Abstract
Transcriptional activation of T4 middle promoters requires σ70-containing E. coli RNA polymerase, the T4 activator MotA, and the T4 co-activator AsiA. T4 middle promoters contain the σ70 –10 DNA element. However, these promoters lack the σ70 –35 element, having instead a MotA box centered at –30, which is bound by MotA. Previous work has indicated that AsiA and MotA interact with region 4 of σ70, the C-terminal portion that normally contacts –35 DNA and the β-flap structure in core. AsiA binding prevents the σ70/β-flap and σ70/-35 DNA interactions, inhibiting transcription from promoters that require a –35 element. To test the importance of residues within σ70 region 4 for MotA and AsiA function, we investigated how σ70 region 4 mutants interact with AsiA, MotA, and the β-flap and function in transcription assays in vitro. We find that alanine substitutions at residues 584-588 (region 4.2) do not impair the interaction of region 4 with the β-flap or MotA, but they eliminate the interaction with AsiA and prevent AsiA inhibition and MotA/AsiA activation. In contrast, alanine substitutions at 551-552, 554-555 (region 4.1) eliminate the region 4/β-flap interaction, significantly impair the AsiA/σ70 interaction, and eliminate AsiA inhibition. However, the 4.1 mutant σ70 is still fully competent for activation if both MotA and AsiA are present. A previous NMR structure shows AsiA binding to σ70 region 4, dramatically distorting regions 4.1 and 4.2 and indirectly changing the conformation of the MotA interaction site at the σ70 C-terminus. Our analyses provide biochemical relevance for the σ70 residues identified in the structure, indicate that the interaction of AsiA with σ70 region 4.2 is crucial for activation, and support the idea that AsiA binding facilitates an interaction between MotA and the far C-terminus of σ70.
Keywords: transcription, σ70, AsiA, MotA, polymerase
Introduction
Prokaryotic RNA polymerase holoenzyme is composed of a core (subunits β, β′, α2, ω) that has RNA synthesizing activity and a σ factor that imparts DNA specificity 1; 2. The primary σ factor in E. coli, σ70, recognizes promoters that are needed for transcription of general housekeeping genes and thus, is responsible for most of the transcription during exponential growth 3. Sequence, structure, and biochemical studies indicate that σ70 shares four regions of similarity with primary σ proteins of other prokaryotes 1; 4. When present in RNA polymerase holoenzyme (Eσ70), σ70 sets the start site for transcription by recognizing various DNA elements, and residues within each of the 4 regions have been shown to interact with sequences in promoter DNA 5; 6; 7; 8; 9. The majority of E. coli promoters have –10 and –35 DNA elements, which are contacted by σ70 regions 2 and 4, respectively 8; 9. Specific base recognition of the -35 sequences arises through a DNA-binding helix-turn-helix (H-T-H) motif in region 4.2 (H3-T-H4 in Fig. 1A). In addition, interactions between region 4 and core, particularly a structure called the β-flap, are required for proper positioning of regions 2 and 4 so they can contact the –10 and –35 elements simultaneously 10; 11; 12; 13 (Fig. 1A).
Fig. 1.
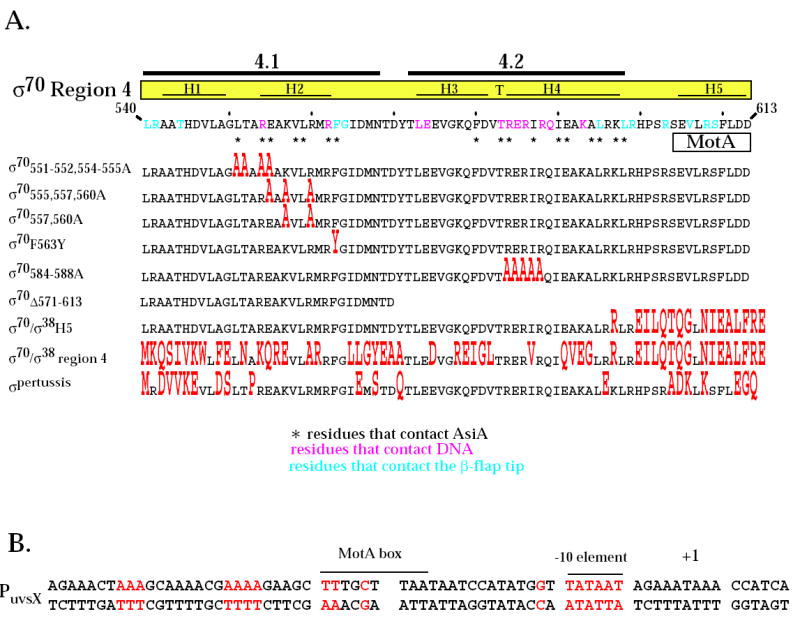
Region 4 of wild type σ70 and mutant σ proteins and the T4 middle promoter PuvsX. A. The C-terminal amino acid sequence of σ70 is shown from residues 540 to 613. Residues in pink contact the -35 element of E. coli promoter DNA 8 while residues in blue contact the β-flap tip 11; 12; 13; 45. Every tenth residue is marked with a small vertical line. Above the sequence is shown the locations of regions 4.1 and 4.2 1 and the positions of the α-helices H1, H2, and H5 and the helix-turn-helix H3-T-H4 structures observed in region 4 of the primary σ of T. aquaticus complexed with -35 element DNA 8. Residues that contact AsiA in the AsiA/σ70 region 4 structure 30 are designated with an asterisk and the region of the MotA contact site 20 is indicated. Below are shown the region 4 sequences of the mutant σ proteins, with residues that differ from σ70 in red. B. The sequence of PuvsX from −61 to +9 is shown with the start of transcription indicated as +1. The locations of the σ70 –10 element and the MotA box are indicated. Sequences within PuvsX that match the canonical polymerase elements σ70 -10 element (TATAAT); σ70 extended -10 element (-15T, -14G); σ70 -35 element (TTGACA); and α UP element (AAAa/ta/tTa/tTTTTnnAAAA) are in red.
The presence of a good match to both the -10 and -35 canonical sequences is usually sufficient for Eσ70 to recognize a promoter without the aid of additional factors. However, activation factors can be needed when either sequence element deviates significantly from the consensus. Many activators in E. coli work through class I or class II activation mechanisms (reviewed in 14; 15). Class I activators interact with residues in the C-terminal domain (CTD) of the α subunit of polymerase whereas a class II activator may also interact with residues in σ70 region 4. MotA/AsiA activation of bacteriophage T4 middle promoters arises through a different mechanism, called σ appropriation (reviewed in 16). MotA protein is a transcriptional activator 17; 18; 19, whose CTD binds to a MotA box element, centered at -30 in T4 middle promoter DNA, and whose NTD interacts with the far C-terminal portion of σ70, 20 (Fig. 1A). AsiA is needed to co-activate transcription from T4 middle promoter 21. However, AsiA by itself is also an inhibitor of σ70-dependent transcription from -10/-35 promoters 22; 23; 24. Several studies have indicated that AsiA binds tightly to σ70 regions 4.1 and 4.2 22; 23; 25; 26; 27; 28; 29; 30; 31; 32 (Fig. 1A). A recent structure of the AsiA/σ70 region 4 complex implicated 17 σ70 residues as AsiA contacts and indicated that AsiA structurally remodels region 4, converting the DNA–binding H3-T-H4 to a continuous helix 30. It has been suggested that this remodeling could also serve to facilitate the interactions of MotA with the MotA box and with the C-terminus of σ70, 20; 30.
Here we have performed mutational analyses to investigate the molecular interface used by σ70 with AsiA and MotA, and to ask whether there is biochemical support for σ70 residues that were identified in the σ70 region 4/AsiA structure. We demonstrate that substitutions of σ70 residues in regions 4.1 and 4.2 that interact with AsiA in the AsiA/σ70 region 4 structure 30 do indeed impair the interaction of AsiA with σ70. We also find that substitutions within region 4.1 that significantly compromise the interaction of AsiA with σ70 impair the ability of AsiA to inhibit transcription. However, in the presence of both MotA and AsiA, polymerase containing the 4.1 substitutions remains fully competent for activation. In contrast, substitutions in σ70 region 4.2 that eliminate the AsiA/σ70 interaction render polymerase immune both to AsiA inhibition and MotA/AsiA activation. Our work indicates that the interaction of AsiA with its contacts in σ70 region 4.2 is crucial for σ appropriation by MotA and AsiA and supports a model in which AsiA binding and remodeling of σ70 region 4 serve to facilitate the interaction of MotA with σ70 and with MotA box DNA.
Results
Rationale
Based on crystal structures using the primary σ factors of T. aquaticus and T. thermophilus and biochemical analyses with σ70 mutants (reviewed in 2; 33), specific residues with σ70 region 4 are thought to be crucial for transcription from σ70-dependent -10/-35 promoters, either because they contact the -35 DNA element or because they interact with the β-flap structure of core. The NMR structure of a complex of AsiA with σ70 region 430 shows that many of these same residues contact AsiA and thus, predicts that these residues may also be important for AsiA inhibition and/or MotA/AsiA activation. In addition, biochemical evidence 20 has suggested that some of the residues within the far C-terminal portion of σ70 that normally interact with the β-flap may coincide with residues that contact MotA during MotA/AsiA activation. (These various residues in regions 4.1 and 4.2 and the C-terminal portion of σ70 are indicated at the top of Fig. 1A.)
