Abstract
C-tail-anchored (C-TA) proteins are anchored to specific organelle membranes by a single transmembrane segment (TMS) at the C-terminus, extruding the N-terminal functional domains into the cytoplasm in which the TMS and following basic segment function as the membrane-targeting signals. Here, we analyzed the import route of mitochondrial outer membrane (MOM) C-TA proteins, Bak, Bcl-XL, and Omp25, using digitonin-permeabilized HeLa cells, which provide specific and efficient import under competitive conditions. These experiments revealed that (i) C-TA proteins were imported to the MOM through a common pathway independent of the components of the preprotein translocase of the outer membrane, (ii) the C-TA protein-targeting signal functioned autonomously in the absence of cytoplasmic factors that specifically recognize the targeting signals and deliver the preproteins to the MOM, (iii) the function of a cytoplasmic chaperone was required if the cytoplasmic domains of the C-TA proteins assumed an import-incompetent conformation, and intriguingly, (iv) the MOM-targeting signal of Bak, in the context of the Bak molecule, required activation by the interaction of its cytoplasmic domain with VDAC2 before MOM targeting.
Keywords: C-tail-anchored proteins, membrane proteins, mitochondria, protein translocation
Introduction
The vast majority of mitochondrial proteins are encoded by nuclear DNA, synthesized on the cytoplasmic ribosomes as preproteins, and transported post-translationally to the mitochondria (Neupert, 1997; Pfanner and Geissler, 2001; Wiedemann et al, 2004). Preproteins destined to the mitochondrial matrix and a number of proteins of the inner membrane and the intermembrane space (IMS) have a cleavable matrix-targeted presequence at the N-terminus. Another class of proteins, which includes mitochondrial outer membrane (MOM) proteins and a number of the IMS and inner membrane proteins, contains an uncleavable internal mitochondrial targeting signal. The preprotein translocation machinery of the mitochondrial outer membrane (TOM complex), composed of the import receptors Tom70, Tom20, and Tom22; the channel component Tom40; and small Tom proteins, Tom5, Tom6, and Tom7 that regulate assembly of the TOM complex, is considered to be responsible for translocation and sorting of virtually all of these preproteins.
MOM proteins are categorized based on their membrane topology. One group comprises the proteins anchored to the MOM through a transmembrane segment (TMS) localized in the N-terminus, extruding the bulk of the polypeptide into the cytoplasm (the N-TA proteins). The preprotein import receptors Tom20 and Tom70 are categorized into this class (Rapaport, 2003). Another group of MOM proteins is the C-tail-anchored (C-TA) proteins, which are composed of three segments: an N-terminal hydrophilic functional domain exposed to the cytoplasm, a TMS, and the following basic hydrophilic short segment supposed to extrude into the IMS. Omp25 (Nemoto and DeCamilli, 1999), a mitochondrial fission-related protein Fis1 (Shaw and Nunnari, 2002), the small Tom proteins (Tom5, Tom6, and Tom7) (Dietmeier et al, 1997; Dembowski et al, 2001), the proapoptotic protein Bak, and the antiapoptotic proteins Bcl-XL and Mcl-1 (Cory and Adams, 2002) are included in this class. Fzo1 in Drosophila and yeast, and its mammalian homologues Mfn1 and Mfn2 (Fritz et al, 2001; Rojo et al, 2002) and peripheral benzodiazepine receptor (Joseph-Liauzun et al, 1998) span the MOM two and five times, respectively, through TMS. Lastly, Tom40, porin, Sam50/Tob55, and Mdm10 are β-barrel proteins that traverse MOM by a series of antiparallel β-strands (Wiedemann et al, 2003; Meisinger et al, 2004; Pfanner et al, 2004).
The TMS with moderate hydrophobicity and length, and the flanking segments rich in basic amino-acid residues function as the mitochondrial-targeting and sorting signal for N-TA and C-TA proteins (Kanaji et al, 2000; Horie et al, 2002; Kaufmann et al, 2003; Rapaport 2003). On the other hand, the information exposed on the folded tertiary structure is thought to function as the mitochondrial targeting signal for β-barrel proteins (Rapaport, 2003).
It is generally thought that the TOM complex is responsible for the targeting and integration of almost all of these proteins. Indeed, the N-TA proteins Tom70 and Tom20 are inserted into the MOM with the assistance of Tom40, but through a unique insertion pathway, a pathway that requires neither the import receptors nor the import pore portion (the general insertion/import pore or GIP) of Tom40 (Ahting et al, 2005). Yeast Tom5 is assembled to the yeast TOM complex through a similar pathway (Horie et al, 2003). Neurospora crassa Tom6 and Tom7 are assembled to the TOM complex through the GIP of Tom40, bypassing the import receptors (Dembowski et al, 2001). On the other hand, the β-barrel protein porin is integrated into the MOM depending on Tom20, Tom22, Tom5, and Tom40 of the TOM machinery via the GIP (Krimmer et al, 2001). Newly synthesized Tom40 is targeted to the import receptors Tom70 and Tom20 and is translocated through the GIP of the pre-existing TOM complex to the IMS, where it is integrated into the TOM complex assisted by IMS small molecular size chaperones and the β-barrel protein Tob55/Sam50 (Paschen et al, 2003; Pfanner et al, 2004; Wiedemann et al, 2004). A similar integration pathway is used during MOM integration and assembly of Tob55/Sam50 and porin (Wiedemann et al, 2004; Habib et al, 2005).
Detailed analyses of protein integration into the MOM have been restricted to the subunits of the TOM complex and β-barrel proteins that are translocated across MOM into the IMS once before assembling into the MOM, and almost no information is available on the membrane integration pathway of the other MOM proteins.
In the present study, we evaluated the MOM C-TA proteins Bak, Omp25, and Bcl-XL, which are unrelated to the components of the TOM complex, and analyzed their insertion pathway using immunofluorescence microscopy in digitonin-permeabilized ‘semi-intact' cells; the assay was performed under competitive conditions in which various organelles were present. This approach, combined with cell fractionation and in vivo analyses, enabled detection of the intracellular localization of target proteins with high sensitivity and fidelity.
We demonstrated that these C-TA proteins initially take a common, but TOM complex-independent, import route that recognizes the MOM-targeting signal. The MOM-targeting signal appeared to function autonomously to target C-TA proteins to the MOM without the help of signal-specific targeting factors in the cytoplasm. Interestingly, MOM targeting of Bak, but not GFP-BakC (GFP fused to the MOM-targeting signal of Bak), was compromised by knockdown of VDAC2, the reported binding partner of Bak, indicating that the MOM-targeting signal of Bak, when transplanted to a correctly folded reporter, was constitutively active, whereas the signal remained inactive in the context of full-length Bak. Interaction of the Bak cytoplasmic segment with VDAC2 seemed to activate the targeting signal before MOM targeting via a common C-TA protein pathway.
Results
Assay design and characterization
First, we established the assay system for mitochondrial import of C-TA proteins in digitonin-permeabilized semi-intact HeLa cells using immunofluorescence microscopy. We chose the proapoptotic effector Bak as a model protein. The N-terminally 3 × FLAG-tagged Bak (3FLAG-Bak) was translated in a rabbit reticulocyte lysate system and incubated with 25 μg/ml digitonin-treated HeLa cells. When the cells were permeabilized by 1% Triton X-100 after fixing and examined by indirect immunofluorescence staining with counter-immunostaining by organelle-specific marker proteins as a reference, 3FLAG-Bak colocalized with mitochondrial Tom22 in punctate and/or tubular structures, indicating that it was transported to the mitochondria (Figure 1A). In contrast, 3FLAG-Bak did not colocalize with either Sec61β, giantin, or Pex14p, marker proteins for the ER, Golgi, and peroxisomes, respectively (Figure 1A). The same results were also obtained with the mitochondrial matrix-targeted precursor Su9-DHFR-HA (Figure 1B). Precise inspection of the semi-intact cells in which 3FLAG-Bak and Su9-DHFR-HA were coexpressed revealed their distinct submitochondrial localization; 3FLAG-Bak localized to the outer layer of the mitochondria, whereas Su9-DHFR-HA localized in the interior region of the mitochondria (Figure 1C). 6myc/3FLAG-BakΔC, in which the mitochondrial targeting signal, consisting of the TMS and the following basic C-segment (referred to as the ‘MOM-targeting signal', hereafter) was deleted, gave very low immunofluorescence signal, indicating that it failed in mitochondria targeting and was removed from the cells in this assay system (Supplementary Figure 1A). Furthermore, the import proceeded in a temperature- and time-dependent manner for both substrates as compared with endogenous mitochondrial Tom22 signal (Supplementary Figure 1B and C). This was further confirmed by immunoblot analysis of the lysates of the cells after the import reaction; time-dependent processing of Su9-DHFR-HA or time-dependent accumulation of 6myc/3FLAG-Bak was clearly observed, indicating that the assay was quantitative (Supplementary Figure 1D).
