Abstract
The contribution of prolactin (PRL) to the pathogenesis and progression of human breast cancer at the cellular, transgenic, and epidemiological levels is increasingly appreciated. Acting at the endocrine and autocrine/paracrine levels, PRL functions to stimulate the growth and motility of human breast cancer cells. The actions of this ligand are mediated by at least six recognized PRL receptor isoforms found on, or secreted by, human breast epithelium. The PRL/PRL receptor complex associates with and activates several signaling networks that are shared with other members of the cytokine receptor superfamily. Coupled with the recently identified intranuclear function of PRL, these networks are integrated into the in vitro and in vivo actions induced by ligand. These findings indicate that antagonists of PRL/PRL receptor interaction or PRL receptor-associated signal transduction may be of considerable utility in the treatment of human breast cancer.
Abbreviations: CIS, Cytokine-inducible inhibitor of signaling; CypB, cyclophilin B; ECD, extracellular domain; EGF, epidermal growth factor; GHR, GH receptor; hPRLR, human PRLR; ICD, intracellular domain; Jak, Janus kinase 2; JNK, c-jun N-terminal kinase; PIAS, peptide inhibitor of activated Stat; PI3K, phosphatidylinositol 3′-kinase; PRL, prolactin; PtdIns, phosphatidylinositol; PRLBP, PRL binding protein; PRLR, PRL receptor; SHP-2, SH2-containing protein tyrosine phosphatase; SOCS, suppressor of cytokine signaling; Stat, signal transducer and activator of transcription
I. Introduction
The function of prolactin (PRL) in mammary neoplasia has been the subject of considerable debate. PRL was first recognized as a hormone that significantly contributed to both the pathogenesis and progression of rodent mammary neoplasia in the 1970s (1). However, subsequent clinical trials in the 1980s on breast cancer patients with pharmacological agents that inhibited the pituitary secretion of PRL were failures. These findings led many oncologists to overlook the potential autocrine/paracrine actions of this hormone during neoplastic progression and to consider PRL as a hormone regulating lactation only (2). Data gathered during the 1990s at the cellular, epidemiological, and transgenic levels, however, have reestablished a contributory role for this hormone during breast oncogenesis. Although the principal focus of this review will examine in detail the action of this hormone in human breast cancer, relevant data from rodent model systems will be discussed where appropriate.
II. Epidemiology of PRL and Human Breast Cancer
A. Correlates of PRL levels
A number of studies have evaluated the association between PRL levels and several well-confirmed breast cancer risk factors such as parity and age at menarche (Table 1). A consistent correlation between PRL levels and these risk factors would raise the possibility that the increase in PRL was at least part of the underlying etiological mechanism between the risk factor and disease and would provide indirect support for a PRL/breast cancer association.
Table 1.
Breast cancer risk factors associated with higher circulating PRL levels in women: a summary of the evidence
Confirmed association with PRL levels
|
Probable association with PRL levels, based on substantial data
|
Probable association with PRL levels, based on limited data
|
Limited data, no current evidence of association with PRL levels
|
1. Parity (childbirth) and age at first birth
A long-lasting reduction in PRL levels after a first pregnancy has been observed in most (3–7), although not all (8), studies. In the one study in which no association was observed (8), only 19 nulliparous women were evaluated and thus an association may have been missed. The association with parity has been observed in both premenopausal and postmenopausal women, suggesting that the reduction in levels is long lasting. The percent reduction in levels has varied substantially among studies with a range of 15–50% when nulliparous were compared with parous women. In the only studies with a large enough sample size to assess the issue in detail (6, 7), PRL levels appeared to decrease, at least modestly, with each additional pregnancy. Also, no independent association between age at first birth and PRL level was seen (6), although this observation requires confirmation in additional studies.
2. Age at menarche and menopause
Overall, no significant associations between PRL and either age at menarche or age at menopause have been reported (6, 8, 9).
3. Family history of breast cancer
In studies of premenopausal women, most (4, 7, 10, 11), but not all (12), investigators observed at least modestly higher PRL levels (examined primarily in the luteal phase) in women with a family history of breast cancer compared with women with no such history. However, in several studies among either adolescents (13–15) or postmenopausal women (6 –8), little if any relationship was observed according to family history of breast cancer. Reasons for these differences by menopausal status are not clear. To our knowledge, the relationship between PRL levels and specific gene mutations (e.g., BRCA1) has not been assessed.
4. Mammographic density
Breast density, defined as the areas of opacity on a mammogram, reflects the amount of breast epithelial and stromal tissue. A strong positive association between mammographic breast density [or breast parenchymal pattern (16)] and breast cancer risk has been consistently observed (17). The association between circulating PRL levels and mammographic breast density has been evaluated in a single published study (6) and two preliminary reports (18, 19). In all three, higher PRL levels were observed in post-menopausal women with higher breast density, suggesting a measurable influence of PRL on breast epithelial and/or stromal proliferation.
5. Ethnic differences
PRL levels have been assessed in adolescents or women defined as being at high or low risk of breast cancer according to breast cancer rates in their country of origin. In general, no substantial differences were observed when average levels in women (or adolescents) from the United States or Britain (defined as high-risk countries) were compared with those in rural Japan or China (defined as low-risk countries) (20 –22). In one recent study, PRL levels during pregnancy were compared in women from the United States and China (23). PRL levels were significantly lower in the US women compared with the Chinese women at both wk 16 and wk 27 of pregnancy, differences that remained after controlling for maternal age and parity.
6. Dietary intake
Relatively few dietary factors have been consistently associated with risk of breast cancer. Alcohol intake has been most consistently related to an increase in risk, but in a single study moderate intake did not correlate with postmenopausal PRL levels (24). Several studies have evaluated PRL levels and either dietary fat (7, 25–29) or soy intake (30), factors hypothesized to influence breast cancer risk, but consistent findings are yet to emerge.
7. Medication use
A number of medications are known to increase (e.g., oral contraceptives, reserpine, haldol, cimetidine, and the phenothiazines) or decrease (e.g., levodopa) plasma PRL levels. Long-term recent use of oral contraceptives increases risk of breast cancer (31). The increase in PRL levels observed with their use (32) could conceivably play a role in this effect. Of the other medications known to influence PRL levels, reserpine, an antihypertensive agent, is the most extensively studied. Reserpine initially causes an acute elevation of PRL; however, long-term use results in about a 50% elevation in plasma levels (33). Although a positive association between reserpine use and breast cancer was noted in several studies (34 –36), no association was observed in a number of subsequent evaluations (37–42). Possible reasons for this inconsistency include the small size of many of the studies and the exposure definition used (e.g., most investigators reported the relationship for “ever use” of reserpine only). If PRL is a promoter of breast cancer, only longer durations of use would be expected to have a discernible influence on risk, as is observed with postmenopausal hormone use (43). Cimetidine also increases PRL levels, but the few studies published have not shown any meaningful link with breast cancer (44, 45). Thus, current evaluations of medications known to influence PRL levels do not indicate any important association with risk of breast cancer; however, further assessments that include a detailed assessment of duration of medication use are warranted.
In interpreting the above results, it is important to keep one limitation in mind. Levels of plasma estrogens and androgens [hormones that are confirmed or probable predictors of breast cancer risk, respectively (46)] also have been evaluated in relation to a variety of breast cancer risk factors (46 –48). With the exception of positive associations between blood estrogens and both body mass index and alcohol intake, consistent relationships have generally not emerged, although many of the studies were small, and modest associations could not be excluded. Ideally, in any analysis of PRL level and breast cancer risk factors, estrogens and androgens also would be assessed, thus allowing an evaluation of each hormone’s independent and joint association with the risk factor. However, with only a few exceptions, this has not been done and, hence, more work in this area is needed.
8. Prolactinomas and breast cancer risk
Women with prolactinomas have greatly elevated PRL levels; thus, rates of breast cancer in this group are of considerable interest. However, just a few case reports of breast cancer in women or men with prolactinomas (49 –53) and a small cohort study of 67 women with prolactinomas (54) have been published to date; therefore, additional data are needed. A limitation in using these data to infer the relationship between PRL levels in the normal or modestly elevated range and breast cancer risk is the frequent occurrence of hypogonadism in women with prolactinomas (55). Lower exposure to estrogens and androgens premenopausally is hypothesized to decrease breast cancer risk, thereby potentially counterbalancing, at least in part, any increase in risk associated with elevated PRL levels.
B. Methodological issues in the evaluation of PRL levels and breast cancer
Several methodological issues arise in the evaluation of PRL levels and risk of breast cancer in human population studies. Because of logistic and financial issues, it is generally only possible to collect a single blood sample per study subject in these studies. Whether a single sample can reflect long-term hormone levels (generally the exposure of greatest etiological interest) is therefore an important issue. Three studies have addressed this topic (56 –58). In premenopausal women, the correlation of repeated PRL assessments in the same women over a 1- to 3-yr period ranged from 0.40 –0.48 and, in postmenopausal women, the correlations ranged from 0.53–0.76. This level of reproducibility is slightly lower than that found for other biological variables, such as blood pressure and serum cholesterol measurements (with correlations of 0.6 –0.8); these parameters are considered to be reasonably well measured and are consistent predictors of disease in epidemiological studies (59). These data thus suggest that epidemiological studies of PRL levels and breast cancer risk using a single blood sample to estimate PRL exposure should be able to detect a moderate to strong association if it exists, although results will be somewhat attenuated. Of note, measurement-error correction methods exist (60, 61) and can be applied in epidemiological studies to provide a more accurate understanding of the strength of the relationship.
Another issue of importance to the study of circulating PRL levels and breast cancer risk is the marked circadian and, to a lesser extent, postprandial and menstrual variation observed. Epidemiological studies must carefully account for time of day, phase of the menstrual cycle, and fasting status in the design or the analysis.
In all epidemiological studies, circulating PRL levels are measured. How well these levels represent exposure at the tissue level, where both autocrine and paracrine production play a role, is unknown. Several lines of evidence from studies of other hormones and breast cancer risk, in which the same issue exists, suggest that circulating hormone levels may have an influence on risk through either direct or indirect mechanisms. For example, although levels of 17β-estradiol in breast tissue are considerably higher than circulating levels (62) and substantial conversion from steroid precursors to 17β-estradiol can occur in the breast tissue (63), circulating 17β-estradiol levels are strong and consistent predictors of subsequent breast cancer risk (46). In addition, it was recently reported that the reduction in breast cancer risk associated with raloxifene use in the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial was particularly great among women with high circulating 17β-estradiol levels (64), again suggesting that circulating levels are providing important information on baseline risk. Finally, recent data using a liver-specific IGF-I-deficient mouse model showed that circulating IGF levels (derived primarily from the liver) can influence tumor development and progression (65). The epidemiological evidence from studies of PRL and breast cancer risk (described below) suggest that circulating PRL levels might be serving as a surrogate marker of exposure at the tissue level; however, much more work in this area is needed. Certainly the ability to better define a woman’s individual risk of breast cancer by using markers such as circulating, rather than tissue, hormone levels (as cholesterol levels are used to help determine an individual’s heart disease risk) would be both feasible and of considerable importance to public health.
The two primary epidemiological study designs used to evaluate the relationship between PRL levels and breast cancer risk have been case-control studies and nested case-control studies. In case-control studies, PRL levels in women with breast cancer are compared with those measured in women without breast cancer. This study design has been used most commonly because it can be conducted relatively quickly and at low cost. However, because PRL secretion can be altered by physical or psychological stress (66 –68), levels in women with breast cancer may not reflect predisease levels, thus biasing study results. Nested case-control studies are conducted within a prospective cohort study. Here, blood samples are collected and archived from a large group of nondiseased women; the women are followed over time, and those who go on to develop breast cancer are identified. Breast cancer cases are each matched to one or more women who did not develop breast cancer, and blood levels from the two groups are measured and compared. The important aspects of this design are that all blood samples were collected before disease diagnosis, and all subjects were selected from the same study population. Although this is methodologically a much stronger study design, because of the cost of prospective studies (and hence nested case-control studies), few have been conducted to date.
C. Epidemiological studies: case control and prospective
The majority of the epidemiological studies have employed a RIA to measure circulating PRL levels. Only a small subset of studies (11, 69) used a bioassay utilizing Nb2 rat lymphoma cells (70). Hence, there are insufficient data to determine whether study results vary according to assay method used.
1. Case-control studies
In six case-control studies, results were reported among premenopausal women specifically (3, 7, 71–74). These studies have ranged in size from 6 cases and 16 controls (72) to 66 cases and 59 controls (73). In three of the studies, a statistically significant positive relationship with risk was reported (71–73); in one, a nonsignificant positive relation was reported (74); and in two, no association was observed (3, 7). In five studies the relation among post-menopausal women was evaluated (47); these studies again were small, comprising 12 cases and 9 controls (72) and 48 cases and 70 controls (7), respectively. In two of these studies, a significant positive association was reported (7, 73); no association was observed in two others; and in one a significant inverse association was seen (72). Finally, in four small studies in which premenopausal and postmenopausal women were combined (69, 75–77), no significant associations were reported. Overall, results from the case-control studies have been inconsistent. However, because of their small size and the assessment of PRL levels in women already diagnosed with breast cancer (which may not be reflective of predisease levels), both important methodological limitations, these studies contribute only modestly to the overall weight of evidence in evaluating PRL levels and breast cancer.
2. Prospective studies
In contrast to the relationship between endogenous estrogen levels and risk of breast cancer where at least nine prospective studies with more than 650 cases have been published (46), relatively few prospective studies of PRL levels and breast cancer have been conducted (Table 2). Three studies of premenopausal levels and risk of breast cancer have been conducted, with 21–71 cases per study (78 –80). In none of the studies was a positive relationship between circulating PRL level and risk observed; however, the studies were so small that a moderate to strong association could not be detected. For example, in the largest of the studies, conducted on the island of Guernsey, 71 cases of breast cancer were diagnosed among 2596 premenopausal women who were followed for up to 22 yr (78). When women in the top vs. bottom 20% of PRL levels were compared, the relative risk of breast cancer was 1.07, suggesting no relationship. However, the 95% confidence limits ranged from 0.51–2.23, indicating that even a 2-fold increase (or decrease) in risk could not be ruled out. The other two studies had even wider confidence intervals. Additional studies are needed among premenopausal women to clarify this relationship.
Table 2.
Prospective epidemiologic studies of the association between plasma PRL levels and risk of breast cancer: study size, characteristics, and summary results
| Study | Study characteristics | No. of cases/controls (ca/co) by menopausal status | Comparison madea | Result relative risk (95% CI)b |
|---|---|---|---|---|
| Wang, 1992 | 1968 –1990 follow-up Guernsey Cohort; controlled for age, parity, height, benign breast disease | Premenopausal 71 ca/2596 co | Top to bottom quintile categories | 1.07 (0.51–2.23) |
| Postmenopausal 40 ca/1180 co | Top to bottom quintile categories | 1.63 (0.57–4.71) | ||
| Helzlsouer, 1994 | 1974 –1991 follow-up Washington County Cohort; controlled for age, time blood drawn, fasting | Premenopausal 21 ca/42 co | Top to bottom tertiles | 1.1 (0.3–4.1) |
| Hankinson, 1999 | 1989 –1994 follow-up Nurses’ Health Study; controlled for age, month and time of day of blood draw, fasting, hormone use, body mass index, family history of breast cancer, parity, age at first birth, age at menarche, age at menopause | Postmenopausal 306 ca/448 co | Top to bottom quartile | 2.03 (1.24 –3.31)
2.64c (1.54 –4.51) |
| Kabuto, 2000 | 1970 –1983 follow-up; controlled for city, age, date of blood, radiation dose | Premenopausal 46 ca/94 co | Unit increase in log10 PRL | 1.01 (.02–47.4) |
| Postmenopausal 26 ca/56 co | Unit increase in log10 PRL | 6.45 (.01–43.9) |
Contrast made in PRL levels to calculate relative risks in last column of this table.
95% Confidence interval.
Invasive cases only (n = 276).
Three prospective studies of PRL levels and breast cancer risk have been conducted among postmenopausal women. In the study by Wang et al. (78), which included 40 cases of breast cancer diagnosed among 1180 postmenopausal women over 22 yr of follow-up, a nonsignificant positive association was observed. The relative risk comparing the top to bottom 20% of the PRL distribution was 1.63 (95% confidence interval, 0.57–4.71). In a second study, conducted among atomic bomb survivors in Japan with follow-up from 1970 –1983, 26 cases and 56 controls were evaluated (80). Investigators observed a nonsignificant increase in risk with a unit increase in log10 PRL level (relative risk [95% confidence interval] = 6.45 [0.01–43.9]). Results of these two studies indicate a possible positive relationship with breast cancer risk, but substantial statistical uncertainty limits the conclusions that can be drawn.
Only one large prospective study has been conducted to date. From 1989 –1990, blood samples were collected and archived from 32,826 members of the Nurses’ Health Study cohort. After 5 yr of follow-up, 306 breast cancer cases and 448 controls were identified and had PRL levels measured (81). All women were postmenopausal, and controls were individually matched to cases by age, month and time of day of blood collection, fasting status, and use of postmenopausal hormones at time of blood collection. A statistically significant positive association was observed between plasma level of PRL and subsequent breast cancer risk: women in the top 25% of levels had a 2-fold higher risk of breast cancer relative to women in the bottom 25% of the distribution [relative risk (95% confidence limits) = 2.03 (1.24 –3.31)]. Women in the top category had PRL levels ranging from 9.7–37.4 ng/ml with a median value of 14 ng/ml. Results were essentially unchanged when women with PRL levels above 20 ng/ml were excluded from the analysis [the relative risk changed from 2.03–1.95 (1.15–3.31)]. Findings appeared slightly stronger among the subset of cases with invasive disease specifically [comparable relative risk = 2.64 (1.54 –4.51)]. Results also were essentially unchanged after the first 2 yr of follow-up were excluded [comparable relative risk 2.39 (1.24 –4.61)], suggesting that presence of the as-yet-undiagnosed breast cancer did not cause the observed association. Finally, the relationship appeared independent of circulating estrogen, androgen, and IGF-I levels. For example, among the subset of women whose steroid hormone levels were also measured, the relative risk for the top vs. bottom quartile of levels was 2.45 when not controlling for 17β-estradiol and 2.35 after controlling for 17β-estradiol in the same statistical model (Fig. 1).
Fig. 1.
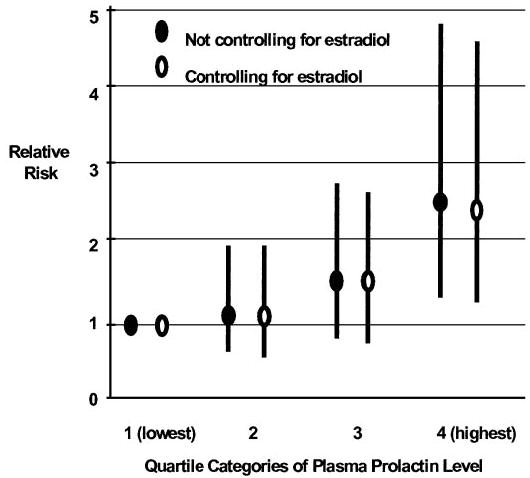
PRL levels and risk of breast cancer. Relative risk (and 95% confidence intervals) of breast cancer by category of plasma PRL level, controlling and not controlling for estradiol. Data are from the only large prospective study (81) of plasma PRL and breast cancer in postmenopausal women and suggest that the observed positive association between PRL levels and breast cancer risk is independent of circulating estradiol level.
D. PRL levels and breast cancer prognosis
In several prospective studies, preoperative (82) and/or postoperative (82–84) plasma PRL levels have been evaluated as predictors of disease-free survival and overall survival. In several studies, a higher postoperative PRL level at least weakly predicted poorer breast cancer prognosis (82, 83), although in another study (84), hyperprolactinemia, assessed 1 wk after surgery, predicted a better prognosis.
III. Endocrine vs. Autocrine/Paracrine Actions of PRL within Mammary Tissues
It is now recognized that both endocrine and autocrine/paracrine sources for PRL exist in mammals. An examination of the regulation of PRL elaborated from endocrine sources, i.e., the pituitary, has been discussed at length in other texts (85, 86) and is beyond the scope of this review. However, it is important to note that the regulation of PRL synthesis and secretion, while incompletely understood, is multifactorial involving both negative (e.g., dopamine) and positive regulators (e.g., estrogen, TRH, etc.). Neuroendocrine regulation contributes to both the daily variation in serum PRL levels and the increase in serum PRL noted during stress (87, 88). As discussed above, these variations are important factors to consider in the design of epidemiological studies aimed at examining the relationship between serum PRL levels and risk of breast cancer.