To investigate the functional significance of several of the σ70 residues that the structural work predicts could be important for AsiA and MotA function, we used σ70 proteins with mutations within regions 4.1 and 4.2 and the C-terminal portion of σ70 (listed in the bottom portion of Fig. 1A). Mutations in region 4.1 included one at F563 (F563Y), which was previously found in a 2-hybrid screen to be somewhat defective for an interaction with AsiA34. However, this and another screen did not reveal an importance for other region 4.1 residues that contact AsiA in the AsiA/region 4 structure 34; 35. Thus, we constructed σ70 mutants with alanine substitutions within region 4.1 that should either affect (σ70551-552, 554-555A) or not affect (σ70557, 560A) the interaction of AsiA with region 4 based on the AsiA/region 4 structure. For region 4.2, we made a σ70 mutant that contained alanine substitutions at residues that directly interact with the –35 DNA element (σ70584-588A). Although residues within this portion of region 4.2 interact with AsiA in the structure, a previous analysis of region 4.2 substitutions had not revealed an importance for this portion of 4.2 for AsiA binding 36. Finally, we compared the functions of a fused σ70/σ38 protein that contains multiple changes within the C-terminal portion of σ70 (σ70/σ38H5) with the function of a σ70/σ38 fusion (σ70/σ38 region 4) that contains multiple changes throughout region 4, including residues that are predicted to interact with AsiA, and with the function of the primary σ of pertussis (σpertussis) that despite multiple changes within region 4 from σ70, retains all the predicted AsiA contact residues. The various σ70 mutants were then tested in assays to determine the effects of the σ70 mutations on AsiA inhibition, MotA/AsiA activation, and the interactions of σ70 region 4 with AsiA, MotA, and the β-flap.
Substitutions within σ70 region 4.1 that impair the interaction of σ70 with AsiA do not affect MotA/AsiA activation at the T4 middle promoter PuvsX
During a T4 infection, transcription from most T4 middle promoters, such as PuvsX (Fig. 1B), is fully dependent on AsiA and MotA. However, in vitro wild type polymerase can use PuvsX in the absence of MotA and AsiA, presumably because of the presence of a perfect σ70 –10 element and partial matches to the σ70 –35 element and to the UP element sequence (for binding by the α subunit of core 37). Consequently, we used transcription from PuvsX to monitor the effect of various σ70 mutations on transcription by polymerase alone and with AsiA and/or MotA (Fig. 2). As has been seen previously 38, polymerase containing wild type σ70 yields a moderate level of PuvsX transcription in vitro, which is completely inhibited by the presence of AsiA. Addition of both AsiA and MotA yields a high level of activated transcription. In addition, in single round transcription assays and under certain conditions, such as the ones used here, we have found about a 2-fold increase in transcription in the presence of MotA without AsiA (Makela and Hinton, manuscript in preparation).
Fig. 2.

Transcriptional activities of polymerases reconstituted with mutant σ proteins using PuvsX in vitro in the presence of AsiA and/or MotA. The indicated σ (0.2 pmol) and AsiAHis6 (3 pmol ) were incubated in 1.8 μl of protein buffer I for 10 min at 37° C. A solution containing 0.05 pmol of core in 1.1 μl of protein buffer II was then added, and the resulting solution was incubated for an additional 10 min at 37° C. Transcription was initiated by adding this solution to 2.1 μl of DNA buffer containing 0.01 pmol DNA. After 37° C for 20 sec, rifampicin (150 ng) was added to limit transcription to a single round. For each indicated σ, [the amount of PuvsX RNA observed]/[the amount of PusvX RNA observed with σ70 polymerase in the absence of MotA and AsiA] is shown using polymerase alone, polymerase with MotA, polymerase with AsiA, and polymerase with AsiA and MotA. Values were determined from two or more transcriptions. Error is indicated by darker shaded area. In some cases where the level of transcription was low, 10-fold values (10 × values) are also shown. In these cases, zero is the bottom of the enclosed box.
Like wild type polymerase, the polymerases reconstituted with any of the σ proteins having substitutions within region 4.1 (σ70551-552,554-555A, σ70557,560A, σ70555,557,560A, σ70F563Y) were fully competent for MotA/AsiA activation at PuvsX (Fig. 2). Thus, none of these mutations impaired σ appropriation at PuvsX. In contrast, AsiA inhibition was not observed when using σ70551-552, 554-555A (Fig. 2A) and as has been reported previously 34, AsiA inhibition was impaired by σ70F563Y (Fig. 2B). (Although the level of transcription with σ70551-552, 554-555A was extremely low, the results were highly reproducible.)
The ability of the σ70 mutants to interact with AsiA and MotA was investigated by two independent assays of protein-protein interactions. In the native gel assay, free σ was incubated with excess AsiA and/or MotA and the formation of protein-protein complexes was detected by a shift in σ migration. Previous work 39; 40 has shown that either AsiA or MotA alone forms a complex with wild type σ70 and a new species is formed in the presence of all three, indicative of a tertiary complex (Fig. 3). In the E. coli 2-hybrid assay, the interaction of σ70 region 4 with a bait protein, here AsiAK20A or the N-terminal half of MotA (MotANTD), which is known to contain the activation domain 20, was monitored by an increase in the level of activity of a reporter gene, lacZ (Fig. 4A). AsiA with the substitution K20A was used in this assay because previous work has shown that AsiAK20A results in a higher level of β-galactosidase activity when tested with σ70 region 4 than does wild type AsiA 34.
Fig. 3.
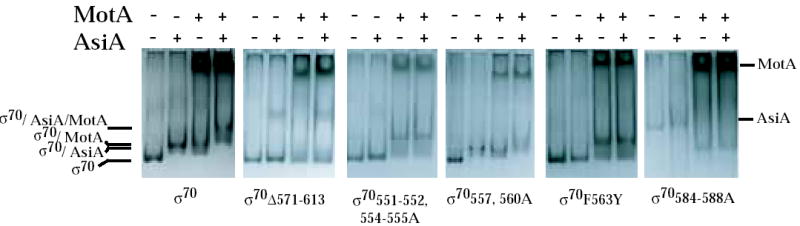
σ/AsiA, σ/MotA, and AsiA/σ/MotA complexes in native protein gels. The indicated σ was incubated with excess His6AsiA and/or MotA and then subjected to electrophoresis through native 6 % acrylamide gels as described in Materials and Methods. The positions of σ, the σ/AsiA complex, the σ/MotA complex, and the AsiA/σ/MotA complex are shown. The positions of free MotA and AsiA are also marked. Excess free MotA protein is the dark band that migrates near the top of the gel in the lanes with added MotA. The excess free AsiA is usually not seen because AsiA is a small protein (10 kDa) and stains poorly.
Fig. 4.
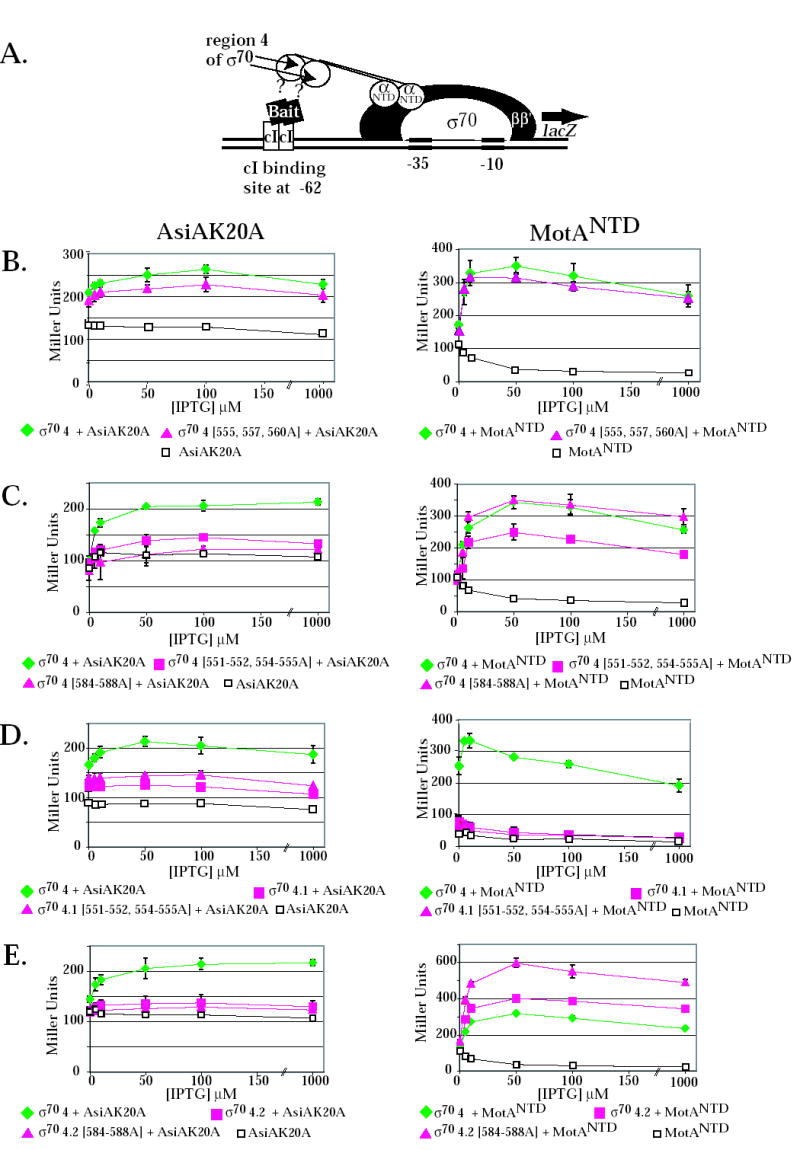
2-hybrid assay and the effect of σ70 substitutions on the interaction of σ70 region 4 with the AsiAK20A and MotANTD. (A) Cartoon shows the test promoter, which has a λ operator (OR) located 62 bp upstream from the initiation site of the lac core promoter. The presence of the α-σ chimera protein, expressed by a pBRα-σ plasmid, and the cI-bait fusion protein, expressed by a pcI-bait plasmid, can result in an increase in lacZ transcription and subsequent β-galactosidase activity if there is an interaction between the σ present in the chimera and the bait protein. The addition of IPTG induces expression of both the α-σ chimera and the cI-bait fusion protein. B-E. Effect of σ70 mutations on the interaction of σ70 region 4 with AsiAK20A (left panels) and MotANTD (right panels). Cultures contained the pcI-AsiAK20A or pcI-MotANTD plasmid and either the control plasmid, pBRα that lacks σ70 region 4, or as indicated, a pBRα-σ70 plasmid that has σ70 region 4 (σ70 4), σ70 region 4.1 (σ70 4.1), σ70 region 4.2 (σ70 4.2) with or without the indicated σ70 substitutions. Graphs show β-galactosidase activity (in Miller units) versus the concentration of IPTG present in the cultures.
