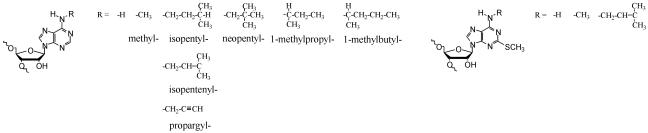Abstract
The N6-alkyladenosines and 2-methylthio-N6-alkyladenosines are the most common modified adenosine nucleosides and transfer ribonucleic acids (tRNA) are particularly rich in these modified nucleosides. They are present at position 37 of the anticodon arm and the contribution of these hypermodified nucleosides to codon–anticodon interactions, as well as translation, are significant, although not fully understood. Herein we described a new chemical synthesis method of the oligoribonucleotides containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines via post-synthetic modifications of precursor oligoribonucleotides. To obtain oligoribonucleotides containing N6-alkyladenosines, the precursor oligoribonucleotide carrying 6-methylthiopurine riboside residue was used, whereas for the synthesis of oligoribonucleotides containing 2-methylthio-N6-alkyladenosines the precursor oligoribonucleotide carrying the 2-methylthio-6-chloropurine riboside was applied. Among the modified oligoribonucleotides of different length and secondary structures, there were several containing naturally occurring modified nucleosides such as: N6-isopentenyladenosine (i6A), N6-methyladenosine (m6A), 2-methylthio-N6-isopentenyladenosine (ms2i6A), and 2-methylthio-N6-methyladenosine (ms2m6A), as well as several unnaturally modified adenosine derivatives.
INTRODUCTION
There are 96 different natural modifications of nucleotides occurring in most types of RNA (1,2). Transfer RNAs (tRNAs) are particularly rich in modified nucleotides and, until now, 80 different modified nucleotides were found. The modifications are mostly present in single stranded regions of tRNA such as hairpin and multi-branch loops. The positions 34 and 37 of the anticodon arm are particularly highly modified.
The functions of modified nucleotides are not clearly defined, but it is known that they affect structure and biological activities of RNA (3,4). It was reported that some modified nucleotides influence the secondary and tertiary interactions of tRNA (5), others change hydrogen bond and stacking interactions as well as puckering of ribose residues (6), and influence divalent cation binding (7).
The study of modified RNA activities is significantly limited by the access to modified oligoribonucleotides. In the literature, syntheses of RNA carrying simple modifications are well described (8). Introduction of nucleoside bearing complex modifications, the so-called hypermodified nucleosides, is still challenging. In 1978, Wiewiórowski and co-workers described the first chemical synthesis of an anticodon loop heptamer containing N6-threonylcarbamoyladenosine (t6A) (9). Recently, several chemical syntheses of anticodon loops and arms containing t6A were published (10–13).
We were interested in the synthesis of oligoribonucleotides containing various N6-alkyladenosines as well as 2-methylthio-N6-alkyladenosines, which are the most common adenosine modifications, especially in tRNA (14–16). These modified oligomers were used to determine the effect of modifications on thermal stability of RNA duplexes and hairpins (17).
In this paper we described new, general methods of chemical synthesis of modified oligoribonucleotides containing N6-alkyl- as well as 2-methylthio-N6-alkyladenosines via post-synthetic modification of support-linked precursor oligoribonucleotides containing 6-methylthiopurine riboside and 2-methylthio-6-chloropurine riboside, respectively. We synthesized the oligoribonucleotides carrying 10 various N6-alkyl- and 2-methylthio-N6-alkyladenosines including such naturally occurring modifications as: N6-isopentenyladenosine (i6A), N6-methyladenosine (m6A), 2-methylthio-N6-isopentenyladenosine (ms2i6A) and 2-methylthio-N6-methyladenosine (ms2m6A) (Fig. 1).
Figure 1.
The structure of N6-alkyl substituents of adenosine and 2-methylthioadenosine incorporated into modified oligoribonucleotides.
MATERIALS AND METHODS
General methods
The chemical syntheses of the precursor oligoribonucleotides were performed on an Applied Biosystems 392 synthesizer using β-cyanoethyl phosphoramidite approach. The synthesis of the 2′,3′,5′-O-triacetyl-6-chloro-2-aminopurine riboside was performed according to the procedure published by Robins and Uznañski (18). The synthesis of 2′,3′,5′-O-triacetyl-6-thioinosine was obtained according to the published procedure, except that Lawesson reagent was used in the reaction (19,20) whereas 2′,3′,5′-O-triacetyl-6-methylthiopurine riboside was obtained according to the procedure published by Wetzel and Eckstein (21).
The 1H, 13C, 31P NMR spectra were measured on a Varian Unity 300 MHz spectrometer using tetramethylsilane as an internal standard for proton and carbon spectra and 85% H3PO4 as an external standard for phosphorous spectra. High performance liquid chromatography (HPLC) was performed on a Hewlett Packard series 1100 using a reverse phase Supelco RP-18 column (4.6 × 250 mm). UV spectra were recorded on DU-640 (Beckman) spectrophotometer. Mass spectrometry spectra were obtained on LC MS Hewlett Packard series 1100 MSD with API-ES detector or AMD 604/402. Thin-layer chromatography (TLC) was carried out on Merck 60 F254 TLC plates with the following systems: A (chloroform:methanol = 9:1 v/v), B (ethyl acetate:hexanes = 1:1 v/v), C (ethyl acetate:hexanes = 4:6 v/v), D (hexanes: acetone:triethylamine = 45:45:10 v/v/v) and silanized 60 F254 TLC plates with the following systems: E (acetone:water = 8:2 v/v) and F (acetone:water = 7:3 v/v).
The chemical synthesis of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside 3′-O-phosphoramidite (1)
Synthesis of 5′-O-dimethoxytrityl-6-methylthiopurine riboside. To 2′,3′,5′-O-triacetyl-6-methylthiopurine riboside (10.27 g, 24.2 mmol), 40 ml pyridine and 20 ml aqueous ammonia were added and left for 16 h at room temperature. TLC analysis indicated complete deprotection of acetyl groups. The reaction mixture was evaporated and co-evaporated with pyridine. The introduction of the 5′-O-dimethoxytrityl group into 6-methylthiopurine riboside was performed according to the general procedure (22). The reaction mixture was purified by silica gel column chromatography using dichloromethane as eluent and methanol to form the gradient (up to 2%). Yield for this reaction: 10.73 g (17.9 mmol, 74%). Rf = 0.40 (system A); MS m/z = 601.5; UV (MeOH): λmax = 215, 226, 280 nm, λmin = 220, 256 nm; 1H NMR (CDCl3): 2.64 (s, 3H, SCH3), 3.31–3.36 (m, 1H, H5′), 3.43–3.48 (m, 1H, H5′′), 3.72 (s, 6H, OCH3), 4.37 (d, 1H, H4′), 4.46–4.49 (m, 1H, H3′), 4.85 (t, 1H, H2′), 6.06 (d, J = 6.0 Hz, 1H, H1′), 6.71–6.74 (d, 4H, DMTr), 7.14–7.28 (m, 9H, DMTr), 8.20 (s, 1H, H2), 8.54 (s, 1H, H8); 13C NMR (CDCl3): 11.84 (SCH3), 55.19 (OCH3), 63.53 (C5′), 72.89 (C3′), 76.15 (C2′), 86.32 (C4′), 91.07 (C1′), 113.17 (DMTr), 126.95 (C5), 127.85, 127.99, 129.14, 129.90, 135.37, 135.47 (DMTr), 140.90 (C8), 144.17 (C4), 151.23 (C2), 158.58 (C6).
Synthesis of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside. The incorporation of the tertbutyldimethylsilyl group into 5′-O-dimethoxytrityl-6-methylthiopurine riboside was performed according to the published procedure (23). The reaction mixture was purified by silica gel column chromatography in toluene with gradient form by ethyl acetate (up to 4%). The fractions containing 2′-isomer were collected, whereas the fractions containing mostly 3′-isomer were evaporated and isomerized. After the isomerization, the mixture was purified as described previously. The 2′-isomer was lyophilized from benzene. Yield for this reaction: 8.29 g (11.6 mmol, 66%). Rf = 0.77 (system B); MS m/z = 715.2; 1H NMR (CDCl3): –0.20, –0.04 (2s, 6H, SiCH3), 0.76 (s, 9H, t-butyl), 2.67 (s, 3H, SCH3), 3.30–3.36 (m, 1H, H5′), 3.43–3.48 (m, 1H, H5′′), 3.73 (s, 6H, OCH3), 4.37 (d, 1H, H4′), 4.45–4.50 (m, 1H, H3′), 4.85 (t, 1H, H2′), 6.06 (d, 1H, H1′), 6.71–6.74 (d, 4H, DMTr), 7.14–7.28 (m, 9H, DMTr), 8.20 (s, 1H, H2), 8.54 (s, 1H, H8); 13C NMR (CDCl3): –5.21, –5.00 (SiCH3), 11.71 (SCH3), 17.85 [C(CH3)], 25.52 [C(CH3)3], 55.19 (OCH3), 63.38 (C5′), 71.57 (C3′), 75.63 (C2′), 84.22 (C4′), 88.24 (C1′), 113.19 (DMTr), 126.92 (C5), 127.88, 128.09, 129.11, 130.05, 131.84, 135.59, (DMTr), 140.29 (C8), 144.52 (C4), 152.00 (C2), 158.55 (C6).
