Abstract
The N6-alkyladenosines and 2-methylthio-N6-alkyladenosines make up over half of the population of all naturally modified adenosines and they are present in the transfer ribonucleic acids (tRNA) at position 37. We measured effects of N6-alkyladenosines and 2-methylthio-N6-alkyladenosines on the thermodynamic stability of RNA duplexes containing a U-AMod base pair at internal and terminal duplex positions, as well as containing modified adenosines as a 3′-terminal unpaired nucleotide. Beside naturally modified adenosines such as N6-isopentenyladenosine (i6A), N6-methyladenosine (m6A), 2-methylthio-N6-isopentenyladenosine (ms2i6A) and 2-methylthio-N6-methyladenosine (ms2m6A), we studied several artificial modifications to evaluate the steric and electronic effects of N6-alkyl substituents. Moreover, some N6-alkyladenosines and 2-methylthio-N6-alkyladenosines were placed in hairpins at positions corresponding to nucleotide 37 of the tRNA anticodon arm, and the thermodynamic stability of those hairpins was studied. The stability of the modified RNA hairpins was measured in standard melting buffer containing 1 M sodium chloride as well as in physiological buffer containing 10 mM magnesium chloride and 150 mM potassium chloride. The results obtained indicate that the nature of the adenosine modification and the position of U-AMod base pairs within the duplex influence the thermodynamic stability of RNA duplexes. For most of the modification, the destabilization of duplexes was observed. Moreover, we found that the buffer composition and the structure of the modified adenosine very significantly affect the thermodynamic stability of RNA.
INTRODUCTION
In natural ribonucleic acids, there have been 96 modified nt found (1). Among them 80 modified nt can be found in transfer ribonucleic acids (tRNA) (2). They are placed mostly at single-stranded fragments of tRNA such as hairpin and multi-branch loops. Some of the modified nucleotides are in helical fragments of tRNA at terminal or penultimate positions.
The function of modified residues in tRNA is not completely understood. It has been reported that the presence of modified nucleotides: (i) stabilizes tRNA structure (3), (ii) affects tRNA aminoacylation (4), (iii) is important for tRNA interactions with initiation and elongation factors (5) and (iv) influences the functioning of codon–anticodon interactions and the accuracy of aminoacyl-tRNA selection (6).
We are interested in the influence of N6-alkyladenosines and 2-methylthio-N6-alkyladenosines on the thermodynamic stability of RNA duplexes and hairpins. These modified adenosines were found in tRNA at position 37, and include N6-isopentenyladenosine (i6A), N6-methyladenosine (m6A), 2-methylthio-N6-isopentenyladenosine (ms2i6A) and 2-methyl thio-N6-methyladenosine (ms2m6A). To evaluate the influence of these modifications (via steric or electronic effects of N6-alkyl substituents) on the thermodynamic stability of hairpins, we first carried out experiments with RNA duplexes having modified adenosines. We were interested in understanding what would happen to RNA thermodynamic stability if the modification were present in a double helix. For this reason we studied the effect of N6-alkyladenosines and 2-methylthio-N6-alkyladenosines on the thermodynamic stability of RNA duplexes when placed at internal (both separated and tandem) and terminal base pair positions, as well as when placed as the terminal unpaired nucleotide (Fig. 1). Moreover, in studying the thermodynamic stability of these modified RNA duplexes we hoped to better understand the factors important for interaction between complementary strands, particularly the contribution of stacking and hydrogen bonding.
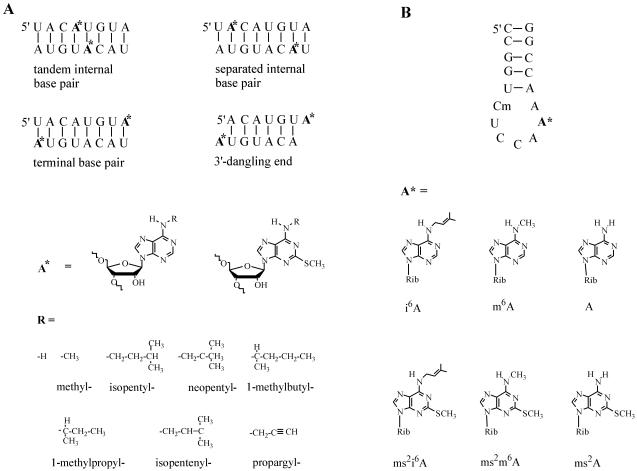
Figure 1.
(A) RNA duplex sequences and the structures of N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. (B) The structure of the anticodon loop of tRNATrp from E.coli, with modified nucleotides placed at position 37 of this sequence (the modified position is indicated by A*, and Cm is the naturally occurring 2′-O-methylcytidine).
The stability of RNA duplexes was studied in standard melting buffer containing 1 M sodium chloride, whereas the stability of RNA hairpins was measured in standard melting buffer and physiological buffer containing 150 mM potassium chloride and 10 mM magnesium chloride.
The data concerning the influence of modified nucleotides on thermodynamic stability are very limited, not only by the type of modified nucleotides, but also by the conditions in which thermodynamic stability of RNA was studied (3).
MATERIALS AND METHODS
Synthesis and purification of modified oligoribonucleotides
Oligoribonucleotides were synthesized on an Applied Biosystems DNA/RNA synthesizer, using β-cyanoethyl phosphoramidite chemistry (7). For synthesis, commercially available phosphoramidites that contained 2′-O-tertbutyldimethylsilyl groups were used. Additionally, precursor amidites such as 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-6-methylthiopurine riboside 3′-O-phosphoramidite and 5′-O-dimethoxytrityl-2′-O-tertbutyldimethylsilyl-2-methylthio- 6-chloropurine riboside 3′-O-phosphoramidite were used. The modification was introduced via a post-synthetic method using an appropriate amine. Commercially available CPG or Fractosil 500 supports were also used and contained protected 6-methylthiopurine riboside or 2-methylthio-6-chloropurine riboside connected via succinyl linker to the solid phase.
The details of post-synthetic transformation, deprotection and purification of modified oligoribonucleotides are described in the preceding paper (8). The purity of modified oligoribonucleotides used for the melting experiments was analyzed by HPLC (C-18 column) and it was found to be >95%.
UV melting of the oligoribonucleotides
The oligoribonucleotides were melted in buffer containing 1 M sodium chloride, 20 mM sodium cacodylate, 0.5 mM Na2EDTA, pH 7.0 and in physiological buffer containing 150 mM potassium chloride, 10 mM magnesium chloride, 20 mM sodium cacodylate and 0.5 mM Na2EDTA, pH 7.0. Oligoribonucleotide single strand concentrations were calculated from high-temperature (>80°C) absorbencies and single strand extinction coefficients approximated by a nearest-neighbor model (9–11). Absorbency versus temperature melting curves were measured at 260 or 280 nm with a heating rate of 1°C/min from 0 to 90°C on a Gilford 250 spectrometer controlled by a Gilford 2527 thermoprogrammer, or a Beckman DU 640 spectrometer with a thermoprogrammer. There were nine melts for duplexes and hairpins in standard buffer and three for hairpins in physiological buffer at concentrations ranging from 10–3 to 10–6 M RNA. Melt curves were analyzed and thermodynamic parameters were calculated using the program MeltWin 3.5.