Interaction assays indicated that alanine substitutions at σ70 residues 555, 557, and 560 had little effect on the interaction of σ70 with AsiA or MotA. The pattern of complexes seen in the native gel with σ70557,560A was very similar to that seen with wild type σ70 (Fig. 3). 2-hybrid assays indicated that the σ70 555, 557, 560A substitutions had no effect on the interaction with MotANTD (Fig. 4B, right) and only a slight decrease in the interaction with AsiAK20A (Fig. 4B, left). These results are consistent with the fact either σ70 555, 557, 560A or σ70 557, 560A yielded transcription patterns that were very similar to that of wild type σ70 (Fig. 2A).
Results obtained in the interaction assays with the other 4.1 mutants, σ70 551-552, 554-555A and σ70 F563Y, were different. Previous results have shown that the σ70 F563Y substitution renders σ70 region 4 defective in its interaction with AsiAK20A in the 2-hybrid assay 34. We found that the 551-552, 554-555A substitutions also impaired the interaction of σ70 with AsiAK20A (Fig.4C, left). In contrast, these substitutions had a more modest effect on the σ70 region 4/MotANTD interaction (Fig. 4C, right). In native gels, σ70 551-552, 554-555 and σ70 F563Y were very slightly retarded when AsiA was added (Fig. 3). In the presence of MotA, a much slower migrating species was formed, consistent with the formation of a stable MotA/σ70 complex. Addition of AsiA did not significantly change the position of this species, although a very slight shift (like that seen with AsiA alone) would not have been detected. We conclude that the 551-552, 554-555A and the F563Y substitutions result in a σ70 that is impaired in its interaction with AsiA but not with MotA. Consequently, σ70 mutant proteins having these substitutions are less susceptible to inhibition by AsiA because they are less able to interact with AsiA. However, the defect for AsiA binding does not impair MotA/AsiA activation. Thus, during transcription either the presence of MotA can improve the defective interaction of AsiA with σ70 551-552, 554-555A or σ70 F563Y or an impaired interaction of AsiA with region 4.1 is still sufficient for AsiA to work as a co-activator.
We also used the two-hybrid assays to investigate the interactions of AsiAK20A and MotANTD with region 4.1 alone (Fig. 4D). We found that AsiAK20A interacts weakly with region 4.1 in the absence of region 4.2. Surprisingly, this interaction was not disrupted by the 551-552, 554-555A substitutions, suggesting that it might arise through different contacts than those used when AsiA interacts with all of region 4. In addition, we found that σ70 region 4.1 alone was not sufficient for an interaction with MotANTD.
Alanine substitutions at residues 584-588 within σ70 region 4.2 render σ70 defective for an interaction with AsiA and for AsiA inhibition and MotA/AsiA activation at PuvsX
The side chains of σ70 4.2 residues R584, E585, and R588 are thought to interact directly with specific base determinants in the –35 sequences of host promoter DNA 8; 35; 41; 42; 43. Residues R584 and I587 were identified as contact residues for AsiA in the AsiA/σ70 region 4 structure 30 (Fig. 1A), suggesting that AsiA prevents the binding of σ70 with the –35 element in part by directly blocking the σ70 region 4/DNA interaction. However, a previous analysis, investigating how single alanine substitutions affect the binding AsiA to σ70 region 4.2 peptides, had found that residues 584-588 were not important for the interaction of AsiA with region 4.2 36. To determine the importance of this portion of region 4.2 when present within all of region 4, we constructed σ70 with alanine substitutions at residues 584-588. In the 2-hybrid assay, these mutations eliminated the interaction of AsiAK20A with σ70 region 4 (Fig. 4C, left), indicating that one or more of residues 584-588 are indeed important for an interaction with AsiA. However, we also found that these 4.2 contacts are not sufficient because the interaction of σ70 residues 569-613 (region 4.2 plus the C-terminal end of σ70) with AsiA was low (Fig. 4E, left). Results with the native gel assay were difficult to interpret because σ70584-588A migrated very differently from wild type σ70 (Fig. 3). Nevertheless, the addition of AsiA did not significantly affect this migration, a result that was consistent with the lack of a detectable interaction between AsiA and σ70 584-588A in the 2-hybrid assay.
In contrast to the results with AsiAK20A, the σ70584-588A did not impair the interaction of σ70 with MotANTD. In 2-hybrid assays with all of σ70 region 4 and MotANTD, the 584-588 alanine substitutions gave results that were similar to wild type region 4 (Fig. 4C, right). When using a portion of σ70 containing residues 569-613 (region 4.2 to the end of σ70) (Fig. 4E, right), the presence of the substitutions even increased its interaction with MotANTD. In native gels, the migration of σ70584-588A was shifted in the presence of MotA without AsiA and the addition of AsiA did not change this migration (Fig. 3). The σ70 584-588A/MotA complex migrated differently from the complex formed with wild type σ70 and MotA, again perhaps because the mutant σ alone migrated aberrantly.
In in vitro transcription assays, PuvsX transcription by Eσ70584-588A was not inhibited by AsiA alone or activated by MotA/AsiA (Fig. 2A). (The 2-fold increase seen with MotA/AsiA is not significant because it is also observed with MotA alone). We conclude that alanine substitutions at σ70 residues 584-588 specifically impair the interaction of AsiA with σ70 region 4, and this impairment then eliminates AsiA inhibition and MotA/AsiA activation at PuvsX.
Mutation of the far C-terminal region of σ70 significantly reduces MotA/AsiA activation
Previous work has indicated that the far C-terminal region of σ70 is needed for an interaction between MotANTD and σ70, 20. Specifically, in the 2-hybrid assay, a σ70/σ38H5 mutation, in which the last 17 residues of σ70 (Helix 5 (H5) in Fig. 1A) have been replaced with the corresponding residues of σ38, eliminates the interaction of MotANTD with σ70 region 4, but decreases the interaction with AsiA by only about 50% 20. When we tested this mutant in transcription assays, we found that AsiA inhibition was normal and the modest increase in transcription observed with MotA alone was present, but MotA/AsiA activation was significantly reduced (Fig. 2B). This result supports the idea that during σ appropriation, the interaction of σ70 H5 with MotA is crucial for activation. However, the increase in transcription observed with MotA alone as well as the low level of MotA/AsiA activation that was observed with σ70/σ38H5 also suggest that either a very poor interaction of σ38 H5 with MotA is still somewhat productive for transcription or there is another interaction site for MotA, besides H5, that can promote transcription.
We also tested σ70Δ571-613, which removes the far C-terminal region of σ70 as well as all of region 4.2. Previous work with a similar deletion (σ70Δ 565-613) had indicated that when only region 4.1 is present, AsiA alone significantly increases transcription from the T4 middle promoter PrIIB2 44. Thus, we wondered whether a similar result would be observed with PuvsX. However, in this case, transcription was unaffected by the addition of AsiA or MotA, and there was only a small, but reproducible increase in transcription when both MotA and AsiA were present (Fig. 2A). As was discussed before, in 2-hybrid assays the interaction of region 4.1 alone with MotANTD was nearly background and the interaction with AsiA was significantly lower than that seen with all of region 4 (Fig. 4D). In native gels, the migration of σ70Δ571-613 was unaffected by the addition of MotA, AsiA, or AsiA and MotA (Fig. 3). These results are consistent with the idea that the deletion of regions 4.2 and H5 significantly impairs the interaction of σ70 with either AsiA or MotA. However, the slight increase in transcription that is observed when both MotA and AsiA are present suggests that a weak interaction of AsiA with region 4.1 and an interaction of MotA with a region other than H5 can weakly promote transcription.
