Abstract
In avian smooth muscles, GTPγS produces a Rho kinase mediated increase in PHI-1 phosphorylation and force, but whether this correlation is causal is unknown. We examined the effect of phosphorylated PHI-1 (P-PHI-1) on force and MLC20 phosphorylation at a constant [Ca2+]. P-PHI-1, but not PHI-1, increased MLC20 phosphorylation and force, and phosphorylation of PHI-1 increased the interaction of PHI-1 with PP1c. Microcystin induced a dose-dependent reduction in the binding of PHI-1 to PP1c. These results suggest PHI-1 inhibits MLCP by interacting with the active site of PP1c to produce a Ca2+ independent increase in MLC20 phosphorylation and force.
Keywords: Ca2+ sensitization, Myosin light chain phosphatase, PP1c, PHI-1, MYPT1, Chicken Gizzard
Introduction
Phosphorylation of the 20 kDa myosin light chain (MLC20) is the hallmark of smooth muscle contraction, and is dependant on the balance between the activities of Ca+2-dependant myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP) [1]. Agonist stimulation leads to an inhibition of the MLCP, or Ca2+ sensitization of smooth muscles, a G-protein mediated process, which involves many different signaling pathways and molecules (reviewed in [2]). MLCP is a trimeric protein consisting of three subunits; a myosin binding subunit (MYPT1), a catalytic subunit (PP1cδ), and a 20 kDa subunit of a unknown function [3]. Although little is known about the physiologically relevant mechanism for MLCP inhibition, two are widely accepted; 1) the phosphorylation of MYPT1 [4,5] and 2) the binding of phosphatase inhibitor proteins to the catalytic subunit of the enzyme [6,7].
We have previously demonstrated that phosphorylation of MYPT1 does not participate in Ca2+ sensitization of avian smooth muscle tissue [8]. These data suggest that a phosphatase inhibitor protein is a likely candidate to mediate Ca2+ sensitization. Phosphatase inhibitor proteins are a family of proteins that specifically inhibit MLCP, and their inhibitory potency for the phosphatase is increased upon phosphorylation [9]. They are termed inhibitor-1, in contrast to inhibitor-2 proteins that are effective without phophorylation [7]. Phosphatase inhibitor-1 proteins include a 17 kDa PKC and/or Rho-kinase potentiated protein (CPI-17), phosphoprotein holoenzyme inhibitor-1 (PHI-1), and dopamine and cAMP regulated phosphoprotein of 32 kDa (DARPP-32) that is expressed in brain [7]. It has previously been demonstrated that CPI-17 is not expressed in chicken smooth muscles [8,10], suggesting that another inhibitor-1 type protein may serve an analogous role. We have previously demonstrated in avian smooth muscle that a Rho-kinase mediated pathway phosphorylates PHI-1 during both G-protein stimulation of skinned smooth muscles and agonist stimulation of intact preparations [8]. However, whether in chicken smooth muscle, PHI-1 phosphorylation mediates Ca2+ sensitization is unknown. In this study, we tested the hypothesis that phosphorylation of PHI-1 leads to a Ca2+ sensitization of chicken smooth muscle.
Materials and Methods
Preparation of phospho-PHI-1
Purified PHI-1 (Upstate Biotechnologies) was phosphorylated using a previously described protocol [7]. Briefly, PHI-1 was phosphorylated in an assay buffer containing: 25mM MOPS-NaOH pH 7.0, 10mM magnesium acetate, 0.3mg/ml PHI-1 (Upstate Biotechnologies), 2μg/ml Rho kinase (Upstate Biotechnologies), and 0.1 mM ATP for 120 minutes at 30°C. Phosphorylation of PHI-1 was confirmed by SDS-PAGE and western blotting using a phosphospecific antibody which recognizes PHI-1 phosphorylated at Thr 57 [7].
