Abstract
Sertoli cells (SC) are instrumental to stem spermatogonia differentiation, a process that critically depends on retinoic acid (RA). We show here that selective ablation of RA receptor alpha (RARalpha) gene in mouse SC, singly (RaraSer−/− mutation) or in combination with RARbeta and RARgamma genes (Rara/b/gSer−/− mutation), abolishes cyclical gene expression in these cells. It additionally induces testis degeneration and delays spermatogonial expression of Stra8, two hallmarks of RA deficiency. As identical defects are generated upon inactivation of RARalpha in the whole organism, our data demonstrate that all the functions exerted by RARalpha in male reproduction are Sertoli cell-autonomous. They further indicate that RARalpha is a master regulator of the cyclical activity of SC and controls paracrine pathways required for spermatogonia differentiation and germ cell survival. Most importantly, we show that the ablation of all RXR (alpha, beta and gamma isotypes) in SC does not recapitulate the phenotype generated upon ablation of all three RARs, thereby providing the first evidence that RARs exert functions in vivo independently of RXRs.
Keywords: biological rhythms, germ cells, heterodimers, nuclear receptors, spermatogenesis
Introduction
The mammalian seminiferous epithelium consists of Sertoli cells (SC) and germ cells. Its development, renewal and functioning, which underlie spermatogenesis, require a complex assortment of hormones and cytokines (Meng et al, 2000; Holdcraft and Braun, 2004). Among these signals, retinoic acid (RA), the active metabolite of vitamin A (retinol), regulates spermatogonia differentiation and spermatid adhesion properties (Ghyselinck et al, 2006; Vernet et al, 2006). RA acts through binding to nuclear retinoic acid receptors (RARα, β and γ isotypes), which are ligand-dependent transcriptional regulators transducing the RA signal in the form of heterodimers with the rexinoid receptors, RXRα, β and γ (Kastner et al, 1997; Chambon, 2005; Mark et al, 2006). During post-natal development and adulthood, each RAR is detected predominantly in a specific cell type of the seminiferous epithelium: RARα in SC, RARβ in round spermatids and RARγ in A spermatogonia (Vernet et al, 2006). Germline inactivation of Rara results in testis degeneration comprising features observed upon dietary vitamin A deficiency (VAD), whereas those of Rarb or Rarg do not cause primary testis defects (Lufkin et al, 1993; Vernet et al, 2006).
Stem spermatogonia have remarkable ability to both self-renew and differentiate. The balance between these two processes is thought to depend on a proper environment, which is provided by the supporting SC (Payne and Braun, 2006; Ryu et al, 2006, and references therein). In addition, SC are essential to initiate spermatogenesis at puberty, and to maintain it after sexual maturity (Sharpe et al, 2003). SC display cyclical changes in morphology, gene expression and biochemical activity (Morales and Clermont, 1993; Parvinen, 1993), which are associated with stages of the seminiferous epithelium cycle, a series of constant germ cell associations reflecting coordination of meiosis and spermiogenesis (i.e., spermatid maturation; Russell et al, 1990). As the cyclical activity of SC is established before any signs of heterogeneity in germ cell populations, it may be involved in initiating spermatogenesis at puberty (Timmons et al, 2002). Given the central role of SC in spermatogonial stem cell self-renewal and spermatogenesis, we were interested in generating SC-specific Rar and Rxr knockouts. Using this genetic approach, we demonstrate that RARα is cell-autonomously instrumental to the cyclical activity of SC and to the structural integrity of the seminiferous epithelium, in contrast to RXRs, which are dispensable.
Results
SC-specific ablation of RARα in mice
Mice carrying loxP-flanked alleles of Rara (Chapellier et al, 2002a) were crossed with mice bearing the Amh-Cre transgene (Lecureuil et al, 2002) to generate RaraSer−/− mutants, in which both alleles of Rara were excised in SC. These crosses also generated control males carrying two loxP-flanked alleles of Rara, which did not display histological defects and are hereafter referred to as wild-type (WT) mice. Importantly, no immunostaining for RARα was detected in the testes of RaraSer−/− adult mice (Supplementary Figure 1), indicating that RaraSer−/− mutants actually lack RARα in SC. Note that, as the Amh–Cre transgene is expressed from embryonic day 15.5 onwards (Lecureuil et al, 2002), excision of Rara occurs before the onset of spermatogenesis at postnatal day 5 (P5) (Bellve et al, 1977).
Ablation of RARα in SC results in reduced spermatogenesis and age-dependent testis degeneration
In young, 9-week-old RaraSer−/− mutants (n=6), spermatogenesis yielded mature spermatids in which nuclear elongation as well as acrosome and flagellum development was complete (St16; Figure 1F and Supplementary Figure 2). However, these mature spermatids failed to align at the luminal side of the seminiferous epithelium (St16; compare Figure 1E with F), and were often not released. They were instead retained within the epithelium (St16r; compare Figure 1G and H). In addition, they were scarce (St16; compare Figure 1E and F), and frequently exhibited ultrastructural abnormalities indicative of necrosis (Supplementary Figure 2B). The seminiferous epithelium of RaraSer−/− mutants also showed large vacuoles (VA; compare Figure 1A and B), and desquamation of round spermatids (black arrowhead in Figure 1B and K, compare with 1A and J). In keeping with these defects, the caudal epididymis contained low spermatozoa stores (compare SZ in Figure 1C and D), but numerous round spermatids (R; Figure 1D); all these cells were necrotic (Supplementary Figure 2J). Altogether, these data are indicative of complete but reduced spermatogenesis and testis degeneration (Holstein et al, 2003). A decreased production and a failure of detachment of mature spermatids contribute to the reduced spermatogenesis in RaraSer−/− mutants. To investigate whether cell death also contributed to this phenotype, TUNEL assays were performed in 9-week-old testes (Table I). Numerous TUNEL-positive spermatocytes and round spermatids were detected in the mutant testes (compare Figure 1K with J), and TUNEL-positive elongated spermatids (yellow arrowheads, Figure 1L–N) were present in 68±1% (mean±s.e.m.; n=3 mutants) of the tubule sections versus 5±1% in WT testes (n=3). Thus, lack of RARα markedly impairs the SC capacity to support survival of meiotic (spermatocytes) and post-meiotic germ cells (spermatids).
Figure 1.