Figure 1.
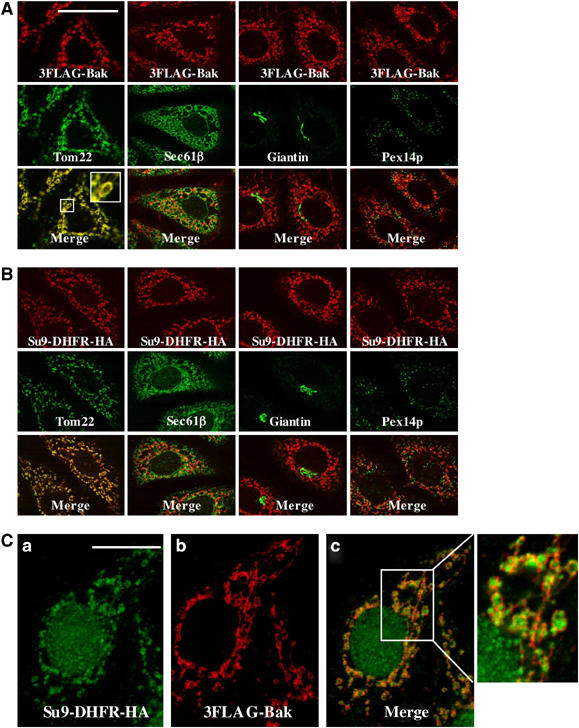
Mitochondrial preprotein import in semi-intact cells. (A, B) HeLa cells grown on coverslips were semi-permeabilized with 25 μg/ml digitonin, and incubated at 26°C for 60 min with the reticulocyte lysate-synthesized 3FLAG-Bak (A) or Su9-DHFR-HA (B). After fixation and permeabilization with 1% Triton X-100 for 5 min, the cells were processed for indirect immunofluorescence microscopy as follows: all the cells were stained with monoclonal anti-FLAG (3FLAG-Bak) and anti-HA (Su9-DHFR-HA) antibodies, or anti-Tom22 (mitochondria), anti-Sec61β (ER), anti-giantin (Golgi), and anti-Pex14p (peroxisomes). 3FLAG-Bak and Su9-DHFR-HA are shown in red and organelle-specific marker proteins are shown in green. Merged images are also shown. × 4 magnification of the central area (outlined by a box) of the micrographs. Magnification, × 630; bar=20 μm. (C) The semi-intact cells were incubated with Su9-DHFR-HA and 3FLAG-Bak as described above. After fixation and 1% Triton X-100 permeabilization, the cells were incubated with rabbit anti-HA antibodies (green) and mouse monoclonal anti-FLAG antibodies (red). Boxed region in (C) is shown at threefold magnification. Note that rabbit polyclonal anti-HA antibodies stained nuclear matrix for unknown reasons. Bar=20 μm.
We then used immunostaining techniques to examine whether the barrier function of the mitochondrial membranes was maintained (Supplementary Figure 2A). After the import reaction with 3FLAG-Bak or Su9-DHFR-HA in the semi-permeabilized condition, Tom20 and HtrA2 immunoreactivity was analyzed. Tom20, the MOM protein extruding the bulk of the molecule to the cytoplasm, but not IMS-localized HtrA2, was immunostained in the absence of Triton X-100 (Supplementary Figure 2B, j and d). HtrA2 was stained only after dissolving the outer and inner membranes with 1% Triton X-100 (c), confirming that MOM integrity was intact in the semi-intact cells. The mitochondria-imported 3FLAG-Bak (b), but not Su9-DHFR-HA (h), was immunostained in the absence of 1% Triton X-100. In contrast, Su9-DHFR-HA was immunostained only after the outer and inner membranes were dissolved with 1% Triton X-100 (g); Su9-DHFR-HA precursor detected by immunoblotting (Supplementary Figure 1D) probably represented the matrix-localized form. Together, these results indicated that in plasma membrane permeabilization with 25 μg/ml digitonin, the barrier function of the outer and inner membranes was intact and 3FLAG-Bak was integrated into the MOM exposing the N-terminal portion to the cytoplasm, whereas Su9-DHFR-HA was imported into the matrix. The membrane integration of 3FLAG-Bak was verified by alkali extraction (pH 11.5) (Supplementary Figure 2C, lanes 1 and 2). Similar results were likewise obtained with in vitro-imported 3FLAG-Bak (Supplementary Figure 2C, lanes 3 and 4). Thus, correct targeting and topogenesis were reconstituted in the semi-intact cells. Taken together, we established a reliable, specific, and quantitative assay to study the targeting and integration of C-TA proteins into the MOM.
Using this system, we analyzed the requirements for cytoplasmic ATP for mitochondrial targeting of C-TA proteins. Mitochondrial import of Su9-DHFR-HA and HA-VDAC2 was strongly inhibited by AMP-PNP (Supplementary Figure 3A and B), confirming that the initial steps of preprotein transfer to the receptor involve cytosolic ATP for most, but not all, preproteins (Neupert, 1997); gel-filtration seemed to be insufficient to completely remove ATP from the protein synthesis reaction mixture, as import of these proteins occurred in the absence AMP-PNP. In contrast, the mitochondrial import of 3FLAG-Bak was not affected at all by AMP-PNP (Supplementary Figure 3A and B). These results were also confirmed for the membrane integration of 3FLAG-Bak as assessed by alkali extraction (Supplementary Figure 3C). The same results were also obtained for Omp25 (data not shown). Together, these results indicated that mitochondrial targeting and integration of C-TA proteins did not depend on the hydrolysis of cytoplasmic ATP. Also, membrane potential (ΔΨ) across the inner membrane was not required for MOM targeting and integration of 3FLAG-Bak and 6myc-Omp25, because the import was not inhibited by a protonophore CCCP (Supplementary Figure 3D and E).
Membrane integration of C-TA proteins shares a common, but TOM complex-independent, import pathway
We then examined whether these C-TA proteins share a common import pathway. The ability of excess 6myc-Bak to compete with the other C-TA proteins for the mitochondrial targeting was examined in semi-intact cells (Figure 2). First of all, the substrate amounts were assessed by immunoblotting (Figure 2A shows 3 μl each (defined as ‘one equivalent') of translation products), and the substrates of 1.7-equivalent each were used for the import reaction (Figure 2B and C). In the absence of 6myc-Bak, the C-TA proteins were imported into the mitochondria. Their import, however, was efficiently inhibited by 13-equivalents of 6myc-Bak. In contrast, excess 6myc-Bak did not inhibit mitochondrial import of a matrix-targeted preprotein Su9-DHFR-HA and a MOM-targeted β-barrel protein HA-VDAC2, nor peroxisomal import of peroxisomal C-TA protein Pex26p (Fang et al, 2004; Jones et al, 2004) (Figure 2B and C). Thus, these mitochondrial C-TA proteins shared a common import pathway that initially recognized the characteristics of the MOM-targeting signal conserved among C-TA proteins; the TMS of 18–22 amino-acid residues, followed by the C-segment of 2–3 basic amino-acid residues (Horie et al, 2002).
Figure 2.
