Abstract
Alternative splicing plays an important role in processes such as development, differentiation and cancer. With the recent increase in the estimates of the number of human genes that undergo alternative splicing from 5 to 35–59%, it is becoming critical to develop a better understanding of its functional consequences and regulatory mechanisms. We conducted a large scale study of the distribution of protein domains in a curated data set of several thousand genes and identified protein domains disproportionately distributed among alternatively spliced genes. We also identified a number of protein domains that tend to be spliced out. Both the proteins having the disproportionately distributed domains as well as those with spliced-out domains are predominantly involved in the processes of cell communication, signaling, development and apoptosis. These proteins function mostly as enzymes, signal transducers and receptors. Somewhat surprisingly, 28% of all occurrences of spliced-out domains are not effected by straightforward exclusion of exons coding for the domains but by inclusion or exclusion of other exons to shift the reading frame while retaining the exons coding for the domains in the final transcripts.
INTRODUCTION
Alternative splicing was first predicted to occur in 1978 (1). With only 32 000 genes predicted from the genome sequence and an estimated 100 000 genes based on EST clustering, alternative splicing may be a major mechanism for producing genomic complexity (2). Prior estimates of the prevalence of alternative splicing were as low as 5% (3) and have been revised to 35–59% of all genes having at least one alternative splice form (4–8). The increased expectation of alternative splicing raises intriguing questions about the identification, functional roles and regulation of alternative splice variants across the whole genome.
Alternative splicing plays a major role in sex determination in Drosophila, antibody response in humans and other tissue or developmental stage-specific processes (9). Coordinated changes in alternative splicing patterns of pre-mRNAs are an integral component of gene expression programs such as those involved in nervous system differentiation (10) and apoptotic cell death (11,12). Alternative splicing is also implicated in a large number of human pathologies, such as cancer and Alzheimer’s disease (13).
The diverse outcomes of alternative splicing fall into two major categories: protein-level alterations and transcript-level modifications. On the protein level, alternative splicing generates splice variants that give rise to different protein products, for example, a shortened protein product due to a frame shift introduced by an alternate exon or a protein product with a different functional domain due to the inclusion of a specific exon from a mutually exclusive group of exons. On the transcript level, alternative splicing produces splice variants that have different translation or stability profiles, for example, a transcript with a longer life span would prolong the availability of the corresponding protein product.
There are many examples of protein-level alterations introduced by alternative splicing (14–20). In some cases, the proteins produced by the splice variants play opposite roles in the cellular processes. These studies tend to focus on specific genes and not on classes of proteins that are, as a group, disproportionately affected by alternative splicing. We would like to find protein domains that are disproportionately distributed among alternatively spliced or constitutively spliced genes. We would also like to find domains that are frequently spliced out among members of a protein family or across protein families.
Most large scale studies of alternative splicing focus on the DNA and RNA level. Loraine et al. identified genes whose alternative transcripts produce different protein domain structures and developed a graphical tool to study protein domain differences for the splice variants of individual genes (21). Their work did not address the distribution of protein domains more generally. In this paper, we used manually reviewed LocusLink and RefSeq records to systematically study protein domains in the context of alternative splicing.
MATERIALS AND METHODS
Data source
The July 26, 2002 release of LocusLink was downloaded from NCBI web site (ftp://ftp.ncbi.nih.gov/refseq/LocusLink/). The flat file was parsed with a parser program and selected fields were loaded into a relational database. Only loci with a ‘reviewed’ status were used for this study. Reviewed status is given to a locus after a careful review by the NCBI staff. Reviewed loci that have more than one RefSeq transcript associated with them constituted the set of reviewed alternatively spliced loci.
Identification of disproportionately distributed protein domains
The NCBI Conserved Domain Database (CDD, http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) protein domain annotations were used to identify protein domains. These annotations are generated by the NCBI staff using the NCBI CDD search tool (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). For each of the CDD protein domains annotated on reviewed loci, the numbers of constitutively spliced and alternatively spliced reviewed loci that were annotated with it were determined. A 2 × 2 contingency table and Fisher’s exact test were used to determine if a protein domain is disproportionately distributed among alternatively spliced and constitutively spliced loci. A sample contingency table for Cadherin domain is shown in Table 1. To avoid false positives generated by the testing of multiple hypothesis, the false discovery rate correction method (22) was applied.