Force
Following an institutionally approved IACUC protocol, the chicken gizzard was removed and placed into cold Ca2+ free saline solution (140mM NaCl, 4.7mM KCl, 1.2mM NaH2PO4, 2.0mM MOPS, 0.02mM EDTA, 1.2mM MgCl2, 5.6mM glucose, and 0.5mM EGTA, pH 7.0). Small pieces of gizzard smooth muscles were cut into strips approximately 200–700μm long, 100–150μm wide, and 50–150μm thick. As previously described [11,12], aluminum foil T-clips were attached to each end of a gizzard strip, and then the preparation was skinned for 30 minutes at 4°C in pCa9 (−log10[Ca2+]) solution containing 1% TritonX-100. Skinned gizzard strips were then transferred to a mechanics workstation (Aurora Scientific, Aurora, Canada). In pCa9 solution, one end was hooked to a force transducer (Akers AE 801 MEMSCAP, San Jose, USA) and the other to a servomotor (Aurora Scientific). The tissue was stretched to L0, the length where force is maximum as previously described [12]. Strips were then moved to pCa6.2 solution, and varying concentrations of PHI-1 or P-PHI were added and the resulting change in force was recorded. Finally, the tissue was moved to pCa4 solution to determine the maximum Ca2+ activated force for each strip. The force at pCa9 was set to zero, and all forces are given relative to the baseline at pCa9.
Western blotting
The level of MLC20 phosphorylation was determined as previously described [8,11–14]. After skinning, tissue was placed in a pCa6.2 solution with and without 3μg/ml PHI-1 or P-PHI-1 for 15 minutes. The tissues were then denatured in 10% TCA in acetone with 10 mM dithiothreitol and stored at −80°C overnight. Samples were removed and brought to room temperature for 1 hour. After centrifuging for 1 minute, the TCA was removed and the tissues were washed three times in acetone with 10mM dithiothreitol. After the final wash, the tissue was dried and cut into fine pieces. MLC20 was solubilized by vortexing the tissue in 8 M urea, 20 mM Tris, 22 mM glycine, pH 8.6, 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl fluoride. The samples were run in the absence of SDS using 19:1 acrylamide:bisacrylamide 10% gels containing 40% v/v glycerol. The running buffer contained 20 mM Tris, 22 mM glycine, 1 mM dithiothreitol, and 1 mM thioglycolic acid, pH 8.6. The proteins were transferred to nitrocellulose membrane and probed for MLC20 using a monoclonal anti-MLC20 antibody (Sigma). The blot was developed using alkaline phosphatase substrate buffer with NitroBlue Tetrazolium and 5-Bromo-4-choloro-3-indolyl phosphate disodium salt. The level of phosphorylation of MLC20 was determined as (MLC20−Pi/(MLC20−Pi+MLC20))×100% using densitometric analysis (Scion Image). All blots were in the linear range of the detection system [8].
The same samples were used to determine the expression of RhoA and Rho-kinase as well as PHI-1 phosphorylation. These proteins were resolved on 29:1 acrylamide:bisacrylamide 12% SDS-PAGE, and were then transferred to nitrocellulose membrane and probed with anti-Rho A (Upstate Biotechnologies), anti-Rho kinase (Upstate Biotechnologies), anti-PHI-1 [7] or anti–Thr57 phosphospecific PHI-1 antibody [7]. Blots were developed with alkaline phosphatase or chemo-luminescence (Amersham).
Co-immunoprecipitation
Adult chicken gizzard was dissected and homogenized in lysis buffer (8M Urea, 10mM Tris-HCl, 0.1 mM EDTA, 1X EDTA-free Complete Protease Inhibitor (Roche), pH 8). The lysate was rotated for 15 minutes at 4°C, spun (14,000 rpm) and the supernatant was collected and stored at −80°C. Aliquots (250–300μl) of the homogenates were added to 1.5 ml of immunoprecipitation buffer (50mM Tris-HCl (pH 8), 7mM MgCl2, 2mM EDTA, and 1mM PMSF). Samples were incubated on ice for 20 minutes under the following conditions: lysates only, lysate + GTPγS (100 μM), and lysates + GTPγS (100 μM) + microcystin LR (2μM or 20μM). The supernatant was rotated for 30 minutes at 4°C, centrifuged and separated from the precipitate. An antibody to PP1c (Transduction Laboratories), the catalytic subunit of MLCP, was added to the lysates and the samples were rotated overnight. Lysates were also rotated overnight in absence of the antibody as a negative control. Samples were centrifuged for 5 minutes at 4°C, and the supernatant was removed. The antibody-protein complex was recovered using Protein G sepharose beads (Amersham Biosciences). Samples were washed twice with 200μl of immunoprecipitation buffer, 40 μl of SDS sample buffer was added, the samples were heated, and the protein was resolved by 29:1, 12% SDS-PAGE, and Western blotted with the antibody to PHI-1 [7]. Blots were developed with alkaline phosphatase or chemiluminescence (Amersham). Protein loading was normalized by the IgG band, and the PHI-1 band intensity was expressed using the following formula: [PHI-1 band intensity/(IgG band intensity)]. Then, the intensity of the band for lysates treated with GTPγS was set to 100%, and for the other conditions, intensities were normalized accordingly.
