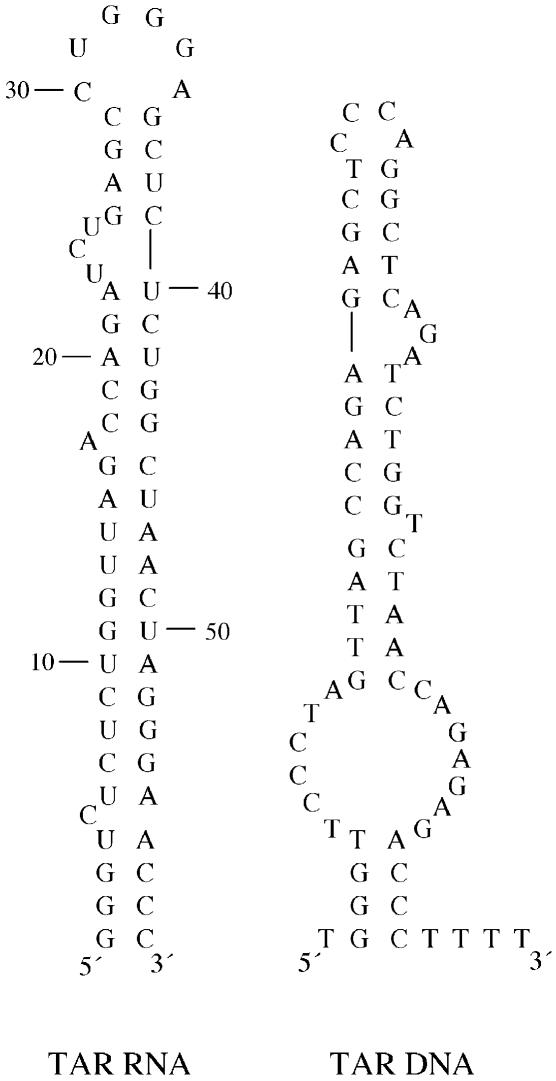Abstract
In the minus-strand transfer step of HIV-1 reverse transcription, the nucleocapsid protein (NC) promotes annealing of the 3′ ‘R’ (repeat) region of the RNA genome to its complementary sequence located in the newly synthesized minus-strand strong-stop DNA. The R region contains the highly stable transactivation response (TAR) RNA hairpin. To gain insights into the molecular details of TAR RNA–NC interactions, we carried out hydroxyl radical footprinting, as well as gel-shift and fluorescence anisotropy binding assays using wild-type and mutant forms of NC. Our results support the conclusion that NC variants with mutations in their zinc finger domains have dramatically altered TAR RNA binding interactions relative to wild-type NC. These data demonstrate that a specific zinc finger architecture is required for optimal TAR RNA binding, and help to explain the requirement for the zinc finger motifs of NC in its role as a nucleic acid chaperone in minus-strand transfer.
INTRODUCTION
HIV-1 nucleocapsid protein (NC) is a highly basic protein consisting of 55 amino acid residues and two zinc finger structures each comprised of a CCHC motif (1–3). The nucleic acid chaperone activity of NC has been demonstrated in vitro to greatly accelerate tRNALys,3 annealing to the primer binding site on the HIV-1 genome (4,5) as well as to facilitate minus- and plus-strand transfer reactions of retroviral reverse transcription (6–12). Although the zinc finger structures of NC are not essential for tRNA primer annealing (13,14), they are responsible for inducing subtle tertiary structural changes and destabilizing helical regions of the tRNA (14,15). In contrast to tRNA annealing, the zinc finger structures are critical for the annealing step in minus-strand transfer (16). In this reaction, minus-strand strong-stop DNA [(–) SSDNA] is annealed to an acceptor RNA containing the highly stable TAR structure, which is part of the R region of the RNA genome (7,10,17). The annealing reaction between the TAR-containing RNA acceptor and (–) SSDNA results in formation of a thermodynamically favored 98-nucleotide (nt) base-paired duplex. NC has also been shown to facilitate minus-strand transfer by blocking a competing intramolecular self-priming reaction, which occurs due to the formation of a stable hairpin structure (‘TAR DNA’) at the 3′-end of (–) SSDNA (7,10,18,19). Although the zinc fingers are known to be critical for minus-strand transfer, their precise role in facilitating this process is not completely understood.
To begin to characterize NC’s interactions with TAR RNA, we carried out hydroxyl radical footprinting of a 59-nt TAR RNA hairpin (Fig. 1, left) incubated with NC both in the absence and presence of a complementary 64-nt TAR DNA hairpin (Fig. 1, right). To explore the role of the zinc finger structures of NC on TAR RNA binding, we performed similar footprinting studies with NC mutants containing changes in one or both zinc finger motifs. A single point mutation of His 23 to Cys in NC converts the CCHC motif in the first finger to CCCC. This variant (CCCC NC) can still bind zinc with high affinity (20). In contrast, SSHS NC, wherein both CCHC motifs have been mutated to SSHS, is unable to bind zinc tightly. Interestingly, both mutants were previously shown to be defective in facilitating the annealing step of minus-strand transfer (16,21) as well as in destabilizing double-stranded DNA as measured by single molecule DNA stretching (22,23). To gain further insights into TAR RNA binding by both wild-type and mutant NC, gel- and fluorescence-based assays were also employed in this work. Taken together, our results strongly suggest that the specific zinc finger architecture of NC plays a critical role in TAR RNA interaction, as well as in NC-induced RNA aggregate formation, and help to explain the zinc finger requirement for optimal NC-facilitated minus-strand transfer.