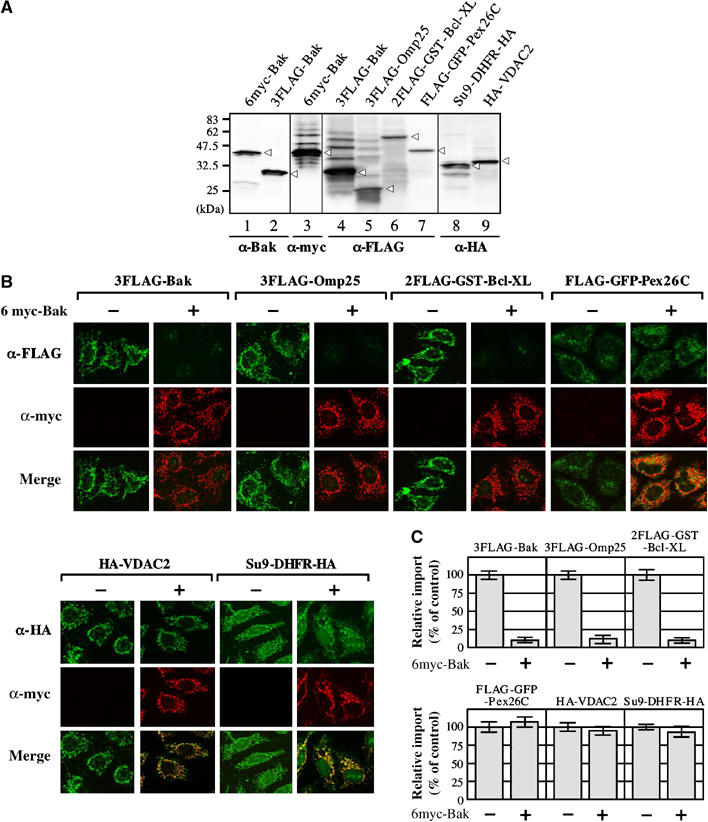
Competitive inhibition of mitochondrial import of C-TA proteins by an excess amount of 6myc-Bak. (A) Reticulocyte lysate-synthesized proteins used in this assay (3 μl each) were analyzed by SDS–PAGE and subsequent immunoblotting using the indicated antibodies. Substrate proteins are indicated by arrowheads. (B) The indicated precursor proteins (5 μl each) were subjected to mitochondrial import reaction (in 120 μl) at 26°C for 45 min in semi-intact cells in the presence or absence of an excess amount (40 μl) of 6myc-Bak. Other conditions were as in Figure 1. Imported preproteins and competitor 6myc-Bak are shown in green and red, respectively. (C) The extent of import was quantified by NIH Image, setting fluorescence intensities in the absence of 6myc-Bak at 100%. Three independent fields (each contains at least 100 cells) in a representative experiment were analyzed.
C-TA proteins are transported to the MOM independently of the TOM components
The TOM complex serves as the central entry site for the general mitochondrial precursor proteins, and is thought to be responsible for the import and sorting of virtually all mitochondrial preproteins synthesized in the cytoplasm. Here, we examined whether the TOM subunits are involved in transport of C-TA proteins to the MOM using knockdown of the subunits according to the procedure depicted in Figure 3A. The Tom components were depleted by approximately 82–95% (Figure 3C), which was clearly reflected in the decreased fluorescence intensity of the Tom proteins (Figure 3B). Of note, knockdown reaction was specific for the target proteins, except for Tom22: Tom22 knockdown significantly lowered the level of Tom20 for unknown reasons (Figure 3C). As expected, knockdown of preprotein import receptors Tom20 and Tom22, and import channel Tom40 clearly inhibited the import of the matrix-destined preprotein Su9-DHFR-HA and the MOM-destined β-barrel protein HA-VDAC2 (Figure 3B and Supplementary Figure 4). These inhibitions were not attributed to the dissipation of ΔΨ, as ΔΨ-dependent dye MitoTracker Red was normally incorporated into mitochondria of the Tom component knockdown cells (Supplementary Figure 4). CCCP treatment inhibited mitochondrial import of Su9-DHFR-HA, concomitant with the inhibition of mitochondrial accumulation of MitoTracker Red (Supplementary Figure 4). In sharp contrast, knockdown of these Tom proteins did not inhibit mitochondrial import of 3FLAG-Bak (Figure 3B and Supplementary Figure 4). Essentially, the same results were also obtained for 3FLAG-Omp25 and 2FLAG-GST-Bcl-XL (data not shown). Knockdown of Tom70 did not affect mitochondrial import of any of these preproteins. Tom70 is a unique import receptor for hydrophobic proteins with internal targeting signals (Brix et al, 1997), although its cellular function remains to be investigated. Together, these experiments revealed that mitochondrial targeting and integration of C-TA proteins proceed in a TOM complex-independent manner.
Figure 3.
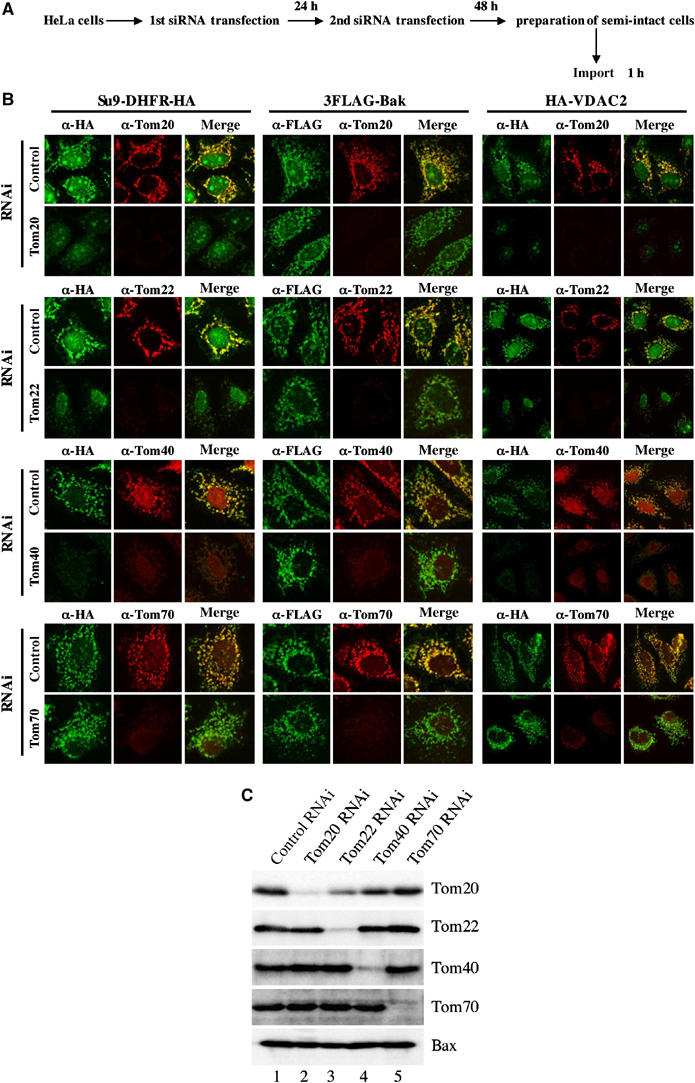
Mitochondrial import of C-TA proteins in the TOM component-depleted semi-intact cells. (A) Schematic representation of small interfering RNA (siRNA) transfection and import assay. (B) HeLa cells were subjected to siRNA transfection for the indicated proteins and following in vitro import assay using the semi-intact cells at 26°C for 60 min. The semi-intact cells were processed for double indirect immunofluorescence microscopy with either anti-HA antibodies (green; for Su9-DHFR-HA and HA-VDAC2) or anti-FLAG antibodies (green; for 3FLAG-Bak) and antibodies against the indicated Tom proteins (red). Merged images are also shown. (C) HeLa cells subjected to RNAi experiments as in (B) (equal protein amounts) were analyzed by SDS–PAGE and subsequent immunoblotting using the indicated antibodies.