Replacement of σ70 region 4 with region 4 of σ38 eliminates interactions with AsiA and MotA while the primary σ of B. pertussis is competent for MotA/AsiA activation
As seen in Fig. 1A, region 4 of σ38 differs from region 4 of σ70 at many residues, including those that interact with AsiA in the AsiA/σ70 structure and those that include the MotA site in H5. The addition of either AsiA alone or MotA and AsiA together had no significant effect on PuvsX transcription when using Eσ70/σ38region 4 (Fig. 2B), indicating that σ38 region 4 is not competent for AsiA inhibition or MotA/AsiA activation. Furthermore, the 2-fold increase in PuvsX transcription seen with MotA alone was also eliminated when using Eσ70/σ38region 4. When combined with the results with σ70/σ38H5 and σ70Δ571-613, these results suggest that another interaction site for MotA may lie within region 4.1.
The primary σ of B. pertussis also varies from σ70 at many residues, including several in region 4 (Fig. 1). However, all of the residues that contact AsiA in the σ70 region 4/AsiA structure are identical in the E. coli and B. pertussis primary σ proteins. We found that Eσpertussis was significantly inhibited by AsiA (Fig. 2B), which is consistent with known AsiA/σ70 contacts. In contrast, the far C-terminal region (H5) of the pertussis σ differs at several residues from that of σ70. Despite these differences, we found that Eσpertussis was significantly activated by MotA/AsiA (Fig. 2B). This result suggests that none of the differences between σpertussis and σ70 in H5 are crucial for the σ70/MotA interaction.
Effect of σ70 mutations on the interaction of σ70 region 4 with the β-flap
Because many residues within region 4 are thought to interact with the β-flap 10; 11; 12; 13 and AsiA is thought to prevent the σ70/β-flap interaction 34, we investigated the effect of two of the σ70 region 4 mutants on the interaction of region 4 with the β-flap in the 2-hybrid assay. Previous work has shown that the interaction of region 4 with the β-flap can be monitored using this assay, provided that σ70 region 4 contains the substitution D581G 13. D581G is thought to stabilize the H-T-H conformation of region 4.2 (H3-T-H4 in Fig. 1A) and does not affect the interaction of region 4 with the -35 DNA element 13; 45. In addition, previous work has also indicated that region 4 of σ38, which contains a glycine at the position corresponding to σ70 D581, is fully competent for the interaction with the β-flap 13. We found here that the alanine substitutions at 551-552, 554-555 eliminated the interaction of σ70 region 4 with the β-flap (Fig. 5A). This result is consistent with the very low level of transcription observed by polymerase containing the σ70 551-552, 554-555A mutant in the absence of AsiA and MotA (Fig. 2A). However, this result was surprising since none of the substituted residues have been implicated in an interaction with the β-flap (Fig.1A) 45; 11; 12. In contrast, the interaction of region 4 with the β-flap was actually increased by the alanine substitutions at 584-588 (Fig. 5B). We conclude that the significant reduction in transcription observed with polymerase containing σ70 584-588A is not due to its inability to interact with the β-flap. Thus, it is likely to stem from the loss of the side chains at R584, E585, and R588, which are thought to interact directly with base determinants in the –35 DNA 8; 35; 42; 43.
Fig. 5.
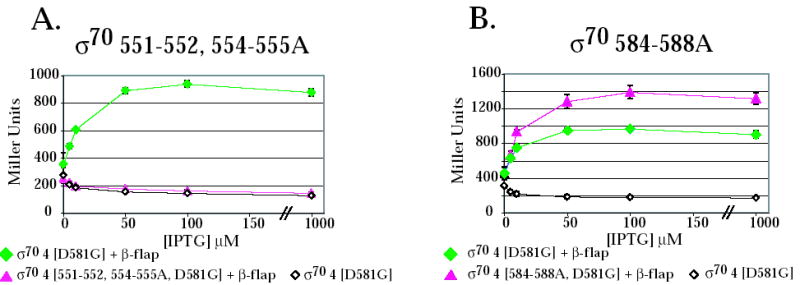
The σ70 substitutions 551-552,554-555A (A) eliminate the interaction of the β-flap with σ70 region 4 whereas substitutions 584-588A (B) do not impair the interaction of σ70 region 4 with the β-flap. Cultures contained a pBRα-σ70 plasmid, which had σ70 region 4 (σ70 4) with the indicated substitutions, and either the control plasmid, pACλcI32, the pcI plasmid that lacks a fused bait protein, or the pcI-β-flap plasmid (β-flap). Graphs show β-galactosidase activity (in Miller units) versus the concentration of IPTG present in the cultures.
In addition to the interaction between σ70 region 4 and the β-flap, contact between σ70 region 4.1 residues 555, 562, and 565 and another region of core, a portion of β′ (residues 393 to 402), has been proposed 10; 46. However, our 2-hybrid assays using pcI-rpoC (357-430) and pBRα-σ70 or pBRα-σ70D581G failed to detect any interaction (data not shown).
Discussion
Interface of σ70 region 4 with AsiA and MotA
T4 middle promoters are hybrid promoters, which contain an excellent match to the σ70 –10 element, but replace the σ70 –35 element with the MotA box sequence centered at –30 (reviewed in 47). The T4 MotA and AsiA proteins are needed to switch the sequence specificity of polymerase for the upstream promoter DNA (reviewed in 16). For host promoters, polymerase recognition of a promoter sequence involves contact with the DNA but also the correct positioning of the DNA-binding domains within polymerase. In E. coli, recognition of -10/-35 promoters requires interaction between residues in σ70 region 2 and a -10 element and between residues in regions 4.1 and 4.2 and a -35 DNA element, respectively 1; 8; 9. (Fig. 6A; highlighted in pink in Fig. 1A). Specific base determinants in the –35 DNA are contacted by 4.2 residues R584 8; 35; 42, E585 8; 43, and perhaps R588 41, which are present in the second helix of H3-T-H4 (Fig. 1A). The interactions between region 4 and the β-flap are also needed to position region 4 properly relative to region 2, allowing simultaneous contact of both the –10 and –35 elements 10; 11; 12; 13; 48. σ70 residues, which are needed for this contact, have been located within region 4.1 and at the C-terminus of σ70, including H5 11; 12; 34; 45 (highlighted in blue in Fig. 1A) Our work suggests that residues 551-552, 554-555 are also important. Alanine substitutions at these positions eliminate the interaction of σ70 with the β-flap in a 2-hybrid assay (Fig. 5A) and result in very low levels of transcription by polymerase alone (Fig. 2A). Thus, assuming that the interaction between the β-flap peptide and σ70 region 4 in the 2-hybrid assay reflects the interaction in holoenzyme, these results argue that residues 551-555 are involved in the σ70/β-flap interaction or they affect the local conformation. The wild type level of activated PuvsX transcription observed with σ70551-552, 554-555A in the presence of AsiA (Fig. 2A) and MotA eliminates the possibility that the mutations are deleterious simply because they result in a protein that is generally misfolded.
Fig. 6.
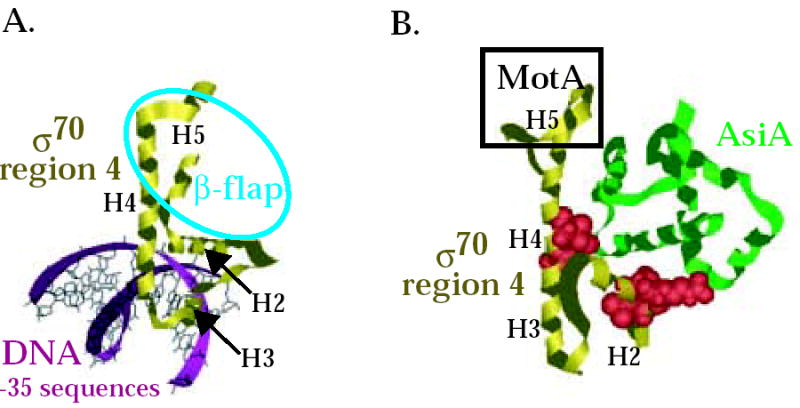
Interaction of σ70 region 4 with -35 element and the β-flap versus its interaction with AsiA and MotA. Structures of (A) T. aquaticus σ region 4 (residues corresponding to E. coli σ70 residues 546-612) with the –35 region of promoter DNA 8 (PDB: 1KU7) and of (B) E. coli σ70 region 4 (residues 546-613) with AsiA (residues 2-90) 30 (PDB: 1TLH). σ70 region 4 is shown in gold, AsiA is shown in green, and the DNA is shown in magenta. Alpha helices H2, H3, H4, and H5 in σ70 (see Fig. 1A) are labeled. Spacefill structures of σ70 residues 551, 554, and 555 in region 4.1 and residues 584 and 587 in region 4.2, which are predicted to interact with AsiA in the structure and whose substitution impairs the σ70/AsiA interaction, are shown in red in (B). The portion of σ70 region 4 that interacts with the β-flap is indicated by the blue oval in (A). H5 of σ70 that interacts with MotA is indicated by the black rectangle in (B). Note that the binding of AsiA to σ70 region 4 converts H3-T-H4 of σ70, which normally interacts with the –35 sequences of promoter DNA, into one continuous helix.