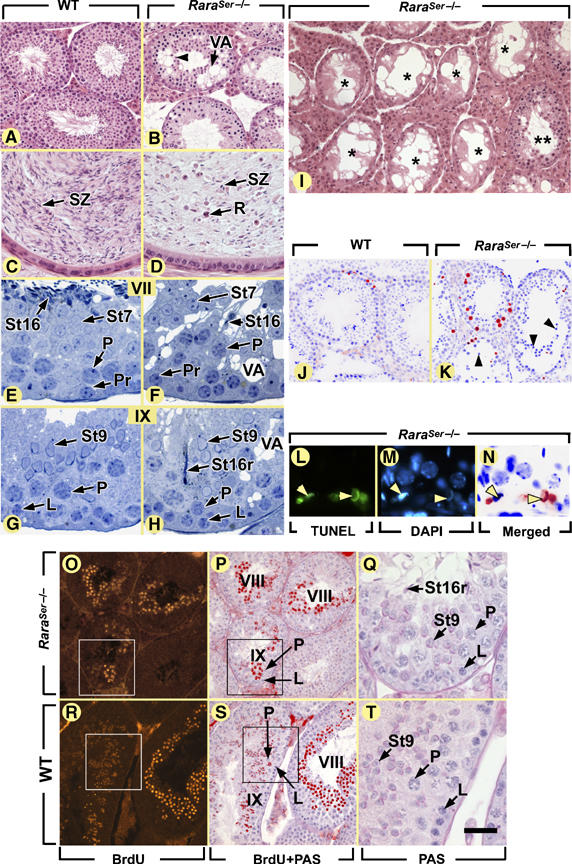
Ablation of RARα in SC yields a progressive testis degeneration resulting from spermatid desquamation and germ cell apoptosis, but not from a disruption of the seminiferous epithelium cycle. (A–I) Histological sections stained with hematoxylin and eosin (A–D, I) or toluidine blue (E–H) through the testes (A, B, E–I) or epididymides (C, D) of 9-week-old (A–H) and 12-month-old (I) mice. (J–N) TUNEL assays: note that in panels J, K and N, the positive signal was converted to a red false color and superimposed with the DAPI nuclear stain (blue false color). (O–T) Identification of the descendants of preleptotene spermatocytes, 18 days following a single injection of BrdU. (P) and (S) are superimpositions of the BrdU-labeled step 8 and 9 spermatids (red false color) with a periodic acid Schiff (PAS) counterstain; (Q) and (T) are high-magnification views of the boxed areas. L, leptotene spermatocytes; P, pachytene spermatocytes; Pr, preleptotene spermatocytes; R, round germ cells; St7, St9, St16, step 7, 9 and 16 spermatids, respectively; St16r, retained step 16 spermatids; SZ, spermatozoa; VA, vacuoles. Asterisks and double asterisks indicate tubules containing SC only and full complement of germ cells, respectively. The black and yellow arrowheads point to round spermatids detaching from the seminiferous epithelium and to TUNEL-positive elongated spermatids, respectively. Roman numerals designate stages of the seminiferous epithelium cycle. Bar (in T): 80 μm (A, B, I–K, O, P, R, S), 30 μm (C, D, Q, T), 20 μm (E–H) and 15 μm (L–N).
Table 1.
Percentage of tubule cross-sections containing TUNEL-positive cells in 9-week-old WT and RaraSer−/− mice, and distribution of apoptotic cell types
| WT | RaraSer−/− | |
|---|---|---|
| Percentage of tubule sections containing TUNEL-positive round germ cells | 18±1 | 58±12 |
| Number of TUNEL-positive round germ cells in a testis cross-section | 46±6 | 225±45 |
| Percentage of spermatocytes | 89±3 | 80±16 |
| Percentage of spermatids | 1±1 | 17±1 |
| Percentage of unidentified | 10±1 | 3±1 |
| Mean±s.e.m.; n=3 in each group of age. The number of tubule sections analyzed in each testis was between 120 and 140. | ||
In 12-month-old RaraSer−/− mutants (n=3), up to 86% of tubule sections contained only SC (asterisks; Figure 1I and Supplementary Table I), indicating that (i) RARα in SC is also necessary for the survival of spermatogonia, and (ii) testis degeneration (i.e., vacuolation of the seminiferous epithelium, desquamation of immature spermatids and germ cell death) becomes more severe upon aging.
Ablation of RARα in SC does not affect their polarity and their density
At the ultrastructural level, SC of RaraSer−/− mutants were essentially normal and the blood testis barrier appeared unaffected (Supplementary Figure 2). As a given SC can support survival and differentiation of only a limited number of germ cells (Sharpe et al, 2003), we assumed that germ cell desquamation and apoptosis observed in RaraSer−/− mutants could be accounted for by a reduced SC density. However, no significant difference in SC density was noted between WT (26.7±0.8 cells/mm; mean±s.e.m.; n=40 tubule sections) and RaraSer−/− testes (28.7±1.1 cells/mm; n=44 tubule sections) at 9 weeks of age (Supplementary Figure 4), indicating that the ratio of SC to germ cells is normal in RaraSer−/− mutants.
Ablation of RARα in SC does not affect the cycle of the seminiferous epithelium or the duration of spermatogenesis
The different generations of germ cells, while synchronously progressing through spermatogenesis, form cellular associations of fixed composition (called epithelial stages) that follow each other according to a stereotyped sequence known as the seminiferous epithelium cycle. Twelve epithelial stages (I–XII) can be identified in the mouse (Russell et al, 1990). In 9-week-old RaraSer−/− testes, the 12 epithelial stages were readily identifiable and each occupied the circumference of a seminiferous tubule cross-section (Figure 1E–H). The duration of meiotic and post-meiotic phases of spermatogenesis was evaluated by identifying, 18 days after a single injection of BrdU, the labeled descendants of preleptotene spermatocytes. In both WT and RaraSer−/− mice, the most advanced BrdU-labeled cells were step 9 spermatids, and there was no labeling in germ cells younger than step 8 spermatids (Figure 1O–T). These data indicate that the absence of RARα in SC does not alter the seminiferous epithelium cycle or the duration of meiosis and spermiogenesis.
Absence of RARα or of RA in SC delays the progression of the prepubertal wave of spermatogenesis
To analyze the impact of the RaraSer−/− mutation on the prepubertal wave of spermatogenesis, which is normally completed by postnatal day 35 (P35), we compared development of the seminiferous epithelium in RaraSer−/− and WT males (n=3 for each genotype and age group) at P5 (i.e., when gonocytes differentiate into primitive A spermatogonia), P10 (i.e., at the onset of meiosis), P20 (i.e., when post-meiotic cells first appear), P25 and P30 (Bellve et al, 1977).