RESULTS AND DISCUSSION
Selection of the model of RNA duplexes and hairpins
The anticodon arm of tRNATrp from Escherichia coli was selected for these studies because it contained ms2i6A at position 37 (Fig. 1). To evaluate the influence of ms2i6A on stability, the thermodynamic stability of this hairpin was compared with others containing modified adenosines at position 37, such as i6A, m6A, ms2m6A, ms2A and adenosine.
The oligoribonucleotide 5′-UACAUGUA-3′ was chosen as the model system for measurement of duplex stability for the following reasons: (i) it was a self-complementary oligonucleotide so it reduced by half the number of model oligoribonucleotides that needed to be synthesized, (ii) the sequence of oligoribonucleotides allowed for the placement of base pairs containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines at internal (both separated and tandem) and terminal positions and (iii) removal of 5′-terminal uridine resulted in a duplex with a modified adenosine as the 3′-terminal unpaired nucleotide.
We were interested in steric and electronic effects of the N6-substituents of adenosine (12). The isopentenyl substituent present in i6A and ms2i6A contains a double bond and two methyl groups at carbon γ. It is known that multi-bonds and steric hindrance affect the thermodynamic stability of RNA duplexes (13–15). To understand the influence of these factors on the thermodynamic stability of RNA duplexes, we studied several analogs of i6A (Fig. 1). In the first group of models we studied the influence of triple, double and single bonds within N6-alkyl substituents, whereas in the second group of oligomers we modulated steric hindrance of N6-alkyl substituents by placing one or more methyl groups on carbons α, β or γ.
The influence of N6-alkyladenosines on the thermodynamic stability of RNA duplexes
The N6-alkyladenosines were placed at internal base pair positions within UR6ACAUGUA (separated) and UAC R6AUGUA (tandem), as well as a terminal base pair position (UACAUGUR6A) and 3′-dangling end (ACAUGUR6A). The influence of the following N6-substitutents of adenosine on thermodynamic stability of RNA duplexes were studied: methyl, neopentyl, 1-methylpropyl, 1-methylbutyl, isopentyl, isopentenyl and propargyl.
The influence of N6-alkyladenosines placed in tandem and separated internal base pair positions on thermodynamic stability of RNA duplexes
The most modified nucleotides in natural RNAs are found in single-stranded regions or at terminal positions within duplexes. Experiments were carried out with duplexes where N6-alkyladenosines were placed in tandem and separated internal base pair positions (Table 1). Free energy values (ΔΔG037) in Table 1 and subsequent tables were calculated for two U-R6A base pairs present in RNA duplex. For U-R6A placed in tandem position analysis of thermodynamic parameters from melt curves and linear correlation Tm–1 versus ln CT indicate that melting of all duplexes, except those containing N6-neopentenyladenosine, undergo a two-state transition. All analyzed N6-substituents of adenosine destabilized the RNA duplexes. The destabilization of N6-alkyladenosines containing duplexes compared with (UACAUGUA)2 was 2.55, 4.87, 4.31, 4.46, 4.25, 3.44 and 3.61 kcal/mol for N6-methyl-, N6-neopentyl-, N6-(1-methylpropyl)-, N6-(1-methylbutyl)-, N6-isopentyl-, N6-isopentenyl- and N6-propargyladenosine, respectively. The results indicate strongly that the destabilization effect results from steric hindrance of N6-substituent of adenosine. This conclusion is particularly clear when comparing the effects of a methyl substituent to more steric substituents such as: neopentyl, 1-methylpropyl, 1-methylbutyl and isopentyl. The free energy difference between (UACAUGUA)2 and duplexes containing m6A was ∼2 kcal/mol. Additional destabilization due to more steric N6-substituents of adenosine amounts to only ∼0.6 kcal/mol, relative to (UACm6AUGUA)2 and (Um6ACAUGUA)2. The two natural modified adenosines, m6A and i6A, were the least destabilizing modifications among those examined.
Table 1. Thermodynamic parameters for helix formation with R6A-U at tandem and separated base pair positionsa,b.
| R = | Tandem internal R6A-U: (UACR6AUGUA)2 | Separated internal R6A-U: (UR6ACAUGUA)2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| –ΔH0 (kcal/mol) | –ΔS0 (eu) | –ΔG037 (kcal/mol) | TMc (°C) | ΔΔG037 (kcal/mol) | –ΔH0 (kcal/mol) | –ΔS0 (eu) | –ΔG037 (kcal/mol) | TMc (°C) | ΔΔG037 (kcal/mol) | |
| Methyl- | 57.2 ± 1.6 | 170.2 ± 5.4 | 4.41 ± 0.07 | 30.3 | 2.55 | 55.7 ± 1.3 | 164.6 ± 4.2 | 4.67 ± 0.04 | 31.5 | 2.25 |
| Neopentyl- | 97.8 ± 0.1d | 322.2 ± 0.4d | 2.09 ± 0.01d | 14.2d | 4.87d | 45.4 ± 3.5 | 133.9 ± 11.5 | 3.86 ± 0.12 | 25.0 | 2.83 |
| 1-Methylpropyl- | 59.4 ± 2.6 | 182.9 ± 8.6 | 2.65 ± 0.09 | 22.0 | 4.31 | 38.6 ± 4.8 | 112.5 ± 15.6 | 3.74 ± 0.19 | 22.2 | 2.95 |
| 1-Methylbutyl- | 58.9 ± 1.7 | 181.8 ± 5.8 | 2.50 ± 0.07 | 21.1 | 4.46 | 43.7 ± 5.0 | 129.6 ± 16.7 | 3.53 ± 0.22 | 22.5 | 3.16 |
| Isopentyl- | 52.1 ± 4.9 | 159.3 ± 16.4 | 2.71 ± 0.19 | 20.3 | 4.25 | 53.1 ± 3.1 | 157.9 ± 10.4 | 4.16 ± 0.15 | 28.4 | 2.53 |
| Isopentenyl- | 48.1 ± 1.4 | 143.7 ± 4.7 | 3.52 ± 0.08 | 23.7 | 3.44 | 40.5 ± 2.1 | 114.8 ± 7.2 | 4.90 ± 0.10 | 31.2 | 2.06 |
| Propargyl- | 47.0 ± 5.1 | 140.8 ± 17.1 | 3.35 ± 0.23 | 22.4 | 3.61 | 45.1 ± 1.6 | 130.7 ± 5.2 | 4.52 ± 0.02 | 29.2 | 2.44 |
| (UACAUGUA)2 | 56.2 ± 1.6 | 158.7 ± 5.2 | 6.96 ± 0.02 | 44.3 | 0 | 56.2 ± 1.6 | 158.7 ± 5.2 | 6.96 ± 0.02 | 44.3 | 0 |
aSolutions are 1 M sodium chloride, 20 mM sodium cacodylate and 0.5 mM Na2EDTA, pH 7.
bThermodynamic parameters were calculated from TM–1 versus log CT plots.
cMelting temperatures were calculated for 10–4 M oligomer concentration.
dNot a two-state transition.
