Abstract
When iron repletes, Schizosaccharomyces pombe cells repress transcription of genes encoding components involved in the reductive iron transport system. Fep1 mediates this transcriptional control by interacting specifically with GATA-type cis-acting elements. To further investigate the role that Fep1 plays in iron homeostasis, we searched for additional Fep1-regulated genes. We found that str1+ is subject to negative transcriptional regulation, which is exerted through binding of Fep1 to a single GATA element in the str1+ promoter. Introduction of str1+ into a Saccharomyces cerevisiae fet3Δ arn1-4Δ strain led to assimilation of iron from ferrichrome, revealing that Str1 functions as a siderophore-iron transporter in S.pombe. We also identified two additional target genes of Fep1, named str2+ and str3+. We demonstrate that the str1+, str2+ and str3+ genes share a common promoter element, 5′-(A/T)GATAA-3′. We found that the N-terminal 241 residue segment of Fep1 expressed in Escherichia coli specifically interacts with the 5′-(A/T)GATAA-3′ element present in each of these promoters. Consistent with this, constitutive high level str1+, str2+ and str3+ gene expression was observed in a fep1Δ mutant strain. Taken together, these results demonstrate that Fep1 occupies a central role in coordinating transcriptional regulation of genes encoding components of the reductive and non-reductive iron transport systems in fission yeast.
INTRODUCTION
The mechanisms by which organisms accumulate iron to maintain intracellular iron homeostasis is a critical aspect of cellular growth control (1). Although iron has been established as an essential redox cofactor for numerous enzymes, excess iron can be deleterious to cells by forming oxygen radicals after reaction with hydrogen peroxide (2). Consequently, iron acquisition and distribution in organisms must be highly regulated to prevent both starvation and toxicity.
Although iron is abundant in the environment, it is rarely present in a soluble form. In an oxidizing environment like air, iron is essentially insoluble as ferric hydroxides. To satisfy iron needs, organisms have elaborated a number of mechanisms of iron assimilation in cells, including enzymatic reduction of ferric iron at the cell surface, heme-iron transport across the cell wall and production of siderophores, which are small organic molecules with a high affinity for iron (3–6). Once secreted, these iron-specific chelators can be recognized and imported into the cell as siderophore-iron complexes, rendering iron bioavailable. In fungal species that produce siderophores, including Ustilago maydis, Neurospora crassa and Aspergillus nidulans, studies have shown that when cells are grown under conditions of iron repletion, negative transcriptional regulators turn off expression of siderophore biosynthesis genes (7). The U.maydis URBS1 (8), N.crassa SRE (9) and A.nidulans SREA (10) gene products are among these repressors.
Although nearly all examined aerobic bacteria and fungi make siderophores, both Saccharomyces cerevisiae and Schizosaccharomyces pombe lack this ability (11). However, both species of yeast can assimilate siderophore-bound iron when secreted into the environment by other microorgan isms (12,13). Under low environmental iron conditions, S.cerevisiae cells can accumulate siderophore-bound iron in their cell wall through anchored mannoproteins, named Fit1, Fit2 and Fit3 (14). Saccharomyces cerevisiae employs two strategies to take up iron from siderophores trapped in the cell wall (3). One pathway requires the action of plasma membrane-associated Cu2+/Fe3+ reductases of the FRE family (15). Fre reductases facilitate release of the iron from the siderophores prior to uptake by the Ftr1-Fet3 permease-oxidase complex (16). Fet4, which is a low affinity iron transporter, can also serve in Fe2+ uptake (17). A second pathway, which is independent of reduction, involves four genes whose products are involved in uptake of siderophore-bound iron. These genes are termed ARN1, ARN2 (TAF1), ARN3 (SIT1) and ARN4 (ENB1) (13,18–22). The siderophore specificities of all four transmembrane proteins are different. Arn1, Arn2 and Arn3 proteins transport hydroxamate-type siderophores. Of these three siderophore transporters, Arn1 displays high specificity for ferrichrome-iron, whereas ferroxiamine B-iron is taken up preferentially through Arn3 protein (4,23). Triacetylfusarinine uptake is facilitated by Arn2 and to a lesser extent by Arn1 and Arn3 proteins (3,4). As opposed to Arn1, Arn2 and Arn3, Arn4 exhibits no uptake activity for hydroxamate-type siderophores. Instead, it appears to facilitate assimilation of enterobactin, which is a siderophore of the catecholate class (3,4). Like FRE1-6, FIT1-3, FET3 and FTR1, the ARN1–ARN4 genes are transcriptionally regulated as a function of iron availability: repressed when cells are grown in the presence of iron and highly activated under iron deprivation conditions (13). This regulation requires the function of the S.cerevisiae transcription factor Aft1 (24). Aft1 binds to the promoters of these genes in the absence of iron by interacting with the 5′-(T/C)(G/A) CACCC(A/G)-3′ element and then trans-activates target gene expression (25).
In S.pombe, three components involved in the reductive iron uptake system have been identified (26,27). Frp1, which shares amino acid sequence similarity with the Fre1 reductase from S.cerevisiae, is required at the cell surface of the fission yeast to reduce Fe3+ (26). Following reduction, ferrous ions are taken up by a permease-oxidase complex, called Fip1-Fio1 (27), and transported into the cell. Iron assimilation in S.pombe is tightly regulated in response to the availability of iron in the growth medium (26,27). frp1+, fip1+ and fio1+ mRNA levels are induced by iron starvation. Conversely, when cells are grown under elevated iron concentrations, the iron-sensing transcription factor Fep1 represses iron uptake genes (28). Fep1 is a member of the GATA factor family that binds to DNA sequences containing 5′-(A/T)GATAA-3′. The N-terminal region of Fep1 has been shown to be required for DNA binding (28). Yeast cells in which fep1+ has been insertionally inactivated display elevated levels of expression of the iron transporter gene fio1+. Furthermore, fep1Δ mutant cells exhibit increased activity of the cell surface metalloreductase(s). Moreover, the fep1Δ disruption strain is hypersensitive to phleomycin, an antibiotic that cleaves nucleic acids in the presence of excess iron. Consistent with a role for Fep1 in repressing the expression of the reductive iron transport genes, two genes, tup11+ and tup12+, known to encode general transcriptional co-repressors, have been identified to play an important role with Fep1 in down-regulation of fip1+ and fio1+ genes (28).
In this study, we have identified a gene (SPBC4F6.09+) that has a consensus Fep1 binding site within its promoter. Expression of SPBC4F6.09+ in S.cerevisiae fet3Δ, arn1-4Δ cells revealed that the protein can mediate ferrichrome-bound iron transport. We named this S.pombe gene str1+ (for siderophore transporter 1). Analysis of regions in the promoter of the str1+ gene demonstrated that a GATA-type regulatory element, 5′-–870AGATAA–865-3′, is necessary for transcriptional repression in response to iron repletion conditions. Furthermore, we found that the N-terminal 241 residue segment of Fep1 expressed in Escherichia coli specifically interacts with the 5′-–870AGATAA–865-3′ element. Based on the similarity of the Str1 protein to two other uncharacterized proteins from S.pombe, we also identified the str2+ and str3+ genes. Experiments revealed that iron-mediated transcriptional repression of the str1+, str2+ and str3+ genes in S.pombe required a functional fep1+ gene. Taken together, these results show that Fep1 is involved in an additional layer of control of iron acquisition in regulating siderophore-bound iron transport gene expression in fission yeast.