The recognition that PRL could act as an autocrine/para-crine factor within mammary tissues came historically late. Research in the 1970s had revealed that PRL significantly contributed to the pathogenesis and progression of rodent mammary cancer (1, 89 –91). Furthermore, treatment of rodent model systems of mammary neoplasia with bromocriptine, a dopamine agonist that inhibits the secretion of PRL from the pituitary, could provide effective prophylaxis against incipient mammary neoplasia, or long-term cure against established carcinomas (1, 92). These observations did not escape human oncologists, and several clinical trials with bromocriptine on human breast cancer patients were performed. Without exception, these trials were failures, with no improvement in long term-survival or disease-free interval (93–95). As a consequence of these trials and the pervading dogma that the only sources for PRL were endocrine in nature, the hypothesis of a contributory role for PRL in the pathogenesis of human breast cancer fell into disfavor (2).
This dogma of PRL as an endocrine-only hormone has been revisited over the past decade, and it appears that the clinical failure of bromocriptine was most likely a consequence of its inability to inhibit the local elaboration of PRL from breast epithelium and other nonendocrine tissues. Evidence from the 1970s indicated that hypophysectomized breast cancer patients had near-normal PRL levels (96), whereas immunohistochemistry studies revealed the expression of immunoreactive PRL protein in human breast epithelium (97). Despite these data, the notion that PRL could be synthesized locally, however, was not considered. Additional studies in the early 1990s indicated that the mRNA for PRL could be found in normal and neoplastic human breast epithelium (98) and mammary epithelium from pregnant rodents (99, 100). These studies extended the precedent immunohistochemical analysis (which could not distinguish between locally synthesized vs. endocytosed PRL) of mammary epithelium, revealing that the synthesis of PRL could occur locally. Furthermore, these studies revealed a fundamental difference between the mammary epithelium in humans vs. rodents, i.e., PRL synthesis in human breast epithelium occurred in both the pregnant and nonpregnant states, whereas in rodents, PRL synthesis in the mammary gland was observed during pregnancy but was not detectable in 6-wk-old virgin mice. Concurrent data also indicated that the local production of PRL was not unique to the mammary gland, as both decidua and T cells synthesize PRL (101–104).
These findings led both the Clevenger and Vonderhaar laboratories (105–107) to hypothesize and subsequently prove that PRL was synthesized and secreted in human breast tissues and cells. These studies revealed that cultured breast cancer cells could synthesize appreciable quantities of PRL into defined medium (≤ 0.3 ng PRL/ml/4 × 105 cells/24 h). Furthermore, the expression of PRL mRNA in both normal and malignant epithelium, but not the underlying stroma, was noted. Indeed, the vast majority, i.e., 98% of human breast cancer synthesize PRL mRNA as detected by in situ hybridization (106). As discussed below, this locally elaborated PRL is thought to interact with its cell surface receptor with subsequent functional consequences. In addition, the local elaboration of PRL by mammary epithelium may provide an alternative mechanism to ligand trancytosis, resulting in the high levels of PRL found in breast milk (108, 109).
IV. PRLR Expression in Breast Tissues
The actions of PRL in the mammary gland require the presence of its cognate cell surface receptor, the PRLR. In vitro cell models of PRL action lacking the PRLR are nonresponsive to ligand (110). In vivo data from PRLR−/− knockout mice reveal marked deficiencies in lobularalveolar differentiation during pregnancy and a marked diminution of milk production (111). Thus, if PRL is contributing to the pathogenesis of mammary neoplasia, it is anticipated that the PRLR would significantly contribute to this process. Given the significant structural and functional differences between the rodent and human PRLR, and the sizable literature attached to each, this review will focus on the quantitative and qualitative aspects of human (h) PRLR expression in normal and malignant breast tissues.
A. Quantitative expression of PRLR in human breast tissues
As with its ligand, our understanding of the quantitative and qualitative expression of the PRLR in human breast cancer has been an evolutionary process, driven by technological advances. From a quantitative prospective, initial studies using radioligand binding approaches revealed that the expression of the PRLR occurred in 30 –60% of human breast cancers, generally in association with the expression of estrogen receptor/progesterone receptor (ER/PR) (112–116). However, these quantitative studies were relatively insensitive and demonstrated poor interlaboratory reproducibility. This may have resulted from the technical difficulty of these assays, requiring removal of endogenous ligand from receptor. Fortunately, advances in immunohistochemistry, in situ hybridization, and RT-PCR enabled a more sensitive estimation of the PRLR in human breast cancer. Initial reports using these technologies (106), subsequently confirmed by other laboratories (117, 118), have revealed that the hPRLR is expressed in up to 98% of all human breast cancers. The studies examining PRLR expression at the mRNA level have suggested an association with either ER/PR expression (118) or neoplasia (117); however, studies at the protein level have not confirmed these observations (106). These discrepancies may relate to the inherent sensitivity of RT-PCR-based assays or variability in the affinity of existing anti-PRLR antibodies.
B. Qualitative expression of the hPRLR isoforms
After the cloning of a human PRLR from human hepatoma and breast cancer cells in 1989 (119), the standing viewpoint for one decade was that this isoform (termed the “long” isoform after similarity to its homologous rat receptor) was the sole PRLR species. This was curious, given repeated observations in other species (rat, mouse, chicken, etc.) of multiple PRLR isoforms (120 –122). In part, the identification of other hPRLR isoforms was hindered by the paucity of high-quality anti-hPRLR antibodies, a situation that has only recently improved.
When examined at the protein level by immunoblot analysis, normal and malignant breast tissues and cells reveal multiple cross-reactive species, most notably at 85, 70, 50, and 30 kDa (105). Using appropriate primers for RT-PCR, five of the largest hPRLR isoforms have been cloned and sequenced, whereas the sixth and shortest isoform appears to be a product of proteolysis. Each of these receptors will be discussed in the order of their chronological identification (Fig. 2).
Fig. 2.
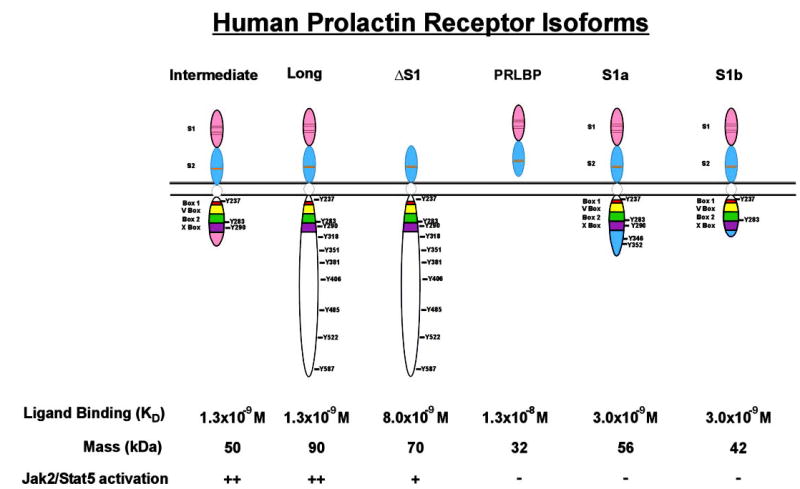
Structure of the hPRLR isoforms. The two type-III fibronectin-like domains are indicated with S1 and S2 with their conserved cysteine residues and WSXWS motif marked by black or orange lines, respectively. The conserved proximal region containing the Box motifs is delineated with the corresponding tyrosine residues in each ICD. The C-terminal domains unique to the intermediate, short 1a, and short 1b hPRLR isoforms, respectively, are also noted. Affinities of receptors for ligand were calculated in all cases by radioligand binding/Scatchard analysis with the exception of the PRLBP, which was determined by biosensor analysis.
The long hPRLR isoform is a classic type I transmembrane receptor and, on the basis of structural homology, a member of the larger family of cytokine receptors (123, 124). Consisting of 211 amino acids, and migrating in SDS-PAGE at approximately 85 kDa, the extracellular domain (ECD) of the long hPRLR contains two type III fibronectin-like domains, termed the S1 and S2 domains. These motifs consist of seven anti-parallel β-strands divided into two β-sheets that are connected by a linker of five amino acids. The N-terminal S1 domain contains both sites of N-linked glycosylation of the PRLR and two pairs of disulfide linkages. The S1 domain contains the majority of ligand contact sites. The S2 domain contains a tryptophanserine-X-tryptophanserine motif conserved across the cytokine receptor family. The S2 domain has a smaller surface area for interacting with ligand but also contains elements responsible for interacting with its partner receptor in the ligand-dimerized complex. These structures impart the relatively high affinity of the hPRLR for hPRL (Fig. 2). Containing 24 amino acids, the function of the transmembrane region during juxtaposition of the intracellular domain (ICD) as a consequence of ligand binding remains unresolved. The ICD contains a juxtamembrane region containing the so-called Box 1, Variable Box (V-Box), Box 2, and Extended Box 2 (X-Box) motifs. These motifs are conserved across the cytokine receptor superfamily, with the highest degree of conservation noted in the Box 1 and 2 domains. The function of the Box 1 and 2 motifs during PRLR signal transduction, however, remains poorly characterized. It is recognized, however, that the Box 1 motif is necessary for the engagement and activation of Janus kinase 2 (Jak2) after ligand stimulation (125–127). The function of the C-terminal region of the hPRLR is even less well understood. Precedent studies in the rat have demonstrated that the most C-terminal tyrosine residue contributes the engagement of signal transducer and activator of transcription 5 (Stat5) (128) and SH2-containing protein tyrosine phosphatase (SHP-2) (129). However, the C terminus of the hPRLR is very different from the corresponding rodent PRLR in the number of tyrosine residues (10 vs. 9), their location, and the surrounding amino acid residues thought to contribute to the functionality of such (phospho)tyrosine residues. Indeed, the tyrosine residues phosphorylated during the activation of the hPRLR remain to be determined.
The intermediate hPRLR isoform is truncated in its C terminus as a consequence of an out-of-frame splicing event (130). This results in a deletion of all coding sequence C terminal to the X-Box and the addition of a novel 13-aminoacid sequence unique in the protein databases, resulting in a protein with a mobility in SDS-PAGE of approximately 50 kDa. The function of this C-terminal motif is currently unknown. Identical in its ECD, the affinity for the intermediate hPRLR is similar to that of the long isoform. Lacking 191 amino acids found in the ICD of the long hPRLR isoform, it was anticipated that the functionality of the intermediate isoform could be distinct. Indeed, when transfected into PRLR-responsive cells, the intermediate isoform demonstrated comparable levels of Jak2 activation with respect to the long hPRLR but was incapable of activating the Fyn tyrosine kinase (130). It was also noted that whereas the intermediate isoform was unable to trigger the proliferation of transfected cells in response to ligand, it was equipotent to the long form in mediating cell survival.
Like the intermediate hPRLR isoform, the ΔS1 isoform also represents a mRNA splice variant. Unlike the intermediate hPRLR, however, the alternative splicing that generates the ΔS1 hPRLR removes exons 4 and 5 in frame from this mRNA species, resulting in the loss of the entire S1 domain of this receptor isoform, and yielding a protein of approximately 70 kDa (131). Thus, as anticipated, the affinity of the ΔS1 homodimer for ligand is reduced by approximately 7-fold, when examined by radioligand binding analysis. Interestingly, the dose-dependent activation of associated signaling cascades after ligand stimulation is only modestly delayed, and unlike the long hPRLR, the ΔS1 isoform does not demonstrate self-antagonism at high ligand concentrations. The basis for these functional differences may be related to the ratios of the relative affinities of the receptor1/receptor2 ligand binding sites in the long (1:12) vs. the ΔS1 (1:4) (131, 132). Alternatively, recent data from the Clevenger laboratory have revealed that the ΔS1 isoform is capable of associating and differentially regulating integrin-associated signaling cascades, a functionality not observed in either the long or intermediate PRLR (our unpublished observations).
The shortest PRLR isoform identified to date, the PRL binding protein (PRLBP), was recently identified in human serum (133). This isoform represents the freely circulating ECD of the PRLR, with a molecular mass of approximately 32 kDa. It is found in human serum at a concentration of approximately 14 ng/ml and is secreted into the medium of cultured human breast cancer cells and hematopoietic cells transfected with the long hPRLR. Given these data, and the absence of a detectable corresponding mRNA, these findings would suggest that the PRLBP arises from a proteolytic event. In vitro, the PRLBP antagonizes the actions of PRL on responsive cells (133). Its function in vivo, where it binds approximately 36% of circulating PRL, remains to be determined. Precedent data with the GHBP transgenic animals (134) would suggest that PRLBP may limit secretion and degradation, increase serum half-life, and enhance in vivo PRL function.
The most recently identified hPRLR species are two short hPRLR isoforms (135). Identified by selective RT-PCR analysis, these isoforms are formed by the differential replacement of some or all of exon 10 with some or all of exon 11. These splicing events result in isoforms termed hPRLR S1a and hPRLR S1b of 56 and 42 kDa, respectively. The S1a isoform contains both the Box 1 and 2 motifs, whereas the S1b PRLR contains only the Box 1 element. Like the corresponding forms identified in rodents, both of the hPRLR short isoforms appear inert from a signaling perspective and may serve as ligand traps that function to either internalize ligand and/or down-regulate PRL-induced signaling.
Given the functional differences that exist between the hPRLr isoforms, the evaluation of the expression and function of the various hPRLRs in breast tissues and cells is an active area of research. Only one study to date has examined the relative levels of PRLR isoform expression in normal and malignant breast tissues, using an anti-PRLR antibody cross-reactive to each of the various isoforms. This preliminary study would indicate that higher levels of the intermediate and ΔS1 hPRLR isoforms are expressed relative to the long isoform in both normal and malignant tissues (105). How the various PRLR isoforms function within the mammary gland remains a difficult question to address, as covariable expression of each of the PRLR isoforms is observed within mammary tissues and cell lines derived thereof, in contrast to the singular expression of individual isoforms obtained in trans-fected model systems (130, 131). Nevertheless, Scatchard and biosensor analysis indicate that each of the PRLR isoforms identified to date is capable of binding PRL found at physiological concentrations, suggesting an in vivo functionality for these receptors.
V. Function of the PRL/PRLR Complex in the Mammary Gland
Once cells have undergone the critical genetic and epigenetic mutations that determine tumorigenic potential, successful neoplastic development and progression require deregulated cell proliferation, increased cellular survival, acquisition of an adequate vascular supply, and escape from constraints on motility. As discussed below, and as summarized in Fig. 3, PRL has been shown to promote all these activities in mammary cells in vitro, consistent with contributions to carcinogenesis in this tissue.
Fig. 3.
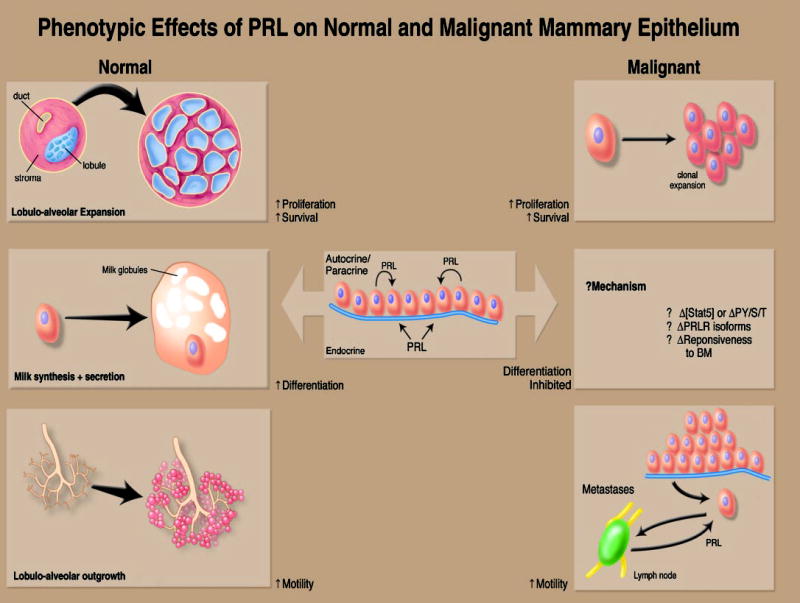
Function of the PRL/PRLR complex in breast tissues. The effects of PRL on normal tissues (left panels) result in the cellular expansion of lobular units and their differentiation and outgrowth into the stroma. These effects are directly related to PRL-induced proliferation, survival, differentiation, and motility of mammary epithelium. Such actions may be due to PRL derived from both local (i.e., adjacent mammary epithelium) and distant (i.e., pituitary) sources. The functions of PRL in malignant tissues (right panels) are less clearly delineated. Although evidence exists that PRL can trigger the growth and motility of human breast cancer cells, the inability of PRL to trigger differentiation (and thereby inhibit the malignant phenotype) remains uncertain. Potential mechanisms for this include alterations in Stat5 levels or phosphorylation, quantitative changes in the expression of the various hPRLR isoforms, or alteration in the malignant epithelial cell’s responsiveness to the basement membrane, which could indirectly impact on PRLR signaling.
The following section describes the actions of PRL in human mammary tumor epithelial cell culture models. Many well-characterized cell lines are available, with differing oncogenic mutations and characterized steroid hormone responsiveness. A striking observation from the literature is that despite this range of phenotypes, PRL activities are evident in many cell lines, consistent with a role in these processes in a wide range of mammary tumors.
A. Cell models
Multiple available human mammary tumor cell lines bind PRL (118, 136). More recent studies have examined some of these cell lines for mRNA for the PRLR using RT-PCR. It is clear that most of these lines express PRLR, although the absolute levels vary. Furthermore, of those examined, all express more than one isoform, and relative levels also vary. In light of the different abilities of the hPRLR isoforms to transmit signals, this could be very significant.
In addition, the highly sensitive RT-PCR method revealed PRL mRNA in most lines examined (137–139). Little is known about the control of PRL expression within these cells, and even less is known for normal mammary epithelial cells. RT-PCR analyses have suggested that at least some of the PRL produced within the mammary cells utilizes the distal PRL promoter best characterized in uterine decidual cells (137), consistent with transcriptional regulation distinct from that in lactotrophs. Furthermore, PRL can be modified post-translationally, and this modification can be influenced by environmental factors including steroid hormones (140, 141). These modifications can alter activity at the target cell as well as biological half-life. Walker and her colleagues (140) developed a molecular mimic of PRL phosphorylated at serine 179, S179D hPRL. Although controversial (142), this molecule has activities reported to be distinct from unmodified hPRL in the mouse mammary cell line, HC11 (143), underscoring the importance of understanding this component of the mammary environment.
These characteristics make human mammary tumor cell lines complex systems in which to explore PRL actions. Expression of more than one PRLR isoform as well as endogenous PRL production have been reported in other species, including the rat, sheep, and goat (139). However, differences in PRLR isoforms among species and the use of a distal PRL promoter unique to primates make these models difficult to translate to human disease.
B. Proliferation
Considering the breadth of genotypes in the mammary tumor cell lines that have been examined, the evidence for a mitogenic action of PRL is remarkably consistent. Exogenously added PRL has modest trophic effects on human tumor tissue and cells in vitro (144). However, it is now clear that this relatively low activity is in part due to PRL synthesized by the mammary cells themselves. Neutralizing PRL antibodies reduced proliferation in both MCF-7 and T47Dco cells (107). The human GH receptor antagonist, G120R, which binds both the PRL and GH receptors but does not permit dimerization, also reduced proliferation of several mammary cells lines (145). Interestingly, the BT-474 line, which expresses PRL but did not respond well to the antagonist, contains transcripts encoding only the ECD of the PRLR. This is consistent with trapping exogenous PRL before it can bind to membrane receptors and points to one way that mammary cells could lose PRL responsiveness.
An antagonist specific for the PRLR, G129R-hPRL, also inhibited proliferation and cell cycle progression in multiple cell lines (146 –148), demonstrating the importance of PRL, rather than GH, in these responses. This specific reagent enabled Goffin and colleagues (147) to confirm that exogenous hPRL increased tyrosine phosphorylation of Stats 1, 3, and 5b, as well as stimulated phosphorylation of ERKs 1 and 2 in several mammary tumor cell lines.
MCF-7-derived sublines with deficient endogenous PRL production have provided a system in which to investigate target genes and signaling pathways more directly. As predicted, these cells exhibited a greater proliferative response to exogenous PRL compared with control cells and demonstrated marked changes in levels of cell cycle regulators (149). The expression of cyclin D1, a critical regulator of the G1/S transition, but not cyclin D3, was increased by PRL. This was associated with hyperphosphorylation of the Rb protein at Ser-780, indicating increased cyclin-dependent kinase 4 activity. Small increases in cyclins E and A were observed, as well as a marked increase in cyclin B1. In contrast, PRL decreased the expression of a Cip/Kip family inhibitor, p21, but not p16 or p27. These studies support a role for PRL in cell cycle progression and identify specific target genes in this process. The pattern of changes induced by PRL is distinct from many mammary mitogens. Although stimulation of cyclin D1 is shared among these factors, many mitogens, including epidermal growth factor (EGF) and IGF-I, increased levels of p21 protein and decreased p27 (150, 151). Estrogens, however, like PRL, reduced p21 (152).
