Abstract
To replicate, HIV-1 capitalizes on endogenous cellular activation pathways resulting in recruitment of key host transcription factors to its viral enhancer. RNA interference has been a powerful tool for blocking key checkpoints in HIV-1 entry into cells. Here we apply RNA interference to HIV-1 transcription in primary macrophages, a major reservoir of the virus, and specifically target the transcription factor NFAT5 (nuclear factor of activated T cells 5), which is the most evolutionarily divergent NFAT protein. By molecularly cloning and sequencing isolates from multiple viral subtypes, and performing DNase I footprinting, electrophoretic mobility shift, and promoter mutagenesis transfection assays, we demonstrate that NFAT5 functionally interacts with a specific enhancer binding site conserved in HIV-1, HIV-2, and multiple simian immunodeficiency viruses. Using small interfering RNA to ablate expression of endogenous NFAT5 protein, we show that the replication of three major HIV-1 viral subtypes (B, C, and E) is dependent upon NFAT5 in human primary differentiated macrophages. Our results define a novel host factor–viral enhancer interaction that reveals a new regulatory role for NFAT5 and defines a functional DNA motif conserved across HIV-1 subtypes and representative simian immunodeficiency viruses. Inhibition of the NFAT5–LTR interaction may thus present a novel therapeutic target to suppress HIV-1 replication and progression of AIDS.
Synopsis
To replicate, viruses exploit the cellular machinery of infected cells in their hosts. Macrophages, blood cells involved in the body's defense against pathogens, are resistant to the toxic effects of certain viruses, such as HIV-1, the AIDS virus. The drawback, however, is that macrophages become an avenue through which viruses can spread to other tissues and organs of the body. Here, Ranjbar, Tsytsykova, and colleagues have identified a new role for a human protein, nuclear factor of activated T cells 5 (NFAT5), as a host factor important for replication of HIV-1 in macrophages. The binding site for NFAT5 on a region of the HIV-1 DNA critical for its replication, the long terminal repeat, is the same across multiple subtypes of HIV-1 and the related virus HIV-2, and even the simian immunodeficiency virus; thus, disrupting the interaction between NFAT5 and the long terminal repeat provides a potential means of inhibiting replication of multiple versions of HIV.
Introduction
The monocyte/macrophage lineage and dendritic cells are the first cells to be infected by HIV-1 [1–3] (reviewed in [4–7]). Monocytes/macrophages are relatively resistant to the cytopathic effects of HIV-1 and thus can serve as a reservoir for persistent HIV-1 infection [3] (reviewed in [7–10]). Furthermore, based on experiments with HIV-1 subtype B, the first HIV-1 subtype identified [11], it has been suggested that signal transduction pathways and transcription factors acting to modulate HIV-1 gene expression through the HIV-1 long terminal repeat (LTR) enhancer region are distinct in the monocyte/macrophage lineage and dendritic cells as compared with T cells [7,12].
A critical step in the replication of HIV-1 is the transcription of viral genes following integration of viral DNA into the genome of the host cell (reviewed in [13]). However, the majority of HIV-1 gene transcription studies have been performed using isolates of subtype B, although multiple HIV-1 subtypes have a major role in the global HIV-1 pandemic, and each subtype has a distinct sequence of transcription factor binding sites in its LTR [14–18]. Differences in the number and arrangement of these activator binding sites influence subtype-specific expression of the virus in vitro [14,19–21]. The ubiquitous transcription factor nuclear factor κB (NF-κB) has long been implicated in HIV-1 viral gene transcription in multiple cell types [22,23] (reviewed in [12,24,25]). Although the NF-κB motifs within the core κB enhancer/basal promoter of the HIV-1 subtype B LTR are canonical binding sites for the NF-κB p50/p65 heterodimer [22], they are also recognized by the calcineurin-dependent proteins of the nuclear factor of activated T cells (NFAT) family [26–32], which shares a structurally conserved DNA-binding motif with the NF-κB proteins, the rel homology region (reviewed in [33–35]).
Nuclear translocation of the two NFAT proteins that have been shown to play a role in HIV-1 gene transcription and replication in T cells, NFATp (also known as NFAT1 or NFATc2), and NFATc (also known as NFAT2 or NFATc1) [26–28,30] (reviewed in [36,37]), is regulated by the calcium-dependent phosphatase calcineurin, as is the case for NFAT3 (also known as NFATc4) and NFAT4 (also known as NFATc3) (reviewed in [33,34,38]). The most recently identified NFAT protein, NFAT5 (also known as TonEBP) [39], is the most evolutionarily divergent member of the NFAT family: NFAT5 has a homolog in Drosophila, while NFATp, NFATc, NFAT3, and NFAT4 are found exclusively in vertebrates [40]. NFAT5 was classified as an NFAT protein on the basis of its rel homology region domain, since it has greater sequence similarity with the NFAT protein family than with NF-κB proteins [39], but it is a unique member in many important respects. Specifically, (i) unlike the other NFAT proteins, NFAT5 binds DNA as an obligate dimer in a fashion similar to the NF-κB proteins; (ii) NFAT5 does not cooperate with the basic region leucine zipper proteins Fos and Jun in gene activation; and (iii) NFAT5 functions independently of calcinuerin [39,41] (reviewed in [33–35]). Finally, NFAT5 activity is regulated by osmotic stress and integrin activation [41–43], although the details of the upstream effects of these and other signal transduction pathways upon NFAT5 remain to be elucidated.
Here, we have identified a role for NFAT5 in the replication of HIV-1 in cells of the monocyte/macrophage lineage. We show that terminally differentiated macrophages have high constitutive levels of NFAT5 mRNA and protein, and that NFAT5 specifically and functionally binds to a site conserved in the LTR of HIV-1 subtypes B, C, and E. Strikingly, this unique NFAT5 site is conserved in all major HIV-1 subtypes, in HIV-2, and in simian immunodeficiency virus (SIV) from multiple primate species. By targeting NFAT5 expression with small interfering RNA (siRNA), we inhibit production of HIV-1 subtypes B, C, and E in human monocyte-derived macrophages (MDMs). Our results thus establish a pivotal role of NFAT5 in HIV-1 replication in a physiological monocyte/macrophage model system of infection. By demonstrating that HIV-1 replication can be inhibited in terminally differentiated macrophages by targeting the transcription factor NFAT5 through RNA interference, we illustrate a novel approach that may be harnessed in suppressing HIV-1 replication in this major viral reservoir.