We further confirmed the above observations in nonpermeabilized intact cultured cells (referred to as the ‘in vivo system' in Supplementary Figure 5). 3FLAG-Bak, Su9-GFP, or HA-tagged human Tom5, a C-TA component of the mammalian TOM complex (Kato and Mihara, unpublished data) was exogenously expressed in Tom component-depleted HeLa cells. Mitochondrial import of Su9-GFP was strongly compromised by knockdown of Tom components other than Tom70, whereas import of HA-hTom5 was compromised only by Tom40 knockdown, confirming our previous results for yeast Tom5 (Horie et al, 2002). Mitochondrial import of 3FLAG-Bak, however, was not affected by these manipulations. Thus, we concluded that C-TA proteins unrelated to the TOM components are imported to the MOM in a TOM component-independent pathway.
Mitochondrial import of C-TA proteins does not require proteinase K-sensitive components of MOM
Having excluded the involvement of the TOM complex, we further examined whether the other membrane components were involved in C-TA protein import. For this purpose, we adopted a limited proteinase K digestion in a semi-intact cell system; HeLa cells were exposed to 30 μg/ml proteinase K at 26°C for 3 min after plasma membrane permeabilization by 25 μg/ml digitonin, washed with buffer containing PMSF, and then subjected to the preprotein import assay (Figure 4A). Immunofluorescence microscopy revealed that Tom20 and FKBP38 (Shirane and Nakayama, 2003), the MOM proteins exposing the bulk portion to the cytoplasm, were removed by this treatment, whereas the IMS-localized proteins HtrA2/Omi and cytochrome c were not digested, clearly indicating that proteinase K specifically removed mitochondrial surface-exposed proteins without affecting the barrier function of MOM (Figure 4B). This was further confirmed by immunoblot analysis of the above treated cells (Figure 4C). Tom20, Tom70, and FKBP38 were completely removed by 30 μg/ml proteinase K in the presence of 25 μg/ml digitonin, whereas cytochrome c and HtrA2 remained undigested. The slight increase in mobility of the protected Tom22 was due to the removal of its cytoplasmic N-terminal domain by proteinase K (Saeki et al, 2000). Tom40 remained undigested in this condition. Thus membrane-embedded segments of MOM remained intact under 25 μg/ml digitonin that is used for preparation of semi-intact cells. Under 400 μg/ml digitonin, Tom40 and the IMS-localized proteins cytochrome c and HtrA2 were digested by proteinase K, whereas the matrix proteins mtHsp70 and Hsp60 were unaffected (data not shown), indicating that this concentration of digitonin selectively permeabilized both the plasma and mitochondrial outer membranes, but not the inner membrane. In agreement with the above observations, the mitochondrial import of Su9-DHFR-HA was greatly reduced by 30 μg/ml proteinase K treatment (Figure 4D). In contrast, this treatment did not abolish the import of C-TA proteins (Figure 4D). Of note, the import of 3FLAG-Bak that depends on VDAC2 (see Figure 5) proceeded normally under this condition, because VDAC2 remained undigested under this condition. Importantly, C-TA proteins thus targeted to the mitochondria were resistant to alkaline extraction, indicating that they were firmly anchored to the proteinase K-shaved MOM (Figure 4E). These findings indicated that the proteinase K-sensitive components on the mitochondrial surface were not essential for the import of C-TA proteins, but were necessary for the import of Su9-DHFR-HA.
Figure 4.
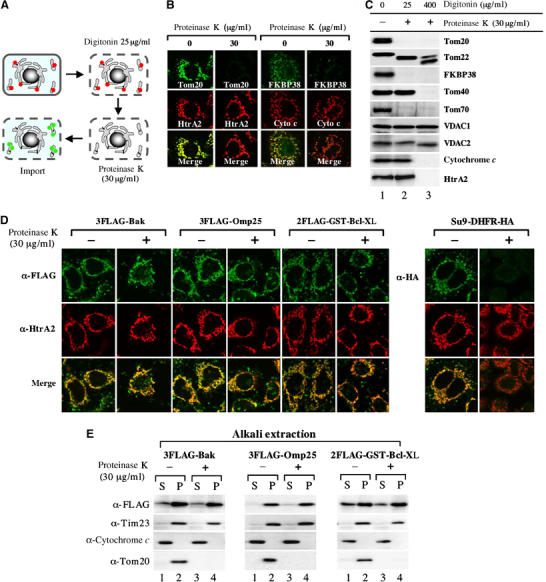
Import of C-TA proteins into proteinase K-treated mitochondria in semi-intact cells. (A) Cartoon of the in vitro import assay using proteinase K-treated semi-intact cells. Red balls represent the cytoplasmic domains of membrane proteins. Green balls represent the imported C-TA proteins. (B) Semi-intact HeLa cells were treated with or without proteinase K as in (A) and processed for indirect immunofluorescence microscopy; the cells were fixed with 4% paraformaldehyde, permeabilized with 1% Triton X-100, and immunostained using antibodies against the indicated proteins. See Materials and methods for details. (C) Semi-intact cells were incubated with or without proteinase K under the indicated digitonin concentrations. All these samples were subjected to SDS–PAGE and subsequent immunoblot analysis using antibodies against the indicated proteins. (D) The indicated substrates were subjected to the import reaction at 26°C for 45 min in proteinase K-treated semi-intact cells. After the import reaction, the cells were processed for double indirect immunofluorescence microscopy with either anti-FLAG antibodies (green; 3FLAG-Bak, 3FLAG-Omp25, and 2FLAG-GST-Bcl-XL) or anti-HA antibodies (green; Su9-DHFR-HA) and antibodies against IMS protein HtrA2 (red). Merged images are also shown. (E) The cells shown in (D) were treated with 100 mM sodium carbonate (pH 11.5) to separate the supernatant (S) and membrane precipitates (P), which were analyzed by SDS–PAGE and subsequent immunoblotting using the indicated antibodies.
Figure 5.
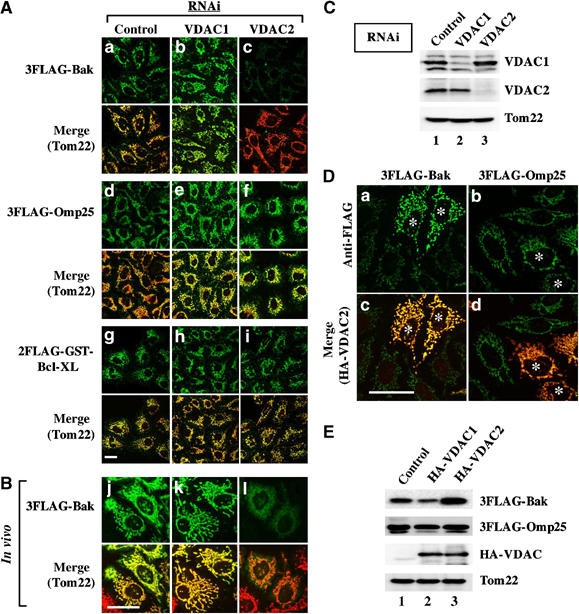
Mitochondrial import of Bak is compromised in VDAC2-depleted semi-intact cells. (A, B) The indicated preproteins were subjected to mitochondrial import in VDAC1- or VDAC2-depleted semi-intact (A) or intact (‘in vivo' (B)) cells. RNAi and the in vitro import assay were performed as described in Figure 3. Cells were processed for double indirect immunofluorescence microscopy with either anti-FLAG antibodies (green; 3FLAG-Bak, 3FLAG-Omp25, and 2FLAG-GST-Bcl-XL) or anti-Tom22 antibodies (red). Merged images are also shown. (C) Cell lysates (equal protein amounts) in (B) were analyzed by SDS–PAGE and subsequent immunoblotting (red) using antibodies against the indicated proteins. (D) The indicated preproteins were subjected to mitochondrial import in VDAC2-overexpressing semi-intact cells. The semi-intact cells were processed for double indirect immunofluorescence microscopy as in (A). The asterisks indicate cells overexpressing HA-VDAC2. (E) Cell lysates (equal protein amounts) prepared from (D) were analyzed by SDS–PAGE and subsequent immunoblotting using the antibodies against the indicated proteins.
