Abstract
cGMP-inhibited cAMP phosphodiesterase 3A (PDE3A) is expressed in mouse oocytes, and its function is indispensable for meiotic maturation as demonstrated by genetic ablation. Moreover, PDE3 activity is required for insulin/insulin-like growth factor-1 stimulation of Xenopus oocyte meiotic resumption. Here, we investigated the cAMP-dependent protein kinase B (PKB)/Akt regulation of PDE3A and its impact on oocyte maturation. Cell-free incubation of recombinant mouse PDE3A with PKB/Akt or cAMP-dependent protein kinase A catalytic subunits leads to phosphorylation of the PDE3A protein. Coexpression of PDE3A with constitutively activated PKB/Akt (Myr-Akt) increases PDE activity as well as its phosphorylation state. Injection of pde3a mRNA potentiates insulin-dependent maturation of Xenopus oocytes and rescues the phenotype of pde3−/− mouse oocytes. This effect is greatly decreased by mutation of any of the PDE3A serines 290–292 to alanine in both Xenopus and mouse. Microinjection of myr-Akt in mouse oocytes causes in vitro meiotic maturation and this effect requires PDE3A. Collectively, these data indicate that activation of PDE3A by PKB/Akt-mediated phosphorylation plays a role in the control of PDE3A activity in mammalian oocytes.
Keywords: oocyte maturation, PDE3A, PKB/Akt, phosphorylation
Introduction
Mammalian and amphibian oocytes progress through the meiotic cell cycle until the prophase of meiosis I, and then are arrested at a G2-like phase. During each reproductive cycle in vivo, resumption of meiosis is triggered by the preovulatory surge of luteinizing hormone (LH). Resumption of meiosis and progression of metaphase II is characterized by germinal vesicle breakdown (GVBD), chromatin condensation and segregation, and extrusion of the first polar body. Unlike the mitotic cell cycle where DNA synthesis occurs during the S phase, oocytes immediately enter a second meiotic division without the S phase and are arrested in metaphase II. Only metaphase II stage oocytes are able to undergo fertilization by spermatozoa, complete the second meiotic maturation, and form pronuclei.
Although it is well known that the LH surge triggers oocyte maturation, the mechanistic details of this regulation remain unclear. Because LH does not act directly on oocytes, intermediate steps must be present to transduce LH action to oocyte maturation. Recently, it has been proposed that the effect of LH in rodent oocytes is mediated by epidermal growth factor-like peptide hormones (EGF-like growth factors), such as epiregulin, amphiregulin, and betacellulin (Park et al, 2004), which function as paracrine signals between mural granulosa and cumulus cells (Conti et al, 2005). However, the signaling mechanisms of how EGF-like growth factors affect rodent oocyte maturation have not been defined. Although its physiological significance is debated, it has been established that insulin-like growth factor-I (IGF-I) and insulin are potent inducers of meiotic resumption in Xenopus oocytes (El-Etr et al, 1979; Maller and Koontz, 1981). IGF-I- or insulin-dependent Xenopus oocyte maturation is mediated by activation of phosphoinositide 3 (PI3)-kinase and cAMP-dependent protein kinase B (PKB)/Akt (Liu et al, 1995; Deuter-Reinhard et al, 1997). In addition, we have shown that activation of PKB/Akt is necessary and sufficient for IGF-I- or insulin-dependent maturation (Andersen et al, 1998, 2003). Thus, PI3-kinase and PKB/Akt activation play an important role in growth factor-dependent oocyte meiotic maturation.
High levels of cyclic AMP (cAMP) are involved in maintaining meiotic arrest in both mammals and amphibians as well as some invertebrate species (Maller and Krebs, 1977; Meijer et al, 1989; Tsafriri et al, 1996; Conti et al, 1998). In both mammalian and amphibian oocytes, considerable evidence indicates that phosphodiesterase type III (PDE3), which hydrolyzes cAMP, plays an important role in regulating resumption of meiosis (Tsafriri et al, 1996; Shitsukawa et al, 2001; Conti et al, 2002). Of the two cGMP-inhibited cAMP phosphodiesterase 3A (PDE3A) and PDE3B isoenzymes, only PDE3A is expressed in the rodent oocyte and is responsible for the regulation of oocyte cAMP concentration (Richard et al, 2001; Shitsukawa et al, 2001). In Xenopus oocytes, insulin-dependent meiotic resumption is completely blocked by cilostamide, a potent PDE3 inhibitor, whereas rolipram, a PDE4 inhibitor, has no effect (Sadler, 1991; Andersen et al, 1998). The spontaneous maturation of rodent oocytes is also blocked by cilostamide treatment and more importantly, the pde3a null female mice are sterile because of a meiotic block at the G2–M transition (Masciarelli et al, 2004). All these data suggest that PDE3A is critical for meiotic maturation. However, the regulatory mechanisms of PDE3A activity and their involvement in oocyte maturation are unclear. Here, we investigated the mechanisms of regulation of PDE3A activity and the potential role of this activity in the control of cAMP levels and GVBD in oocytes. Our data demonstrate that PDE3A is phosphorylated by PKB/Akt and that this phosphorylation plays a role during meiotic maturation induced by growth factors.
Results
PDE3A is phosphorylated and activated by PKB/Akt and PKA
Given the observation that the paralog PDE3B is a substrate of cAMP-dependent protein kinase A (PKA) and PKB/Akt (Movsesian, 2002), we used several strategies to elucidate whether PDE3A also is phosphorylated by these kinases. Immunoprecipitated recombinant PDE3A was incubated with radiolabeled ATP in the presence or absence of purified active PKB/Akt or PKA catalytic subunit. PDE3A was phosphorylated in the presence of either kinase in this cell-free system (Figure 1A). To determine whether PDE3A is phosphorylated by PKB/Akt also in intact cells, cells expressing PDE3A were cotransfected with different Akt constructs, including wildtype (WT), kinase dead mutant (K179M) PKB/Akt (KD-Akt), constitutively active ΔPH myristoylated PKB/Akt (myr-Akt), or the form of myr-Akt (A2myr-Akt) that is modified in the myristoylation signal (Kohn et al, 1996; Figure 1B). After immunoprecipitation with HA antibodies, the phosphorylation of PDE3A was determined by SDS–PAGE and blotting with phospho-Akt substrate antibodies. Coexpression with myr-Akt caused a major increase in the phosphorylation of PDE3A, whereas minor phosphorylation with WT-Akt (Supplementary Figure 1) or no phosphorylation (KD-Akt, A2myr-Akt) was observed (Figure 1C). These results further suggest that PDE3A is a physiological substrate of PKB/Akt in the intact cells.
Figure 1.