Synthesis of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside 3′-O-phosphoramidite (1). The 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methyl thiopurine riboside (1.10 g, 1.5 mmol) was dried under vacuum for several hours and dissolved in 7.5 ml of anhydrous acetonitrile. Diisopropylethylamine (0.41 ml, 2.3 mmol) and dropwise 2-cyanoethyl diisopropylchlorophosphoramidite (0.43 ml, 1.8 mmol) were added to the solution and left for 1 h at room temperature. The reaction mixture was worked up with saturated aqueous solution of sodium bicarbonate. The mixture was extracted three times (20 ml) with dichloromethane containing 1% of triethylamine. The combined layers were dried with anhydrous sodium sulfate and evaporated. The reaction mixture was purified by silica gel column chromatography in hexane containing 1% triethylamine with gradient form by ethyl acetate (up to 40%). The fractions containing 3′-O-phosphoramidite were evaporated and lyophilized from benzene. Yield for this reaction: 1.20 g (1.3 mmol, 85%). Rf = 0.73 (system D), 0.28 (RP, system E); 1H NMR (CDCl3): –0.22, –0.04 (2d, 6H, SiCH3), 0.75 (s, 9H, t-butyl), 1.04–1.21 (m, 12H, 2iPr), 2.73 (s, 3H, SCH3), 3.28–3.35 (m, 1H, H5′), 3.49–3.66 (m, 5H, H5′′, CH2CH2), 3.78 (s, 6H, OCH3), 3.85–4.00 (m, 2H, 2CH), 4.34–4.44 (m, 2H, H4′, H3′), 5.08 (t, J = 5.6 Hz,1H, H2′), 6.01, 6.07 (dd, 1H, H1′), 6.79–6.83 (m, 4H, DMTr), 7.21–7.48 (m, 9H, DMTr), 8.19 (d, 1H, H2), 8.61 (d, 1H, H8); 13C NMR (CDCl3): –5.17, –4.70 [Si(CH3)], 11.69 (SCH3), 17.89 [C(CH3)], 20.03, 20.42, 24.63, [NC(CH3)2], 25.59 [C(CH3)3], 42.94, 43.50 [NC(CH3)2], 55.19 (OCH3), 57.65, 58.84 (CH2CH2O-), 63.28 (C5′), 72.75 (C3′), 75.17 (C2′), 73.43, 74.52 (CH2CH2O-), 83.90 (C4′), 88.15 (C1′), 113.20 (DMTr), 117.37 (CN), 126.91 (C5), 127.88, 128.09, 129.11, 130.04, 131.95, 135.57, (DMTr), 140.65 (C8), 144.51 (C4), 151.94 (C2), 158.55 (C6); 31P NMR (CDCl3): 149.36, 150.78.
The chemical synthesis of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio-6-chloropurine riboside 3′-O-phosphoramidite (2)
Synthesis of 2′,3′,5′-O-triacetyl-2-methylthio-6-chloropurine riboside. The 2′,3′,5′-O-triacetyl-6-chloro-2-aminopurine riboside (7.23 g, 16.9 mmol) was dissolved in 140 ml of anhydrous acetonitrile followed by the addition of dimethyldisulfide (15.2 ml, 169.0 mmol) and isoamyl nitrite (4.53 ml, 33.8 mmol). The reaction mixture was heated at 60°C for 45 min. The reaction mixture was concentrated to ∼25 ml and worked-up with a saturated aqueous solution of sodium bicarbonate. The mixture was extracted three times with dichloromethane, and the organic layers were combined, dried with sodium sulfate and evaporated. The reaction mixture was purified by silica gel column chromatography using dichloromethane as eluent and methanol to form the gradient (up to 2%). Yield for this reaction: 4.13 g (9.0 mmol, 53%). Rf = 0.64 (system A), 0.70 (RP, system E); MS m/z = 459.2; UV (MeOH): λmax = 233, 263, 306 nm, λmin = 248, 281 nm; 1H NMR (CDCl3): 2.10, 2.11, 2.15 (3s, 9H, CH3), 2.65 (s, 3H, SCH3), 4.29–4.48 (m, 3H, H4′,5′,5′′), 5.65 (t, 1H, H3′), 5.99 (t, 1H, H2′), 6.12 (d, 1H, H1′), 8.10 (s, 1H, H8); 13C NMR (CDCl3): 14.78 (SCH3), 20.33, 20.43, 20.65 [C(O)CH3], 62.67 (C5′), 70.11 (C3′), 72.90 (C2′), 80.01 (C4′), 87.03 (C1′), 120.32 (C5), 142.15 (C8), 151.23 (C4), 151.92 (C6), 167.33 (C2), 169.25, 169.43, 170.22 [C(O)CH3].
Synthesis of 5′-O-dimethoxytrityl-2-methylthio-6-chloropurine riboside. To the 2′,3′,5′-O-triacetyl-2-methylthio-6-chloropurine riboside (11.0 g, 17.3 mmol), a mixture of methanol/triethylamine (9:1 v/v) was added and left at room temperature for 48 h. TLC analysis indicated complete deprotection of acetyl groups. The reaction mixture was evaporated and co-evaporated with anhydrous pyridine. The introduction of 5′-O-dimethoxytrityl group into 2-methylthio-6-chloropurine riboside was performed according to the general procedure (22). The reaction mixture was purified by silica gel column chromatography using dichloromethane as eluent and methanol to form a gradient (up to 2%). Yield for this reaction: 9.72 g (13.0 mmol, 75%). Rf = 0.43 (RP, system F), 0.59 (system A); MS: m/z = 635.3; 1H NMR (CDCl3): 2.49 (s, 3H, SCH3), 3.37–3.40 (m, 2H, H5′,5′′), 3.74 (s, 6H, OCH3), 4.34 (q, 1H, H4′), 4.46–4.49 (m, 1H, H3′), 4.85 (t, 1H, H2′), 6.01 (d, 1H, H1′), 6.74 (d, 4H, DMTr), 7.17–7.24 (m, 9H, DMTr), 8.14 (s, 1H, H8); 13C NMR (CDCl3): 14.82 (SCH3), 55.24 (OCH3), 63.49 (C5′), 72.42 (C3′), 75.59 (C2′), 81.43 (C4′), 86.84 (C1′), 113.17 (DMTr), 127.07 (C5), 127.84, 129.12, 129.92, 135.31, 139.46, (DMTr), 142.36 (C8), 144.14 (C4), 147.32 (C6), 158.64 (C2).
Synthesis of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio-6-chloropurine riboside. The 5′-O-dimethoxytrityl-2-methylthio-6-chloropurine riboside (5.44 g, 8.6 mmol) was co-evaporated with N,N-dimethylformamide and dissolved in 70 ml of N,N-dimethylformamide. Imidazole (1.50 g, 21.4 mmol) and tertbutyldimethylsilyl chloride (1.54 g, 10.3 mmol) were added to the solution and left for 5.5 h at room temperature. TLC analysis indicated almost complete disappearance of the substrate and the formation of three compounds. The reaction mixture was concentrated to ∼25 ml and worked-up with a saturated aqueous solution of sodium bicarbonate. The mixture was extracted three times with dichloromethane, the organic layers were washed with saturated aqueous solution of sodium dihydrogenphosphate, and combined organic layers were dried with anhydrous sodium sulfate and evaporated. The residue was co-evaporated several times with toluene. The reaction mixture was purified by silica gel column chromatography in toluene with a gradient formed by ethyl acetate (up to 5%). The fractions containing 2′-isomer were collected, whereas fractions containing mostly 3′-isomer were evaporated and isomerized. After the isomerization, the mixture was purified as described previously. The 2′-isomer was lyophilized from benzene. Yield for this reaction: 3.66 g (4.9 mmol, 57%). Rf = 0.70 (system C); MS m/z = 749.4, 1H NMR (CDCl3): –0.15, 0.12 (2s, 6H, SiCH3), 0.85 (s, 9H, t-butyl), 2.55 (s, 3H, SCH3), 3.41–3.52 (m, 2H, H5′,5′′), 3.79 (s, 6H, OCH3), 4.27 (q, 1H, H4′), 4.37 (q, 1H, H3′), 4.85 (t, 1H, H2′), 6.08 (d, 1H, H1′), 6.80 (d, 4H, DMTr), 7.22–7.43 (m, 9H, DMTr), 8.20 (s, 1H, H8); 13C NMR (CDCl3): –5.15, –4.95 [Si(CH3)], 14.66 (SCH3), 17.86 [C(CH3)], 25.49 [C(CH3)3], 55.21 (OCH3), 63.41 (C5′), 71.65 (C3′), 75.27 (C2′), 84.28 (C4′), 88.09 (C1′), 113.27 (DMTr), 127.06 (C5), 127.97, 128.02, 130.02, 135.31, 135.41, (DMTr), 142.13 (C8), 150.94 (C4), 158.65 (C6), 166.86 (C2).