Materials and Methods
Cell Culture
HeLa-CD4 [44] cells were obtained from the United States National Institutes of Health AIDS Research and Reference Reagent Program (http://www.aidsreagent.org) and were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, http://www.invitrogen.com) supplemented with 10% FCS, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine, and 500 μg/ml G418. One day before experimental manipulation, cells were washed and incubated in medium without antibiotics and plated at ~70% confluence. THP-1 cells were obtained from the AIDS Research and Reference Reagent Program and were maintained in RPMI 1640 medium (Gibco) supplemented with 10% FCS, 50 U/ml penicillin, 50 μg /ml streptomycin, and 2 mM L-glutamine. Transfection of THP-1 cells was performed as described previously [45], and luciferase assays were performed according to the manufacturer's instructions (Dual Luciferase Reporter Assay System; Promega, http://www.promega.com) using a Dynex luminometer (http://www.dynextechnologies.com), with Renilla luciferase (pRL-TK) as an internal control.
Preparation of Human MDMs
Human monocytes were isolated from buffy coats prepared from healthy volunteer donors. Peripheral blood mononuclear cells isolated by Ficoll-Hypaque (Pharmacia Corporation, http://www.pfizer.com) density gradient centrifugation were seeded at 6 × 106/2 ml in 12-well plates in RPMI 1640 medium and supplemented with 10% heat-inactivated human AB serum (Nabi Biopharmaceuticals, http://www.nabi.com), 50 U/ml penicillin, 50 μg /ml streptomycin, and 2 mM L-glutamine. The cell cultures were incubated at 37 °C and 5% CO2 overnight, after which nonadherent cells were removed by repeated gentle washing with warm medium. The cells were further incubated for 4 d in fresh medium before manipulation. More than 95% of the adherent cells obtained with this technique were CD14+ macrophages.
siRNA Target Sequences and Transfection of Cell Lines and MDMs
siRNAs were constructed (Ambion, http://www.ambion.com) to target sequences of NFAT5: NFAT5#1, 5′-CAACATGCCTGGAATTCAA-3′ (nucleotides [nt] 335 to 353), and NFAT5#2, 5′-CCAGTTCCTACAATGATAA-3′ (nt 3981 to 3999). As a control for nonspecific siRNA effects, we used siRNA targeting the green fluorescent protein (GFP), 5′-GGCTACGTCCAGGAGCGCACC-3′. HeLa-CD4 cells (1 × 104) were transfected in 24-well plates with cationic lipid complexes, prepared by incubating 200 nM of the indicated siRNA in 3 μl siPORT NeoFX transfection reagent (Ambion), prepared as recommended by the manufacturer, in a final volume of 500 μl DMEM containing 10% FCS. After 24 h of incubation, cells were washed and resuspended in fresh medium. MDMs were seeded in 12-well plates and were transfected with 1 μM siRNA in 4 μl of siPORT NeoFX transfection reagent (Ambion), prepared as recommended by the manufacturer, and added to the cells in a final volume of 750 μl RPMI containing 10% human serum. The cultures were incubated overnight and then washed and incubated in fresh medium.
Viruses
HIV-1Bal and HIV-193TH64 were obtained from the AIDS Research and Reference Reagent Program. HIV-1LAI and HIV-198IN22 were obtained from the Centralised Facility for AIDS Reagents (http://www.nibsc.ac.uk/spotlight/aids.html), United Kingdom National Institute for Biological Standards and Control. High titer of virus stock was prepared by infection of a pool of phytohemagglutinin (Sigma, http://www.sigmaaldrich.com) and IL-2-stimulated (AIDS Research and Reference Reagent Program) normal donor peripheral blood mononuclear cells with each virus for approximately 2 wk. Supernatants were aliquoted and stored at −70 °C.
HIV-1 Infection
HeLa-CD4 cells were infected with 100 TCID50 of HIV-1LAI in 1 ml of medium. The cells were incubated overnight, after which they were washed and 1 ml fresh medium was added to each culture. After viral challenge, the cells were analyzed for p24 expression by flow cytometry and real-time PCR. Cell-free viral production was measured in supernatants using the HIV-1 p24 ELISA (enzyme-linked immunosorbent assay) kit (Perkin Elmer, http://las.perkinelmer.com). MDMs were infected with 50 TCID50 of HIV-1Bal, or 100 TCID50 of HIV-198IN22 or HIV-193TH64 in 1 ml of medium. The cells were incubated for 24 h, after which they were washed and fresh medium was added. Supernatants were collected at different intervals and kept frozen at −70 °C for further analysis.
Flow Cytometry
Uninfected cells or cells infected with HIV-1 were washed with HBSS, and fixed and permeabilized using the Fix and Perm kit from Caltag Laboratories (http://www.invitrogen.com). The cells were stained with p24-RD1 conjugate (Beckman Coulter, http://www.beckmancoulter.com). Intracellular NFAT5 levels were determined using NFAT5 antibody (Oncogene Research Products, http://www.emdbiosciences.com/g.asp?f=CBC/home.html) labeled with phycoerythrin and analyzed by flow cytometry on a FACSCalibur instrument with CellQuest software (Becton Dickinson, http://www.bdbiosciences.com) according to standard techniques [46].
Quantitative PCR
NFAT5 and HIV-1 expression level was determined by real-time PCR using SYBR green (Applied Biosystems, http://www.appliedbiosystems.com). The reaction conditions were 95 °C for 10 min to activate the DNA polymers followed by 40 cycles of 95 °C for 20 s and 60.5 °C for 1 min. The results were normalized using β-actin as an internal control.
LTR Molecular Clone Construction
LTR molecular clones were constructed by inserting −208 to +64 nt relative to the transcriptional initiation site of HIV-1LAI, HIV-1BAL (B subtype), HIV-198IN22, HIV-198CH01 (C subtype), HIV-193TH64, and HIV-1KR25 (E subtype) into the pGL3 vector (Promega, http://www.promega.com) using Xho I and Hind III restriction enzyme sites. Sequences were aligned and analyzed by using ClustalW (http://www.ebi.ac.uk/clustalw). The HIV-1LAI NFAT5 mutant (N5-Mut) was created by using standard PCR-based mutagenesis methods [47].
DNase I Footprinting and Electrophoretic Mobility Shift Assay
DNase I footprinting of HIV-1 LTR fragments was performed using recombinant NF-κB (p50/p65), NFATp (generous gifts from D. Thanos), and NFAT5 (a generous gift from A. Rao). Briefly, the −208 to +64–nt fragment of HIV-1 LTRs from subtypes B (HIV-1LAI), C (HIV-198IN22), and E (HIV-193TH64) were end-labeled with [−32 P]ATP using T4 polynucleotide kinase and incubated with increasing amounts of recombinant proteins. After 30 min of incubation, the samples were digested with 0.3 units of DNase I for exactly 1 min, resolved on an 8% denaturing polyacrylamide gel, and exposed overnight on film. In the electrophoretic mobility shift assay (EMSA) experiment with recombinant proteins, we used the NF-κB, NFATp, and NFAT5 recombinant proteins described above with oligonucleotides end-labeled with [−32 P]ATP as described [48]. For the EMSA with nuclear extracts from unstimulated THP-1 cells (Figure S1), the wild-type LTR oligonucleotide was used along with a specific anti-NFAT5 antibody (anti-NFAT5; Santa Cruz Biotechnology, http://www.scbt.com) or a control antibody (anti-Ig control, Santa Cruz Biotechnology).