VDAC2, but not VDAC1, is important for the mitochondrial import of Bak
The multi-domain proapoptotic molecule Bak is required to initiate the mitochondrial pathway of apoptosis (Cheng et al, 2003). In viable cells, Bak is complexed with VDAC2 in MOM to maintain the inactive conformation. We therefore examined whether knockdown of VDAC2 affected mitochondrial import of Bak, and found that this was indeed the case. RNA interference (RNAi) for VDAC1 and VDAC2 resulted in ∼95% reduction of both proteins in HeLa cells at 72 h after transfection (Figure 5C). The cells were then semi-permeabilized and subjected to an import reaction of 3FLAG-Bak, 3FLAG-Omp25, 2FLAG-GST-Bcl-XL, and Tom40-HA (Figure 5A; Tom40-HA not shown). Mitochondrial import of these proteins proceeded normally in VDAC1 RNAi cells as compared with control RNAi cells (Figure 5A, a, b, d, e, g, and h). Strikingly, however, mitochondrial import of 3FLAG-Bak, but not the other substrates, was markedly compromised in VDAC2 RNAi cells (c). Knockdown of the major MOM component VDAC1 did not affect the import (b). The same results were obtained in nonpermeabilized cultured cells, although the inhibitory effect was less pronounced (Figure 5B, l). Conversely, exogenous expression of HA-VDAC2, but not HA-VDAC1, significantly increased the mitochondrial accumulation of 3FLAG-Bak without affecting mitochondrial targeting of 3FLAG-Omp25 (Figure 5D and E). Together, these results indicated that the Bak-VDAC2 interaction was essential for the mitochondrial import of Bak. Considering that C-TA proteins were targeted to the MOM via a common MOM-targeting route using the MOM-targeting signal, these results suggested that VDAC2 specifically affected the MOM-targeting signal activity in the context of the Bak molecule (see below).
MOM targeting of GFP-BakC is not affected by the VDAC2 expression level
We then examined the reason why only Bak, but not Omp25 and Bcl-XL, depended on VDAC2 for MOM localization. For this purpose, the MOM-targeting signal of Bak was fused to the C-terminus of GFP to create GFP-BakC and used for the mitochondrial transport assay. This chimeric protein was targeted to the mitochondria as efficiently as wild-type Bak in control cells (Supplementary Figure 6A, a and e). Intriguingly, the chimeric construct was efficiently targeted to the mitochondria even in VDAC2 knockdown cells (b). In contrast, the import of GFP-Bak in which GFP was fused to the N-terminus of full-size Bak was strongly compromised by VDAC2 knockdown (f). Furthermore, mitochondrial targeting of GFP-Bak was stimulated significantly in semi-intact VDAC2-overexpressed cells, whereas the import of GFP-BakC was not at all affected (Supplementary Figure 6B and C). These results suggested that the MOM-targeting signal of Bak was inactivated or masked within the molecule, and required activation or unmasking by interaction of the cytoplasmic domain of Bak with VDAC2 before MOM targeting, a situation similar to that of the targeting signals of Bax and Bcl-XL (Jeong et al, 2004; Schinzel et al, 2004).
Mitochondrial C-TA proteins are autonomously targeted to MOM independently of cytosolic factors
In peroxisomal protein import, Pex19p functions as a chaperone that is specific for nascent peroxisomal membrane proteins, including peroxisomal C-TA proteins such as Pex26p, to deliver them from the cytoplasm to the Pex3p of peroxisomal membranes (Jones et al, 2004; Fang et al, 2004). Little is known, however, about whether mitochondrial C-TA proteins are delivered to the mitochondrial surface (receptors) by cytoplasmic mobile factor(s) that specifically recognize the MOM-targeting signal. Here we examined this point using the semi-intact cell system.
It should be noted that during the preparation of semi-intact cells for the in vitro import assay, a 5-min incubation at room temperature was included after plasma membrane permeabilization with 25 μg/ml digitonin to allow for leakage of the cytosolic components. Indeed, most of the cytoplasmic marker protein LDH and the cytoplasmic factors involved in mitochondrial preprotein transport such as Hsp90, Hsc70, Hsp40, MSF, and AIP (Mihara and Omura, 1996; Yano et al, 2003; Young et al, 2003) were released by this procedure (Figure 6B, lane 2). To investigate the involvement of cytosolic factor(s) in the mitochondrial import of C-TA proteins, we used the Escherichia coli PURESYSTEM, which does not contain cytoplasmic chaperones that might interact with nascent proteins. Model C-TA proteins GFP-BakC, GFP-Bcl-XLC, and GFP-Omp25C, in which the MOM-targeting signals of Bak, Bcl-XL, and Omp25 were ligated to the C-terminus of GFP, respectively, were translated in the PURESYSTEM and subjected to mitochondrial import in the presence or absence of rabbit reticulocyte lysate (Figure 6A). These substrates were all efficiently transported to the mitochondria irrespective of the presence or absence of the reticulocyte lysate. In contrast, the PURESYSTEM-synthesized Su9-DHFR-HA was not imported into the mitochondria (data not shown), confirming the results that the import of matrix-targeted preproteins depends on cytoplasmic factors (Mihara and Omura, 1996). To rule out the possibility that the remaining targeting factor(s) associated with the intracellular membranes were involved in this targeting, HeLa cells were permeabilized under stronger conditions with 100 μg/ml digitonin, which efficiently removed the above described cytoplasmic proteins (Figure 6B, lane3). GFP-BakC, however, was efficiently imported to the mitochondria (Supplementary Figure 7). In contrast, the transport of a model peroxisomal C-TA protein, GFP-Pex26C, to the peroxisomes clearly depended upon co-translationally expressed Pex19p (Figure 6C and D). Co-translationally synthesized Pex19p did not affect mitochondrial targeting of GFP-BakC (Figure 6E). Thus, it is most likely that the MOM-targeting signal exposed to the surface of C-TA molecules is directly recognized by mitochondrial surface elements without the assistance of cytoplasmic targeting factor(s), although we could not rigorously rule out the possibility that the semi-permeabilization procedure was insufficient to remove the essential cytosolic factor(s).
Figure 6.
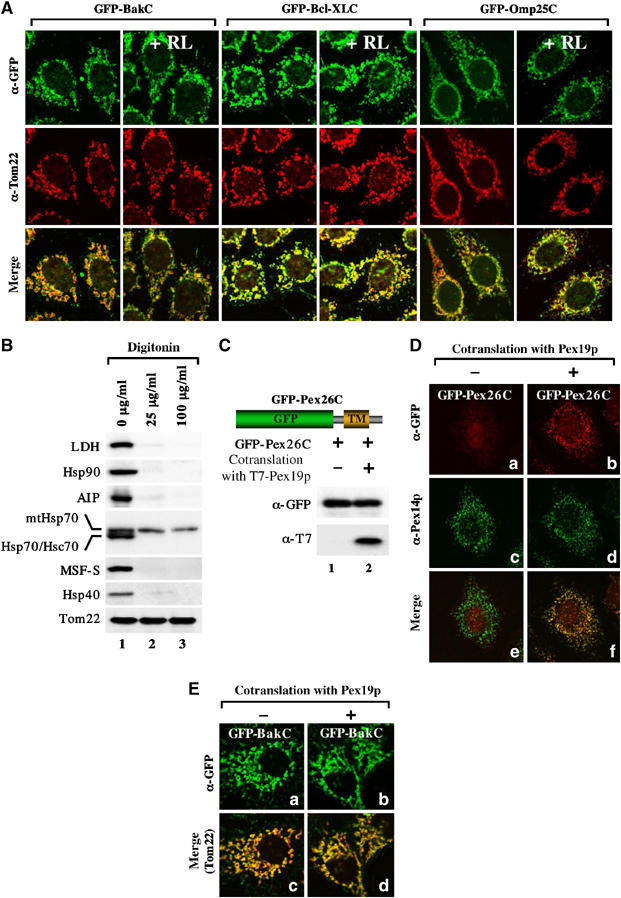
Mitochondrial import of the PURESYSTEM-synthesized chimeric proteins consisting of GFP and MOM-targeting signals of C-TA proteins proceeds in the absence of cytoplasmic factors. (A) GFP-BakC, GFP-Bcl-XLC, and GFP-Omp25C were synthesized in the PURESYSTEM and subjected to mitochondrial import in semi-intact cells in the presence or absence of rabbit reticulocyte lysate (20 mg/ml (+RL)). Cells were processed for double indirect immunofluorescence microscopy with anti-GFP antibodies (green) and anti-Tom22 antibodies (red). Merged images are also shown. (B) HeLa cells were permeabilized with 25 μg/ml (lane 2) or 100 μg/ml digitonin (lane 3). After 5 min at 26°C, the semi-intact cells were washed and subjected to SDS–PAGE and subsequent immunoblot analysis using the indicated antibodies. (C) GFP-Pex26C was cotranslated with or without T7-tagged Pex19p in the PURESYSTEM. The reaction mixtures were analyzed by SDS–PAGE and subsequent immunoblotting using the indicated antibodies. (D) In vitro-synthesized GFP-Pex26C as in (C) was incubated with semi-intact HeLa cells and the cells were processed for double indirect immunofluorescence microscopy with anti-GFP antibodies (red) and anti-Pex14p antibodies (peroxisome marker; green). Merged images are also shown. (E) GFP-Bak was cotranslated in PURESYSTEM with (+) or without (−) Pex19p, then subjected to mitochondrial import as described in (D).