At P5, RaraSer−/− and WT seminiferous cords were morphologically indistinguishable (not shown), although the cords expressing the spermatogonia differentiation marker Stra8 were significantly fewer in RaraSer−/− testes (Figure 2A and Supplementary Figure 5). At P10, leptotene spermatocytes, which represented the most advanced germ cell type, were also fewer in RaraSer−/− testes (Figure 2B). At P20, a majority of RaraSer−/− tubule sections did not contain spermatocytes beyond the zygotene stage, whereas the vast majority of their WT counterparts displayed more advanced pachytene and diplotene spermatocytes (Figure 2C). Along the same lines, post-meiotic round spermatids were absent in RaraSer−/− testes at P20, but were always present in age-matched WT testes (Figure 2C). These data are indicative of a delay in spermatogenesis, which interestingly is not related to testis degeneration as increase in germ cell apoptosis (Figure 2D) and vacuolation of the seminiferous epithelium (not shown) were not observed in RaraSer−/− testes before P20 and P25, respectively.
Figure 2.
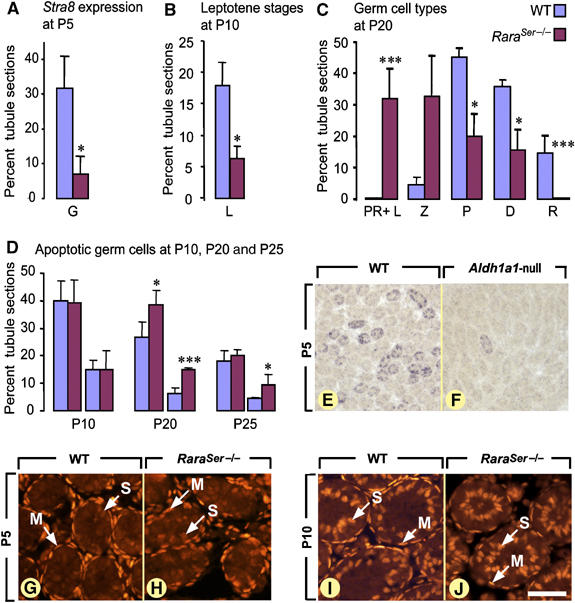
Ablation of RA signaling in SC delays the first spermatogenic cycle without altering the normal timing of androgen receptor expression. (A) Percentage of seminiferous cord cross-sections containing Stra8-positive spermatogonia in WT (blue bars) and in RaraSer−/− (purple bars) testes at P5. (B, C) Percentages of seminiferous cord or tubule cross-sections in which leptotene spermatocytes (L), preleptotene/leptotene (PR+L), zygotene (Z), pachytene (P) and diplotene (D) spermatocytes and round spermatids (R) represent the most advanced germ cell types in WT (blue bars) and RaraSer−/− (purple bars) testes at P10 (B) and P20 (C). (D) Percentages of seminiferous cord or tubule cross-sections containing at least one (first set of bars) or at least three (second set of bars) apoptotic germ cell in WT (blue bars) and in RaraSer−/− (purple bars) testes at P10, P20 and P25. Note that in panels A–D, the bars represent mean±s.e.m. (n=3–5); the asterisks indicate a significant difference (*P<0.05; ***P<0.001). (E, F) Detection of Stra8 transcripts at P5 in WT and Aldh1a1-null testes: although histologically indistinguishable at this developmental stage, the seminiferous cord sections containing Stra8-positive spermatogonia are much less abundant in the Aldh1a1-null than in the WT testis. (G–J) Immunodetection of androgen receptor (red signal) at the onset of spermatogenesis (i.e., P5) and at the beginning of meiosis (i.e., P10). At P5, the androgen receptor is detected in all peritubular myoid cell precursors, as well as occasionally and weakly in immature SC. At P10, the androgen receptor is expressed in peritubular myoid cells and in all immature SC. G, spermatogonia; PR, L, Z, P, D, preleptotene, leptotene, zygotene, pachytene and diplotene spermatocytes, respectively; M, peritubular myoid cells. S, immature Sertoli cells; R, spermatids. Bar (in J): 200 μm (E, F) and 50 μm (G–J).
Androgen and FSH signaling pathways in SC play essential functions in testis development, as inactivation of androgen and FSH receptors (Ar and Fshr, respectively) delay the prepubertal wave of spermatogenesis (Chang et al, 2004; De Gendt et al, 2004; Johnston et al, 2004). However, these two pathways are not involved in the delay of prepubertal spermatogenesis in RaraSer−/− mutants as the expression pattern of Ar (Figure 2G–J) and Fshr (Supplementary Figure 5) were normal in immature SC of RaraSer−/− testes.
To investigate whether the delay in spermatogonia differentiation observed in testes lacking RARα could be mimicked upon decreasing RA availability, we analyzed expression of Stra8 in the testes of Aldh1a1-null mice lacking retinaldehyde dehydrogenase 1 (Matt et al, 2005), which is the main RA-synthesizing enzyme in SC at P5 (Vernet et al, 2006). In Aldh1a1-null testes at P5, only 4±3% (mean±s.e.m.; n=3) of seminiferous cords contained spermatogonia expressing Stra8 versus 28±3% (mean±s.e.m.; n=3) in WT littermates (compare Figure 2E with F). Therefore, a RA-liganded RARα in SC is required for proper spermatogonia differentiation during the prepubertal wave of spermatogenesis.
Cyclical expression of numerous genes is lost in SC lacking RARα
The cyclical activity of SC was investigated through analysis of genes known to display SC-restricted cyclical expression, such as the androgen receptor (AR) whose expression peaks at stage VI–VIII (Figure 3A–D; Zhou et al, 2002), and the membrane protein STRA6 expressed at stages VII–VIII and IX (Figure 4A and B; Bouillet et al, 1997). In RaraSer−/− adults, all SC displayed similar levels of AR (Figure 3E–H), and low, uniform, levels of STRA6 from stage I to stage XII (Figure 4C and D). Along these lines, the stage-dependent variations of expression of GATA1 (Yomogida et al, 1994), galectin-1 (Timmons et al, 2002), clusterin (Morales et al, 1987) and procathepsin L (Wright et al, 2003) were all lost in the RaraSer−/− seminiferous epithelium (Supplementary Figures 6 and 7). Altogether, these data indicate that the cyclical activity of SC is abolished in RaraSer−/− mutants. Importantly, the cyclical expression of Stra8 in spermatocytes and A spermatogonia (Figure 4E–H; Supplementary Figure 7) and Rxra in round spermatids (not shown) were not modified in RaraSer−/− testes. These observations are in keeping with our histological findings that ablation of RARα in SC does not alter the seminiferous epithelium cycle (Figure 1E–H).
Figure 3.