Table 1. Sample contingency table for use in Fisher’s test.
| Cadherin domain | Constitutively spliced | Alternatively spliced | Total |
|---|---|---|---|
| With domain | 50 | 50 | 100 |
| Without domain | 3566 | 882 | 4448 |
| Total | 3616 | 932 | 4548 |
Characterization of gene ontology (GO) annotations for a set of genes
GO terms and annotations were obtained in a mySQL database developed by BDGP from http://www.godatabase.org/dev/database/. We downloaded the December 2002 release and imported it into the Oracle database previously loaded with LocusLink data. Given a set of genes, all the GO terms for two sub-ontologies of GO (molecular function and biological process) were extracted from the GO database. The count of the number of genes for each GO term was recorded. We defined the sub-tree covering a given set of GO terms as the one rooted by the deepest common ancestor of all the given GO terms. For gene coverage, the number of genes represented by the sub-tree rooted in a GO term was divided into the total number of annotated genes to arrive at the percentage coverage. For GO term coverage, the number of GO terms represented by the sub-tree rooted in a GO term was divided into the total number of unique GO terms to arrive at the percentage coverage.
The P-value assigned to each GO term was calculated as described on the ProToGo website (http://www.protonet.cs.huji.ac.il/ProToGO/Introduction.html). First, the number of reviewed loci assigned to each GO term was calculated. This count includes all the loci assigned to a GO term and those assigned to its descendents. Duplicated loci were counted only once. This served as the background distribution. Then, for a given set of loci, a similar calculation was performed to derive the number of loci from this set assigned to each GO term. The P-value calculated for each GO term is expressing the probability to receive such a count from the given set, or higher, assuming the null hypothesis under hypergeometric distribution.
Identification of spliced-out protein domains
To identify spliced-out protein domains, we used CDD protein domain annotations. In particular, annotations corresponding to each splice variant were extracted from the database. Domains that were not present on all splice variants were considered spliced-out protein domains.
Classification of transcript level modifications to spliced-out domains
Two types of transcript level modifications can be employed to produce a spliced variant without a protein domain. One is to exclude one or more exons coding for the domain from the processed transcript. The other is to retain all the exons coding for the domain in the transcript but shift the reading frame resulting in the loss of the domain. To estimate the prevalence of each type of modification, we classified each occurrence of alternatively spliced domains in our data set. For each locus that has a specific spliced-out domain, all the transcripts for the locus were first aligned with BLAT against the June 2002 release of the human genome draft sequence at UCSC (http://genome.ucsc.edu). Only transcripts that align to the draft genome without internal gaps and with at most 50 nt missing at either end were kept. The location of the domain was determined using the NCBI CDD search tool available at http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml for the transcripts having the domain. The location information was then used to extract the exons coding for the domain. Exon structures of the transcripts without the domain were compared with the exons coding the domain to classify the type of modifications.
RESULTS
Disproportionately distributed protein domains
In our study, all disproportionately distributed domains are domains that are disproportionately distributed in the direction of alternatively spliced genes. They are not necessarily the domains that are spliced in or out. Thus, these domains can be considered ‘innocent bystanders’ of the splicing process which affects other areas of the genes.
The July 26, 2002 release of LocusLink contains 47 310 human loci. 4548 of these have a ‘reviewed’ status. 932 of the reviewed loci have more than one RefSeq transcript associated. Thus, 20% of all the reviewed loci are annotated with alternative splicing. A total of 2501 unique CDD protein domains were annotated on all the human loci in LocusLink. From these, a total of 1675 were annotated on all the reviewed loci, or 67%.
Disproportionately distributed domains are summarized in Table 2. Twenty four domains were found to be disproportionately distributed and all of them showed higher frequency of occurrences in alternatively spliced loci. The proteins harboring these domains are engaged in diverse cellular processes such as adhesion and morphogenesis (23) (cadherin domains), growth and differentiation (24) (phosphatase domains), transcription regulation (25) (B-box zinc finger domain), apoptosis (26) (caspase domains) and intracellular Ca2+ signaling (SPRY domain).
Table 2. Protein domains disproportionately distributed among alternatively spliced genes.