Solutions
Calcium solutions were prepared using a computer program designed to give a set of free ion concentrations that are adjusted for both temperature and ionic strength [15]. The ionic strength for all solutions was 200mM and the experiments were carried out at a temperature of 22°C. The relaxing solution (pCa9.0) contained (in mM): 25 BES, 10 EGTA, 0.02 CaCl2, 7.2 MgCl2, 5.5 ATP, 25 creatine phosphate, 56.5 KMS, pH to 7.0 with 1M KOH and pCa4.0 solution (in mM): 25 BES, 10 EGTA, 10.2 CaCl2, 6.9 MgCl2, 5.6 ATP, 25 creatine phosphate, 35.8 KMS, pH to 7.0 with 1M KOH. The solution of pCa6.2 was prepared by proportionate mixing of pCa9.0 and pCa4.0 solutions.
Statistics
All values are given as the mean ± SEM of between three and six experiments. Means were compared with an ANOVA and the Tukey HSD test, and statistical significance was taken at p < 0.05.
Results
These experiments were designed to investigate whether phosphorylation of PHI-1 leads to force enhancement at a constant Ca2+, or Ca2+ sensitization in avian smooth muscle strips. We phosphorylated PHI-1, in vitro, with Rho kinase using a previously published protocol [7], and confirmed PHI-1 phosphorylation with Western blotting (Figure 1). As is demonstrated, the anti–Thr57 phosphospecific PHI-1 antibody [7] does not recognize the nonphosphorylated, purified protein, while after Rho kinase treatment, Thr57 phosphorylated PHI-1 is easily detected. Further, both RhoA and Rho kinase are retained after skinning of the avian smooth muscle strips with TritonX-100 (Figure 2).
Figure 1. Rho kinase phosphorylates PHI-1.
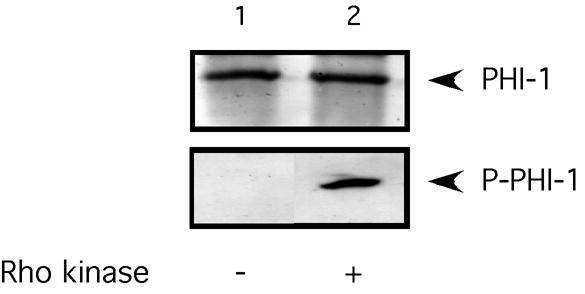
Purified PHI-1 was phosphorylated by Rho kinase (see text for details). Lane 1 shows the western blot of the purified protein, and lane two after Rho kinase treatment. Western blots were probed with anti-PHI-1 and antiphospho-PHI-1 antibodies. The Thr57 phospho-specific antibody only recognized the protein after phosphorylation.
Figure 2. Rho signaling is intact after Triton skinning.
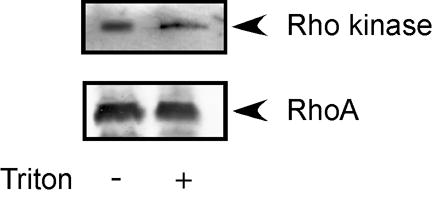
The expression of RhoA and Rho kinase was determined in intact smooth muscle and Triton skinned smooth muscle. As demonstrated, both RhoA and Rho kinase are retained following skinning of the smooth muscle.