The influence of separated internal U-R6A base pairs is also illustrated in Table 1. In (UR6ACAUGUA)2, U-R6A base pairs were separated by four canonical Watson–Crick base pairs, and observed effects were consequences of the interaction within U-R6A and with adjacent Watson–Crick base pairs only. In this series, melting of all duplexes resulted in two-state transitions, and all N6-substituents of adenosine destabilized the RNA duplexes. The stability difference between the least (N6-methyl) and the most (N6-isopentenyl) destabilizing of RNA duplex was 0.91 kcal/mol, while for duplexes with U-R6A at tandem positions the stability difference was 2.32 kcal/mol. The destabilization of RNA duplexes due to the presence of N6-methyl-, N6-neopentyl-, N6-(1-methylpropyl)-, N6-(1-methylbutyl)-, N6-isopentyl-, N6-isopentenyl- and N6-propargyladenosine was 2.55, 2.83, 2.95, 3.16, 2.53, 2.06 and 2.44 kcal/mol, respectively. For U-R6A placed as separated internal base pairs, methyl placed at position α of N6-substituents was the most destabilizing, and moving a methyl group to carbon β and γ reduced steric hindrance and the destabilization effect. The multi-bond N6-substituents did not affect significantly the stability of RNA duplexes. This is best illustrated by the similar thermodynamic stability of oligoribonucleotides containing N6-isopentyl- and N6-propargyladenosine, where destabilization effects were 2.53 and 2.44 kcal/mol, respectively. m6A and i6A were the least destabilizing RNA duplexes studied, similar to results for tandem internal base pairs.
The influence of N6-alkyladenosines placed in terminal base pair positions on the thermodynamic stability of RNA duplexes
The same set of N6-alkyladenosines was placed within (UACAUGUR6A)2, and their influence on thermodynamic stability was measured; for all of these duplexes, melting was two-state. The data collected in Table 2 demonstrate that N6-alkyladenosines destabilized RNA duplexes relative to unmodified terminal base pairs, and that this effect resulted from steric hindrance due to N6-substituents. The most destabilization was observed for 1-methylbutyl (1.63 kcal/mol). The destabilization was 0.76, 1.02, 1.50 and 0.86 kcal/mol for other oligoribonucleotides containing N6-methyl-, N6-neopentyl-, N6-(1-methylpropyl)- and N6-isopentyladenosine, respectively. The unsaturated substituents N6-isopentenyl- and N6-propargyladenosine destabilized the duplex by 0.94 and 0.98 kcal/mol, respectively. The destabilization is related to the position of the methyl within an N6-substituent in the same pattern as is seen for the separated internal base pairs. The presence of multiple bonds does not affect the thermodynamic stability of RNA duplexes in terminal U-R6A base pairs. Again, naturally occurring N6-alkyladenosines were the least destabilizing to RNA duplexes.
Table 2. Thermodynamic parameters for helix formation with R6A-U at terminal base pair position and R6A as 3′-dangling endsa,b.
| R = | Terminal R6A-U: (UACAUGUR6A)2 | 3′-dangling end R6A: (ACAUGUR6A)2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| –ΔH0 (kcal/mol) | –ΔS0 (eu) | –ΔG037 (kcal/mol) | TMc (°C) | ΔΔG037 (kcal/mol) | –ΔH0 (kcal/mol) | –ΔS0 (eu) | –ΔG037 (kcal/mol) | TMc (°C) | ΔΔG037 (kcal/mol) | |
| Methyl- | 57.5 ± 1.4 | 165.4 ± 4.6 | 6.20 ± 0.01 | 39.9 | 0.76 | 62.0 ± 1.8 | 181.3 ± 5.9 | 5.79 ± 0.02 | 37.6 | –0.09 |
| Neopentyl- | 62.0 ± 2.5 | 180.8 ± 8.2 | 5.94±0.04 | 38.3 | 1.02 | 60.4 ± 2.0 | 177.0 ± 6.7 | 5.50 ± 0.04 | 36.1 | 0.20 |
| 1-Methylpropyl- | 59.3 ± 2.5 | 173.6 ± 8.2 | 5.46 ± 0.05 | 35.9 | 1.50 | 61.3 ± 1.8 | 181.5 ± 5.9 | 4.96 ± 0.04 | 33.4 | 0.74 |
| 1-Methylbutyl- | 59.5 ± 2.0 | 174.7 ± 6.4 | 5.33 ± 0.04 | 35.2 | 1.63 | 63.3 ± 1.8 | 188.7 ± 6.0 | 4.76 ± 0.05 | 32.6 | 0.94 |
| Isopentyl- | 60.9 ± 1.8 | 176.8 ± 5.9 | 6.10 ± 0.02 | 39.2 | 0.86 | 59.0 ± 1.7 | 172.2 ± 5.5 | 5.60 ± 0.03 | 36.6 | 0.10 |
| Isopentenyl- | 53.0 ± 3.0 | 151.3 ± 9.9 | 6.02 ± 0.06 | 39.0 | 0.94 | 52.2 ± 3.1 | 149.6 ± 9.9 | 5.85 ± 0.06 | 38.1 | –0.15 |
| Propargyl- | 62.4 ± 1.6 | 181.8 ± 5.3 | 5.98 ± 0.02 | 38.5 | 0.98 | 65.5 ± 1.9 | 193.3 ± 6.2 | 5.52 ± 0.03 | 36.3 | 0.18 |
| (UACAUGUA)2 | 56.2 ± 1.6 | 158.7 ± 5.2 | 6.96 ± 0.02 | 44.3 | 0 | |||||
| (ACAUGUA)2 | 50.9 ± 1.0 | 145.8 ± 3.1 | 5.70 ± 0.01 | 37.1 | 0 | |||||
aSolutions are 1 M sodium chloride, 20 mM sodium cacodylate and 0.5 mM Na2EDTA, pH 7.
bThermodynamic parameters were calculated from TM–1 versus log CT plots.
cMelting temperatures were calculated for 10–14 M oligomer concentration.