Existing evidence suggests a participatory role for cyclin D1 during the pathogenesis of mammary carcinoma. Targeted overexpression of cyclin D1 induced mammary tumors in transgenic mice (153), and cyclin D1 is overexpressed in more than 50% of human tumors (154 –156). Recently, Sicin-ski and colleagues (157) reported that this regulator was critical for v-Ha-ras- and c-neu-, but not c-myc- and Wnt-1-, induced carcinogenesis in transgenic models. Antisense oli-gonucleotides to cyclin D1 were able to inhibit the proliferative response to PRL in the mammary tumor cells deficient in PRL production, suggesting a key role for this protein in PRL-induced proliferation as well, at least in vitro (149). Use of selective inhibitors to examine pathways contributing to the PRL-induced increase in cyclin D1 protein in this model indicated that ERKs 1 and 2, p38 and/or c-jun N-terminal kinase (JNK) kinases, and the phosphatidylinositol 3′-kinase (PI3K) pathways were involved at some point in the regulatory pathway (149). Actinomycin D also prevented the PRL-induced increase in cyclin D1 levels, indicating transcriptional control. In the more defined Chinese hamster ovary cell system, PRL was able to activate a cyclin D1 promoter (containing the 944 bp before the site of transcription initiation) through the long form of the receptor via a Jak2-dependent pathway (158). Stat5 was critical for both basal levels of transcription, as well as PRL-induced activation, an effect mediated via a γ-interferon-activated sequence site at present at position −465 within this promoter. This pathway is similar to the action of another cytokine, IL-3, in hematopoietic cells (159). However, it is clear that PRL signaling to this promoter is more complex; a more proximal region of the promoter was also implicated, and Stat3 and, to a lesser extent, Stat1, also contributed to the PRL response. Moreover, Stats did not appear to bind DNA directly in the proximal region, suggesting the involvement of other transcriptional regulators (160).
C. Survival
In addition to stimulation of proliferation, PRL may also actively inhibit apoptosis of mammary tumor cells. Although the ability of PRL to promote cell survival is clear in the Nb2 lymphoma model system (110, 161), evidence for a similar activity in mammary epithelial cells is only beginning to emerge. Chen et al. (148) reported that hPRL-G129R, but not hPRL, induced apoptosis in T47D cells as measured by the terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling assay. Subsequent work demonstrated that hPRL-G129R treatment activated caspase-3 in these cells (162, 163). However, little is known about the pathways that regulate these events. The importance of the serine/threonine kinase Akt in apoptosis (164, 165) and suppressing mammary involution in vivo (166), in combination with the ability of PRL to stimulate Akt in several mammary tumor cell lines (167), points to an obvious possibility. These observations suggest fertile areas for additional research.
D. Motility
Several epidemiological studies have indicated that PRL may also function as a progression factor for human breast cancer (82–84, 168). Because enhanced motility is one aspect of the metastatic process, one recent study (169) has questioned whether PRL could serve as a chemoattractant for human breast cancer in vitro. When analyzed by monolayer wounding, time-lapse video microscopy, and Boyden chamber analyses, PRL was found to significantly enhance the motility of ER+ and ER− cell lines. This motion was noted to follow the PRL gradient and resulted in significant alterations in the cytoskeleton, with the PI3K-dependent formation of lamellipodia and stress fibers. Coupled with precedent studies examining the effects of PRL on the progression of rodent mammary carcinoma (1, 92), these findings would suggest that PRL may contribute significantly to the meta-static phenotype of breast cancer.
E. Angiogenesis
Although not a direct effect of PRL on mammary epithelial cells themselves, PRL may also influence mammary carcinogenesis by modulating vascularization. Neoplastic cells must secure an adequate blood supply for successful tumor growth and progression. Furthermore, secretion of antiangiogenic agents by the primary tumor inhibits growth of micrometastases (170 –174). The murine placental PRL-related hormones, proliferin and proliferin-related protein, which are angiogenic and antiangiogenic, respectively, modulate this process in the developing placenta (175, 176). Recently, it was shown that hPRL itself, as well as human GH and the placental hormones, human placental lactogen and hGH-V, could also stimulate formation of capillaries in the chicken chorioallantoic membrane assay (177). In contrast, a proteolytic cleavage product of PRL, 16K-PRL, is a potent antiangiogenic agent in vivo and in vitro (177–179). This N-terminal cleavage product of PRL inhibited endothelial cell proliferation in response to vascular endothelial growth factor and basic fibroblast growth factor by inhibiting the Ras-Raf1-MAPK pathway and increasing expression of type 1 plasminogen activator inhibitor (177, 180, 181). These activities appear to be mediated by a receptor distinct from the PRLR (182). 16K-PRL can be produced by mammary cell extracts, presumably by cathepsin D, and is found in the serum of the human, mouse, and rat (141, 183). Taken together, these data indicate that PRL may contribute to the control of neovascularization in the tumor environment by the balance of angiogenic intact hormone and antiangiogenic cleavage product. This promises to continue to be a highly interesting area for additional studies.
VI. PRL/PRLR Signaling and Endocytosis
A. Signaling
The web of kinases, adaptors, and transcription factors that connects PRL with control of cellular gene expression has received considerable study in multiple cell types. Many of the studies to dissect these pathways in cultured cells have employed cells of the immune system, especially the lym-phoma line, Nb2. This cell line proliferates robustly in response to PRL (184, 185) and is exquisitely sensitive because of high levels of an alternatively spliced isoform of the PRLR with a higher affinity for this hormone (186, 187). Other preferred model cell lines, such as Chinese hamster ovary, COS, or human embryonic kidney 293 cells, have the advantages of 1) low or nondetectable levels of PRLR, so that the isoform complement may be dictated by the investigator; and 2) low levels of intermediates of some signaling cascades, so that this, also, may be controlled at will. Mammary epithelial cells, especially human cells, present a more complex target due to endogenous PRL expression and a complex complement of PRL isoforms (see above). These features make them relatively insensitive to exogenous PRL, and fewer studies have been done in these cells. However, accumulating data make clear that the actions of growth factors, cytokines, and hormones may differ with cell type and genetic background, in addition to environment, underscoring the importance of examination of PRL actions in the cell type of interest. Tumor cells, of course, achieve this state by multiple routes, and thus, predictably, mammary tumors and derived cell lines differ widely with respect to oncogenic mutations in signaling pathways. Therefore, appropriate caution must be exerted when extrapolating from model systems.
The following section will focus on the relatively few studies of mediators of PRL action in human mammary tumor cells (partially schematized in Fig. 4). The reader is urged to consult other excellent reviews for a more comprehensive view of PRL signal transduction (188 –190). Our growing understanding of the complex relationships between these signaling elements in other systems points to the importance of interpreting these studies as glimpses into a complex network, rather than hierarchically.
Fig. 4.
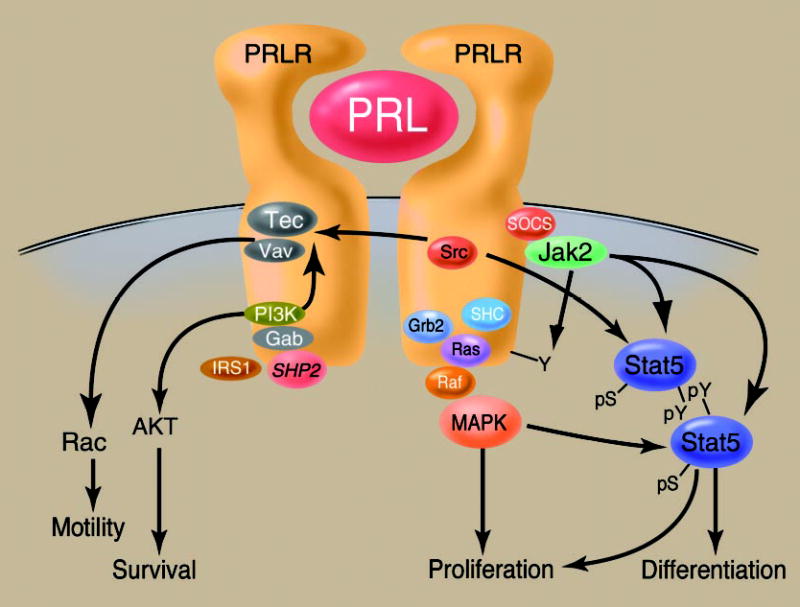
Aspects of PRLR signaling as related to mammary gland function. Relationships between some of the salient PRLR-associated transduction cascades are demonstrated. PRL-induced receptor dimerization induces the association of the Jak2 kinase, resulting in the activation of Jak2, PRLR phosphorylation, and the association and phosphorylation of Stat5. This triggers Stat5 dimerization and nuclear translocation and events necessary for PRL-triggered mammary differentiation. Signaling through the SHC/GRB2/Ras/Raf/MEK/MAPK pathway also directly stimulates proliferation and modulates Stat activity. Furthermore, the complex between the Tec tyrosine kinase and the Vav family of guanine nucleotide exchange factors also inducibly associates with ligand-bound PRLR. This results in the exchange of GDP for GTP on the small G protein Rac, resulting in its activation and stimulation of cellular motility. Activation of Tec and the kinase Akt are directly tied to the PRL-induced activation of PI3K. The phosphatase SHP-2 also associates with the PRLR and potentiates its activity.
1. Jak2 activation
Like other cytokines, PRL activates a member of the Jak family, primarily Jak2, upon receptor dimerization. Although it is not clear that all PRL signaling requires Jak2 as a proximal intermediate (189, 191, 192), a great deal of evidence in many cell types supports a key role for this kinase in many actions of PRL (188, 190, 193, 194). Jak2, like its other family members, is a promiscuous kinase and phosphorylates multiple substrates, including the PRLR and Jak2 itself. This provides docking sites for proteins with SH2 domains, including Stats. The interaction of Jak2 with the PRLR appears to be mediated by an interaction of the membrane-proximal Box 1/Box 2 motif of the PRLR (126, 127) with the N terminus of Jak2 (195, 196). Extensive study of the actions of Jak family kinases in response to cytokine signaling in other systems has linked them to multiple additional downstream pathways, such as Src family kinases, Ras-MAPKs, and PI3K (191). As discussed below, the actions of Jak2 are attenuated by members of the suppressor of cytokine signaling/cytokine-inducible inhibitor of signaling (SOCS/CIS) family.
2. Stats
One of the best studied consequences of activation of Jak2 by PRL is tyrosine phosphorylation of Stat family members. This pathway has been extensively studied in the COMMA-D-derived murine mammary epithelial cell line, HC11, where it mediates PRL’s signals to milk protein genes (197). In commonly studied mammary tumor cell lines, including T47D, MCF-7, and BT-20, PRL treatment results in increased tyrosine phosphorylation of Stats 1, 3, and 5 (147, 198, 199). Several studies have demonstrated increased levels of Stats 1 and 3 in primary mammary tumors (200, 201), and the incidence of elevated Stat5 activation in other tumor types (201) suggests a high probability that these Stats may be elevated in mammary tumors as well. However, their target genes in oncogenic processes, the relative importance of PRL in their regulation, and differences from the normal mammary gland are not understood. Both Stats 3 and 5 are involved in PRL activation of the cyclin D1 promoter (158), suggesting at least one target of PRL through this pathway that could contribute to tumorigenesis. However, their activities are likely to be complex. In numerous cell models other than mammary cells, Stat1 is frequently growth inhibitory, whereas Stats 3, 5a, and 5b are growth promoting. However, it is now clear that their activities in proliferation, apoptosis, and differentiation depend both on the level of activation and cell context (201–203). Their roles in the normal mammary gland in vivo reflect this complexity. Stat3, in particular, does not follow the growth-promoting generalization. Levels of all four Stats are altered dramatically over the stages of mammary function, and genetic deletions of Stats 3, 5a, and 5b have demonstrated critical roles for Stat3 in involution (204) and for Stat5 in normal lobuloalveolar development (205, 206). Despite common actions in many transfection models, Stats 5a and 5b are not completely redundant. Kazansky and Rosen (207) have demonstrated that Stat5b, but not Stat5a, is a potent mediator of Src-induced tumorigenesis. This coupled with the observation of delayed or absent oncogene-induced mammary tumorigenesis (see Section VII) indicate an important role for the Stat family in mammary pathology.
Levels and activities of the Stats are altered by multiple hormones, growth factors, and signaling cascades, pointing to an obvious role they may play in cross-talk with many other agents important in mammary carcinogenesis. For example, Horwitz and colleagues (151) demonstrated that progestins were able to up-regulate Stats 3 and 5 protein levels in T47Dco cells, sensitizing these cells to the effects of both EGF and PRL. EGF family members also activated Stats in mammary tumor cells (208), and overexpression of a TGF α mammary transgene in vivo altered levels and activities of these factors as well (209, 210). However, utilization of common mediators does not necessarily translate to signaling cross-talk. Although type 1 interferons also activated Stats 1 and 3 in mammary tumor cells, cotreatment with PRL did not interfere with interferon α/β signals (199).
As discussed above, Stat activation requires tyrosine phosphorylation by a receptor-associated Jak2 kinase (211, 212). This results in the dimerization/multimerization and nuclear retrotranslocation of the Stat complex where it engages its cognate DNA binding sequence, resulting in promoter transactivation under appropriate conditions (213). In addition to the SOCS/CIS family regulating the tyrosine phosphorylation status of Stat proteins, the PIAS (peptide inhibitors of activated Stat) family of proteins has been found to block the DNA binding of activated Stats (214 –216). In addition, serine phosphorylation, in part mediated by MAPK, has been found to modulate the activity of Stat5 (217, 218). Other signaling pathways may also impact on Stat activation. For instance, the EGF-induced activation of Stat5a in vitro required c-Src (219). No studies to date link PRL signaling with Src family kinases in mammary tumor cells. However, PRL has been shown to activate Src in a variety of cell types, including rat liver (220), transfected chick embryonic fibroblasts (221), as well as cells of the immune system (222). This family also played a modest role in PRL signaling to the β-casein promoter in HC11 cells (223). Both Stats 3 and 5 can be activated by Src family members (192, 224, 225). However, the pattern of Stat5 activation by c-Src is distinct from that of PRL, at least in COS-1 and HeLa cells. Whereas PRL stimulated tyrosine phosphorylation and nuclear translocation of both Stats 5a and 5b, Src activation resulted in tyrosine phosphorylation of both Stats 5a and 5b, but nuclear translocation of only Stat 5b (192). Cross-talk with this pathway in a mammary tumor context may be important.
3. Ras-Raf-MAPK pathway
A second pathway that has received focused attention in mammary tumor cells is the Ras-Raf-MAPK pathway. PRL has been shown to activate this pathway in a number of PRL-dependent models (226) and mammary tumor cell lines (147, 227, 228), as well as normal mouse mammary epithelial cells (227, 228). In T47D cells, this was associated with increased association of Shc with Jak2, as well as Grb2 and Sos, indicating a role for Jak2 in this cascade. The p42/44 MAPKs are linked to proliferation for many growth factors in many systems (229 –231) and also appear to be linked to PRL-induced proliferation of mammary tumor cells. In PRL-deficient MCF-7 cells, the MEK1 inhibitor PD98059 decreased proliferation of unstimulated cells. EGF, but not PRL, was able to overcome this inhibition (149), indicating a critical role for this pathway in PRL, but not EGF, -stimulated proliferation. PRL also can synergistically activate this pathway, via cross-talk with other growth factors, depending on the phenotype of the tumor cell. PRL-induced activation of Jak2 resulted in tyrosine phosphorylation of erbB2, thereby increasing association with Grb2, and activating the Ras-MAPK pathway (232). p42/44 MAPKs are believed to exert these effects on proliferation via multiple mechanisms, including phosphorylation of Ets transcription factors, increasing synthesis of the fos gene family (c-fos, Fra-1,2, c-jun, JunB), phosphorylation of carbamoyl phosphate synthetase II, leading to increased DNA synthesis, as well as many other protein kinases and other substrates in the cytoplasm, indirectly modulating downstream activity. Cross-talk between the Stat and MAPK pathways at other points is well documented for many cytokines, including PRL (233). MAPKs are able to phosphorylate Stats on serine and threonine residues, which augments the activities of Stats 1 and 3 (218, 235). However, the role of p42/44 MAPKs in serine phosphorylation of Stats 5a and 5b in response to PRL appears to be more complex (217, 233, 236).
Other MAPK families have been shown to be involved in regulation of proliferation and differentiation, as well as apoptosis, in multiple cell types (229 –231). However, less is known about these pathways in PRL action. PRL is able to activate JNK in T47D cells (237), as well as bovine mammary epithelial cells (238), Nb2 (237), and PC12 (239) cells. Inhibition of this rise in activity in Nb2 and PC12 cells prevented PRL-induced increases in proliferation and, in the case of the Nb2 cells, also increased apoptosis. JNK family members are able to phosphorylate c-jun, and indeed, in bovine mammary epithelial cells, the PRL-induced activation of JNK was associated with AP-1 activation, suggesting one mechanism for the effect on proliferation (238). The SB203580 inhibitor at 10 μM prevented the PRL-induced rise in cyclin D1 levels associated with cellular proliferation in PRL-deficient MCF-7 cells (149). This inhibitor was first thought to be selective for p38; however, recent studies have shown that at higher concentrations, in the range frequently used by investigators (10 μM), it can also inhibit JNK.
4. PI3K and downstream pathways
Activation of PI3K by a variety of G protein-coupled receptors and receptors with intrinsic or associated tyrosine kinase activity generates phosphoinositides that serve as second messengers for molecules containing pleckstrin-homology domains. Class I PI3Ks consist of a p110 catalytic subunit and a p85 regulatory subunit. They generate the metabolites, phosphatidylinositol (PtdIns) (3)P, PtdIns (3,4)P2, and PtdIns (3,4,5)P3, which can regulate multiple pathways important in oncogenesis, including proliferation and cytoskeletal rearrangements, as well as inhibition of apoptosis and angiogenesis (240 –243). Although activation of this pathway has not been dissected in mammary tumor cells, the p85 subunit became associated with the PRLR after ligand exposure in transfected human embryonic kidney 293, COS, and Chinese hamster ovary cells (244, 245). Furthermore, use of inhibitors, such as LY294002 and wortmannin, point toward a role in PRL-induced cell motility (169). PRLR association with Src family members contributed to PI3K activation in Nb2 cells (246). PI3K could potentially be activated by PRL through multiple additional pathways. It can be a target of Ras (247), and the p85 regulatory subunit has been shown to associate with several downstream effectors and adaptors of cytokine and growth factor receptors, including Stat5, Stat3, IRS 1, Gab1 and Gab 2, and SHP-2, (248 –251), all of which have been shown to be activated by PRL, or are associated with the activated PRLR (129, 244, 245) in some system.
PI3K-generated phosphoinositides provide docking sites for Akt (protein kinase B), as well as its upstream kinases PtdIns-dependent kinase 1 and 2, which activate Akt by threonine/serine phosphorylation. This pathway initiates survival, inhibits proapoptotic signals (164, 165), and also modulates regulators of cell cycle progression such as E2-F, and cyclin D1 (253, 254). Indeed, expression of activated Akt retarded mammary involution (166) and contributed to mammary tumor progression in vivo (255). A preliminary report from Anderson and colleagues (167) demonstrated that PRL can activate this kinase in a variety of mammary tumor cell lines.
Phosphoinositide metabolites may also bind to the pleckstrin homology domains of a family of guanine nucleotide exchange factors, including Vav, as well as Tec, a member of a larger family of tyrosine kinases including Tec, Btk, Itk, and Bmx. A constitutive complex of Tec and Vav (256) associates with the PRLR in ligand-stimulated T47D (257). Activation of this pathway permits exchange of GDP for GTP on Rho family members, including Rac1 and RhoA, which ultimately results in formation of stress fibers and lamellipodia, an observed response to PRL in several mammary tumor cell lines (169). In addition to modulation by PI3K, members of the Jak and Src families are both able to up-regulate activity of Tec family members (256), pointing out obvious sites for cross-talk. PRL stimulated tyrosine phosphorylation of focal adhesion kinase and paxillin in T47D and MCF-7 cells (258), additional molecules important in cell adhesion and migration. However, the pathway leading to this activation in these cells is unclear.