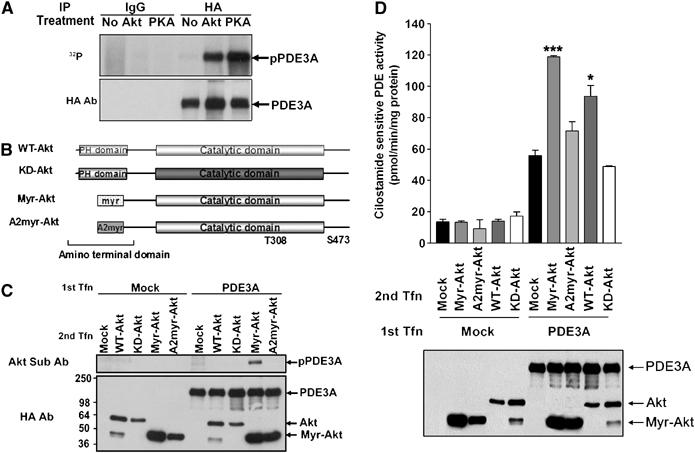
PDE3A is phosphorylated by PKB/Akt and PKA. (A) Immunoprecipitated PDE3A was incubated with or without recombinant PKB/Akt or PKA in the presence of [γ-32P]ATP. The reaction products were subjected to SDS–PAGE and phosphorylated PDE3A (upper panel; pPDE3A) was detected after treatment of PKA or PKB/Akt. The amount of expressed PDE3A was monitored by Western blot analysis using anti-HA antibodies (lower panel; PDE3A). (B) Diagram of the constructs of PKB/Akt used in this study. (C) PDE3A-transfected (PDE3A) or empty vector-transfected (Mock) cells were cotransfected with different Akt constructs including WT, kinase-dead mutant (K179M) PKB/Akt (KD-Akt), constitutively active ΔPH myristoylated PKB/Akt (myr-Akt), or myr-Akt (A2myr-Akt). Immunoprecipitates were subjected to SDS–PAGE followed by Western blot analysis using phospho-Akt substrate antibodies. Coexpression with myr-Akt caused an increase in the phosphorylation of PDE3A (pPDE3A). The expression of PDE3A and various Akt constructs was confirmed by Western blot using anti-HA antibodies (PDE3A, Akt, Myr-Akt). (D) Coexpression of PDE3A with PKB/Akt enhances PDE3A activity. A first transfection (1st Tfn) with empty vector (Mock) or PDE3A-HA (PDE3A) was followed by a second transfection (2nd Tfn) using one of PKB/Akt constructs. PDE3A activity was enhanced by WT- or myr-Akt by 1.5- to 2-fold, but not by KD-Akt and A2myr-Akt. Expression of PDE3A and Akt constructs was analyzed by Western blot using anti-HA antibodies (PDE3A, Akt, Myr-Akt). A representative experiment of the three performed is reported. * and *** represent values of P<0.05 and P<0.005, respectively, compared to control.
To test whether the PKB/Akt-mediated phosphorylation is associated with changes in PDE3A activity, sequential transfection was performed with PDE3A and one of the PKB/Akt constructs including WT-Akt, KD-Akt, myr-Akt, or A2myr-Akt. PDE3A was significantly activated by WT- or myr-Akt by 1.5- to 2-fold, respectively (Figure 1D, upper panel). This finding suggests that kinase activity of PKB/Akt is necessary for PDE3A activation. The amount of PDE3A expressed was not affected by myr-Akt transfection (Figure 1D, lower panel), ruling out that the increase in activity is due to increasing amounts of PDE3A protein. Because Akt-related kinase serum- and glucocorticoid-inducible kinase (Sgk) shares a similar structure with PKB/Akt (without the PH domain) and phosphorylates similar substrates (Murray et al, 2005), we tested whether this kinase has a similar effect on PDE3A. However, a constitutively active Sgk mutant did not activate PDE3A (data not shown). This set of experiments demonstrated that PDE3A is a substrate for PKB/Akt but not Sgk, even though these two kinases have a similar catalytic domain, further supporting the specificity of the Akt effects observed.
Serine 290–292 phosphorylation of PDE3A is important for activation
The activation of PDE3A by PKB/Akt is likely mediated by direct phosphorylation as there are at least five potential PKB/Akt phosphorylation motifs (RxRxxS/T) in the mouse PDE3A sequence (S35, S271, S291, S292, and S462; Figure 2A). An alignment search revealed that three of them, S291, S292, and S462, are conserved among mouse, human, and rat PDE3A sequences (Figure 2A; Supplementary Figure 2). To investigate whether these phosphorylation sites at the amino terminus of the protein are important for PKB/Akt-dependent activation of PDE3A, we generated a truncated form of PDE3A (ΔNcoI-PDE3A). The construct lacks the amino-terminal domain that contains all the putative phosphorylation sites with the exception of S462. This mutant was not activated by PKB/Akt, confirming that the amino-terminal region of PDE3A is important for mediating the PKB/Akt effects (Figure 2B).
Figure 2.
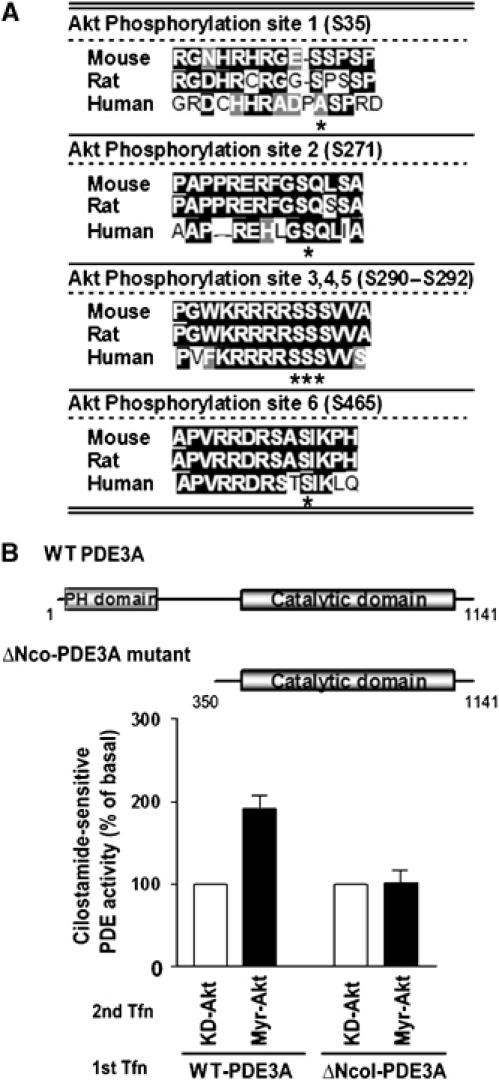
An amino-terminus deletion mutant of PDE3A is not activated by PKB/Akt. (A) Putative PKB/Akt phosphorylation sites in the PDE3A amino-acid sequence. Serines marked with asterisk indicate the residues that are likely to be phosphorylated by PKB/Akt. PKB/Akt phosphorylation sites 3, 4, 5, and 6 are conserved among mouse, rat, and human PDE3A sequences. (B) MA10 cells were transfected with full-length PDE3A-HA (WT-PDE3A) or amino-terminus truncated PDE3A-HA (ΔNcoI-PDE3A) (1st Tfn), and constitutively active PKB/Akt (myr-Akt) or kinase-dead PKB/Akt (KD-Akt) was transfected after 16 hours (2nd Tfn). Nineteen hours later, cilostamide-sensitive PDE activity was measured on total cell extracts. Only full length of PDE3A not ΔNcoI-PDE3A mutant is activated by myr-Akt transfection.