MATERIALS AND METHODS
Yeast strains and growth conditions
The S.pombe strains used in this study were the wild-type FY435 (h+ his7-366 leu1-32 ura4-Δ18 ade6-M210) and a fep1Δ disruption strain (h+ his7-366 leu1-32 ura4-Δ18 ade6-M210 fep1Δ::ura4+) (28). Under non-selective conditions, S.pombe cells were grown in YES medium plus supplements (225 mg/l adenine, histidine, leucine, uracil and lysine), unless otherwise stated. When plasmid maintenance was required, fission yeast cells were grown in Edinburgh minimal medium3 (EMM3) as described previously (28), except that the medium contained only 74 nM FeCl3. Iron repletion or iron deprivation was carried out by adding the indicated amount of FeCl3 or bathophenanthroline disulfonic acid (BPS) to cells grown to mid-logarithmic phase (A600 nm ≈ 1.0) as described previously (28). For ectopic expression of the str1+ gene in S.cerevisiae, the fet3Δ arn1-4Δ strain [MATa ura3-52 lys2-801 (amber) ade2-101 (ochre) trp1-63Δ his3-200Δ leu2-1Δ fet3Δ::HIS3 arn1Δ::HISG arn3Δ::HISG arn4Δ::HISG-URA3-HISG arn2Δ::HISG-URA3-HISG] (29,30) was used to ensure the presence of Str1 as the sole protein with the ability of transporting siderophores. As a control, the wild-type strain YPH499 [MATa ura3-52 lys2-801 (amber) ade2-101 (ochre) trp1-63Δ his3-200Δ leu2-1Δ] was used. Saccharomyces cerevisiae cells were grown either in YPD medium (1% yeast extract, 2% bactopeptone and 2% dextrose) or under conditions of moderate iron starvation using a modified SD minimal medium containing 0.67% yeast nitrogen base minus copper and iron (Q-BIOgene, Carlsbad, CA), 2% dextrose and 50 mM MES buffer (pH 6.1) (31). This latter SD minimal medium was prepared with necessary auxotrophic requirements and made iron-limited by the addition of 30 µM BPS or iron-replete by addition of 50 µM FeCl3. For plate assays in the presence of siderophore-iron complexes, the siderophores ferrichrome and ferroxiamine B (both from Sigma, St Louis, MO) were prepared in the presence of FeCl3 and then assayed with bakers’ yeast cells expressing either the S.pombe str1+ or S.cerevisiae ARN1 gene.
Plasmids
The plasmid pSP1str1+-966lacZ harbors the str1+ promoter region up to –966 from the initiator codon of the str1+ gene in addition to the E.coli lacZ gene. This latter plasmid was constructed via three-piece ligation by simultaneously introducing the EcoRI–StuI fragment of YEp357R and the BamHI–EcoRI fragment from the str1+ promoter containing 966 bp of the 5′-non-coding region and the first 10 codons of the str1+ gene into BamHI + SmaI digested pSP1 vector (32). Five plasmids (pSP1str1+-846lacZ, pSP1str1+-747lacZ, pSP1str1+-602lacZ, pSP1str1+-455lacZ and pSP1str1+-296lacZ) harboring sequential deletions from the 5′ end of the str1+ promoter were created from plasmid pSP1str1+-966lacZ using the exonuclease III/mung bean nuclease method as described previously (28). As a control for iron-regulated gene expression, the plasmid pSP1fio1+-884lacZ was used as described previously (28). To create both the wild-type and mutant str1+-CYC1-lacZ fusions, a DNA fragment (positions –966 to –846) encompassing the 5′-–870AGATAA–865-3′ element from the S.pombe str1+ regulatory region was amplified by PCR to obtain both wild-type and GATA mutant sequences. Both types of fragments were inserted into pCF83 (32) using the SmaI and XhoI sites. To generate the p415GPDstr1+ and p415GPDARN1 plasmids, the str1+ and ARN1 genes were isolated by PCR from S.pombe FY435 and S.cerevisiae YPH499 genomic DNA, respectively. The primers were made corresponding to the start and stop codons of the str1+ and ARN1 genes. A plasmid, which contained either the str1+ or ARN1 gene in the proper orientation, was transformed into the S.cerevisiae fet3Δ arn1-4Δ strain for ectopic expression in the presence of selected siderophores.
RNA analysis methods
For northern blot analyses, the str1+, fio1+ and fip1+ genes were isolated by PCR using primers that corresponded to the start and stop codons for each of these three ORFs. PCR products were purified using the GFX gel band purification kit (Amersham, Piscataway, NJ). Subsequently, 32P-labeled probes were made from the isolated DNA fragments using the Random primed labeling kit (Roche, Laval, Canada) and purified using the Quick spin probe purification column system (Roche). Hybridizations were carried out according to the Schleicher & Schuell (Keene, NH) protocol. The S.pombe act1+ probe (32) was used as an internal control. For RNase protection analyses, three plasmids were constructed for making antisense RNA probes. Plasmid pSKstr1+ was created by inserting a 202 bp BamHI–EcoRI fragment of the str1+ gene into the same sites of pBluescript SK. The antisense RNA hybridizes to the first nucleotide of the initiator codon of str1+ down to +202. To generate pSKstr2+, a 179 bp fragment of the str2+ gene was amplified from S.pombe (FY435) genomic DNA and cloned into the BamHI and EcoRI sites of pBluescript SK. The resulting antisense RNA probe hybridizes to the first nucleotide of the initiator codon of str2+ down to +179. To create the pSKstr3+ plasmid, a 188 bp DNA fragment which hybridizes to the S.pombe str3+ gene positions +1 to +188 was amplified and cloned into the pBluescript SK vector. Plasmids pKSlacZ and pSKact1+ were described previously (32).
Binding assays
For binding analyses, the maltose binding protein (MBP)-Fep1 fusion protein was purified from E.coli cells as described previously (28). Electrophoretic mobility shift assays were conducted as described previously (28), except that 32P-end-labeled DNA oligonucleotides encompassed the str1+ promoter 5′-AGATAA-3′ element located between –870 and –865 relative to the first nucleotide of the translational start codon of the str1+ ORF. Binding assays were also performed using 32P-end-labeled DNA oligonucleotides that correspond to the str2+ (positions –92 to –42) and str3+ (positions –663 to –608) upstream regions within which lie a consensus 5′-(A/T)GATAA-3′ element. When indicated, competitors to concentrations specified in Figures 4 and 7 were added together with the probe. For DNase I footprinting assays, purified recombinant Fep1 protein (∼280 ng) was incubated for 20 min at 25°C with ∼1 ng of str1+ promoter fragment 5′-end-labeled either at position –980 (upper strand) or at position –757 (lower strand) relative to the start point of translation. Reactions were carried out using 1× binding buffer that contained 12.5 mM HEPES (pH 7.9), 75 mM NaCl, 4 mM MgCl2, 1 mM EDTA, 10% glycerol, 4 mM Tris–HCl (pH 7.9), 0.6 mM dithiothreitol, 1 µg poly(dI·dC)2, 5 µM ZnSO4 and 5 µM FeCl3, unless stated otherwise. DNase I freshly diluted in 2.5 mM MgCl2, 25 mM Tris–HCl (pH 7.9), 10% glycerol and 100 µg/ml acetylated BSA was added to the binding reaction and incubated at 25°C for 2 min. Reactions were stopped with 150 µl of 200 mM NaCl, 20 mM EDTA, 1% SDS, 250 µg/ml yeast tRNA and 150 µg/ml proteinase K (Roche). Samples were incubated for 60 min at 50°C, extracted with an equal volume of phenol/chloroform and ethanol precipitated. DNA pellets were dried, resuspended in 80% formamide, heated at 95°C for 5 min and loaded on a 6% polyacrylamide, 8.3 M urea sequencing gel. Gels were fixed, dried and exposed to a Molecular Dynamics screen.