Statistical Analysis
Where applicable, results are expressed as a mean +SEM, and comparison between two groups was performed using the unpaired Student's t-test with the aid of Microsoft Excel software. p < 0.05 was considered significant.
Results
Isolation and Sequence Analysis of Representative HIV-1 Subtype B, C, and E LTRs
To examine the regulation of HIV-1 subtypes by NFAT proteins, we first cloned and sequenced the LTR region (−208 to +64 nt) of representative primary HIV-1 C (HIV-198IN22, India and HIV-1CH01, China) and E (HIV-1KR25, Cambodia) and HIV-193TH64 (Thailand)) isolates. We analyzed at least ten clones from each isolate, determined the dominant sequences, and compared these results with LTR sequences that we cloned from the lab-adapted B subtype strains HIV-1BAL and HIV-1LAI (Figure 1). Strikingly, we found an NFAT5 consensus binding motif (5′-RNRNNTTTCCA-3′) [39] that was completely conserved in all of the HIV-1 isolates examined. It overlaps the NF-κB site proximal to the start site of transcription in the subtype B isolates, the single NF-κB site in the subtype E isolates, and the central NF-κB site in the subtype C isolates (Figure 1). We note that these NFAT5 binding motifs also coincide with NFATp/NFATc binding sites previously detected in the LTRs of the HIV-1 B, C, and E subtypes [17,27,30].
Figure 1. Alignment of LTR Promoter and Enhancer Sequences in Representative Isolates of Subtypes B, C, and E.
Sequence variation within the NF-κβ/NFATp, SP1, and TATA box regions that distinguish the C and E subtypes from the B subtype are indicated in the boxes. Sequences were aligned and analyzed by using ClustalW (http://www.ebi.ac.uk/clustalw).
NFAT5 Binds to a Conserved Element within the LTRs of HIV-1 Subtypes B, C, and E
To establish the pattern of NFAT5 binding to B, C, and E LTRs, we next compared the binding of recombinant NF-κB p50/p65, NFATp, and NFAT5 proteins to the LTRs (−208 to +64 nt relative to the transcription start site) from HIV-1 subtypes B (HIV-1LAI), C (HIV-1CH01 and HIV-198IN22), and E (HIV-193TH64), using quantitative DNase I footprinting analysis.
We detected binding of p50/p65 at the two consensus sites in the B subtype LTR and the single consensus site in the E subtype; furthermore, p50/p65 bound to these sites with similar affinities (Figure 2A, panels 1 and 3; also see summary in Figure 2B). Interestingly, although the C subtype has three putative NF-κB binding motifs and the B subtype has two, approximately the same number of nucleotides is protected from DNase cleavage using the C and B LTRs, since binding of p50/p65 does not extend to the 3′ κB site in the subtype C LTR (Figure 2B). The lack of binding of p50/p65 to the 3′ site in the C subtypes can be ascribed to the presence of a thymine or cytosine in place of the purine that is typically in the fourth position of most NF-κB binding motifs (reviewed in [49,50]). We note that a cytosine has been observed in the fourth position of putative NF-κB motifs in other C isolates [17,29].
Figure 2. NFAT5 Binds Distinctly to a Motif Conserved in HIV-1 Subtype B, C, and E LTR Promoter Regions.
(A) NF-κβ, NFATp, and NFAT5 bind to distinct and overlapping sequences in the HIV-1 LTR enhancer/promoter region of HIV-1 subtypes B, C, and E. Quantitative DNase I footprinting analysis is shown using HIV-1 LTR fragments (−208 to +64 nt relative to the transcription start site) from representative subtype B, C, and E viral isolates and increasing concentrations of recombinant NF-κB (p50/p65), NFATp (20 ng, 100 ng, 400 ng, and 20 μg), or NFAT5 (10 μg, 50 μg, 200 μg, and 1 mg) as shown. The low DNA-binding affinity of NFAT5 relative to NFATp has been noted previously [60]. The regions that are protected from DNase cleavage by the binding of NF-κB, NFATp, and NFAT5 are indicated with bars. We note that in the case of NFAT5, distinct cleavage patterns (including a hypersensitive site 5′ to the region of DNase I protection) are evident at 200 μg.
(B) Summary of DNase footprinting results with NF-κβ, NFATp, and NFAT5, and alignment of HIV-1 LTR nucleotide sequences from representative isolates from subtypes B, C, and E. The diagram illustrates the sequences protected from DNase cleavage by the binding of NF-κβ (blue), NFATp (green), and NFAT5 (red). The NF-κβ, NFATp, and NFAT5 binding sites are boxed. The broken boxes indicate sites that have sequence similarity to NF-κβ, NFATp, and NFAT5 sites, but which do not bind the proteins in the footprinting assay displayed in (A). The GGA repeats in the NFATp core dimer binding region are shown in bold.
Consistent with previous reports [17,29,51], NFATp binds to sequences that overlap the NF-κB sites of the three subtypes (Figure 2A and 2B). In contrast to our results with p50/p65, the number of nucleotides protected by NFATp in each of the isolates is approximately the same, although NFATp exhibits the lowest relative affinity for the E subtype. This can be explained by further inspection of the LTR sequences (Figure 2B). The B and C subtypes contain two identical copies of NF-κB sites (both κB sites in B and the 5′ and central κB sites in C); previous studies based on the B subtype LTR sequence have shown that NFATp binds as a dimer to this site [28,52]. These sites are 9-bp (base pair) inverted repeats of GGA core motifs (bold black font in Figure 2B), a consensus sequence that preferentially binds the NFATp dimer (J. Falvo, C. Lin, A. Tsytsykova, P. Hwang, D. Thanos, et al., unpublished data); this pattern does not appear in the 3′ NF-κB-like site in the C subtype, which fails to bind NFATp or NF-κB p50/p65. The E subtype LTR has one copy of the conserved NF-κB site and an 8-bp inverted repeat of the GGA core motif (see dotted line boxed sequences and green font in Figure 2B), which is not optimal spacing for NFATp dimer binding (J. Falvo, C. Lin, A. Tsytsykova, P. Hwang, D. Thanos, et al., unpublished data).
We next investigated whether NFAT5 could bind to the three LTRs, given that they contain a consensus NFAT5 binding site, 5′-RNRNNTTTCCA-3′ [39], overlapping the NF-κB/NFATp dimer binding site proximal to the transcription start site (Figure 2A, panels 7–9). We found that NFAT5 protected an approximately equal number of nucleotides in each LTR (Figure 2B), footprinting at the B isolate LTR (Figure 2A, panel 7) more weakly than the C and E isolates (Figure 2A, panels 8 and 9). NFAT5 selectively binds the 3′ NF-κB/NFATp motif (5′-GGGACTTTCCA-3′, conserved in all subtypes examined) instead of the immediately upstream NF-κB/NFATp motif (5′-GGGACTTTCCG-3′), which varies by only a single base pair (underlined). Thus, NFAT5, unlike p50/p65, discriminates between the tandem NF-κB motifs of the B and C subtype LTRs (Figure 2B).