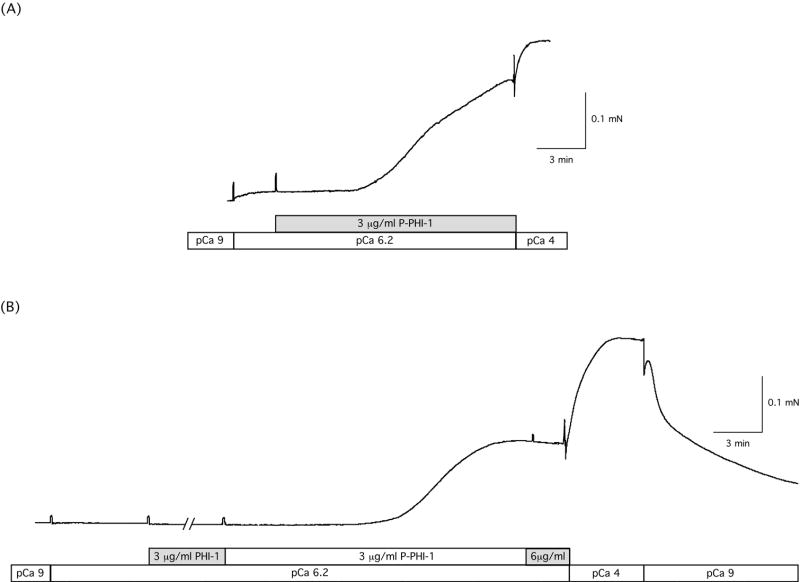
The effect of PHI-1 and P-PHI-1 on force in skinned gizzard strips is demonstrated in Figure 3 and summarized in Table I. When skinned gizzard strips were placed in pCa6.2 solution, there was no increase in force compared to pCa9.0. Further at pCa6.2, the addition of 3μg/ml PHI-1 did not lead to force enhancement (0.0 ± 3.4 mN/mm2, n=5, p>0.05). However at pCa6.2, 3μg/ml of P-PHI-1 increased force by 7.4 ± 1.7 mN/mm2 (n=5, p<0.05), which is ~ 50% of the level for maximal Ca2+ activation (18.4 ± 4.4 mN/mm2, p<0.05). In some instances, adding higher concentrations of PHI-1 also produced an increase in force. However, unlike P-PHI-1, which always increased force at 3μg/ml, higher concentrations of PHI-1 (> 6μg/ml) produced an increase in force only in 2 of 8 cases. Because the force increase at high concentrations of unphosphorylated PHI-1 occurred infrequently, it did not reach statistical significance.
Figure 3. P-PHI-1 leads to Ca2+ sensitization.
(A) A skinned gizzard strip was placed in relaxing solution (pCa9) and then at the first transferred to pCa6.2. P-PHI-1 (3 μg/ml) was added to the pCa6.2 and the preparation was then transferred to pCa4 solution (without PHI-1). The pCa solutions changes and the addition of P-PHI-1 are indicated below the data trace. (B) A skinned gizzard strip was placed in relaxing solution (pCa9) and then transferred to pCa6.2. PHI-1 (3 μg/ml) and then P-PHI-1 (1st, 3 μg/ml and then 6 μg/ml) was added to the pCa6.2 solution. The fiber was then transferred to pCa4 solution (without PHI-1 or P-PHI-1) and then to pCa9 solution (without PHI-1 or P-PHI-1). The pCa solutions changes and the addition of PHI-1 and P-PHI-1 are indicated below the data trace. As is demonstrated, the addition of P-PHI-1, but not PHI-1, resulted in a significant increase in force.
Table I.
Force
| (mN/mm2) | |
|---|---|
| pCa 6.2 | 0 ± 0 |
| pCa 6.2 + PHI-1 | 0.0 ± 3.4 |
| pCa 6.2 + P-PHI-1 | 7.4 ± 1.7 |
| pCa4 | 18.4 ± 4.4 |
Adult gizzard tissues were skinned with 1% Triton-X100, and stimulated with pCa 6.2 alone or pCa 6.2+PHI-1, or pCa 6.2+P-PHI-1. Force is given as the mean ± SEM (n = 4–5). P-PHI-1 significantly (p<0.05) increased force, but not to the level achieved with maximal Ca2+ activation (pCa 4).
MLC20 phosphorylation is the hallmark of smooth muscle contraction, and to determine if phosphorylation of PHI-1 enhances force by increasing MLC20 phosphorylation, we measured MLC20 phosphorylation in skinned gizzard smooth muscle strips at pCa6.2 alone, pCa6.2 + PHI-1, or pCa6.2 + P-PHI-1. At pCa6.2, the level of MLC20 phosphorylation in skinned gizzard strips was 45 ± 3.9% (n=4), and the addition of PHI-1 did not result in an increase in MLC20 phosphorylation (45 ± 1.9%, n=4, p> 0.05). In contrast, at pCa6.2, the addition of 3μg/ml P-PHI-1 increased MLC20 phosphorylation to 56 ± 3% (n=4, p<0.05), compared to 63 ± 4.3% (n=3, p< 0.05) at pCa4.0 (Figure 4 and Table II). This result is consistent with P-PHI-1 enhancing force by producing an increase in MLC20 phosphorylation.
Figure 4. P-PHI-1 increases MLC20 phosphorylation.