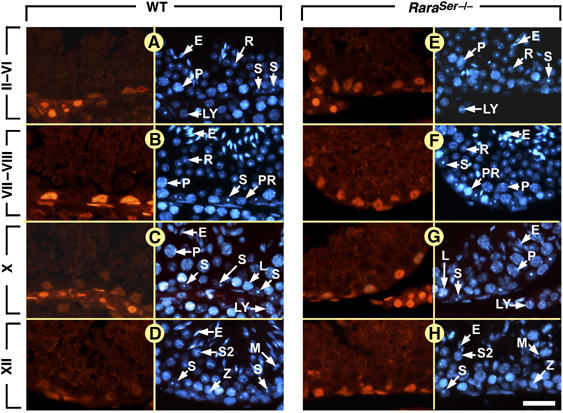
Ablation of RARα in SC abrogates the epithelial stage-dependent variations of AR expression. Immunohistochemical detection of AR in the seminiferous epithelium at 9 weeks of age. (A–D) In WT testis, immunolabeling for AR is strong in SC nuclei at stages VII and VIII and weak at other epithelial stages. (E–H) In RaraSer−/− mutant testis, AR is expressed at similar levels in all SC nuclei, irrespective of the epithelial stage. Note that (i) the left side of each panel corresponds toe staining using the anti-AR antibody and the right side to a DAPI nuclear counterstain and (ii) the histological sections from WT males and from RaraSer−/− mutants were processed in parallel for immunohistochemistry, and identical exposure times were used to acquire the fluorescence pictures. E, elongated spermatids; L, leptotene spermatocytes; LY, Leydig cell; M, spermatocytes in metaphase; P, pachytene spermatocytes; PR, preleptotene spermatocytes; R, round spermatids; S, Sertoli cells; S2, type 2 spermatocytes; Z, zygotene spermatocytes. Roman numerals designate stages of the seminiferous epithelium cycle: II–VI, stage II, III, IV, V or VI; VII–VIII, stage VII or VIII. Bar: 30 μm (A–H).
Figure 4.
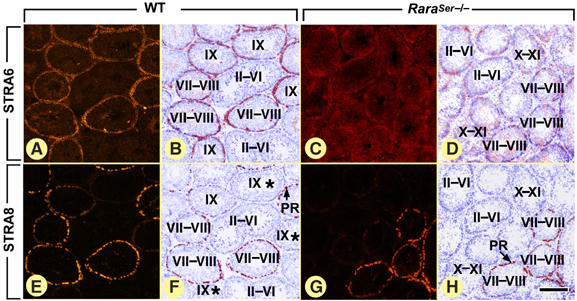
Ablation of RARα in SC abrogates the epithelial stage-dependent variations of Stra6 expression, but does not alter cyclic expression of the germ cell marker Stra8 in adult testes. Immunostaining for STRA6 (A–D) and STRA8 (E–H) in WT and RaraSer−/− testes, as indicated. In WT males, STRA6 protein is present at epithelial stages VII–IX, and its level peaks at stage VIII. In WT testes, immunolabeling for STRA8 is strong in preleptotene spermatocytes (present at stages VII–VIII of the seminiferous epithelium cycle) and weak in leptotene spermatocytes (present at stages IX–X), and STRA8-containing spermatocytes co-distribute with STRA6-containing SC. Note that STRA8 is also expressed in spermatogonia (see Supplementary Figure 7). In RaraSer−/− testes, the epithelial stage-specific expression of STRA6 is lost; in contrast, STRA8 distribution is unaffected. Note that (i) panels A, B and panels E, F correspond to consecutive sections of a WT testis and (ii) that panels C, D and panels G, H correspond to consecutive sections of a RaraSer−/− testis: PR, preleptotene spermatocytes. Roman numerals designate stages of the seminiferous epithelium cycle: II–VI, stage II, III, IV, V or VI; VII–VIII, stage VII or VIII; X–XI, stage X or XI. Asterisks indicate tubule sections containing STRA8-positive leptotene spermatocytes, which are not visible at the illustrated magnification. In panels B, D, F and H, the immunohistochemical signals were converted to a red false color and superimposed with the DAPI nuclear counterstain (blue false color). Bar (in H): 160 μm (A–H).
We also analyzed the cyclical activity of SC before the appearance of the seminiferous epithelium cycle. In WT males at P5 and P10, distribution of STRA6 protein, and the cellular retinol-binding protein CRBP1 (Rbp1) and galectin-1 (Lgals1) transcripts varied between seminiferous cord sections (Figure 5A–C and G–I). In contrast, expression of these genes appeared uniform in all seminiferous cords of RaraSer−/− mutants (Figure 5D–F and J–L). Importantly, the total amounts of Stra6, Lgals1 and Rbp1 transcripts measured by quantitative RT–PCR in RaraSer−/− testes at P5 did not significantly differ from those in WT testes (Supplementary Figure 5), indicating that changes in their expression were only qualitative. The GATA1 immunolabeling at P10 varied markedly between individual seminiferous cords in WT testes, whereas it was strong in all SC nuclei in RaraSer−/− testes (compare Figure 5M and N). This abnormal, uniform, expression of GATA1 was also observed in RaraSer−/− testes at P20 (compare Figure 5O and P), indicating that it was not related to the developmental delay of the mutant testis (see above). Altogether, these data demonstrate that the cyclical activity of SC is disrupted in RaraSer−/− mutants, already at the onset of pubertal testis development.
Figure 5.
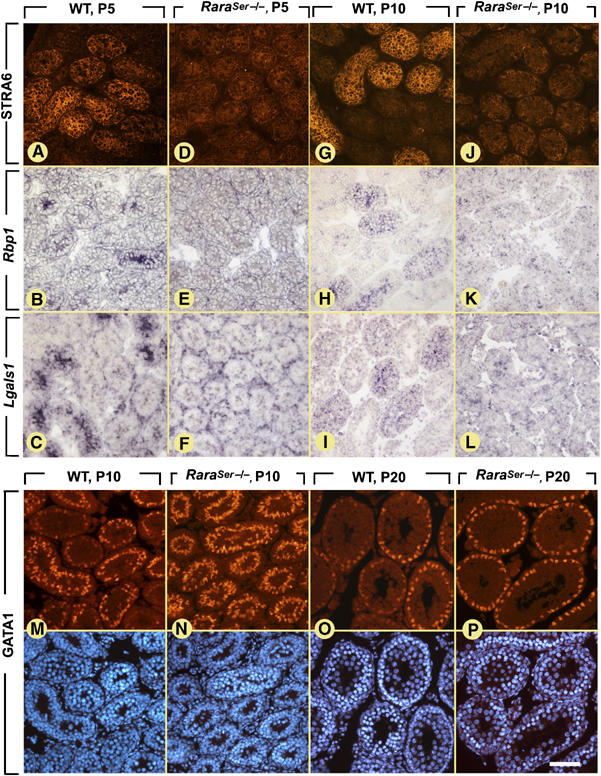
Ablation of RARα in SC abrogates the epithelial stage-dependent variations of gene expression in prepubertal testes. (A, D, G, J) Immunostaining for STRA6. (B, C, E, F, H, I, K, L) ISH analyses using Rbp1 (B, E, H, K) and Lgals1 (C, F, I, L) antisense probes; the positive signals for transcripts are violet. (M–P) Immunostaining for GATA1. (A, D, G, J, M–P) The positive signal for STRA6 and GATA1 is red. The DAPI counterstain is also illustrated in panels M–P. Bar (in P): 50 μm (A–F) and 80 μm (G–P).