Previous work has indicated that in its interaction with AsiA, σ70 uses a broad interface that includes multiple residues in regions 4.1 and 4.2 (reviewed in 16). The interface revealed by the σ70 region 4/AsiA structure was quite surprising since it showed a previously unseen conformation of region 4, a dramatic reconfiguration that changes the DNA-binding H3-T-H4 to one continuous helix. Prior biochemical analyses that had determined specific AsiA residues needed for the AsiA/σ70 interaction 31; 49 were in good agreement with the structure (30 reviewed in 16), providing support for the AsiA interface. The biochemical analyses here now provide support for several σ70 residues implicated by the structure. Alanine substitutions at σ70 residues that interact with AsiA in the structure [L551, R554 and E555 (σ70 551-552, 554-555A mutant) or R584 and I587 (σ70 584-588A mutant)] seriously impair AsiA/σ70 binding (Fig. 4C) and AsiA inhibition (Fig. 2A). Substitution of F563, which is also predicted to interact with AsiA, also decreases the AsiA/region 4 interaction and AsiA inhibition (Fig. 2B and 34). In contrast, σ70 mutants with substitutions of residues that are not predicted to interact (the σ70567, 560A and σpertussis mutants) behave like wild type σ70 (Fig. 2A and B, Fig. 3). Previous work has also shown that a single alanine substitution at T552 is somewhat defective for the σ70 region 4/AsiA interaction and for AsiA inhibition 35. Although T552 is not a contact point in the structure, it is adjacent to the implicated contact residue, L551.
The work here also indicates that the interaction of AsiA with region 4 does not appear to be a simple addition of its contacts with regions 4.1 and 4.2. AsiA does not interact with σ70 region 4.1 or region 4.2 alone in a yeast 2-hybrid assay 50, and we have seen only a low level of interaction with either region alone in the E. coli 2-hybrid assay (Fig. 4D and E). In addition, we found that the effect of the 551-552, 554-555A mutations in the 2-hybrid with AsiA differed whether we used region 4.1 alone (Fig. 4D) or all of region 4 (Fig. 4C). These results might explain the differences between the AsiA contacts reported in the NMR structure of AsiA with all of region 430 and an NMR analysis performed using AsiA and separate σ70 peptides for region 4.1 (residues 540 to 565) and region 4.2 (residues 570 to 599) 29.
Presently there is not a structure detailing the molecular interface between MotA and σ70. Previous biochemical evidence has identified H5 as a region of contact for MotA 20. We show here that MotA/AsiA activation is significantly reduced when using Eσ70/σ38H5, but not when using Eσpertussis (Fig. 2B). These results suggest that L607, S609, F610, and/or L611, the H5 residues that are identical between the E. coli and the pertussis primary σ factors, may be contact residues for MotA. In addition, the weak transcription observed with AsiA and MotA even when using σ70Δ571-613 (Fig. 2A), in which H5 is missing, or σ70/σ38H5 (Fig. 2B), in which σ70 H5 has been replaced with σ38 H5, suggests that it is region 4.1 that may contain additional contacts for MotA.
Model for AsiA/MotA activation at PuvsX
Based on structural 30 and biochemical 20; 31 work, a model for how MotA and AsiA together activate transcription at PuvsX has emerged. AsiA associates with polymerase by binding tightly to free σ70, and then remaining stably bound after σ70 binds to core 24; 51; 52; 53. The dramatic conformational change of region 4 that is seen once AsiA is bound would then be incompatible with the interaction of σ70 with the –35 DNA and with the β-flap. Thus, AsiA alone acts as an inhibitor of transcription from promoters that require an interaction with the –35 DNA. Because AsiA prevents the interactions of σ70 with the β-flap and the –35 DNA, AsiA binding also makes σ70 H5 and the MotA box, centered at –30, available for MotA. Thus, AsiA binding could facilitate MotA activation simply because of its inhibition function. However, our results with the σ70551-552, 554-555A mutant suggest that eliminating σ70 interactions with the β-flap and with the –35 DNA is not sufficient for MotA activation at PuvsX. When the 551-552, 554-555A mutations were present, we did not detect an interaction between region 4 and the β-flap (Fig. 5A), and transcription was severely compromised (Fig. 2A). These results argue that when polymerase contains σ70551-552, 554-555A, the σ70/β-flap interaction is disrupted and as a consequence, the σ70 region 4/-35 DNA interaction is also lost. However, despite these defects, AsiA was still required for MotA activation (Fig. 2A). Our results suggest that AsiA provides an additional function for MotA apart from disrupting the region 4/β-flap and region 4/DNA contacts. The AsiA-induced conformational change of H5, observed in the AsiA/σ70 structure 30, would be an additional feature of AsiA binding that could be needed to facilitate MotA interaction with H5 and indirectly the interaction of MotA with MotA box DNA.
Previous work has shown that in the absence of MotA, AsiA alone increases in vitro transcription from the T4 middle promoter PrIIB2 when using a σ70 that is truncated after region 4.1 at residue 565 44. This result suggested that MotA may function to remove the interaction of σ70 H5 with the β-flap, allowing the interaction of AsiA with σ70 region 4.1, which then activates transcription. Thus, when only region 4.1 is present, AsiA activates without MotA. However, we found that σ70 truncated just after region 4.1 (Δ571-613) did not interact with AsiA in the native gel assay (Fig. 3) and did not yield AsiA activation of PuvsX in the absence (or presence) of MotA (Fig. 2A). Thus, we do not detect activation by AsiA alone at PuvsX like that seen at PrIIB2. Further studies will be needed to test differences between various T4 middle promoters.
No other activators besides MotA are known to interact with σ70 H5. While certain class II activators have been shown to interact with σ70 region 4, they use the C-terminal half of H4 in region 4.2 54; 55; 56. However, H5 of σ38 may be involved in regulating the activity of Eσ38 at σ38-dependent promoters needed to express genes required to cope with conditions of high salt 57; 58. A thorough understanding of how MotA interacts with σ70 H5 may shed light on how the C-terminal portion of σ factors can be used as a target for regulation.
Materials and Methods
DNA and Buffers
Plasmids were constructed using standard cloning procedures. Sequences of primers are available upon request. DNA sequencing (CBR-DNA Sequencing Facility of the University of Maryland, MWG Biotech, or UMDNJ (University of Medicine and Dentistry of New Jersey) DNA Core facility) throughout the cloned regions confirmed the presence of only the desired sequences.
pDKT90, which contains PuvsX, has been described 59. pBRα-σ70, 60 and pBRα-σ70 D581G 61 direct transcription of an α-σ chimera gene, composed of residues 1-248 of α fused in frame to residues 528-613 of σ70, with or without the substitution at D581G. In each case, the gene is under the control of tandem promoters lpp and isopropyl-β-D-thiogalatoside (IPTG)-inducible lacUV5. pBRα 60 encodes the N-terminal domain of wild type α (residues 1-248). pACλcI32 62 encodes residues 1-236 of the bacteriophage λ cI protein under the control of the IPTG-inducible lacUV5 promoter. pACΔ-35λcI-AsiAK20A 34 expresses a cI-AsiA fusion protein which contains the K20A substitution within the AsiA protein, and pcI-MotANTD 20 expresses a cI-MotANTD protein in which the N-terminal domain of motA (encoding residues 1 to 97, followed by a non-coded serine) has been fused in frame to cI in pACλcI32. The K20A substitution in AsiA was used because this substitution results in a higher level of β-galactosidase activity in the 2-hybrid assay 34. pACλcI-β-flap contains the rpoB gene encoding residues 858-946 of the β subunit of RNAP (the β-flap moiety) fused in frame to cI in pACλcI32 and has been described 13. pcI-rpoC (357-430), in which a portion of the rpoC gene encoding residues 357 to 430 of the β’ subunit of RNAP is fused in frame to cI in pACλcI32 was constructed by first obtaining a PCR product containing this region of rpoC using chromosomal DNA from E. coli XL1-Blue (Stratagene), a primer that annealed to rpoC DNA encoding residues 357 to 362 preceded by a NotI site, a primer that annealed to rpoC DNA encoding residues 430 to 425 preceded by a BglII site, and Pfu polymerase (Stratagene). After digestion with BglII and NotI, the fragment was ligated with pACλcI32 that had been digested with BglII and NotI, resulting in pcI-rpoC (357-430).