| Domain name | Domain ID | InterPro ID | No. of constitutively spliced loci with this domain | No. of alternatively spliced loci with this domain | No. of constitutively spliced loci without this domain | No. of alternatively spliced loci without this domain | P-value |
|---|---|---|---|---|---|---|---|
| Cadherin domain | pfam00028 | IPR002126 | 50 | 50 | 3566 | 882 | 0 |
| Cadherin repeats | smart00112 | IPR002126 | 47 | 43 | 3569 | 889 | 0 |
| Protein tyrosine phosphatase, catalytic domain | smart00194 | IPR000242 | 18 | 21 | 3598 | 911 | 0.000004 |
| Protein tyrosine phosphatase, catalytic domain motif | smart00404 | IPR003595 | 20 | 21 | 3596 | 911 | 0.000011 |
| Protein-tyrosine phosphatase | pfam00102 | IPR000242 | 22 | 22 | 3594 | 910 | 0.000011 |
| B-box zinc finger | pfam00643 | IPR000315 | 12 | 16 | 3604 | 916 | 0.000021 |
| Caspase, interleukin-1 beta converting enzyme (ICE) homologs | smart00115 | IPR002398 | 2 | 9 | 3614 | 923 | 0.000023 |
| ICE-like protease (caspase) p10 domain | pfam00655 | IPR002138 | 2 | 9 | 3614 | 923 | 0.000023 |
| ICE-like protease (caspase) p20 domain | pfam00656 | IPR001309 | 2 | 9 | 3614 | 923 | 0.000023 |
| Domain in SPla and the RYanodine receptor | smart00449 | IPR003877 | 6 | 12 | 3610 | 920 | 0.000028 |
| Ankyrin repeats | smart00248 | IPR002110 | 5 | 11 | 3611 | 921 | 0.00004 |
| B-box-type zinc finger | smart00336 | IPR000315 | 12 | 15 | 3604 | 917 | 0.000061 |
| Dual specificity phosphatase, catalytic domain | smart00195 | IPR000340 | 12 | 15 | 3604 | 917 | 0.000061 |
| Fibronectin type III domain | smart00060 | IPR003961 | 16 | 17 | 3600 | 915 | 0.000071 |
| Ank repeat | pfam00023 | IPR002110 | 14 | 15 | 3602 | 917 | 0.000179 |
| Dual specificity phosphatase | pfam00782 | IPR000340 | 18 | 17 | 3598 | 915 | 0.000181 |
| Ring finger | smart00184 | IPR001841 | 16 | 16 | 3600 | 916 | 0.000183 |
| SPRY domain | pfam00622 | IPR003877 | 11 | 13 | 3605 | 919 | 0.000269 |
| Somatotropin hormone family | pfam00103 | IPR001400 | 0 | 5 | 3616 | 927 | 0.000358 |
| Zinc-binding domain seen in both chromatinic and cytoskeletal proteins | LOAD_zz | 0 | 5 | 3616 | 927 | 0.000358 | |
| Serine/threonine protein kinases, catalytic domain | smart00220 | IPR002290 | 46 | 28 | 3570 | 904 | 0.000406 |
| Tyrosine kinase, catalytic domain | smart00219 | IPR001245 | 46 | 28 | 3570 | 904 | 0.000406 |
| Double-stranded RNA binding motif | pfam00035 | IPR001159 | 1 | 6 | 3615 | 926 | 0.000422 |
| Zinc finger, C3HC4 type (ring finger) | pfam00097 | IPR001841 | 16 | 15 | 3600 | 917 | 0.000459 |
Process and function GO annotations for alternatively spliced genes with disproportionately distributed protein domains
To more accurately assess the roles played by alternatively spliced genes with disproportionately distributed protein domains, we extracted GO annotations on these genes and sought to identify common themes from both the perspective of GO terms and that of genes. The 24 domains identified are found on 169 alternatively spliced genes. For the biological process sub-ontology of GO, 108 genes were annotated with 75 unique GO terms (229 total).
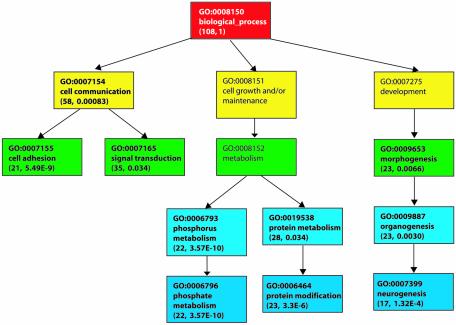
Figure 1 shows the top 10 significant terms in the biological process hierarchy with respect to gene coverage. Gene coverage allows the identification of processes that a majority of these genes participate in. The top three significant terms—cell communication, signal transduction and protein metabolism—account for 53, 32 and 25% of this set of genes, respectively. The 10 most significant terms ranked by P-value are shown in Table 3. All these processes require exquisite control of responses to extracellular or intracellular signals and it is our belief that alternative splicing is one of the regulatory mechanisms employed.
Figure 1.
Top 10 significant terms in the biological process hierarchy with respect to gene coverage for alternatively spliced genes having the disproportionately distributed domains. The terms are color coded according to their depth in the hierarchy and the numbers in parentheses after the name of each term are the number of genes covered in the sub-tree with the term as the root and the P-value. The terms in bold font are the top terms. The terms not in bold and with no numbers of genes and P-values are shown because they are on the path leading to the top significant terms.