Similar to the adult situation (see above), loss of Stra6 cyclical expression in prepubertal RaraSer−/− testes (Figure 5D and J) did not abolish the cyclical expression of Stra8 (not shown). This finding was quite unexpected, as in prepubertal WT testes we found a strong positive, temporal and spatial, correlation between SC displaying high levels of STRA6- and STRA8-positive spermatogonia and spermatocytes (Supplementary Figure 8). The coordinated expression of Stra6 and Stra8 is therefore uncoupled upon ablation of Rara in SC.
Additional ablation of RARβ and RARγ in SC does not increase the severity of the phenotype resulting from RARα ablation
A striking variability in the extent of the seminiferous epithelium vacuolation was observed not only in different RaraSer−/− mutants but also within a given mutant in different tubule segments (Figure 1I and Supplementary Figure 3). Although RARα is the only RAR evidenced in SC using immunohistochemistry (Vernet et al, 2006), the variability in seminiferous epithelium degeneration left open the possibility that stochastic variations of RARβ and/or RARγ possibly present in low (i.e., undetectable) amounts could compensate for the RARα loss of function. To investigate this possibility, we generated Rara/b/gSer−/− mice lacking RARα, RARβ and RARγ in SC (see Supplementary information). Testes from 9-week-old Rara/b/gSer−/− mutants (n=3) displayed alterations that were indistinguishable from those found in RaraSer−/− mice (Supplementary Figure 9), indicating that RARα is the sole functional RAR in SC.
Ablation of all RXRs in SC does not recapitulate the RaraSer−/− phenotype
RXRβ is the predominant RXR in SC (Vernet et al, 2006). Selective ablation of Rxrb in SC (RxrbSer−/− mutation) yielded, at 9 weeks of age, testis defects identical to those generated upon inactivation of RXRβ function in the whole organism (Kastner et al, 1996; Mascrez et al, 2004), namely an accumulation of lipids in SC and a failure of spermiation (our unpublished data). The latter is also generated upon ablation of Rara (see above). On the other hand, testis degeneration (i.e., vacuolation, desquamation of immature round spermatids, increased germ cell apoptosis) and loss of Stra6 cyclical expression, which are hallmarks of age-matched RaraSer−/− mutants, were never observed in RxrbSer−/− mutants (not shown). To exclude the possibility that RXRα and/or RXRγ present at low amounts in SC could compensate for the loss of RXRβ, mice lacking RXRα, RXRβ and RXRγ in SC (i.e., Rxra/bSer−/−/Rxrg-null mutants, see Supplementary information) were analyzed. At 9 weeks of age, these mutants (n=3) recapitulated the defects of RxrbSer−/− mutants (Figure 6D), but did not exhibit degeneration of the seminiferous epithelium (Figure 6B and F) and loss of Stra6 cyclical expression (Figure 6H; not shown). Interestingly, none of the mutations altered the cyclical expression of Stra8 in germ cells (Figure 6I and J). Therefore, in contrast to RARα, RXRs are dispensable for the structural integrity of the seminiferous epithelium and for the cyclical activity of SC.
Figure 6.
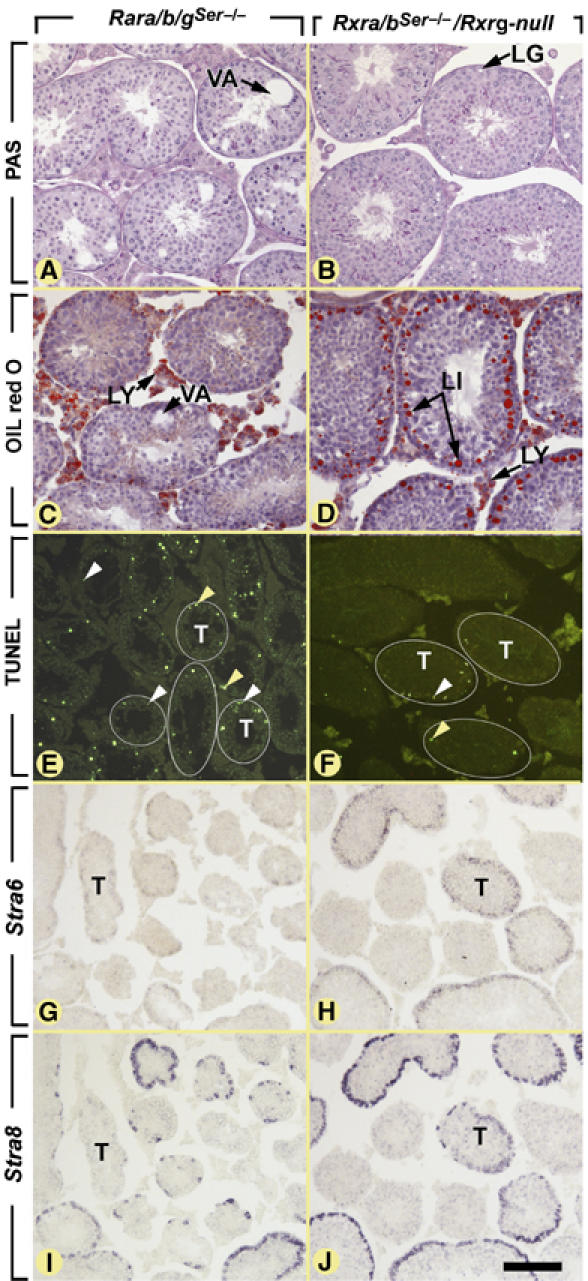
Ablations of all three RARs (i.e., Rara/b/gSer−/− mutants) or of all three RXRs (i.e., Rxra/bSer−/−/Rxrg-null mutants) yield very different abnormalities. Histological sections of testes at 9 weeks of age stained by (A, B) the PAS method, (C, D) oil red O for detection of lipids droplets (red dots), (E, F) the TUNEL method to detect apoptopic cells (green fluorescent signals) and (G–J) ISH using antisense probes to (G, H) Stra6 and (I, J) Stra8 (purple signals). (A, C, E, G, I) The Rara/b/gSer−/− mutant testis displays (i) large, lipid-free, vacuoles (VA in panel A), (ii) numerous apoptotic round germ cells (some indicated by yellow arrowheads in panel E) and elongated spermatids (indicated by white arrowheads in panel E) in numerous seminiferous tubules sections (T) and (iii) low and uniform expression of Stra6 in all seminiferous tubules that does not follow the cyclical expression of Stra8 shown on an consecutive section. (B, D, F, H, J) In contrast, the Rxra/bSer−/−/Rxrg-null mutant testis displays (i) numerous lipid inclusions (LI in panel D) whose extraction during paraffin embedding yields ‘lipid ghosts' (LG in panel B) (ii) a normal, low, proportion of apoptotic round germ cells (yellow arrowheads in panel F), but an increase in the proportion of TUNEL-positive elongated spermatids (white arrowheads; see Mascrez et al, 2004 for further details), when compared to WT mice and (iii) normal cyclic expression of Stra6 in seminiferous tubules superimposed with that of Stra8 shown on an adjacent section. (E, F) Some tubule sections were highlighted by a thin white line. LG, lipid ghost; LI, lipid inclusion; LY, Leydig cell; T, seminiferous tubule; VA, vacuoles. Bar (in J): 80 μm (A–D) and 160 μm (E–J).