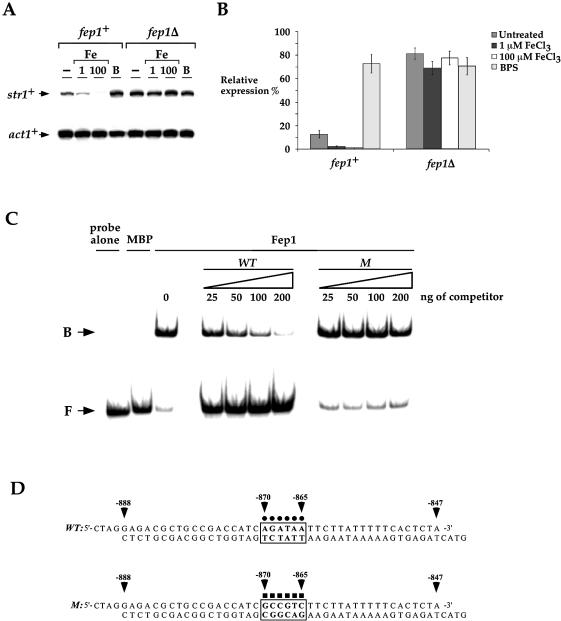
Figure 4.
Down-regulation of str1+ in response to iron is controlled at the transcriptional level by the GATA factor Fep1. (A) The wild-type (fep1+) and isogenic fep1Δ strains were grown to mid-logarithmic phase in YES medium and subjected to control (–) or 1 and 100 µM FeCl3 (Fe) or 100 µM BPS (B) treatment at 30°C for 90 min. str1+ and act1+ (as a control) mRNA steady-state levels were analyzed by RNase protection assay. (B) Graphic representation of quantitation of three independent RNase protection assays, including the experiment shown in (A). Values are the averages of triplicate determinations ± SD. (C) Electrophoretic mobility shift analysis was carried out using chromatographic fractions prepared from E.coli cells expressing either the plasmid alone (MBP) or the MBP-fep1+ fusion allele (Fep1). Affinity purified Fep1 was incubated with 32P-labeled DNA fragment corresponding to the wild-type 5′-AGATAA-3′ element derived from the str1+ promoter. The amount of competitor used in each reaction is indicated over the lanes. The probe concentration was ∼1 ng/reaction. B, bound probe DNA; F, free probe DNA. (D) Sequences of the synthetic oligomers used. The box marked with dots indicates that the GATA element is wild type (WT), whereas the box marked with squares indicates that the GATA sequence contains six substitutions (M). The nucleotide numbers refer to the position relative to the A of the initiator codon of the str1+ ORF.
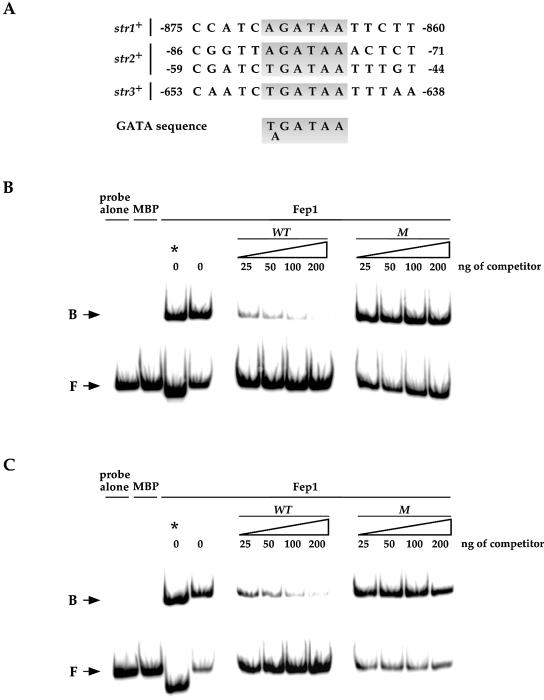
Figure 7.
Fep1 interaction with GATA-type binding sites in the str2+ and str3+ promoters. (A) Like str1+, potential Fep1 consensus binding sites are present within the predicted regulatory regions of the str2+ and str3+ genes. Shaded areas indicate a GATA-like element. The nucleotide numbers refer to the position relative to the A of the initiator codon of the indicated ORF. Electrophoretic mobility shift assays were performed with probes derived from the str2+ (B) and str3+ (C) promoter sequences indicated in (A). Binding reactions were conducted using affinity purified Fep1 prepared from E.coli cells expressing either the plasmid alone (MBP) or the MBP-fep1+ fusion allele (Fep1). Bound (B) and free (F) species are indicated by arrows. Competitors harboring wild-type (WT) and mutant (M) GATA sequences derived from the str2+ or str3+ promoter were assayed. The amount of competitor used in each reaction is shown over the lanes. The probe concentration was ∼1 ng/reaction. The asterisk indicates one electrophoretic mobility shift reaction using the wild-type probe derived from the str1+ promoter shown in Figure 4 and reproduced here as a control.
RESULTS
Str1 is a S.pombe siderophore-iron transporter
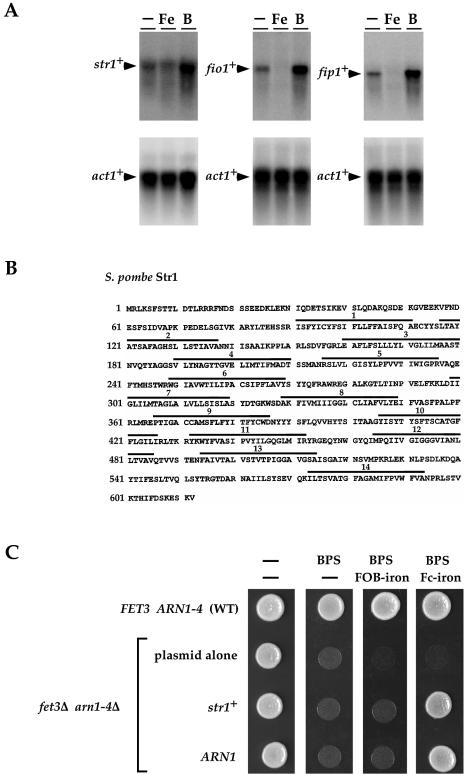
Using a promoter motif analysis program (33), Fep1 binding sites were identified in the non-coding sequences located upstream of genes of the S.pombe genome. One locus, SPBC4F6.09, which we termed str1+, was selected for further analysis because of its similarity to the ARN1- to ARN4-encoded siderophore-iron transporters from S.cerevisiae. To determine whether a correlation exists between the presence of a potential iron-responsive element within the str1+ locus and str1+ mRNA expression, we measured str1+ mRNA levels when cells were treated with iron and the iron chelator BPS. As shown in Figure 1A, the expression of str1+ mRNA was indeed iron regulated, exhibiting repression upon addition of iron and induction in the presence of BPS. As expected, fio1+ and fip1+ mRNA levels (assayed as a control) were down- and up-regulated after treatment with iron and BPS, respectively (Fig. 1A). The str1+ ORF encodes a polypeptide of 612 amino acids with a predicted molecular mass of 68.2 kDa (Fig. 1B). As mentioned above, analysis of the str1+-encoded protein revealed that the sequence and topology of this S.pombe protein harbors a significant similarity to four previously identified siderophore transporters: the Arn1, Arn2, Arn3 and Arn4 proteins from S.cerevisiae (13). The overall sequence homology with the S.cerevisiae Arn1 (31.2%), Arn2 (30.4%), Arn3 (29.3%) and Arn4 (28.6%) proteins was noteworthy, especially within the putative transmembrane domains. Like the Arn1–Arn4 proteins, the str1+-encoded protein contains 14 transmembrane regions according to TopPred II (34) and both the N- and C-termini are predicted to be inside. Consistent with Str1 functioning as a putative siderophore-iron transporter in S.pombe, when expressed in S.cerevisiae cells defective in the uptake of siderophore iron, the str1+-encoded protein restored growth in the presence of the hydroxamate-type siderophore ferrichrome-iron (Fig. 1C). Growth rescue appeared to be highly ferrichrome-iron specific because utilization of media supplemented with ferroxiamine B-iron (Fig. 1C) or rhodotorulic acid-iron (data not shown) resulted in no detectable growth under iron-limited conditions. As observed for Str1, defective growth on medium containing the iron chelator BPS can be corrected by returning a wild-type copy of the S.cerevisiae ARN1 gene expressed from a centromeric plasmid (Fig. 1C). Interestingly, expression of the str1+ gene fostered remediation of growth with an efficacy similar to that found with the ARN1 gene (Fig. 1C and data not shown). Taken together, the results presented here are consistent with Str1 functioning as a ferrichrome-iron transport protein in S.pombe.