Figure 1.
Predicted secondary structures of the 59-nt TAR RNA (left) and complementary 64-nt TAR DNA (right) used in this study. The four thymidine residues at the 3′-end of the TAR DNA are not part of the actual R region sequence in HIV-1. Structures were determined at 37°C using the program mfold (31).
MATERIALS AND METHODS
Nucleic acid preparation
DNA templates encoding the 59-nt TAR RNA hairpin were generated by PCR amplification of chemically synthesized DNA oligonucleotides using a 5′-primer containing the T7 promoter sequence (5′-ATATGTAATACGACTCACTATAGGGTCTCTCTGGTTAGACCA-3′) and the following 3′-primer: 5′-GGGTTCCCTAGTTAGCCAGA-3′. The RNA was transcribed in vitro with T7 RNA polymerase (24) and purified by 10% denaturing polyacrylamide gel electrophoresis (PAGE). The RNA was 5′-end-labeled with γ-[32P]ATP using T4 polynucleotide kinase (New England Biolabs, Beverly, MA) following dephosphorylation with calf intestinal alkaline phosphatase (New England Biolabs). The 64-nt TAR DNA was chemically synthesized by the Microchemical Facility (University of Minnesota).
A 63-nt 5′- indocarbocyanine (Cy3)-labeled TAR RNA variant (5′-Cy3-TAR RNA) containing a four-residue uuuu overhang at the 3′ end was used in fluorescence anisotropy measurements. This dye-labeled RNA was chemically synthesized, purified on 12% PAGE, and determined to be >98% pure by matrix-assisted laser-desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Dharmacon, CO).
Protein preparation
All of the proteins used in this work were prepared essentially as previously described: wild-type NC (NL 4-3 isolate) (25), SSHS NC (16) and CCCC and 1-1 NC (26). In the case of wild-type NC, the protein was further purified by reversed-phase HPLC (Beckman model 126) using a Vydac C18 column (25 × 100 mm) as described previously (27).
Hydroxyl radical footprinting
Fe(II)–EDTA cleavage reactions were carried out as previously described (28) with slight modifications. A solution of 200 nM or 1 µM TAR RNA was refolded by mixing 0.5 µl of 4 or 20 µM 32P-labeled TAR RNA, 2.5 µl of HK buffer containing 200 mM 4-(2-hydroxylethyl)-1-piperazineethanesulfonic acid (HEPES, pH 7.5) and 250 mM KCl, and 1.5 µl of diethyl pyrocarbonate (DEPC)-treated water. The RNA solution was heated at 80°C for 2 min and cooled to 25°C over 5 min. To this solution was added 1 µl of 100 mM MgCl2 and 1 µl of 50 mM DTT/200 mM NaCl. The folded RNA was preincubated with various concentrations (see Fig. legends) of NC or mutant NC at 37°C for 30 min prior to cleavage reactions. Some reactions contained 1.5 µM TAR DNA. Cleavage reaction components including 0.5 µl of 0.8% H2O2 (v/v), 0.5 µl of 100 mM sodium ascorbate and 1 µl of Fe(II)–EDTA [10 mM Fe(NH4)2SO4/12.5 mM EDTA, pH 8] were added sequentially to the inside wall of the reaction tubes and quickly mixed. Reactions were allowed to proceed at 25°C for 1 min. Reactions were terminated by the addition of 2 vol of 10 mM thiourea. SDS was added to 1% final concentration (v/v) and solutions were incubated at 37°C for 5 min, followed by two phenol-chloroform extractions and ethanol precipitation. Pellets were resuspended in gel-loading solution (50% formamide, 0.01% bromophenol blue and 0.01% xylene cyanol), and cleavage products were resolved by 10% denaturing PAGE. Results were analyzed and quantified using a Bio-Rad Molecular Imager FX and Quantity One software.
Fluorescence anisotropy assays
Fluorescence anisotropy measurements were performed at 20°C using a PTI spectrofluorometer (Photon Technology International, model QM-2000) equipped with Glan-Thompson polarizers. Excitation and emission wavelengths were set at 550 and 570 nm, respectively. Measurements were performed on samples (65 µl) containing 10 nM 5′-Cy3-TAR RNA in HEPES–NaCl (50 mM HEPES, pH 7.5, 120 mM NaCl) in the absence and presence of various concentrations (0–20 µM) of the wild type, CCCC and SSHS NC. Anisotropy (A) was calculated with Felix32 software (PTI) using the equation A = (Iv – Ih)/(Iv + 2Ih), where Iv and Ih are the intensities detected through vertical and horizontal polarizers when vertically polarized light is used to excite the sample (29). Average anisotropy values were obtained using data from three independent experiments.