Mitochondrial C-TA proteins require cytoplasmic factors with chaperone function depending on the conformation of the N-terminal cytoplasmic domains
Because the above results were obtained using the fusion proteins between GFP and the MOM-targeting signal of the C-TA proteins, we examined the requirement of cytoplasmic factor(s) using full-length Bak and Bcl-XL. Reticulocyte lysate-synthesized 3FLAG-Bak or 2FLAG-GST-Bcl-XL was immunopurified with anti-FLAG IgG-conjugated beads and used for the import. Mitochondrial import of full-length Bak and Bcl-XL clearly depended on the dose of rabbit reticulocyte lysate added (Supplementary Figure 8A and B). In contrast, 3FLAG-Bak synthesized in a PURESYSTEM failed to be transported to the mitochondria even in the presence of rabbit reticulocyte lysate (Supplementary Figure 8C). Interestingly, however, the PURESYSTEM-synthesized GFP-Bak, in which GFP was fused to the N-terminus of full-size Bak, was imported to the mitochondria even in the absence of reticulocyte lysate (Supplementary Figure 8D).
These results indicated that the import competence of C-TA proteins depends largely on the folded state of the N-terminal reporter domains. 3FLAG-Bak synthesized in the PURESYSTEM might be in an incorrectly folded state that sequestered the MOM-targeting signal because the protein synthesis system did not contain molecular chaperones (Supplementary Figure 8C). In the case of GFP-Bak, however, the GFP domain probably functioned as a folding template, and the substrate attained import-competent folding states (Supplementary Figure 8D). Reticulocyte lysate-synthesized and immunopurified 3FLAG-Bak and 2FLAG-GST-Bcl-XL were in the folding states that can reversibly assume the import-competent conformation, where the MOM-targeting signal can be exposed depending on the amounts of reticulocyte lysate added. These results together indicated that mitochondrial C-TA proteins require cytoplasmic factors with chaperone function depending on the folded states of the N-terminal cytoplasmic domains, but that the MOM-targeting signal autonomously functioned to deliver the C-TA molecules to the mitochondria, in contrast to the case of Pex19p in peroxisomal import of C-TA proteins.
Discussion
Although it has long been known that C-TA proteins (e.g., cytochrome b5) can bind in vitro to protein-free liposomes or to various isolated organelle membranes (‘insertion sequences'; Blobel, 1980), the requirements for physiologically relevant insertion and a molecular basis for the membrane selectivity observed in vivo have remained a matter of debate (Kutay et al, 1995; Borgese et al, 2003; Yabal et al, 2003; Abell et al, 2004). Despite the wide functional diversity and current interest in C-TA proteins, membrane components and cytosolic factors that influence or regulate their post-translational targeting and insertion have been elusive. This has been due in large part to the lack of assays with sufficient sensitivity and specificity to allow for reliable analysis. Furthermore, assessment of the correct topogenesis has been difficult because the precursor proteins carry uncleavable organelle-targeting signals. In this study, we established a reliable and specific assay to study the targeting of C-TA proteins into the MOM using semi-intact cells; protein import under competitive conditions that is distinct from the commonly used system: in vitro import into the isolated mitochondria. This assay revealed several novel properties of the C-TA import process.
How are the characteristics of the MOM-targeting signals of C-TA proteins decoded and delivered correctly to the outer membrane? In the present study, we demonstrated that E. coli PURESYSTEM-synthesized GFP-BakC, GFP-Bcl-XLC, and GFP-Omp25C were all efficiently and specifically targeted to the mitochondria in cytosol-depleted semi-intact cells; the import proceeded even under harsh digitonin-permeabilization conditions that might efficiently remove cytoplasmic import factors loosely associated with the membranes (Figure 6). In contrast, peroxisomal import of GFP-Pex26C strictly depended on Pex19p, a cytosolic chaperone specific for peroxisomal membrane proteins. In the overexpression condition, GFP-Pex26p was mistargeted to the mitochondria in HeLa cells (data not shown). Coexpression of Pex19p with GFP-Pex26p strikingly enhanced the fidelity of the import of GFP-Pex26p to peroxisomes (data not shown); mitochondria might be the default destination for the peroxisomal membrane proteins. Together, these results suggested that the MOM-targeting signals of mitochondrial C-TA proteins function autonomously to direct reporter proteins to the MOM in the absence of the cytoplasmic targeting factor(s) that specifically recognizes the signal and delivers the substrate to the mitochondrial surface.
In vitro experiments examining the involvement of the TOM components were performed using the model protein Bcl-2. These studies have yielded contradictory results, indicating that Bcl-2 is imported to the protease-treated mitochondria (Janiak et al, 1994) on the one hand, and that Bcl-2 binding to yeast mitochondria is partially dependent on Tom20 (Motz et al, 2002) on the other hand. These contradictory results might be due to the use of a yeast heterologous system to analyze the intracellular localization. In the present study, we analyzed the involvement of the TOM complex in semi-intact cells using dual color immunofluorescence as a rigorous criterion. The results of these experiments strongly indicated that the import of C-TA proteins (Bak, Bcl-XL, and Omp25) occurs by a novel mechanism that does not involve the TOM complex (Figure 3 and Supplementary Figure 4). It should be clarified whether this is the general import pathway for the mitochondrial C-TA proteins.
It also remains to be clarified whether the insertion of these C-TA proteins into the MOM is facilitated by protein components of the membrane that specifically recognize the MOM-targeting signal and integrate them into the membrane, or if they are directly inserted into the lipid bilayer as previously thought (Blobel, 1980). In the latter case, the characteristics of the MOM-targeting signal of the C-TA proteins as described above might be recognized by specific lipid compositions (Borgese et al, 2003). In this relation, contradictory results are reported for membrane integration of the ER-destined C-TA proteins: Sec61 complex-dependent insertion (Abell et al, 2004), the involvement of membrane protein(s) distinct from Sec61 (Kutay et al, 1995), or the possibility of direct insertion into lipid bilayers (Borgese et al, 2003; Yabal et al, 2003). Biochemical approaches using reconstitution into proteoliposomes and genetic approaches are both required to answer these questions regarding mitochondrial transport of C-TA proteins.