Figure 1.
Identification of a S.pombe ferrichrome transporter. (A) A RNA blot of str1+, fio1+ and fip1+ mRNA steady-state levels. (Bottom) act1+ mRNA as a control. Total RNA was isolated from cells that were incubated in the absence (–) or presence of 1 µM FeCl3 (Fe) or presence of 100 µM BPS (B). (B) The Str1 amino acid sequence depicted in single letter code. The bars marked with numbers refer to putative transmembrane domains. (C) Wild-type (WT) strain YPH499 transformed with p415GPD alone was used as a control. A fet3Δ, arn1-4Δ mutant strain, transformed with plasmid p415GPD alone (plasmid alone), plasmid p415GPDstr1+ (str1+) or plasmid p415GPDARN1 (ARN1) was spotted onto a modified minimal medium, depleted of iron by the addition of BPS (30 µM) and containing no siderophore (–), ferroxiamine B (FOB)-iron (1 µM) or ferrichrome (Fc)-iron (1 µM). Plates were photographed after 3 days incubation at 30°C.
str1+ gene expression is negatively regulated by iron through a conserved GATA-type element
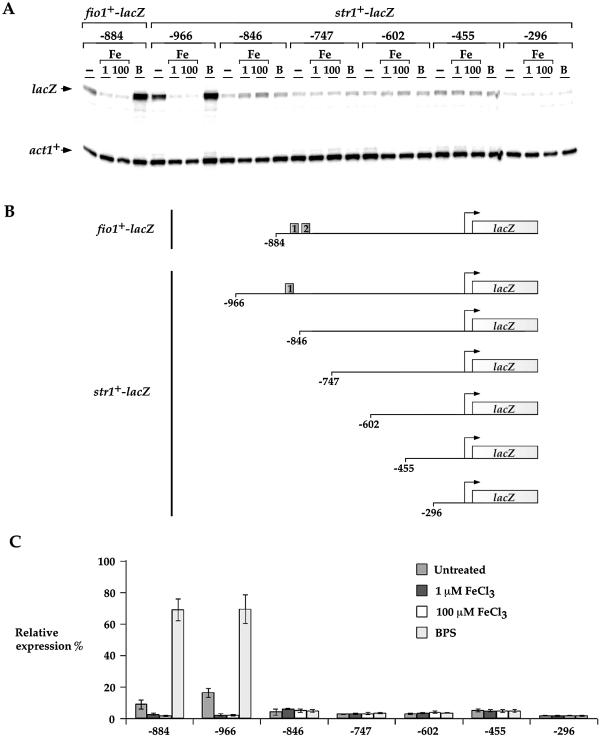
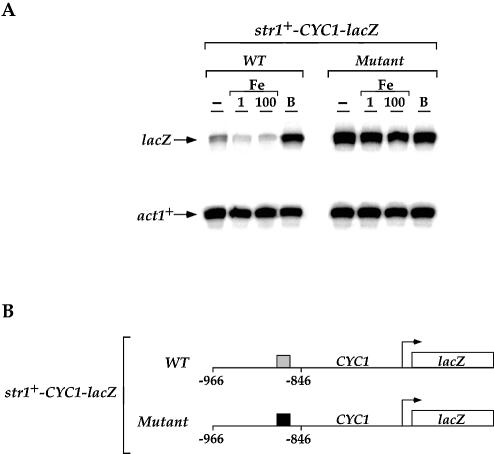
To examine whether this iron-dependent regulation of str1+ gene expression required the cis-acting element 5′-(T/A)GA TAA-3′ identified for appropriate regulation of fio1+ gene expression (28), we fused 966 bp of the 5′-non-coding region and the first 10 codons of str1+ in-frame with the E.coli lacZ gene. The str1+-966lacZ fusion expression was repressed in the presence of iron (∼7-fold) and induced in the presence of BPS (∼4-fold) (Fig. 2). Removal of the str1+ upstream region between –966 and –846 abolished the iron-dependent regulation of the str1+-lacZ fusion. Similarly, further deletions to positions –747, –602 and –455 gave low levels of gene expression with complete failure to respond to the presence of iron. When the str1+ promoter was further deleted to position –296, the overall magnitude further decreased as compared with the other str1+-lacZ promoter derivatives, indicating the presence of a potential activating promoter element between –455 and –296. Due to the observation that the integrity of the region between –966 and –846 was necessary for fostering iron repression and iron starvation-induced activation of the str1+-lacZ fusion, we examined whether this sequence could regulate a heterologous reporter gene in an iron-dependent manner. A short DNA segment derived from the str1+ promoter (positions –966 to –846) was inserted in its natural orientation into the minimal promoter of the iso-1-cytochrome c (CYC1) gene fused to lacZ in pCF83. As shown in Figure 3, this fusion promoter was able to repress (∼2- to 3-fold) lacZ mRNA expression in the presence of iron. Conversely, under iron deprivation conditions, lacZ mRNA expression was strongly derepressed (∼12-fold) as compared with the level of transcript detected from a control culture (Fig. 3A, untreated). Within this 120 bp str1+ promoter DNA fragment, we noted the presence of a sequence, 5′-AGATAA-3′ (positions –870 to –865), that is similar to the binding site for the fission yeast iron-sensing transcription factor Fep1 (28). To ascertain whether this element plays a role in str1+ regulation by iron, all six of these nucleotides were substituted and the cells carrying the str1+-CYC1-lacZ mutant fusion plasmid were assayed for iron-regulated expression of lacZ mRNA (Fig. 3). Multiple point mutations in the 5′-–870AGATAA–865-3′ element gave rise to a high level of str1+-CYC1-lacZ fusion gene expression without any change in response to the presence of a wide range of iron concentrations (Fig. 3 and data not shown). The str1+-CYC1-lacZ mutant fusion gene was highly derepressed by ∼18- to 20-fold as compared with the basal level of str1+-CYC1-lacZ fusion transcript detected with the wild-type promoter segment. Interestingly, this result suggests that a putative region at the 5′ end of the 5′-–870AGATAA–865-3′ element is required for high levels of str1+ gene expression in response to iron deprivation. Taken together, these data suggest that, like the fio1+ gene which encodes a component of the reductive iron transport machinery in fission yeast, str1+ is down-regulated at the transcriptional level in response to iron repletion by the promoter GATA-like sequence 5′-–870AGATAA–865-3′.
Figure 2.
Mapping of the str1+ promoter region necessary for iron-dependent repression. (A) lacZ fusion genes containing wild-type DNA fragments derived from the fio1+ (as a control) (28) and str1+ promoters were analyzed by RNase protection assay. Total RNA was extracted from aliquots of cultures incubated in the absence (–) or presence of 1 and 100 µM FeCl3 (Fe) or 100 µM BPS (B) for 90 min at 30°C. The lacZ and act1+ mRNA steady-state levels are indicated by arrows. (B) A schematic representation of nested 5′ deletions of str1+ promoter sequences. The boxes shown in the fio1+ and str1+ promoter regions indicate the location of the 5′-(A/T)GATAA-3′ elements. The nucleotide numbers refer to the position relative to the A of the initiator codon of the fio1+ and str1+ ORFs. (C) Graphic representation of quantitation of three independent RNase protection assays, including the experiment shown in (A). The values are the means of three replicates ± SD.
Figure 3.
The str1+ promoter GATA element confers iron responsiveness to the minimal promoter CYC1-lacZ. (A) CYC1-lacZ fusion genes harboring a wild-type (WT) DNA fragment and a GATA mutant (Mutant) derived from the str1+ promoter were assayed. The lacZ and act1+ mRNA levels are indicated by arrows. Results shown are representative of three independent experiments. (B) The gray box represents the wild-type 5′-AGATAA-3′ element and the filled box indicates the mutant element containing multiple point substitutions (5′-GCCGTC-3′ instead of 5′-AGATAA-3′). The nucleotide numbers refer to the position relative to the A of the ATG codon of the str1+ ORF.