Table 3. Ten most significant biological process GO terms for alternatively spliced genes with disproportionately distributed domains.
| GO term accession | GO term name | No. of genes annotated | Depth of term | Percent of gene occurrences | P-value |
|---|---|---|---|---|---|
| GO:0006470 | Protein dephosphorylation | 14 | 6 | 12 | 9.96E–11 |
| GO:0016311 | Dephosphorylation | 14 | 5 | 12 | 9.96E–11 |
| GO:0006796 | Phosphate metabolism | 22 | 4 | 20 | 3.57E–10 |
| GO:0006793 | Phosphorus metabolism | 22 | 3 | 20 | 3.57E–10 |
| GO:0007155 | Cell adhesion | 21 | 2 | 19 | 5.49E–09 |
| GO:0006464 | Protein modification | 23 | 4 | 21 | 3.33E–06 |
| GO:0007185 | Transmembrane receptor protein tyrosine phosphatase signaling pathway | 3 | 5 | 2 | 7.28E–05 |
| GO:0007399 | Neurogenesis | 17 | 4 | 15 | 1.32E–04 |
| GO:0007154 | Cell communication | 58 | 1 | 53 | 8.30E–04 |
| GO:0007089 | Mitotic start control point | 3 | 5 | 2 | 0.001327 |
Top terms with respect to term coverage were also identified. Term coverage allows the identification of processes that these genes frequently participate in without being biased by the number of genes annotated by each term. The top two terms—cell growth and/or maintenance and cell communication—account for 52 and 28% of all biological processes participated in by this set of genes, respectively.
Similar analyses of these genes were carried out for the molecular function sub-ontology. Out of 169 alternatively spliced genes, 121 were annotated with 69 unique GO terms (176 total). For term coverage, three first level terms—enzyme, binding and signal transducer—account for 50, 27 and 18% of all molecular functions carried out by this set of genes, respectively. For gene coverage, the top three significant terms—enzyme, hydrolase and protein kinase—account for 51, 30 and 20% of all the genes, respectively. These functions are critical for the processes identified above. The 10 most significant terms ranked by P-value are shown in Table 4. They include seven terms for phosphatase and one term each for serine/threonine kinase, cell adhesion molecule and caspase. Proteins with these functions are involved in signal transduction, development and apoptosis.
Table 4. Ten most significant molecular function GO terms for alternatively spliced genes with disproportionately distributed domains.
| GO term accession | GO term name | No. of genes annotated | Depth of term | Percent of gene occurrences | P-value |
|---|---|---|---|---|---|
| GO:0004721 | Protein phosphatase | 22 | 5 | 18 | 2.07E–16 |
| GO:0004725 | Protein tyrosine phosphatase | 19 | 6 | 15 | 1.40E–14 |
| GO:0016302 | Phosphatase | 22 | 2 | 18 | 1.92E–14 |
| GO:0016791 | Phosphoric monoester hydrolase | 22 | 4 | 18 | 2.75E–14 |
| GO:0005001 | Transmembrane receptor protein tyrosine phosphatase | 11 | 7 | 9 | 3.01E–11 |
| GO:0019198 | Transmembrane receptor protein phosphatase | 11 | 6 | 9 | 3.01E–11 |
| GO:0004674 | Protein serine/threonine kinase | 18 | 6 | 14 | 9.69E–10 |
| GO:0005194 | Cell adhesion molecule | 22 | 1 | 18 | 1.27E–09 |
| GO:0016788 | Hydrolase, acting on ester bonds | 22 | 3 | 18 | 1.84E–09 |
| GO:0004199 | Caspase | 7 | 6 | 5 | 2.83E–09 |
Spliced-out protein domains
Spliced-out domains, in contrast to disproportionately distributed domains, are those which not only occur in alternatively spliced genes, but are also the target of splicing in or out.
Out of 932 reviewed loci that have more than one associated RefSeq transcript, we identified 202 domains as spliced out. This represents 26% of a total of 773 domains. 139 loci (15%) were found to have one or more of these domains. Two interesting subsets are shown in Tables 5 and 6. Table 5 shows 14 domains that are present on four or more alternatively spliced loci. Table 6 shows 13 domains that are present on at least two loci and are always alternatively spliced. Fourteen of the 24 disproportionately distributed domains were also identified as spliced-out domains.
Table 5. Spliced-out domains that are present on at least four loci.
| Domain name | Domain ID | InterPro ID | No. of alternatively spliced loci where this domain is spliced out | No. of alternatively spliced loci where this domain is present | Percent of loci where this domain is spliced out |
|---|---|---|---|---|---|
| Domain in SPla and the RYanodine receptor | smart00449 | IPR003877 | 7 | 12 | 58 |
| SPRY domain | pfam00622 | IPR003877 | 7 | 13 | 54 |
| Intermediate filament proteins | pfam00038 | IPR001664 | 6 | 11 | 55 |
| ICE-like protease (caspase) p20 domain | pfam00656 | IPR001309 | 5 | 9 | 56 |
| Eukaryotic protein kinase domain | pfam00069 | IPR000719 | 5 | 42 | 12 |
| EGF-like domain. pfam00053 is very similar | pfam00008 | IPR006209 | 4 | 6 | 67 |
| Ezrin/radixin/moesin family | pfam00769 | IPR000798 | 4 | 9 | 44 |
| ICE-like protease (caspase) p10 domain | pfam00655 | IPR002138 | 4 | 9 | 44 |
| Immunoglobulin domain | pfam00047 | IPR003006 | 4 | 14 | 29 |
| Serine/threonine protein kinases, catalytic domain | S_TKc | IPR002290 | 4 | 15 | 27 |
| Tyrosine kinase, catalytic domain | TyrKc | 4 | 15 | 27 | |
| Dual specificity phosphatase | pfam00782 | IPR000340 | 4 | 17 | 24 |
| Fibronectin type III domain | pfam00041 | IPR003961 | 4 | 18 | 22 |
| Tyrosine kinase, catalytic domain | smart00219 | IPR001245 | 4 | 28 | 14 |
Table 6. Domains that are spliced out for all alternatively spliced genes in which they are found.