5. Modulatory pathways
PRLR activation also stimulates positive and negative regulatory molecules that modulate the strength and duration of PRL-induced signals. Certainly, dysregulation of systems resulting in signal prolongation or attenuation would have consequences for mammary tumor-igenesis. In general, these feedback mechanisms have been examined more extensively for other cytokines, and very few studies have been reported on the role of these proteins in modulating PRL action in mammary tumor cells. Some of those directly linked to the PRLR in other systems are summarized below. Additional studies of PRL action at this target will doubtless reveal other major regulators of its signaling pathways.
Among these are SHPs. One of these, SHP-2 (also known as PTP-1D), up-regulates cytokine and growth factor signaling by removal of inhibitory phosphotyrosines (259). SHP-2 itself was shown to be phosphorylated on tyrosines in response to PRL in model cell systems, as well as murine mammary cell lines, and increased activation of the Jak2-Stat pathway to the β-casein promoter (129, 260, 261). This protein can also act as an adaptor to multiple other cellular regulators (129), influencing signals through a number of pathways.
Protein tyrosine phosphatases also negatively regulate signaling through a variety of cytokine signaling systems (259, 262, 263). PTP1B overexpression decreased PRL-induced tyrosine phosphorylation of both Stats 5a and 5b, reduced their nuclear translocation, and also reduced β-casein promoter activity (264). Additional phosphatases likely to be important include MAPK phosphatases and lipid phosphatases, such as the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which terminates signaling through PI3K-mediated pathways.
Members of the CIS family, also known as SOCS, also modulate cytokine signaling, including that of PRL, at a variety of targets (262, 263, 265). These proteins are rapidly induced after cytokine stimulation with distinct time courses depending on the CIS/SOCS family member, and ligand/receptor system. Depending on the family member and receptor, they interact with the receptors and/or Jaks, and either inhibit signaling or relieve suppression. SOCS-1 and -3 were transiently induced by PRL, and SOCS-1, in particular, was a potent inhibitor of Stat5-dependent transcription (266 –268). In contrast, SOCS-2 and CIS were induced more slowly, and SOCS-2, but not CIS, could relieve the SOCS-3-induced inhibition. Although the mechanisms of these effects have been examined only in model cell systems, the kinetics of induction were examined in both liver in vivo and T47D cells (266). Although the general patterns of SOCS-1 and SOCS-3 expression were similar, SOCS-2 was not elevated in T47D cells before 24 h after PRL stimulation, whereas induction of SOCS-2 was readily apparent within 15 min in the liver. These data point to the importance of examining targets of interest, in addition to easily manipulated model systems.
6. Genomic actions of PRL
A growing body of evidence has indicated a functional role for PRL within the nucleus (269, 270). These data stand in contrast with the classic theory that dictates that peptide hormone action only occurs at a distance as mediated by cell surface receptors. Although considerable data have indicated that PRL and GH can be endocytosed and retrotranslocated to the nucleus after receptor binding (271–273), the intranuclear function of such ligand had remained elusive. Recent data, however, have revealed a role for the peptidyl prolyl isomerase cyclophilin B (CypB), in the nuclear transport and function of PRL (270). As such, these data indicate that a complex between PRL and CypB exists in human serum that binds to the PRLR and is endocytosed during receptor internalization. CypB facilitates the nuclear transport of PRL via its N-terminal nuclear localization sequence. As schematized in Fig. 5, within the nucleus the PRL/CypB complex acts as a transcriptional inducer by facilitating the interaction of Stat5 with DNA by inducing the release of a repressor of Stat5, namely PIAS3 (216). These observations indicate that considerable parallels exist between steroid and peptide hormones in their respective genomic and nongenomic actions and, like steroid hormones, may suggest that the PRL ligand contributes to its signaling specificity through its intranuclear functions.
Fig. 5.
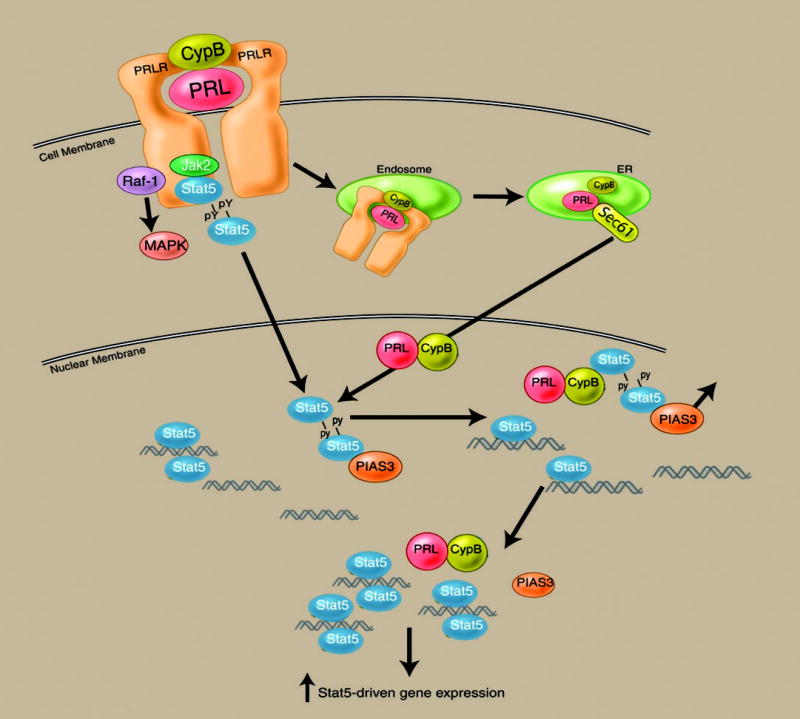
Nuclear actions of the PRL/CypB complex. After endocytosis mediated by the PRLR, the PRL/CypB complex is retrotranslocated to the endoplasmic reticulum/Golgi, where the complex associates with the Sec61 transporter. After transport into the cytoplasm, the nuclear translocation signal sequence in the N terminus of CypB facilitates nuclear import. Within the nucleus, the PRL/CypB encounters the Stat5 dimer. Stat5, when bound to the endogenous pool of PIAS3 repressor, is unable to bind to its corresponding DNA promoter sequences. Binding of the PRL/CypB complex to the Stat5 dimer results in the release of PIAS3 (an event requiring the isomerase activity of CypB), enabling Stat5 to engage its DNA binding sequence. The binding of DNA by the Stat5 dimer results in the release of the PRL/CypB complex. Blockade of the nuclear retrotransport of PRL or inactivation of the isomerase activity of CypB significantly down-modulates PRL-driven gene expression and function.
It has been reasoned that if the intranuclear actions of PRL contribute to the growth of PRL-responsive tissues, then interference with this pathway may prove useful in the treatment of PRL-responsive malignancies. To test this hypothesis, an enzymatically inactive mutant of CypB was synthesized by recombinant technique and introduced into the culture medium of human breast cancer cells. This treatment resulted in a significant inhibition of the growth of such cells (216), at concentrations 100- to 1000-fold less than any previously reported PRL antagonist (145). Collectively, these data would suggest that pharmacological manipulation of both genomic and nongenomic PRL/PRLR-associated signaling pathways may be of therapeutic utility in the treatment of breast cancer.
B. Endocytosis
Ligand binding to many membrane receptors initiates internalization of both ligand and receptor. This process has been shown to result in multiple potential fates, including 1) recycling of receptors back to the surface, 2) degradation of ligand and/or receptor by lysosomes or proteasomes resulting in down-regulation of displayed receptor or, possibly, 3) transport of ligand and/or receptors or fragments thereof to other cellular compartments, such as the nucleus, where they may directly alter gene expression. Internalization is therefore a major modulator of surface expression and, consequently, cell responsiveness over the short term and also may be directly linked to cell signaling processes. We are only beginning to understand the molecular events regulating these processes for other membrane receptors, and relatively little is known about PRLR and other receptors of the cyto-kine superfamily.
Ligand-induced endocytosis of both the rat and bovine PRLR isoforms has been examined in defined in vitro systems. Both of these species express a long form of the PRLR as well as short isoforms generated by alternative splicing. These differences in cytoplasmic domains result in differences in the rate of internalization of the PRLR isoforms in these species (274, 275). This would lead to an altered complement of PRLR isoforms on target cells expressing both isoforms after exposure to ligand. The different ability of the isoforms to transmit signal suggests that this would lead to altered responsiveness of the remaining receptor population.
Dileucine motifs in the proximal cytoplasmic domain are critical for internalization of the short PRLR and GH receptor (GHR) isoforms in both the rat and the cow (274, 276). Motifs critical for ligand-induced internalization of the bovine long PRLR isoform have been localized to two regions (274). The first is unique to the long PRLR isoform and is highly conserved across species. A phenylalanine residue (F290) and a nearby dileucine pair (LL286/287) contribute cooperatively to internalization. This phenylalanine is in a context similar to the ubiquitin-dependent endocytosis motif identified for the GHR (277); however, the GHR has no dileucine-like sequences in a similar position. In addition, the long isoform requires some of the dileucine motifs in the proximal cytoplasmic region.
Similar studies have not been performed using the hPRLR. However, the high homology in these regions across species makes it likely that the long form of the hPRLR utilizes many of the same mechanisms as the bovine PRLR. The intermediate form of the hPRLR (130) is identical with the long hPRLR isoform in the regions encoding the characterized endocytosis motifs; therefore, differences from the long isoform do not appear likely unless additional regulatory sequences are identified in the C terminus (see Fig. 2). The two recently identified isoforms with more drastically truncated cytoplasmic domains and unique C termini (135), however, will prove more interesting. The S1b isoform diverges from the long isoform at amino acid 261, similar to the rodent and ruminant PRLRs, and consequently would be predicted to rely on the proximal dileucine motifs for internalization. However, the S1a diverges only after amino acid 337, leaving the characterized endocytic motifs intact. Moreover, its unique 39-amino-acid C terminus contains two regions that mediate enhanced degradation in the absence of ligand (135). Further studies are necessary to explore trafficking of these primate PRLR isoforms.
Clathrin-coated pathways are involved in internalization of both the long and short rodent and ruminant isoforms (274). The ligand-bound rat short PRLR coprecipitates with α-adaptin, a component of adaptor protein-2 (275), which links cargo to clathrin-coated pits and so facilitates endocytosis. Dileucine residues are able to bind directly to adaptor protein-2 subunits (278, 279), suggesting that these dipeptides in the PRLR may contribute to internalization by this means.
Receptor trafficking after ligand-induced internalization has not been examined in detail. Total PRLR is down-regulated in response to ligand both in the mammary gland in vivo (280) and mammary explant systems in vitro (281). In the latter system, lysosomotropic agents prevented much of this decline, indicating that much of the internalized receptor is degraded in lysosomes. In Chinese hamster ovary cells stably expressing the long isoform of the rat PRLR, cycloheximide prevented the reappearance of much of the receptor at the cell surface, indicating that recycling was not a major sequela in this system (282). However, other pathways and differences between the PRLR isoforms have not yet been systematically investigated. Transport to other compartments, such as the nucleus as discussed above, remains a possibility.
At present, it is unclear how ligand binding triggers endocytosis. Activation of Jak2 is not essential (283). However, the ability of the PRLR to activate both pathways regulating Rho family members (256), which may mediate cytoskeletal alterations, and Src family members (189), which have been shown to be involved in internalization of other membrane receptors (284, 285), suggests obvious possibilities for investigation.
In addition to moderating surface expression of receptors by sorting for degradation, recycling, or other intracellular transport, data for several other membrane receptor families have shown that the ligand-stimulated internalization process is intimately connected to downstream effectors as well. Many receptors are associated with caveolae, microdomains in the plasma membrane that are enriched in cholesterol, glycosphingolipids, and lipid-anchored membrane proteins. These regions have been proposed to spatially coordinate signaling, although many details remain to be worked out (286, 287). Our understanding of the relationship of internalization to signaling for receptors belonging to the cytokine superfamily, including the PRLR, is still at an early stage. However, understanding the endocytotic mechanisms and connections to signaling pathways, differences between receptor isoforms, and how these events are altered in tumor cells will increase our understanding of the role PRL plays in mammary carcinogenesis and potentially open new avenues for treatment.
VII. Mouse Models of PRL Action and PRL-Induced Signaling
In rodents, PRL exposure enhances the development of chemically induced mammary cancers (1, 288 –292). Development of chemically induced mammary cancers is dependent upon the number of terminal end buds and the degree of cell proliferation at the time of chemical exposure (293). Terminal end buds disappear with differentiation of the mammary gland. Differentiation of the mammary gland correlates with the onset of sexual maturity. A differentiated mammary gland that contains fewer terminal end buds is less susceptible to the action of chemical carcinogens (294). Exposure to estrogen with secondary increases in circulating PRL levels is able to restore susceptibility to chemical carcinogens in parous mice (295). In summary, numerous studies point to a role for PRL in increasing receptiveness to chemical carcinogens in rodent mammary glands.
In the mammary gland, PRL stimulates phosphorylation and activation of Jak2 and the Stat5a and Stat5b proteins. Stat5a plays a more prominent role than Stat5b in the mammary gland (296). Jak2 is the major Janus kinase activated by PRL in mammary epithelial cells (297–301). The SOCS family of proteins act in a classical negative feed-back loop to down-regulate PRL-induced Jak/Stat activation (267). Activation of Stat5a and Stat5b in the mammary gland also can be controlled by other mechanisms. For example, local factors, and not changes in circulating levels of PRL secretion, are responsible for the inactivation of Stat5a and Stat5b during mammary gland involution (303). Finally, PRL also can signal through the MAPK pathway with stimulation of mammary epithelial cell proliferation (147, 227, 232).
The development of transgenic technology offered the opportunity to study the role of PRL in mammary gland cancer development through gain-of-function and loss-of-function mouse models. In gain-of-function mouse models, a trans-gene encoding a selected protein either can be overexpressed in a tissue that normally demonstrates expression of that particular protein or introduced into a tissue that does not normally express that particular protein. In loss-of-function models, the function of a selected protein is lost by preventing expression of the protein through a germ-line disruption of the gene encoding the protein or through expression of a dominant negative form of the selected protein that interrupts gene function. These models permit study of specific elements within the PRL-signaling pathway in the context of an intact animal (Table 3). To date, specific investigations have focused on gain and loss of PRL function, loss of PRLR function, loss of Jak2 function, loss of Stat5a and Stat5b function, and gain and loss of SOCS1 activity. Germ-line loss of Jak2 function results in late embryonic lethality, necessitating the use of embryonic mammary gland transplants for study of its specific role in mammary gland development and carcinogenesis (205, 206). Redundancy in the MAPK pathways complicates study of the role of specific proteins in PRL-related cancer development in the intact animal.
Table 3.
Effects on specific signaling molecules in the PRL pathway on mammary gland development and mammary gland cancer in mouse models
| Mouse model | Signaling molecule | Mammary gland development | Mammary gland cancer | Refs. |
|---|---|---|---|---|
| Germ-line disruption of the PRL gene | Loss of PRL | Arrested mammary gland development | Reduced growth of polyoma middle T antigen-induced mammary cancers | 313–315 |
| Metallothionein promoterrat PRL transgene | PRL overexpression in mammary gland | Development of lactation type morphology in virgin mice | Mammary cancers at 11–15 months of age | 309 |
| Germ-line disruption of the PRLR gene | Loss of functional PRLR | Loss of one functional PRLR gene results in impaired lactation at the first pregnancy.
Loss of two functional PRLR genes results in loss of alveolar development during pregnancy. Loss of functional PRLR in mammary stroma impairs differentiation of wild-type mammary epithelial cells during puberty. |
111, 316 | |
| Germ-line disruption of the Jak2 gene | Loss of Jak2 | No alveolar development during pregnancy | 212, 318 | |
| Germ-line disruption of the Stat5a gene | Loss of Stat5a | Impaired mammary gland development and lactation
Decreased survival of mammary epithelial cells |
Decreased survival of premalignant and malignant mammary epithelial cells
Reduced tumor growth of TGFα and SV40 TAg induced cancers |
205, 210, 319 |
| Germ-line disruption of both Stat5a and Stat5b genes | Loss of functional Stat5a and Stat5b | No alveolar development during pregnancy | 320 | |
| β -Actin-CIS1/SOCS1/SSI1 transgene | Overexpression of CIS1/SOCS1/SSI1 in mammary gland | Impaired mammary gland development and lactation | 321 | |
| Germ-line disruption of CIS1/SOCS1/SSI1 | Loss of functional CIS1/SOCS1/SSI1 | Accelerated mammary gland development during pregnancy
Loss of one functional CIS1/SOCS1/SSI1 gene rescues the lactation defect found in mice that carry only one functional PRLR gene. |
308 |
Studies of specific signaling molecules in the PRL pathway have illustrated the dose responsiveness of the signaling cascade in the intact animal. For example, loss of one functional copy of the PRLR gene is sufficient to interrupt lactation after a first pregnancy (306). Loss of Stat5a function through germ-line disruption of the Stat5a gene results in impaired lactation after the first pregnancy, but lactation can be recovered with increased activation of the Stat5b gene during subsequent lactation periods (307). Similarly, the first pregnancy-associated lactation failure found in mice carrying only one functional copy of the PRLR gene is rescued by a germ-line deletion of one SOCS1 allele (308).
Overexpression of PRL in transgenic mice with increased activation of the PRLR is sufficient to induce the formation of mammary cancers at 11–15 months of age (309, 310). In contrast, no tumors were noted in parallel transgenic controls expressing bovine GH (309), suggesting that unlike PRL, the contribution of GH to mammary neoplasia may be indirect. Supporting this hypothesis is the phenotype observed in lit−/− mice. Lit−/− mice have a functional mutation in the GnRH receptor, demonstrate markedly decreased levels of both GH and IGF, and evidence significant reductions in the growth of mammary tumor xenografts (311, 312).
Loss of PRL function through germ-line disruption of the PRL gene aborts mammary gland development by impairing ductal arborization and lobular budding and reduces the growth of Polyoma middle T antigen-induced mammary cancers (313–315).
In mice, germ-line disruption of only one PRLR allele is sufficient to impair lactation after the first pregnancy (111). Loss of PRLR function in mammary epithelial cells by disruption of both PRLR gene impairs mammary lobular development during pregnancy (316). Significantly, loss of PRLR function also can have an indirect effect on mammary gland development. Mammary gland transplant experiments have demonstrated that wild-type mammary epithelial cells transplanted into the mammary fat pad of mice carrying germ-line deletions of the PRLR genes do not undergo normal development during puberty (316). These mice now can be used to study the specific role of the PRLR in mammary cancer development. The use of mammary gland transplant experiments will allow investigators to isolate the role of the PRLR in mammary epithelial cells from systemic effects resulting from loss of PRLR function in other tissues (317).
The role of Jak2 in mammary epithelium was studied using mammary transplants of Jak2-null epithelium into the mammary gland fat pads of wild-type mice (318). Loss of Jak2 function through disruption of both Jak2 genes in the mammary epithelium results in impaired mammary gland development during pregnancy. Although ductal tissues formed normally, there was no development of secretory epithelium during pregnancy. This indicates that Jak2 is required for pregnancy-induced mammary gland development through the placental lactogen- and PRL-signaling pathways.
Germ-line disruption of the Stat5a gene and complete loss of Stat5a expression not only impair lactation but also result in decreased survival of mammary epithelial cells (210). Complete loss of Stat5a promotes apoptosis of TGFα over-expressing mammary epithelial cells during mammary gland involution and delays development of TGFα -induced mammary hyperplasia and cancer in a mouse model (210). Germ-line disruption of just one Stat5a allele results in reduced Stat5a expression levels in mammary epithelial cells (319). Reduced Stat5a expression levels result in significantly increased levels of apoptosis of mammary adenocarcinoma cells and delay tumorigenesis in the whey acidic protein-TAg mouse model of mammary gland cancer progression (319). Germ-line disruption of both Stat5a and Stat5b genes results in the loss of both Stat5a and Stat5b in mammary epithelial cells (320); as a consequence, these cells fail to differentiate into alveolar cells during pregnancy.
Gain of SOCS1 function through expression of a SOCS1-encoding transgene in mammary epithelial cells results in decreased levels of Stat5 activation and impaired lactation (321). Loss of SOCS1/CIS1/SSI1 function by germ-line disruption of the SOCS1/CIS1 genes increases levels of Stat5 activation and accelerates mammary gland development during pregnancy (308). Haploid loss of SOCS1 function is sufficient to rescue the lactation defect in haploid-deficient PRLR mice (308). Changes in either proliferation or survival of mammary epithelial cells were not determined directly in the gain of SOCS1/CIS1/SSI1 function mouse model and no molecules in the MAPK pathway were studied. In contrast, the MAPK pathway has been examined in the loss of SOCS1 function mice. In these mice a decrease in phosphorylated ERK1/2 was reported. Thus, further analysis of these signaling pathways and associated functions in these mouse models should further delineate the role of SOCS1 in mammary cancer development.