Synthesis of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio-6-chloropurine riboside 3′-O-phosphoramidite (2). The 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio-6-chloropurine riboside (0.51 g, 0.7 mmol) was dried under vacuum for several hours and dissolved in 3.5 ml of anhydrous acetonitrile. To this solution diisopropylethylamine (0.18 ml, 1.0 mmol) and dropwise 2-cyanoethyl diisopropylchlorophosphoramidite (0.19 ml, 0.8 mmol) were added and left for 1 h at room temperature. The reaction mixture was worked up with saturated aqueous solution of sodium bicarbonate. The mixture was extracted three times (20 ml) with dichloromethane containing 1% triethylamine. The combined layers were dried with anhydrous sodium sulfate and evaporated. The reaction mixture was purified by silica gel column chromatography in hexane containing 1% triethylamine with a gradient formed by ethyl acetate (up to 40%). The fractions containing 3′-O-phosphoramidite were evaporated and lyophilized from benzene. Yield for this reaction: 0.55 g (0.6 mmol, 84%). Rf = 0.72 (system D), 0.02 (RP, system F), 0.32 (RP, system E); 1H NMR (CDCl3): –0.20, –0.04 (2s, 6H, SiCH3), 0.78 (s, 9H, t-butyl), 1.16–1.22 (m, 12H, 2iPr), 2.55 (s, 3H, SCH3), 3.33–3.40 (m, 1H, H5′), 3.45–3.50 (m, 1H, H5′′), 3.58–3.70 (m, 4H, -CH2CH2-), 3.79 (d, 6H, OCH3), 3.84–4.02 (m, 2H, 2CH), 4.33–4.41 (m, 2H, H4′, H3′), 4.87–4.91 (m, 1H, H2′), 6.12 (d, 1H, H1′), 6.80–6.84 (m, 4H, DMTr), 7.22–7.45 (m, 9H, DMTr), 8.22 (s, 1H, H8); 13C NMR (CDCl3): –5.11, –4.73 [Si(CH3)], 14.60 (SCH3), 17.89 [C(CH3)], 20.37, 20.45, 24.62, [NC(CH3)2], 25.54 [C(CH3)3], 42.91, 43.46 [NC(CH3)2], 55.22 (OCH3), 57.55, 58.73 (CH2CH2O-), 63.42 (C5′), 72.79 (C3′), 73.61, 75.32 (CH2CH2O-), 76.12 (C2′), 83.95 (C4′), 87.69 (C1′), 113.30 (DMTr), 117.45 (CN), 127.07 (C5), 128.00, 128.14, 130.04, 135.22, 135.49, (DMTr), 142.19 (C8), 150.82 (C4), 158.66 (C6), 166.69 (C2); 31P NMR (CDCl3) 149.68, 150.70.
The chemical synthesis of modified oligoribonucleotides containing N6-alkyladenosines or 2-methylthio-N6-alkyladenosines via the transformation of precursor oligoribonucleotides
The synthesis of support-linked precursor oligoribonucleotides. The chemical synthesis of the precursor oligoribonucleotides by the phosphoramidite method on solid support was performed on an Applied Biosystems 392 synthesizer using commercially available 3′-O-phosphoramidites with 2′-O-tertbutyldimethylsilyl group as well as 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside 3′-O-phosphoramidite (1) and 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio-6-chloropurine riboside 3′-O-phosphoramidite (2). For the synthesis, commercially available CPG or Fractosil 500 support or support containing protected 6-methythiopurine riboside or 2-methylthio-6-chloropurine riboside bond via succinyl linker to the support were used.
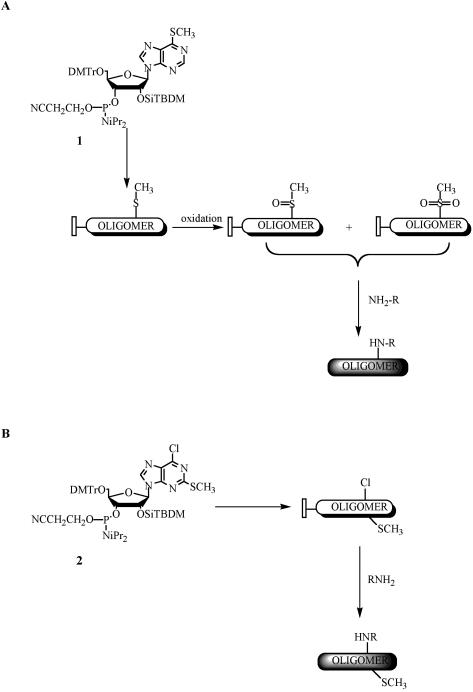
The post-synthetic modification of precursor oligoribonucleotides carrying 6-methylthiopurine riboside into oligoribonucleotides containing N6-alkyladenosines. In this protocol, we described the synthesis of the modified oligoribonucleotides containing m6A, i6A and N6-isopentyladenosine (p6A) residues as representative for this method of post-synthetic modification (Fig. 2A).
Figure 2.
The general scheme of the synthesis of the oligoribonucleotides containing N6-alkyladenosines (A) and 2-methylthio-N6-alkyladenosines (B) via the post-synthetic modification of solid support-linked precursor oligonucleotides.
To support-linked precursor of the oligoribonucleotide with 6-methylthiopurine riboside residue (225 mg, 7.5 µmol), 20 mM solution of magnesium salt of monoperoxyphtalic acid (7.5 ml, 0.15 mmol) in dioxane/water (9:1 v/v) was added. The mixture was left at room temperature for 2.5 h. The next support was filtered off and washed three times with 10 ml of dioxane/water (9:1 v/v), followed by acetonitrile and dried.
The support was split into three even samples. To the first sample (to get an oligomer containing m6A) 2 M methylamine (1 ml, approximately 800 equivalents) in tetrahydrofuran was added and left for 12 h at room temperature. To the second sample (to get an oligomer containing i6A) 1 ml of anhydrous pyridine, isopentenylamine hydrochloride (64 mg, 213 equivalents) and triethylamine (0.13 ml, 426 equivalents) were added and left for 12 h at 55°C. Finally, to the third sample (to get an oligomer containing p6A) a mixture of acetonitrile/isoamylamine (9:1 v/v) (1.25 ml, 527 equivalents of amine) was added and left for 12 h at room temperature.
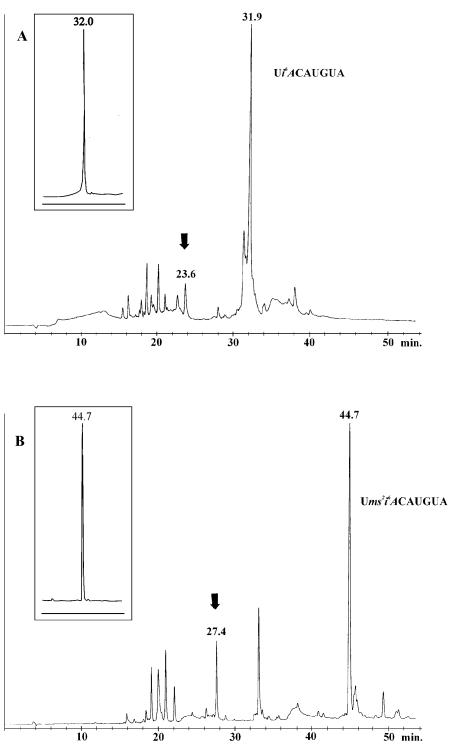
After that, the samples were evaporated and to each sample 2 ml of aqueous ammonia/ethanol (3:1 v/v) was added and left for 8 h at 55°C. Next, the support was filtered off and washed twice (1 ml each) with water and evaporated to dryness. To the solid residue 0.25 ml of pyridine was added and evaporated, followed by the addition of 0.50–0.75 ml of 1 M pirydinium solution of triethylammonium fluoride and left for 48 h at 55°C. The pyridine was evaporated and the mixture was desalted by Sep-pak column. Finally, the oligoribonucleotides were purified as described earlier (24). The purity of TLC purified N6-alkylated oligoribonucleotides was at least 95% (HPLC, C-18 column) (Fig. 3A, insert).
Figure 3.
The HPLC analysis of the crude reaction mixture of the post-synthetic modification of precursors oligoribonucleotides into Ui6ACAUGUA (A) and Ums2i6ACAUGUA (B). Arrows indicate the positions of UACAUGUA (A) and Ums2ACAUGUA (B). Inserts indicate HPLC analysis of TLC-purified Ui6ACAUGUA (A) and Ums2i6ACAUGUA (B).