To confirm whether these putative Akt phosphorylation sites are phosphorylated by myr-Akt, the following seven PDE3A mutants were generated: M1–6 mutant (S35A, S271A, S290A, S291A, S292A, S462A), M3 mutant (S290A), M4 mutant (S291A), M5 mutant (S292A), M6 mutant (S462A), M3–5 mutant (S290A, S291A, S292A), or M3–6 mutant (S290A, S291A, S292A, S462A) (Figure 3A). After serial transfection of myr-Akt and one of the PDE3A mutants, the immunoprecipitated complexes were blotted with phospho-Akt substrate antibodies. All of the immunoprecipitated mutants from intact cells expressing myr-Akt were phosphorylated except M1–6, which is a mutant of all of the putative sites (Figure 3B). Next, by measuring the PDE3A activity in the lysates of double-transfected cells, we defined whether PKB/Akt-dependent phosphorylation of PDE3A is important for activation. Only the WT and M6 mutant PDE3As were activated by myr-Akt (Figure 3C). Because all single mutated PDE3As in the M3, M4, and M5 sites were not activated by PKB/Akt (Figure 3C), we surmised that the three serines in the mouse PDE3A sequence, S290–292, are required for PKB/Akt-dependent activation, whereas the other putative phosphorylation sites, although perhaps physiologically relevant, do not impact on the PDE activity. This conclusion is further supported by the analysis of an additional construct mutated in S35, S271, and S462 to alanine (M1, 2, 6) but retaining the Akt putative sites (Supplementary Figure 3). After myr-Akt cotransfection, this mutant was phosphorylated to a lesser extent than the WT PDE3A, but was activated in a manner similar to WT PDE3A (Supplementary Figure 3).
Figure 3.
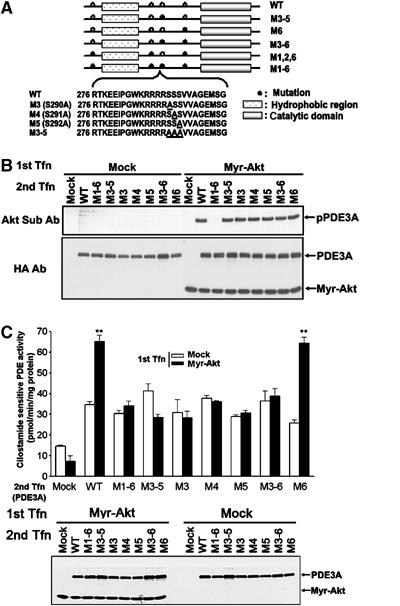
S290–292 serine residues in the PDE3A sequence are important for PKB/Akt-induced PDE3A activation. (A) M3, M4, M5, M6, M3–5, M3–6, M1, 2, 6, or M1–6 mutant PDE3A-HAs were generated as described in Materials and methods. The open circle indicates the site likely to be phosphorylated by PKB/Akt. The closed circle indicates the site that was mutated from serine to alanine. Amino-acid sequences of the S290–S292 region of PDE3A and mutant M3, M4, M5, and M3–5 are shown. Underlined alanine indicates the Ser/Ala mutation introduced. (B) The phosphorylation state of the various PDE3A mutants was determined using phospho-Akt substrate antibody after cotransfection with myr-Akt and immunoprecipitation. All of the immunoprecipitated mutants were phosphorylated except M1–6, which is a mutant of all of the putative sites. (C) Cilostamide-sensitive PDE activity was measured after serial transfection of Myr-Akt or empty vector (1st Tfn) and various PDE3A mutants (2nd Tfn). All single mutated PDE3As in the M3, M4, and M5 site were not activated by PKB/Akt and only the WT and M6 mutant PDE3As were activated by myr-Akt. ** represents P<0.01 compared to mock control. The expression levels of PDE3A and myr-Akt were confirmed with HA antibodies (lower panel; PDE3A, Myr-Akt).
PDE3A phosphorylation mediates the insulin effects on Xenopus oocyte maturation
To determine whether this PDE3A phosphorylation and activation by Akt is functionally significant in an intact cell model, we used oocyte maturation as a downstream readout for PDE activation. We have shown previously that PKB/Akt injection in Xenopus oocytes causes a two- to three-fold increase in PDE activity (Andersen et al, 1998). Increasing concentrations of mouse pde3a mRNA were injected into Xenopus oocytes and percentage of GVBD was measured. The induction of GVBD by overexpression of PDE3A was confirmed by activation (phosphorylation) of MAP kinase as reported (Figure 4A). Oocytes were then injected with 5 ng of WT pde3a mRNA followed by insulin treatment at a concentration that has a submaximal effect on GVBD (Andersen et al, 2003). Approximately 80–90% of the oocytes underwent GVBD (Figure 4B), and an approximate two-fold increase in PDE activation was measured in the oocyte extracts. Conversely, only 40% of GVBD was induced by insulin after injection of 10 ng of the M3–5 mutant form of pde3a mRNA (Figure 4B). When 10 ng mRNA was injected into the Xenopus oocytes, only the WT and M6 mutant induced more than 90% of GVBD with insulin, whereas less than 50% of GVBD was detected with the injection of the other mutants, a value comparable to water injection as a control (Figure 4C, middle panel). The induction of maturation was associated with an approximate two- to four-fold increase of PDE activity in Xenopus oocytes (Figure 4C, upper panel). Together with our previous report, these results strongly suggested that the PKB/Akt-stimulated PDE activity is required for insulin-dependent oocyte maturation in Xenopus oocytes.
Figure 4.
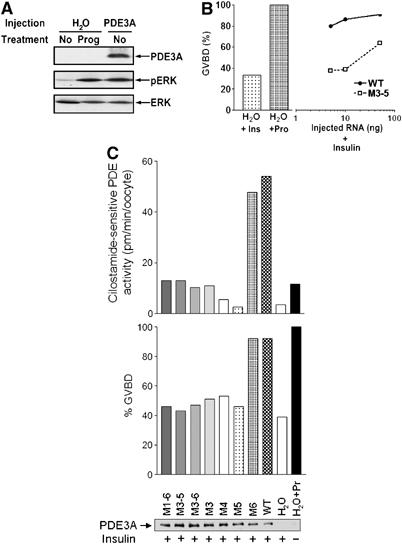
A two- to four-fold increase in PDE3A activity is sufficient to induce oocyte maturation. (A) Fifty nanograms of pde3a mRNA or H2O (vehicle) was injected into Xenopus oocytes. One group of H2O-injected oocytes was treated with 500 nM progesterone to induce oocyte maturation. Treatment of progesterone in the H2O-injected oocytes or PDE3A expression induces the phosphorylation of ERK (pERK), which is an established oocyte maturation marker. Protein expression (PDE3A) and the amount of loaded protein (ERK) were monitored by Western blot analysis. (B) Xenopus oocytes were injected with different amounts of pde3a mRNA (0.1–50 ng/oocyte) and treated with insulin. Twelve hours later, oocyte maturation (GVBD) was scored by the appearance of a white spot on the animal pole of oocytes. By injection with 5 ng WT pde3a mRNA (WT), more than 70% oocytes undergo GVBD, whereas only 40% of GVBD occurred by injection of 10 ng of the M3–5 mutant form (M3–5). The capacity of oocyte maturation is tested in H2O-injected oocyte by insulin or progesterone treatment (H2O+Ins, H2O+Prog). (C) Xenopus oocytes were injected with 10 ng of various mutants of PDE3A and treated with 1 μM of insulin. The maturation of the oocytes was scored and PDE3A activity was measured using the oocyte lysate. The WT and M6 mutant induced more than 90% of GVBD (middle panel) and this induction of maturation was associated with an approximate two- to four-fold increase of PDE activity (upper panel). Expression levels of the PDE3As were confirmed with HA antibodies (lower panel, PDE3A).