VIII. Conclusions and Future Directions
To date, the epidemiological data suggest a relatively strong positive association between circulating PRL levels and breast cancer risk in postmenopausal women. However, this is based only upon one large prospective study and two small ones; hence, additional assessments to confirm and better quantify these observations are needed. Insufficient data currently exist to judge whether an association is also present among premenopausal women. To assess the independent effect of PRL on risk, future studies must also include measurement of other plasma hormones such as the steroids and IGFs. The evaluation of a recently reported PRL binding protein (133), which is thought to influence tissue bioavailability, may also provide new insights to this relationship. In addition, the relationship between plasma and tissue PRL levels is not well understood—further delineation of this relationship will require work from both epidemiologists and laboratory scientists. Parous women have been consistently observed to have lower PRL levels than nulliparous women. With the possible exception of family history of breast cancer, no consistent associations have been observed for other breast cancer risk factors, although in several cases the available data remain limited. For mammographic density, a strong and consistent breast cancer risk factor, recent data suggest a positive association but, once again, few detailed studies have been published.
Although the functional role of PRL in nonlactating mammary tissues is increasingly recognized, it is not always known whether the effects of PRL are direct, or whether PRL induces expression of another factor(s) that may modulate, or more directly mediate, the observed outcome. For example, Chen and colleagues (163) observed recently that PRL increased TGFα and decreased TGFβ 1 in T47D cells. In vivo, of course, mammary function is regulated by complex interactions among hormones, including PRL, estrogens, and progestins, as well as local growth factors, such as EGF family members, IGFs, and TGFα, and the different cell types present in the mammary tumor environment. These factors can amplify or inhibit one another’s signals to the epithelial cells by several mechanisms, including altering expression of receptors, influencing the level or activities of signaling pathways, and activating paracrine modulators via action on different cell types (118, 322, 323). These complex opportunities for cross-talk are only beginning to be examined in these in vitro systems. Recent findings emphasize the importance of understanding PRL actions in cells of varying phenotype and environmental context. For example, in PRL-deficient MCF-7 cells, both PRL and EGF increased cyclin D1 expression, but the PRLR and erbB1, the primary EGF receptor family member expressed in these cells, did not appear to cooperate (149). However, Yamauchi et al. (232) demonstrated that endogenous PRL increased the constitutive tyrosine phosphorylation of erbB2 expressed in another mammary tumor cell line, SK-BR-3, leading to enhanced proliferation via the Ras-MAPK cascade. Additional work exploring these kinds of interactions in vitro and extending them to in vivo models of tumorigenesis is crucial. In addition, we do not yet appreciate the factors that determine the distinct responses to PRL in normal cells at different times in mammary function and in tumor cells. Relatively little work has been done in nontumor human cell lines. The murine mammary epithelial cell line, HC11, a clonal derivative of COMMA-D cells, has been extensively studied. These cells proliferate in response to growth factors. However, PRL, in combination with glucocorticoids, causes these cells to grow more slowly, and differentiate, as characterized by milk protein synthesis (197, 324 –326). This is an especially important area for study, which will increase our understanding of this hormone and its interactions with other factors in normal and pathogenic processes.
Clearly, much more work is needed to understand the signaling pathways used by PRL to promote tumorigenesis in mammary cells, interactions of these signaling cascades and their complex regulatory loops with different oncogenes, growth factors and hormones important in mammary carcinogenesis, and differences in PRL actions between normal and tumor cells. However, it is clear that already-identified signaling pathways employed by PRL are connected to processes of proliferation, survival, and motility, both in cell cultures in vitro as well as in vivo. Moreover, it is also clear that these pathways within the cytoplasm and nucleus present rich sites for cross-talk between PRL and other growth factor and hormonal regulators that may contribute to tumor development and progression.
The development of genetically altered mouse models has allowed investigators to study the roles of specific molecules in the PRL-signaling cascade in the intact animal. At the present time the use of mammary gland transplants enables investigators to separate stromal from epithelial specific effects and local, as opposed to systemic, effects of gene deletion. Future application of mammary epithelial-specific gene deletion to individual molecules within the PRL-signaling cascade should complement currently available approaches (327). Additional experiments will be required to delineate how specific molecules in the PRL-signaling cascade influence mammary cancer development. To date, relatively few cancer models have been examined in the context of specific interventions in PRL signaling. Moreover, experiments in cancer progression can be complicated by the interruption of mammary gland development that occurs with deletion of specific molecules in the PRL pathway. Thus, strategies such as those utilizing xenografts of human breast cancer into immunocompromised mouse models may provide alternative approaches to delineating the role of PRL/PRLR signaling in this disease.
The ongoing development of PRLR-specific antagonists holds promise at blocking the actions of PRL at the endocrine and autocrine/paracrine levels within the breast. Like their corresponding GHR antagonists, however, many of these functional PRLR antagonists need to be used in the micromolar to millimolar range (146 –148), thereby limiting their potential utility. Continued evolution of these antagonists using mutagenic approaches, however, may increase their potency. Alternatively, antagonists directed against specific components of the PRL/PRLR signaling networks (216) may demonstrate even greater utility in this regard. Thus, the development of an effective PRL/PRLR antagonist, such as tamoxifen for estrogen receptor and Herceptin for EGF receptor, may yield a novel therapeutic treatment for human breast cancer and simultaneously validate the perceived function of this hormone in the pathogenesis of this disease.
Acknowledgments
This work was supported by NIH Grants R01 CA-69294, DK-92265, CA-49449, CA-87969, CA-67262, CA-8312, and T32 HD-07259; Department of Defense Breast Cancer Research Program Grants BC000716 and BC97285; and American Cancer Society Grant RPG00307 TBE.
Contributor Information
CHARLES V. CLEVENGER, Department of Pathology and Laboratory Medicine.
PRISCILLA A. FURTH, University of Pennsylvania, Philadelphia, Pennsylvania 19104; Lombardi Cancer Center
SUSAN E. HANKINSON, Department of Oncology, Georgetown University, Washington, D.C. 20057; Channing Laboratories, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, and Department of Epidemiology
LINDA A. SCHULER, Harvard School of Public Health, Cambridge, Massachusetts 02115; and Department of Comparative Bioscience, University of Wisconsin, Madison, Wisconsin 53706
References
- 1.Welsch CW, Nagasawa H. Prolactin and murine mammary tumorigenesis: a review. Cancer Res. 1977;37:951–963. [PubMed] [Google Scholar]
- 2.Dickson RB, Johnson MD, Bano M, Shi E, Kurebayashi J, Ziff B, Martinez-Lacaci I, Amundadottir LT, Lippman ME. Growth factors in breast cancer: mitogenesis to transformation. J Steroid Biochem Mol Biol. 1992;43:69 –78. doi: 10.1016/0960-0760(92)90189-p. [DOI] [PubMed] [Google Scholar]
- 3.Love RR, Rose DR, Surawicz TS, Newcomb PA. Prolactin and growth hormone levels in premenopausal women with breast cancer and healthy women with a strong family history of breast cancer. Cancer. 1991;68:1401–1405. doi: 10.1002/1097-0142(19910915)68:6<1401::aid-cncr2820680637>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Kwa HG, Wang DY. An abnormal luteal-phase evening peak of plasma prolactin in women with a family history of breast cancer. Int J Cancer. 1977;20:12–14. doi: 10.1002/ijc.2910200104. [DOI] [PubMed] [Google Scholar]
- 5.Musey VC, Collins DC, Musey PI, Martino-Saltzman D, Preedy JR. Long-term effect of a first pregnancy on the secretion of prolactin. N Engl J Med. 1987;316:229 –234. doi: 10.1056/NEJM198701293160501. [DOI] [PubMed] [Google Scholar]
- 6.Wang DY, De Stavola BL, Bulbrook RD, Allen DS, Kwa HG, Verstraeten AA, Moore JW, Fentiman IS, Hayward JL, Gravelle IH. The permanent effect of reproductive events on blood prolactin levels and its relation to breast cancer risk: a population study of postmenopausal women. Eur J Cancer Clin Oncol. 1988;24:1225–1231. doi: 10.1016/0277-5379(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 7.Ingram DM, Nottage EM, Roberts AN. Prolactin and breast cancer risk. Med J Aust. 1990;153:469 –473. [PubMed] [Google Scholar]
- 8.Hankinson SE, Colditz GA, Hunter DJ, Manson JE, Willett WC, Stampfer MJ, Longcope C, Speizer FE. Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses’ Health Study (United States) Cancer Causes Control. 1995;6:217–224. doi: 10.1007/BF00051793. [DOI] [PubMed] [Google Scholar]
- 9.Apter D, Reinila M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783–787. doi: 10.1002/ijc.2910440506. [DOI] [PubMed] [Google Scholar]
- 10.Levin PA, Malarkey WB. Daughters of women with breast cancer have elevated mean 24-hour prolactin (PRL) levels and a partial resistance of PRL to dopamine suppression. J Clin Endocrinol Metab. 1981;53:179 –183. doi: 10.1210/jcem-53-1-179. [DOI] [PubMed] [Google Scholar]
- 11.Love RR, Rose DP. Elevated bioactive prolactin in women at risk for familial breast cancer. Eur J Cancer Clin Oncol. 1985;21:1553–1554. doi: 10.1016/0277-5379(85)90251-2. [DOI] [PubMed] [Google Scholar]
- 12.Fishman J, Fukushima D, O’Connor J, Rosenfeld RS, Lynch HT, Lynch JF, Guirgis H, Maloney K. Plasma hormone profiles of young women at risk for familial breast cancer. Cancer Res. 1978;38:4006 –4011. [PubMed] [Google Scholar]
- 13.Pike MC, Casagrande JT, Brown JB, Gerkins V, Henderson BE. Comparison of urinary and plasma hormone levels in daughters of breast cancer patients and controls. J Natl Cancer Inst. 1977;59:1351–1355. doi: 10.1093/jnci/59.5.1351. [DOI] [PubMed] [Google Scholar]
- 14.Henderson BR, Gerkins V, Rosario I, Casagrande J, Pike MC. Elevated serum levels of estrogen and prolactin in daughters of patients with breast cancer. N Engl J Med. 1975;293:790 –795. doi: 10.1056/NEJM197510162931602. [DOI] [PubMed] [Google Scholar]
- 15.Boffard K, Clark GM, Irvine JB, Knyba RE, Bulbrook RD, Wang DY, Kwa HG. Serum prolactin, androgens, oestradiol and progesterone in adolescent girls with or without a family history of breast cancer. Eur J Cancer Clin Oncol. 1981;17:1071–1077. doi: 10.1016/0014-2964(81)90290-5. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486 –2492. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87:1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 18.Boyd NF, Stone J, Martin L, Minkin S, Yaffe M Sunnybrook and Women’s College Hospital. Mammographic densities and the growth hormone-IGF-1 prolactin axis. Proc 92nd Meeting of the American Association for Cancer Research; New Orleans, LA. 2001. p. p 558. (Abstract) [Google Scholar]
- 19.Byrne C, Hankinson SE, Colditz GA, Willett WC, Speizer FE Channing Laboratory. Plasma prolactin and mammographic density in postmenopausal women. Proc 92nd Meeting of the American Association for Cancer Research; New Orleans, LA; 2001. p. p 153. (Abstract) [Google Scholar]
- 20.Key TJ, Chen J, Wang DY, Pike MC, Boreham J. Sex hormones in women in rural China and in Britain. Br J Cancer. 1990;62:631–636. doi: 10.1038/bjc.1990.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayward JL, Greenwood FC, Glober G, Stemmerman G, Bulbrook RD, Wang DY, Kumaokas S. Endocrine status in normal British, Japanese and Hawaiian-Japanese women. Eur J Cancer. 1978;14:1221–1228. doi: 10.1016/0014-2964(78)90228-1. [DOI] [PubMed] [Google Scholar]
- 22.Gray GE, Pike MC, Hirayama T, Tellez J, Gerkins V, Brown JB, Casagrande JT, Henderson BE. Diet and hormone profiles in teenage girls in four countries at different risk for breast cancer. Prev Med. 1982;11:108 –113. doi: 10.1016/0091-7435(82)90010-x. [DOI] [PubMed] [Google Scholar]
- 23.Lipworth L, Hsieh CC, Wide L, Ekbom A, Yu SZ, Yu GP, Xu B, Hellerstein S, Carlstrom K, Trichopoulos D, Adami HO. Maternal pregnancy hormone levels in an area with a high incidence (Boston, USA) and in an area with a low incidence (Shanghai, China) of breast cancer. Br J Cancer. 1999;79:7–12. doi: 10.1038/sj.bjc.6690003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longcope C, Speizer FE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 25.Crighton IL, Dowsett M, Hunter M, Shaw C, Smith IE. The effect of a low-fat diet on hormone levels in healthy pre- and postmenopausal women: relevance for breast cancer. Eur J Cancer. 1992;12:2024 –2027. doi: 10.1016/0959-8049(92)90252-w. [DOI] [PubMed] [Google Scholar]
- 26.Shultz TD, Wilcox RB, Spuehler JM, Howie BJ. Dietary and hormonal interrelationships in premenopausal women: evidence for a relationship between dietary nutrients and plasma prolactin levels. Am J Clin Nutr. 1987;46:905–911. doi: 10.1093/ajcn/46.6.905. [DOI] [PubMed] [Google Scholar]
- 27.Hill P, Wynder F. Diet and prolactin release. Lancet. 1976;2:806 –807. doi: 10.1016/s0140-6736(76)90644-9. [DOI] [PubMed] [Google Scholar]
- 28.Hill P. Diet and plasma prolactin. Am J Clin Nutr. 1981;34:1162–1163. doi: 10.1093/ajcn/34.6.1162. [DOI] [PubMed] [Google Scholar]
- 29.Gray GE, Pike MC, Henderson BE. Dietary fat and plasma prolactin. Am J Clin Nutr. 1981;34:1160 –1162. doi: 10.1093/ajcn/34.6.1160. [DOI] [PubMed] [Google Scholar]
- 30.Martini MC, Dancisak BB, Haggans CJ, Thomas W, Slavin JL. Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr Cancer. 1999;34:133–139. doi: 10.1207/S15327914NC3402_2. [DOI] [PubMed] [Google Scholar]
- 31.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:3106 –3108. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 32.Mishell DR, Jr, Kletzky OA, Brenner PF, Roy S, Nicoloff J. The effect of contraceptive steroids on hypothalamicpituitary function. Am J Obstet Gynecol. 1977;128:60 –74. doi: 10.1016/0002-9378(77)90295-2. [DOI] [PubMed] [Google Scholar]
- 33.Ross RK, Paganini-Hill A, Krailo MD, Gerkins VR, Henderson BE, Pike MC. Effects of reserpine on prolactin levels and incidence of breast cancer in postmenopausal women. Cancer Res. 1984;44:3106 –3108. [PubMed] [Google Scholar]
- 34.Boston Collaborative Drug Surveillance Program BUMC. Reserpine and breast cancer. Lancet. 1974;2:669 –671. [Google Scholar]
- 35.Heinonen OP, Shapiro S, Tuominen L, Turunen MI. Reserpine use in relation to breast cancer. Lancet. 1974;2:675–677. doi: 10.1016/s0140-6736(74)93259-0. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong B, Skegg D, White G, Doll R. Rauwolfia derivatives and breast cancer in hypertensive women. Lancet. 1976;2:8 –12. doi: 10.1016/s0140-6736(76)92966-4. [DOI] [PubMed] [Google Scholar]
- 37.Mack TM, Henderson BE, Gerkins VR, Arthur M, Baptista J, Pike MC. Reserpine and breast cancer in a retirement community. N Engl J Med. 1975;292:1366 –1371. doi: 10.1056/NEJM197506262922603. [DOI] [PubMed] [Google Scholar]
- 38.Aromaa A, Hakama M, Hakulinen T, Saxen E, Teppo L, Idanpaan-Heikkila J. Breast cancer and use of rauwolfia and other antihypertensive agents in hypertensive patients: a nationwide case-control study in Finland. Int J Cancer. 1976;18:727–738. doi: 10.1002/ijc.2910180603. [DOI] [PubMed] [Google Scholar]
- 39.Laska EM, Meisner M, Siegel C, Fischer S, Wanderling J. Matchedpairs study of reserpine use and breast cancer. Lancet. 1975;2:296 –301. doi: 10.1016/s0140-6736(75)92731-2. [DOI] [PubMed] [Google Scholar]
- 40.Curb JD, Hardy RJ, Labarthe DR, Borhani NO, Taylor JO. Reserpine and breast cancer in the hypertension detection and follow-up program. Hypertension. 1982;4:307–311. doi: 10.1161/01.hyp.4.2.307. [DOI] [PubMed] [Google Scholar]
- 41.Labarthe DR, O’Fallon WM. Reserpine and breast cancer. A community-based longitudinal study of 2,000 hypertensive women. JAMA. 1980;243:2304 –2310. doi: 10.1001/jama.243.22.2304. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro S, Parsells JL, Rosenburg L, Kaufman DW, Stolley PD, Schottenfeld D. Risk of breast cancer in relation to the use of rauwolfia alkaloids. Eur J Clin Pharmacol. 1984;26:143–146. doi: 10.1007/BF00630277. [DOI] [PubMed] [Google Scholar]
- 43.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative re-analysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 44.Colin-Jones DG, Langman MJ, Lawson DH, Logan RF, Paterson KR, Vessey MP. Postmarketing surveillance of the safety of cimetidine: 10 year mortality report. Gut. 1992;33:1280 –1284. doi: 10.1136/gut.33.9.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossing MA, Scholes D, Cushing-Haugen KL, Voigt LF. Cimetidine use and risk of prostate and breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:319 –323. [PubMed] [Google Scholar]
- 46.Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: collaborative reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606 –616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk: epidemiologic reviews. Epidemiol Rev. 1993;15:48 –79. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 48.Verkasalo PK, Thomas HV, Appleby PN, Davey GK, Key TJ. Circulating levels of sex hormones and their relation to risk factors for breast cancer: a cross-sectional study in 1902 pre- and post-menopausal women (United Kingdom) Cancer Causes Control. 2001;12:47–59. doi: 10.1023/a:1008929714862. [DOI] [PubMed] [Google Scholar]
- 49.Strungs I, Gray RA, Rigby HB, Strutton G. Two case reports of breast carcinoma associated with prolactinoma. Pathology. 1997;29:320 –323. doi: 10.1080/00313029700169205. [DOI] [PubMed] [Google Scholar]
- 50.Volm MD, Talamonti MS, Thangavelu M, Gradishar WK. Pituitary adenoma and bilateral male breast cancer: an unusual association. J Surg Oncol. 1997;64:74 –78. doi: 10.1002/(sici)1096-9098(199701)64:1<74::aid-jso14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 51.Buytaert P, Viaene P. Amenorrhea, glactorrhea, hyperprolac-tinaemia syndrome and breast carcinoma in a young women. Eur J Gynaecol Reprod Biol. 1985;11:341–346. doi: 10.1016/0028-2243(81)90035-6. [DOI] [PubMed] [Google Scholar]