Fep1 is required for iron-mediated repression of str1+ gene expression
Because the cis-acting element 5′-–870AGATAA–865-3′ conferred iron responsiveness to the str1+ promoter fused to lacZ, we tested whether str1+ gene regulation by iron is dependent on the iron sensor Fep1 (28). The S.pombe fep1+ gene encodes a transcription factor that exhibits a high level of homology to four previously identified iron transcriptional repressors of siderophore biosynthesis in other fungi (9,10,35,36). Our previous studies in S.pombe revealed that fep1Δ cells accumulate iron in excess of the physiological requirement, thereby implicating Fep1 in iron metabolism (28). Using isogenic strains harboring a wild-type fep1+ gene and an insertionally inactivated fep1 allele (fep1Δ), we ascertained whether fep1+ plays an essential role in str1+ gene regulation as a function of cellular iron status by RNase protection analysis (Fig. 4A and B). In the wild-type strain, basal level of str1+ transcript was visible. However, in the presence of 1 or 100 µM FeCl3 the steady-state level of str1+ mRNA was strongly repressed ∼5- and ∼10-fold under basal level, respectively. Conversely, in response to iron starvation conditions (100 µM BPS), the str1+ gene was highly derepressed by ∼8-fold with respect to the basal level. In the fep1Δ mutant strain, the str1+ gene was strongly and constitutively expressed (∼8- to 10-fold as compared with the basal level of str1+ mRNA observed in the wild-type strain) and was unregulated by cellular iron status (Fig. 4A and B).
Based upon the gene expression data we obtained, we predicted that the Fep1 transcription factor directly interacts with the sequence 5′-AGATAA-3′ found in the str1+ promoter between positions –870 and –865. To determine if such interaction occurs, we produced the N-terminal 241 amino acids of Fep1 harboring two GATA-type zinc finger motifs in E.coli using the MBP fusion protein expression system with subsequent affinity purification and cleavage with protease (37). As shown in Figure 4C by a representative electrophoretic mobility shift assay, the wild-type 32P-labeled 46 bp str1+ promoter fragment, which contains the above mentioned GATA sequence, forms a DNA–protein complex in the presence of Fep1. To ascertain the specificity of this complex formation, we conducted competition experiments with unlabeled oligomers using either wild-type GATA or GATA with multiple point mutations within the 46 bp DNA fragment (Fig. 4D). Formation of the DNA–protein complex was inhibited by incubation with excess wild-type oligomer, but not by the mutant competitor (Fig. 4C), indicating that the complex was formed by sequence-specific interactions.
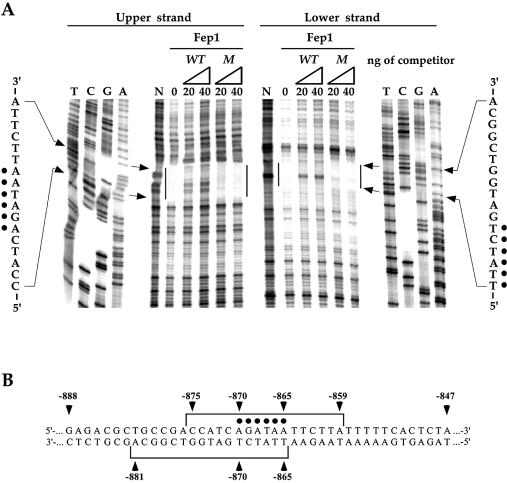
In DNase I footprinting analyses, Fep1 bound to a region encompassing and flanking the 5′-–870AGATAA–865-3′ element in the str1+ promoter (Fig. 5). The protected pattern covered 17 bases on each strand starting at –875 to –859 on the upper strand and starting at –865 to –881 on the lower strand (oriented in the opposite direction relative to the direction of transcription). Specifically, the residues corresponding to positions –870 and –865 that constitute the 5′-AGATAA-3′ element were strongly protected from DNase I cleavage. To assess the binding specificity of purified Fep1, competition experiments were performed by DNase I footprinting analyses. Formation of the complex at the level of the GATA sequence was inhibited by incubation with excess of the specific wild-type oligomer (WT, Fig. 5A) but not by the mutant competitor (M, Fig. 5A). Taken together, these data demonstrate 5′-AGATAA-3′ DNA binding activity for Fep1, which is consistent with a role for Fep1 in repressing str1+ gene expression under conditions of iron adequacy.
Figure 5.
DNA protection pattern of Fep1. (A) DNase I footprinting analysis of affinity purified Fep1 following competition with the synthetic oligomers presented in Figure 4D. In each case, 0, 20 and 40 ng of competitors were used. N, probes incubated in the absence of Fep1. Dots indicate the position of the residues that compose the 5′-AGATAA-3′ element. Vertical bars refer to the protected regions on each strand. Shown to the left and right sides are the reference DNA sequencing markers for the upper and lower strand, respectively. Results illustrated are representative of three independent experiments. (B) Schematic representation of the data shown in (A). Brackets indicate the region protected from DNase I by Fep1 in the str1+ promoter on both strands. The numbers refer to the position relative to the A of the start codon of str1+ ORF.
Analogous to str1+, the str2+ and str3+ genes, in which GATA elements are found, are negatively regulated by iron through Fep1
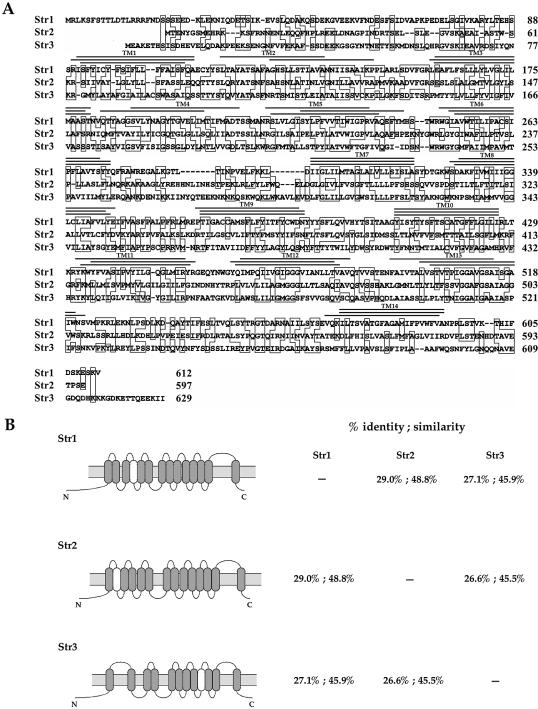
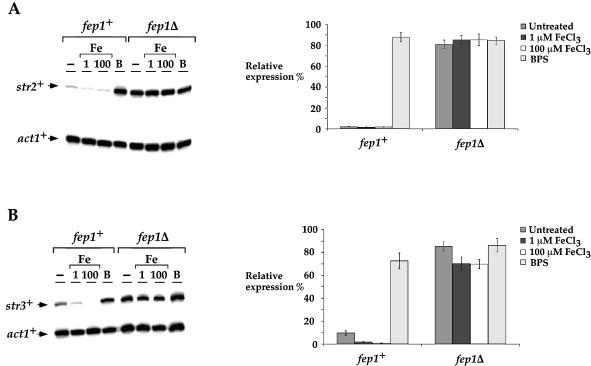
Analysis of genomic DNA sequences from the S.pombe Genome Project revealed two other ORFs (SPCC622.20c and SPAC1F8.03c) related to str1+. We termed these latter proteins Str2 and Str3, respectively. All of these are predicted to be highly homologous members of a subfamily of the major facilitator superfamily (MFS) of transporters. The amino acid identity between Str1 and the putative siderophore transporters Str2 and Str3 is 29.0 and 25.1%, respectively. Furthermore, the amino acid sequence of Str1 displays 48.8 and 45.9% similarity to the Str2 and Str3 proteins, respectively. A hallmark of Str1, Str2 and Str3 is that all exhibit very similar hydropathy profiles (data not shown). Consistently, the proposed topological structure of these proteins displays high resemblance, as shown in Figure 6. As would be expected for genes involved in iron acquisition, we observed the presence of putative fep1+ consensus binding sites, 5′-(A/T)GATAA-3′, in the str2+ and str3+ promoters (Fig. 7A). Based on this observation, we ascertained whether these potential regulatory elements could complex with Fep1. GATA oligomer probes derived from the str2+ (positions –92 to –42) and str3+ (positions –663 to –608) promoters were able to form a complex with Fep1 (Fig. 7B and C). To ascertain the specificity of this complex formation, we carried out competition experiments with unlabeled oligomers, either wild type or containing point mutations in GATA motifs. Excess wild-type oligomers inhibited complex formation with Fep1. In contrast, such inhibition of complex formation was not observed using mutant competitors. To evaluate whether str2+ and str3+ gene expression is regulated by cellular iron status through Fep1, we examined the str2+ and str3+ mRNA steady-state levels in cells grown in the presence of iron or starved for iron. As shown in Figure 8A and B, the S.pombe str2+ and str3+ mRNA expression in a wild-type strain was repressed (∼2- to ∼10-fold) when cells were exposed to the presence of iron. Conversely, when cells were grown in the presence of the iron chelator BPS, the str2+ and str3+ transcript levels were derepressed ∼7- to ∼40-fold over basal levels. Furthermore, using isogenic strains harboring a wild-type fep1+ gene and an insertionally inactivated fep1 allele, we found that the iron-dependent regulation of str2+ and str3+ mRNAs required the Fep1 protein (Fig. 8A and B). Indeed, in the absence of Fep1, the str2+ and str3+ genes were highly expressed (∼37- to 39-fold for str2+ and ∼7- to 9-fold for str3+ with respect to the basal level detected in the wild-type strain) and were virtually unresponsive to iron repression. These results demonstrate that Fep1 is an essential trans-acting component for appropriate regulation of the str2+ and str3+ genes. Furthermore, Fep1 appears to negatively control gene expression through binding to a consensus 5′-(T/A)GATAA-3′ element present in the promoters of these target genes.