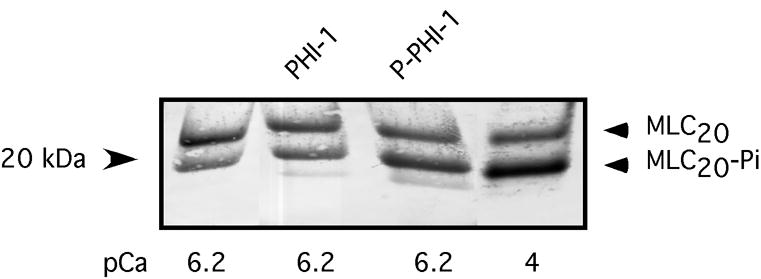
MLC20 phosphorylation was determined in skinned gizzard strips (n=4 for each condition) at pCa6.2 alone, pCa6.2 with PHI-1, pCa6.2 with P-PHI-1, as well as pCa4 (see Table II).
Table II.
MLC20 Phosphorylation (%)
| (%) | |
|---|---|
| pCa 6.2 | 45 ± 4 |
| pCa 6.2 + PHI-1 | 45 ± 2 |
| pCa 6.2 + P-PHI-1 | 56 ± 3 |
| pCa4 | 63 ± 4 |
Adult gizzard tissues were skinned with 1% Triton-X100, and stimulated with pCa 6.2 alone, pCa 6.2+PHI-1, or pCa 6.2+P-PHI-1 (n=3–4). The samples were then resolved and analyzed (see the text for details). P-PHI-1 stimulation significantly (p<0.05) increased the level of MLC20 phosphorylation compared to pCa 6.2, and MLC20 phosphorylation was further increased at pCa 4 (p<0.05).
Two mechanisms have been reported for MLCP inhibition. The first mechanism is phosphorylation of MYPT1 [4,5,16,17], and the second mechanism is direct binding of phosphatase inhibitors to the catalytic subunit of the enzyme [6,7,18]. Since we have previously demonstrated that MYPT1 phosphorylation does not change during GTPγS stimulation in avian smooth muscles [8], this suggests that P-PHI-1 could inhibit MLCP activation by binding to PP1c, similar to CPI-17 [18]. To investigate this possibility, we utilized a co-immunoprecipitation protocol. The data show that PHI-1 and PP1c interact in avian smooth muscles (Figure 5). G-protein stimulation is known to enhance the inhibitory potency of PHI-1 by Thr57 phosphorylation [7], which could occur if G-protein stimulation enhanced the binding of PHI-1 to the catalytic subunit. To study this, we added GTPγS, which increases PHI-1 phosphorylation [8], to the tissue lysates prior to co-immunoprecipitation. The addition of GTPγS resulted in an increase in the interaction of PHI-1 and PP1c (p<0.05), as evidenced by increase in the signal detected after probing with PHI-1 antibody (Figure 5). The increase in the interaction of PHI-1 and PP1c was not due to an increase in PP1c that is immunoprecipitated, as PP1c was similar before and after GTPγS treatment (Supplemental Figure).
Figure 5. P-PHI-1 binds to the active site of PP1c.
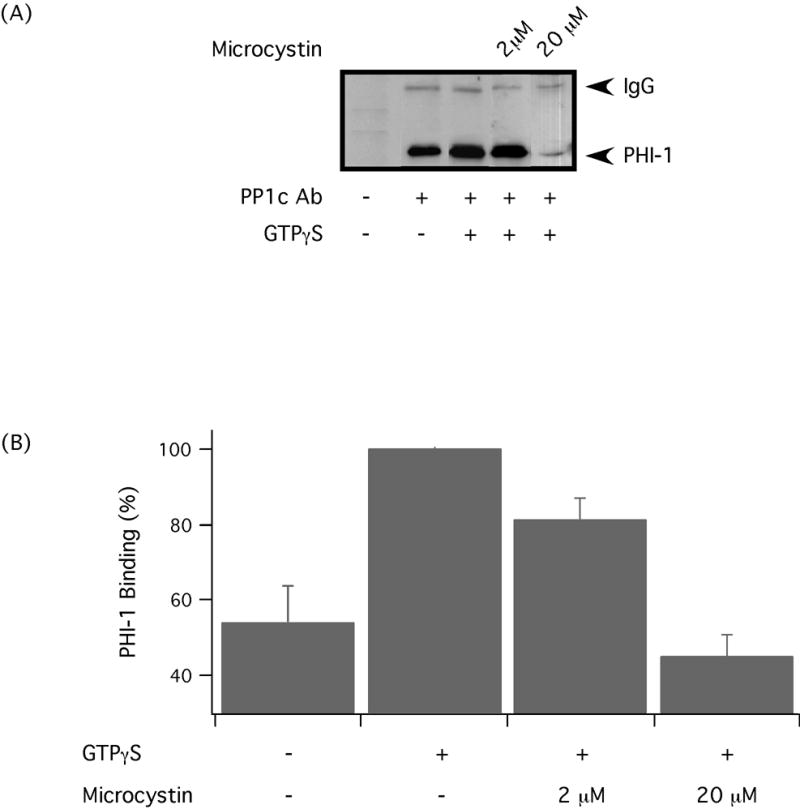
(A) Representative immunoprecipitation with anti-PP1c antibody and Western blot for PHI-1, which demonstrate the interaction of PP1c and PHI-1. Immunoprecipitation was also performed following the addition of GTPγS and microcytin to the tissue lysates. (B) Western blots were analyzed using densitometry, and the bar graph summarizing the results (n=3–6). The immunoprecipitations demonstrate an interaction between PP1c and PHI-1, which increases with phosphorylation of PHI-1. The addition of Microcystin LR results in a dose dependent decrease in the interaction of PP1c and PHI-1.