| Domain name | Domain ID | InterPro ID | No. of alternatively spliced loci where this domain is spliced out | No. of alternatively spliced loci where this domain is present | Percent of loci where this domain is spliced out |
|---|---|---|---|---|---|
| Calcium-binding EGF-like domain | EGF_CA | IPR001881 | 3 | 3 | 100 |
| Domain abundant in complement control proteins | CCP | IPR000436 | 2 | 2 | 100 |
| Epidermal growth factor-like domain | EGF | IPR006209 | 2 | 2 | 100 |
| Conserved domain seen in Groovin and Gas2 proteins | LOAD_gas2groo | 2 | 2 | 100 | |
| Growth-arrest-specific protein 2 domain | smart00243 | IPR003108 | 2 | 2 | 100 |
| Growth-arrest-specific protein 2 domain | pfam02187 | IPR003108 | 2 | 2 | 100 |
| Elongation factor Tu GTP binding domain. This domain contains a P-loop motif | pfam00009 | IPR000795 | 2 | 2 | 100 |
| von Willebrand factor (vWF) type C domain | smart00214 | IPR001007 | 2 | 2 | 100 |
| von Willebrand factor type C domain | pfam00093 | IPR001007 | 2 | 2 | 100 |
| Immunoglobulin C-type | smart00407 | IPR003597 | 2 | 2 | 100 |
| Neuregulin family | pfam02158 | IPR002154 | 2 | 2 | 100 |
| Zinc-binding domain present in Lin-11, Isl-1, Mec-3 | smart00132 | IPR001781 | 2 | 2 | 100 |
| Shikimate kinase | pfam01202 | IPR000623 | 2 | 2 | 100 |
GO term analyses were carried out for the 40 alternatively spliced genes with spliced-out domains shown in Table 5. These results are fundamentally the same as previous analyses on genes with disproportionately distributed domains. For the biological process sub-ontology, 28 genes were annotated with 40 unique GO terms (58 total). With respect to gene coverage, the top three significant terms—protein metabolism, development and organogenesis—account for 35, 35 and 28% of all the genes, respectively. Protein metabolism and organogenesis are also the top significant terms for genes with disproportionately distributed domains as shown in Figure 1.
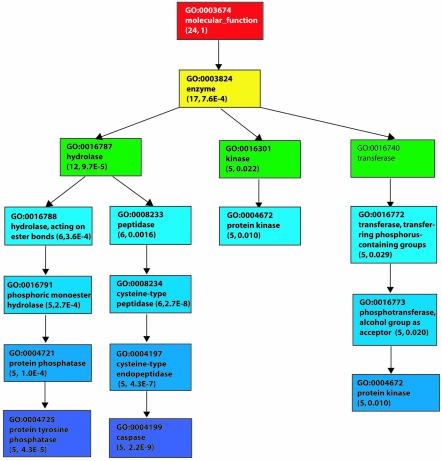
For the molecular function sub-ontology, 24 genes were annotated with 31 unique GO terms (38 total). Figure 2 shows the top 10 significant terms with respect to gene coverage for this set of alternatively spliced genes. The top three significant terms—enzyme, hydrolase and cysteine-type peptidase—account for 70, 50 and 25% of all the genes, respectively. Table 7 shows the 10 most significant molecular function GO terms ranked by P-value. They include terms that lead from hydrolase to signaling caspase and tyrosine phosphatase as shown in Figure 2. Proteins with these functions are involved in apoptosis and signal transduction.
Figure 2.
Top 10 significant terms in the molecular function hierarchy with respect to gene coverage for alternatively spliced genes having the 14 spliced-out domains that are present on four or more alternatively spliced loci. The terms are color coded according to their depth in the hierarchy and the number in parentheses after the name of each term is the number of genes covered in the sub-tree with the term as the root and the P-value. The terms in bold font are the top terms. The terms not in bold and with no numbers of genes and P-values are shown because they are on the path leading to the top significant terms. More than 10 terms are shown because of ties.