Another important finding of the present study is that the interaction of the C-TA proteins with the membrane components sometimes influenced C-TA protein import. Indeed, VDAC2 specifically affected Bak import into the MOM. Because Bak interacts with VDAC2 in MOM to regulate apoptosis (Cheng et al, 2003), the overall import efficiency of Bak might be regulated by the Bak–VDAC2 interaction. The data indicated that the MOM-targeting signal spliced from Bak and transplanted to GFP was active in mitochondrial targeting in the VDAC2-knockdown cells, whereas full-size Bak failed to be transported to the mitochondria in the VDAC2-knockdown cells (Figure 5 and Supplementary Figure 6), clearly indicating that the MOM-targeting signal of Bak was somehow masked within the Bak molecule, and the signal was activated by Bak–VDAC2 interaction just before or shortly after the mitochondrial binding. Considering that a weak import of 3FLAG-Bak was still observed in VDAC2-knockdown (by ∼95%) cells, 3FLAG-Bak might contain a small fraction of the activated, import-competent population, which might be imported into the mitochondria in a VDAC2-independent, bypass pathway (Figure 5 and Supplementary Figure 6). C-TA proteins Bax and Bcl-XL have similarly regulated targeting. Bax has the N-terminal apoptosis-regulated targeting domain and the MOM-targeting signal at the C-terminus. Its MOM-targeting signal is housed within a hydrophobic pocket in healthy cells. During apoptosis, Bax undergoes a conformational change to expose both domains, resulting in mitochondrial targeting (Schinzel et al, 2004). A significant fraction of Bcl-XL is localized in the cytosol in healthy cells as homodimers formed via the C-terminal MOM-targeting signal. Binding of the proapoptotic factor Bad to Bcl-XL dissociates the homodimers to expose the targeting signal and triggers Bcl-XL targeting to the MOM (Jeong et al, 2004). The mechanism of activation of Bak-targeting/integration is thus distinct from those of the other Bcl-2 family proteins. How the MOM-targeting signal of Bak is inactivated within the context of the Bak molecule and how VDAC2 activates the signal, remains to be elucidated.
Materials and methods
SDS–PAGE and immunoblot analysis
Immunoblotting was performed using anti-FLAG (Sigma), anti-Hsp40 (Santa Cruz Biotechnology), anti-Hsp70/Hsc70 (StressGen), anti-Hsp90 (StressGen), anti-AIP (Yano et al, 2003), anti-MSF-S (Alam et al, 1994), anti-LDH (Sigma), anti-cytochrome c (Pharmingen), anti-Tom22 (Sigma), anti-HA (Covance), anti-Tom20 (Santa Cruz Biotechnology), anti-FKBP38 (Shirane and Nakayama, 2003), anti-Tom40 (Santa Cruz Biotechnology), anti-Tom70 (Suzuki et al, 2002), anti-VDAC1 (Abcam), anti-VDAC2 (Abcam), anti-HtrA2 (R&D systems), anti-GFP (MBL), anti-T7 (Novagen), anti-myc (Upstate), anti-Tim23 (Ishihara and Mihara, 1998), and anti-mtHsp70 (StressGen) primary antibodies followed by peroxidase-coupled, goat anti-rabbit or anti-mouse secondary antibodies (Biosource). Immunodetection was performed by ECL (Amersham).
Cell culture, cDNA transfection, and morphologic analysis
HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum under 5% CO2 and 95% air. DNA transfection to cells was performed using Fugene6 (Roche) as recommended by the manufacturer. For immunocytochemistry analysis, HeLa cells were seeded onto glass slides in the mounting medium and observed by confocal fluorescence microscopy. Cells were fixed with 4% paraformaldehyde and then permeabilized with 1% Triton X-100 in phosphate-buffered saline at room temperature for 5 min and immunostained with the appropriate antibodies. Antigen–antibody complexes were detected using Alexa 488- or Alexa 568-labeled goat anti-rabbit or anti-mouse IgG antibody (Molecular Probes). Importantly, in these experiments, no Alexa 568 signal was detected in the 488-nm (green) Alexa 488 channel, and vice versa. Immunofluorescence images were captured with the same detection sensitivity and processed with the Adobe Photoshop 8.0.1 software (Adobe System Inc.).
Cell-free synthesis of proteins
All proteins were synthesized separately in vitro using the respective cDNA in the pcDNA3.1 vector and TNT Quick Coupled Transcription/Translation System (Promega). GFP-BakC, GFP-Bak, GFP-Bcl-XLC, and GFP-Omp25C were also synthesized in vitro using the respective cDNA and PURESYSTEM (POST GENOME INSTITUTE, Co., LTD) according to the manufacturer's protocol. Translation mixtures were cleared by centrifugation at 100 000 g for 20 min at 4°C before use in the import assays.
Immunofluorescence-based in vitro import assay
The immunofluorescence-based in vitro import assay was performed as follows. HeLa cells (∼80% confluent in 18 mm coverslips) were permeabilized with 25 μg/ml digitonin (normal condition) or 100 μg/ml digitonin (stronger condition) in an in vitro import buffer (20 mM HEPES–KOH buffer (pH 7.5) containing 0.25 M sucrose, 2.5 mM magnesium acetate, 25 mM KCl, 2.5 mM EGTA, and 1 μM Taxol) at 26°C for 5 min. Semi-intact cells were incubated with in vitro-translated proteins for 60 min at 26 or 4°C in the import buffer of final 120 μl. The immunofluorescence-based in vitro import assay using proteinase K-treated semi-intact cells was performed as follows. The semi-intact cells were incubated with 30 μg/ml proteinase K for 3 min at 26°C. The reaction was stopped by the addition of 1 mM PMSF in the import buffer and then incubated with in vitro-synthesized proteins for 45 min at 26°C in the in vitro import buffer containing 1 mM PMSF. After the import reaction, all these cells were washed with the import buffer and processed for indirect immunofluorescence microscopy using anti-FLAG (Sigma), anti-myc (Upstate), anti-HA (Covance), anti-Tom22 (Sigma), anti-Sec61β (Upstate), anti-giantin (Sohda et al, 2001), anti-Pex14p (Santa Cruz Biotechnology), anti-Tom20 (Santa Cruz Biotechnology), anti-Tom40 (Suzuki et al, 2000), anti-Tom70 (Suzuki et al, 2002), anti-FKBP38 (Shirane and Nakayama, 2003), anti-GFP (Molecular Probes), or anti-GFP (MBL) primary antibodies.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figures 3 and 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7, 8 and 9
Supplementary Data
Supplementary Methods
Supplementary Information
Acknowledgments
We thank T Matsuzaki (Kyushu University) for valuable discussion during this study. We also thank M Shirane and K Nakayama (Kyushu University), Y Ikehara and Y Misumi (Fukuoka University), and M Yano and M Mori (Kumamoto University) for providing anti-FKBP38, anti-giantin, and anti-AIP antibodies, respectively, and members of the Mihara laboratory for helpful discussion. This work was supported by grants from the Ministry of Education, Science, and Culture of Japan, from the Human Frontier Science Program and Takeda Science Foundation.
