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K, et al. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dube C, et al. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K, et al. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 13.Dube C, et al. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Dempsey TJ, et al. The effect of temperature reduction on respiratory rate in febrile illnesses. Arch Dis Child. 1993;68:492–495. doi: 10.1136/adc.68.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor JA, Del Beccaro M, Done S, Winters W. Establishing clinically relevant standards for tachypnea in febrile children younger than 2 years. Arch Pediatr Adolesc Med. 1995;149:283–287. doi: 10.1001/archpedi.1995.02170150063011. [DOI] [PubMed] [Google Scholar]
- 16.Gadomski AM, Permutt T, Stanton B. Correcting respiratory rate for the presence of fever. J Clin Epidemiol. 1994;47:1043–1049. doi: 10.1016/0895-4356(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 17.Mariak Z, White MD, Lewko J, Lyson T, Piekarski P. Direct cooling of the human brain by heat loss from the upper respiratory tract. J Appl Physiol. 1999;87:1609–1613. doi: 10.1152/jappl.1999.87.5.1609. [DOI] [PubMed] [Google Scholar]
- 18.Mortola JP, Frappell PB. Ventilatory responses to changes in temperature in mammals and other vertebrates. Annu Rev Physiol. 2000;62:847–874. doi: 10.1146/annurev.physiol.62.1.847. [DOI] [PubMed] [Google Scholar]
- 19.Cameron YL, Merazzi D, Mortola JP. Variability of the breathing pattern in newborn rats: effects of ambient temperature in normoxia or hypoxia. Pediatr Res. 2000;47:813–818. doi: 10.1203/00006450-200006000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Kaila K, Ransom BR. pH and Brain Function 1–688. Wiley-Liss, Inc.; New York: 1998. [Google Scholar]
- 21.Balestrino M, Somjen GG. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol (Lond) 1988;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarolimek W, Misgeld U, Lux HD. Activity dependent alkaline and acid transients in guinea pig hippocampal slices. Brain Res. 1989;505:225–232. doi: 10.1016/0006-8993(89)91447-9. [DOI] [PubMed] [Google Scholar]
- 23.Banke TG, Dravid SM, Traynelis SF. Protons trap NR1/NR2B NMDA receptors in a nonconducting state. J Neurosci. 2005;25:42–51. doi: 10.1523/JNEUROSCI.3154-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Taira T, Pihlaja P, Ransom BR, Kaila K. Effects of CO2 on excitatory transmission apparently caused by changes in intracellular pH in the rat hippocampal slice. Brain Res. 1996;706:210–216. doi: 10.1016/0006-8993(95)01214-1. [DOI] [PubMed] [Google Scholar]
- 25.Wirrell EC, et al. Will a critical level of hyperventilation-induced hypocapnia always induce an absence seizure? Epilepsia. 1996;37:459–462. doi: 10.1111/j.1528-1157.1996.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 26.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 27.de Curtis M, Manfridi A, Biella G. Activity-dependent pH shifts and periodic recurrence of spontaneous interictal spikes in a model of focal epileptogenesis. J Neurosci. 1998;18:7543–7551. doi: 10.1523/JNEUROSCI.18-18-07543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong ZQ, Saggau P, Stringer JL. Activity-dependent intracellular acidification correlates with the duration of seizure activity. J Neurosci. 2000;20:1290–1296. doi: 10.1523/JNEUROSCI.20-04-01290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prole DL, Lima PA, Marrion NV. Mechanisms underlying modulation of neuronal KCNQ2/KCNQ3 potassium channels by extracellular protons. J Gen Physiol. 2003;122:775–793. doi: 10.1085/jgp.200308897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aram JA, Lodge D. Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-D-aspartate. Neurosci Lett. 1987;83:345–350. doi: 10.1016/0304-3940(87)90112-1. [DOI] [PubMed] [Google Scholar]
- 31.Baulac S, et al. Fever, genes, and epilepsy. Lancet Neurol. 2004;3:421–430. doi: 10.1016/S1474-4422(04)00808-7. [DOI] [PubMed] [Google Scholar]
- 32.Mulley JC, Scheffer IE, Harkin LA, Berkovic SF, Dibbens LM. Susceptibility genes for complex epilepsy. Hum Mol Genet. 2005;14:R243–R249. doi: 10.1093/hmg/ddi355. Spec No. 2. [DOI] [PubMed] [Google Scholar]
- 33.Haut SR, Veliskova J, Moshe SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3:608–617. doi: 10.1016/S1474-4422(04)00881-6. [DOI] [PubMed] [Google Scholar]
- 34.Baram TZ, Gerth A, Schultz L. Febrile seizures: an appropriate-aged model suitable for long-term studies. Brain Res Dev Brain Res. 1997;98:265–270. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voipio J, Tallgren P, Heinonen E, Vanhatalo S, Kaila K. Millivolt-scale DC shifts in the human scalp EEG: evidence for a nonneuronal generator. J Neurophysiol. 2003;89:2208–2214. doi: 10.1152/jn.00915.2002. [DOI] [PubMed] [Google Scholar]
- 36.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 37.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- 38.Saiki C, Mortola JP. Effect of CO2 on metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J Physiol (Lond) 1996;491:261–269. doi: 10.1113/jphysiol.1996.sp021213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respir Physiol Neurobiol. 2005;149:165–179. doi: 10.1016/j.resp.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg AT, Shinnar S. Complex febrile seizures. Epilepsia. 1996;37:126–133. doi: 10.1111/j.1528-1157.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Scheffer IE, Crossland K, Berkovic SF. Generalized epilepsy with febrile seizures plus: a common childhood-onset genetic epilepsy syndrome. Ann Neurol. 1999;45:75–81. doi: 10.1002/1531-8249(199901)45:1<75::aid-art13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 42.Mantegazza M, et al. Identification of an Nav1.1 sodium channel (SCN1A) loss-of-function mutation associated with familial simple febrile seizures. Proc Natl Acad Sci USA. 2005;102:18177–18182. doi: 10.1073/pnas.0506818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang JQ, Shen W, Macdonald RL. Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J Neurosci. 2006;26:2590–2597. doi: 10.1523/JNEUROSCI.4243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahtinen H, et al. Postnatal development of rat hippocampal gamma rhythm in vivo. J Neurophysiol. 2002;88:1469–1474. doi: 10.1152/jn.2002.88.3.1469. [DOI] [PubMed] [Google Scholar]
- 45.Yi DK, Barr GA. The suppression of formalin-induced fos expression by different anesthetic agents in the infant rat. Dev Psychobiol. 1996;29:497–506. doi: 10.1002/(SICI)1098-2302(199609)29:6<497::AID-DEV2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 46.Vanhatalo S, Voipio J, Kaila K. Full-band EEG (fbEEG): a new standard for clinical electroencephalography. Clin EEG Neurosci. 2005;36:311–317. doi: 10.1177/155005940503600411. [DOI] [PubMed] [Google Scholar]
- 47.Voipio J, Kaila K. Interstitial PCO2 and pH in rat hippocampal slices measured by means of a novel fast CO2/H+-sensitive microelectrode based on a PVC-gelled membrane. Pflugers Arch. 1993;423:193–201. doi: 10.1007/BF00374394. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan-Jones RD, Kaila K. The sensitivity of liquid sensor, ion-selective microelectrodes to changes in temperature and solution level. Pflugers Arch. 1986;406:641–644. doi: 10.1007/BF00584033. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz D, Mellor J, Breustedt J, Nicoll RA. Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nat Neurosci. 2003;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- 50.Hajos N, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