Isoforms of PDE3A expressed in mouse oocyte
It has been reported that at least three different immunoreactive forms of human PDE3As are present in cardiac myocytes (Movsesian, 2002). However, it is unclear whether these forms are generated by proteolysis, alternate splicing during transcription, or different methionine usage during translation. Previous report with oocytes had indicated the presence of multiple transcripts (Shitsukawa et al, 2001). Western blot analysis was performed with available PDE3A antibodies on extracts from WT and pde3−/− oocytes. As shown in Figure 5, an immunoreactive band of approximately 136 kDa is detected only in WT but not pde3−/− oocytes. The migration of this band corresponds to the size of full-length recombinant PDE3A. A more prominent smaller band of 118–120 kDa is expressed only in WT oocytes but not pde3−/− oocytes (Figure 5). Additional bands were considered nonspecific because they were still present in pde3a−/− oocytes. The same expression pattern of PDE3A was detected in WT and pde3−/− mice lung lysates (Figure 5), known to express the PDE3A. The experiments with two different antibodies showed comparable results (data not shown). On the basis of these findings, we surmised that the full-length PDE3A is expressed in mouse oocytes, together with a smaller form; however, the properties and the mechanism by which the lower form is generated are unclear.
Figure 5.
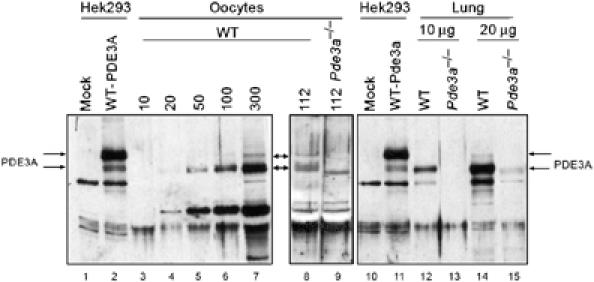
PDE3A expression in mouse oocytes. Increasing number of WT oocytes (lanes 3–7), WT (lane 8) or pde3−/− oocytes (lane 9, 112 oocytes/lane), and different amounts of lung lysate (lanes 12–15) were subjected to SDS–PAGE and protein detected with a PDE3A antibody (PDE3ACY). Different forms of PDE3A are expressed in the mouse oocyte. The double arrows indicate the 136 and 120 kDa specific bands. These two bands were not detected in oocyte (lane 9) and lung from pde3−/− animals (lanes 13, 15). Full-length recombinant PDE3A-transfected Hek293 cell lysate was used as size control (lanes 2, 11).
Activation of Akt and PDE3A precedes activation of the MPF complex during in vivo maturation of mouse oocytes
We investigated the time course of the activation of the Akt and maturation promoting factor (MPF) complexes (Cdc2/CyclinB) during in vivo mouse oocyte maturation. Mice were primed with PMSG for 44 h and then injected with hCG to induce oocyte maturation. Oocytes were collected and the activation of PKB/Akt was determined in extracts using Akt phospho-S473-specific antibodies. The phosphorylation of PKB/Akt reached a maximum 2 h after hCG injection and preceded MPF activation by 1 h (Figure 6A) and GVBD by 1.5–2 h. To determine whether PDE3A expressed in the oocyte is activated during the same time frame, extracts were immunoprecipitated with PDE3A antibody and activity measured in the immunoprecipitation pellet. The PDE3A activity recovered 2.5 h after hCG was significantly increased approximately by 30% (Figure 6B). Thus, activation of the PKB/Akt–PDE module does occur during oocytes maturation also in vivo.
Figure 6.
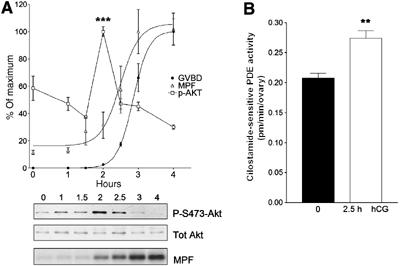
PKB/Akt and PDE3A activation precedes activation of the MPF complex. (A) Phosphorylation state of PKB/Akt at indicated hours after hCG injection was determined using Akt phosho-S473-specific antibodies (P-S473-Akt) and the amount of loaded protein was confirmed with Akt-specific antibodies (Tot Akt). Highly phosphorylated state of Akt is detected after 2 h from hCG injection. The value of p-Akt reports the ratio of phosphorylated Akt and total Akt (square). Activity of MPF complex (Cdc2/CyclinB) was measured with five randomly selected oocytes at each time point (triangle). The phosphorylation of histone H1, which is a substrate of MPF complex, started at 2 h after hCG injection and reached a maximum at 3 h (MPF). GVBD was counted by disappearance of the germinal vesicle or extrusion of the first polar body (filled circle). A representative of the three independent experiments performed is reported. *** represents P<0.005 compared to 0 h. (B) The mouse ovaries were removed and lysates immunoprecipitated with PDE3A antibody. The PDE3A activity was measured in the immune complex. After 2.5 h from hCG injection, 30% increase in PDE3A activity over basal was detected. The data are the mean±s.e.m. of three independent experiments. ** represents P<0.01 compared to 0 h.
Expression of PDE3A rescues the phenotype of the pde3a−/− oocytes
To investigate the impact of PDE3A phosphorylation during mammalian oocyte maturation, we injected WT and M3T mutant pde3a mRNA into oocytes from pde3a−/− mice. In oocytes where PDE3A is absent, PKA is maintained in an activated state by the elevated cAMP, thus preventing maturation in vitro and in vivo (Masciarelli et al, 2004). The injection of WT pde3a mRNA caused meiotic resumption in approximately 75% of oocytes, whereas only 30% of oocytes resumed meiosis when a similar concentration of the M3–5 mutant mRNA was used (Figure 7A, left panel). When M1, 2, 6 mutant was injected in the pde3−/− oocytes, 60% of oocytes underwent GVBD, at a rate similar to the WT PDE3A injection, but only 24% of M1–6 mutant resumed meiosis (Figure 7A, right panel). The expression of WT and mutant proteins was comparable (Figure 7A). Furthermore, injection of myr-Akt in mouse oocytes maintained in meiotic arrest with hypoxanthine caused meiotic resumption in approximately 80% of the oocytes and two-fold increase in PDE activity (Figure 7B). This effect is dependent on the expression of PDE3A because myr-Akt failed to induce meiotic maturation in pde3a null oocytes (Figure 7B, left panel). Furthermore, PDE3A activity is required for the Akt effect because inclusion of cilostamide prevents meiotic resumption (data not shown). Consistent with the observation in Xenopus oocytes, these findings confirm that PDE3A plays a role in mouse oocyte maturation, and that the putative PKB/Akt phosphorylation sites identified above (serines 290–292) are implicated in the regulation of PDE3A activity in these oocytes.
Figure 7.