The influence of N6-alkyladenosines placed as 3′-unpaired terminal nucleotides on the thermodynamic stability of RNA duplexes
We were particularly interested in the thermodynamic effect of N6-alkyladenosines placed as 3′-unpaired nucleotides (16–18). In particular, we hoped to test Grosjean’s hypothesis, which states that modified purine nucleotides placed at the 3′-dangling end of a mini-helix formed by codon–anticodon interactions modulate the stability of this interaction in a sequence-dependent fashion (19).
The duplex (ACAUGUR6A)2 was used to study the effect of N6-alkyladenosines placed as 3′-unpaired nucleotides, and data concerning thermodynamic stability are collected in Table 2. The thermodynamic parameters demonstrated two-state melting for all duplexes studied. The analyses indicate that both naturally occurring nucleotides (m6A and i6A) stabilized RNA duplexes, whereas the remaining N6-alkyladenosines destabilized RNA duplexes. The free energy of (ACAUGUR6A)2, relative to (ACAUGUA)2, changed by –0.09, 0.20, 0.74, 0.94, 0.11, –0.15 and 0.18 kcal/mol for N6-methyl-, N6-neopentyl-, N6-(1-methylpropyl)-, N6-(1-methylbutyl)-, N6-isopentyl-, N6-isopentenyl- and N6-propargyladenosine, respectively. The adenosine derivatives carrying the most sterically hindering N6-substituents (1-methylpropyl and 1-methylbutyl) destabilized duplexes to the greatest extent. Modified adenosines at 3′-dangling ends destabilized RNA duplexes less relative to other positions within duplexes. Additionally, it is interesting that the two natural substituents, N6-methyl- and N6-isopentyl-, stabilized duplexes. The effect of the multi-bond N6-substituents of adenosine on thermodynamic stability of RNA duplexes was negligible.
Comparison of the influence of N6-alkyladenosines at various positions on the thermodynamic stability of RNA duplexes
Analyses of the thermodynamic stability of RNA duplexes containing N6-alkyladenosines clearly indicate that steric hindrance from N6-substituents is mostly responsible for destabilization of RNA duplexes. In particular, a large destabilization was observed between unmodified duplex and those containing m6A, whereas increasingly bulky adenosine substituents increase destabilization to a smaller extent. In contrast, the comparison of single, double and triple bond substituents revealed that the contribution of electronic components is less important. This may be due to the distance of the multiple bonds from the purine ring.
Collected data lead to the following conclusions: (i) duplex destabilization is correlated with steric hindrance of N6-alkyl substituent. This is particularly clear upon comparison of differences in destabilization between the least (N6-methyl) and the most (N6-neopentyl) destabilizing substituents. For these substituents, the difference of free energy difference between tandem internal and terminal positions of U-R6A is 1.79 and 3.85 kcal/mol, respectively. (ii) The positions of N6-alkyladenosines within the duplex have a large influence on thermal stability of RNA duplexes. Placing U-R6A base pairs closer to the center of a duplex (or closer to one another) destabilizes duplexes to a greater extent for all N6-substituents. (iii) The destabilization effects are not linearly dependent upon changing of the U-R6A position within the duplex. Moving U-R6A base pair from tandem to separated internal positions (i.e. 2 bp from the center) results in a change in free energy difference by (on average) 1.3 kcal/mol, whereas moving from separated internal to terminal positions (i.e. by 1 bp) changes free energy difference by (on average) 1.5 kcal/mol. (iv) The naturally occurring modified nucleosides, m6A and i6A, are the least destabilizing substituents for any position of U-R6A base pairs within the RNA duplex. Moreover, these two modified adenosines are the only derivatives that stabilize RNA duplexes when placed as 3′-dangling ends.
Similar behavior, with lower destabilization at the end of the RNA duplexes relative to the center, has been observed for single mismatches (20) and unsymmetrical internal loops (21). Presumably, the base pairs at the ends of duplex are less tight and N6-substituent can adopt more optimal orientation within the duplex.
The influence of 2-methylthio-N6-alkyladenosines on thermodynamic stability of RNA duplexes
The second group of purine nucleotide modifications studied was derivatives of 2-methylthio-N6-alkyladenosines. The U-ms2R6A base pairs (where ms2R6A means 2-methylthio-N6-alkyladenosine) were placed at the same position within RNA duplexes as discussed earlier. For these studies, ms2i6A and ms2m6A naturally occurring in tRNA as well as unnatural ms2A were selected to evaluate the influence of 2-methylthio groups alone and synergistically with N6-alkyl substituents.
The influence of 2-methylthio-N6-alkyladenosines placed at internal and terminal base pair positions as well as 3′-dangling ends on the thermodynamic stability of RNA duplexes
The measurement of thermodynamic stability of (UACms2R6AUGUA)2 demonstrated non-two-state melting character. The difference between thermodynamic parameters calculated from the curve fit and Tm–1 versus log CT plots were >15%, and for this reason they are not discussed in detail herein. The U-ms2R6A substituent in tandem positions destabilized duplexes, and this destabilization is greater than for U-R6A. However, when the same 2-methylthio-N6-alkyladenosines were placed into separated internal base pair positions, terminal base pairs, or as 3′-dangling ends the two-state melting transition was observed. The data collected in Table 3 for separated internal positions demonstrated that ms2i6A and ms2m6A destabilized duplexes by 1.48 and 1.98 kcal/mol, respectively. At the same time, ms2A destabilized the same core duplex by 2.81 kcal/mol. It was surprising to find that the 2-methylthio substituent alone destabilized duplexes more than when it was associated with the N6-isopentenyl and N6-methyl substituents.
Table 3. Thermodynamic parameters for helix formation with 2-methylthio-N6-alkyladenosine–uridine base pairs at various positions within oligomers and 2-methylthio-N6-alkyladenosine as 3’-dangling enda,b.