The post-synthetic modification of precursor oligoribonucleotides carrying 2-methylthio-6-chloropurine riboside into oligoribonucleotides containing 2-methylthio-N6-alkyladenosines. In this protocol, we describe the synthesis of the modified oligoribonucleotides containing ms2m6A, ms2i6A and ms2A residues as representative for this method of post-synthetic modification (Fig. 2B).
The support containing precursor oligoribonucleotide with 2-methylthio-6-chloropurine riboside residue (165 mg, 6 µmol) was split into three even samples. To the first sample (to get an oligomer containing ms2m6A) 2 M methylamine (1 ml, approximately 1000 equivalents) in tetrahydrofuran was added. To the second sample (to get an oligomer containing ms2i6A) 1 ml anhydrous pyridine, isopentenylamine hydrochloride (52 mg, 213 equivalents) and triethylamine (0.13 ml, 426 equivalents) were added. Finally, to the third sample (to get an oligomer containing ms2A) 1.5 ml of 32% aqueous ammonia was added and left for 16 h at 55°C. The first and second samples were left 2–5 h at 55°C.
The further procedure of the deprotection and work-up was the same as described for the synthesis of oligoribonucleotides containing N6-alkyladenosines. The purity of TLC-purified 2-methylthio-N6-alkylated oligoribonucleotides was at least 95% (HPLC, C-18 column) (Fig. 3B, insert).
RESULTS AND DISCUSSION
The rationale behind our study was to prepare the precursor form of oligoribonucleotides, which could be post-synthetically converted into oligoribonucleotides containing modified or hypermodified adenosine residues. Several approaches were used to synthesize modified oligonucleotides via post-synthetic modification (9,15,25–31). They were based on the application of triazolyl (27), 2,4-dinitrophenyl (28) and pentafluorophenyl (29) as leaving groups for purine and pyrimidine series. The most important requirements for the leaving group in the precursor oligonucleotide are: (i) quantitative transformation into modified derivative, (ii) stability during chemical synthesis of phosphoramidite and precursor oligonucleotides, (iii) if leaving group is activated before substitution, activation must be selective and quantitative and (iv) conditions of transformation of precursor must be inert for entire fragments of oligomers such as nucleobases, ribose residues and internucleotide bonds.
Below, we report two methods based on: (i) chemical transformation of 6-methylthiopurine riboside and (ii) use of 2-methylthio-6-chloropurine riboside as a new precursor to the synthesis of modified oligoribonucleotides containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines, respectively.
The chemical synthesis of oligoribonucleotides containing N6-alkyladenosines via the post-synthetic modification of precursor oligonucleotides
The conversion of 6-alkylthiopurine derivatives into N6-isopentenyladenosine and synthesis of tetramer AAAi6A were reported (14,15). This result as well as several others suggest that 6-methylthio could be the group of choice for the transformation of those precursor oligoribonucleotides into modified oligoribonucleotides carrying N6-alkyladenosine residues (14,15,21,32,33).
To test the applicability of the 6-methylthio group, we analyzed the following processes: (i) oxidation of 6-methylthiopurine riboside (ms6A) in solution and on polymer support; (ii) substitution of 6-methylsulfoxide and 6-methylsulfone of nucleosides with various primary amines; (iii) stability of the 6-methylthio group during automatic synthesis of oligoribonucleotides by the phosphoramidite approach and (iv) stability of precursor of oligoribonucleotides during activation and substitution of the leaving group.
To analyze the properties of the 6-methylthiopurine residue, both on monomer and oligoribonucleotide level, we synthesized protected derivatives of 6-methylthiopurine riboside and 3′-O-phosphoramidite of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside (1) and used the latter in the synthesis of the model pentamer CGUms6AA.
Synthesis of 3′-O-phosphoramidite (1) started with the conversion of 2′,3′,5′-O-triacetylinosine into 2′,3′,5′-O-triacetyl-6-thioinosine with Lawesson reagent. In the next step, the last derivative was treated with methyl iodide followed by the removal of acetyl groups by aqueous ammonia in pyridine to prepare 6-methylthiopurine riboside. This last derivative was converted into 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside by the consequent treatment of dimethoxytrityl chloride and tertbutyldimethylsilyl chloride. In the final step, this last derivative was treated with 2-cyanoethyl diisopropylchlorophosphoramidite and converted into the 3′-O-phosphoramidite of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside (1).
Oxidation of 6-methylthiopurine riboside
To become a good leaving group, the 6-methylthio substituent must be activated by oxidation, which results in the formation of a mixture of 6-methylsulfoxide and 6-methylsulfone derivatives. Various reagents can be used for the oxidation (21,32,33); however, we used a 20 mM solution of magnesium salt of monoperoxyphtalic acid in dioxane/water (9:1 v/v) (34). The experiments with 5′-O-dimethoxytrityl-2′,3′-bis(tertbutyldimethylsilyl)-6-methylthiopurine riboside and 5′-O-dimethoxytrityl-6-methylthiopurine riboside demonstrated that the treatment of both derivatives with 20 mM solution of magnesium salt of monoperoxyphtalic acid lead to a mixture of 6-methylsulfoxide and 6-methylsulfone. The 6-methylsulfoxide was formed first and in the following reaction it was converted into the 6-methylsulfone derivative. The ratio of 6-methylsulfoxide and 6-methylsulfone depends on the excess of magnesium salt of monoperoxyphtalic acid and the time of the reaction. Using 1.8 equivalents of oxidant for 15 min at room temperature results in the quantitative oxidation of the 6-methylthio group and the reaction mixture then it contains 90% 6-methylsulfoxide and 10% 6-methylsulfone derivatives, whereas 18 equivalents of magnesium salt of monoperoxyphtalic acid result mostly in the formation of 6-methylsulfone derivatives. The structure of the oxidation products was confirmed by spectroscopic methods and mass spectrometry. The 6-methylthio group resonates at 2.73 (1H NMR) and 11.84 p.p.m. (13C NMR), the 6-methylsulfoxide derivative at 3.12 (1H NMR) and 38.80 p.p.m. (13C NMR), and the 6-methylsulfone at 3.47 (1H NMR) and 41.11 p.p.m. (13C NMR). Moreover, the infrared spectra (IR) demonstrate the presence of one peak at 1071 cm–1 for 6-methylsulfoxide and the peaks at 1300 and 1071 cm–1 for 6-methylsulfone derivatives.
To transform 6-methylthio groups on support-linked oligoribonucleotides, we tested the oxidation of the 6-methylthiopurine derivative linked to solid support as well. For this experiment, 5′-O-dimethoxytrityl-2′-O-acetyl-6-methylthiopurine riboside linked to Fractosil 500 via succinyl linker was used. The solid support carrying the 6-methylthiopurine riboside derivative was treated with 18 equivalents of 20 mM solution of magnesium salt of monoperoxyphtalic acid in dioxane/water (9:1 v/v). After 0, 5, 15, 30 and 60 min, as well as 2, 8 and 24 h, the samples of solid support were collected. The samples were treated with aqueous ammonia and the reaction mixtures were analyzed by HPLC. The analysis demonstrated that after 2 h treatment 6-methylthio group was oxidized quantitatively.
This observation demonstrates that the oxidation of 6-methylthiopurine riboside linked to solid support proceed to the same products; however, the process is 5–10 times slower than oxidation of the same derivative in the solution. It is presumably related to the slow diffusion of the solution of oxidant into the structure of support.
Transformation of 6-methylsulfoxide and 6-methylsulfone purine riboside into N6-alkylated adenosine derivatives
The crucial step in the synthesis of modified oligoribonucleotides containing N6-alkyladenosines is aminolysis of the oxidized precursor oligoribonucleotide into modified oligoribonucleotide. To evaluate the influence of steric and electronic factors of N6-substituent, the reactivity of various primary amines with 6-methylsulfoxide and 6-methylsulfone purine riboside were tested.
The experiments (acetonitrile/amine = 9:1 v/v, 50–70 equivalents of amine, room temperature) on 5′-O-dimethoxytrityl-6-methylsulfoxide purine riboside, which is two to three times less reactive than 6-methylsulfone derivative, provides the following conclusions: (i) all tested primary amines, except tertbutylamine, reacted and formed appropriate 5′-O-dimethoxytrityl-N6-alkyladenosines; (ii) reactivity of primary amines is a function of pKa and steric hindrance. The order of reactivity was as follows: methylamine (<0.5 h) > isopentylamine (0.5 h) > neopentylamine (1 h) > 1-methylbutylamine (2 h) > 1-methylpropylamine (3h) > propargylamine (4 h); (iii) reaction with isopentenylamine hydrochloride/triethylamine at 55°C was completed within 0.5 h and (iv) after 48 h the appropriate 5′-O-dimethoxytrityl-N6-alkyladenosines were the only products.