Discussion
All functions of RARα in the mouse testis are cell-autonomously exerted in SC
RARα expression studies had left open the possibility that it could have exerted functions on testis physiology by acting in germ cells (Akmal et al, 1997; Gaemers et al, 1997) or in cells of the hypothalamus–pituitary axis (Krezel et al, 1999). However, the present study demonstrates that the failure of spermiation, epithelial vacuolation, germ cell desquamation and apoptosis displayed by RaraSer−/− and by Rara-null mutants are indistinguishable (Lufkin et al, 1993). In addition, the onset of degeneration at a prepubertal stage following cessation of SC proliferation (Vergouwen et al, 1991), its progression with aging to ultimately yield seminiferous tubules with only SC and the delay in the first spermatogenic cycle are identical in RaraSer−/− and Rara-null mutant mice. These observations therefore demonstrate that all functions of RARα in testis development and spermatogenesis are cell-autonomously exerted in SC.
An RARα-mediated RA signaling pathway in prepubertal but not adult SC promotes spermatogonia differentiation
RaraSer−/− mutants exhibit a delay in the progression of the first spermatogenetic wave. This delay is established during spermatogonia differentiation, as indicated by the retarded expression of Stra8. It is unlikely to additionally affect meiosis and spermiogenesis, as the time required to generate elongated spermatids from preleptotene spermatocytes is normal in the sexually mature RaraSer−/− testis. Moreover, it cannot be accounted for by the increase in germ cell apoptosis, which occurs later during development of the RaraSer−/− testis. On the other hand, RA binding to RARα is most likely required, as a developmental delay in the appearance of Stra8-positive spermatogonia is observed in Aldh1a1-null mice. Thus, a cell-autonomous effect of RA-liganded RARα in immature SC is required to promote spermatogonia differentiation during the prepubertal spermatogenic wave.
As mentioned above, RARα promotes differentiation of spermatogonia during prepuberty. In contrast, spermatogonia differentiation arrest during adulthood, which is the hallmark of the VAD-induced testis degeneration, is not observed upon RAR ablation in SC. For instance, retention of BrdU in spermatogonia and disappearance of entire germ cell layers in the seminiferous tubule sections (Ghyselinck et al, 2006) are absent in RaraSer−/− and Rara/b/gSer−/− mutants (present report). In keeping with these observations, expression of the RA target gene Stra8, which is required for meiotic initiation, is maintained in RaraSer−/− and Rara/b/gSer−/− testes, whereas it is abolished in spermatogonia from VAD testes (Bowles et al, 2006; Ghyselinck et al, 2006; Koubova et al, 2006 and present report). Therefore, inhibition of RAR signaling solely in SC cannot account for the VAD-induced arrest in spermatogonia differentiation.
RARα signaling is a master regulator required to initiate the cyclical activity of SC, which is however not instrumental to the seminiferous epithelium cycle
That expression of Lgals1, Rbp1, Stra6 and Gata1 is no longer cyclical in postnatal mitotic SC lacking RARα indicates that this nuclear receptor may initiate the cyclical activity of these cells. In addition, adult post-mitotic SC lacking RARα have also lost their capacity to modulate expression of Ar, Ctsl and Clu, strongly suggesting thereby that RARα acts on top of a genetic cascade orchestrating the cyclical activity of SC.
It has been proposed that the progression of germ cells through the stages of the seminiferous epithelium cycle modulates expression of specific genes in SC, which, in turn, may ultimately influence the development of the surrounding germ cells (Yomogida et al, 1994; Griswold, 1995; Bitgood et al, 1996; Zhao et al, 1996; Zabludoff et al, 2001). As a matter of fact, we show that the 12 stages of the seminiferous epithelium cycle are easily identified in RaraSer−/−, Rara/b/gSer−/− and Rara-null mutants and, as for WT testes, each stage occupies the whole circumference of a tubule section. In addition, the normal, epithelial stage-specific expression of the germ cell markers Stra8 and Rxra is maintained in RaraSer−/− mutants, and BrdU incorporated into premeiotic spermatocytes of RaraSer−/− mutants is transferred to homogeneous cohorts of spermatids at the same step of maturation as in WT mice. These observations do not support the view that RAR signaling in SC is critical for the maintenance of proper germ cell associations (Chung et al, 2004), and clearly indicate that, unexpectedly, cyclical gene expression by SC is not essential to coordinate the cyclical progression of germ cells through meiosis and spermiogenesis. We additionally show that co-distribution of SC expressing Stra6 and germ cells expressing Stra8 is set up along the seminiferous cords already at P5 (see Supplementary Figure 8). This indicates that coordination between germ cell differentiation and cyclical activity of SC precedes the onset of spermatogonia differentiation, and therefore occurs much earlier than previously thought (Timmons et al, 2002).
In any events ablation of RAR signaling in SC causes germ cell apoptosis and desquamation. These seminiferous epithelium dysfunctions may be accounted for by the disruption of SC cyclical gene expression, which precedes testis degeneration by about 2 weeks. In this context, ablations of Clu, Ctsl and disruption of androgen signaling cause defects of spermatogenesis, which, when taken altogether, are reminiscent of those displayed by RaraSer−/− mutants (Bailey et al, 2002; Wright et al, 2003; Chang et al, 2004; De Gendt et al, 2004). On the other hand, despite the fact that targeted inactivation of Gata1, Lgals1 and Rbp1 does not yield reproductive phenotypes (Poirier and Robertson, 1993; Ghyselinck et al, 1999; Lindeboom et al, 2003), the possibility exists that their simultaneous ablation may impair SC functions.