These results thus define the binding site selectivity for NF-κB p50/p65, NFATp, and NFAT5 at the LTRs of representatives of three different HIV-1 subtypes. Different activators are able to bind in overlapping, yet distinct patterns within these enhancer regions, similar to inducer- and cell type–specific recruitment of different activators to the tumor necrosis factor promoter [47,53–55]. In the case of the HIV-1 LTR in three major viral subtypes, a single nucleotide difference, an adenine immediately 3′ of the NF-κB sites, confers selective binding of NFAT5.
The NFAT5 Binding Site Is Highly Conserved in HIV-1, HIV-2, and SIV LTRs
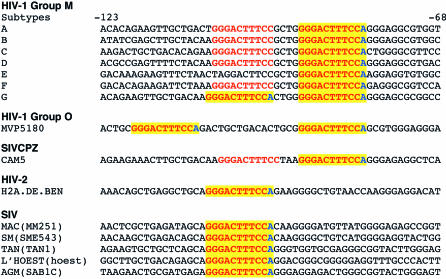
We next examined the Los Alamos HIV Sequence Database (http://hiv-web.lanl.gov/content/hiv-db/mainpage.html) for LTRs from representatives of HIV-1 Group M (for main) subtypes A through G, HIV-1 Group O, HIV-2, and five SIV LTRs [17,56], including SIVCPZ, the closest relative to HIV-1 [57], to determine whether the NFAT5 binding site we had discovered was present in other viral subtypes and in SIVs. Remarkably, we found complete conservation of the NFAT5 binding motif, including the 3′ adenine that confers selective NFAT5 binding over closely related NF-κB sites, in each of these LTRs (Figure 3).
Figure 3. The NFAT5 Binding Motif Is Conserved in HIV-1, HIV-2, and SIV LTRs.
Sequence analysis of HIV-1 Group M subtypes A through G and HIV-1 Group O as well as HIV-2 and selected SIV LTR enhancer/promoter regions. Numbering is based on B subtype (HIV-1LAI) sequences. The conserved NFAT5 binding motif is highlighted in yellow; the sequence corresponding to the canonical NF-κB site is in red, and the unique 3′ terminal adenine is in blue. Sequences were obtained from the Los Alamos HIV Sequence Database (http://hiv-web.lanl.gov/content/hiv-db/mainpage.html) [17,56] and aligned using HIV-1 subtype B sequences spanning −123 to −68 nt relative to the viral transcription start site as a reference.
Specific Disruption of NFAT5 Binding Impairs LTR Activation
We next examined the functional impact of NFAT5 binding upon LTR-mediated activation of transcription. Inspection of the NF-κB/NFATp/NFAT5 shared binding element suggested that we could disrupt NFAT5 but not NF-κB binding by mutating two of the thymines to cytosines in this motif in the HIV-1LAI subtype B LTR (N5-Mut in Figure 4A); in fact, these mutations convert the site to a canonical NF-κB p50/p65 site that is found in the major histocompatibility complex class I H2-κB enhancer and other promoter regions (reviewed in [50]). An EMSA using the wild-type and NFAT5 (N5-Mut) mutant oligonucleotides confirmed that changing the CC dinucleotide to TT in the N5-Mut did indeed significantly inhibit NFAT5 binding without disrupting the binding of p50/p65. In fact, the binding of p50/p65 when the site was converted to the major histocompatibility complex class I H2-κB site was enhanced (Figure 4A).
Figure 4. Specific Disruption of NFAT5 Binding Impairs LTR–Reporter Gene Activity in Monocytic THP-1 Cells.
(A) Disruption of NFAT5 binding does not impair NF-κβ (p50/p65) binding. An EMSA is shown using recombinant NFAT5 and NF-κB proteins with an oligonucleotide matching the NFAT5/NF-κβ site from the HIV-1LAI (wild-type [WT]) or an oligonucleotide with a mutation in the nt predicted to be required for NFAT5 binding (N5-Mut), but not NF-κB binding (shown at the bottom of the figure).
(B) Mutation of the NFAT5 binding site significantly reduces HIV-1LAI LTR reporter activity in THP-1 cells. HIV-1LAI LTR-Luc reporter constructs that contained −208 to +64 nt relative to the transcriptional initiation site of HIV-1LAI, and that were isogenic except for the specific N5 mutation, were transfected into THP-1 cells with the Renilla luciferase (pRL-TK) control plasmid. Luciferase activity was determined 26 h later. The LTR mutation, which abolishes NFAT5 binding to the promoter/enhancer region, significantly (**, p < 0.01) suppressed LTR-dependent activity of transcription compared with WT in THP-1 cells.
We next were interested in determining whether this specific mutation of the NFAT5 binding site resulted in a change in LTR-mediated gene activation. We thus introduced the CC to TT change in the context of an HIV-1 B subtype (HIV-1LAI) LTR-luciferase reporter gene spanning −208 to +64 nt relative to the transcription start site, transfected the wild-type and NFAT-5 mutant LTR reporter genes into the human monocytic cell line THP-1, and measured LTR driven luciferase activity 36 h later. We note that NFAT5 is present in nuclear extracts from unstimulated THP-1 monocytic cells and binds to the wild-type LTR oligonucleotide (Figure S1). The NFAT5-specific mutation, which does not impact NF-κB binding to this site, inhibited HIV-1LAI LTR expression by approximately 65% (p < 0.01) (Figure 4B). These data thus provide a direct link between the binding of NFAT5 at the core NFAT5/NF-κB/NFATp element of the HIV-1LAI LTR and the ability of this LTR to activate transcription.