pETσfl was obtained by inserting the EcoRI/HindIII fragment containing σ70 DNA from pσfl 63 into pET21(+) (Novagen) that had been digested with EcoRI and HindIII. The resulting plasmid expresses His6-tagged σ70 protein, which has the sequence MRGSHHHHHHGSSGLVPRGSELGTRL fused to the start of σ70. This σ70 also contains the substitutions V36L and N149D, but we have not found any consequence of these substitutions in numerous assays (data not shown). To make pETσ584-588A we used the Quikchange procedure (Stratagene) and primers that introduced codons for alanines at σ70 residues 584-588 as well as introduced a PstI site. The presence of the desired substitutions in the resulting plasmids were screened by PstI digestion and DNA sequencing. The ClaI/HindIII fragment containing the 3′ end of σ70 DNA was then obtained from a plasmid with the desired change, and this fragment was inserted into pETσfl that had been digested with ClaI and HindIII, resulting in pETσ584-588A. To construct pETσ551-552,554-555A, we first deleted the DNA coding for σ70 residues 551-555 in pETσfl using the Quikchange procedure and primers that surrounded the DNA for residues 551-555, but lacked the 551-555 codons. Using the resulting deleted plasmid, we then used the Quikchange procedure with primers that surrounded the DNA for residues 551-555, but now had alanine codons for residues 551-555. After sequencing to identify a plasmid with the desired change, the XhoI fragment containing the 3′ end of the σ70 gene was obtained and ligated to pETσfl that had been digested with XhoI, resulting in pETσ551-552,554-555A. pETσ70/σ38region 4 encodes a σ70/σ38 fusion protein in which σ70 residues 1-529 are followed by σ38 region 4 (residues 245-330). To construct this plasmid, we used the pBRα-σ38; 60, in which DNA encoding region 4 of σ38 had been fused to DNA encoding the N-terminal domain of the α subunit of RNA polymerase. An XhoI site had also been placed at the α-σ junction in this plasmid. We performed PCR using pBRα-σ38, a primer that annealed to the DNA at the α-σ38 junction, and a primer that annealed to the DNA at the end of the σ38 sequence and introduced a XhoI site. The resulting PCR product was digested with XhoI and ligated into pETσfl that had been digested with XhoI, resulting in pETσ70/σ38region 4. pETσ70/σ38H5 was constructed by ligating pETσfl that had been previously digested with ClaI and HindIII with the ClaI/HindIII fragment containing the σ38 sequences from pBRα-σ70/σ38; 20. pBRα-σ70/σ38 is identical to pBRα-σ70 except that the σ70 sequences encoding amino acids 597-613 have been replaced with the comparable σ38 sequences (encoding amino acids 312-330). pETσΔ571-613 was constructed by ligating pETσfl that had been digested with ClaI and ScaI with the ClaI/ScaI fragment containing the 3′ end of σ70 DNA from pσ1-570, 20.
To construct pET15b-557A560A and pET15b-555A557A560A, we used a plasmid pET15b-σ70, in which the rpoD gene is cloned between NdeI and EcoRI sites. We performed two-step PCR site-directed mutagenesis and obtained PCR products containing the corresponding mutations and BamHI and EcoRI sites at the ends of the each PCR fragment. As the pET15b-σ70 contains unique BamHI site in the rpoD gene, the plasmids pET15b-557A560A and pET15b-555A557A560A were obtained by inserting the corresponding BamHI/EcoRI fragments into the pET15b-σ70 that had been digested with BamHI and EcoRI. These plasmids produce N-terminally His6-tagged σ70 with the indicated mutations.
To construct pBRα-σ4.1 we obtained a PCR product with pETσfl and a primer that annealed to DNA encoding σ70 residues 528-534 preceded by an XhoI site and a primer that annealed to DNA encoding 562-568 followed by a BamHI site. After digestion of the product with BamHI and XhoI, the fragment was ligated with pBRα-σ70 that had also been digested with BamHI and XhoI. The resulting plasmid produces an α-σ4.1 fusion protein, in which α residues 1-248 are followed by σ70 residues 528-568. pBRα-σ4.2 produces an α-σ4.2 fusion protein, in which α residues 1-248 are followed by the protein sequence LEL (brought in by the linker DNA) and then σ70 residues 569-613. It was constructed like pBRα-σ4.1 except that the primers annealed to σ70 residues 569-574 and 608-613. pBRα-σ4.2584-588A, pBRα-σ4.1551-552, 554-555A, and pBRα-σ470555,557,560A were constructed following similar procedures except that the PCR templates were pETσ70584-588A, pETσ70551-552, 554-555A, and pET15b-555A557A560A, respectively.
Protein buffer I contained 47 mM Tris-Cl (pH 7.9), 83 mM NaCl, 45% glycerol, 0.9 mM EDTA, 0.09 mM DTT, 0.009% Triton X-100, and 22 mM immidazole. Protein buffer II contained 5.2 mM Tris-Cl (pH 7.9), 72 mM Tris-acetate (pH 7.9), 26 mM NaCl, 5.2% glycerol, 0.2 mM EDTA, 0.3 mM DTT, 270 mM potassium glutamate, 7.2 mM magnesium acetate, and 180 μg/ml BSA. DNA buffer contained 53 mM Tris-acetate (pH 7.9), 6 mM NaCl, 0.2% glycerol, 0.6 mM EDTA, 0.1 mM DTT, 200 mM potassium acetate, 5.3 mM magnesium acetate, 130 μg/ml BSA, 0.48 mM each ATP, GTP, and CTP, and 24 μM [α-32P]UTP (6 × 104 dpm/pmol).
Proteins
The purification of wild type σ70 (not His6-tagged), which was used for the native gel assays, has been described 39. Purification of the His6-tagged proteins, σ70, σ70551-552,554-555A, σ70/σ38H5, and σ70Δ571-613, was done using BL21(DE3)/pLysE 64 cultures containing the desired pETσ plasmid as described 65 by denaturation of inclusion bodies containing the protein, Ni++ resin affinity chromatography under denaturing conditions, and then a slow renaturation of the protein. His6-tagged σ70/σ38region 4 was purified by a similar procedure except that after sonication of the cells, this protein was found in the 17,400 × g supernatant rather than in inclusion bodies and consequently, it was subjected to Ni++ resin chromatography under native conditions. 584-588A was purified by both procedures. Transcription assays His6-tagged σ70 indicated that the His6-tagged proteins σ70551-552, 554-555A, σ70584-588A, σ70/σ38region 4, σ70Δ571-613, and the σ70 saturated core at a ratio of ~2:1 (data not shown). σ70F563Y, σpertussis, and the σ70 used as the control for the σ70F563Y and σpertussis proteins (Fig. 5B) were gifts of Phil Boucher and Scott Stibitz (FDA). E. coli RNA polymerase core was purchased from Epicentre Technologies. MotA was purified as described 38. The purifications of N-terminally His6-tagged AsiA, His6AsiA 31, and C-terminally His6-tagged AsiA, AsiAHis6 40 have been described. We have not observed any significant difference in activity between wild type AsiA, AsiAHis6, and His6AsiA.
To isolate the His6-tagged proteins, σ70555, 557, 560A and σ70557, 560A, E. coli BL21(DE3) containing the appropriate plasmid was grown in LB broth with 100 μg/ml ampicillin at 37° C to an OD600 of 0.8-1.0, and synthesis of the mutant σ70 was induced by the addition of IPTG to 0.5 mM. After 6 more hours of growth, cells were harvested by centrifugation, resuspended in buffer A (20 mM Tris-HCl, pH 8.0; 500 mM NaCl; 5% glycerol; 2 mM imidazole, pH 8.0) and lysed by sonication. After centrifugation, the cleared lysate was loaded onto a 5 ml chelating Hi-trap Sepharose column attached to syringe. The column was then washed with buffer A containing 20 mm imidazole, and the proteins were eluted with 100 mm imidazole in the same buffer. The proteins were concentrated to 1–2 mg/ml by dialyzing against buffer B that contained 20 mM Tris-HCl, pH 8.0; 100 mM NaCl, 1 mM β–mercaptoethanol, 50 % glycerol, and stored at −20°C.
Two-hybrid assays
β-galactosidase assays were performed as described previously 31 using cultures of E. coli KS1 66 containing the indicated plasmids except that cultures were grown for 3 hr in LB media supplemented with the appropriate antibiotics at the following concentrations: carbenicillin (50 ug/ml), chloramphenicol (25 ug/ml), and kanamycin (50 ug/ml). β-galactosidase values represent the averages of duplicate assays. Error bars are shown in Fig. 4 and 5, but in some cases they are not seen because they are smaller than the symbols.
Native Protein Gels
Protein-protein complexes were separated from free proteins by gel electrophoresis on native, 6% polyacrylamide gels as described 39. Reactions were composed of 24 pmol of His6AsiA in 2 μl of a buffer containing 20 mM Tris-Cl (pH 7.9), 500 mM NaCl, 5% glycerol, and 100 mM immidazole); 4.6 pmol of σ70 in 2 μl of a buffer containing 50 mM Tris-Cl (pH 8.0), 50 mM NaCl, 50% glycerol, 1 mM EDTA, 0.1 mM DTT, and 0.01% Triton X-100; and/or 17 pmol of MotA in 1 μl of a buffer containing 20 mM Tris-Cl (pH 7.9), 270 mM NaCl, 10% glycerol, 1 mM EDTA, and 1 mM 2-mercaptoethanol. Reactions were incubated at 37° C for 10 min before electrophoresis. After electrophoresis, proteins were detected using SilverXpress® (Invitrogen).
In vitro transcriptions
Transcription reactions were assembled as indicated in the figure legends. Upon addition of rNTPs, reactions were incubated at 37° C for 20 sec, rifampicin (0.5 μl of 300 ng/μl) was added, and then the reactions were incubated for an additional 7 min before being collected on dry ice. Gel load solution (1 × TBE, 7 M urea, 0.1% bromphenol blue, 0.1% xylene cyanol FF) was added at a volume of 5 times that of the reaction aliquots, and the solution was heated at 95° C for two minutes before electrophoresis on 4% polyacrylamide, 7 M urea denaturing gels run in 1/2 × TBE. After autoradiography, films were scanned using a Powerlook 2100XL densitometer and quantification was performed using Quantity One software from Bio-Rad, Inc.