Table 7. Ten most significant molecular function GO terms for alternatively spliced genes with spliced-out domains shown in Table 5.
| GO term accession | GO term name | No. of genes annotated | Depth of term | Percent of gene occurrences | P-value |
|---|---|---|---|---|---|
| GO:0004199 | Caspase | 5 | 6 | 20 | 2.23E–09 |
| GO:0004200 | Signaling (initiator) caspase | 4 | 7 | 16 | 2.65E–08 |
| GO:0008234 | Cysteine-type peptidase | 6 | 4 | 25 | 2.68E–08 |
| GO:0004197 | Cysteine-type endopeptidase | 5 | 5 | 20 | 4.34E–07 |
| GO:0004725 | Protein tyrosine phosphatase | 5 | 6 | 20 | 4.34E–05 |
| GO:0016787 | Hydrolase | 12 | 2 | 50 | 9.66E–05 |
| GO:0004721 | Protein phosphatase | 5 | 5 | 20 | 1.04E–04 |
| GO:0016302 | Phosphatase | 5 | 2 | 20 | 2.51E–04 |
| GO:0016791 | Phosphoric monoester hydrolase | 5 | 4 | 20 | 2.69E–04 |
| GO:0016788 | Hydrolase, acting on ester bonds | 6 | 3 | 25 | 3.56E–04 |
Transcript level modifications to splice out protein domains
The straightforward way to splice out a protein domain would be to exclude the exons coding the domain. However, we found several examples of another transcript level modification to achieve this, i.e. inclusion or exclusion of other exons to shift the reading frame (15,17,18).
We performed a systematic analysis of the types of transcript level modification used to splice out domains. Somewhat surprisingly, out of a total of 314 instances of a domain being spliced out, only 225 instances (72%) were effected with the straightforward exclusion of one or more exons coding for the domain, while 89 instances (28%) were effected by retaining all the exons coding for the domain but shifting the reading frame to remove the original amino acid sequence of the domain from the final protein product.
DISCUSSION
One of the direct effects of alternative splicing is the production of different protein products. The current study identifies two types of protein domain that are of special interest. The first type includes protein domains disproportionately distributed among the alternatively or constitutively spliced genes (the ‘innocent bystanders’). The second includes spliced-out protein domains (the ‘victims’). The disproportionately distributed and spliced-out domains are associated with genes playing important roles in processes such as signal processing, development, differentiation and apoptosis. Alternative splicing may be an important mechanism to regulate the functions of these genes. It has long been hypothesized that alternative splicing plays a regulatory role in these processes. Our results provide objective evidence to support this hypothesis.
Although we understand the significance of some domains on our list of disproportionately distributed and spliced-out domains, the functional significance of all the domains is not well understood. For example, caspase catalytic domains, elongation factor Tu GTP binding domain and von Willebrand factor (vWF) type C domain play important roles in the modification of protein functions, especially across members of a protein family. Isoforms with or without these domains may be involved in the development of diseases such as leukemia, neuroblastoma and gastric carcinoma (14–18,27,28). The cadherin domain, a disproportionately distributed domain, is present on all members of the large protocadherin family, whose splice variants play important roles during nervous system development, and the exact functions of the variant cytoplasmic domains are active areas of research (29). Our results suggest that genes containing these domains are more likely than others to use splicing as a regulatory mechanism.
Somewhat surprisingly, a high percentage (28%) of the spliced-out domains were not effected by straightforward exclusion of exons coding the affected domain. Presumably, some exons coding the domains are under very high selection pressure to be conserved, so other exons either upstream or in the middle of these exons are used to modulate the exclusion of the domains in protein isoforms. As such, it is very important to supplement the traditional exon structure study of alternative splicing with studies from the perspective of protein domains. Otherwise, the changes to protein sequences as a result of reading frame shifts while retaining most of the exon structures will not be readily detected. This phenomenon demonstrates the importance of proteomics since genome and transcriptome studies may not readily detect such changes. It also presents an interesting challenge to gene prediction and eventually alternative splicing prediction algorithms as most algorithms use reading frame consistency as a guide to predict the exon structures.
The current study adopted a protein domain centric approach as opposed to a gene centric approach. We looked at the functional diversity of proteins grouped together with common patterns of alternative splicing via GO molecular function annotations. We found that some groups had similar and consistent annotations (e.g. proteins having a cadherin domain) and others that had diverse annotations (e.g. proteins having a B-box zinc finger domain or a Fibronectin type III domain). The significance of these findings is difficult to assess because function assignments are made to multi-domain genes.
A relatively small percentage of domains (1.4%) were found to be disproportionately distributed among the alternatively spliced and constitutively spliced loci. The number of occurrences of each domain among reviewed loci is very low. As more loci are reviewed, the number of occurrences of each domain will increase correspondingly and more domains could be found to be disproportionately distributed.