We also studied the substitution of activated 5′-O-dimethoxytrityl-2′-O-acetyl-6-methylthiopurine riboside linked via succinyl linker to Fractosil 500. The support was treated with 20 mM solution of magnesium salt of monoperoxyphtalic acid in dioxane/water (9:1 v/v) for 2 h. To support the mixture of acetonitrile/isopentylamine, 9:1 v/v, 500 equivalents of amine was added and left for 16 h at room temperature followed by the aqueous ammonia treatment (16 h, 55°C). The HPLC analysis of the reaction mixture demonstrated the presence of only 5′-O-dimethoxytrityl-N6-isopentyladenosine.
The final experiments of this set were performed on pentamer CGUms6AA, which was oxidized as described above. The oxidized support containing pentamer was split and the samples were treated with isopentylamine, neopentylamine, 1-methylbutylamine, 1-methylpropylamine and propargylamine in acetonitrile (1:9 v/v, 500–700 equivalents of amines) for 16 h at room temperature. To convert 6-methylthio residue within the pentamer into the N6-methyl derivative, the sample was treated with 2 M methylamine in tetrahydrofuran. Meanwhile, precursor pentamer was converted into N6-isopentenyl pentamer by placing the mixture of isopentenylamine hydrochloride and triethylamine in acetonitrile for 16 h at 55°C. In the next step, all mixtures were treated with the aqueous ammonia and fluoride, and analyzed by HPLC.
The yield of CGUR6AA (where R6A means N6-alkyladenosine) oscillates between 59 and 67%. Only for the least reactive amine (propargylamine) yield was 42%. Also, pentamer CGUi6AA was formed with 39% yield. The reason for the lower yield is probably due to low solubility of isopentenylamine hydrochloride in acetonitrile. To improve yield, the next experiments were performed in pyridine.
The stability of the 6-methylthio group during the automatic synthesis of oligoribonucleotides and base protection groups as well as the internucleotide bond during aminolysis
During the synthesis of oligoribonucleotides by the phosphoramidite approach, trivalent phosphorous is oxidized with iodine into pentavalent phosphorus (35). Even partial oxidation of the 6-methylthio group could result in the side reaction. To test the stability of the 6-methylthio group, 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside was treated with the mixture used for oxidation during oligonucleotide synthesis. The aliquots were taken after 10 and 30 s as well as 1, 3, 5, 15 and 30 min and analyzed by TLC. The analysis demonstrated the stability of 6-methylthio group over 30 min, which was equivalent to 60 oxidation steps during an oligonucleotides synthesis. The same result was obtained when 6-methylthiopurine riboside linked to support was used in the experiment.
The next step was the analysis of a stability of base labile protecting groups during the aminolysis with primary amines. The experiments indicated that commonly used nucleobase protection groups were removed when treated with isoamylamine/acetonitrile (1:9 v/v) for 5 h at room temperature. Meanwhile, succinyl linker is cleaved only 50% after 16 h at 55°C treatment. This experiment clearly demonstrated that after the reaction with primary amines, it is necessary to treat the reaction mixture with aqueous ammonia to completely remove the protecting groups and hydrolyze the succinyl linker.
The last element of the analysis concerns the stability of the phosphodiester bond during the primary amines treatment. To test the internucleotide bond stability, dimer rA2SipT (where rA2Si means adenosine carrying 2′-O-tertbutyldimethylsilyl group) was synthesized and half of the sample was treated with acetonitrile/isopentylamine (9:1 v/v), while the second with isopentenylamine hydrochloride and triethylamine in pyridine. Both samples were heated at 55°C and after 0, 0.5, 1, 2, 4, 8, 24 and 48 h samples were taken and analyzed by HPLC. The quantitative analysis demonstrated that the stability of 2′-O-tertbutyldimethylsilyl group in the presence of isopentylamine and isopentenylamine generated in situ from isopentenylamine hydrochloride is similar. In standard conditions (500 equivalents of amine, 16 h, room temperature), each internucleotide bond present within oligoribonucleotide is cleaved no more than 0.3%.
We also tested the stability of N6-isopentenyl group during the synthesis of oligoribonucleotides. That is related to early reports indicating instability of N6-isopentenyladenosine in the presence of various oxidants (36). The derivative of N6-isopentenyladenosine covalent linked to solid support was used to the synthesis of pseudodecamer on a synthesizer, however, instead of 3′-O-phosphoramidites, acetonitrile was added to the column. After the deprotection with aqueous ammonia, the reaction mixture was analyzed by TLC and HPLC and indicated the presence of N6-isopentenyladenosine only. It demonstrated that N6-isopentenyladenosine is stable during RNA chemical synthesis and can be incorporated into oligoribonucleotide using this modified phosphoramidite.
The chemical synthesis of oligoribonucleotides containing N6-alkyladenosines via post-synthetic modification
The final test of our approach was the synthesis of oligoribonucleotides containing N6-alkyladenosines via post-synthetic modification of precursor oligonucleotides. These oligoribonucleotides (heptamers, octamers and heptadecamers) were necessary to study the thermodynamic stability of modified RNA duplexes and hairpins (17).
For the synthesis of precursor oligoribonucleotides, we used commercially available 3′-O-phosphoramidites of 2′-O-tertbutyldimethylsilylated ribonucleotides and 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside 3′-O-phosphoramidite (1) (35). The coupling yields of precursor phosphoramidite (1) were compatible with standard phosphoramidites. The synthesis was performed several times in 1 µmol scale and the support was combined together to get a homogeneous mixture of precursor oligonucleotides, so the differences in the synthesis of modified oligonucleotides could be related to the modification step only. The support carrying oligonucleotides was oxidized as described above. The support was split and each sample was treated with mixture amine/acetonitrile (1:9 v/v, 500–1000 equivalents of amine) and left for 12 h at room temperature. To get the oligomers containing N6-isopentenyladenosine, the mixture of isopentenylamine hydrochloride (213 equivalents) and triethylamine (426 equivalents) in pyridine was used and left for 12 h at 55°C. Next, the solutions were evaporated and residues treated with the aqueous ammonia/ethanol (3:1 v/v) for 12 h at 55°C. The solution was evaporated again and 2′-O-tertbutyldimethylsilyl groups were removed by the triethylammonium fluoride as described earlier (24). The reaction mixtures were analyzed by HPLC and a typical chromatogram is shown in Figure 3A. The retention time of this modified oligoribonucleotide, Ui6ACAUGUA was 31.9 min, whereas UACAUGUA was 23.6 min. The last oligoribonucleotide was obtained from the activated precursor oligoribonucleotide, which was not transformed into Ui6ACAUGUA, but during the ammonia treatment it was converted into UACAUGUA. The comparison of these two peaks indicates that the transformation of precursor oligonucleotide was ∼90%. However, the overall yield was 41%, which is the sum of yields of the chemical synthesis of precursor oligoribonucleotides, two steps of modification and deprotection.
The chemical synthesis of heptadecamers—CCGGUC mUCCAXAACCGG (where X was N6-methyladenosine or N6-isopentenyladenosine) via the post-synthetic modification of precursor oligoribonucleotides were less sufficient (yield below 10%).
The overall yields of all obtained modified oligoribonucleotides are listed in Table 1 and oscillate between 25 and 91%. Moreover, the synthesis yield of oligoribonucleotides containing N6-alkyladenosines depends simultaneously on the steric hindrance and the reactivity of amines used in substitution. The yields are not dependent on position of modification within the oligoribonucleotides and the yield of oligonucleotides containing N6-methyl-, N6-isopentenyl- and N6-propargyladenosine were the lowest. The lower yield of N6-methylated oligomers can result from the volatility of methylamine, meanwhile the yield of N6-isopentenyladenosine oligomers can be related to the fact that the isopentenylamine was generated in situ from hydrochloride, which is very insoluble and generates many manual problems during the work-up of the reaction mixture after modification. The propargylamine was the least reactive in many model reactions and that could be related to its pKB value (5.83).
Table 1. The synthesis yields of oligoribonucleotides containing N6-alkyladenosines via post-synthetic modification of precursor form of oligonucleotides.
| UACR6AUGUA | UR6ACAUGUA | UACAUGUR6A | ACAUGUR6A | |||||
|---|---|---|---|---|---|---|---|---|
| Yielda | MSb | Yielda | MSb | Yielda | MSb | Yielda | MSb | |
| R = H- | 64% | 2494.99 | 74% | 2188.35 | ||||
| R = methyl- | 47% | 2508.92 | 40% | 2508.93 | 51% | 2508.61 | 49% | 2202.32 |
| R = neopentyl- | 51% | 2564.87 | 47% | 2564.93 | 58% | 2564.90 | 69% | 2258.49 |
| R = 1-methylpropyl- | 72% | 2550.88 | 42% | 2550.90 | 77% | 2551.05 | 75% | 2244.46 |
| R = 1-methylbutyl- | 71% | 2565.01 | 61% | 2564.95 | 51% | 2564.84 | 91% | 2258.52 |
| R = isopentyl- | 45% | 2564.94 | 52% | 2564.78 | 51% | 2564.78 | 55% | 2258.47 |
| R = isopentenyl- | 56% | 2562.92 | 41% | 2563.75 | 25% | 2563.30 | 65% | 2256.11 |
| R = propargyl- | 39% | 2532.98 | 40% | 2532.64 | 49% | 2532.81 | 42% | 2226.46 |
| CCGGUCmUCCAXAACCGG | ||||||||
| X = A | X = m6Ac | X = i6Ac | ||||||
| 34% | 5398.72 | 29% | 5413.92 | 13% | 5467.45 | |||
aBased on C-18 HPLC analysis.
bMeasured molecular weight of the oligoribonucleotides.
cObtained from modified phosphoramidites.