Fluorescence anisotropy data analysis
The dissociation constants (Kd) were determined by non-linear curve fitting using either the one- (equation 1) (30) or two-site binding model (equation 2).
A = Amin + [([RNA]T + [NC]T + Kd) – {([RNA]T + [NC]T + Kd)2 – (4[RNA]T[NC]T)}1/2](Amax – Amin)/(2[RNA]T)1
A = Amin + [([RNA]T + [NC]T + [Kd1 + Kd2]/2) – ({([RNA]T + [NC]T + Kd1)2 – (4[RNA]T[NC]T)}1/2 + {([RNA]T + [NC] + Kd2)2 – (4[RNA]T[NC]T)}1/2)/2](Amax – Amin)/(2[RNA]T)2
where A = measured anisotropy, Amin = minimum anisotropy of free RNA and Amax = maximum anisotropy of bound RNA at saturation.
Gel-shift assays
A solution of 3 nM 32P-labeled TAR RNA was refolded as described for the footprinting assays and preincubated with various concentrations (see Figure legends) of NC or mutant NC at 37°C for 30 min. Native gel loading buffer (30% glycerol, 0.01% bromophenol blue and 0.01% xylene cyanol) was added and samples were run on 8% native PAGE at 4°C for 2 h under constant voltage (200 V). Results were analyzed and quantified as described above.
RESULTS
TAR RNA/DNA annealing assays with wild-type and mutant NC
Using an in vitro minus-strand annealing reaction, Levin et al. previously showed that disruption of the zinc finger structures dramatically reduces the chaperone activity of NC (16,21). This previous work utilized a 131-nt (–) SSDNA strand and a 148-nt acceptor RNA. In the present work, a truncated acceptor RNA containing only the core TAR RNA hairpin was used, as well as a complementary DNA strand derived from the (–) SSDNA (Fig. 1). Using mfold (31) the DNA was also predicted to fold into a hairpin structure, and we will refer to this as ‘TAR DNA’ (Fig. 1). To confirm a critical role for the zinc finger motifs of NC in our truncated TAR RNA/DNA system, we performed annealing assays with wild-type NC and two NC mutants, CCCC and SSHS NC. Annealing assays were conducted using both low (20 nM) and high (200 nM) TAR RNA concentrations. Similar annealing profiles were observed under both sets of conditions; however, as expected, the extent of total annealing was greater when higher nucleic acid concentrations were used (data not shown). Whereas the initial rate of annealing was ∼2.4-fold reduced with SSHS NC compared to wild-type NC, CCCC NC consistently displayed levels of activity that were below the level of annealing observed in the absence of NC. These results are in accord with trends observed with these same mutants in previous studies using longer RNA and DNA constructs (16,21). Taken together, our results further support the critical role of the CCHC motif in the N-terminal zinc finger domain for NC-mediated TAR RNA/DNA annealing.
Fe–EDTA footprinting of NC bound to TAR RNA and to the annealed TAR RNA/DNA binary complex
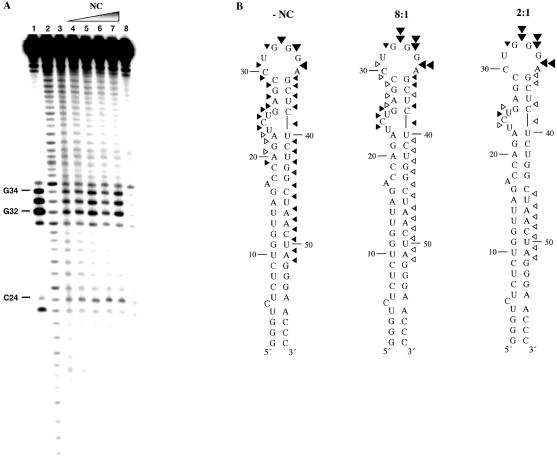
Hydroxyl radical footprinting is a useful method to probe RNA tertiary structure and RNA–protein interactions. The utility of this method for predicting solvent accessible regions of RNA has been proven by comparison of hydroxyl radical cleavage profiles and three-dimensional structures of RNA solved by X-ray crystallography (28,32–35). Here, we employed this method to better understand TAR RNA–NC interactions by examining Fe–EDTA cleavage profiles of TAR RNA (1 µM) in the absence and presence of varying amounts of NC (Fig. 2A). In the absence of NC, approximately half the TAR RNA hairpin was accessible to hydroxyl radical cleavage, with moderate cleavage observed in the 3′ stem, stronger cleavage in the loop region, and moderate cleavage at the top of the 5′ stem (Fig. 2A, lane 3 and B, left). In the presence of NC at an 8:1 nt:NC ratio, mild protection was observed at almost every position of the 3′ and 5′ stems, with enhanced cleavage in the loop (Fig. 2A, lane 5 and B, middle). At intermediate concentrations of NC (4:1 nt:NC), additional protection is observed in the helical regions, with less cleavage in the loop (Fig. 2A, lane 6). When higher concentrations of NC were present (2:1 nt:NC), strong cleavage was again apparent in the loop, whereas complete protection from cleavage was observed in certain helical regions, most notably G26–C29 and U40–G44 (Fig. 2A, lane 7 and B, right). Surprisingly, little or no protection was observed in the single-stranded regions of the RNA including the bulge (U23–U25) and loop (C30–A35). We postulate that the enhanced cleavage observed in the loop region at certain nt:NC ratios is due to a conformational change in the loop induced upon binding of NC to neighboring helical regions. The lack of protection from hydroxyl radical cleavage in the single-stranded regions may also be due to the high off-rate of NC binding (5–6 s–1), which has previously been measured for binding to the SL2 and SL3 stem–loop packaging signals (36).