| –ΔH0 (kcal/mol) | –ΔS0 (eu) | –ΔG037 (kcal/mol) | TMc (°C) | ΔΔG037d (kcal/mol) | |
|---|---|---|---|---|---|
| UACXUGUA | |||||
| AUGUXCAU | |||||
| X = ms2i6Ae | 101.4 ± 14.9 | 328.2 ± 50.4 | 0.44 ± 0.80 | 19.3 | 6.52 |
| X = ms2m6Ae | 117.0 ± 20.3 | 379.5 ± 68.5 | 0.69 ± 0.98 | 21.0 | 6.27 |
| X = ms2Ae | 65.2 ± 3.4 | 200.5 ± 11.2 | 3.03 ± 0.10 | 24.9 | 3.93 |
| UXCAUGUA | |||||
| AUGUACXU | |||||
| X = ms2i6A | 50.6 ± 8.5 | 147.0 ± 27.2 | 4.98 ± 0.09 | 33.1 | 1.98 |
| X = ms2m6A | 46.8 ± 3.5 | 133.3 ± 11.1 | 5.48 ± 0.10 | 35.7 | 1.48 |
| X = ms2A | 53.2 ± 1.3 | 158.2 ± 4.1 | 4.15 ± 0.02 | 28.4 | 2.81 |
| UACAUGUX | |||||
| XUGUACAU | |||||
| X = ms2i6A | 61.6 ± 6.9 | 177.5 ± 22.3 | 6.52 ± 0.07 | 41.8 | 0.44 |
| X = ms2m6A | 62.8 ± 1.8 | 179.8 ± 5.8 | 7.01 ± 0.02 | 43.7 | –0.05 |
| X = ms2A | 62.5 ± 1.4 | 179.5 ± 4.6 | 6.86 ± 0.01 | 43.0 | 0.1 |
| X = A | 56.2 ± 1.6 | 158.7 ± 5.2 | 6.96 ± 0.02 | 44.3 | 0 |
| ACAUGUX | |||||
| XUGUACA | |||||
| X = ms2i6A | 62.0 ± 1.9 | 180.1 ± 6.2 | 6.14 ± 0.02 | 39.4 | –0.44 |
| X = ms2m6A | 65.0 ± 2.8 | 188.0 ± 8.8 | 6.73 ± 0.03 | 42.1 | –1.03 |
| X = ms2A | 57.6 ± 2.1 | 164.3 ± 6.8 | 6.66 ± 0.02 | 42.4 | –0.96 |
| X = A | 50.9 ± 1.0 | 145.8 ± 3.1 | 5.70 ± 0.01 | 37.1 | 0 |
aSolutions are 1 M sodium chloride, 20 mM sodium cacodylate and 0.5 mM Na2EDTA, pH 7.
bThermodynamic parameters were calculated from TM–1 versus log CT plots.
cMelting temperatures were calculated for 10–4 M oligomer concentration.
dCalculated relative to the stability of an oligomer containing adenosine.
eNot a two-state transition.
For terminal positions of the U-ms2R6A base pair, within (UACAUGUms2R6A)2, the effect of ms2m6A and ms2A was negligible, whereas ms2i6A destabilized the RNA duplex (Table 3). The free energy difference for ms2i6A, ms2m6A and ms2A was 0.44, –0.05 and 0.10 kcal/mol, respectively.
It is interesting to compare the free energy difference of RNA duplexes resulting from the presence of N6-alkyl- and 2-methylthio-N6-alkyladenosines. The derivatives carrying both substituents stabilized duplexes by 0.50 and 0.81 kcal/mol for ms2i6A and ms2m6A compared with i6A and m6A, respectively.
Data in Table 3 demonstrated that 2-methylthio-N6-alkyladenosines and ms2A placed at the 3′-dangling end of duplexes enhanced the thermodynamic stability of RNA duplexes. The free energy difference, relative to (ACA UGUA)2, was –0.44, –1.03 and –0.96 kcal/mol for RNA duplexes containing ms2i6A, ms2m6A and ms2A, respectively. The presence of both 2-methylthio and N6-alkyl groups stabilized ms2i6A by 0.29 kcal/mol compared with the duplex containing only i6A. Similarly, ms2m6A stabilized duplex by 0.94 kcal/mol relative to m6A.
Comparison of the influence of 2-methylthio-N6-alkyladenosines at various positions on the thermodynamic stability of RNA duplexes
The studies of thermodynamic stability of RNA duplexes carrying 2-methylthio-N6-alkyladenosines and ms2A at various positions lead to several general conclusions. At tandem internal base pair positions, modified nucleotides destabilized RNA duplexes so much that melts performed lacked two-state character. This departure is proportional to the size of N6-substituent. The lack of the two-state melting of RNA duplexes is pretty often reported due to various reasons including the presence of nucleobase substituents (14,22,23). When the base pair U-ms2R6A is moved towards the end of the duplex the destabilization is significantly reduced, whereas at a 3′-dangling end position 2-methylthio-N6-alkyladenosines stabilized RNA duplexes. The thermodynamic effect of 2-methylthio-N6-alkyladenosines in separated internal and terminal positions is significantly lower than the sum of 2-methylthio and N6-alkyl substituent effects separately. For example, at separated internal base pair positions ms2A and i6A destabilized duplexes by 2.84 and 2.06 kcal/mol, respectively. The measured destabilization effect of ms2i6A was 1.92 kcal/mol, which was 2.92 kcal/mol less than calculated from the sum of the destabilization effects of 2-methylthio and N6-isopentenyl substituents separately. The same trend can be observed for ms2m6A.
Based on the thermodynamic stability of oligoribonucleotides carrying 2-methylthio-N6-alkyladenosine, we can conclude that 2-methylthio substituents affect RNA duplex thermodynamic stability in two different ways. First, the presence of a 2-methylthio group interferes with the pairing to complementary uridine, likely because of a steric clash and weakened hydrogen bond interactions. Secondly, 2-methylthio groups enhance the stacking interactions with adjacent base pairs and stabilize the duplexes. As a consequence, the measured influence of 2-methylthio group within 2-methylthio-N6-alkyladenosine results from both effects and it is very dependent on the position of U-ms2R6A within RNA duplexes. Moving U-ms2R6A base pairs to the end of RNA duplexes results in weaker steric interactions and stabilizing effects are more pronounced.
The thermodynamic stability of hairpins containing N6-alkyladenosines or 2-methylthio-N6-alkyladenosines
At position 37 of the tRNA anticodon arm there are naturally occurring modified purine nucleotides including N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. For our studies, we selected the anticodon hairpin of tRNATrp from E.coli (Fig. 1) (24). This hairpin contains ms2i6A at position 37 and 2′-O-methylcytidine (Cm) at position 32. In other tRNAs i6A, m6A and ms2m6A are found at position 37 (2). Besides four hairpins with the modifications listed above at position 37, hairpins containing ms2A and adenosine were studied to evaluate the influence of substituents at positions 2 and/or 6 on the thermodynamic stability of anticodon hairpins.
The thermodynamic stability of anticodon hairpins in the presence of 1 M sodium chloride
The data concerning the thermodynamic stability of modified hairpins are collected in Table 4 and demonstrate that melting temperature is concentration independent. The melts were performed in standard melting buffer containing 1 M sodium chloride, 20 mM sodium cacodylate and 0.5 mM Na2EDTA, pH 7. The analysis of data indicated that the thermodynamic stability of hairpins containing ms2i6A, i6A, m6A, ms2m6A, ms2A and adenosine was very similar. The difference of free energy between the least stable hairpin (ms2i6A) and the most stable hairpin (m6A) was 1.03 kcal/mol. Hairpins containing N6-alkyladenosines were more stable than those carrying 2-methylthio-N6-alkyladenosines. Hairpins containing ms2i6A and ms2m6A were 0.63 kcal/mol less stable than their corresponding N6-alkyladenosines. Moreover, comparison of effects for ms2A and N6-alkyladenosine substitutions with the influence of 2-methylthio-N6-alkyladenosines indicate that the last derivative destabilized hairpins by ∼0.8 kcal/mol more than that calculated from the sum of both derivatives separately.