Acknowledgments
We thank A. Makela for the construction of pETσfl and the purification of σ70/σ38H5. We thank A. Hochschild and B. Gregory for gifts of pACλcI-β-flap and pACΔ-35λcI-AsiAK20A and P. Boucher and S. Stibitz for the gifts of σpetussis and σ70F563Y. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (K.B., J.L., and D. H.) and NIH grant R01 GM59295 (L.M. and K.S.)
References
- 1.Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–66. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 2.Murakami KS, Darst SA. Bacterial RNA polymerases: the wholo story. Curr Opin Struct Biol. 2003;13:31–9. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 3.Paget MS, Helmann JD. The sigma70 family of sigma factors. Genome Biol. 2003;4:203. doi: 10.1186/gb-2003-4-1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonetto M, Gribskov M, Gross CA. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–9. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barne KA, Bown JA, Busby SJ, Minchin SD. Region 2.5 of the Escherichia coli RNA polymerase sigma70 subunit is responsible for the recognition of the ‘extended-10’ motif at promoters. Embo J. 1997;16:4034–40. doi: 10.1093/emboj/16.13.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: an additional recognition element for RNA polymerase. Cell. 2006;125:1069–82. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Feklistov A, Barinova N, Sevostyanova A, Heyduk E, Bass I, Vvedenskaya I, Kuznedelov K, Merkiene E, Stavrovskaya E, Klimasauskas S, Nikiforov V, Heyduk T, Severinov K, Kulbachinskiy A. A Basal Promoter Element Recognized by Free RNA Polymerase sigma Subunit Determines Promoter Recognition by RNA Polymerase Holoenzyme. Mol Cell. 2006;23:97–107. doi: 10.1016/j.molcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Campbell EA, Muzzin O, Chlenov M, Sun JL, Olson CA, Weinman O, Trester-Zedlitz ML, Darst SA. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol Cell. 2002;9:527–39. doi: 10.1016/s1097-2765(02)00470-7. [DOI] [PubMed] [Google Scholar]
- 9.Murakami KS, Masuda S, Campbell EA, Muzzin O, Darst SA. Structural basis of transcription initiation: an RNA polymerase holoenzyme-DNA complex. Science. 2002;296:1285–90. doi: 10.1126/science.1069595. [DOI] [PubMed] [Google Scholar]
- 10.Mekler V, Kortkhonjia E, Mukhopadhyay J, Knight J, Revyakin A, Kapanidis AN, Niu W, Ebright YW, Levy R, Ebright RH. Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase-promoter open complex. Cell. 2002;108:599–614. doi: 10.1016/s0092-8674(02)00667-0. [DOI] [PubMed] [Google Scholar]
- 11.Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science. 2002;296:1280–4. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- 12.Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature. 2002;417:712–9. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 13.Kuznedelov K, Minakhin L, Niedziela-Majka A, Dove SL, Rogulja D, Nickels BE, Hochschild A, Heyduk T, Severinov K. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science. 2002;295:855–7. doi: 10.1126/science.1066303. [DOI] [PubMed] [Google Scholar]
- 14.Lawson CL, Swigon D, Murakami KS, Darst SA, Berman HM, Ebright RH. Catabolite activator protein: DNA binding and transcription activation. Curr Opin Struct Biol. 2004;14:10–20. doi: 10.1016/j.sbi.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnard A, Wolfe A, Busby S. Regulation at complex bacterial promoters: how bacteria use different promoter organizations to produce different regulatory outcomes. Curr Opin Microbiol. 2004;7:102–8. doi: 10.1016/j.mib.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Hinton DM, Pande S, Wais N, Johnson XB, Vuthoori M, Makela A, Hook-Barnard I. Transcriptional takeover by sigma appropriation: remodelling of the sigma70 subunit of Escherichia coli RNA polymerase by the bacteriophage T4 activator MotA and co-activator AsiA. Microbiology. 2005;151:1729–40. doi: 10.1099/mic.0.27972-0. [DOI] [PubMed] [Google Scholar]
- 17.Mattson T, Richardson J, Goodin D. Mutant of bacteriophage T4D affecting expression of many early genes. Nature. 1974;250:48–50. doi: 10.1038/250048a0. [DOI] [PubMed] [Google Scholar]
- 18.Hinton DM. Transcription from a bacteriophage T4 middle promoter using T4 motA protein and phage-modified RNA polymerase. J Biol Chem. 1991;266:18034–44. [PubMed] [Google Scholar]
- 19.Schmidt RP, Kreuzer KN. Purified MotA protein binds the -30 region of a bacteriophage T4 middle-mode promoter and activates transcription in vitro. J Biol Chem. 1992;267:11399–407. [PubMed] [Google Scholar]
- 20.Pande S, Makela A, Dove SL, Nickels BE, Hochschild A, Hinton DM. The bacteriophage T4 transcription activator MotA interacts with the far-C-terminal region of the sigma70 subunit of Escherichia coli RNA polymerase. J Bacteriol. 2002;184:3957–64. doi: 10.1128/JB.184.14.3957-3964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouhammouch M, Adelman K, Harvey SR, Orsini G, Brody EN. Bacteriophage T4 MotA and AsiA proteins suffice to direct Escherichia coli RNA polymerase to initiate transcription at T4 middle promoters. Proc Natl Acad Sci U S A. 1995;92:1451–5. doi: 10.1073/pnas.92.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colland F, Orsini G, Brody EN, Buc H, Kolb A. The bacteriophage T4 AsiA protein: a molecular switch for sigma 70-dependent promoters. Mol Microbiol. 1998;27:819–29. doi: 10.1046/j.1365-2958.1998.00729.x. [DOI] [PubMed] [Google Scholar]
- 23.Pahari S, Chatterji D. Interaction of bacteriophage T4 AsiA protein with Escherichia coli sigma70 and its variant. FEBS Lett. 1997;411:60–2. doi: 10.1016/s0014-5793(97)00668-6. [DOI] [PubMed] [Google Scholar]
- 24.Severinova E, Severinov K, Darst SA. Inhibition of Escherichia coli RNA polymerase by bacteriophage T4 AsiA. J Mol Biol. 1998;279:9–18. doi: 10.1006/jmbi.1998.1742. [DOI] [PubMed] [Google Scholar]
- 25.Severinova E, Severinov K, Fenyo D, Marr M, Brody EN, Roberts JW, Chait BT, Darst SA. Domain organization of the Escherichia coli RNA polymerase sigma 70 subunit. J Mol Biol. 1996;263:637–47. doi: 10.1006/jmbi.1996.0604. [DOI] [PubMed] [Google Scholar]
- 26.Severinov K, Muir TW. Expressed protein ligation, a novel method for studying protein-protein interactions in transcription. J Biol Chem. 1998;273:16205–9. doi: 10.1074/jbc.273.26.16205. [DOI] [PubMed] [Google Scholar]
- 27.Sharma UK, Ravishankar S, Shandil RK, Praveen PV, Balganesh TS. Study of the interaction between bacteriophage T4 asiA and Escherichia coli sigma(70), using the yeast two-hybrid system: neutralization of asiA toxicity to E. coli cells by coexpression of a truncated sigma(70) fragment. J Bacteriol. 1999;181:5855–9. doi: 10.1128/jb.181.18.5855-5859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbauer JL, Adelman K, Urbauer RJ, Simeonov MF, Gilmore JM, Zolkiewski M, Brody EN. Conserved regions 4.1 and 4.2 of sigma(70) constitute the recognition sites for the anti-sigma factor AsiA, and AsiA is a dimer free in solution. J Biol Chem. 2001;276:41128–32. doi: 10.1074/jbc.M106400200. [DOI] [PubMed] [Google Scholar]
- 29.Simeonov MF, Bieber Urbauer RJ, Gilmore JM, Adelman K, Brody EN, Niedziela-Majka A, Minakhin L, Heyduk T, Urbauer JL. Characterization of the interactions between the bacteriophage T4 AsiA protein and RNA polymerase. Biochemistry. 2003;42:7717–26. doi: 10.1021/bi0340797. [DOI] [PubMed] [Google Scholar]
- 30.Lambert LJ, Wei Y, Schirf V, Demeler B, Werner MH. T4 AsiA blocks DNA recognition by remodeling sigma(70) region 4. Embo J. 2004;23:2952–62. doi: 10.1038/sj.emboj.7600312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pal D, Vuthoori M, Pande S, Wheeler D, Hinton DM. Analysis of regions within the bacteriophage T4 AsiA protein involved in its binding to the sigma70 subunit of E. coli RNA polymerase and its role as a transcriptional inhibitor and co-activator. J Mol Biol. 2003;325:827–41. doi: 10.1016/s0022-2836(02)01307-4. [DOI] [PubMed] [Google Scholar]
- 32.Stevens A, Rhoton JC. Characterization of an inhibitor causing potassium chloride sensitivity of an RNA polymerase from T4 phage-infected Escherichia coli. Biochemistry. 1975;14:5074–9. doi: 10.1021/bi00694a007. [DOI] [PubMed] [Google Scholar]
- 33.Young BA, Gruber TM, Gross CA. Views of transcription initiation. Cell. 2002;109:417–20. doi: 10.1016/s0092-8674(02)00752-3. [DOI] [PubMed] [Google Scholar]