All oligoribonucleotides were analyzed by mass spectrometry and showed very good agreement of the calculated and measured mass (difference was <1D) (Table 1). Some oligomers were digested by snake venom phosphodiesterase (SVPDE) followed by calf intestine phosphatase (CIP). The HPLC analysis of the reaction mixture confirmed the expected ratio of nucleosides.
The chemical synthesis of oligoribonucleotides containing 2-methylthio-N6-alkyladenosines via the post-synthetic modification of precursor oligomer
Among seven N6-modified adenosines, five are analogous which beside the same modification at position 6 carry additional methylthio group at position 2 (1). The function of these modified nucleosides is even less understood than N6-modifed. The chemical synthesis of oligoribonucleotides with these modifications was not reported.
The synthesis and preliminary analysis of the precursor forms of nucleosides
Similarly, as reported earlier for oligoribonucleotides containing N6-alkyladenosines, we developed a synthesis method of these modified oligomers via the transformation of precursor form of oligoribonucleotide. We decided to use as precursor 2-methylthiopurine riboside containing a substituent at position 6, which could be a good leaving group during amine nucleophile substitution. Since the 2-methylthio group undergoes the oxidation faster than 6-methylthio, 2,6-dimethylthiopurine riboside as precursor nucleoside was eliminated (37). As the potential precursor nucleoside, we considered several 6-substituted purine derivatives such as: dinitrothiophenyl- (28), pentafluorophenyl- (29) and chloro- (38,39) substituted nucleosides. The crucial points of selection were the reactivity of the 6-substituted derivative with primary amines and the introduction of a 2-methylthio group into the nucleotide. To select the best precursor nucleoside, 2′,3′,5′-O- triacetyl-2-methylthio-6-S-(2,4-dinitrophenyl)-purine riboside, 2′,3′,5′-O-triacetyl-2-methylthio-6-O-pentafluorophenylpurine riboside and 2′,3′,5′-O-triacetyl-2-methylthio-6-chloropurine riboside were synthesized and tested.
The synthesis of 2′,3′,5′-O-triacetyl-2-methylthio-6-S-(2,4-dinitrophenyl)-purine riboside started with 2′,3′,5′-O-triacetylguanosine which in the reaction with Lawesson reagent was converted into acetylated 6-thioguanosine (19,20). The last derivative in the reaction with 2,4-dinitrofluorobenzene was transformed into 2′,3′,5′-O-triacetyl-6-S-(2,4-dinitrophenyl)-guanosine (28). The next reaction was the conversion of the 2-amino group of this last derivative into 2-methylthio with dimethyldisulfide in the presence of isoamyl nitrite (40,41). Unfortunately, the main product of the reaction was not 2′,3′,5′-O-triacetyl-2-methylthio-6-S-(2,4-dinitrophenyl)-purine riboside, but 2′,3′,5′,N2-tetraacetyl-6-S-(2,4-dinitrophenyl)-guanosine, which was formed with 50–80% yield.
To synthesize the second potential precursor nucleo side, 2′,3′,5′-O-triacetyl-2-methylthio-6-O-pentafluorophenylpurine riboside, guanosine was converted into 6-O-pentafluorophenylguanosine according to the Jones procedure (29) and later into 2′,3′,5′-O-triacetyl-2-methylthio-6-O-pentafluorophenylguanosine. To convert the 2-amino group into 2-methylthio, the same procedure was used as described above (40,41). Similarly, as described earlier, the formation of two products was observed. One of them was 2′,3′,5′-O-triacetyl-2-methylthio-6-O-pentafluorophenylpurine riboside, while the second was 2′,3′,5′,N2-tetraacetyl-2-methylthio-6-O-pentafluorophenylguanosine (with 40–60% yield).
The synthesis of the third potential precursor nucleoside, 2′,3′,5′-O-triacetyl-2-methylthio-6-chloropurine riboside start ed with the transformation of 2′,3′,5′-O-triacetylguanosine into a 6-chloro-2-aminopurine riboside derivative according to the published procedure (18). This last derivative was converted into 2′,3′,5′-O-triacetyl-2-methylthio-6-chloropurine riboside by the deamination with dimethyldisulfide in the presence of isoamyl nitrite (40,41). In this case, the expected product was obtained with ∼90% yield whereas the N2-acetylated side product was formed in ∼10%.
The structures of 2′,3′,5′-O-triacetyl-2-methylthio-6-substituted purine ribosides as well as 2′,3′,5′,N2-tetraacetyl-6-substituted guanosines were confirmed by 1H and 13C NMR, and mass spectrometry, as well as by the comparison with authentic samples. The mechanism of formation of side products is not clear at that moment, particularly the source of acetyl group.
Substitution of 2′,3′,5′-O-triacetyl-2-methylthio-6-substituted purine ribosides with various amines
The conversion of potential precursor nucleosides, 2′,3′,5′- O-triacetyl-2-methylthio-6-S-(2,4-dinitrophenyl)-purine riboside, 2′,3′,5′-O-triacetyl-2-methylthio-6-O-pentafluorophenylpurine riboside, and 2′,3′,5′-O-triacetyl-2-methylthio-6-chloropurine riboside into 2-methylthio-N6-alkyladenosines were tested by the reaction with isopentylamine [30 equivalents of isopentylamine in acetonitrile (1:9.v/v), room temperature, 48 h]. Next, the reaction mixtures were treated with aqueous ammonia (16 h, 55°C) and analyzed by TLC.
When substrates of the reaction were 6-O-pentafluorophenyl- and 6-chloropurine riboside the product of substitution was 2-methylthio-N6-isopentyladenosine. Whereas 6-S-(2,4-dinitrophenyl) derivative treated under the same conditions resulted in the formation of 2-methylthio-6-thiopurine riboside (28).
Based on the structure of the products formed in the reaction with isopentylamine and reactivity during the incorporation of the 2-methylthio group into nucleoside, we selected a 2-methylthio-6-chloropurine riboside derivative for the intensive studies. To perform the experiments, we synthesized 3′-O-phosphoramidite of 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio-6-chloropurine riboside (2). The synthesis of 2′,3′,5′-O-triacetyl-2-methylthio-6-chloropurine riboside was performed as described above (40,41). The acetyl groups were removed by treatment with a mixture of triethylamine and methanol (5:95 v/v). The fully deprotected nucleoside was transformed into 5′-O-dimethoxytrityl- and later into 2′-O-tertbutyldimethylsilyl-derivative and finally into 3′-O-phosphoramidite (2).
The 5′-O-dimethoxytrityl-2-methylthio-6-chloropurine riboside was used to test the transformation into various 2-methylthio-N6-alkyladenosine derivatives. The substrate was treated with 70 equivalents of appropriate amine and acetonitrile (1:9 v/v) at 55°C. At the same time, to obtain 2-methylthio-N6-isopentenyladenosine and 2-methylthio-N6-methyladenosine, isopentenylamine hydrochloride/triethylamine or 2 M solution of methylamine in tetrahydrofuran were used, respectively.
The results of this set of experiments demonstrated that: (i) reactivity of 5′-O-dimethoxytrityl-2-methylthio-6-chloropurine riboside with primary amines changed in the following order: isopentylamine (2 h) > neopentenylamine (2.5 h) > 1-methylbutylamine (3 h) > 1-methylpropylamine (4 h) > propargylamine (5 h); (ii) treatment of the substrate with isopentenylamine hydrochloride/triethylamine yields ∼95% of 2-methylthio-N6-isopentenyladenosine within 1.5 h at 55°C; (iii) 2 M methylamine in tetrahydrofuran converted quantitatively into 2-methylthio-N6-methyladenosine derivative within 3–4 h at room temperature; (iv) treatment of 5′-O-dimethoxytrityl-2-methylthio-6-chloropurine riboside with aqueous ammonia in pyridine yield in 90% of 5′-O-dimethoxytrityl-2-methylthioadenosine formation and (v) reactivity of 2-methylthio-6-chloropurine riboside is 5–10 times lower than 6-methylsulfoxide purine riboside derivatives.
The analysis of stability of the 2-methylthio group during the synthesis of oligoribonucleotides by the phosphoramidite approach
As it was mentioned earlier, the 2-methylthio group is oxidized more easily than 6-methylthio (37). To analyze the stability of the 2-methylthio group, 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio-6-chloropurine riboside was treated with an oxidizing mixture used during the chemical synthesis of oligonucleotides by the phosphoramidite approach (35). The analysis of the reaction mixture after 30 min treatment indicated complete stability of the 2-methylthio group. This conclusion was confirmed by the experiment in which a derivative of 2-methylthio-N6-isopentenyladenosine covalent linked to the solid support was used in the experiment.