To ensure the quality of our analyses with limited data, our study focused on the set of reviewed loci in LocusLink. Reviewed status is given to a locus after a careful review by the NCBI staff. Such a focus probably improves the quality of the annotation but introduces potential biases in our findings. For example, the reviewers could prioritize their reviews based on the availability of publications, and researchers could be more interested in studying genes involved in signal transduction, development and apoptosis. Since the percentage of loci that have multiple RefSeq annotation among the provisional and predicted loci is low, the choice of reviewed loci should not result in any significant losses of domains from either the disproportionately distributed list or the spliced-out list.
The percentage (20%) of alternatively spliced loci among reviewed loci in LocusLink is much lower than those estimates (40–60%) produced by several studies of alternative splicing based on mRNA and/or EST data. Since ESTs are only partial fragments of the full-length transcripts, it is difficult to reliably infer the full-length transcripts from a collection of ESTs. As our results show, full-length transcripts are required before alternate protein sequences can reliably be determined. Methods that find and annotate alternative splicing and generate full-length transcripts are needed before all the disproportionately distributed and alternatively spliced domains can be identified. There are some promising recent developments in this area (5,30–37).
Given the important roles alternative splicing is playing, large scale studies of its functional consequences and regulatory mechanisms can provide insights into many important normal and abnormal processes such as differentiation and cancer development and provide us with a better understanding of why nature chooses to play the combinatorial game.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Drs Sam Karlin, Michael Walker, Robert Tibshirani and Luciano Brocchieri for comments and suggestions. R.A. and S.L. are supported by NIH GM61374. S.L. was a Stanford Graduate Fellowship James Clark Fellow and is also supported by NIH LM07033. We thank SUN, Inc. for a hardware grant.
REFERENCES
- 1.Gilbert W. (1978) Why genes in pieces? Nature, 271, 501. [DOI] [PubMed] [Google Scholar]
- 2.Brett D., Pospisil,H., Valcarcel,J., Reich,J. and Bork,P. (2002) Alternative splicing and genome complexity. Nature Genet., 30, 29–30. [DOI] [PubMed] [Google Scholar]
- 3.Sharp P.A. (1994) Split genes and RNA splicing. Cell, 77, 805–815. [DOI] [PubMed] [Google Scholar]
- 4.Modrek B. and Lee,C. (2002) A genomic view of alternative splicing. Nature Genet., 30, 13–19. [DOI] [PubMed] [Google Scholar]
- 5.Modrek B., Resch,A., Grasso,C. and Lee,C. (2001) Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res., 29, 2850–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kan Z., Rouchka,E.C., Gish,W.R. and States,D.J. (2001) Gene structure prediction and alternative splicing analysis using genomically aligned ESTs. Genome Res., 11, 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brett D., Hanke,J., Lehmann,G., Haase,S., Delbruck,S., Krueger,S., Reich,J. and Bork,P. (2000) EST comparison indicates 38% of human mRNAs contain possible alternative splice forms. FEBS Lett., 474, 83–86. [DOI] [PubMed] [Google Scholar]
- 8.Mironov A.A., Fickett,J.W. and Gelfand,M.S. (1999) Frequent alternative splicing of human genes. Genome Res., 9, 1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez A.J. (1998) Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu. Rev. Genet., 32, 279–305. [DOI] [PubMed] [Google Scholar]
- 10.Grabowski P.J. (1998) Splicing regulation in neurons: tinkering with cell-specific control. Cell, 92, 709–712. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Z.H. and Wu,J.Y. (1999) Alternative splicing and programmed cell death. Proc. Soc. Exp. Biol. Med., 220, 64–72. [DOI] [PubMed] [Google Scholar]
- 12.Wu J.Y., Tang,H. and Havlioglu,N. (2003) Alternative pre-mRNA splicing and regulation of programmed cell death. Prog. Mol. Subcell. Biol., 31, 153–185. [DOI] [PubMed] [Google Scholar]
- 13.Ho L., Guo,Y., Spielman,L., Petrescu,O., Haroutunian,V., Purohit,D., Czernik,A., Yemul,S., Aisen,P.S., Mohs,R. et al. (2001) Altered expression of a-type but not b-type synapsin isoform in the brain of patients at high risk for Alzheimer’s disease assessed by DNA microarray technique. Neurosci. Lett., 298, 191–194. [DOI] [PubMed] [Google Scholar]
- 14.Droin N., Bichat,F., Rebe,C., Wotawa,A., Sordet,O., Hammann,A., Bertrand,R. and Solary,E. (2001) Involvement of caspase-2 long isoform in Fas-mediated cell death of human leukemic cells. Blood, 97, 1835–1844. [DOI] [PubMed] [Google Scholar]