Figure 6.
Alignment of Str1 and the two homologs identified in the S.pombe proteome. (A) Amino acid residues identical in at least two of the compared proteins are boxed. Potential transmembrane spanning domains (TM1–TM14) are indicated with a triple line above. The upper, middle and lower lines specify the positions where transmembrane regions are predicted in Str1, Str2, and Str3, respectively. (B) Topological models of the Str1, Str2 and Str3 proteins predicted by TopPred II (left). The predicted transmembrane domains are depicted in gray (certain) and white (putative). The amino acid identity and similarity between Str1, Str2 and Str3 are indicated (right).
Figure 8.
Regulation of str2+ and str3+ expression by iron and Fep1. (A) Total RNA from control (–), 1 and 100 µM FeCl3 (Fe) or 100 µM BPS (B) cultures was isolated and analyzed. Shown is a representative RNase protection assay of str2+ and act1+ mRNA steady-state levels. On the right side is the quantitation of str2+ mRNA levels after treatment. The data are the means of three replicates ± SD. (B) RNase protection analysis as described in (A), except that str3+ and act1+ mRNA levels were assayed and quantitated.
DISCUSSION
In this study, we have identified a conserved regulatory element, 5′-(A/T)GATAA-3′, present in each of the str1+, str2+ and str3+ promoters. Our in vivo sequential deletions from the 5′ end of the str1+ promoter clearly demonstrated that the integrity of the 5′-AGATAA-3′ element, from position –870 to position –865, is essential for mediating iron repression. Furthermore, using two independent assays for DNA binding, electrophoretic mobility shift and DNase I footprinting, Fep1 was shown to bind specifically to the 5′-–870AGATAA–865-3′ element of str1+ (Figs 4 and 5). In U.maydis (8), N.crassa (38,39) and A.nidulans (7,10), when iron is in excess, siderophore transporter gene expression is negatively regulated at the transcriptional level by Urbs1, Sre and Srea, respectively. Like Fep1, these GATA factors have two Cys2/Cys2-type zinc fingers found within their DNA-binding domains, which constitute a distinct structural feature of this class of GATA binding transcription factors (40). Furthermore, analogous to the situation for Fep1, in vitro the Urbs1 and Srea proteins can specifically interact with a single GATA motif (8,10). However, for in vivo function, these regulators require two GATA boxes when compared to Fep1. This may indicate differences in the utilization of amino acids that serve to interact with DNA between Fep1 and the above mentioned proteins. Although in A.nidulans the iron- dependent transcriptional repression of siderophore gene expression is controlled by Srea, the iron-dependent repression of freA (41), a novel gene encoding a putative metalloreductase orthologous to S.cerevisiae Fre reductases, is independent of Srea. However, whether FreA is a specific component of a reductive iron uptake system in A.nidulans is still unclear. In S.pombe, an explicit mechanistic link exists between the transcriptional regulation of fission yeast genes encoding components of the reductive and non-reductive iron transport systems. By combining our previous studies (28) and this work, a number of experimental results have identified Fep1 as an essential regulatory protein in coordinating expression of genes involved in reductive and non-reductive iron uptake in fission yeast. First, Fep1 is required for the iron-mediated repression of fio1+, fip1+, str1+, str2+ and str3+ gene expression. Second, fep1Δ mutant cells display a very high basal expression of these genes and do not repress the reductive and non-reductive iron transport genes in response to high iron levels. Third, Fep1 has been demonstrated to interact directly with the 5′-(A/T)GATAA-3′ elements found in the fio1+, fip1+, str1+, str2+ and str3+ promoter regions.
The problems of mobilizing iron and preventing its accumulation to toxic levels require tight regulation as a function of iron availability by the organisms. While Fep1 acts as a repressor of transcription by interacting with GATA-like sequences in the presence of iron, Aft1 of S.cerevisiae functions as a transcriptional activator and interacts with the 5′-(T/C)(G/A)CACCC(A/G)-3′ element in the absence of iron. BLAST searches for Aft1 homologs in the S.pombe and C.albicans genome databases revealed no S.pombe or C.albicans proteins with significant identity. Interestingly, in C.albicans, when iron is in excess, at least three genes (CFL95, CaFTR1 and CaFET99) involved in reductive iron uptake and the CaARN1-encoded ferrichrome transporter are repressed by iron (30,42). This may involve a Fep1 ortholog that functions as a repressor for down-regulation of genes encoding components of the reductive and non-reductive iron transport systems. Consistently, recent studies (42) have demonstrated that iron-mediated regulation of genes involved in reductive iron uptake in C.albicans requires the co-repressor Tup1 protein orthologous to S.pombe Tup11 and Tup12. Importantly, these latter proteins have been shown to be required in addition to Fep1 for repression of the fio1+ iron transport gene in fission yeast (28). Because of this analogy, the fission yeast S.pombe may represent an attractive model system for understanding how C.albicans cells establish and maintain normal iron homeostasis.