Figure 2.
(Opposite and above) Fe–EDTA footprinting of [32P]-TAR RNA in the presence of NC and with or without TAR DNA. (A) Footprinting experiments performed in the absence of TAR DNA. Lanes 1 and 2, RNase T1 and alkaline hydrolysis ladders of radiolabeled TAR RNA, respectively. Lane 3, Fe–EDTA cleavage profile of 1 µM [32P]-TAR RNA in the absence of NC. Lanes 4–7, cleavage profiles of 1 µM [32P]-TAR RNA in the presence of the following concentrations of NC: 3.7 (lane 4), 7.4 (lane 5), 14.8 (lane 6) and 29.5 µM (lane 7) to achieve 16:1, 8:1, 4:1 and 2:1 nt:NC ratios, respectively. Lane 8, control reaction carried out in the absence of NC and Fe–EDTA. (B) Quantification of the Fe–EDTA footprinting results shown in (A) mapped onto the backbone of TAR RNA. Results of experiments in the absence of NC (left) and in the presence of 8:1 (middle) and 2:1 (right) nt:NC ratios are shown. Symbols represent the following extent of cleavage: 0.20–0.40% (open arrowheads), 0.41–0.70% (closed small arrowheads), 0.71–0.85% (closed large arrowheads) and 0.86–1.10% (double arrowheads). (C) Footprinting experiments performed in the presence of TAR DNA. Lanes 1 and 2, RNase T1 and alkaline hydrolysis ladders, respectively. Lanes 3 and 4, cleavage profiles of 1 µM [32P]-TAR RNA in the absence and presence of 1.5 µM TAR DNA, respectively. Lane 5, cleavage profile of TAR RNA in a heat-annealed TAR RNA/DNA complex. Lanes 6–8, TAR RNA was preincubated for 30 min with TAR DNA and the following concentrations of NC prior to Fe–EDTA cleavage: 9.7 (16:1 nt:NC), 19 (8:1 nt:NC) and 39 µM (4:1 nt:NC). Lane 9, control reaction carried out in the absence of NC and Fe–EDTA. (D) Quantification of the Fe–EDTA footprinting results shown in (C), lanes 5 and 8, mapped onto the backbone of TAR RNA for the NC-annealed (4:1 nt:NC ratio, left) and heat-annealed (right) TAR RNA/DNA complex. Symbols have the same meaning as in (B).
We have shown that NC promotes TAR RNA/DNA complex formation in the annealing assays (data not shown), and hydroxyl radical footprinting studies provide direct evidence for TAR RNA–NC interaction (Fig. 2A and B). However, an open question is whether NC remains bound to the TAR RNA/DNA duplex even after binary complex formation. To address this question, we carried out footprinting experiments in the presence of TAR DNA. In the absence of NC, similar cleavage profiles were observed for TAR RNA upon incubation with TAR DNA in the absence of heat and following heat-annealing (Fig. 2C, lanes 4 and 5, D, right). Under these conditions, the extent of binary complex formation determined via gel-shift annealing assays performed in the absence of heat is 50% following 1 h incubation, while essentially complete annealing is observed with heat- annealing (data not shown). Based on these data, we conclude that the RNA is still readily cleaved by hydroxyl radicals in the annealed binary complex. In fact, the cleavage profiles in the presence of TAR DNA with or without heat-annealing were very similar to that observed in the absence of TAR DNA (Fig. 2C, lane 3, B, left).
We next carried out hydroxyl radical cleavage of the NC-annealed TAR RNA/DNA binary complex. In the presence of TAR DNA and NC at a 16:1 nt:NC ratio, the cleavage profile of TAR RNA is nearly identical to that of the heat-annealed complex (Fig. 2C, lanes 6 and 5, respectively). As more NC was bound, protection from cleavage was observed throughout the TAR RNA structure, with the exception of the loop region (Fig. 2C, lanes 7 and 8, D, left). Since the annealing extent at a nt:NC ratio of 4:1 is ∼90% (data not shown), the protection profile observed under these conditions (Fig. 2C, lane 8) strongly suggests that NC remains bound to the TAR RNA even after heteroduplex formation.