Supplemental Figure. GTPγS does not affect immunoprecipitation of PP1c.
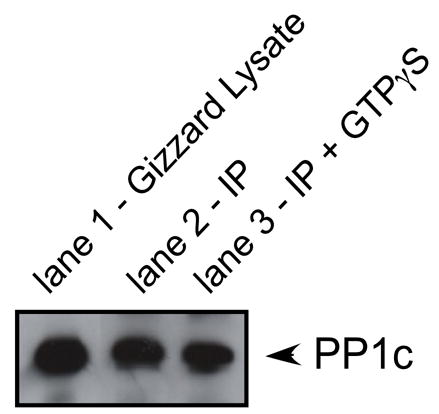
Western blot for PP1c in gizzard lysate (lane 1). Endogenous PP1c was immunoprecipitated from gizzard lysates in the absence (lane 2) and presence (lane 3) of GTPγS. Similar amounts of PP1c were detected in the immunoprecipitation with and without GTPγS.
To further investigate the mechanism of PHI-1 inhibition of MLCP, we used microcystin LR. Microcystin has been demonstrated to inhibit MLCP by binding to the active site of PP1c [18,19], which results in a Ca2+-independent increase in MLC20 phosphorylation and force [20,21]. Lysates were treated with microcystin after adding GTPγS. The addition of microcystin to the co-immunoprecipitation experiments resulted in a dose dependent reduction (p<0.05) in the signal detected with the PHI-1 antibody (Figure 5), which suggests that microcystin and PHI-1 could compete for the same binding site on PP1c.
Discussion
G-protein stimulation has been shown to inhibit MLCP with a resultant increase in MLC20 phosphorylation and force [22]. Phosphorylation of MYPT1 has been identified both in vivo and in vitro as a mechanism for the inhibition of MLCP [4,5,16,23,24]. However, a G-protein stimulation induced increase in MYPT1 phosphorylation appears to be species, tissue and agonist dependent [4,8,16,23–29]. In chicken smooth muscle, we have demonstrated that MYPT1 phosphorylation does not change during GTPγS stimulation [8]. These results suggest that a mechanism, other than MYPT1 phosphorylation, mediates Ca2+ sensitization in avian smooth muscle. One possibility is that a phosphatase inhibitor protein could inhibit MLCP to produce Ca2+ sensitization. Phosphorylation of protein-1 phosphatase inhibitors increases their inhibitory potency towards the phosphatase [6,7], and it has been previously demonstrated that the small phosphatase inhibitor protein, CPI-17, is phosphorylated by both PKC and Rho kinase mediated pathways [29,30]. Phosphorylation of CPI-17 results in CPI-17 binding to the active site of PP1c [18], and this leads to an inhibition of MLCP. However, CPI-17 expression is tissue and species specific [10], and is not expressed in avian smooth muscle tissue [8,10]. These findings raise the possibility that another phosphatase inhibitor protein exists in tissues with low levels of CPI-17 expression. PHI-1 belongs to the family of protein 1 phosphatase inhibitors and is homologous to CPI-17, with 29% sequence identity [7]. We have previously demonstrated that in chicken smooth muscles, a Rho-kinase mediated pathway leads to an increase in PHI-1 phosphorylation at Thr 57 during both agonist stimulation of intact smooth muscles, and GTPγS stimulation of skinned smooth muscle strips [8]. Phosphorylation of PHI-1 at Thr 57 is known to increase the inhibition of MLCP [7]. Our data demonstrate that the addition of P-PHI-1 to skinned avian smooth muscle results in a Ca2+ independent increase in force, and are similar to results reported for rat tail arterial strips [31].