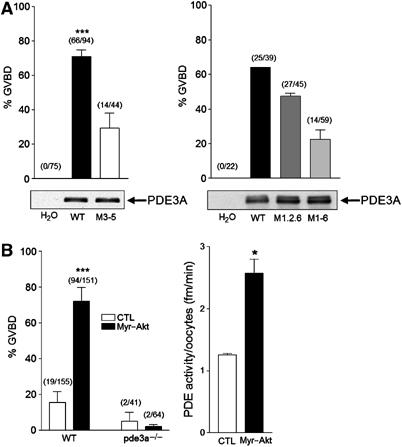
PDE3A is required for Akt-induced mouse oocyte maturation. (A) Oocytes from pde3−/− mice were injected with mRNA of WT PDE3A, M3–5, M1,2,6, or M1–6 mutant form. Vehicle (H2O) was injected as a control. Maturation of mouse oocytes was monitored by disappearance of the germinal vesicle or extrusion of the first polar body 19 h after mRNA injection. The injection of WT or M1,2,6 PDE3A mRNA induced meiotic resumption around 60–75% of oocytes, whereas about 30% of oocytes resumed meiosis when M3–5 or M1–6 mRNA was used. Numbers above the bars indicate the number of GVBD stage oocytes out of total injected oocytes. Protein expression of the injected mRNAs was compared with HA antibodies using 44 oocytes per each lane (left panel) and using 39 oocytes with PDE3A antibody (right panel). (B) Oocytes from WT and pde3a−/− mice were collected in M2 media, and WT oocytes maintained in meiotic arrest with 3.5 mM hypoxanthine. Left panel: Oocytes were injected with myr-Akt mRNA or H2O as a control. Injection of myr-Akt causes approximately 80% of meiotic resumption in WT oocytes but not in pde3−/− mouse oocytes. Right panel: Total PDE activity was measured with injected oocytes as described in Materials and methods. Approximately, two-fold increase in PDE activity was detected in myr-Akt-injected oocytes in three independent experiments. *** represents P<0.005 and * represents P<0.05 compared to control.
Discussion
PKB/Akt activation is a critical intermediate step downstream of PI3-kinase in insulin and IGF-I signaling. Several PKB/Akt substrates have been identified including FKHR, GSK3, and BAD (Toker and Newton, 2000; Scheid and Woodgett, 2001; Downward, 2004), thus demonstrating the pleiotropic effects of this signaling cascade on cell functions such as metabolism, growth, and survival. In this study, we have identified the residues of PDE3A required for PKB/Akt-mediated phosphorylation. Ablation of these sites prevents Akt phosphorylation of the enzyme and blocks its activation in vitro and in intact cells. Moreover, PDE3A lacking these sites is functionally impaired in its ability to promote oocyte maturation. Because Akt promotes maturation in frog and mouse oocytes, and this effect requires PDE3A, we hypothesize that PKB/Akt and PDE3A regulation represents a signaling module involved in the regulation of cAMP in amphibian and rodent oocytes. We also infer that this regulatory loop may be directly or indirectly involved in signaling meiotic resumption.
Using available motif scanning utilities (http://scansite.mit.edu), we established that there are at least five predicted PKB/Akt phosphorylation sites in the mouse PDE3A major open reading frame (S35, S271, S291, S292, S462) (Figures 2A and 3A). We found that PDE3A is phosphorylated in vitro by PKB/Akt and PKA (Figure 1A). In addition to PKA activation of other PDEs such as PDE4 (Sette and Conti, 1996; Conti et al, 1998; Oki et al, 2000; Murthy et al, 2002), it has been reported that PDE3A is activated by PKA phosphorylation in human platelets, and it functions as a negative feedback loop to regulate cAMP concentration and therefore aggregation (Grant et al, 1988; Macphee et al, 1988; Kitamura et al, 1999; Murthy et al, 2002). In our experimental paradigm, PKA also phosphorylated PDE3A mainly in serine 291 (Supplementary Figure 4) and this phosphorylation regulated the activity of PDE3A. Even though Akt also phosphorylates this serine (Figure 3B) as well as other sites, there is no synergic effect on the PDE activity between PKA and Akt (Supplementary Figure 5). Because it has been known that high concentration of cAMP maintains the PKA as an active form in the GV oocyte, there is a possibility that PDE3A is phosphorylated and activated by PKA in the GV oocyte. However, this phosphorylation does not prevent Akt-mediated activation both in Hek293 cells and oocytes, and the two regulations are clearly additive. It is also possible that PKA and PDE3A are sequestered in different compartments and PKA phosphorylation occurs only during GVBD (Newhall et al, 2006).
After cotransfection of myr-Akt and PDE3A in Hek293 cells, or by insulin treatment in Xenopus oocytes, the activity of PDE3A is increased at least two-fold by phosphorylation (Figures 1D, 2B, 3C and 4C). This activation by phosphorylation may be caused by conformational changes in PDE3A or may depend on binding of regulatory proteins. It is established that the Akt phosphorylation domains in PDE3B bind 14-3-3β protein (Onuma et al, 2002). In addition, it has been reported that PDE3A once phosphorylated by PKC binds 14-3-3 protein (Pozuelo Rubio et al, 2005). Furthermore, PDE3A can bind plectin-1, which is a linker of the intracellular cytoskeleton network, in a phosphorylation-independent manner (Pozuelo Rubio et al, 2005). This latter result suggests that the localization of PDE3A is important for its function. It is established that three different forms of human PDE3A are generated by alternative splicing or by usage of different starting codons in cardiac myocytes (Movsesian, 2002). The subcellular localization of these three forms is dissimilar. The longest form is localized on the membrane via its two hydrophobic regions in the amino terminus (NHR), whereas the intermediate form, which has only one NHR, is recovered in both the membrane and cytosol. The shortest form, with no NHR, is entirely in the cytosol (Kenan et al, 2000; Shakur et al, 2000; Wechsler et al, 2002). In oocytes, the PDE3 activity is mostly recovered in the soluble fraction (Richard et al, 2001). At least two different transcripts were detected by Northern blot analysis (Shitsukawa et al, 2001). By using three different independently generated polyclonal antibodies, we confirmed the expression of the longest PDE3A open reading frame in mouse oocyte (Figure 5). As an increase in PDE3A activity was observed in the oocyte after Akt ectopic expression, we surmise that these forms can be phosphorylated and activated by Akt. It is not clear whether the sequence of the lower form corresponds to the isoforms detected in the human heart (Movsesian, 2002) and whether this latter form can be activated by PKB/Akt. When in vivo PDE activation by hCG was measured, 30% of increase in PDE3A activity was observed 2.5 h after hCG stimulation (Figure 6B). As only the fraction of oocytes in preovulatory follicles are able to respond to the maturation signals triggered by hCG, and in the experiments performed PDE3A was immunoprecipitated from all oocytes, the small stimulation may be due to the large background of PDE3A in immature oocytes. It is also possible that the 118–120 kDa form is not activated during maturation and the increase in PDE activity observed is entirely due to activation of the 134 kDa form.
In adipocytes, it has been shown that PDE3B (Liu and Maurice, 1998) is phosphorylated and activated by insulin stimulation, thereby controlling the levels of cAMP (Wijkander et al, 1998; Kitamura et al, 1999; Rondinone et al, 2000). This activation of PDE3B is mediated by PI3-kinase because it is completely blocked by the addition of the PI3-kinase inhibitor, wortmannin. In addition, mutagenesis studies indicated that phosphorylation of the S273 site in PDE3B is critical for activation. Alignment analysis indicated that this phosphorylation site corresponds to serine 290 in PDE3A, which is one of the putative PKB/Akt phosphorylation sites defined in this study. Our mutagenic data suggest that the three serine residues are all-important for PKB/Akt-dependent PDE3A activation (Figures 4C and 5C). These serines are conserved among several species analyzed including human, rat, mouse, and Xenopus (Supplementary Figure 2), thereby re-emphasizing the importance of this amino-acid motif in PDE3A regulation.