Zinc finger-dependence of NC–TAR RNA binding
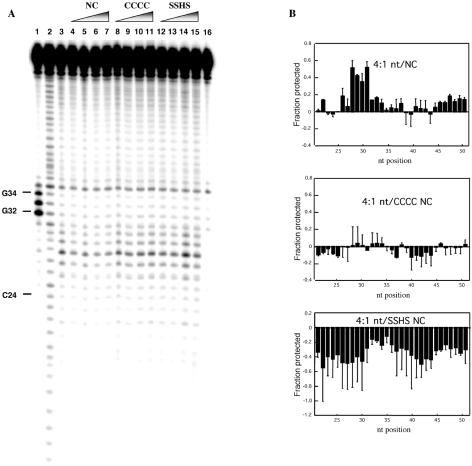
To elucidate the role of the zinc finger domains on NC–TAR RNA binding, we carried out footprinting experiments with CCCC and SSHS NC. In these experiments we employed 5-fold lower TAR RNA concentration (200 nM) than in the experiments shown in Figure 2. Under these conditions differences between the wild-type and mutant forms of NC were clearly evident in annealing assays (data not shown). The TAR RNA cleavage results in the presence of wild-type and mutant forms of NC are shown in Figure 3A. These data were quantified and the results are depicted in the bar graphs shown in Figure 3B. The footprinting profile in the presence of wild-type NC under these conditions is similar to the results obtained using higher RNA concentrations (Fig. 2), with some differences observed in the loop region. In particular, moderate protection is observed toward the top of the 5′ stem and along most of the 3′ stem (Fig. 3B, top). We also observed significant protection in the stem–loop region (G28–G34), but not in the bulge (U23–U25). The protection from cleavage observed at loop nucleotides G32–G34 is in contrast to the results obtained at higher RNA concentrations, where enhanced cleavage was observed at these positions upon NC addition (Fig. 2A, lanes 5–7). Although the same nt:NC ratio was maintained under both sets of conditions, the dependence of the cleavage pattern in the loop on RNA concentration suggests a difference in the extent of NC binding directly to the loop and/or to adjacent helical regions.
Figure 3.
Fe–EDTA footprinting of [32P]-TAR RNA in the presence of wild-type and mutant NC. (A) Denaturing polyacrylamide gel showing the Fe–EDTA cleavage profiles of 200 nM [32P]-TAR RNA in the presence of NC (lanes 4–7), CCCC NC (lanes 8–11) and SSHS NC (lanes 12–15) present at the following nt:protein ratios: 16:1 (lanes 4, 8 and 12), 8:1 (lanes 5, 9 and 13), 4:1 (lanes 6, 10 and 14) and 2:1 (lanes 7, 11 and 15). Lane 3, cleavage pattern of TAR RNA in the absence of NC. Lanes 1 and 2, RNase T1 and alkaline hydrolysis ladders, respectively. Lane 16, control reaction carried out in the absence of NC and Fe–EDTA. (B) Bar graph representation of the protection profiles of TAR RNA based on the data shown in (A). Data are plotted for the protection observed in the presence of a 4:1 nt:protein ratio in each case. Positive and negative values indicate protection from cleavage and enhanced cleavage, respectively, compared to radiolabeled TAR RNA cleaved in the absence of mutant or wild-type NC. Data are based on quantification of two gels with the average value shown by the solid bar and the standard deviation indicated.
In contrast to the protection pattern observed with wild-type NC, only very minor protection and some enhancement of cleavage was observed with CCCC NC (Fig. 3B, middle). The difference with SSHS NC was even more dramatic. Instead of protection from cleavage, only enhanced cleavage was observed in the case of this mutant (Fig. 3B, bottom). These results suggest a substantially different mode of interaction with TAR RNA for the mutant NC proteins. Binding of these proteins appears to alter the TAR RNA conformation such that its phosphate backbone is somewhat more exposed to solvent, with SSHS NC binding resulting in a larger effect than CCCC NC.
Characterization of NC–TAR RNA binding by fluorescence anisotropy
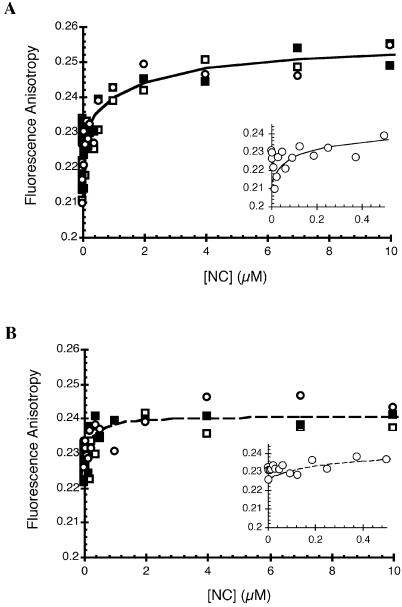
In an attempt to gain further insights into NC–TAR RNA binding parameters, we employed fluorescence anisotropy to determine relative binding affinities of wild-type, CCCC and SSHS NC to a 63-nt 5′-Cy3-TAR RNA variant. The starting anisotropy value measured for the free RNA in solution was 0.231 ± 0.002. Upon titration of wild-type and CCCC NC to TAR RNA, an initial decrease in anisotropy was observed to 0.213 and 0.226, respectively (Fig. 4A and B, insets). Although the nature of this decrease in anisotropy at the low concentrations of NC is unclear, it may be due to NC-induced destabilization of the end of the helix, which affects local probe mobility. Following the initial decrease, increases in anisotropy were observed with plateau values of ∼0.25 and ∼0.24 for wild-type and CCCC NC, respectively (Fig. 4). Thus, despite the fact that NC has an approximately three times lower molecular mass than TAR RNA, protein addition significantly perturbs the rotational mobility of the TAR hairpin, suggesting that binding interactions are detectable by this method. Although it is likely that multiple NCs are bound to the TAR RNA, for wild-type NC, the anisotropy data could be fit to a two-site model, with apparent Kd values of 60 ± 4 and 2200 ± 10 nM. We cannot rule out the possibility that RNA aggregation may have contributed to the observed increase in anisotropy at the higher NC concentrations (see below). Although the changes in anisotropy were smaller upon CCCC NC binding, the data could be fit to a single site model with an apparent Kd value of 200 ± 40 nM. These results suggest that wild-type NC has an ∼3-fold higher affinity for TAR RNA relative to CCCC NC.