Table 4. Thermodynamic parameters for the formation of hairpins having the sequence CCGGUCmUCCAXAACCGG.
| RNA hairpins | 1 M sodium chloride, 20 mM sodium cacodylate, 0.5 mM Na2EDTA, pH 7 | 10 mM magnesium chloride, 150 mM potassium chloride, 20 mM sodium cacodylate, 0.5 mM Na2EDTA, pH 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| –ΔH0 (kcal/mol) | –ΔS0 (eu) | –ΔG037 (kcal/mol) | TM (°C) | ΔΔG037 (kcal/mol) | –ΔH0 (kcal/mol) | –ΔS0 (eu) | –ΔG037 (kcal/mol) | TM (°C) | ΔΔG037 (kcal/mol) | |
| X = ms2i6A | 45.9 ± 4.2 (62.4 ± 4.6) | 130.9 ± 11.9 (185.3 ± 9.9) | 5.35 ± 0.54 (4.97 ± 0.36) | 77.9 (63.8) | 0.36 (–0.21) | 71.3 ± 3.1 | 202.6 ± 7.9 | 8.46 ± 0.62 | 78.8 | –3.41 |
| X = i6A | 51.6 ± 7.2 (46.9 ± 1.0) | 146.9 ± 21.2 (137.5 ± 2.7) | 5.98 ± 0.61 (4.26 ± 0.2) | 77.7 (68.0) | –0.27 (0.50) | 60.5 ± 8.0 | 170.7 ± 23.6 | 7.54 ± 0.71 | 81.2 | –2.49 |
| X = ms2m6A | 51.2 ± 3.4 (56.9 ± 1.8) | 146.4 ± 9.9 (166.5 ± 5.0) | 5.75 ± 0.40 (5.24 ± 0.30) | 76.3 (68.5) | –0.04 (–0.48) | 59.7 ± 6.6 | 168.9 ± 20.2 | 7.29 ± 0.50 | 80.2 | –2.24 |
| X = m6A | 58.0 ± 6.3 (61.8 ± 0.3) | 166.5 ± 17.9 (181.7 ± 2.0) | 6.38 ± 0.79 (5.48 ± 0.28) | 75.3 (67.2) | –0.67 (–0.72) | 53.3 ± 3.5 | 152.4 ± 12.6 | 6.01 ± 0.42 | 76.4 | –0.96 |
| X = ms2A | 52.9 ± 3.0 (56.5 ± 3.7) | 151.7 ± 8.8 (165.9 ± 9.9) | 5.87 ± 0.28 (5.07 ± 0.12) | 75.7 (67.5) | –0.16 (–0.31) | 56.4 ± 7.0 | 160.9 ± 20.6 | 6.49 ± 0.84 | 77.4 | –1.44 |
| X = A | 52.0 ± 4.9 (51.8 ± 5.5) | 149.4 ± 14.4 (151.7 ± 9.7) | 5.71 ± 0.47 (4.76 ± 0.64) | 75.2 (68.4) | 0 (0) | 43.9 ± 14.5 | 125.1 ± 42.1 | 5.05 ± 1.45 | 77.3 | 0 |
The thermodynamic parameters in parenthesis concern the melting in 100 mM sodium chloride, 20 mM sodium cacodylate, 0.5 mM Na2EDTA, pH 7.0.
Our results are difficult to compare with others, since the data in literature are very limited. It was reported that the presence of N6-threonylcarbamoyladenosine at tRNA position 37 destabilized these hairpins by 0.7–0.9 kcal/mol (25), whereas 1-methylguanosine destabilized by 1.6 kcal/mol (26). Recently, Nikonowicz and co-workers reported that presence of i6A in the anticodon stem–loop of E.coli tRNAPhe reduced the melting temperature by 3°C relative to a hairpin containing adenosine at that position (27).
The thermodynamic stability of anticodon hairpins in the presence of magnesium chloride
Beside the standard solution conditions, buffer containing 1 M sodium chloride, we measured thermodynamic stability of hairpins in physiological buffer. There are several physiological buffers with different amounts of salts, particularly potassium and magnesium. For our studies, we used buffer containing 150 mM potassium chloride, 10 mM magnesium chloride, 20 mM sodium cacodylate and 0.5 mM Na2EDTA, pH 7. The concentration of potassium and magnesium salts in this buffer was similar to that present in intercellular fluid (28). Since magnesium cations present in buffer resulted in the partial cleavage of RNA during melting of RNA hairpins, thermodynamic parameters were calculated for three different concentrations, whereas RNA hairpins in 1 M sodium chloride were melted in nine different concentrations (29).
The measurements of thermodynamic stability of RNA hairpins are collected in Table 4. Analyses of the data demonstrate that in physiological buffer thermodynamic stability of the hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines was very dependent on modification. The hairpin containing ms2i6A, the same as in the anticodon arm of tRNATrp from E.coli, was the most stable. The remaining five hairpins containing unnatural modified adenosines or adenosine were less stable by up to 3.41 kcal/mol. The thermodynamic stability (ΔG037) of the hairpins changes in the following order: ms2i6A (–8.46 kcal/mol), i6A (–7.54 kcal/mol), ms2m6A (–7.29 kcal/mol), ms2A (–6.49 kcal/mol), m6A (–6.01 kcal/mol) and adenosine (–5.05 kcal/mol). These data clearly demonstrate that increasingly modified adenosine at position 37 resulted in more thermodynamically stable RNA hairpins. The presence of a 2-methylthio group in nucleotides additionally increased stability of RNA hairpins relative to hairpins containing only N6-alkyladenosine or adenosine. This stability difference was 1.48 kcal/mol between hairpins containing ms2i6A and i6A, 1.28 kcal/mol between hairpins containing ms2m6A and m6A and finally 0.92 kcal/mol between hairpins containing ms2A and adenosine.
The comparison of thermodynamic stability of RNA hairpins in 1 M sodium chloride and physiological buffer indicates that the melting temperatures (TM) are very similar, whereas more favorable free energy in physiological conditions is related to the changes of the enthalpy and entropy of RNA hairpins.
To better evaluate the influence of magnesium cations on the thermodynamic stability of RNA hairpins, the stability of one hairpin was measured at various magnesium concentrations. For these studies, the natural hairpin of tRNATrp from E.coli containing ms2i6A at position 37 was selected. The thermodynamic stability of the hairpin was measured in physiological buffer containing 0, 1, 3, 5, 10 and 30 mM magnesium chloride. The results were collected in Table 5. The stability of the RNA hairpin increased non-linearly with the amount of magnesium present in the buffer. The free energy increased by 1.01, 0.92, 1.27, 3.46 and 3.40 kcal/mol for measurements in buffer containing 1, 3, 5, 10 and 30 mM magnesium chloride, respectively. The increase in hairpin stability was related to stability in physiological buffer without magnesium chloride. Interestingly, particularly large increases of the hairpin stability were observed between measurements in buffers containing 0–1 mM (1.01 kcal/mol) and 5–10 mM (2.19 kcal/mol) magnesium chloride. The increase of hairpin stability from 1–5 mM magnesium chloride was only 0.26 kcal/mol, whereas above 10 mM magnesium chloride the hairpin stability was very similar.