- 15.Droin N., Beauchemin,M., Solary,E. and Bertrand,R. (2000) Identification of a caspase-2 isoform that behaves as an endogenous inhibitor of the caspase cascade. Cancer Res., 60, 7039–7047. [PubMed] [Google Scholar]
- 16.Eckhart L., Henry,M., Santos-Beneit,A.M., Schmitz,I., Krueger,A., Fischer,H., Bach,J., Ban,J., Kirchhoff,S., Krammer,P.H. et al. (2001) Alternative splicing of caspase-8 mRNA during differentiation of human leukocytes. Biochem. Biophys. Res. Commun., 289, 777–781. [DOI] [PubMed] [Google Scholar]
- 17.Horiuchi T., Himeji,D., Tsukamoto,H., Harashima,S., Hashimura,C. and Hayashi,K. (2000) Dominant expression of a novel splice variant of caspase-8 in human peripheral blood lymphocytes. Biochem. Biophys. Res. Commun., 272, 877–881. [DOI] [PubMed] [Google Scholar]
- 18.Waltereit R. and Weller,M. (2002) The role of caspases 9 and 9-short (9S) in death ligand- and drug-induced apoptosis in human astrocytoma cells. Brain Res. Mol. Brain Res., 106, 42. [DOI] [PubMed] [Google Scholar]
- 19.Ando S., Sarlis,N.J., Krishnan,J., Feng,X., Refetoff,S., Zhang,M.Q., Oldfield,E.H. and Yen,P.M. (2001) Aberrant alternative splicing of thyroid hormone receptor in a TSH-secreting pituitary tumor is a mechanism for hormone resistance. Mol. Endocrinol., 15, 1529–1538. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Z.H., Zhang,W.J., Rao,Y. and Wu,J.Y. (1998) Regulation of Ich-1 pre-mRNA alternative splicing and apoptosis by mammalian splicing factors. Proc. Natl Acad. Sci. USA, 95, 9155–9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loraine A.E., Helt,G.A., Cline,M.S. and Siani-Rose,M.A. (2002) Protein based analysis of alternative splicing in the human genome. IEEE Computer Society Bioinformatics Conference. IEEE Computer Society, pp. 118–124. [PubMed] [Google Scholar]
- 22.Benjamini Y. and Hochberg,Y. (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Statist. Soc. B, 57, 289–300. [Google Scholar]
- 23.Takeichi M. (1990) Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem., 59, 237–252. [DOI] [PubMed] [Google Scholar]
- 24.Fischer E.H., Charbonneau,H. and Tonks,N.K. (1991) Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science, 253, 401–406. [DOI] [PubMed] [Google Scholar]
- 25.Borden K.L. (1998) RING fingers and B-boxes: zinc-binding protein–protein interaction domains. Biochem. Cell Biol., 76, 351–358. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson D.W. and Thornberry,N.A. (1997) Caspases: killer proteases. Trends Biochem. Sci., 22, 299–306. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S., Sugimachi,K., Saeki,H., Kinoshita,J., Ohga,T., Shimada,M. and Maehara,Y. (2001) A novel variant of WISP1 lacking a Von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene, 20, 5525–5532. [DOI] [PubMed] [Google Scholar]
- 28.Schurmann A., Brauers,A., Massmann,S., Becker,W. and Joost,H.G. (1995) Cloning of a novel family of mammalian GTP-binding proteins (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. J. Biol. Chem., 270, 28982–28988. [DOI] [PubMed] [Google Scholar]
- 29.Frank M. and Kemler,R. (2002) Protocadherins. Curr. Opin. Cell Biol., 14, 557–562. [DOI] [PubMed] [Google Scholar]
- 30.Fairbrother W.G., Yeh,R.F., Sharp,P.A. and Burge,C.B. (2002) Predictive identification of exonic splicing enhancers in human genes. Science, 297, 1007–1013. [DOI] [PubMed] [Google Scholar]
- 31.Lee C., Atanelov,L., Modrek,B. and Xing,Y. (2003) ASAP: the Alternative Splicing Annotation Project. Nucleic Acids Res., 31, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Q., Modrek,B. and Lee,C. (2002) Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res., 30, 3754–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spingola M., Grate,L., Haussler,D. and Ares,M.,Jr. (1999) Genome-wide bioinformatic and molecular analysis of introns in Saccharomyces cerevisiae. RNA, 5, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamm S., Zhu,J., Nakai,K., Stoilov,P., Stoss,O. and Zhang,M.Q. (2000) An alternative-exon database and its statistical analysis. DNA Cell Biol., 19, 739–756. [DOI] [PubMed] [Google Scholar]
- 35.Davuluri R.V., Grosse,I. and Zhang,M.Q. (2001) Computational identification of promoters and first exons in the human genome. Nature Genet., 29, 412–417. [DOI] [PubMed] [Google Scholar]
- 36.Clark T.A., Sugnet,C.W. and Ares,M., Jr. (2002) Genomewide analysis of mRNA processing in yeast using splicing-specific microarrays. Science, 296, 907–910. [DOI] [PubMed] [Google Scholar]
- 37.Lim L.P. and Burge,C.B. (2001) A computational analysis of sequence features involved in recognition of short introns. Proc. Natl Acad. Sci. USA, 98, 11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]