Figure 4.
Fluorescence anisotropy analysis of the binding of wild-type and CCCC NC to 5′-Cy3-TAR RNA. Data were analyzed as described in Materials and Methods. Three independent binding curves are shown as open squares, closed squares and open circles for (A) wild-type and (B) CCCC NC. The insets show an expanded view of the binding curves at the lower NC concentrations for a single data set. The dashed and solid lines correspond to the fit of the data to one- (B) and two-site (A) binding models, respectively.
In contrast to results obtained with wild-type and CCCC NC, no change in anisotropy was observed upon addition of SSHS NC (data not shown), suggesting either a binding mode that does not result in detectable changes in the rotational mobility of the RNA hairpin, or extremely weak binding of this mutant to the TAR RNA. The latter is consistent with the lack of protection observed in the hydroxyl radical footprinting experiments.
Zinc finger-dependent TAR RNA aggregation
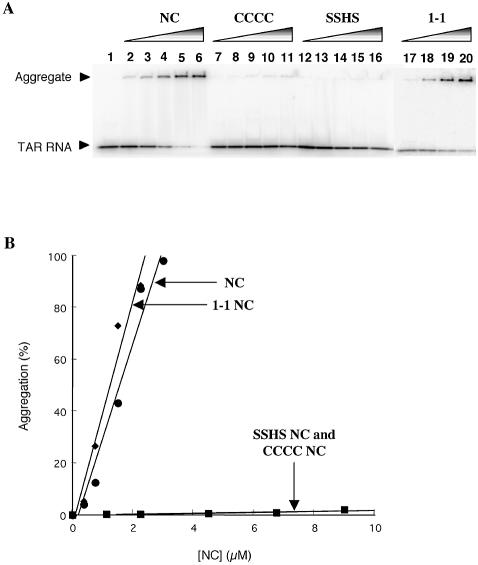
Previous annealing assays (16,21) along with the present studies suggest that the CCHC motif in the first zinc finger domain is critical for NC-mediated TAR RNA/DNA complex formation. The footprinting results (Fig. 3) and fluorescence anisotropy analysis (Fig. 4) support a role for this structural motif in NC–TAR RNA interaction. To further characterize TAR RNA binding by NC, we carried out gel mobility-shift assays with wild-type NC and three zinc finger mutants: CCCC, SSHS and 1-1 NC. In the latter variant, the C-terminal zinc finger domain of wild-type NC is replaced with the sequence present in the N-terminal domain to generate a first finger repeat mutant. The activity of the 1-1 NC mutant was previously shown to be only slightly lower than that of wild-type NC in both annealing and minus-strand transfer assays (21).
As seen in the gel shown in Figure 5A, rather than observing discrete bands corresponding to distinct protein–RNA complexes, in the presence of wild-type NC, we observed a gradual increase in NC–TAR RNA aggregate formation as evidenced by the appearance of a band at the top of the sample well (lanes 2–6). A similar trend was observed for 1-1 NC (lanes 17–20). In contrast, no significant aggregates formed in the presence of CCCC or SSHS NC even when concentrations as high as 1:50 nt:NC ratio were used (Fig. 5A, lanes 7–11 and 12–16, respectively). Figure 5B is a graphical representation of the gel-shift results. Taken together, these data demonstrate that the first zinc finger motif is vital for NC–TAR RNA aggregate formation, although it cannot be ruled out that the second finger is also required.
Figure 5.
NC-induced TAR RNA aggregation assay. (A) Native 8% polyacrylamide gel showing aggregation of TAR RNA in the presence of wild-type and mutant NC. [32P]-TAR RNA (3 nM) was incubated in the absence of NC (lane 1), or in the presence of 0.37, 0.75, 1.5, 2.2 and 3.0 µM NC (lanes 2–6), 1.1, 2.2, 4.5, 6.7 and 9.0 µM CCCC NC (lanes 7–11), 1.1, 2.2, 4.5, 6.7 and 9.0 µM SSHS NC (lanes 12–16), and 0.37, 0.75, 1.5 and 2.25 µM 1-1 NC (lanes 17–20). The arrowheads show the position of free TAR RNA and the TAR RNA–NC aggregate. (B) Graph showing concentration-dependent TAR RNA aggregation in the presence of wild-type and mutant NC. Data points are based on quantification of the gel shown in (A).