- 34.Gregory BD, Nickels BE, Garrity SJ, Severinova E, Minakhin L, Urbauer RJ, Urbauer JL, Heyduk T, Severinov K, Hochschild A. A regulator that inhibits transcription by targeting an intersubunit interaction of the RNA polymerase holoenzyme. Proc Natl Acad Sci U S A. 2004;101:4554–9. doi: 10.1073/pnas.0400923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregory BD, Nickels BE, Darst SA, Hochschild A. An altered-specificty DNA-binding mutant of E. coli σ70 facilitates the analysis of σ70 function in vivo. Molec Microbiol. 2005 doi: 10.1111/j.1365-2958.2005.04624.x. in press. [DOI] [PubMed] [Google Scholar]
- 36.Minakhin L, Camarero JA, Holford M, Parker C, Muir TW, Severinov K. Mapping the molecular interface between the sigma(70) subunit of E. coli RNA polymerase and T4 AsiA. J Mol Biol. 2001;306:631–42. doi: 10.1006/jmbi.2001.4445. [DOI] [PubMed] [Google Scholar]
- 37.Ross W, Ernst A, Gourse RL. Fine structure of E. coli RNA polymerase-promoter interactions: alpha subunit binding to the UP element minor groove. Genes Dev. 2001;15:491–506. doi: 10.1101/gad.870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinton DM, March-Amegadzie R, Gerber JS, Sharma M. Bacteriophage T4 middle transcription system: T4-modified RNA polymerase; AsiA, a sigma 70 binding protein; and transcriptional activator MotA. Methods Enzymol. 1996;274:43–57. doi: 10.1016/s0076-6879(96)74007-7. [DOI] [PubMed] [Google Scholar]
- 39.Gerber JS, Hinton DM. An N-terminal mutation in the bacteriophage T4 motA gene yields a protein that binds DNA but is defective for activation of transcription. J Bacteriol. 1996;178:6133–9. doi: 10.1128/jb.178.21.6133-6139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pineda M, Gregory BD, Szczypinski B, Baxter KR, Hochschild A, Miller ES, Hinton DM. A family of anti-sigma70 proteins in T4-type phages and bacteria that are similar to AsiA, a Transcription inhibitor and co-activator of bacteriophage T4. J Mol Biol. 2004;344:1183–97. doi: 10.1016/j.jmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Gardella T, Moyle H, Susskind MM. A mutant Escherichia coli sigma 70 subunit of RNA polymerase with altered promoter specificity. J Mol Biol. 1989;206:579–90. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 42.Siegele DA, Hu JC, Walter WA, Gross CA. Altered promoter recognition by mutant forms of the sigma 70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989;206:591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- 43.Keener J, Nomura M. Dominant lethal phenotype of a mutation in the -35 recognition region of Escherichia coli sigma 70. Proc Natl Acad Sci U S A. 1993;90:1751–5. doi: 10.1073/pnas.90.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minakhin L, Niedziela-Majka A, Kuznedelov K, Adelman K, Urbauer JL, Heyduk T, Severinov K. Interaction of T4 AsiA with its target sites in the RNA polymerase sigma70 subunit leads to distinct and opposite effects on transcription. J Mol Biol. 2003;326:679–90. doi: 10.1016/s0022-2836(02)01442-0. [DOI] [PubMed] [Google Scholar]
- 45.Nickels BE, Garrity SJ, Mekler V, Minakhin L, Severinov K, Ebright RH, Hochschild A. The interaction between sigma70 and the beta-flap of Escherichia coli RNA polymerase inhibits extension of nascent RNA during early elongation. Proc Natl Acad Sci U S A. 2005;102:4488–93. doi: 10.1073/pnas.0409850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharp MM, Chan CL, Lu CZ, Marr MT, Nechaev S, Merritt EW, Severinov K, Roberts JW, Gross CA. The interface of sigma with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev. 1999;13:3015–26. doi: 10.1101/gad.13.22.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller ES, Kutter E, Mosig G, Arisaka F, Kunisawa T, Ruger W. Bacteriophage T4 genome. Microbiol Mol Biol Rev. 2003;67:86–156. doi: 10.1128/MMBR.67.1.86-156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geszvain K, Gruber TM, Mooney RA, Gross CA, Landick R. A hydrophobic patch on the flap-tip helix of E.coli RNA polymerase mediates sigma(70) region 4 function. J Mol Biol. 2004;343:569–87. doi: 10.1016/j.jmb.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 49.Sharma UK, Praveen PV, Balganesh TS. Mutational analysis of bacteriophage T4 AsiA: involvement of N- and C-terminal regions in binding to sigma(70) of Escherichia coli in vivo. Gene. 2002;295:125–34. doi: 10.1016/s0378-1119(02)00831-4. [DOI] [PubMed] [Google Scholar]
- 50.Sharma UK, Chatterji D. Both regions 4.1 and 4.2 of E. coli sigma(70) are together required for binding to bacteriophage T4 AsiA in vivo. Gene. 2006 doi: 10.1016/j.gene.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Hinton DM, Vuthoori S. Efficient inhibition of Escherichia coli RNA polymerase by the bacteriophage T4 AsiA protein requires that AsiA binds first to free sigma70. J Mol Biol. 2000;304:731–9. doi: 10.1006/jmbi.2000.4113. [DOI] [PubMed] [Google Scholar]
- 52.Hinton DM, Vuthoori S, Mulamba R. The Bacteriophage T4 Inhibitor and Coactivator AsiA Inhibits Escherichia coli RNA Polymerase More Rapidly in the Absence of {sigma}70 Region 1.1: Evidence that Region 1.1 Stabilizes the Interaction between {sigma}70 and Core. J Bacteriol. 2006;188:1279–85. doi: 10.1128/JB.188.4.1279-1285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972;69:603–7. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhodius VA, Busby SJ. Transcription activation by the Escherichia coli cyclic AMP receptor protein: determinants within activating region 3. J Mol Biol. 2000;299:295–310. doi: 10.1006/jmbi.2000.3736. [DOI] [PubMed] [Google Scholar]
- 55.Lonetto MA, Rhodius V, Lamberg K, Kiley P, Busby S, Gross C. Identification of a contact site for different transcription activators in region 4 of the Escherichia coli RNA polymerase sigma70 subunit. J Mol Biol. 1998;284:1353–65. doi: 10.1006/jmbi.1998.2268. [DOI] [PubMed] [Google Scholar]
- 56.Landini P, Busby SJ. The Escherichia coli Ada protein can interact with two distinct determinants in the sigma70 subunit of RNA polymerase according to promoter architecture: identification of the target of Ada activation at the alkA promoter. J Bacteriol. 1999;181:1524–9. doi: 10.1128/jb.181.5.1524-1529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenthal AZ, Hu M, Gralla JD. Osmolyte-induced transcription: -35 region elements and recognition by sigma38 (rpoS) Mol Microbiol. 2006;59:1052–61. doi: 10.1111/j.1365-2958.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 58.Ohnuma M, Fujita N, Ishihama A, Tanaka K, Takahashi H. A carboxy-terminal 16-amino-acid region of sigma(38) of Escherichia coli is important for transcription under high-salt conditions and sigma activities in vivo. J Bacteriol. 2000;182:4628–31. doi: 10.1128/jb.182.16.4628-4631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.March-Amegadzie R, Hinton DM. The bacteriophage T4 middle promoter PuvsX: analysis of regions important for binding of the T4 transcriptional activator MotA and for activation of transcription. Mol Microbiol. 1995;15:649–60. doi: 10.1111/j.1365-2958.1995.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 60.Dove SL, Huang FW, Hochschild A. Mechanism for a transcriptional activator that works at the isomerization step. Proc Natl Acad Sci U S A. 2000;97:13215–20. doi: 10.1073/pnas.97.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nickels BE, Dove SL, Murakami KS, Darst SA, Hochschild A. Protein-protein and protein-DNA interactions of sigma70 region 4 involved in transcription activation by lambda cI. J Mol Biol. 2002;324:17–34. doi: 10.1016/s0022-2836(02)01043-4. [DOI] [PubMed] [Google Scholar]
- 62.Hu JC, Kornacker MG, Hochschild A. Escherichia coli one- and two-hybrid systems for the analysis and identification of protein-protein interactions. Methods. 2000;20:80–94. doi: 10.1006/meth.1999.0908. [DOI] [PubMed] [Google Scholar]
- 63.Wilson C, Dombroski AJ. Region 1 of sigma70 is required for efficient isomerization and initiation of transcription by Escherichia coli RNA polymerase. J Mol Biol. 1997;267:60–74. doi: 10.1006/jmbi.1997.0875. [DOI] [PubMed] [Google Scholar]
- 64.Studier FW. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991;219:37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- 65.Vuthoori S, Bowers CW, McCracken A, Dombroski AJ, Hinton DM. Domain 1.1 of the sigma(70) subunit of Escherichia coli RNA polymerase modulates the formation of stable polymerase/promoter complexes. J Mol Biol. 2001;309:561–72. doi: 10.1006/jmbi.2001.4690. [DOI] [PubMed] [Google Scholar]
- 66.Dove SL, Joung JK, Hochschild A. Activation of prokaryotic transcription through arbitrary protein-protein contacts. Nature. 1997;386:627–30. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]