We found that PDE3A is not activated by Sgk. Even though PKB/Akt and Sgk have a similar catalytic domain (68% homology) and both kinases are activated by PDK1 and 2 (Park et al, 1999), several differences in these kinases have been reported. First, PKB/Akt is ubiquitously expressed whereas the expression of Sgk is restricted with expression reported in rat oocytes (Webster et al, 1993; Alliston et al, 2000). Second, Sgk does not have a PH domain, which is thought to be important for membrane targeting (Webster et al, 1993). Third, it is likely that Sgk has significantly more restricted substrates compared to PKB/Akt, which phosphorylates the RxRxxS/T motif. Park et al (1999) reported that Sgk preferentially phosphorylates KRxRRxS/T motif. We have shown that the addition of a myristoylation signal at the amino terminus of Sgk still cannot activate PDE3A; thus, the different effects of the two kinases are not due to their localization, implying that differences in substrate recognition are more relevant.
The present data indicate that a two- to three-fold increase in PDE activity is sufficient to induce GVBD in Xenopus oocytes (Figure 4C). Therefore, it is likely that PKB/Akt-dependent Xenopus oocyte maturation is mediated mainly by PDE3A activation as suggested by our studies (Andersen et al, 2003). Similarly, a two-fold increase in PDE activity was observed by ectopic expression of myr-Akt in the mouse oocyte (Figure 7B) and Akt induction of meiosis requires PDE3A activity. These findings suggest that this PKB/Akt–PDE3A module can signal meiotic resumption. Okumura et al (2002) indicated an alternative mechanism of PKB/Akt-dependent induction of oocyte maturation in starfish. According to their findings, PKB/Akt directly phosphorylates and inactivates Myt1, which has been shown to phosphorylate and inactivate MPF (Okumura et al, 2002). It remains unclear if this mechanism is conserved in mammals or amphibians because the Akt phosphorylation site reported in starfish Myt1 is not conserved in human, mouse, or Xenopus Myt1 sequence. Moreover, the Akt phosphorylation site in PDE3A could not be identified in sea urchin PDE3-like sequences.
The phenotype of the pde3a−/− mice clearly shows that PDE3A is indispensable for LH-mediated maturation (Masciarelli et al, 2004); however, it cannot be distinguished whether this phenotype is due simply to the increase of cAMP above a threshold that precludes maturation induced by other signals, or whether PDE3A ablation removes an important effector of LH stimulation. Our data show that Akt overexpression induces maturation in mouse oocytes and that this effect requires PDE3A activity. We have detected an increase in PDE3 activity in rat cumulus–oocyte complex (COC) 2–2.5 h after LH stimulation (Richard et al, 2001) in vitro or in vivo (Figure 6B) and an increase in cilostamide-sensitive PDE activity approximately 70 min after oocytes were removed from the follicle. An increase in Akt phosphorylation and activity that precedes MPF activation and GVBD has been reported during spontaneous and LH-induced maturation (Kalous et al, 2006), a finding reproduced in this study. It is then possible that the increase in PDE activity we have observed is caused by Akt phosphorylation. How the Akt activation in the oocyte is brought about is however not known clearly. In the same vein, it is unclear whether this Akt/PDE3A module plays a role in the physiological resumption of mammalian meiosis triggered by the LH surge in vivo. To date, there is no evidence that LH stimulation causes granulosa cells to produce a signal that activates the PI3-kinase/Akt pathway in oocytes. EGF-like growth factors have recently been implicated in LH action at the time of ovulation; however the presence of EGFR, which activates PI3-kinase on oocytes, is debated (Wiley et al, 1992; Park et al, 2004) and the effect on denuded oocytes of these growth factors is still controversial (Goud et al, 1998; Smitz et al, 1998). PI3-kinase inhibitors also do not block and at best delay LH-induced oocyte maturation in follicles (Hoshino et al, 2004), even though the interpretation of these findings is confounded by the fact that these drugs have marked toxic effects on the oocyte (Conti, personal communication). Although the exact role of the PI3-kinase/Akt/PDE3A cascade requires further investigation, an alternative scenario may reconcile these conflicting results. It is possible that Akt/PDE3A regulation is not the primary switch for oocytes maturation, but serves as an amplifying loop to decrease cAMP when maturation is initiated. According to this view, an initial decrease in cAMP is induced by other mechanisms. This decrease is associated with or causes activation of PKB/Akt, which in turn activates PDE3A leading to a further decrease in cAMP. A crosstalk between cAMP and Akt has been demonstrated in cell lines (Kim et al, 2001).
In conclusion, we have identified the mechanism through which PDE3A is activated by PKB/Akt-dependent phosphorylation. Additionally, our findings strongly indicated that S290–292 phosphorylation of PDE3A is necessary for activation. Ectopic overexpression of activated Akt induces mouse oocyte maturation through PDE3A. Thus, PKB/Akt-dependent phosphorylation and regulation of PDE3A should be included among the mechanisms potentially involved in induction of Xenopus and mouse oocyte maturation.
Materials and methods
Plasmids and mutagenesis
The construct encoding the WT Akt, myr-Akt, A2 mutated myristoylated Akt (A2myr-Akt), and kinase-dead Akt (K179M-Akt) in the pECE vector have been described (Kohn et al, 1996). All Akt constructs have the hemagglutinin (HA) epitope tag at the carboxyl terminus to monitor protein expression. The pcDNA3.1-PDE3A construct has been described previously (Shitsukawa et al, 2001). To create HA tagged pcDNA3.1-PDE3A, HA tag was introduced into the 3′ end of PDE3A cDNA. Amino-terminus-deleted PDE3A-HA (Δ-NcoI PDE3A-HA mutant) was created by using NcoI sites on the 5′-multicloning site and the internal mouse PDE3A sequence that is located near amino acid 350. Serines (Ser) in the consensus Akt phosphorylation sites (RxRxxS/T) of mouse PDE3A were mutated to alanine (Ala) using a QuickchangeTM site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's protocol. The following mouse PDE3A mutants were generated: M3 (Ser290Ala); M4 (Ser291Ala); M5 (Ser292Ala); M6 (Ser462Ala); M3–5 (Ser290Ala, Ser291Ala, Ser292Ala); M3–6 (Ser290Ala, Ser291Ala, Ser292Ala, Ser462Ala); M1,2,6 (Ser35Ala, Ser271Ala, Ser462Ala); and M1–6 (Ser35Ala, Ser271Ala, Ser290Ala, Ser291Ala, Ser292Ala, Ser462Ala).
In vitro phosphorylation of PDE3A
PDE3A-transfected Hek293 cells were harvested using Tris–NP40 lysis buffered solution (50 mM Tris (pH 7.6), 150 mM NaCl, 1 mM EDTA, 0.1% NP-40, 4 μg/ml aprotinin, 0.7 μg/ml pepstatin, 0.2 mM PMSF, 0.5 μg/ml leupeptin, 1 μM microcystin-LR, 1 mM NaVO4, and 1 mM NaF), and immunoprecipitated with 1 μg of anti-HA antibody. Immunoprecipitates were then incubated with phosphorylation buffered solution (50 mM Tris–HCl (pH 7.6), 5 mM MgCl2, 1 mM ATP, 4 μg/ml aprotinin, 0.7 μg/ml pepstatin, 0.2 mM PMSF, 0.5 μg/ml leupeptin, and 500 μCi/ml [γ-32P]ATP (3000 Ci/mmol, Amersham-Pharmacia Biotech) with or without the PKA catalytic subunit (Promega Corp., Madison, WI) or activated Akt (Upstate, Billerica, MA), then fractionated by 8% SDS–PAGE. Radiolabeled PDE3A was detected by autoradiography. Total amount of PDE3A was measured by Western blot analysis using anti-HA antibody.