DISCUSSION
HIV-1 NC is a nucleic acid chaperone protein that facilitates unwinding and hybridization of complementary nucleic acid strands (5,37–41). The role of the zinc finger motifs of NC in its various functions has been the subject of extensive investigation both in vivo (42–47) and in vitro (16,21–23,48). The zinc fingers are dispensable for tRNA primer annealing to the primer binding site (14) and for the annealing step in plus-strand transfer (16). Both of these reactions involve formation of a relatively short 18-nt base pairing interaction. In contrast, the zinc finger motifs of NC are critical for the destabilization and subsequent annealing of the highly structured TAR RNA element and its complementary DNA (16,21). This annealing reaction, which is part of the minus-strand transfer step of reverse transcription, results in formation of a 98-nt base paired binary complex. In agreement with the critical role of the N-terminal zinc finger in minus-strand transfer, single molecule DNA stretching studies showed that the capability of NC to destabilize λ-DNA and to lower the cooperativity of the helix–coil transition depends on the presence of a wild-type first zinc finger motif (22,23). Taken together, these previous results suggested that the zinc finger structures are critical for facilitating the melting and reannealing of long and/or highly structured nucleic acid helices.
NC binding to TAR DNA has recently been characterized by UV absorbance and fluorescence spectroscopy (49,50). These studies showed that NC binding to TAR DNA alone, results in a shift in the population distribution of TAR DNA molecules towards less folded states. It has been estimated that under these conditions as many as eight base pairs of TAR DNA are destabilized upon NC binding at a nt:NC ratio of 5:1 (49). A more substantial conformational change of TAR DNA occurs in the presence of both NC and the TAR RNA acceptor (50).
Destabilization of TAR RNA upon NC binding has previously been shown to be significantly less than that of TAR DNA (49). In this work, our aim was to continue to characterize the interactions of NC with TAR RNA and to determine what role, if any, the zinc fingers play in these interactions. In accordance with previous studies using longer TAR RNA and DNA constructs, our annealing assays using only the core TAR RNA hairpin and the complementary TAR DNA sequence showed a significant dependence on the presence of the wild-type zinc finger motifs for optimal chaperone activity (data not shown).
Using the truncated TAR RNA system, hydroxyl radical footprinting revealed that wild-type NC binds to TAR RNA and remains bound to the annealed TAR RNA/DNA binary complex. In the absence of NC, approximately half of the TAR RNA was susceptible to hydroxyl radical cleavage. Upon addition of NC, a distinct protection pattern was observed on the TAR RNA molecule (Fig. 2A and B). When TAR DNA was also present, we observed protection from hydroxyl radical cleavage in the case of a NC-annealed RNA/DNA complex but not in the case of a heat-annealed complex (Fig. 2C and D). This result suggests that NC does not dissociate, but rather remains bound to the TAR RNA/DNA binary complex. In contrast to the protection from cleavage observed with wild-type NC, we observed little protection, but rather mild to moderate enhancement of cleavage upon binding of CCCC and SSHS NC to TAR RNA (Fig. 3). These results indicate that the binding interactions of wild-type and mutant NC proteins with TAR RNA are considerably different. Although the CCCC mutant consistently displays lower chaperone activity than SSHS NC (21, this work), based on the fluorescence anisotropy data reported here, the latter mutant binds more weakly to TAR RNA than the former. Taken together, these data suggest that the defect in chaperone activity observed for some NC zinc finger mutants is not necessarily directly correlated with lower binding affinity.
NC presumably enhances nucleic acid annealing rates by destabilizing helices and by facilitating duplex nucleation. The latter effect is related to the ability of NC to aggregate nucleic acids. Previously, using quasielastic light scattering and optical density measurements, it was shown that NC-induced aggregation of poly(A) and ribosomal RNA depends on the basic regions of NC but not the zinc finger motifs (51,52). In contrast, our data suggest that the zinc finger motifs of NC, particularly the N-terminal zinc finger domain, are critical for aggregation of TAR RNA. Using gel-shift assays, aggregation of TAR RNA was only observed in the presence of wild-type and 1-1 NC, but not upon addition of high concentrations of CCCC and SSHS NC (Fig. 5). Although additional NC mutants were not analyzed here, previous reports using these and other zinc finger mutants established the importance of the N-terminal zinc finger of NC for optimal chaperone activity (21,23). A recent study using 1-1 NC as well as 2-2 NC, a mutant wherein finger 2 replaces finger 1, suggested that the first finger was more important for helix destabilization than finger 2 (48). Our observations using CCCC NC suggest that the N-terminal finger also plays a critical role in nucleic acid aggregation.