References
- Abell BM, Pool MR, Schlenker O, Sinning I, High S (2004) Signal recognition particle mediates post-translational targeting in eukaryotes. EMBO J 23: 2755–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahting U, Weizenegger T, Neupert W, Rapaport D (2005) Signal-anchor proteins follow a unique insertion pathway into the outer membrane of mitochondria. J Biol Chem 280: 48–53 [DOI] [PubMed] [Google Scholar]
- Alam R, Hachiya N, Sakaguchi M, Kawabata S, Iwanaga S, Kitajima M, Mihara K, Omura T (1994) cDNA cloning and characterization of mitochondrial import stimulation factor (MSF) purified from rat liver cytosol. J Biochem 116: 416–425 [DOI] [PubMed] [Google Scholar]
- Blobel G (1980) Intracellular protein topogenesis. Proc Natl Acad Sci USA 77: 1496–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N, Colombo S, Pedrazzini E (2003) The tale of tail-anchored proteins: coming from the cytosol and looking for a membrane. J Cell Biol 161: 1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix J, Dietmeier K, Pfanner N (1997) Differential recognition of preproteins by the purified cytosolic domains of the mitochondrial import receptors Tom20, Tom22, and Tom70. J Biol Chem 272: 20730–20735 [DOI] [PubMed] [Google Scholar]
- Cheng EH-Y, Sheiko TV, Fisher KJ, Craigen JW, Korsmeyer SJ (2003) VDAC2 inhibits Bak activation and mitochondrial apoptosis. Science 301: 513–517 [DOI] [PubMed] [Google Scholar]
- Cory S, Adams A (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2: 647–656 [DOI] [PubMed] [Google Scholar]
- Dembowski M, Künkele K-P, Nargang FE, Neupert W, Rapaport D (2001) Assembly of Tom6 and Tom7 into the TOM core complex of Neurospora crassa. J Biol Chem 276: 17679–17685 [DOI] [PubMed] [Google Scholar]
- Dietmeier K, Hönlinger A, Bömer U, Dekker PJT, Eckerskorn C, Lottspeich F, Kübrich M, Pfanner N (1997) Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature 388: 195–200 [DOI] [PubMed] [Google Scholar]
- Fang Y, Morrell JC, Jones JM, Gould SJ (2004) PEX3 functions as a PEX19 docking factor in the import of class I peroxisomal membrane proteins. J Cell Biol 164: 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz S, Rapaport D, Klanner E, Neupert W, Westermann B (2001) Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organeller function. J Cell Biol 152: 683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib SJ, Waizenegger T, Lech M, Neupert W, Rapaport D (2005) Assembly of the TOB complex of mitochondria. J Biol Chem 280: 6434–6440 [DOI] [PubMed] [Google Scholar]
- Horie C, Suzuki H, Sakaguchi M, Mihara K (2002) Characterization of signal that directs C-tail-anchored proteins to mammalian mitochondrial outer membrane. Mol Biol Cell 13: 1615–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie C, Suzuki H, Sakaguchi M, Mihara K (2003) Targeting and assembly of mitochondrial tail-anchored protein Tom5 to the TOM complex depend on a signal distinct from that of tail-anchored proteins dispersed in the membrane. J Biol Chem 278: 41462–41471 [DOI] [PubMed] [Google Scholar]
- Ishihara N, Mihara K (1998) Identification of the protein import components of the rat mitochondrial inner membrane, rTIM17, rTIM23, and rTIM44. J Biochem 123: 722–732 [DOI] [PubMed] [Google Scholar]
- Janiak F, Leber B, Andrews DW (1994) Assembly of Bcl-2 into microsomal and outer mitochondrial membranes. J Biol Chem 269: 9842–9849 [PubMed] [Google Scholar]
- Jeong S-Y, Gaume B, Lee Y-J, Hsu Y-T, Ryu S-W, Yoon S-H, Youl RJ (2004) Bcl-XL sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J 23: 2146–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Morrell JC, Gould SJ (2004) PEX19 is a predominantly cytosolic chaperone and import receptor for class I peroxisomal membrane proteins. J Cell Biol 164: 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph-Liauzun E, Delmas P, Shire D, Ferrara P (1998) Topological analysis of the peripheral benzodiazepine receptor in yeast mitochondrial membrane supports a five-transmembrane structure. J Biol Chem 273: 2146–2152 [DOI] [PubMed] [Google Scholar]
- Kanaji S, Iwahashi J, Kida Y, Sakaguchi M, Mihara K (2000) Characterization of the signal that directs Tom20 to the mitochondrial outer membrane. J Cell Biol 151: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C (2003) Characterization of the signal that directs Bcl-XL, but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol 160: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimmer T, Rapaport D, Ryan MT, Meisinger C, Kassenbrock CK, Blachly-Dyson E, Forte M, Douglas MG, Neupert W, Nargang FE, Pfanner N (2001) Biogenesis of porin of the outer mitochondrial membrane involves an import pathway via receptors and the general import pore of the TOM complex. J Cell Biol 152: 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Ahnert-Hilgen G, Hartmann E, Wiedemann B, Papoport TA (1995) Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J 14: 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger C, Rissler M, Chacinska A, Kozjak V, Schönfisch B, Lohaus C, Meyer HE, Yaffe MP, Guiard B, Wiedemann N, Pfanner N (2004) The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev Cell 7: 61–71 [DOI] [PubMed] [Google Scholar]
- Mihara K, Omura T (1996) Cytoplasmic chaperones in precursor targeting to mitochondria: the role of MSF and hsp70. Trends Cell Biol 6: 104–108 [DOI] [PubMed] [Google Scholar]
- Motz C, Martin H, Krimmer T, Rassow J (2002) Bcl-2 and porin follow different pathways of TOM-dependent insertion into the mitochondrial outer membrane. J Mol Biol 323: 729–738 [DOI] [PubMed] [Google Scholar]
- Nemoto Y, DeCamilli P (1999) Recruitment of an alternatively spliced form of synaptojanin 2 to mitochondria by the interaction with the PDZ domain of a mitochondrial outer membrane protein. EMBO J 18: 2991–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W (1997) Protein import into mitochondria. Annu Rev Biochem 66: 863–917 [DOI] [PubMed] [Google Scholar]
- Paschen SA, Weizenegger T, Stan T, Preuss M, Cyrklaff M, Hell K, Rapaport D, Neupert W (2003) Evolutionary conservation of biogenesis of β-barell membrane proteins. Nature 426: 862–866 [DOI] [PubMed] [Google Scholar]
- Pfanner N, Geissler A (2001) Versatility of the mitochondrial protein import machinery. Nat Rev Mol Cell Biol 2: 339–349 [DOI] [PubMed] [Google Scholar]
- Pfanner N, Wiedemann N, Meisinger C, Lithgow T (2004) Assembling the mitochondrial outer membrane. Nat Struct Mol Biol 11: 1044–1048 [DOI] [PubMed] [Google Scholar]
- Rapaport D (2003) Finding the right organelle, targeting signals in mitochondrial outer-membrane proteins. EMBOR 4: 948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo M, Legres F, Chateu D, Lombes A (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of transmmbrane GTPase Fzo. J Cell Sci 115: 1663–1674 [DOI] [PubMed] [Google Scholar]
- Saeki K, Suzuki H, Tsuneoka M, Maeda M, Iwamoto R, Hasuwa H, Shida S, Takahashi T, Sakaguchi M, Endo T, Mihara K (2000) Identification of mammalian TOM22 as a subunit of the preprotein translocase of the mitochondrial oute membrane. J Biol Chem 275: 31996–32002 [DOI] [PubMed] [Google Scholar]
- Schinzel A, Kaufmann T, Schuler M, Martinalbo J, Grubb D, Borner C (2004) Conformational control of Bax localization and apoptotic activity by Pro168. J Cell Biol 164: 1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JM, Nunnari J (2002) Mitochoppndrial dynamics and division in budding yeast. Trends Cell Biol 12: 178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane M, Nakayama K (2003) Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochndria and inhibits apoptosis. Nat Cell Biol 5: 28–37 [DOI] [PubMed] [Google Scholar]
- Sohda M, Misumi Y, Yamamoto A, Yano A, Nakamura N, Ikehara Y (2001) Identification and characterization of a novel Golgi protein, BC60, that interacts with the integral membrane protein giantin. J Biol Chem 276: 45298–45306 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Maeda M, Mihara K (2002) Characterization of rat TOM70 as a receptor of the preprotein translocase of the mitochondrial outer membrane. J Cell Sci 115: 1895–1905 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Okazawa Y, Komiya T, Saeki K, Mekada E, Kitada S, Ito A, Mihara K (2000) Characterization of rat TOM40, a central component of the proprotein translocase of the mitochondrial outer membrane. J Biol Chem 275: 37930–37936 [DOI] [PubMed] [Google Scholar]
- Wiedemann N, Frazer AE, Pfanner N (2004) The protein import machinery of mitochondria. J Biol Chem 79: 14473–14476 [DOI] [PubMed] [Google Scholar]
- Wiedemann N, Kozjak V, Chacinska A, Schönfish B, Rospert S, Ryan MT, Pfanner N, Meisinger C (2003) Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424: 565–571 [DOI] [PubMed] [Google Scholar]
- Yabal M, Brambillasca S, Soffientini P, Pedrazzini E, Borgese N, Makarow M (2003) Translocation of the C-terminus of a tail-anchored protein across the endoplasmic reticulum membrane in yeast mutant defective in signal peptide-driven translocation. J Biol Chem 278: 3489–3496 [DOI] [PubMed] [Google Scholar]
- Yano M, Terada K, Mori M (2003) AIP is a mitochondrial import mediator that binds to both import receptor Tom20 and preproteins. J Cell Biol 163: 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor. Cell 112: 41–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figures 3 and 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7, 8 and 9
Supplementary Data
Supplementary Methods
Supplementary Information