In this study, we have examined whether the first member of the Str proteins in fission yeast can transport siderophore-bound iron. We have used a S.cerevisiae yeast system in which the endogenous FET3 and ARN1–ARN4 genes were inactivated (30,43,44). By using this approach, we sought to ensure the presence of Str1 as the sole protein with the ability to capture siderophore-iron complexes. Importantly, the expression of str1+ was placed under the control of the glyceraldehyde 3-phosphate dehydrogenase (GPD) gene promoter (45), which is an iron-independent promoter (B.Pelletier and S.Labbé, unpublished data). This enables us to ensure that the ferrichrome-iron transport activity observed when the str1+ gene was expressed in a heterologous context was not due to other proteins regulated by Aft1. The ability of the S.pombe Str1 transporter to facilitate the uptake of ferrichrome-bound iron is consistent with the fact that among the Arn1–Arn4 family members, Str1 displays the highest sequence identity (31.2%) with the Arn1 protein, which is a ferrichrome transporter. Because Str1 failed to transport siderophores structurally related to ferrichrome, such as the peptide-linked hydroxamates ferroxiamine B and rhodotorulic acid, we have not tested more distantly related siderophores, such as triacetylfusarinine C and enterobactin. In a separate study, we have observed that S.cerevisiae fet3Δ arn1-4Δ mutant cells expressing the Str2 protein were able to take up both ferrichrome- and ferroxiamine B-iron (B.Pelletier and S.Labbé, unpublished data). However, a fet3Δ arn1-4Δ strain harboring the str3+ allele failed to grow when ferrichrome- or ferroxiamine B-iron was the iron source. Based on this observation, it appears that S.pombe possesses three siderophore transporters, which are different in terms of substrate specificity. The inability of S.pombe to secrete siderophores may be compensated for by the utilization of transporters that have a broader range of substrate specificity for these iron-specific chelators.
Recently, three siderophore-iron transporters, MirA, MirB and MirC, have been identified in A.nidulans (44). When expressed in a S.cerevisiae fet3Δ arn1-4Δ mutant strain, iron uptake assays from different siderophores revealed that MirA and MirB mobilize iron bound to enterobactin and triacetylfusarinine C, respectively. Although MirC may participate in the mobilization of iron bound to siderophores, its substrate specificity has not been determined (44). The S.pombe Str1 protein exhibits 28.9, 32.5 and 34.9% overall identity to A.nidulans MirA, MirB and MirC, respectively. The putative siderophore transporter Str2 bears 27.0, 28.0, and 35.9% identity to MirA, MirB and MirC, respectively. The amino acid identity between Str3 and MirA, MirB and MirC is 26.8, 27.5 and 27.8%, respectively. From these sequence comparisons, the S.pombe Str proteins are similar to but not more structurally related to A.nidulans Mir than to S.cerevisiae Arn proteins. A direct alignment of the amino acid sequences of Str1 with Str2 and Str3 identified extended amino acid sequence homology, especially with respect to several residues within the regions that encompass the predicted transmembrane domains. Str1 and Str2 harbor 14 membrane-spanning regions identified using the TopPred II program (34), whereas 12 were predicted for Str3. Although the exact number must await biochemical verification, in all cases, this is sufficient to form a functional translocation path, which contains, in general, 12 ± 2 transmembrane domains within transport proteins (46). Interestingly, both the N- and C-termini of Str1, Str2 and Str3 proteins are predicted to be located on the same side relative to the membrane. Recent studies in S.cerevisiae have demonstrated that the Arn1 protein moves from the endosome to the plasma membrane in response to low ferrichrome concentrations (47). However, in response to elevated ferrichrome levels, the Arn1 protein rapidly undergoes endocytosis (47). Whether this latter form of regulation plays a role in iron delivery to the interior of the cell or provides a mechanism to reduce potential iron toxicity by controlling the number of active siderophore-iron transporters at the cell surface has not yet been resolved. Because of the importance of maintaining iron homeostasis in cells, studies to further understand the mechanisms by which siderophore-bound iron transporters function represent a pertinent area for future study.
Acknowledgments
ACKNOWLEDGEMENTS
This study was supported by Natural Sciences and Engineering Research Council of Canada Grant 238238-01 to S.L. Infrastructure equipment essential for performing this investigation was obtained through Canada Foundation for Innovation Grant NOF-3754 to S.L. B.P. was supported in part by the Fondation Dr Étienne Lebel and Fonds de la Recherche en Santé du Québec. S.L. is a New Investigator Scholar from the Canadian Institutes of Health Research.
REFERENCES
- 1.Van Ho A., Ward,D.M. and Kaplan,J. (2002) Transition metal transport in yeast. Annu. Rev. Microbiol., 56, 237–261. [DOI] [PubMed] [Google Scholar]
- 2.Touati D. (2000) Iron and oxidative stress in bacteria. Arch. Biochem. Biophys., 373, 1–6. [DOI] [PubMed] [Google Scholar]
- 3.Philpott C.C., Protchenko,O., Kim,Y.W., Boretsky,Y. and Shakoury-Elizeh,M. (2002) The response to iron deprivation in Saccharomyces cerevisiae: expression of siderophore-based systems of iron uptake. Biochem. Soc. Trans, 30, 698–702. [DOI] [PubMed] [Google Scholar]
- 4.Winkelmann G. (2002) Microbial siderophore-mediated transport. Biochem. Soc. Trans, 30, 691–696. [DOI] [PubMed] [Google Scholar]
- 5.Kosman D.J. (2003) Molecular mechanisms of iron uptake in fungi. Mol. Microbiol., 47, 1185–1197. [DOI] [PubMed] [Google Scholar]
- 6.Mazmanian S.K., Skaar,E.P., Gaspar,A.H., Humayun,M., Gornicki,P., Jelenska,J., Joachmiak,A., Missiakas,D.M. and Schneewind,O. (2003) Passage of heme-iron across the envelope of Staphylococcus aureus. Science, 299, 906–909. [DOI] [PubMed] [Google Scholar]
- 7.Oberegger H., Zadra,I., Schoeser,M., Abt,B., Parson,W. and Haas,H. (2002) Identification of members of the Aspergillus nidulans SREA regulon: genes involved in siderophore biosynthesis and utilization. Biochem. Soc. Trans, 30, 781–783. [DOI] [PubMed] [Google Scholar]
- 8.An Z., Mei,B., Yuan,W.M. and Leong,S.A. (1997) The distal GATA sequences of the sid1 promoter of Ustilago maydis mediate iron repression of siderophore production and interact directly with Urbs1, a GATA family transcription factor. EMBO J., 16, 1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L.W., Haas,H. and Marzluf,G.A. (1998) Isolation and characterization of a new gene, sre, which encodes a GATA-type regulatory protein that controls iron transport in Neurospora crassa. Mol. Gen. Genet., 259, 532–540. [DOI] [PubMed] [Google Scholar]
- 10.Haas H., Zadra,I., Stoffler,G. and Angermayr,K. (1999) The Aspergillus nidulans GATA factor SREA is involved in regulation of siderophore biosynthesis and control of iron uptake. J. Biol. Chem., 274, 4613–4619. [DOI] [PubMed] [Google Scholar]
- 11.Neilands J.B. (1995) Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem., 270, 26723–26726. [DOI] [PubMed] [Google Scholar]
- 12.Lesuisse E. and Labbé,P. (1989) Reductive and non-reductive mechanisms of iron assimilation by the yeast Saccharomyces cerevisiae. J. Gen. Microbiol., 135, 257–263. [DOI] [PubMed] [Google Scholar]
- 13.Yun C.W., Ferea,T., Rashford,J., Ardon,O., Brown,P.O., Botstein,D., Kaplan,J. and Philpott,C.C. (2000a) Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J. Biol. Chem., 275, 10709–10715. [DOI] [PubMed] [Google Scholar]