Blocking NFAT5 by siRNA Inhibits HIV-1 Production in HeLa-CD4 Cells
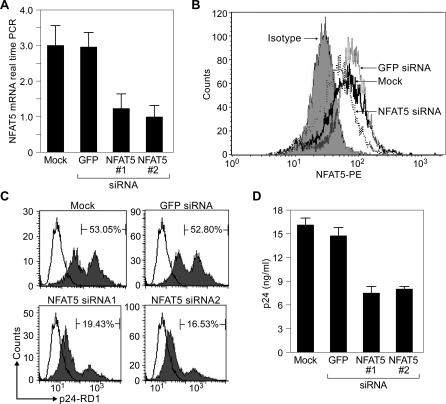
Consistent with the findings above in monocytic THP-1 cells and with our previous studies in fibroblast cells [48], we also detected constitutive levels of NFAT5 mRNA and protein in HeLa-CD4 cells (Figure 5A and 5B). Notably, HeLa-CD4 cells can be infected with and sustain replication of HIV-1LAI and have been successfully used in studies employing RNA interference [46,58]. These cells thus allowed us to determine whether NFAT5 played a functional role in HIV-1 replication using RNA interference. We designed small interfering RNA (siRNA) directed against NFAT5 and used these to block NFAT5 mRNA expression (Figure 5A) and protein production (Figure 5B) in HeLa-CD4 cells. To confirm the specificity of NFAT5 RNA inhibition, we designed another distinct NFAT5-specific siRNA targeting a different exon of NFAT5. We performed four independent experiments and demonstrated that both siRNAs significantly inhibited (p < 0.01) NFAT5 mRNA expression in HeLa-CD4 cells (Figure 5A). We also investigated the impact of siRNA directed against NFAT5 upon NFAT5 protein production using intracellular staining for NFAT5. As shown in the experiment displayed in Figure 5B (which is representative of four independent experiments), NFAT5 protein expression was lower in the cells transfected with an NFAT5 siRNA than in those mock transfected or transfected with the GFP siRNA.
Figure 5. NFAT5 Plays an Important Functional Role in HIV-1 Replication in HeLa-CD4 Cells.
(A) Real-time PCR showing that HeLa-CD4 cells constitutively express NFAT5 mRNA. Using siRNA-targeting NFAT5, we were able to reduce NFAT5 mRNA levels significantly (p < 0.01) compared with that of GFP siRNA or mock-transfected cells. To confirm that NFAT5 knockdown was specific, we used two NFAT5 siRNAs (NFAT5#1 and NFAT#2) targeting two different exons of the NFAT5 gene. We chose the sequence siRNA to GFP because this sequence has been validated and shown to have no nonspecific effects upon HIV replication [46,58]. Results show the average of four independent experiments.
(B) Flow cytomety analysis of intracellular NFAT5 levels in HeLa-CD4 cells. A representative experiment of a flow cytometry analysis using a monoclonal antibody to NFAT5 demonstrated that intracellular NFAT5 protein levels were lower in the cells transfected with NFAT5 siRNA than those that were transfected with GFP siRNA or mock transfected. The experiment is representative of four independent experiments.
(C) Flow cytometry analysis of intracellular p24 levels in HIV-1LAI infected HeLa-CD4 cells. Intracellular HIV-1 levels were determined using a p24-RD1 conjugate in a representative experiment. Three days following virus infection, intracellular p24 was lower in cells transfected with NFAT5 siRNA targeting two different exons of NFAT5 (NFAT5#1 and NFAT5#2; 33.3% and 36.27% inhibition, respectively) than in cells transfected with a GFP control siRNA or mock transfected. This experiment is representative of four independent experiments.
(D) Cell-free p24 production in supernatants of HIV-1LAI infected HeLa-CD4 cells. HIV-1 replication as assessed by p24 ELISA is significantly (p < 0.04) lower in cellular cultures transfected with NFAT5 siRNA targeting two different exons of NFAT5 (NFAT5#1 and NFAT5#2) than in cells mock transfected or transfected with the GFP control siRNA.
We next transfected HeLa-CD4 cells with each of the two NFAT5-specific siRNAs or with GFP siRNA as a control. The cells were then infected with HIV-1LAI. At day 3 post-infection, we performed internal staining with HIV-1 p24-RD1 conjugated antibody and analyzed the cells by flow cytometry (Figure 5C). No reduction in intracellular virus level was observed when the cells were transfected with GFP siRNA as compared with mock-transfected cells. However, there were 33.3% and 36.27% reductions in intracellular p24 levels in cells transfected with these specific NFAT5 siRNAs relative to mock-transfected cells and cells transfected with GFP siRNA (Figure 5C). Furthermore, using a p24 ELISA assay, we demonstrated that virus replication was significantly (p < 0.04) reduced in the supernatants of cells transfected with either one of the specific NFAT5 siRNAs compared with virus replication in supernatants from cells that were transfected with GFP siRNA or mock transfected (Figure 5D).
NFAT5 Is Expressed in Primary Macrophages and Plays an Important Role in the Replication of HIV-1 Subtypes B, C, and E in MDMs
Given that macrophages are a major virus reservoir as infection progresses, we next determined whether NFAT5 is produced in terminally differentiated primary macrophages and whether it plays a role in virus replication. We generated MDMs from normal donor peripheral blood mononuclear cells and found that NFAT5 mRNA (Figure 6A) and protein (Figure 6B) are constitutively expressed in these cells.
Figure 6. MDMs Constitutively Express NFAT5, Which Is Required for Replication of HIV-1 Representative Isolates from Subtypes B, C, and E.
(A) NFAT5 expression was detected in human MDMs using real-time PCR. NFAT5 mRNA expression was significantly inhibited 6 d after transfection with NFAT5 siRNA (NFAT5#1) compared with cells transfected with GFP siRNA or mock transfected (p < 0.02).
(B) Flow cytomety analysis of intracellular NFAT5 levels in MDMs. A representative experiment is shown of a flow cytometry analysis of MDM using a monoclonal antibody to NFAT5. Intracellular NFAT5 protein levels were lower in the cells transfected with NFAT5 siRNA than in those transfected with GFP siRNA or those mock transfected. The experiment is representative of five independent experiments.
(C) Flow cytometry analysis of intracellular p24 levels in HIV-1BAL–infected MDMs. Mock- or siRNA-transfected MDMs were infected with HIV-1Bal, and intracellular p24 levels were determined on day 4 post-infection with flow cytometry using the p24-RD1 conjugated antibody. Intracellular virus levels were 26.9% lower in the cells transfected with NFAT5 siRNA than in GFP siRNA–transfected cells.
(D) Knockdown of NFAT5 RNA inhibits replication of HIV-1 representative isolates from subtypes B, C, and E. Cell-free virus replication was determined by p24 ELISA at day 3, 6, 9, and 13 post-infection of MDMs by subtype B (HIV-1BAL), C (HIV-198IN22), or E (HIV-193TH64) viruses as indicated. Free virus levels were lower in the cells transfected with NFAT5 siRNA compared with the cells transfected with GFP siRNA (control) in cellular supernatants at all the time points monitored and indicated in the figure. Notably, on day 13 post-infection, inhibition of free virus levels in the NFAT5 siRNA–transfected cultures was on average 1.9-fold lower for HIV-1BAL, 2-fold lower for HIV-198IN22, and 2.1-fold lower for HIV-193TH64 compared with GFP siRNA–transfected cultures.
We performed five independent experiments and demonstrated that NFAT5 siRNA (NFAT5#1) significantly inhibited (p < 0.02) NFAT5 mRNA expression in MDM cells (Figure 6A). We note that the second specific NFAT5 siRNA behaved identically in two experiments where it was tested (unpublished data). We also investigated the impact of siRNA directed against NFAT5 upon NFAT5 protein production using intracellular staining for NFAT5. As shown in the experiment displayed in Figure 6B, (which is representative of five independent experiments), NFAT5 protein expression was lower in the cells transfected with an NFAT5 siRNA than in those mock transfected or transfected with GFP siRNA.