Table 5. Thermodynamic parameters for the formation of hairpins having the sequence CCGGUCmUCCAXAACCGG at various concentration of MgCl2a.
| X = ms2i6A | –ΔH0 (kcal/mol) | –ΔS0 (eu) | –ΔG037 (kcal/mol) | TM (°C) | ΔΔG037 (kcal/mol) |
|---|---|---|---|---|---|
| 150 mM KCl | 59.3 ± 7.4 | 175.0 ± 22.5 | 5.00 ± 0.47 | 67.6 | 0 |
| 1 mM MgCl2, 150 mM KCl | 69.9 ± 6.5 | 205.8 ± 19.8 | 6.01 ± 0.30 | 67.5 | –1.01 |
| 3 mM MgCl2, 150 mM KCl | 57.7 ± 5.1 | 166.8 ± 15.4 | 5.92 ± 0.28 | 75.3 | –0.92 |
| 5 mM MgCl2, 150 mM KCl | 61.1 ± 21.4 | 176.7 ± 59.5 | 6.27 ± 2.91 | 74.9 | –1.27 |
| 10 mM MgCl2, 150 mM KCl | 71.3 ± 3.1 | 202.6 ± 7.9 | 8.46 ± 0.62 | 78.8 | –3.46 |
| 30 mM MgCl2, 150 mM KCl | 72.6 ± 14.4 | 207.0 ± 42.0 | 8.40 ± 1.38 | 79.5 | –3.40 |
aBesides magnesium chloride and potassium chloride, the buffer contained 20 mM sodium cacodylate, 0.5 mM Na2EDTA, pH 7.
The measurements of thermodynamic stability of the hairpins in buffer containing 100 mM sodium chloride, 20 mM sodium cacodylate and 0.5 mM Na2EDTA, pH 7, additionally confirmed that the enhancement of free energy is related to the presence of magnesium cations in physiological buffer (Table 4).
Moreover, when RNA hairpins were melted at various magnesium chloride concentrations, an additional low temperature transition was observed. This transition was concentration dependent, suggesting that it resulted from the interaction of two molecules. The thermodynamic stability of this two-molecule structure was reduced with increased complexity of adenosine modification present at position 37. At this moment, we cannot unambiguously determine the structure of this molecule. It could be due to kissing hairpins (30) or a duplex containing an internal loop formed by two self-complementary oligoribonucleotides (31).
CONCLUSIONS
The collected data indicate that modified adenosines, including the natural derivatives i6A, m6A, ms2i6A and ms2m6A reduced the thermodynamic stability of RNA duplexes. This destabilization is likely related to steric hindrance of the N6-substituent of N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. The influence of modified adenosines placed as 3′-terminal unpaired nucleotides was more complex. Most N6-alkyladenosines destabilized duplexes, relative to the unmodified adenosine RNA duplexes; i6A and m6A did not significantly affect duplex stability. At the same position ms2i6A, ms2m6A and ms2A stabilized duplexes by 0.5–1.0 kcal/mol.
The N6-alkyladenosines and 2-methylthio-N6-alkyladenosines were found only in tRNA at single strand regions (1,2). One reason could be that these modifications destabilize RNA duplexes, mostly due to steric hindrance of N6-substituents and weakness of hydrogen bonding. However, it was observed that among all studied N6-alkyladenosines, the naturally modified derivatives i6A and m6A destabilized RNA duplexes the least at any position within the helix. The fact that natural N6-alkyladenosines and 2-methylthio-N6-alkyladenosines placed at 3′-dangling ends stabilized duplexes could mean that the stabilization effect of modified adenosines is related primarily to stacking interactions. Similar interactions were observed by Davis and co-workers (32) and Agris and co-workers (33) based on NMR studies.
We believe that the results presented herein will help to understand the process of protein biosynthesis, particularly when recent studies suggest that this process proceeds due to the catalytic activity of RNA present in ribosome (34).
Acknowledgments
ACKNOWLEDGEMENTS
The Polish State Committee for Scientific Research (KBN) supported this work, Grant No. 3 T09A 140 19 to R.K. and NIH grant 1R03 TW1068 to R.K. and D. H. Turner. We would like to thank David J. Proctor (The Pennsylvania State University) for comments and suggestions on this manuscript.
REFERENCES
- 1.Limbach P.A., Crain,P.F. and McClosky,J.A. (1994) Summary: the modified nucleosides of RNA. Nucleic Acids Res., 22, 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjork G.R. (1995) tRNA: structure, biosynthesis and function. In Soll,D. and RajBhandary,U. (eds), Biosynthesis and Function of Modified Nucleosides. ASM Press, Washington DC, pp. 165–206. [Google Scholar]
- 3.Agris P.F. (1996) The importance of being modified: role of modified nucleosides and Mg+2 in RNA structure and function. Prog. Nucleic Acid Res. Mol. Biol., 53, 79–129. [DOI] [PubMed] [Google Scholar]
- 4.Grosjean H., Houssier,C., Romby,P. and Marquet,R. (1998) Modulatory role of modified nucleotides in RNA loop-loop interaction. In Grosjean,H. and Benne,R. (eds.), Modification and Editing of RNA. ASM Press, Washington DC, pp. 113–133. [Google Scholar]
- 5.Persson B.C. (1993) Modification of tRNA as a regulatory device. Mol. Microbiol., 8, 1011–1016. [DOI] [PubMed] [Google Scholar]
- 6.Sundaram M., Durant,P.C. and Davis,D.R. (2001) Hypermodified nucleosides in anticodon of tRNALys stabilize a canonical U-turn structure. Biochemistry, 39, 12575–12584. [DOI] [PubMed] [Google Scholar]
- 7.McBride L.J. and Caruthers,M.H. (1983) An investigation of several deoxynucleosides phosphoramidites useful for synthesizing oligodeoxynucleotides. Tetrahedron Lett., 24, 245–249. [Google Scholar]
- 8.Kierzek E. and Kierzek,R. (2003) The synthesis of oligoribonucleotides containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines via post-synthetic modifications of precursor oligomers. Nucleic Acids Res., 31, 4461–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freier S.M., Kierzek,R., Jaeger,J.A., Sugimoto,N., Caruthers,M.H., Neilson,T. and Turner,D.H. (1986) Improved free-energy parameters for predictions of RNA duplex stability. Proc. Natl Acad. Sci. USA, 83, 9373–9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borer P.N. (1975) Nucleic acids. In Fasman,G.D. (ed.), Handbook of Biochemistry and Molecular Biology. CRC Press, Cleveland, Vol. I, pp. 597. [Google Scholar]