Measurement of phosphodiesterase activity
To check the PDE activity in the mouse ovary, mice were primed with PMSG for 44 h and then with hCG for further 2.5 h. The ovaries were removed and lysates were immunoprecipitated with PDE3A antibody. Phosphodiesterase activity was measured according to the method of Thompson et al (1979). In brief, samples were assayed in a reaction mixture of 200 μl containing 40 mM Tris–HCl (pH 8.0), 10 mM MgCl2, 5 mM β-mercaptoethanol, 1 μM cAMP (Sigma-Aldrich, St Louis, MO), 0.75 mg/ml bovine serum albumin (Fraction V), and 0.1 μCi of [3H]cAMP (Perkin-Elmer Life Science, Boston, MA) for 20 min at 33°C in the absence or presence of 10 μM cilostamide. The reaction was terminated by heat inactivation in boiling water for 1 min. The PDE reaction product, 5′-AMP, was then hydrolyzed by incubation of the assay mixture with 50 μg of Crotalus atrox snake venom (Sigma-Aldrich) for 20 min at 33°C, and the resulting adenosine was separated by anion exchange chromatography using 1 ml of AG1-X8 resin (Sigma-Aldrich) and quantitated by scintillation counting.
Western blot analysis
The expression of each protein was analyzed by Western blot analysis using the same method described previously (Andersen et al, 2003). Immunoblotting to detect recombinant proteins was performed by 2 h incubation with an anti-HA.11 monoclonal antibody (Covance, Princeton, NJ); phosphorylation of PDE3A was monitored with phosho-Akt substrate antibody, which recognizes (R/K)X(R/K)XX(pT/pS) (Cell Signaling #9611). To detect endogenous PDE3A protein, PDE3A polyclonal antibodies provided by Dr Yan (University of Rochester) (PDE3ACY), an antibody generated in our laboratory (Andersen et al, 2003) (PDE3ACA), and an antibody purchased from FabGennix Inc. (Frisco., TX) (PDE3AFG) were used. The antibody provided by Dr Yan was generated using the 432–660 region of mouse PDE3A. This epitope is present in all PDE3A variants characterized in the heart (Wechsler et al, 2002). Specific proteins were visualized after subsequent 1:5000 dilution of anti-mouse IgG conjugated to horseradish peroxidase (Amersham-Pharmacia Biotech) and ECL procedure (Amersham-Pharmacia Biotech). For Western blotting of mouse oocytes, indicated number of oocytes per lane were used with HA, Akt phospho-S473-specific antibody (Cell Signaling) and total Akt (Cell Signaling), antibodies.
Collection of mouse oocytes for injection of mRNA
All animal procedures were approved by and followed the guidelines of the Stanford University Administrative Panel on Laboratory Animal Care (A-PLAC). Mouse oocytes were collected as described previously (Han et al, 2005). Using an IM-300 Microinjector (Narishige) and sterile pipettes, the mRNA was microinjected into the cytoplasm of denuded GV oocytes in M2 medium with 0.4% BSA with or without 3.5 mM hypoxanthine. Following microinjection, oocytes were incubated overnight at 37°C. After incubation, morphologically normal oocytes were scored for meiotic progression using an Olympus inverted microscope (Center Valley, PA) fitted with a Hoffman contrast lens at X200.
Collection of mouse oocytes for PKB/Akt phosphorylation and histone H1 assay
Twenty-two-day-old mice were injected with PMSG, and 44 h later with hCG. The ovary was isolated in M2 media, and cumulus–oocyte complexes were collected at the indicated timepoint after hCG injection. Oocytes were separated from cumulus cells in M2 media using a pulled Pasteur pipette. Fifty oocytes were collected and frozen for Western blot assay with Akt phosho-S473-specific antibodies or Akt-specific antibodies. Activity of MPF was measured with five randomly selected oocytes at each timepoint. The kinase assay followed published procedures (Svoboda et al, 2001).
Statistical analysis
Values were compared by Student's t-test, and P<0.05 was considered statistically significant.
Supplementary Material
Supplementary data
Acknowledgments
We thank Dr David Pearce for the mSgk construct, Dr Julie C Baker for help with injecting Xenopus oocytes, Dr Chen Yan, and Kye-Im Jeon for PDE3A antibody, and Caren Spencer for editorial work on the manuscript. This work was supported by the National Institute of Child Health and Human Development, National Institutes of Health, through a cooperative agreement U54-HD31398 to MC as part of the Specialized Cooperative Centers Program in Reproduction Research.
References
- Alliston TN, Gonzalez-Robayna IJ, Buse P, Firestone GL, Richards JS (2000) Expression and localization of serum/glucocorticoid-induced kinase in the rat ovary: relation to follicular growth and differentiation. Endocrinology 141: 385–395 [DOI] [PubMed] [Google Scholar]
- Andersen CB, Roth RA, Conti M (1998) Protein kinase B/Akt induces resumption of meiosis in Xenopus oocytes. J Biol Chem 273: 18705–18708 [DOI] [PubMed] [Google Scholar]
- Andersen CB, Sakaue H, Nedachi T, Kovacina KS, Clayberger C, Conti M, Roth RA (2003) Protein kinase B/Akt is essential for the insulin- but not progesterone-stimulated resumption of meiosis in Xenopus oocytes. Biochem J 369: 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A (2002) Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol 187: 153–159 [DOI] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard FJ, Shitsukawa K, Tsafriri A (1998) Role of cyclic nucleotide phosphodiesterases in resumption of meiosis. Mol Cell Endocrinol 145: 9–14 [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ (2005) Role of the EGF network in ovarian follicles. Mol Endocrinol 20: 715–723 [DOI] [PubMed] [Google Scholar]
- Deuter-Reinhard M, Apell G, Pot D, Klippel A, Williams LT, Kavanaugh WM (1997) SIP/SHIP inhibits Xenopus oocyte maturation induced by insulin and phosphatidylinositol 3-kinase. Mol Cell Biol 17: 2559–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J (2004) PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol 15: 177–182 [DOI] [PubMed] [Google Scholar]
- El-Etr M, Schorderet-Slatkine S, Baulieu EE (1979) Meiotic maturation in Xenopus laevis oocytes initiated by insulin. Science 205: 1397–1399 [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, Dhont M (1998) In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod 13: 1638–1644 [DOI] [PubMed] [Google Scholar]
- Grant PG, Mannarino AF, Colman RW (1988) cAMP-mediated phosphorylation of the low-Km cAMP phosphodiesterase markedly stimulates its catalytic activity. Proc Natl Acad Sci USA 85: 9071–9075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M (2005) Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 15: 1670–1676 [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yokoo M, Yoshida N, Sasada H, Matsumoto H, Sato E (2004) Phosphatidylinositol 3-kinase and Akt participate in the FSH-induced meiotic maturation of mouse oocytes. Mol Reprod Dev 69: 77–86 [DOI] [PubMed] [Google Scholar]
- Kalous J, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J (2006) PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol Cell 98: 111–123 [DOI] [PubMed] [Google Scholar]
- Kenan Y, Murata T, Shakur Y, Degerman E, Manganiello VC (2000) Functions of the N-terminal region of cyclic nucleotide phosphodiesterase 3 (PDE 3) isoforms. J Biol Chem 275: 12331–12338 [DOI] [PubMed] [Google Scholar]