- 52.Olsson H, Alm P, Kristoffersson U, Landin-Olsson M. Hypophyseal tumor and gynecomastia preceding bilateral breast cancer development in a man. Cancer. 1984;53:1974 –1977. doi: 10.1002/1097-0142(19840501)53:9<1974::aid-cncr2820530928>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 53.Theodorakis S, Tedesco IIIV, Sutherland C. Breast cancer in a patient with prolactinoma. Surgery. 1985;98:367–369. [PubMed] [Google Scholar]
- 54.Popovic V, Damjanovic S, Micic D, Nesovic M, Djurovic M, Petakov M, Obradovic S, Zoric S, Simic M, Penezic Z, Marinkovic J. Increased incidence of neoplasia in patients with pituitary adenomas. The Pituitary Study Group. Clin Endocrinol (Oxf) 1998;49:441–445. doi: 10.1046/j.1365-2265.1998.00536.x. [DOI] [PubMed] [Google Scholar]
- 55.Webster J. Clinical management of prolactinomas. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:395–408. doi: 10.1053/beem.1999.0030. [DOI] [PubMed] [Google Scholar]
- 56.Koenig KL, Toniolo P, Bruning PF, Bonfrer JMG, Shore RE, Pasterneck BS. Reliability of serum prolactin measurements in women. Cancer Epidemiol Biomarkers Prev. 1993;2:411–414. [PubMed] [Google Scholar]
- 57.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a two to three year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649 –654. [PubMed] [Google Scholar]
- 58.Muti P, Trevisan M, Micheli A, Krogh VBG, Sciajno RBF. Reliability of serum hormones in premenopausal and postmenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev. 1996;5:917–922. [PubMed] [Google Scholar]
- 59.Willett WC. Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 60.Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65(Suppl):1179S–1186S. doi: 10.1093/ajcn/65.4.1179S. [DOI] [PubMed] [Google Scholar]
- 61.Thurigen D, Spiegelman D, Blettner M, Heuer C, Brenner H. Measurement error correction using validation data: a review of methods and their applicability in case-control studies. Stat Methods Med Res. 2000;9:447–474. doi: 10.1177/096228020000900504. [DOI] [PubMed] [Google Scholar]
- 62.van Landeghem AAJ, Poortman J, Nabuurs M, Thijssen JHH. Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res. 1985;45:2900 –2906. [PubMed] [Google Scholar]
- 63.Thijssen JHH, Blankenstein MA, Miller WR, Milewicz A. Estrogens in tissues: uptake from the peripheral circulation or local production. Steroids. 1987;50:297–306. doi: 10.1016/0039-128x(83)90079-x. [DOI] [PubMed] [Google Scholar]
- 64.Cummings S, Duong T, Kenyon E, Cauley J, Whitehead M, Krueger K. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216 –220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- 65.Yakar S, Green J, LeRoith D. Serum IGF-1 levels as a risk marker in mammary tumors. Program of the 83rd Meeting of The Endocrine Society; Denver, CO. 2001. p. p 201. (Abstract P1-242) [Google Scholar]
- 66.Berger RL, Joison J, Braverman LE. Lactation after incision on the thoracic cage. N Engl J Med. 1966;274:1493–1495. doi: 10.1056/NEJM196606302742609. [DOI] [PubMed] [Google Scholar]
- 67.Herman V, Kalk WJ, de Moor NG, Levin J. Serum prolactin after chest wall surgery: elevated levels after mastectomy. J Clin Endocrinol Metab. 1981;52:148 –151. doi: 10.1210/jcem-52-1-148. [DOI] [PubMed] [Google Scholar]
- 68.Yen S, Jaffe R, Barbieri R. Reproductive endocrinology: physiology, pathophysiology, and clinical management. 4th ed. Philadelphia: W.B. Saunders Co; 1999. [Google Scholar]
- 69.Anderson E, Morten H, Wang DY, Burns P, Birch J, Howell A. Serum bioactive lactogenic hormone levels in women with familial breast cancer and their relatives. Eur J Cancer Clin Oncol. 1989;25:1719 –1725. doi: 10.1016/0277-5379(89)90340-4. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka T, Shiu RPC, Gout PW, Beer CT, Nobel RL, Friesen HG. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J Clin Endocrinol Metab. 1980;51:1058 –1063. doi: 10.1210/jcem-51-5-1058. [DOI] [PubMed] [Google Scholar]
- 71.Cole EN, England PC, Sellwood RA, Griffiths K. Serum prolactin concentrations throughout the menstrual cycle of normal women and patients with recent breast cancer. Eur J Cancer. 1977;13:677–684. doi: 10.1016/0014-2964(77)90053-6. [DOI] [PubMed] [Google Scholar]
- 72.Malarkey WB, Schroeder LL, Stevens VC, James AG, Lanese RR. Disordered nocturnal prolactin regulation in women with breast cancer. Cancer Res. 1977;37:4650 –4654. [PubMed] [Google Scholar]
- 73.Rose DP, Pruitt BT. Plasma prolactin levels in patients with breast cancer. Cancer. 1981;48:2687–2691. doi: 10.1002/1097-0142(19811215)48:12<2687::aid-cncr2820481221>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 74.Meyer F, Brown JB, Morrison AS, MacMahon B. Endogenous sex hormones, prolactin, and breast cancer in premenopausal women. J Natl Cancer Inst. 1986;77:613–616. doi: 10.1093/jnci/77.3.613. [DOI] [PubMed] [Google Scholar]
- 75.Sheth NA, Ranadive KJ, Suraiya JN, Sheth AR. Circulating levels of prolactin in human breast cancer. Br J Cancer. 1975;32:160 –167. doi: 10.1038/bjc.1975.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drafta D, Schindler AE, Milcu SM, Keller E, Stroe E, Horodniceanu E, Balanescu I. Plasma hormones in pre- and post-menopausal breast cancer. J Steroid Biochem. 1980;13:793–802. doi: 10.1016/0022-4731(80)90231-9. [DOI] [PubMed] [Google Scholar]
- 77.Wilson RG, Buchan R, Roberts MM, Forrest AP, Boyns AR, Cole EN, Griffiths K. Plasma prolactin and breast cancer. Cancer. 1974;33:1325–1327. doi: 10.1002/1097-0142(197405)33:5<1325::aid-cncr2820330517>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 78.Wang DY, De Stavola BL, Bulbrook RD, Allen DS, Kwa HG, Fentiman IS, Hayward JL, Millis RR. Relationship of blood prolactin levels and the risk of subsequent breast cancer. Int J Epidemiol. 1992;21:214 –221. doi: 10.1093/ije/21.2.214. [DOI] [PubMed] [Google Scholar]
- 79.Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detection Prev. 1994;18:79 –85. [PubMed] [Google Scholar]
- 80.Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiol Biomarkers Prev. 2000;9:575–579. [PubMed] [Google Scholar]
- 81.Hankinson SE, Willett WC, Michaud DS, Manson JE, Colditz GA, Langcope C, Rosner B, Speizer FE. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91:629 –634. doi: 10.1093/jnci/91.7.629. [DOI] [PubMed] [Google Scholar]
- 82.Wang DY, Stepniewska KA, Allen DS, Fentiman IS, Bulbrook RD, Kwa HG, De Stavola BL, Reed MJ. Serum prolactin levels and their relationship to survival in women with operable breast cancer. J Clin Epidemiol. 1995;48:959 –968. doi: 10.1016/0895-4356(94)00201-z. [DOI] [PubMed] [Google Scholar]
- 83.Holtkamp W, Nagel GA, Wander HE, Rauschecker HF, von Heyden D. Hyperprolactinemia is an indicator of progressive disease and poor prognosis in advanced breast cancer. Int J Cancer. 1984;34:323–328. doi: 10.1002/ijc.2910340307. [DOI] [PubMed] [Google Scholar]
- 84.Lissoni P, Barni S, Cazzaniga M, Ardizzoia A, Rovelli F, Tancini G, Brivio F, Frigerio F. Prediction of recurrence in operable breast cancer by postoperative changes in prolactin secretion. Oncology. 1995;52:439 –442. doi: 10.1159/000227507. [DOI] [PubMed] [Google Scholar]
- 85.Ben-Jonathan N. Hypothalamic control of prolactin synthesis and secretion. In: Horseman ND, editor. Prolactin. Norwell, MA: Kluwer Academic Publishers; 2001. pp. 1–24. [Google Scholar]
- 86.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 87.Kjaer A, Knigge U, Olsen L, Vilhardt H, Warberg J. Mediation of the stress-induced prolactin release by hypothalamic histaminergic neurons and the possible involvement of vasopressin in this response. Endocrinology. 1991;128:103–110. doi: 10.1210/endo-128-1-103. [DOI] [PubMed] [Google Scholar]
- 88.Kant GJ, Bauman RA, Anderson SM, Mougey EH. Effects of controllable vs. uncontrollable chronic stress on stress-responsive plasma hormones. Physiol Behav. 1992;51:1285–1288. doi: 10.1016/0031-9384(92)90323-t. [DOI] [PubMed] [Google Scholar]
- 89.Welsch CW. Host factors affecting the growth of carcinogen-induced rat mammary carcinomas: a review and tribute to Charles Brenton Huggins. Cancer Res. 1985;45:3415–3443. [PubMed] [Google Scholar]
- 90.Welsch CW, Jenkins TW, Meites J. Increased incidence of mammary tumors in the female rat grafted with multiple pituitaries. Cancer Res. 1970;30:1024 –1029. [PubMed] [Google Scholar]
- 91.Welsch CW. Interaction of estrogen and prolactin in spontaneous mammary tumorigenesis of the mouse. J Toxicol Environ Health. 1976;1:161–175. [PubMed] [Google Scholar]
- 92.Welsch CW. Prophylaxis of spontaneously developing mammary carcinoma in C3H/HeJ female mice by suppression of prolactin. Cancer Res. 1973;33:2939 –2946. [PubMed] [Google Scholar]
- 93.Anderson E, Ferguson JE, Morten H, Shalet SM, Robinson EL, Howell A. Serum immunoreactive and bioactive lactogenic hormones in advanced breast cancer patients treated with bromocriptine and octreotide. Eur J Cancer. 1993;29A:209 –217. doi: 10.1016/0959-8049(93)90178-i. [DOI] [PubMed] [Google Scholar]
- 94.McMurray RW, Weidensaul D, Allen SH, Walker SE. Efficacy of bromocriptine in an open label therapeutic trial for systemic lupus erythematosus. J Rheumatol. 1995;22:2084 –2091. [PubMed] [Google Scholar]
- 95.Bonneterre J, Mauriac L, Weber B, Roche H, Fargeot P, Tubiana-Hulin M, Sevin M, Chollet P, Cappelaere P. Tamoxifen plus bromocriptine vs. tamoxifen plus placebo in advanced breast cancer: results of a double blind multicenter clinical trial. Eur J Cancer. 1988;24:1851–1853. doi: 10.1016/0277-5379(88)90097-1. [DOI] [PubMed] [Google Scholar]
- 96.Lachelin GCL, Yen SSC, Alksne JFN. Hormonal changes following hypophysectomy in humans. Obstet Gynecol. 1977;50:333–339. [PubMed] [Google Scholar]
- 97.Nolin JM, Witorsch RJ. Detection of endogenous immunoreactive prolactin in rat mammary epithelial cells during lactation. Endocrinology. 1976;99:949 –958. doi: 10.1210/endo-99-4-949. [DOI] [PubMed] [Google Scholar]
- 98.Fields K, Kulig E, Lloyd RV. Detection of prolactin messenger RNA in mammary and other normal and neoplastic tissues by polymerase chain reaction. Lab Invest. 1993;68:354 –360. [PubMed] [Google Scholar]
- 99.Kurtz A, Bristol LA, Toth BE, Lazar-Wesley E, Takacs L, Kacsoh B. Mammary epithelial cells of lactating rats express prolactin messenger ribonucleic acid. Biol Reprod. 1993;48:1095–1103. doi: 10.1095/biolreprod48.5.1095. [DOI] [PubMed] [Google Scholar]
- 100.Steinmetz RW, Grant AL, Malven PV. Transcription of prolactin gene in milk secretory cells of the rat mammary gland. J Endocrinol. 1993;136:271–276. doi: 10.1677/joe.0.1360271. [DOI] [PubMed] [Google Scholar]
- 101.Montogomery DW, LeFevre JA, Ulrich ED, Adamson CR, Zukoski CF. Identification of prolactin-like proteins synthesized by normal murine lymphocytes. Endocrinology. 1990;127:2601–2603. doi: 10.1210/endo-127-5-2601. [DOI] [PubMed] [Google Scholar]
- 102.Kenner JR, Holaday JW, Bernton EW, Smith PF. Prolactin-like protein in murine splenocytes: morphologic and biochemical evidence. Prog Neuroendocrinimmunol. 1991;3:188 –195. [Google Scholar]
- 103.Gellersen B, Kempf R, Telgmann R, DiMattia GE. Nonpituitary human prolactin gene transcription is independent of pit-1 and differentially controlled in lymphocytes and in endometrial stoma. Mol Endocrinol. 1994;8:356 –373. doi: 10.1210/mend.8.3.8015553. [DOI] [PubMed] [Google Scholar]
- 104.Clevenger CV, Freier DO, Kline JB. Prolactin receptor signal transduction in cells of the immune system. J Endocrinol. 1998;157:187–197. doi: 10.1677/joe.0.1570187. [DOI] [PubMed] [Google Scholar]
- 105.Clevenger CV, Chang W-P, Ngo W, Pasha TLM, Montone KT, Tomaszewski JE. Expression of prolactin and prolactin receptor in human breast carcinoma: evidence for an autocrine/paracrine loop. Am J Pathol. 1995;146:695–705. [PMC free article] [PubMed] [Google Scholar]
- 106.Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV. Distribution of prolactin and its receptor in human breast carcinoma. Endocrinology. 1997;138:5555–5560. doi: 10.1210/endo.138.12.5605. [DOI] [PubMed] [Google Scholar]
- 107.Ginsburg E, Vonderhaar BK. Prolactin synthesis and secretion by human breast cancer cells. Cancer Res. 1995;55:2591–2595. [PubMed] [Google Scholar]
- 108.Ellis LA, Picciano MF. Bioactive and immunoreactive prolactin variants in human milk. Endocrinology. 1995;136:2711–2720. doi: 10.1210/endo.136.6.7750496. [DOI] [PubMed] [Google Scholar]
- 109.Ellis LA, Mastro AM, Picciano MF. Milkborne prolactin and neonatal development. J Mammary Gland Biol Neoplasia. 1996;1:259 –269. doi: 10.1007/BF02018079. [DOI] [PubMed] [Google Scholar]
- 110.Clevenger CV, Thickman K, Ngo W, Chang W-P, Takayama S, Reed JC. Role of Bag-1 in the survival and proliferation of the cytokine-dependent lymphocyte lines, Ba/F3 and Nb2. Mol Endocrinol. 1997;11:608 –618. doi: 10.1210/mend.11.5.9925. [DOI] [PubMed] [Google Scholar]
- 111.Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 112.Bonneterre J, Peyrat JP, Beuscart R, Demaille A. Biological and clinical aspects of prolactin receptors in human breast cancer. J Steroid Biochem Mol Biol. 1990;37:977–981. doi: 10.1016/0960-0760(90)90453-r. [DOI] [PubMed] [Google Scholar]
- 113.Bonneterre J, Peyrat JP, Beuscart R, Lefebvre J, Demaille A. Prognostic significance of prolactin receptors in human breast cancer. Cancer Res. 1987;47:4724 –4728. [PubMed] [Google Scholar]
- 114.De Placido S, Gallo C, Perrone F, Marinelli A, Pagliarula C, Carlomagno C, Petrella G, D’Istria M, Delrio G, Bianco AR. Prolactin receptor does not correlate with oestrogen and progesterone receptors in primary breast cancer and lacks prognostic significance. Ten year results of the Naples adjuvant (GUN) study. Br J Cancer. 1990;62:643–646. doi: 10.1038/bjc.1990.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turcot-Lemay L, Kelly PA. Prolactin receptors in human breast tumors. J Natl Cancer Inst. 1982;68:381–383. [PubMed] [Google Scholar]
- 116.L’Hermite-Baleriaux M, Casteels S, Vokaer A, Loriaux C, Noel G, L’Hermite ML. Prolactin and prolactin receptors in human breast disease. Prog Cancer Res Ther. 1984;31:325–334. [Google Scholar]
- 117.Mertani H, Garcia-Cabellero T, Lambert A, Gerard F, Palayer C, Boutin JM, Vonderhaar BK, Waters MJ, Lobie PE, Morel G. Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer. 1998;79:202–211. doi: 10.1002/(sici)1097-0215(19980417)79:2<202::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 118.Ormandy CJ, Hall RE, Manning DL, Robertson JFR, Blamey RW, Kelly PA, Nicholson RI, Sutherland RL. Coexpression and cross-regulation of the prolactin receptor and sex steroid hormone receptors in breast cancer. J Clin Endocrinol Metab. 1997;82:3692–3699. doi: 10.1210/jcem.82.11.4361. [DOI] [PubMed] [Google Scholar]
- 119.Boutin JM, Edery M, Shirota M, Jolicoeur C, Lesueur L, Ali S, Gould D, Djiane J, Kelly PA. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol. 1989;3:1455–1461. doi: 10.1210/mend-3-9-1455. [DOI] [PubMed] [Google Scholar]
- 120.Mai JNC, Burnside J, Li L, Tang J, Davolos C, Cogburn LA. Characterization of unique truncated prolactin receptor transcripts, corresponding to the intracellular domain, in the testis of the sexually mature chicken. Endocrinology. 1999;140:1165–1174. doi: 10.1210/endo.140.3.6603. [DOI] [PubMed] [Google Scholar]
- 121.Ali S, Edery M, Pellegrini I, Paly J, Djiane J, Kelly PA. Functional activity of three different forms of the rat prolactin receptor. Program of the 73rd Annual Meeting of The Endocrine Society; Washington DC. 1991. p. p 73. (Abstract 245A) [Google Scholar]
- 122.Davis JA, Linzer DIH. Expression of multiple forms of the prolactin receptor in mouse liver. Mol Endocrinol. 1989;3:674 –680. doi: 10.1210/mend-3-4-674. [DOI] [PubMed] [Google Scholar]
- 123.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934 –6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bazan JF. Haematopoietic receptors and helical cytokines. Immunol Today. 1990;11:350 –354. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- 125.DaSilva L, Howard OMZ, Rui H, Kirken RA, Farrar WL. Growth signaling and JAK2 association mediated by membrane-proximal cytoplasmic regions of prolactin receptors. J Biol Chem. 1994;269:18267–18270. [PubMed] [Google Scholar]
- 126.Lebrun J-J, Ali S, Ullrich A, Kelly PA. Proline-rich sequence-mediated Jak2 association to the prolactin receptor is required but not sufficient for signal transduction. J Biol Chem. 1995;270:10664 –10670. doi: 10.1074/jbc.270.18.10664. [DOI] [PubMed] [Google Scholar]
- 127.Pezet A, Buteau H, Kelly PA, Edery M. The last proline of box 1 is essential for association with JAK2 and functional activation of the prolactin receptor. Mol Cell Endocrinol. 1997;129:199 –208. doi: 10.1016/s0303-7207(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 128.Pezet A, Ferrag F, Kelly PA, Edery M. Tyrosine docking sites of the rat prolactin receptor required for association and activation Stat5. J Biol Chem. 1997;272:25043–25050. doi: 10.1074/jbc.272.40.25043. [DOI] [PubMed] [Google Scholar]
- 129.Ali S, Ali S. Recruitment of the proteintyrosine phosphatase SHP-2 to the C-terminal tyrosine of the prolactin receptor and to the adaptor protein Gab2. J Biol Chem. 2000;275:39073–39080. doi: 10.1074/jbc.M007478200. [DOI] [PubMed] [Google Scholar]
- 130.Kline JB, Roehrs H, Clevenger CV. Functional characterization of the intermediate isoform of the human prolactin receptor. J Biol Chem. 1999;274:35461–35468. doi: 10.1074/jbc.274.50.35461. [DOI] [PubMed] [Google Scholar]
- 131.Kline JB, Rycyzyn MA, Clevenger CV. Characterization of a novel and functional human prolactin receptor isoform (ΔS1PRLr) containing only one fibronectin-like domain. Mol Endocrinol. 2002;16:2310 –2322. doi: 10.1210/me.2001-0033. [DOI] [PubMed] [Google Scholar]
- 132.Kinet S, Bernichtein S, Kelly PA, Martial JA, Goffin V. Biological properties of human prolactin analogs depend not only on global hormone affinity, but also on the relative affinities of both receptor binding sites. J Biol Chem. 1999;274:16033–26043. doi: 10.1074/jbc.274.37.26033. [DOI] [PubMed] [Google Scholar]
- 133.Kline JB, Clevenger CV. Identification and characterization of the prolactin-binding protein (PRLBP) in human serum and milk. J Biol Chem. 2001;276:24760 –24766. doi: 10.1074/jbc.M011786200. [DOI] [PubMed] [Google Scholar]
- 134.Baumann G, Amburn KD, Buchanan TA. The effect of circulating growth hormone-binding protein on metabolic clearance, distribution, and degradation of human growth hormone. J Clin Endocrinol Metab. 1987;64:657–660. doi: 10.1210/jcem-64-4-657. [DOI] [PubMed] [Google Scholar]
- 135.Hu Z, Meng J, Dufau ML. Isolation and characterization of two novel forms of the human prolactin receptor generated by alternative splicing of a newly identified exon 11. J Biol Chem. 2001;276:41086 –41094. doi: 10.1074/jbc.M102109200. [DOI] [PubMed] [Google Scholar]
- 136.Shiu RPC. Prolactin receptors in human breast cancer cells in long term tissue culture. Cancer Res. 1979;39:4381–4386. [PubMed] [Google Scholar]
- 137.Shaw-Bruha CM, Pirrucello SJ, Shull JD. Expression of the prolactin gene in normal and neoplastic human breast tissues and human breast cell lines: promoter usage and alternative mRNA splicing. Breast Cancer Res Treat. 1997;44:243–253. doi: 10.1023/a:1005879103367. [DOI] [PubMed] [Google Scholar]