Our results are the first demonstration that PHI-1 increases force and mediates Ca2+ sensitization in avian smooth muscle, and further extend the results of Deng et al. [31] by demonstrating that the P-PHI-1 induced increase in force is concomitant with an increase in MLC20 phosphorylation. Further, we have demonstrated that P-PHI-1 inhibits MLCP by interacting with the active site of PP1c. Our data demonstrate that PHI-1 can also interact with PP1c (Figure 5), consistent with data suggesting that both PHI-1 and P-PHI-1 inhibit MLCP. However, phosphorylation of PHI-1 increases the interaction of PHI-1 with PP1c (Figure 5). This is consistent with the results of the demonstrating that phosphorylation of PHI-1 at Thr57 increases the inhibitory potency of PHI-1 towards MLCP [7], as well as our data showing that 3 μg/ml of P-PHI-1, but not PHI-1, increases force, while higher concentrations (>6 μg/ml) of PHI-1 can occasionally lead to force enhancement.
We have previously demonstrated that a RhoA/Rho kinase pathway mediates the increase in MLC20 and PHI-1 phosphorylation during Ca2+ sensitization of skinned and intact avian smooth muscles [8]. Similarly, agonist stimulation of intact smooth muscle has been demonstrated to result in PHI-1 phosphorylation [8,32], suggesting that PHI-1 is part of a physiologically relevant pathway for force regulation. In the present study, the data demonstrate that the increase in PHI-1 phosphorylation increases its interaction with the active site of PP1c to inhibit MLCP and increase MLC20 phosphorylation and force.
In heart failure, there is an increase in renin angiotensin system activity together with the increase in vascular tone, which is thought to be an initial compensatory mechanism for the failing heart [33]. With further progression of the disease, this mechanism and others will contribute to remodeling of the failing heart. However, the molecular mechanism for the increase in vascular tone in heart failure is unknown. An increase in Angiotensin II activity could lead to a generalized increase in PHI-1 phosphorylation, Ca2+ sensitization, and a resting vasoconstriction, which could contribute to the increase in vascular tone observed in heart failure.
Acknowledgments
This study was supported by a grant from the NIH (HL64137; FVB).
References
- 1.Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem. 1992;267:14662–14668. [PubMed] [Google Scholar]
- 2.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 3.Hartshorne DJ, Ito M, Erdîdi F. Myosin light chain phosphatase: Subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem. 1996;271:4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- 5.Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002;527:101–104. doi: 10.1016/s0014-5793(02)03175-7. [DOI] [PubMed] [Google Scholar]
- 6.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem. 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- 7.Eto M, Karginov A, Brautigan DL. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999;38:16952–16957. doi: 10.1021/bi992030o. [DOI] [PubMed] [Google Scholar]
- 8.El-Touhky A, Given AM, Cochard A, Brozovich FV. PHI-1 induced enhancement of myosin phosphorylation in chicken smooth muscle. FEBS Lett. 2005;579:4271–4277. doi: 10.1016/j.febslet.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 9.Erdodi F, Kiss E, Walsh MP, Stefansson B, Deng JT, Eto M, Brautigan DL, Hartshorne DJ. Phosphorylation of protein phosphatase type-1 inhibitory proteins by integrin-linked kinase and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 2003;306:382–387. doi: 10.1016/s0006-291x(03)00976-8. [DOI] [PubMed] [Google Scholar]
- 10.Kitazawa T, Polzin AN, Eto M. CPI-17-deficient smooth muscle of chicken. J Physiol. 2004;557:515–528. doi: 10.1113/jphysiol.2004.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogut O, Brozovich FV. Determinants of the contractile properties in the embryonic chicken gizzard and aorta. Am J Physiol. 2000;279:C1722–C1732. doi: 10.1152/ajpcell.2000.279.6.C1722. [DOI] [PubMed] [Google Scholar]
- 12.Rhee AY, Brozovich FV. The smooth muscle cross-bridge cycle studied using sinusoidal length perturbations. Biophys J. 2000;79:1511–1523. doi: 10.1016/S0006-3495(00)76402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facemire C, Brozovich FV, Jin JP. The maximal velocity of vascular smooth muscle shortening is independent of the expression of calponin. J Muscle Res Cell Motil. 2000;21:367–373. doi: 10.1023/a:1005680614296. [DOI] [PubMed] [Google Scholar]