- Kim S, Jee K, Kim D, Koh H, Chung J (2001) Cyclic AMP inhibits Akt activity by blocking the membrane localization of PDK1. J Biol Chem 276: 12864–12870 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kitamura Y, Kuroda S, Hino Y, Ando M, Kotani K, Konishi H, Matsuzaki H, Kikkawa U, Ogawa W, Kasuga M (1999) Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol Cell Biol 19: 6286–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Takeuchi F, Roth RA (1996) Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem 271: 21920–21926 [DOI] [PubMed] [Google Scholar]
- Liu H, Maurice DH (1998) Expression of cyclic GMP-inhibited phosphodiesterases 3A and 3B (PDE3A and PDE3B) in rat tissues: differential subcellular localization and regulated expression by cyclic AMP. Br J Pharmacol 125: 1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Sorisky A, Zhu L, Pawson T (1995) Molecular cloning of an amphibian insulin receptor substrate 1-like cDNA and involvement of phosphatidylinositol 3-kinase in insulin-induced Xenopus oocyte maturation. Mol Cell Biol 15: 3563–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee CH, Reifsnyder DH, Moore TA, Lerea KM, Beavo JA (1988) Phosphorylation results in activation of a cAMP phosphodiesterase in human platelets. J Biol Chem 263: 10353–10358 [PubMed] [Google Scholar]
- Maller JL, Koontz JW (1981) A study of the induction of cell division in amphibian oocytes by insulin. Dev Biol 85: 309–316 [DOI] [PubMed] [Google Scholar]
- Maller JL, Krebs EG (1977) Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem 252: 1712–1718 [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V (2004) Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest 114: 196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Dostmann W, Genieser HG, Butt E, Jastorff B (1989) Starfish oocyte maturation: evidence for a cyclic AMP-dependent inhibitory pathway. Dev Biol 133: 58–66 [DOI] [PubMed] [Google Scholar]
- Movsesian MA (2002) PDE3 cyclic nucleotide phosphodiesterases and the compartmentation of cyclic nucleotide-mediated signalling in cardiac myocytes. Basic Res Cardiol 97 (Suppl 1): I83–I90 [DOI] [PubMed] [Google Scholar]
- Murray JT, Cummings LA, Bloomberg GB, Cohen P (2005) Identification of different specificity requirements between SGK1 and PKBalpha. FEBS Lett 579: 991–994 [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H, Makhlouf GM (2002) PKA-dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am J Physiol Cell Physiol 282: C508–C517 [DOI] [PubMed] [Google Scholar]
- Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKnight GS (2006) Dynamic anchoring of PKA is essential during oocyte maturation. Curr Biol 16: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki N, Takahashi SI, Hidaka H, Conti M (2000) Short term feedback regulation of cAMP in FRTL-5 thyroid cells. Role of PDE4D3 phosphodiesterase activation. J Biol Chem 275: 10831–10837 [DOI] [PubMed] [Google Scholar]
- Okumura E, Fukuhara T, Yoshida H, Hanada Si S, Kozutsumi R, Mori M, Tachibana K, Kishimoto T (2002) Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat Cell Biol 4: 111–116 [DOI] [PubMed] [Google Scholar]
- Onuma H, Osawa H, Yamada K, Ogura T, Tanabe F, Granner DK, Makino H (2002) Identification of the insulin-regulated interaction of phosphodiesterase 3B with 14-3-3 beta protein. Diabetes 51: 3362–3367 [DOI] [PubMed] [Google Scholar]
- Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA (1999) Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J 18: 3024–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M (2004) EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303: 682–684 [DOI] [PubMed] [Google Scholar]
- Pozuelo Rubio M, Campbell DG, Morrice NA, Mackintosh C (2005) Phosphodiesterase 3A binds to 14-3-3 proteins in response to PMA-induced phosphorylation of Ser428. Biochem J 392: 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard FJ, Tsafriri A, Conti M (2001) Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod 65: 1444–1451 [DOI] [PubMed] [Google Scholar]
- Rondinone CM, Carvalho E, Rahn T, Manganiello VC, Degerman E, Smith UP (2000) Phosphorylation of PDE3B by phosphatidylinositol 3-kinase associated with the insulin receptor. J Biol Chem 275: 10093–10098 [DOI] [PubMed] [Google Scholar]
- Sadler SE (1991) Type III phosphodiesterase plays a necessary role in the growth-promoting actions of insulin, insulin-like growth factor-I, and Ha p21ras in Xenopus laevis oocytes. Mol Endocrinol 5: 1939–1946 [DOI] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR (2001) PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol 2: 760–768 [DOI] [PubMed] [Google Scholar]
- Sette C, Conti M (1996) Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem 271: 16526–16534 [DOI] [PubMed] [Google Scholar]
- Shakur Y, Takeda K, Kenan Y, Yu ZX, Rena G, Brandt D, Houslay MD, Degerman E, Ferrans VJ, Manganiello VC (2000) Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3). Two N-terminal domains are required for the efficient targeting to, and association of, PDE3 with endoplasmic reticulum. J Biol Chem 275: 38749–38761 [DOI] [PubMed] [Google Scholar]
- Shitsukawa K, Andersen CB, Richard FJ, Horner AK, Wiersma A, van Duin M, Conti M (2001) Cloning and characterization of the cyclic guanosine monophosphate-inhibited phosphodiesterase PDE3A expressed in mouse oocyte. Biol Reprod 65: 188–196 [DOI] [PubMed] [Google Scholar]
- Smitz J, Cortvrindt R, Hu Y (1998) Epidermal growth factor combined with recombinant human chorionic gonadotrophin improves meiotic progression in mouse follicle-enclosed oocyte culture. Hum Reprod 13: 664–669 [DOI] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Schultz RM (2001) RNAi in mouse oocytes and preimplantation embryos: effectiveness of hairpin dsRNA. Biochem Biophys Res Commun 287: 1099–1104 [DOI] [PubMed] [Google Scholar]
- Thompson WJ, Terasaki WL, Epstein PM, Strada SJ (1979) Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res 10: 69–92 [PubMed] [Google Scholar]
- Toker A, Newton AC (2000) Cellular signaling: pivoting around PDK-1. Cell 103: 185–188 [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M (1996) Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol 178: 393–402 [DOI] [PubMed] [Google Scholar]
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL (1993) Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 13: 2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J, Choi YH, Krall J, Ahmad F, Manganiello VC, Movsesian MA (2002) Isoforms of cyclic nucleotide phosphodiesterase PDE3A in cardiac myocytes. J Biol Chem 277: 38072–38078 [DOI] [PubMed] [Google Scholar]
- Wijkander J, Landstrom TR, Manganiello V, Belfrage P, Degerman E (1998) Insulin-induced phosphorylation and activation of phosphodiesterase 3B in rat adipocytes: possible role for protein kinase B but not mitogen-activated protein kinase or p70 S6 kinase. Endocrinology 139: 219–227 [DOI] [PubMed] [Google Scholar]
- Wiley LM, Wu JX, Harari I, Adamson ED (1992) Epidermal growth factor receptor mRNA and protein increase after the four-cell preimplantation stage in murine development. Dev Biol 149: 247–260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