- 14.Protchenko O., Ferea,T., Rashford,J., Tiedeman,J., Brown,P.O., Botstein,D. and Philpott,C.C. (2001) Three cell wall mannoproteins facilitate the uptake of iron in Saccharomyces cerevisiae. J. Biol. Chem., 276, 49244–49250. [DOI] [PubMed] [Google Scholar]
- 15.Yun C.W., Bauler,M., Moore,R.E., Klebba,P.E. and Philpott,C.C. (2001) The role of the FRE family of plasma membrane reductases in the uptake of siderophore-iron in Saccharomyces cerevisiae. J. Biol. Chem., 276, 10218–10223. [DOI] [PubMed] [Google Scholar]
- 16.Stearman R., Yuan,D.S., Yamaguchi-Iwai,Y., Klausner,R.D. and Dancis,A. (1996) A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science, 271, 1552–1557. [DOI] [PubMed] [Google Scholar]
- 17.Dix D., Bridgham,J., Broderius,M. and Eide,D. (1997) Characterization of the FET4 protein of yeast. Evidence for a direct role in the transport of iron. J. Biol. Chem., 272, 11770–11777. [DOI] [PubMed] [Google Scholar]
- 18.Lesuisse E., Simon-Casteras,M. and Labbé,P. (1998) Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SIT1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology, 144, 3455–3462. [DOI] [PubMed] [Google Scholar]
- 19.Heymann P., Ernst,J.F. and Winkelmann,G. (1999) Identification of a fungal triacetylfusarinine C siderophore transport gene (TAF1) in Saccharomyces cerevisiae as a member of the major facilitator superfamily. Biometals, 12, 301–306. [DOI] [PubMed] [Google Scholar]
- 20.Heymann P., Ernst,J.F. and Winkelmann,G. (2000) A gene of the major facilitator superfamily encodes a transporter for enterobactin (Enb1p) in Saccharomyces cerevisiae. Biometals, 13, 65–72. [DOI] [PubMed] [Google Scholar]
- 21.Heymann P., Ernst,J.F. and Winkelmann,G. (2000) Identification and substrate specificity of a ferrichrome-type siderophore transporter (Arn1p) in Saccharomyces cerevisiae. FEMS Microbiol. Lett., 186, 221–227. [DOI] [PubMed] [Google Scholar]
- 22.Lesuisse E., Blaiseau,P.L., Dancis,A. and Camadro,J.M. (2001) Siderophore uptake and use by the yeast Saccharomyces cerevisiae. Microbiology, 147, 289–298. [DOI] [PubMed] [Google Scholar]
- 23.Moore R.E., Kim,Y. and Philpott,C.C. (2003) The mechanism of ferrichrome transport through Arn1p and its metabolism in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 100, 5664–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi-Iwai Y., Dancis,A. and Klausner,R.D. (1995) AFT1: a mediator of iron regulated transcriptional control in Saccharomyces cerevisiae. EMBO J., 14, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi-Iwai Y., Stearman,R., Dancis,A. and Klausner,R.D. (1996) Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J., 15, 3377–3384. [PMC free article] [PubMed] [Google Scholar]
- 26.Roman D.G., Dancis,A., Anderson,G.J. and Klausner,R.D. (1993) The fission yeast ferric reductase gene frp1+ is required for ferric iron uptake and encodes a protein that is homologous to the gp91-phox subunit of the human NADPH phagocyte oxidoreductase. Mol. Cell. Biol., 13, 4342–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Askwith C. and Kaplan,J. (1997) An oxidase-permease-based iron transport system in Schizosaccharomyces pombe and its expression in Saccharomyces cerevisiae. J. Biol. Chem., 272, 401–405. [DOI] [PubMed] [Google Scholar]
- 28.Pelletier B., Beaudoin,J., Mukai,Y. and Labbé,S. (2002) Fep1, an iron sensor regulating iron transporter gene expression in Schizosaccharomyces pombe. J. Biol. Chem., 277, 22950–22958. [DOI] [PubMed] [Google Scholar]
- 29.Yun C.W., Tiedeman,J.S., Moore,R.E. and Philpott,C.C. (2000) Siderophore-iron uptake in Saccharomyces cerevisiae. Identification of ferrichrome and fusarinine transporters. J. Biol. Chem., 275, 16354–16359. [DOI] [PubMed] [Google Scholar]
- 30.Ardon O., Bussey,H., Philpott,C., Ward,D.M., Davis-Kaplan,S., Verroneau,S., Jiang,B. and Kaplan,J. (2001) Identification of a Candida albicans ferrichrome transporter and its characterization by expression in Saccharomyces cerevisiae. J. Biol. Chem., 276, 43049–43055. [DOI] [PubMed] [Google Scholar]
- 31.Dancis A., Yuan,D.S., Haile,D., Askwith,C., Eide,D., Moehle,C., Kaplan,J. and Klausner,R.D. (1994) Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell, 76, 393–402. [DOI] [PubMed] [Google Scholar]
- 32.Beaudoin J. and Labbé,S. (2001) The fission yeast copper-sensing transcription factor Cuf1 regulates the copper transporter gene expression through an Ace1/Amt1-like recognition sequence. J. Biol. Chem., 276, 15472–15480. [DOI] [PubMed] [Google Scholar]
- 33.Van Helden J., André,B. and Collado-Vides,J. (2000) A web site for the computational analysis of yeast regulatory sequences. Yeast, 16, 177–187. [DOI] [PubMed] [Google Scholar]
- 34.Claros M.G. and von Heijne,G. (1994) TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci., 10, 685–686. [DOI] [PubMed] [Google Scholar]
- 35.Haas H., Angermayr,K. and Stoffler,G. (1997) Molecular analysis of a Penicillium chrysogenum GATA factor encoding gene (sreP) exhibiting significant homology to the Ustilago maydis urbs1 gene. Gene, 184, 33–37. [DOI] [PubMed] [Google Scholar]
- 36.Voisard C., Wang,J., McEvoy,J.L., Xu,P. and Leong,S.A. (1993) urbs1, a gene regulating siderophore biosynthesis in Ustilago maydis, encodes a protein similar to the erythroid transcription factor GATA-1. Mol. Cell. Biol., 13, 7091–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellermann O.K. and Ferenci,T. (1982) Maltose-binding protein from Escherichia coli. Methods Enzymol., 90, 459–463. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L.W. and Marzluf,G.A. (1999) Functional analysis of the two zinc fingers of SRE, a GATA-type factor that negatively regulates siderophore synthesis in Neurospora crassa. Biochemistry, 38, 4335–4341. [DOI] [PubMed] [Google Scholar]
- 39.Harrison K.A. and Marzluf,G.A. (2002) Characterization of DNA binding and the cysteine rich region of SRE, a GATA factor in Neurospora crassa involved in siderophore synthesis. Biochemistry, 41, 15288–15295. [DOI] [PubMed] [Google Scholar]
- 40.Scazzocchio C. (2000) The fungal GATA factors. Curr. Opin. Microbiol., 3, 126–131. [DOI] [PubMed] [Google Scholar]
- 41.Oberegger H., Schoeser,M., Zadra,I., Schrettl,M., Parson,W. and Haas,H. (2002) Regulation of freA, acoA, lysF and cycA expression by iron availability in Aspergillus nidulans. Appl. Environ. Microbiol., 68, 5769–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knight S.A.B., Lesuisse,E., Stearman,R., Klausner,R.D. and Dancis,A. (2002) Reductive iron uptake by Candida albicans: role of copper, iron and the TUP1 regulator. Microbiology, 148, 29–40. [DOI] [PubMed] [Google Scholar]
- 43.Heymann P., Gerads,M., Schaller,M., Dromer,F., Winkelmann,G. and Ernst,J.F. (2002) The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect. Immun., 70, 5246–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas H., Schoeser,M., Lesuisse,E., Ernst,J.F., Parson,W., Abt,B., Winkelmann,G. and Oberegger,H. (2003) Characterization of the Aspergillus nidulans transporters for the siderophores enterobactin and triacetylfusarinine C. Biochem. J., 371, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mumberg D., Muller,R. and Funk,M. (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene, 156, 119–122. [DOI] [PubMed] [Google Scholar]
- 46.Veenhoff L.M., Heuberger,E.H. and Poolman,B. (2002) Quaternary structure and function of transport proteins. Trends Biochem. Sci., 27, 242–249. [DOI] [PubMed] [Google Scholar]
- 47.Kim Y., Yun,C.W. and Philpott,C.C. (2002) Ferrichrome induces endosome to plasma membrane cycling of the ferrichrome transporter, Arn1p, in Saccharomyces cerevisiae. EMBO J., 21, 3632–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]