Additionally, the last experiment shows stability of N6-isopentenyl group during iodine oxidation and it demonstrates that 2-methylthio-N6-isopentenyladenosine can be incorporated into oligoribonucleotide using this modified phosphoramidite (36).
The chemical synthesis of oligoribonucleotides containing 2-methylthio-N6-alkyladenosine via the post-synthetic modification
To study the thermodynamic stability of modified RNA duplexes and hairpins, we were interested in the chemical synthesis of heptamers, octamers and heptadecamers containing 2-methylthio-N6-methyl-, 2-methylthio-N6-isopentenyl- and 2-methylthioadenosine (17). To solve some thermodynamic aspects, the precursor nucleoside residue was placed at various positions within oligonucleotides. The precursor oligoribonucleotides containing 2-methylthio-6-chloropurine riboside residue were synthesized using commercially available phosphoramidites and 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio-6-chloropurine riboside 3′-O-phosphoramidite (2).
The solid support-linked precursor oligoribonucleotides with 2-methylthio-6-chloropurine riboside were treated with 2 M solution of methylamine in tetrahydrofuran (1000 equivalents). The other sample was treated with the mixture of isopentenylamine hydrochloride/triethylamine (213 and 426 equivalents, respectively) in pyridine, both for 2–5 h at 55°C. Next, the aqueous ammonia was added and the samples were left for an additional 18 h. To convert precursor oligomer into oligoribonucleotide containing 2-methylthioadenosine, support-linked precursor oligoribonucleotide was treated with the aqueous ammonia for 16 h at 55°C. The 2′-O-tertbutyldimethylsilyl group was removed as described previously (24). The reaction mixture was purified on silica gel TLC plates and analyzed by HPLC reverse-phase column. The typical chromatogram of the crude mixture from the synthesis of Ums2i6ACAUGUA is shown in Figure 3B. The retention time of Ums2i6ACAUGUA is 44.7 min while the peaks at 27.4 min represents Ums2ACAUGUA. This last oligonucleotide is formed during the aqueous ammonia treatment from the part of the precursor oligoribonucleotide that was not converted into 2-methylthio-N6-isopentenyladenosine carrying oligonucleotide. The synthesis yields of various 2-methylthio-modified oligoribonucleotides via the post-synthetic modification of precursor oligonucleotides are collected in Table 2. The yields of synthesis range from 20 to 65%.
Table 2. The synthesis yield of oligoribonucleotides containing 2-methylthio-N6-alkyladenosines via post-synthetic modification of precursor form of oligonucleotides.
| UACXUGUA | UXCAUGUA | UACAUGUX | ACAUGUX | |||||
|---|---|---|---|---|---|---|---|---|
| Yielda | MSb | Yielda | MSb | Yielda | MSb | Yielda | MSb | |
| X = ms2A | 48% | 2541.02 | 49% | 2540.91 | 27% | 2541.01 | 35% | 2234.65 |
| X = ms2m6A | 64% | 2555.04 | 35% | 2554.79 | 54% | 2555.00 | 53% | 2249.03 |
| X = ms2i6A | 41% | 2608.93 | 24% | 2608.97 | 65% | 2608.57 | 20% | 2302.92 |
| CCGGUCmUCCAXAACCGG | ||||||||
| X = ms2A | X = ms2m6A | X = ms2i6Ac | ||||||
| 16% | 5445.18 | 24% | 5459.53 | 22% | 5513.76 | |||
aBased on C-18 HPLC analysis.
bMeasured molecular weight of the oligoribonucleotides.
cObtained from modified phosphoramidite.
The heptadecamers, CCGGUCmUCCAXAACCGG (where X was 2-methylthioadenosine or 2-methylthio-N6-methyladenosine) were obtained via the post-synthetic modification of the precursor oligoribonucleotides, with 16 and 24% yield, respectively.
Higher yield was obtained for oligoribonucleotides containing 2-methylthio-N6-methyladenosine residue, with lower yields for oligoribonucleotides with 2-methylthio-N6-isopentenyladenosine residue. This could result from the insolubility of isopentenylamine hydrochloride and thermal instability of isopentenylamine. It is important to mention that the yields of synthesis reported in Table 2 are the sum of the yields of chemical synthesis of oligoribonucleotides, transformation of precursor oligomer into 2-methylthio-N6-alkyl or 2-methylthio oligoribonucleotides and deprotection of oligonucleotides.
All oligoribonucleotides were analyzed by mass spectrometry and showed very good agreement of calculated and measured mass (difference was <1D) (Table 2). Some of the oligomers were digested by SVPDE followed by CIP. The HPLC analysis of reaction mixture confirmed the expected ratio of nucleosides.
CONCLUSIONS
The modified oligoribonucleotides are important tools to better understand the various functions of RNA. The chemical synthesis of modified oligoribonucleotides via the post-synthetic transformation of precursor oligoribonucleotides seems to be the method of choice for the preparation of the similarly modified oligoribonucleotides. The application as precursor solid support-linked oligomers containing 6-methylthio- or 2-methylthio-6-chloropurine ribosides allows an efficient synthesis of oligoribonucleotides containing N6-alkyladenosines or 2-methylthio-N6-alkyladenosines, respectively. Using this approach, many modified oligoribonucleotides, up to heptadecamers, carrying various N6-substituents were obtained. Among them were several oligonucleotides with naturally modified adenosines such as: i6A, m6A, ms2i6A and ms2m6A, as well as many carrying unnaturally modified adenosine derivatives. The appropriate primary amines were used for the substitution of solid support-linked precursor oligoribonucleotides. However, the reactivity of these amines depended on the steric hindrance of alkyl chain and nucleophilic character of the amino group. The nucleosides used as precursor synthons were resistant during the synthesis of 3′-O-phosphoramidites and the synthesis of oligoribonucleotides by phosphoramidite approach. The conditions of post-synthetic modification of precursor oligoribonucleotides were safe to nucleobases, ribose residues and internucleotide bonds.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by Polish State Committee for Scientific Research (KBN) grant No. 3 T09A 140 19 to R.K. and NIH grant 1R03 TW1068 to R.K. and D. H. Turner. We would like to thank Brent Znosko (University of Rochester) and Professor Ryszard W. Adamiak (Institute of Bioorganic Chemistry PAS) for comments and suggestions on this manuscript.