We next transfected MDMs with NFAT5 siRNA or GFP siRNA (control) and then 4 d later infected the cells with HIV-1Bal (B subtype) and monitored virus replication. At day 4 post-infection, we performed intracellular staining with HIV-1 p24-RD1 conjugated antibody and analyzed the cells by flow cytometry. As shown in Figure 6C, there was a 26.9% reduction in intracellular p24 levels in cells transfected with NFAT5 siRNA compared with the cells transfected with GFP siRNA–transfected cells.
MDMs also provided us the opportunity to test the effect of NFAT5 inhibition upon the replication of the M-tropic HIV-1 subtype C and E primary isolates. We therefore transfected MDMs with NFAT5 siRNA or GFP siRNA (control) and then 4 d later infected the cells with HIV-198IN22 (C subtype), HIV-193TH64 (E subtype), or HIV-1Bal (B subtype) and performed a p24 ELISA to determine free virus levels in MDM cultures infected with the three viruses in the presence or absence of NFAT5 siRNA over time. The p24 levels from MDMs infected with the representative B, C, and E isolates were inhibited in the cultures transfected with NFAT5 siRNA compared with the control cultures transfected with GFP siRNA or mock transfected over the period of time monitored (Figure 6D). On day 13 post-infection, we analyzed the inhibition caused by NFAT5 siRNA versus transfection with the GFP siRNA or mock transfection. We observed that for the subtype B, C, and E isolates, specific inhibition by knocking down NFAT5 was on average 1.9-, 2-, and 2.1-fold, respectively, at this time point (Figure 6D). We obtained similar results using each of the two specific siRNAs targeting two different exons of NFAT5 in all of the experiments in MDMs (unpublished data).
To verify that infection by HIV-1 itself was not causing an increase in NFAT5 mRNA levels, we infected MDMs with live or heat-inactivated HIV-198IN22, HIV-193TH64, or HIV-1Bal and measured NFAT5 levels by real-time PCR at 24 and 48 h after infection. There was no change in NFAT5 levels at either time point after infection of cells with live virus or incubation with heat inactivated viruses as compared with uninfected cells (unpublished data). Taken together, these results demonstrate that NFAT5 plays a significant role in the replication of HIV-1 from subtype B, C, and E isolates in primary differentiated macrophages.
Discussion
In this study, we have combined in vitro and ex vivo studies with sequence analysis approaches to reveal a new function for NFAT5 in the replication of major subtypes of HIV-1 in cells of the monocyte/macrophage lineage. Previous studies have demonstrated that a range of transcription factors can interact with the LTR of HIV-1 subtype B (reviewed in [12]), and it has been proposed that distinct sets of host factors may direct transcription driven by the HIV-1 LTR in T cells and macrophages [7]. Remarkably, the functional NFAT5 site, 5′-GGGACTTTCCA-3′, that we have identified in the LTR of isolates of HIV-1 subtypes B, C, and E, is conserved not only across HIV-1 Group M subtypes A through G, but also in HIV-1 Group O and HIV-2 isolates, as well as SIV isolates from multiple primate species (Figure 3).
The evolutionary pressure toward conservation of this NFAT5 site is particularly striking given the variability of LTR sequences between subtypes and HIV species [17,56], including the number and arrangement of transcription factor binding sites, as illustrated by the binding of NF-κB proteins to HIV-1 subtype B, C, and E LTRs in this study (Figure 2A). Furthermore, we have shown that a single nucleotide difference, a 3′ adenine, between the NFAT5 site and an upstream NF-κB binding site, confers selective binding of NFAT5 to this conserved enhancer element (Figure 2).
NFAT5 is unique among the NFAT proteins both in its DNA-binding properties and in the upstream signal transduction pathways that regulate its activity. Unlike the other NFAT family members, NFAT5 binds as an obligate dimer and does not act coordinately with Fos and Jun proteins. While the nuclear translocation of other NFAT proteins is tightly regulated by the calcium-dependent phosphatase calcinuerin, the activity of NFAT5 has been shown to be regulated by osmotic stress and integrin activation [41–43]. Our findings that NFAT5 is involved in HIV-1 replication in cells of the monocyte/macrophage lineage, as well as in a nonlymphoid model cell line, and that its functional DNA binding site is highly conserved, provide an initial insight into other potential upstream regulatory mechanisms that further distinguish the physiological role of this evolutionarily divergent NFAT protein from its calcineurin-responsive counterparts. It is interesting to note that the HIV-1 LTR can be activated by osmotic stress in HeLa cells [59]; thus, the upstream signaling pathways involved in NFAT5-dependent HIV-1 replication may overlap with those responsible for osmotic stress-induced transcription of NFAT5-dependent cellular genes. The features of these signaling pathways remain to be determined [34]. Working backward from this functional binding site may thus reveal critical, evolutionarily conserved mechanisms through which constitutive or activated signals are propagated from the cell membrane, resulting in NFAT5-dependent gene expression that is critical in maintaining HIV-1 replication in the monocyte/macrophage lineage.
Even though both NF-κB and NFAT proteins interact with the conserved NFAT5 site, our results have demonstrated a specific role for NFAT5 in the replication of HIV-1 in cells of the monocyte/macrophage lineage. We have established that NFAT5 mRNA and protein are constitutively present in primary human macrophages. Strikingly, replacement of the NFAT5 site with a canonical NF-κB site inhibits activity of the HIV-1 LTR, and direct inhibition of NFAT5 using siRNA inhibits replication of HIV-1 subtypes B, C, and E in primary human macrophages. By examining multiple HIV-1 subtypes, we have discovered a function for a binding site conserved across viral types and even across primate species; this finding, together with the importance of macrophages as a viral reservoir through which HIV-1 can persistently infect multiple tissues, underscores the potential of the NFAT5–LTR interaction as a therapeutic target to suppress virus replication and disease progression.
Supporting Information
An EMSA with nuclear extracts from unstimulated THP-1 monocytic cells and the wild-type LTR oligonucleotide is shown. Reactions were untreated (-) or incubated with a specific anti-NFAT5 antibody (anti-NFAT5) or a control antibody (anti-Ig control). Note that the NFAT5–DNA complex (indicated by the arrow) is ablated by the anti-NFAT5 antibody.
(1.0 MB TIF)
Acknowledgments
The authors would like to thank Dimitris Thanos for the generous gift of recombinant NF-κB and NFATp and Anjana Rao for the generous gift of NFAT5.