RARα in SC exerts physiological functions, even in the absence of RXRs
The convergence of phenotypes generated upon Rar and Rxr ablations has clearly shown that RXR/RAR heterodimers are the functional units transducing the RA signal in the mouse, both during development and in adult tissues (Chapellier et al, 2002b; Calleja et al, 2006; Mark et al, 2006). In the present study, we definitely demonstrate that there is no functional redundancy among RAR isotypes and among RXR isotypes in SC as it is the case in embryonic tissues (reviewed in Mark et al, 2006), and therefore that RARα and RXRβ are the sole functional RA receptors in SC. Interestingly, except for spermiation defects, RaraSer−/− and RxrbSer−/− testes exhibit distinct sets of abnormalities. Those observed in RxrbSer−/− mutants can be ascribed to a loss of RXRβ/LXRβ-mediated events (Mascrez et al, 2004). On the other hand, the testis degeneration observed in RaraSer−/− but not in RxrbSer−/− mutants reveals for the first time that RARα can exert functions in vivo, independently of RXRs. This conclusion could have not been reached without having inactivated all three Rxr genes in SC. This was actually the only experimental way to definitely rule out a functional compensation of RXRβ functions by RXRα and/or RXRγ, which might have been expressed at low, undetectable levels in these cells. This finding opens up new perspectives on physiological functions of RARs, which, as suggested from in vitro studies, may involve regulation of gene expression through homodimers, heterodimers with vitamin D3 and thyroid hormone receptors, or repression of the AP-1 transcription complexes activity (Chambon, 1996; Garcia-Villalba et al, 1996; Benkoussa et al, 2002; Conde et al, 2004; Lee and Privalsky, 2005 and references therein).
Understanding spermatogenesis is the prerequisite to develop concepts for reprogramming spermatogonia to therapeutic stem cells (Guan et al, 2006). RA plays crucial roles in the differentiation of stem germ cells (Bowles et al, 2006; Koubova et al, 2006). In this context, our results provide the first evidence that this developmental hormone signal controls in-SC processes that are required for spermatogenesis. They also demonstrate that RARα on its own, that is, in the absence of an RXR heterodimeric partner, is a master transcriptional regulator of the cyclical activity of SC.
Materials and methods
Histology
Histological observations, as well as analyses involving immunohistochemistry (IHC) and in situ hybridization (ISH), were repeated on at least three mice per age group. Testes and epididymides destined for paraffin embedding were fixed in Bouin's fluid for 48 h; sections were stained with either hematoxylin and eosin or the periodic acid Schiff (PAS) method. Testes and epididymides destined for epon embedding were perfusion-fixed with 2.5% glutaraldehyde (w/v) in PBS and processed as described (Kastner et al, 1996); semi-thin (i.e., 1 μm thick) sections were stained with toluidine blue. Detection of BrdU incorporation and apoptotic cells were as described (Ghyselinck et al, 2006; Vernet et al, 2006).
Immunohistochemistry and in situ hybridization analyses
For immunodetection of STRA6, 10-μm-thick sections of freshly frozen testes were post-fixed for 5 min in cold acetone at −20°C, air-dried, hydrated in PBS and fixed for a second time in ice-cold 4% (w/v) paraformaldehyde in PBS. For immunodetection of STRA8, the fixation step in acetone was omitted. For immunodetection of AR and GATA1, testes were fixed by intracardiac perfusion of ice-cold 4% (w/v) paraformaldehyde in PBS, then kept in the same fixative overnight at 4°C, washed in PBS, dehydrated and embedded in paraffin. The sections were incubated overnight at 4°C with the anti-STRA6 antibody (Bouillet et al, 1997) diluted 1:100 in PBS, anti-STRA8 antibody (Oulad-Abdelghani et al, 1996) diluted 1:500 anti-AR rabbit polyclonal antibody (sc-816; Santa Cruz Biotechnologies) diluted 1:500, or anti-GATA1 rat monoclonal antibody (sc-265; Santa Cruz Biotechnologies) diluted 1:100. Detection of bound primary antibodies was achieved by incubating the section for 45 min at room temperature using either a Cy3-conjugated goat anti-rabbit IgG (Biomol Immuno Research Laboratories, Exeter, UK) diluted 1:500, or a Cy3-conjugated goat anti-rat IgG (Jackson Immunoresearch Laboratories, Baltimore, PA) diluted 1:200. As a control of specificity, the anti-AR antibody was incubated with a histological section from an Ar-null mutant (not shown).
ISH with digoxigenin-labeled probes was as described (Vernet et al, 2006). The plasmids containing full-length Rbp1 (650 bp long) and Stra8 (1180 bp long) cDNAs, or parts of Stra6 (244 bp long; exons 5–7), Lgals1 (366 bp long; exons 2–4), Clu (942 bp long; exons 5–9) and Ctsl (925 bp long; exons 2–8) cDNAs were linearized and used as templates for the synthesis of sense or antisense riboprobes.
Supplementary Material
Supplementary Materials and methods
Acknowledgments
We thank B Féret, B Weber, A Gansmuller and the staff of IGBMC–ICS common services for their technical assistance. This work was supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Hôpital Universitaire de Strasbourg. NV was supported by Institut Universitaire de France (IUF) and Association pour la Recherche sur le Cancer (ARC) fellowships.