REFERENCES
- 1.Limbach P.A., Crain,P.F. and McClosky,J.A. (1994) Summary: the modified nucleosides of RNA. Nucleic Acids Res., 22, 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjork G.R. (1995) tRNA: structure, biosynthesis and function. In Soll,D. and RajBhandary,U. (eds.), Biosynthesis and Function of Modified Nucleosides. ASM Press, Washington, DC, pp. 165–206. [Google Scholar]
- 3.Persson B.C. (1993) Modification of tRNA as a regulatory device. Mol. Microbiol., 8, 1011–1016. [DOI] [PubMed] [Google Scholar]
- 4.Grosjean H., Houssier,C., Romby,P. and Marquet,R. (1998) Modulatory role of modified nucleotides in RNA loop-loop interaction. In Grosjean,H. and Benne,R. (eds.), Modification and Editing of RNA. ASM Press, Washington DC, pp. 113–133. [Google Scholar]
- 5.Sundaram M., Durant,P.C. and Davis,D.R. (2001) Hypermodified nucleosides in anticodon of tRNALys stabilize a canonical U-turn structure. Biochemistry, 39, 12575–12584. [DOI] [PubMed] [Google Scholar]
- 6.Agris P.F., Sierzputowska-Gracz,H., Smith,W., Malkiewicz,A., Sochacka,E. and Nawrot,B. (1992) Thiolation of uridine carbon-2 restricts the motional dynamics of the transfer RNA wobble position nucleoside. J. Am. Chem. Soc., 114, 2652–2656. [Google Scholar]
- 7.Agris P.F. (1996) The importance of being modified: role of modified nucleosides and Mg+2 in RNA structure and function. Prog. Nucleic Acid Res. Mol. Biol., 53, 79–129. [DOI] [PubMed] [Google Scholar]
- 8.Grasby J.A. and Gait,M.J. (1994) Synthetic oligoribonucleotides carrying site-specific modifications for RNA structure-function analysis. Biochimie, 76, 1223–1234. [DOI] [PubMed] [Google Scholar]
- 9.Adamiak R.W., Biala,E., Grzeskowiak,K., Kierzek,R., Kraszewski,A., Markiewicz,W.T., Okupniak,J., Stawinski,J. and Wiewiorowski,M. (1978) The chemical synthesis of the anticodon loop of an eukaryotic initiator tRNA containing the hypermodified nucleoside N-(N-threonylcarbamoyl)adenosine. Nucleic Acids Res., 5, 1889–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sochacka E. (1999) The chemical synthesis of E.coli tRNALys anticodon loop fragment and its analogues. Nucl. Nucl., 17, 327–338. [DOI] [PubMed] [Google Scholar]
- 11.Stuart J.W., Gdaniec,Z., Guenther,R., Marszalek,M., Sochacka,E., Malkiewicz,A. and Agris,P.F. (2000) Functional anticodon architecture of human tRNALys3 includes disruption of intraloop hydrogen bonding by the naturally occurring amino acid modification, t6A. Biochemistry, 39, 13396–13404. [DOI] [PubMed] [Google Scholar]
- 12.Boudou V., Langridge,J., Van Aerchot,A., Hendrix,C., Millar,A., Weiss,P. and Herdewijn,P. (2000) Synthesis of the anticodon hairpin tRNAfMet containing N-{[9-(β-d-ribofuranosyl)-9H-purin-6-yl]carbamoyl}-l-threonine. Helvetica Chim. Acta, 83, 152–161. [Google Scholar]
- 13.Sundaram M., Crain,P.F. and Davis,D.R. (2000) Synthesis and characterization of the native anticodon domain of E. coli. Simultaneous incorporation of modified nucleosides mnm5s2U, t6A and pseudouridine using phosphoramidite chemistry. J. Org. Chem., 65, 5609–5614. [DOI] [PubMed] [Google Scholar]
- 14.Swiderski P., Dembek,P., Antkowiak,W.Z., Biala,E. and Adamiak,R.W. (1987) Studies directed towards introduction of N6-isopentenyladenosine and its 2-methylthio analogue into oligoribonucleotides. Nucleic Acids Res. Symp. Ser., 18, 105–108. [PubMed] [Google Scholar]
- 15.Swiderski P., Antkowiak,W.Z. and Adamiak,R.W. (1991) First, solid-support-aided introduction of isopentenyladenosine. Hypermodified nucleoside of tRNA into oligoribonucleotide chain. Nucl. Nucl., 10, 599–600. [Google Scholar]
- 16.Kierzek E. and Kierzek,R. (2001) Influence of N6-isopentenyladenosine (i6A) on thermal stability of RNA duplexes. Biophys. Chem., 91, 135–140. [DOI] [PubMed] [Google Scholar]
- 17.Kierzek E. and Kierzek,R. (2003) The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res., 31, 4472–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robins M.J. and Uznañski,B. (1981) Nucleic acid related compounds. 33. Conversions of adenosine and guanosine to 2,6-dichloro, 2-amino-6-chloro, and derived purine nucleosides. Can. J. Chem., 59, 2601–2606. [Google Scholar]
- 19.Cava M.P. and Levinson,M.J. (1985) Thionation reactions of Lawesson’s reagents. Tetrahedron, 41, 5061–5087. [Google Scholar]
- 20.Morisawa H., Utagawa,T., Miyoshi,T., Yoshinaga,F., Yamazaki,A. and Mitsugi,K. (1980) A new method for synthesis of some 9-β-d-arabinofuranosylpurines by a combination of chemical and enzymatic reactions. Tetraherdon Lett., 21, 479–482. [Google Scholar]
- 21.Wetzel R. and Eckstein,F. (1975) Synthesis and reactions of 6-methylsulfonyl-9-β-d-ribofuranosylpurine. J. Org. Chem., 40, 658–660. [DOI] [PubMed] [Google Scholar]
- 22.Smith M., Rammler,D.H., Goldberg,I.H. and Khorana,H.G. (1962) Studies on polynucleotides. XIV. Specific synthesis of the C3′-C5′ interribonucleotide linkage. Synthesis of uridyl-(3′-5′)-uridine and uridyl-(3′-5′)-adenosine. J. Am. Chem. Soc., 84, 430–440. [Google Scholar]
- 23.Usman N., Ogilvie,K.K., Jiang,M.-Y. and Cedergren,R.J., (1987) Automated chemical synthesis of long oligoribonucleotides using 2′-O-silylated ribonucleoside 3′-O-phosphoramidites on a controlled-pore glass support: synthesis of a 43-nucleotide sequence similar to the 3′-half molecule of an E.coli formylmethionine tRNA. J. Am. Chem. Soc., 109, 7845–7854. [Google Scholar]
- 24.Xia T., SantaLucia,J.,Jr, Burkard,M.E., Kierzek,R., Schroeder,S.J., Jiao,X., Cox,C. and Turner,D.H. (1998) Parameters for expended nearest-neighbor model for formation of RNA duplexes with Watson–Crick base pair. Biochemistry, 37, 14719–14735. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y-Z. and Swann,P.F. (1990) A simple method for the solid phase synthesis of deoxynucleotides containing O4-alkylthymine. Nucleic Acids Res., 18, 4061–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacMillan A.M. and Verdine.G.L. (1991) Engineering tethered DNA molecules by the convertible nucleoside approach. Tetrahedron, 47, 2603–2616. [Google Scholar]
- 27.Xu Y-Z, Zhang,Q. and Swann,P.F. (1992) Synthesis of DNA containing modified bases by postsynthetic substitution. Synthesis of oligomers containing 4-substituted thymine: O4-alkylthymine, 5-methylcytosine, N4-(dimethylamino)-5-methylcytosine and 4-thiothymine. J. Org. Chem., 57, 3839–3845. [Google Scholar]
- 28.Xu Y-Z, Zhang,Q. and Swann,P.F. (1992) Synthesis by post-synthetic substitution of oligomers containing guanine modified at 6-position with S-, N-, O-derivatives. Tetrahedron, 48, 1729–1740. [Google Scholar]
- 29.Gao H., Fathi,R., Gaffney,B.L., Goswami,B., Kung,P-P., Rhee,Y., Jin,R. and Jones,R.A. (1992) 6-O-(Pentaflurorphenyl)-2′-deoxyguanosine: a versatile synthon for nucleoside and oligonucleotide synthesis. J. Org. Chem., 57, 6954–6959. [Google Scholar]
- 30.Xu Y.-Z., Zhang,Q. and Swann,P.F. (1992) Synthesis and duplex stability of oligodeoxynucleotides containing 6-mercaptopurine. Tetrahedron Lett., 33, 5837–5840. [Google Scholar]
- 31.Coleman R.S. and Kesicki,E.A. (1994) Synthesis and postsynthetic modification oligodeoxynucleodides containing 4-thio-2′-deoxyuridine. J. Am. Chem. Soc., 116, 11636–11642. [Google Scholar]
- 32.Wayne C. and Robins.R.K. (1959) Potential purine antagonists. XX. The preparation and reactions of some methylthiopurines. J. Am. Chem. Soc., 81, 5997–6007. [Google Scholar]
- 33.Villar J.D.F. and Motta,M.A. (2000) Synthesis of 6-alkyl and arylamino-9-(tetrahydro-2-pyranyl)purines via 6-methylsulfonylpurine. Nucl. Nucl. Nucleic Acids, 19, 1005–1015. [DOI] [PubMed] [Google Scholar]
- 34.Burdzy A., Skalski,B., Biala,E., Kowalewski,A., Paszyc,S. and Adamiak,R.W. (1995) Post-synthetic transformations of oligodeoxynucleotides originated at 6-methylthiopurine site. Nucl. Nucl., 14, 979–982. [Google Scholar]
- 35.McBride L.J. and Caruthers,M.H. (1983) An investigation of several deoxynucleosides phosphoramidites useful for synthesizing oligodeoxynucleotides. Tetrahedron Lett., 24, 245–249. [Google Scholar]
- 36.Hall R.H. (1970) N6-Isopentenyladenosine: chemical reactions, biosynthesis, metabolism and significance to the structure and function of tRNA. Prog. Nucleic Acid Res. Mol. Biol., 10, 57–86. [DOI] [PubMed] [Google Scholar]
- 37.Swiderski P. (1989) The synthesis and chemical properties of the hypermodified nucleoside N6-isopentenyladenosine and oligoribonucleotides containing this modification. PhD thesis. Adam Mickiewicz University, Poznañ, Poland.
- 38.Hecht S.M., Helgeson,J.P. and Fujii,T. (1968) Combination of 6-chloropurine with primary amine. In Zorbach,W.W. and Tipson,R.S. (eds.), Synthetic Procedures in Nucleic Acids Chemistry. Wiley and Sons, New York, NY, pp. 8–10. [Google Scholar]
- 39.Ikehara M. and Uno,H. (1965) Futher studies on the chlorination of inosine derivatives with dimethylformamide-thionyl chloride complex. Chem. Pharm. Bull., 13, 221–223. [DOI] [PubMed] [Google Scholar]
- 40.Nair V. and Hattrick,B.J. (1988) Sulfone of the antibiotic, nebularine: synthesis and conversion to novel analogues of nebularine. Tetrahedron, 44, 7001–7006. [Google Scholar]
- 41.Nair V. and Fasbender,A.J. (1993) C-2 functionalized N6-cyclosubstituted adenosines: highly selective agonists for the adenosine A1 receptor. Tetrahedron, 49, 2169–2184. [Google Scholar]