- 14.Richards CT, Ogut O, Brozovich FV. Agonist-induced force enhancement. The role of isoforms and phosphorylation of the myosin-targeting subunit of myosin light chain phosphatase. J Biol Chem. 2002;277:4422–4427. doi: 10.1074/jbc.M111047200. [DOI] [PubMed] [Google Scholar]
- 15.Brozovich FV, Yamakawa M. Thin filament regulation of force activation is not essential in single vascular smooth muscle cells. Am J Physiol. 1995;268:C237–C242. doi: 10.1152/ajpcell.1995.268.1.C237. [DOI] [PubMed] [Google Scholar]
- 16.Trinkle-Mulcahy L, Ichikawa K, Hartshorne DJ, Siegman MJ, Butler TM. Thiophosphorylation of the 130-kDa subunit is associated with a decreased activity of myosin light chain phosphatase in alpha-toxin-permeabilized smooth muscle. J Biol Chem. 1995;270:18191–18194. doi: 10.1074/jbc.270.31.18191. [DOI] [PubMed] [Google Scholar]
- 17.Borman MA, MacDonald JA, Muranyi A, Hartshorne DJ, Haystead TA. Smooth muscle myosin phosphatase-associated kinase induces Ca2+ sensitization via myosin phosphatase inhibition. J Biol Chem. 2002;277:23441–23446. doi: 10.1074/jbc.M201597200. [DOI] [PubMed] [Google Scholar]
- 18.Eto M, Kitazawa T, Brautigan DL. Phosphoprotein inhibitor CPI-17 specificity depends on allosteric regulation of protein phosphatase-1 by regulatory subunits. Proc Natl Acad Sci. 2004;101:8888–8893. doi: 10.1073/pnas.0307812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg J, Huang HB, Kwon YG, Greengard P, Nairn AC, Kuriyan J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature. 1995;376:745–753. doi: 10.1038/376745a0. [DOI] [PubMed] [Google Scholar]
- 20.Kitazawa T, Kobayashi S, Horiuti K, Somlyo AV, Somlyo AP. Receptor coupled, permeabilized smooth muscle: Role of the phosphatidylinositol cascade, G-proteins and the modulation of the contractile response to Ca2+ 1989;264:5339–5342. [PubMed] [Google Scholar]
- 21.Ikebe M, Brozovich FV. Protein kinase C increases force and slows relaxation in smooth muscle: Evidence for regulation of myosin light chain phosphatase. Biochem Biophys Research Comm. 1996;225:370–376. doi: 10.1006/bbrc.1996.1182. [DOI] [PubMed] [Google Scholar]
- 22.Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1992;266:1708–1715. [PubMed] [Google Scholar]
- 23.Sward K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J Physiol. 2000;522:33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macdonald JA, Borman MA, Muranyi A, Somlyo AV, Hartshorne DJ, Haystead TA. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci. 2001;98:2419–2424. doi: 10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niiro N, Ikebe M. Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J Biol Chem. 2001;276:29567–29574. doi: 10.1074/jbc.M102753200. [DOI] [PubMed] [Google Scholar]
- 26.Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin-binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J. 2003;369:117–128. doi: 10.1042/BJ20021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–9900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- 28.Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol. 2003;546:879–89. doi: 10.1113/jphysiol.2002.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama M, Ito M, Feng J, Seko T, Shiraki K, Hartshorne DJ, Nakano T. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle phosphatase, by Rho-kinase. FEBS Lett. 2001;475:197–200. doi: 10.1016/s0014-5793(00)01654-9. [DOI] [PubMed] [Google Scholar]
- 30.Masuo M, Reardon S, Ikebe M, Kitazawa T. A novel mechanism for the Ca2+-sensitizing effect of protein kinase C on vascular smooth muscle: Inhibition of myosin light chain phosphatase. J Gen Physiol. 1994;104:265–286. doi: 10.1085/jgp.104.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1 by integrin-linked kinase. Biochem J. 2002;367:517–24. doi: 10.1042/BJ20020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang H, Guo Z, Su W, Xie Z, Eto M, Gong MC. RhoA-Rho kinase pathway mediates thrombin- and U-46619-induced phosphorylation of a myosin phosphatase inhibitor, CPI-17, in vascular smooth muscle cells. Am J Physiol. 2005;289:C352–C360. doi: 10.1152/ajpcell.00111.2005. [DOI] [PubMed] [Google Scholar]
- 33.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]