- 138.Vonderhaar BK. Prolactin: the forgotten hormone of human breast cancer. Pharmacol Ther. 1998;79:169 –178. doi: 10.1016/s0163-7258(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 139.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639 –669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 140.Kuo CB, Coss D, Walker AM. Prolactin receptor antagonists. Endocrine. 1998;9:121–131. doi: 10.1385/endo:9:2:121. [DOI] [PubMed] [Google Scholar]
- 141.Sinha YN. Structural variants of prolactin: occurrence and physiological significance. Endocr Rev. 1995;16:354 –369. doi: 10.1210/edrv-16-3-354. [DOI] [PubMed] [Google Scholar]
- 142.Bernichtein S, Kinet S, Jeay S, Llovera M, Madern D, Martial JA, Kelly PA, Goffin V. S179D-human PRL, a pseudophosphorylated human PRL analog, is an agonist and not an antagonist. Endocrinology. 2001;142:3950 –3963. doi: 10.1210/endo.142.9.8369. [DOI] [PubMed] [Google Scholar]
- 143.Wu W, Xu X, Walker AM. Differential modulation of the expression of the long and short form of the PRL receptor and β-casein by unmodified PRL and a molecular mimic of phosphorylated PRL suggests that the short form of the receptor does not act as a dominant negative for signaling resulting in β-casein expression. Program of the 83rd Annual Meeting of The Endocrine Society; Denver, CO. 2001. p. p 340. (Abstract P2–219) [Google Scholar]
- 144.Vonderhaar BK. Prolactin in human breast cancer development. In: Ethier SP, editor. Endocrine oncology. Totowa, NJ: Humana Press; 2000. pp. 101–120. [Google Scholar]
- 145.Fuh G, Wells JA. Prolactin receptor antagonists that inhibit the growth of breast cancer cell lines. J Biol Chem. 1995;270:13133–13137. doi: 10.1074/jbc.270.22.13133. [DOI] [PubMed] [Google Scholar]
- 146.Goffin V, Kinet S, Ferrag F, Binart N, Martial JA, Kelly PA. Antagonistic properties of human prolactin analogs that show paradoxical agonistic activity in the Nb2 bioassay. J Biol Chem. 1996;271:16573–16579. doi: 10.1074/jbc.271.28.16573. [DOI] [PubMed] [Google Scholar]
- 147.Llovera M, Pichard C, Bernichtein S, Jeay S, Touraine P, Kelly PA, Goffin V. Human prolactin (hPRL) antagonists inhibit hPRL-activated signaling pathways involved in breast cancer cell proliferation. Oncogene. 2000;19:4695–4705. doi: 10.1038/sj.onc.1203846. [DOI] [PubMed] [Google Scholar]
- 148.Chen WY, Ramamoorthy P, Chen N, Sticca R, Wagner TE. A human prolactin antagonist, hPRL-G129R, inhibits breast cancer cell proliferation through induction of apoptosis. Clin Cancer Res. 1999;5:3583–3593. [PubMed] [Google Scholar]
- 149.Schroeder MD, Symowicz J, Schuler LA. Prolactin modulates cell cycle regulators in mammary tumor epithelial cells. Mol Endocrinol. 2002;16:45–57. doi: 10.1210/mend.16.1.0762. [DOI] [PubMed] [Google Scholar]
- 150.Dupont J, Karas M, LeRoith D. The potentiation of estrogen on insulin-like growth factor I action in MCF-7 human breast cancer cells includes cell cycle components. J Biol Chem. 2000;275:35893–35901. doi: 10.1074/jbc.M006741200. [DOI] [PubMed] [Google Scholar]
- 151.Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB. Convergence of progesterone with growth factor and cytokine signaling in breast cancer—progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273:31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 152.Carroll JS, Prall OWJ, Musgrove EA, Sutherland RL. A pure estrogen antagonist inhibits cyclin E-Cdk2 activity in MCF-7 breast cancer cells and induces accumulation of p130 –E2F4 complexes characteristic of quiescence. J Biol Chem. 2000;275:38221–38229. doi: 10.1074/jbc.M004424200. [DOI] [PubMed] [Google Scholar]
- 153.Wang TC, Cardiff RD, Zukerberg L, Lees M, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669 –671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 154.Gillett CE, Lee AHS, Millis RR, Barnes DM. Cyclin D1 and associated proteins in mammary ductal carcinoma in situ and atypical ductal hyperplasia. J Pathol. 1998;184:396 –400. doi: 10.1002/(SICI)1096-9896(199804)184:4<396::AID-PATH1259>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 155.Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- 156.Van Diest PJ, Michalides RJAM, Jannink I, Van der Valk P, Peterse HL, De Jong JS, Meijer CJLM, Baak JPA. Cyclin D1 expression in invasive breast cancer—correlations and prognostic value. Am J Pathol. 1997;150:705–711. [PMC free article] [PubMed] [Google Scholar]
- 157.Yu QY, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 158.Brockman JL, Schroeder MD, Schuler LA. Prolactin activates the cyclin D1 promoter via the JAK2-STAT pathway. Mol Endocrinol. 2002;16:774 –784. doi: 10.1210/mend.16.4.0817. [DOI] [PubMed] [Google Scholar]
- 159.Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell RG, Kanakura Y. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brockman JL, Schuler LA. Analysis of cyclin D1 promoter regions that support prolactin-induced promoter activity. Program of the 83rd Annual Meeting of The Endocrine Society; Denver, CO. 2001. p. p 431. (Abstract P2–640) [Google Scholar]
- 161.Krumenacker JS, Montgomery DW, Buckley DJ, Gout PW, Buckley AR. Prolactin receptor signaling—shared components with the T-cell antigen receptor in Nb2 lymphoma cells. Endocrine. 1998;9:313–320. doi: 10.1385/ENDO:9:3:313. [DOI] [PubMed] [Google Scholar]
- 162.Beck MT, Peirce SK, Chen WY. Regulation of bcl-2 gene expression in human breast cancer cells by prolactin and its antagonist, hPRL-G129R. Oncogene. 2002;21:5047–5055. doi: 10.1038/sj.onc.1205637. [DOI] [PubMed] [Google Scholar]
- 163.Ramamoorthy P, Sticca R, Wagner TE, Chen WY. In vitro studies of a prolactin antagonist, hPRL-G129R in human breast cancer cells. Int J Oncol. 2001;18:25–32. doi: 10.3892/ijo.18.1.25. [DOI] [PubMed] [Google Scholar]
- 164.Kops GJ, de Ruiter ND, Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630 –634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 165.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-κB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 166.Schwertfeger KL, Richert MM, Anderson SM. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol Endocrinol. 2001;15:867–881. doi: 10.1210/mend.15.6.0663. [DOI] [PubMed] [Google Scholar]
- 167.Richert MM, Decker K, Anderson SM. Mechanisms underlying constitutive activation of Akt in breast cancer cell lines. p 551. Program of the 83rd Annual Meeting of The Endocrine Society; Denver, CO. 2001. (Abstract P3–478) [Google Scholar]
- 168.Bhatavdekar JM, Shah NG, Balar DB, Patel DD, Bhaduri A, Trivedi SN, Karelia NH, Ghosh N, Shukla MK, Giri DD. Plasma prolactin as an indicator of disease progression in advanced breast cancer. Cancer. 1990;65:2028 –2032. doi: 10.1002/1097-0142(19900501)65:9<2028::aid-cncr2820650924>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 169.Maus MV, Reilly SC, Clevenger CV. Prolactin as a chemoat-tractant for human breast carcinoma. Endocrinology. 1999;140:5447–5450. doi: 10.1210/endo.140.11.7245. [DOI] [PubMed] [Google Scholar]
- 170.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 171.Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L. Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999;59:4770 –4775. [PubMed] [Google Scholar]
- 172.Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 173.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994 –1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 174.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 175.Jackson D, Volpert OV, Bouck N, Linzer DIH. Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science. 1994;266:1581–1584. doi: 10.1126/science.7527157. [DOI] [PubMed] [Google Scholar]
- 176.Bengtson NW, Linzer DIH. Inhibition of tumor growth by the antiangiogenic placental hormone, proliferin-related protein. Mol Endocrinol. 2000;14:1934 –1943. doi: 10.1210/mend.14.12.0573. [DOI] [PubMed] [Google Scholar]
- 177.Struman I, Bentzien F, Lee H, Mainfroid V, D’Angelo G, Goffin V, Weiner RI, Martial JA. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA. 1999;96:1246 –1251. doi: 10.1073/pnas.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ferrara N, Clapp C, Weiner R. The 16K fragment of prolactin specifically inhibits basal or fibroblast growth factor stimulated growth of capillary endothelial cells. Endocrinology. 1991;129:896 –900. doi: 10.1210/endo-129-2-896. [DOI] [PubMed] [Google Scholar]
- 179.Clapp C, Martial JA, Guzman RC, Rentier-Delrue F, Weiner RI. The 16-kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology. 1993;133:1292–1299. doi: 10.1210/endo.133.3.7689950. [DOI] [PubMed] [Google Scholar]
- 180.D’Angelo G, Martini JF, Iiri T, Fantl WJ, Martial J, Weiner RI. 16K human prolactin inhibits vascular endothelial growth factor-induced activation of Ras in capillary endothelial cells. Mol Endocrinol. 1999;13:692–704. doi: 10.1210/mend.13.5.0280. [DOI] [PubMed] [Google Scholar]
- 181.Lee H, Struman I, Clapp C, Martial J, Weiner RI. Inhibition of urokinase activity by the antiangiogenic factor 16K prolactin: activation of plasminogen activator inhibitor 1 expression. Endocrinology. 1998;139:3696 –3703. doi: 10.1210/endo.139.9.6194. [DOI] [PubMed] [Google Scholar]
- 182.Clapp C, Weiner R. A specific, high affinity, saturable binding site for the 160 kilodalton fragment of prolactin on capillary endothelial cells. Endocrinology. 1992;130:1380 –1386. doi: 10.1210/endo.130.3.1311239. [DOI] [PubMed] [Google Scholar]
- 183.Goffin V, Touraine P, Pichard C, Bernichtein S, Kelly PA. Should prolactin be reconsidered as a therapeutic target in human breast cancer? Mol Cell Endocrinol. 1999;151:79 –87. doi: 10.1016/s0303-7207(99)00023-4. [DOI] [PubMed] [Google Scholar]
- 184.Gout PW, Beer CT, Noble RL. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980;40:2433–2436. [PubMed] [Google Scholar]
- 185.Gout PW, Noble RL, Beer CT. Cultured Nb rat lymphoma cells in endocrine and cancer research. Mol Endocrinol. 1986;64:659 –666. doi: 10.1139/o86-091. [DOI] [PubMed] [Google Scholar]
- 186.Ali S, Pellegrini I, Kelly PA. A prolactin-dependent immune cell line (Nb2) expresses a mutant form of prolactin receptor. J Biol Chem. 1991;266:20110 –20117. [PubMed] [Google Scholar]
- 187.O’Neal KD, Yu-Lee L. Differential signal transduction of the short, Nb2, and long prolactin receptors. Activation of interferon regulatory factor-1 and cell proliferation. J Biol Chem. 1994;269:26076 –26082. [PubMed] [Google Scholar]
- 188.Yu-Lee LY, Luo GY, Book ML, Morris SM. Lactogenic hormone signal transduction. Biol Reprod. 1998;58:295–301. doi: 10.1095/biolreprod58.2.295. [DOI] [PubMed] [Google Scholar]
- 189.Clevenger CV, Kline JB. Prolactin receptor signal transduction. Lupus. 2001;10:706 –718. doi: 10.1191/096120301717164949. [DOI] [PubMed] [Google Scholar]
- 190.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 191.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–5679. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 192.Kazansky AV, Kabotyanski EB, Wsyzomierski SL, Mancini MA, Rosen JM. Differential effects of prolactin and src/abl kinases on the nuclear translocation of STAT5B and STAT5A. J Biol Chem. 1999;274:22484 –22492. doi: 10.1074/jbc.274.32.22484. [DOI] [PubMed] [Google Scholar]
- 193.Rui H, Lebrun J-J, Kirken RA, Kelly PA, Farrar WL. JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology. 1994;135:1299 –1306. doi: 10.1210/endo.135.4.7925093. [DOI] [PubMed] [Google Scholar]
- 194.Lebrun JJ, Ali S, Sofer L, Ullrich A, Kelly PA. Prolactin-induced proliferation Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J Biol Chem. 1994;269:14021–14026. [PubMed] [Google Scholar]
- 195.Frank SJ, Yi W, Zhao Y, Goldsmith JF, Gilliland G, Jiang J, Sakai I, Kraft AS. Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J Biol Chem. 1995;270:14776 –14785. doi: 10.1074/jbc.270.24.14776. [DOI] [PubMed] [Google Scholar]
- 196.Zhao Y, Wagner F, Frank SJ, Kraft AS. The aminoterminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulated factor receptor βc chain. J Biol Chem. 1995;270:13814 –13818. doi: 10.1074/jbc.270.23.13814. [DOI] [PubMed] [Google Scholar]
- 197.Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B. Prolactin regulation of β-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988;7:2089 –2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.DaSilva L, Rui H, Erwin RA, Howard OM, Kirken RA, Malabarba MG, Hackett RH, Larner AC, Farrar WL. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol Cell Endocrinol. 1996;117:131–140. doi: 10.1016/0303-7207(95)03738-1. [DOI] [PubMed] [Google Scholar]
- 199.Schaber JD, Fang H, Xu J, Grimley PM, Rui H. Prolactin activates Stat1 but does not antagonize Stat1 activation and growth inhibition by type I interferons in human breast cancer cells. Cancer Res. 1998;58:1914 –1919. [PubMed] [Google Scholar]
- 200.Watson CJ, Miller WR. Elevated levels of members of the STAT family of transcription factors in breast carcinoma nuclear extracts. Br J Can. 1995;71:840 –844. doi: 10.1038/bjc.1995.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474 –2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 202.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468 –2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 203.Levy DE, Gilliland DG. Divergent roles of STAT1 and STAT5 in malignancy as revealed by gene disruptions in mice. Oncogene. 2000;19:2505–2510. doi: 10.1038/sj.onc.1203480. [DOI] [PubMed] [Google Scholar]
- 204.Chapman RS, Lourenco P, Tonner E, Flint D, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. The role of Stat3 in apoptosis and mammary gland involution—conditional deletion of Stat3. Adv Exp Med Biol. 2000;480:129 –138. doi: 10.1007/0-306-46832-8_16. [DOI] [PubMed] [Google Scholar]
- 205.Liu X, Robinson GW, Wagner K-U, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179 –186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 206.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 207.Kazansky AV, Rosen JM. Signal transducers and activators of transcription 5B potentiates v-Src-mediated transformation of NIH-3T3 cells. Cell Growth Differ. 2001;12:1–7. [PubMed] [Google Scholar]
- 208.Sartor CI, Dziubinski ML, Yu CL, Jove R, Ethier SP. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997;57:978 –987. [PubMed] [Google Scholar]
- 209.Schroeder MD, Rose-Hellekant T, Sandgren EP, Schuler LA. Dysregulation of signal transducers and activators of transcription 1, 3, and 5 and prolactin receptors by overexpression of mammary TGFα. Mol Cell Endocrinol. 2001;175:173–183. doi: 10.1016/s0303-7207(01)00385-9. [DOI] [PubMed] [Google Scholar]
- 210.Humphreys RC, Hennighausen L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ. 1999;10:685–694. [PubMed] [Google Scholar]
- 211.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69 –74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 212.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, Grosveld G, Ihle JN. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 213.Liu X, Robinson GW, Gouilleux F, Groner B, Henninghausen L. Cloning and expression of Stat5 and an additional homologue (Stat 5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA. 1995;92:8831–8835. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 215.Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD, Shuai K. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci USA. 1998;95:10626 –10631. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rycyzyn MA, Clevenger CV. The intranuclear prolactin/cyclophilin B complex as a transcriptional inducer. Proc Natl Acad Sci USA. 2002;99:6790 –6795. doi: 10.1073/pnas.092160699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yamashita H, Xu J, Erwin RA, Farrar WL, Kirken RA, Rui H. Differential control of the phosphorylation state of proline-juxtaposed serine residues Ser725 of Stat5a and Ser730 of Stat5b in prolactin-sensitive cells. J Biol Chem. 1998;273:30218 –30224. doi: 10.1074/jbc.273.46.30218. [DOI] [PubMed] [Google Scholar]
- 218.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628 –2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 219.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of Stat transcription factors is mediated by src tyrosine kinases. J Biol Chem. 1999;274:17209 –17218. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- 220.Berlanga JJ, Vara JAF, Martín-Pérez J, García-Ruiz JP. Prolactin receptor is associated with c-src kinase in rat liver. Mol Endocrinol. 1995;9:1461–1467. doi: 10.1210/mend.9.11.8584023. [DOI] [PubMed] [Google Scholar]
- 221.Meng L, Lin L, Zhang H, Nassiri M, Morales AR, Nadji M. Multiple mutations of the p53 gene in human mammary carcinoma. Mutat Res DNA Repair. 1999;435:263–269. doi: 10.1016/s0921-8777(99)00053-1. [DOI] [PubMed] [Google Scholar]
- 222.Clevenger CV, Medaglia MV. The protein tyrosine kinase p59fyn is associated with prolactin receptor and is activated by prolactin stimulation of T-lymphocytes. Mol Endocrinol. 1994;8:674 –681. doi: 10.1210/mend.8.6.7935483. [DOI] [PubMed] [Google Scholar]
- 223.Sorensen P, Sheffield LG. Involvement of c-src in β-casein expression by mammary epithelial cells. Biochem Biophys Res Commun. 1997;241:710 –713. doi: 10.1006/bbrc.1997.7879. [DOI] [PubMed] [Google Scholar]
- 224.Zhang Y, Turkson J, Carter-Su C, Smithgall T, Levitzki A, Kraker A, Krolewski JJ, Medveczky P, Jove R. Activation of Stat3 in v-Src-transformed fibroblasts requires cooperation of Jak1 kinase activity. J Biol Chem. 2000;275:24935–24944. doi: 10.1074/jbc.M002383200. [DOI] [PubMed] [Google Scholar]
- 225.Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Clevenger CV, Torigoe T, Reed JC. Prolactin induces rapid phosphorylation and activation of prolactin receptor associated Raf-1 kinase in a T-cell line. J Biol Chem. 1994;269:5559 –5565. [PubMed] [Google Scholar]
- 227.Das R, Vonderhaar BK. Activation of raf-1 MEK, and MAP kinase in prolactin responsive mammary cells. Breast Cancer Res Treat. 1996;40:141–149. doi: 10.1007/BF01806209. [DOI] [PubMed] [Google Scholar]
- 228.Das R, Vonderhaar BK. Involvement of SHC, GRB2, SOS and RAS in prolactin signal transduction in mammary epithelial cells. Oncogene. 1996;13:1139 –1145. [PubMed] [Google Scholar]
- 229.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 230.Roovers K, Assoian RK. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818 –826. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 231.Pearson G, Robinson F, Beers GT, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 232.Yamauchi T, Yamauchi N, Ueki K, Sugiyama T, Waki H, Miki H, Tobe K, Matsuda S, Tsushima T, Yamamoto T, Fujita T, Taketani Y, Fukayama M, Kimura S, Yazaki Y, Nagai R, Kadowaki T. Constitutive tyrosine phosphorylation of ErbB-2 via Jak2 by auto-crine secretion of prolactin in human breast cancer. J Biol Chem. 2000;275:33937–33944. doi: 10.1074/jbc.M000743200. [DOI] [PubMed] [Google Scholar]
- 233.Gao J, Horseman ND. Prolactin-independent modulation of the β-casein response element by Erk2 MAP kinase. Cell Signal. 1999;11:205–210. doi: 10.1016/s0898-6568(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 234.Deleted in proof