Abbreviations
- bp
base pair
- ELISA
enzyme-linked immunosorbent assay
- EMSA
electrophoretic mobility shift assay
- GFP
green fluorescent protein
- LTR
long terminal repeat
- MDM
monocyte-derived macrophage
- N5-Mut
HIV-1LAI NFAT5 mutant
- NF-κB
nuclear factor κB
- NFAT
nuclear factor of activated T cells
- nt
nucleotides
- siRNA
small interfering RNA
- SIV
simian immunodeficiency virus
Footnotes
¤ Current address: CBR Institute for Biomedical Research, Harvard Medical School, Boston, Massachusetts, United States of America
Competing interests. The authors have declared that no competing interests exist.
Author contributions. SR designed the experiments, interpreted the data, isolated all of the viruses, and performed siRNA and plasmid transfection and virus infection experiments. AVT performed in vitro footprinting analysis and EMSA. SKL designed the siRNA primers and participated in the FACS experiments. RR performed the RT-PCR analyses. JVF participated in interpretation of data and preparation of the manuscript. JL and PS participated in the design of experiments and interpretation of data. AG actively participated in the design of experiments, interpretation of data, and preparation of the manuscript.
Funding. This work was supported by grants from the US National Institutes of Health to AEG (R21AI060433–01 and AI065285-01A1) and PS (AI065342), and the Harvard Center for AIDS Research (P30 A060354) and the Campbell Foundation to SR.
References
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- Kulkosky J, Pomerantz RJ. Approaching eradication of highly active antiretroviral therapy–persistent human immunodeficiency virus type 1 reservoirs with immune activation therapy. Clin Infect Dis. 2002;35:1520–1526. doi: 10.1086/344959. [DOI] [PubMed] [Google Scholar]
- Aquaro S, Calio R, Balzarini J, Bellocchi MC, Garaci E, et al. Macrophages and HIV infection: Therapeutical approaches toward this strategic virus reservoir. Antiviral Res. 2002;55:209–225. doi: 10.1016/s0166-3542(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Crowe SM. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem. 2002;9:1893–1903. doi: 10.2174/0929867023368935. [DOI] [PubMed] [Google Scholar]
- Collman RG, Perno CF, Crowe SM, Stevenson M, Montaner LJ. HIV and cells of macrophage/dendritic lineage and other non-T cell reservoirs: New answers yield new questions. J Leukoc Biol. 2003;74:631–634. doi: 10.1189/jlb.0703357. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. Pathogenesis of macrophage tropic HIV-1. Curr HIV Res. 2005;3:53–60. doi: 10.2174/1570162052772951. [DOI] [PubMed] [Google Scholar]
- Verani A, Gras G, Pancino G. Macrophages and HIV-1: Dangerous liaisons. Mol Immunol. 2005;42:195–212. doi: 10.1016/j.molimm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Meltzer MS, Skillman DR, Hoover DL, Hanson BD, Turpin JA, et al. Macrophages and the human immunodeficiency virus. Immunol Today. 1990;11:217–223. doi: 10.1016/0167-5699(90)90086-o. [DOI] [PubMed] [Google Scholar]
- Roulston A, Lin R, Beauparlant P, Wainberg MA, Hiscott J. Regulation of human immunodeficiency virus type 1 and cytokine gene expression in myeloid cells by NF-kappa B/Rel transcription factors. Microbiol Rev. 1995;59:481–505. doi: 10.1128/mr.59.3.481-505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestra E, Perno CF, Aquaro S, Panti S, Bertoli A, et al. Macrophages: A crucial reservoir for human immunodeficiency virus in the body. J Biol Regul Homeost Agents. 2001;15:272–276. [PubMed] [Google Scholar]
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Pereira LA, Bentley K, Peeters A, Churchill MJ, Deacon NJ. A compilation of cellular transcription factor interactions with the HIV-1 LTR. Nuc Acids Res. 2000;28:663–668. doi: 10.1093/nar/28.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs FC, Hogan TH, Quiterio S, Gartner S, Wigdahl B. Kuiken C, Foley B, Hahn B, Marx P, McCutchan F, et al. HIV sequence compendium 2001. Los Alamos (New Mexico): Theoretical Biology and Biophysics, Los Alamos National Laboratory; 2001. Lentiviral LTR-directed expression, sequence variation, and disease pathogeneis; pp. 29–70. In: [Google Scholar]
- Esparza J, Bhamarapravati N. Accelerating the development and future availability of HIV-1 vaccines: Why, when, where, and how? Lancet. 2000;355:2061. doi: 10.1016/S0140-6736(00)02360-6. [DOI] [PubMed] [Google Scholar]
- Girard MP, Osmanove SK, Kieny MP. A review of vaccine research and development: The human immunodeficiency virus (HIV) Vaccine. 2006;24:4692–4700. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Osmanov S, Pattou C, Walker N, Schwardlander B, Esparza J. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J Acquir Immune Defic Syndr. 2002;29:184–190. doi: 10.1097/00042560-200202010-00013. [DOI] [PubMed] [Google Scholar]
- Kuiken C, Foley B, Hahn B, Marx P, McCutchan F, et al. HIV sequence compendium 2000. Los Alamos (New Mexico): Theoretical Biology and Biophysics, Los Alamos National Laboratory; 2000. pp. 193–362. [Google Scholar]
- Jeeninga RE, Hoogenkamp M, Armand-Ugon M, de Baar M, Verhoef K, et al. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G. J Virol. 2000;74:3740–3751. doi: 10.1128/jvi.74.8.3740-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef K, Sanders RW, Fontaine V, Kitajima S, Berkhout B. Evolution of the human immunodeficiency virus type 1 long terminal repeat promoter by conversion of an NF-kappaB enhancer element into a GABP binding site. J Virol. 1999;73:1331–1340. doi: 10.1128/jvi.73.2.1331-1340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano MA, Nixon CP, Ndung'u T, Bussmann H, Novitsky VA, et al. Elevated tumor necrosis factor-alpha activation of human immunodeficiency virus type 1 subtype C in Southern Africa is associated with an NF-kappaB enhancer gain-of-function. J Infect Dis. 2000;181:76–81. doi: 10.1086/315185. [DOI] [PubMed] [Google Scholar]
- van Opijnen T, Jeeninga RE, Boerlijst MC, Pollakis GP, Zetterberg V, et al. Human immunodeficiency virus type 1 subtypes have a distinct long terminal repeat that determines the replication rate in a host-cell–specific manner. J Virol. 2004;78:3675–3683. doi: 10.1128/JVI.78.7.3675-3683.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Israel N, Hazan U, Alcami J, Munier A, Arenzana-Seisdedos F, et al. Tumor necrosis factor stimulates transcription of HIV-1 in human T lymphocytes, independently and synergistically with mitogens. J Immunol. 1989;143:3956–3960. [PubMed] [Google Scholar]
- Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992;6:347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Kwon H, Genin P. Hostile takeovers: Viral appropriation of the NF-kappaB pathway. J Clin Invest. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Chen BK, Kaneshima H, Nolan GP. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, et al. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- Macian F, Rao A. Reciprocal modulatory interaction between human immunodeficiency virus type 1 Tat and transcription factor NFAT1. Mol Cell Biol. 1999;19:3645–3653. doi: 10.1128/mcb.19.5.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux AM, Pare ME, Audet B, Legault E, Lefort S, et al. T-cell activation leads to poor activation of the HIV-1 clade E long terminal repeat and weak association of nuclear factor-kappaB and NFAT with its enhancer region. J Biol Chem. 2004;279:52949–52960. doi: 10.1074/jbc.M409896200. [DOI] [PubMed] [Google Scholar]
- Cron RQ, Bartz SR, Clausell A, Bort SJ, Klebanoff SJ, et al. NFAT1 enhances HIV-1 gene expression in primary human CD4 T cells. Clin Immunol Immunopathol. 2000;94:179–191. doi: 10.1006/clim.1999.4831. [DOI] [PubMed] [Google Scholar]
- Robichaud GA, Barbeau B, Fortin JF, Rothstein DM, Tremblay MJ. Nuclear factor of activated T cells is a driving force for preferential productive HIV-1 infection of CD45RO-expressing CD4+ T cells. J Biol Chem. 2002;277:23733–23741. doi: 10.1074/jbc.M201563200. [DOI] [PubMed] [Google Scholar]
- McCaffrey PG, Luo C, Kerppola TK, Jain J, Badalian TM, et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: Key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Serfling E, Berberich-Siebelt F, Avots A, Chuvpilo S, Klein-Hessling S, et al. NFAT and NF-kappaB factors-the distant relatives. Int J Biochem Cell Biol. 2004;36:1166–1170. doi: 10.1016/j.biocel.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Pessler F, Cron RQ. Reciprocal regulation of the nuclear factor of activated T cells and HIV-1. Genes Immun. 2004;5:158–167. doi: 10.1038/sj.gene.6364047. [DOI] [PubMed] [Google Scholar]
- Cron RQ. HIV-1, NFAT, and cyclosporin: Immunosuppression for the immunosuppressed? DNA Cell Biol. 2001;20:761–767. doi: 10.1089/104454901753438570. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: Choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci U S A. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef IA, Gastier JM, Francke U, Crabtree GR. Evolutionary relationships among Rel domains indicate functional diversification by recombination. Proc Natl Acad Sci U S A. 2001;98:5740–5745. doi: 10.1073/pnas.101602398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, et al. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15:47–58. doi: 10.1016/s1074-7613(01)00165-0. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauliac S, Lopez-Rodriguez C, Shaw LM, Brown LF, Rao A, et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4:540–544. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
- Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, et al. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Ranjbar S, Rajsbaum R, Goldfeld AE. Transactivator of transcription from HIV type 1 subtype E selectively inhibits TNF gene expression via interference with chromatin remodeling of the TNF locus. J Immunol. 2006;176:4182–4190. doi: 10.4049/jimmunol.176.7.4182. [DOI] [PubMed] [Google Scholar]
- Lee SK, Dykxhoorn DM, Kumar P, Ranjbar S, Song E, et al. Lentiviral delivery of short hairpin RNAs protects CD4 T cells from multiple clades and primary isolates of HIV. Blood. 2005;106:818–826. doi: 10.1182/blood-2004-10-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsytsykova AV, Goldfeld AE. Inducer-specific enhanceosome formation controls tumor necrosis factor alpha gene expression in T lymphocytes. Mol Cell Biol. 2002;22:2620–2631. doi: 10.1128/MCB.22.8.2620-2631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esensten JH, Tsytsykova AV, Lopez-Rodriguez C, Ligeiro FA, Rao A, et al. NFAT5 binds to the TNF promoter distinctly from NFATp, c, 3 and 4, and activates TNF transcription during hypertonic stress alone. Nucleic Acids Res. 2005;33:3845–3854. doi: 10.1093/nar/gki701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli G, Saccani S, Bosisio D, Marazzi I. Interactions of NF-kappaB with chromatin: The art of being at the right place at the right time. Nat Immunol. 2005;6:439–445. doi: 10.1038/ni1196. [DOI] [PubMed] [Google Scholar]
- Grilli M, Chiu JJ, Lenardo MJ. NF-kappa B and Rel: Participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Montano MA, Novitsky VA, Blackard JT, Cho NL, Katzenstein DA, et al. Divergent transcriptional regulation among expanding human immunodeficiency virus type 1 subtypes. J Virol. 1997;71:8657–8665. doi: 10.1128/jvi.71.11.8657-8665.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffin MJ, Stroud JC, Bates DL, von Koenig KD, Hardin J, et al. Structure of NFAT1 bound as a dimer to the HIV-1 LTR kappa B element. Nat Struct Biol. 2003;10:800–806. doi: 10.1038/nsb981. [DOI] [PubMed] [Google Scholar]
- Falvo JV, Uglialoro AM, Brinkman BM, Merika M, Parekh BS, et al. Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor alpha gene promoter. Mol Cell Biol. 2000;20:2239–2247. doi: 10.1128/mcb.20.6.2239-2247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, et al. A lipopolysaccharide-specific enhancer complex involving ets, elk-1, sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol. 2000;20:6084–6094. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsytsykova AV, Goldfeld AE. Nuclear factor of activated T cells transcription factor NFATp controls superantigen-induced lethal shock. J Exp Med. 2000;192:581–586. doi: 10.1084/jem.192.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken C, Foley B, Hahn B, Marx P, McCutchan F, et al. HIV Sequence compendium 2001. Los Alamos (New Mexico): Theoretical Biology and Biophysics, Los Alamos National Laboratory; 2001. pp. 307–309. [Google Scholar]
- Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Lee SK, Dykxhoorn DM, Novina C, Zhang D, et al. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77:7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Orsini MJ, Lee JC, McDonnell PC, Debouck C, et al. Activation of the HIV-1 long terminal repeat by cytokines and environmental stress requires an active CSBP/p38 MAP kinase. J Biol Chem. 1996;271:30864–30869. doi: 10.1074/jbc.271.48.30864. [DOI] [PubMed] [Google Scholar]
- Stroud JC, Lopez-Rodriguez C, Rao A, Chen L. Structure of a TonEBP-DNA complex reveals DNA encircled by a transcription factor. Nat Struct Biol. 2002;9:90–94. doi: 10.1038/nsb749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An EMSA with nuclear extracts from unstimulated THP-1 monocytic cells and the wild-type LTR oligonucleotide is shown. Reactions were untreated (-) or incubated with a specific anti-NFAT5 antibody (anti-NFAT5) or a control antibody (anti-Ig control). Note that the NFAT5–DNA complex (indicated by the arrow) is ablated by the anti-NFAT5 antibody.
(1.0 MB TIF)