- 11.Richards E.G. (1975) Nucleic acids. In Fasman,G.D. (ed.), Handbook of Biochemistry and Molecular Biology. CRC Press, Cleveland, Vol. I, pp. 597. [Google Scholar]
- 12.Kierzek E. and Kierzek,R. (2001) Influence of N6-isopentenyladenosine (i6A) on thermal stability of RNA duplexes. Biophys. Chem., 91, 135–140. [DOI] [PubMed] [Google Scholar]
- 13.Barnes T.W. and Turner,D.H. (2001) C5-(1-propynyl)-2′-deoxy-pyrimidines enhance mismatch penalties of DNA:RNA duplex formation. Biochemistry, 40, 12738–12745. [DOI] [PubMed] [Google Scholar]
- 14.Ziomek K., Kierzek,E., Biala,E. and Kierzek.R. (2002) The thermal stability of RNA duplexes containing modified base pairs placed at internal and terminal positions of the oligoribonucleotides. Biophys. Chem., 97, 233–241. [DOI] [PubMed] [Google Scholar]
- 15.Ziomek K., Kierzek,E., Biala,E. and Kierzek.R. (2002) The influence of various modified nucleotides placed as 3′-dangling end on thermal stability of RNA duplexes. Biophys. Chem., 97, 243–249. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto N., Kierzek,R. and Turner,D.H. (1987) Sequence dependence for the energetics of dangling ends and terminal base pairs in ribonucleic acid. Biochemistry, 26, 4554–4559. [DOI] [PubMed] [Google Scholar]
- 17.Burkard M.E., Kierzek,R. and Turner,D.H. (1999) Thermodynamics of unpaired terminal nucleotides on short RNA helixes correlates with stacking at helix termini in larger RNAs. J. Mol. Biol., 290, 967–982. [DOI] [PubMed] [Google Scholar]
- 18.Kierzek E., Biala,E. and Kierzek.R. (2001) Elements of thermodynamics in RNA evolution. Acta Biochim. Polonica, 48, 485–493. [PubMed] [Google Scholar]
- 19.Weissenbach J. and Grosjean,H. (1981) Effect of threonylcarbamoyl modification (t6A) in yeast tRNA Arg III on codon-anticodon and anticodon-anticodon interactions. A thermodynamic and kinetic evolution. Eur. J. Biochem., 116, 207–216. [DOI] [PubMed] [Google Scholar]
- 20.Kierzek R. Burkard,M.E. and Turner,D.H. (1999) The thermodynamics of single mismatches in RNA duplexes. Biochemistry, 38, 14214–14223. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder S.J. and Turner,D.H. (2000) Factors affecting the thermodynamic stability of small asymetric internal loops in RNA. Biochemistry, 39, 9257–9274. [DOI] [PubMed] [Google Scholar]
- 22.Xia T., SantaLucia,J., Burkard,M.E., Kierzek,R., Schroeder,S.J., Cox,C. and Turner.D.H. (1998) Parameters for expanded nearest-neighbor model for formation of RNA duplexes with Watson–Crick base pair. Biochemistry, 37, 14719–14735. [DOI] [PubMed] [Google Scholar]
- 23.Freier S.M., Kierzek,R., Jaeger,J.A., Sugimoto,N., Caruthers,M.H., Neilson,T. and Turner,D.H., (1986) Improved free-energy parameters for predictions of RNA duplex stability. Proc. Natl Acad. Sci. USA, 83, 9373–9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberg S.P., Soll,L. and Yarus,M. (1979) The purification and sequence of a temperature-sensitive tryptophan tRNA. J. Biol. Chem., 254, 5562–5566. [PubMed] [Google Scholar]
- 25.Yarian C., Marszalek,M., Sochacka,E., Malkiewicz,A., Guenther,R., Miskiewicz,A. and Agris,P.F. (2000) Modified nucleoside dependent Watson–Crick and wobble codon binding by tRNALysuuu species. Biochemistry, 39, 13390–13395. [DOI] [PubMed] [Google Scholar]
- 26.Agris P.F., Guenther,R., Sochacka,E., Newman,W., Czerwinska.G., Liu,G., Ye,W. and Malkiewicz,A. (1999) Thermodynamic contribution of nucleoside modifications to yeast tRNAPhe anticodon stem loop analogs. Acta Biochim. Polonica, 46, 163–172. [PubMed] [Google Scholar]
- 27.Cabello-Villagas J., Winkler,M.E. and Nikonowicz,E.P. (2002) Solution conformations of unmodified and A37N6-dimethylallyl modified anticodon stem–loop of Escherichia coli tRNAPhe. J. Mol. Biol., 319, 1015–1034. [DOI] [PubMed] [Google Scholar]
- 28.Feig A.L. and Uhlenbeck,O. (1999) The role metal ions in RNA biochemistry. In Gesteland,R.F., Cech,T.R. and Atkins,J.F. (eds), RNA World. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 287–319. [Google Scholar]
- 29.Wintermeyer W. and Zachau,G. (1973) Mg+2 katalysierte spezifische spaltung von tRNA. Biophys. Biochem. Acta, 299, 82–90. [PubMed] [Google Scholar]
- 30.Chang K.-Y. and Tinoco,I.,Jr (1994) Characterization of a ‘kissing’ hairpins complex derived from the human immunodeficiency virus genome. Proc. Natl Acad. Sci. USA, 91, 8705–8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proctor D.J., Schaak,J.E., Bevilacqua,J.M., Falzone,C.J. and Bevilacqua,P.C. (2002) Isolation and characterization of a family of stable RNA tetraloops with the motif YNMG that participate in tertiary interactions. Biochemistry, 41, 12062–12075. [DOI] [PubMed] [Google Scholar]
- 32.Sundaram M., Durant,P.C. and Davis,D.R. (2000) Hypermodified nucleosides in the anticodon of tRNALys stabilize a canonical U-turn structure. Biochemistry, 39, 12575–12584. [DOI] [PubMed] [Google Scholar]
- 33.Stuart J.W., Gdaniec,Z., Guenther,R., Marszalek,M., Sochacka,E., Malkiewicz,A. and Agris,P.F. (2000) Functional anticodon architecture of human tRNALys3 includes disruption of intraloop hydrogen bonding by the naturally occurring amino acid modification, t6A. Biochemistry, 39, 13396–13404. [DOI] [PubMed] [Google Scholar]
- 34.Nissen P., Ban,N., Hansen,J., Moore,P.B. and Steitz,T.A. (2000) A structural basis of ribosome activity in peptide bond synthesis. Science, 289, 920–930. [DOI] [PubMed] [Google Scholar]