References
- Akmal KM, Dufour JM, Kim KH (1997) Retinoic acid receptor alpha gene expression in the rat testis: potential role during the prophase of meiosis and in the transition from round to elongating spermatids. Biol Reprod 56: 549–556 [DOI] [PubMed] [Google Scholar]
- Bailey RW, Aronow B, Harmony JA, Griswold MD (2002) Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod 66: 1042–1053 [DOI] [PubMed] [Google Scholar]
- Benkoussa M, Brand C, Delmotte MH, Formstecher P, Lefebvre P (2002) Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter. Mol Cell Biol 22: 4522–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M (1977) Spermatogenic cells of the prepuberal mouse isolation and morphological characterization. J Cell Biol 74: 68–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP (1996) Sertoli cell signaling by desert hedgehog regulates the male germline. Curr Biol 6: 298–304 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P (1997) Developmental expression pattern of Stra6 a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev 63: 173–186 [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P (2006) Retinoid signaling determines germ cell fate in mice. Science 312: 596–600 [DOI] [PubMed] [Google Scholar]
- Calleja C, Messaddeq N, Chapellier B, Yang H, Krezel W, Li M, Metzger D, Mascrez B, Ohta K, Kagechika H, Endo Y, Mark M, Ghyselinck NB, Chambon P (2006) Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev 20: 1525–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P (1996) A decade of molecular biology of retinoic acid receptors. FASEB J 10: 940–954 [PubMed] [Google Scholar]
- Chambon P (2005) The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol Endocrinol 19: 1418–1428 [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S (2004) Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in sertoli cells. Proc Natl Acad Sci USA 101: 6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapellier B, Mark M, Garnier JM, LeMeur M, Chambon P, Ghyselinck NB (2002a) A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis 32: 87–90 [DOI] [PubMed] [Google Scholar]
- Chapellier B, Mark M, Messaddeq N, Calleja C, Warot X, Brocard J, Gérard C, Li M, Metzger D, Ghyselinck NB, Chambon P (2002b) Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J 21: 3402–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Sung W, Wang X, Wolgemuth DJ (2004) Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn 230: 754–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde I, Paniagua R, Fraile B, Ruiz A, Arenas MI (2004) Expression of vitamin D3 receptor and retinoid receptors in human breast cancer: identification of potential heterodimeric receptors. Int J Oncol 25: 1183–1191 [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G (2004) Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101: 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaemers IC, van Pelt AM, van der Saag PT, Hoogerbrugge JW, Themmen AP, de Rooij DG (1997) Effect of retinoid status on the messenger ribonucleic acid expression of nuclear retinoid receptors alpha beta and gamma and retinoid X receptors alpha beta and gamma in the mouse testis. Endocrinology 138: 1544–1551 [DOI] [PubMed] [Google Scholar]
- Garcia-Villalba P, Jimenez-Lara AM, Aranda A (1996) Vitamin D interferes with transactivation of the growth hormone gene by thyroid hormone and retinoic acid. Mol Cell Biol 16: 318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghyselinck NB, Bavik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson CB, Hakansson H, Sauvant P, Azais-Braesco V, Frasson M, Picaud S, Chambon P (1999) Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J 18: 4903–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, Viville S, Mark M (2006) Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn 235: 1608–1622 [DOI] [PubMed] [Google Scholar]
- Griswold MD (1995) Interactions between germ cells and sertoli cells in the testis. Biol Reprod 52: 211–216 [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G (2006) Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440: 1199–1203 [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE (2004) Androgen receptor function is required in sertoli cells for the terminal differentiation of haploid spermatids. Development 131: 459–467 [DOI] [PubMed] [Google Scholar]
- Holstein AF, Schulze W, Davidoff M (2003) Understanding spermatogenesis is a prerequisite for treatment. Reprod Biol Endocrinol 1: 107–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O'Shaughnessy PJ (2004) Regulation of sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 145: 318–329 [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona JM, Chambon P (1997) Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development 124: 313–326 [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Decimo D, Krezel W, Dierich A, Chambon P (1996) Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev 10: 80–92 [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC (2006) Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA 103: 2474–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezel W, Kastner P, Chambon P (1999) Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience 89: 1291–1300 [DOI] [PubMed] [Google Scholar]
- Lecureuil C, Fontaine I, Crepieux P, Guillou F (2002) Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 33: 114–118 [DOI] [PubMed] [Google Scholar]
- Lee S, Privalsky ML (2005) Heterodimers of retinoic acid receptors and thyroid hormone receptors display unique combinatorial regulatory properties. Mol Endocrinol 19: 863–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom F, Gillemans N, Karis A, Jaegle M, Meijer D, Grosveld F, Philipsen S (2003) Tissue-specific knockout reveals that Gata1 is not essential for sertoli cell function in the mouse. Nucleic Acids Res 31: 5405–5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P (1993) High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci USA 90: 7225–7229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P (2006) Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 46: 451–480 [DOI] [PubMed] [Google Scholar]
- Mascrez B, Ghyselinck NB, Watanabe M, Annicotte JS, Chambon P, Auwerx J, Mark M (2004) Ligand-dependent contribution of RXRbeta to cholesterol homeostasis in sertoli cells. EMBO Rep 5: 285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB (2005) Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132: 4789–4800 [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H (2000) Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287: 1489–1493 [DOI] [PubMed] [Google Scholar]
- Morales C, Clermont Y (1993) Structural changes of the sertoli cell during the cycle of the seminiferous epithelium. In The Sertoli Cell, Russell LD, Griswold MD (eds) pp 306–329. Clearwater: Cache River Press [Google Scholar]
- Morales C, Hugly S, Griswold MD (1987) Stage-dependent levels of specific mRNA transcripts in sertoli cells. Biol Reprod 36: 1035–1046 [DOI] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, Bronner S, Lutz Y, Chambon P (1996) Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8 a novel retinoic acid-responsive gene. J Cell Biol 135: 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen M (1993) Cyclic functions of sertoli cells. In The Sertoli Cell, Russell LD, Griswold MD (eds) pp 331–347. Clearwater: Cache River Press [Google Scholar]
- Payne C, Braun RE (2006) Glial cell line-derived neurotrophic factor maintains a POZ-itive influence on stem cells. Proc Natl Acad Sci USA 103: 9751–9752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier F, Robertson EJ (1993) Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development 119: 1229–1236 [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin APS, Clegg RA (1990) Histological and Histopathological Evaluation of the Testis. Clearwater, FL, USA: Cache River Press [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL (2006) Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24: 1505–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS (2003) Proliferation and functional maturation of sertoli cells and their relevance to disorders of testis function in adulthood. Reproduction 125: 769–784 [DOI] [PubMed] [Google Scholar]
- Timmons PM, Rigby PW, Poirier F (2002) The murine seminiferous epithelial cycle is prefigured in the sertoli cells of the embryonic testis. Development 129: 635–647 [DOI] [PubMed] [Google Scholar]
- Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG (1991) Proliferative activity of gonocytes sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil 93: 233–243 [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M (2006) Retinoic acid metabolism and signalling pathways in the adult and developing mouse testis. Endocrinology 147: 96–110 [DOI] [PubMed] [Google Scholar]
- Wright WW, Smith L, Kerr C, Charron M (2003) Mice that express enzymatically inactive cathepsin L exhibit abnormal spermatogenesis. Biol Reprod 68: 680–687 [DOI] [PubMed] [Google Scholar]
- Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, Engel JD, Yamamoto M (1994) Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse sertoli cells. Development 120: 1759–1766 [DOI] [PubMed] [Google Scholar]
- Zabludoff SD, Charron M, DeCerbo JN, Simukova N, Wright WW (2001) Male germ cells regulate transcription of the cathepsin l gene by rat sertoli cells. Endocrinology 142: 2318–2327 [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Deng K, Labosky PA, Liaw L, Hogan BL (1996) The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev 10: 1657–1669 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA (2002) Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23: 870–881 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and methods