- 235.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by stat1 and stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 236.Beuvink I, Hess D, Flotow H, Hofsteenge J, Groner B, Hynes NE. Stat5a serine phosphorylation—serine 779 is constitutively phosphorylated in the mammary gland, and serine 725 phosphorylation influences prolactin-stimulated in vitro DNA binding activity. J Biol Chem. 2000;275:10247–10255. doi: 10.1074/jbc.275.14.10247. [DOI] [PubMed] [Google Scholar]
- 237.Schwertfeger KL, Hunter S, Heasley LE, Levresse V, Leon RP, DeGregori J, Anderson SM. Prolactin stimulates activation of c-jun N-terminal kinase (JNK) Mol Endocrinol. 2000;14:1592–1602. doi: 10.1210/mend.14.10.0536. [DOI] [PubMed] [Google Scholar]
- 238.Olazabal I, Munoz J, Ogueta S, Obregon E, Garcia-Ruiz JP. Prolactin (PRL)-PRL receptor system increases cell proliferation involving JNK (c-Jun amino terminal kinase) and AP-1 activation: inhibition by glucocorticoids. Mol Endocrinol. 2000;14:564 –575. doi: 10.1210/mend.14.4.0442. [DOI] [PubMed] [Google Scholar]
- 239.Cheng Y, Zhizhin I, Perlman RL, Mangoura D. Prolactin-induced cell proliferation in PC12 cells depends on JNK but not ERK activation. J Biol Chem. 2000;275:23326 –23332. doi: 10.1074/jbc.M001837200. [DOI] [PubMed] [Google Scholar]
- 240.Roymans D, Slegers H. Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem. 2001;268:487–498. doi: 10.1046/j.1432-1327.2001.01936.x. [DOI] [PubMed] [Google Scholar]
- 241.Toker A. Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol Pharmacol. 2000;57:652–658. [PubMed] [Google Scholar]
- 242.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 243.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439 –1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 244.Berlanga JJ, Gualillo O, Buteau H, Applanat M, Kelly PA, Edery M. Prolactin activates tyrosyl phosphorylation of insulin receptor substrate 1 and phosphatidylinositol-3-OH kinase. J Biol Chem. 1997;272:2050 –2052. doi: 10.1074/jbc.272.4.2050. [DOI] [PubMed] [Google Scholar]
- 245.Yamauchi T, Kaburagi Y, Ueki K, Tsuji Y, Stark GR, Kerr IM, Tsushima T, Akanuma Y, Komuro I, Tobe K, Yazaki Y, Kadowaki T. Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1, -2, and -3, their association with p85 phosphatidylinositol 3-kinase (PI3-kinase), and concomitantly PI3-kinase activation via JAK2 kinase. J Biol Chem. 1998;273:15719 –15726. doi: 10.1074/jbc.273.25.15719. [DOI] [PubMed] [Google Scholar]
- 246.Al Sakkaf KA, Dobson PR, Brown BL. Prolactin induced tyrosine phosphorylation of p59fyn may mediate phospha-tidylinositol 3-kinase activation in Nb2 cells. J Mol Endocrinol. 1997;19:347–350. doi: 10.1677/jme.0.0190347. [DOI] [PubMed] [Google Scholar]
- 247.Rodriguez-Viciana P, Marte BM, Warne PH, Downward J. Phosphatidylinositol 3′ kinase: one of the effectors of Ras. Philos Trans R Soc Lond B Biol Sci. 1996;351:225–231. doi: 10.1098/rstb.1996.0020. [DOI] [PubMed] [Google Scholar]
- 248.Constantino S, Santos R, Lacronique V, Bouchaert I, Monni R, Bernard O, Gisselbrecht S, Gouilleux F. Constitutively active STAT5 variants induce growth and survival of hematopoietic cells through a PI 3-kinase/Akt dependent pathway. Oncogene. 2001;20:2080 –2090. doi: 10.1038/sj.onc.1204308. [DOI] [PubMed] [Google Scholar]
- 249.Craddock BL, Hobbs J, Edmead CE, Welham MJ. Phosphoinositide 3-kinase-dependent regulation of interleukin-3-induced proliferation: involvement of mitogen-activated protein kinases, SHP2 and Gab2. J Biol Chem. 2001;276:24274 –24283. doi: 10.1074/jbc.M009098200. [DOI] [PubMed] [Google Scholar]
- 250.Lecoq-Lafon C, Verdier F, Fichelson S, Chretien S, Gisselbrecht S, Lacombe C, Mayeux P. Erythropoietin induces the tyrosine phosphorylation of GAB1 and its association with SHC, SHP2, SHIP, and phosphatidylinositol 3-kinase. Blood. 1999;93:2578 –2585. [PubMed] [Google Scholar]
- 251.Pfeffer LM, Mullersman JE, Pfeffer SR, Murti A, Shi W, Yang CH. STAT3 as an adapter to couple phosphatidylinositol 3-kinase to the IFNAR1 chain of the type I interferon receptor. Science. 1997;276:1418 –1420. doi: 10.1126/science.276.5317.1418. [DOI] [PubMed] [Google Scholar]
- 252.Deleted in proof
- 253.Muise-Helmericks RC, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, Rosen N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 1998;273:29864 –29872. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- 254.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 255.Hutchinson J, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol Cell Biol. 2001;21:2203–2212. doi: 10.1128/MCB.21.6.2203-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kline JB, Moore DJ, Clevenger CV. Activation and association of the Tec tyrosine kinase with the human prolactin receptor: mapping of a Tec/Vav1-receptor binding site. Mol Endocrinol. 2001;15:832–841. doi: 10.1210/mend.15.5.0631. [DOI] [PubMed] [Google Scholar]
- 257.Clevenger CV, Ngo W, Luger SM, Gewirtz AM. Vav is necessary for prolactin-stimulated proliferation and is translocated into the nucleus of a T-cell line. J Biol Chem. 1995;270:13246 –13253. doi: 10.1074/jbc.270.22.13246. [DOI] [PubMed] [Google Scholar]
- 258.Canbay E, Norman M, Kilic E, Goffin V, Zachary I. Prolactin stimulates the JAK2 and focal adhesion kinase pathways in human breast carcinoma T47-D cells. Biochem J. 1997;324:231–236. doi: 10.1042/bj3240231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Huyer G, Alexander DR. Immune signalling: SHP-2 docks at multiple ports. Curr Biol. 1999;9:R129 –R132. doi: 10.1016/s0960-9822(99)80080-3. [DOI] [PubMed] [Google Scholar]
- 260.Ali S, Chen ZJ, Lebrun JJ, Vogel W, Kharitonenkov A, Kelly PA, Ullrich A. PTP1D is a positive regulator of the prolactin signal leading to β-casein promoter activation. EMBO J. 1996;15:135–142. [PMC free article] [PubMed] [Google Scholar]
- 261.Berchtold S, Volarevic S, Moriggl R, Mercep M, Groner B. Dominant negative variants of the SHP-2 tyrosine phosphatase inhibit prolactin activation of Jak2 (janus kinase 2) and induction of Stat5 (signal transducer and activator of transcription 5)-dependent transcription. Mol Endocrinol. 1998;12:556 –567. doi: 10.1210/mend.12.4.0086. [DOI] [PubMed] [Google Scholar]
- 262.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 263.Gisselbrecht S. The CIS/SOCS proteins: a family of cytokine-inducible regulators of signaling. Eur Cytokine Netw. 1999;10:463–470. [PubMed] [Google Scholar]
- 264.Aoki N, Matsuda T. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J Biol Chem. 2000;275:39718 –39726. doi: 10.1074/jbc.M005615200. [DOI] [PubMed] [Google Scholar]
- 265.Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci. 2000;113:2813–2819. doi: 10.1242/jcs.113.16.2813. [DOI] [PubMed] [Google Scholar]
- 266.Pezet A, Favre H, Kelly PA, Edery M. Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J Biol Chem. 1999;274:24497–24502. doi: 10.1074/jbc.274.35.24497. [DOI] [PubMed] [Google Scholar]
- 267.Tomic S, Chughtai N, Ali S. SOCS-1,-2,-3: selective targets and functions downstream of the prolactin receptor. Mol Cell Endocrinol. 1999;158:45–54. doi: 10.1016/s0303-7207(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 268.Helman D, Sandowski Y, Cohen Y, Matsumoto A, Yoshimura A, Merchav S, Gertler A. Cytokine-inducible SH2 protein (CIS3) and JAK2 binding protein (JAB) abolish prolactin receptor-mediated STAT5 signaling. FEBS Lett. 1998;441:287–291. doi: 10.1016/s0014-5793(98)01555-5. [DOI] [PubMed] [Google Scholar]
- 269.Clevenger CV, Altmann SW, Prystowsky MB. Requirement of nuclear prolactin for interleukin-2-stimulated proliferation of T lymphocytes. Science. 1991;253:77–79. doi: 10.1126/science.2063207. [DOI] [PubMed] [Google Scholar]
- 270.Rycyzyn MA, Reilly SC, O’Malley K, Clevenger CV. Role of cyclophilin B in PRL signal transduction and nuclear retrotrans-location. Mol Endocrinol. 2000;14:1175–1186. doi: 10.1210/mend.14.8.0508. [DOI] [PubMed] [Google Scholar]
- 271.Clevenger CV, Sillman AL, Prystowsky MB. Interleukin-2 driven nuclear translocation of prolactin in cloned T-lymphocytes. Endocrinology. 1990;127:3151–3159. doi: 10.1210/endo-127-6-3151. [DOI] [PubMed] [Google Scholar]
- 272.Lobie PE, Mertani H, Morel G, Marales-Bustos O, Norstedt G, Waters MJ. Receptor-mediated nuclear translocation of growth hormone. J Biol Chem. 1994;269:21330 –21339. [PubMed] [Google Scholar]
- 273.Rao Y-P, Buckley DJ, Olson MD, Buckley AR. Nuclear translocation of prolactin: Collaboration of tyrosine kinase and protein kinase C activation in rat Nb2 node lymphoma cells. J Cell Physiol. 1995;163:266 –276. doi: 10.1002/jcp.1041630207. [DOI] [PubMed] [Google Scholar]
- 274.Lu J-C, Scott P, Strous GJ, Schuler LA. Multiple internalization motifs differentially used by prolactin receptor isoforms mediate similar endocytic pathways. Mol Endocrinol. 2002;16:2515–2527. doi: 10.1210/me.2002-0077. [DOI] [PubMed] [Google Scholar]
- 275.Vincent V, Goffin V, Rozakis-Adcock M, Mornon JP, Kelly PA. Identification of cytoplasmic motifs required for short prolactin receptor internalization. J Biol Chem. 1997;272:7062–7068. doi: 10.1074/jbc.272.11.7062. [DOI] [PubMed] [Google Scholar]
- 276.Ross RJM, Esposito N, Shen XY, Von Laue S, Chew SL, Dobson PRM, Postel-Vinay MC, Finidori J. A short isoform of the human growth hormone receptor functions as a dominant negative inhibitor of the full-length receptor and generates large amounts of binding protein. Mol Endocrinol. 1997;11:265–273. doi: 10.1210/mend.11.3.9901. [DOI] [PubMed] [Google Scholar]
- 277.Strous GJ, Govers R. The ubiquitin-proteasome system and endocytosis. J Cell Sci. 1999;112:1417–1423. doi: 10.1242/jcs.112.10.1417. [DOI] [PubMed] [Google Scholar]
- 278.Kirchhausen T. Clathrin. Annu Rev Biochem. 2000;69:699 –727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- 279.Heilker R, Spiess M, Crottet P. Recognition of sorting signals by clathrin adaptors. Bioessays. 1999;21:558 –567. doi: 10.1002/(SICI)1521-1878(199907)21:7<558::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 280.Djiane J, Clauser H, Kelly PA. Rapid down-regulation of prolactin receptors in mammary gland and liver. Biochem Biophys Res Commun. 1979;90:1371–1378. doi: 10.1016/0006-291x(79)91187-2. [DOI] [PubMed] [Google Scholar]
- 281.Djiane J, Kelly PA, Houdebine L-M. Effects of lysosomotropic agents, cytochalasin B and colchicine on the “down-regulation” of prolactin receptors in mammary gland explants. Mol Cell Endocrinol. 1980;18:87–98. doi: 10.1016/0303-7207(80)90084-2. [DOI] [PubMed] [Google Scholar]
- 282.Genty N, Paly J, Edery M, Kelly PA, Djiane J, Salesse R. Endocytosis and degradation of prolactin and its receptor in Chi-nese hamster ovary cells stably transfected with prolactin receptor cDNA. Mol Cell Endocrinol. 1994;99:221–228. doi: 10.1016/0303-7207(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 283.Perrot-Applanat M, Gualillo O, Pezet A, Vincent V, Edery M, Kelly PA. Dominant negative and cooperative effects of mutant forms of prolactin receptor. Mol Endocrinol. 1997;11:1020 –1032. doi: 10.1210/mend.11.8.9954. [DOI] [PubMed] [Google Scholar]
- 284.Schmid RS, Pruitt WM, Maness PF. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J Neurosci. 2000;20:4177–4188. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for β2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 286.Fielding CJ. Caveolae and signaling. Curr Opin Lipidol. 2001;12:281–287. doi: 10.1097/00041433-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 287.Schlegel A, Lisanti MP. Caveolae and their coat proteins, the caveolins: from electron microscopic novelty to biological launching pad. J Cell Physiol. 2001;186:329 –337. doi: 10.1002/1097-4652(2001)9999:9999<000::AID-JCP1045>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 288.Snyderwine EG. Mammary gland carcinogenesis by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rats: possible mechanisms. Cancer Lett. 1999;143:211–215. doi: 10.1016/s0304-3835(99)00127-5. [DOI] [PubMed] [Google Scholar]
- 289.Welsch CW. Prolactin and the development and progression of early neoplastic mammary gland lesions. Cancer Res. 1978;38:4054 –4058. [PubMed] [Google Scholar]
- 290.Welsch CW, Goodrich-Smith M, Brown CK, Roth L. The prophylaxis of rat and mouse mammary gland tumorigenesis by suppression of prolactin secretion: a reappraisal. Breast Cancer Res Treat. 1981;1:225–232. doi: 10.1007/BF01806262. [DOI] [PubMed] [Google Scholar]
- 291.Welsch CW, Brown CK, Goodrich-Smith M, Chiusano J, Moon RC. Synergistic effect of chronic prolactin suppression and retinoid treatment in the prophylaxis of N-methyl-N-nitrosourea-induced mammary tumorigenesis in female Sprague-Dawley rats. Cancer Res. 1980;40:3095–3098. [PubMed] [Google Scholar]
- 292.Welsch CW, Brown CK, Goodrich-Smith M, Van J, Denenberg B, Anderson TM, Brooks CL. Inhibition of mammary tumori-genesis in carcinogen-treated Lewis rats by suppression of prolactin secretion. J Natl Cancer Inst. 1979;63:1121–1124. [PubMed] [Google Scholar]
- 293.Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast Cancer Res Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- 294.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 295.Thordarson G, Van Horn K, Guzman RC, Nandi S, Talamantes F. Parous rats regain high susceptibility to chemically induced mammary cancer after treatment with various mammotropic hormones. Carcinogenesis. 2001;22:1027–1033. doi: 10.1093/carcin/22.7.1027. [DOI] [PubMed] [Google Scholar]
- 296.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1:467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 297.Hynes NE, Cella N, Wartmann M. Prolactin mediated intra-cellular signaling in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 1997;2:19 –27. doi: 10.1023/a:1026317428542. [DOI] [PubMed] [Google Scholar]
- 298.Deleted in proof
- 299.Han Y, Watling D, Rogers NC, Stark GR. JAK2 and STAT5, but not JAK1 and STAT1, are required for prolactin-induced β-lactoglobulin transcription. Mol Endocrinol. 1997;11:1180 –1188. doi: 10.1210/mend.11.8.9952. [DOI] [PubMed] [Google Scholar]
- 300.Gao J, Hughes JP, Auperin B, Buteau H, Edery M, Zhuang H, Wojchowski DM, Horseman ND. Interactions among Janus kinases and the prolactin (PRL) receptor in the regulation of a PRL response element. Mol Endocrinol. 1996;10:847–856. doi: 10.1210/mend.10.7.8813725. [DOI] [PubMed] [Google Scholar]
- 301.Campbell GS, Argetsinger LS, Ihle JN, Kelly PA, Rillema JA, Carter-Su C. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci USA. 1994;91:5232–5236. doi: 10.1073/pnas.91.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Deleted in proof
- 303.Li M, Liu X, Robinson G, Bar-Peled U, Wagner KU, Young WS, Hennighausen L, Furth PA. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc Natl Acad Sci USA. 1997;94:3425–3430. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Deleted in proof
- 305.Deleted in proof
- 306.Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- 307.Liu X, Gallego MI, Smith GH, Robinson GW, Hennighausen L. Functional release of Stat5a-null mammary tissue through the activation of compensating signals including Stat5b. Cell Growth Differ. 1998;9:795–803. [PubMed] [Google Scholar]
- 308.Lindeman GJ, Wittlin S, Lada H, Naylor MJ, Santamaria M, Zhang JG, Starr R, Hilton DJ, Alexander WS, Ormandy CJ, Visvader J. SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Genes Dev. 2001;15:1631–1636. doi: 10.1101/gad.880801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wennbo H, Gebre-Medhin M, Griti-Linde A, Ohlsson C, Isaksson OGP, Tornell J. Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest. 1997;100:2744 –2751. doi: 10.1172/JCI119820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wennbo H, Tornell J. The role of prolactin and growth hormone in breast cancer. Oncogene. 2000;19:1072–1076. doi: 10.1038/sj.onc.1203349. [DOI] [PubMed] [Google Scholar]
- 311.Holly JMP, Gunnell DJ, Smith GD. Growth hormone, IGF-1 and cancer. Less intervention to avoid cancer? More intervention to prevent cancer? J Endocrinol. 1999;162:321–330. doi: 10.1677/joe.0.1620321. [DOI] [PubMed] [Google Scholar]
- 312.Pollak M. IGF-1 physiology and breast cancer. Recent Res Cancer Res. 1998;152 doi: 10.1007/978-3-642-45769-2_6. [DOI] [PubMed] [Google Scholar]
- 313.Vomachka AJ, Pratt SL, Lockefeer JA, Horseman ND. Prolactin gene-disruption arrests mammary gland development and retards T-antigen-induced tumor growth. Oncogene. 2000;19:1077–1084. doi: 10.1038/sj.onc.1203348. [DOI] [PubMed] [Google Scholar]
- 314.Horseman ND. Prolactin and mammary gland development. J Mammary Gland Biol Neoplasia. 1999;4:79 –88. doi: 10.1023/a:1018708704335. [DOI] [PubMed] [Google Scholar]
- 315.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926 – 6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Weinberg RA, Kelly PA, Ormandy CJ. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol. 1999;210:96 –106. doi: 10.1006/dbio.1999.9271. [DOI] [PubMed] [Google Scholar]
- 317.Furth PA. Conditional control of gene expression in the mammary gland. J Mammary Gland Biol Neoplasia. 1997;2:373–383. doi: 10.1023/a:1026399329934. [DOI] [PubMed] [Google Scholar]
- 318.Shillingford JM, Miyoshi K, Grimm SL, Neubauser H, Pfeffer K, Hennighausen L. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol Endocrinol. 2002;16:563–570. doi: 10.1210/mend.16.3.0805. [DOI] [PubMed] [Google Scholar]
- 319.Furth PA, Ren S, Cai H, Li M. Loss of Stat5a delays mammary cancer progression in a mouse model. Oncogene. 2002;21:4335–4339. doi: 10.1038/sj.onc.1205484. [DOI] [PubMed] [Google Scholar]
- 320.Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson G, Hennighausen L. Signal transducer and activator of transcription Stat5 controls the proliferation and differentiation of mammary epithelium. J Cell Biol. 2001;155:531–542. doi: 10.1083/jcb.200107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Matsumoto A, Seki Y, Kubo M, Ohtsuka S, Suzuki A, Hayashi I, Tsuji K, Nakahata T, Okabe M, Yamada S, Yoshimura A. Suppression of STAT5 functions in liver, mammary glands, and T cells in cytokine-inducible SH2-containing protein 1 transgenic mice. Mol Cell Biol. 1999;19:6396 –6407. doi: 10.1128/mcb.19.9.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Lange CA, Richer JK, Horwitz KB. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or anti-proliferative signals. Mol Endocrinol. 1999;13:829 –836. doi: 10.1210/mend.13.6.0290. [DOI] [PubMed] [Google Scholar]
- 323.Yee D, Lee AV. Crosstalk between the insulin-like growth factors and estrogens in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5:107–115. doi: 10.1023/a:1009575518338. [DOI] [PubMed] [Google Scholar]
- 324.Danielson KG, Oborn CJ, Durban EM, Butel JS, Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci USA. 1984;81:3756 –3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Doppler W, Groner B, Ball RK. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat β-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci USA. 1989;86:104 –108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Taverna D, Groner B, Hynes NE. Epidermal growth factor receptor, platelet-derived growth factor receptor, and c-erbB-2 receptor activation all promote growth but have distinctive effects upon mouse mammary epithelial cell differentiation. Cell Growth Differ. 1991;2:145–154. [PubMed] [Google Scholar]
- 327.Wagner KU, Wall RJ, St Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]