Taken together, these data support a significantly altered mode of TAR RNA binding for the NC mutants. In particular, the wild-type mode of binding is clearly dependent on the presence of the zinc finger structures. These data help to explain the requirement for a specific zinc finger architecture for optimal NC-facilitated annealing in the minus-strand transfer step of reverse transcription.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Dr Matthew Fivash (the National Cancer Institute, NIH) for many helpful suggestions on the fluorescence anisotropy data analysis. We would also like to thank Drs Soobong Park and Natalia Tretyakova (University of Minnesota) for carrying out the mass spectrometry of the 5′-Cy3-TAR RNA, and Donald G. Johnson and Cathy V. Hixson for their assistance in producing the recombinant mutant NC proteins. This research was supported by an NIH postdoctoral grant F32 GM64973 (N.L.) and NIH grant AI65056 (K.M.-F). R.J.G. acknowledges support from the National Cancer Institute, NIH, under contract number N01-CO-12400 with SAIC-Frederick.
REFERENCES
- 1.South T.L., Kim,B., Hare,D.R. and Summers,M.F. (1990) Zinc fingers and molecular recognition: structure and nucleic acid binding studies of an HIV zinc finger-like domain. Biochem. Pharmacol., 40, 123–129. [DOI] [PubMed] [Google Scholar]
- 2.Summers M.F., Henderson,L.E., Chance,M.R., Bess,J.W.,Jr, South,T.L., Blake,P. R., Sagi,I., Perez-Alvarado,G., Sowder,R.C.,III, Hare,D.R. et al. (1992) Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci., 1, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg J.M. (1986) Potential metal-binding domains in nucleic acid binding proteins. Science, 232, 485–487. [DOI] [PubMed] [Google Scholar]
- 4.de Rocquigny H., Gabus,C., Vincent,A., Fournie-Zaluski,M.C., Roques,B.P. and Darlix,J.L. (1992) Viral RNA annealing activities of human immunodeficiency virus type 1 nucleocapsid protein require only peptide domains outside the zinc fingers. Proc. Natl Acad. Sci. USA, 89, 6472–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darlix J.L., Lapadat-Tapolsky,M., de Rocquigny,H. and Roques,B.P. (1995) First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J. Mol. Biol., 254, 523–537. [DOI] [PubMed] [Google Scholar]
- 6.Allain B., Lapadat-Tapolsky,M., Berlioz,C. and Darlix,J.L. (1994) Transactivation of the minus-strand DNA transfer by nucleocapsid protein during reverse transcription of the retroviral genome. EMBO J., 13, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You J.C. and McHenry,C.S. (1994) Human immunodeficiency virus nucleocapsid protein accelerates strand transfer of the terminally redundant sequences involved in reverse transcription. J. Biol. Chem., 269, 31491–31495. [PubMed] [Google Scholar]
- 8.DeStefano J.J. (1995) Human immunodeficiency virus nucleocapsid protein stimulates strand transfer from internal regions of heteropolymeric RNA templates. Arch. Virol., 140, 1775–1789. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Rodriguez L., Tsuchihashi,Z., Fuentes,G.M., Bambara,R.A. and Fay,P. J. (1995) Influence of human immunodeficiency virus nucleocapsid protein on synthesis and strand transfer by the reverse transcriptase in vitro. J. Biol. Chem., 270, 15005–15011. [DOI] [PubMed] [Google Scholar]
- 10.Guo J., Henderson,L.E., Bess,J., Kane,B. and Levin,J.G. (1997) Human immunodeficiency virus type 1 nucleocapsid protein promotes efficient strand transfer and specific viral DNA synthesis by inhibiting TAR-dependent self-priming from minus-strand strong-stop DNA. J. Virol., 71, 5178–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auxilien S., Keith,G., Le Grice,S.F. and Darlix,J.L. (1999) Role of post-transcriptional modifications of primer tRNALys,3 in the fidelity and efficacy of plus strand DNA transfer during HIV-1 reverse transcription. J. Biol. Chem., 274, 4412–4420. [DOI] [PubMed] [Google Scholar]
- 12.Wu T., Guo,J., Bess,J., Henderson,L.E. and Levin,J.G. (1999) Molecular requirements for human immunodeficiency virus type 1 plus-strand transfer: analysis in reconstituted and endogenous reverse transcription systems. J. Virol., 73, 4794–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y., Khorchid,A., Gabor,J., Wang,J., Li,X., Darlix,J.L., Wainberg,M.A. and Kleiman,L. (1998) The role of nucleocapsid and U5 stem/A-rich loop sequences in tRNA(3Lys) genomic placement and initiation of reverse transcription in human immunodeficiency virus type 1. J. Virol., 72, 3907–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hargittai M.R., Mangla,A.T., Gorelick,R.J. and Musier-Forsyth,K. (2001) HIV-1 nucleocapsid protein zinc finger structures induce tRNA(Lys,3) structural changes but are not critical for primer/template annealing. J. Mol. Biol., 312, 985–997. [DOI] [PubMed] [Google Scholar]
- 15.Tisné C., Roques,B.P. and Dardel,F. (2001) Heteronuclear NMR studies of the interaction of tRNA(Lys)3 with HIV-1 nucleocapsid protein. J. Mol. Biol., 306, 443–454. [DOI] [PubMed] [Google Scholar]
- 16.Guo J., Wu,T., Anderson,J., Kane,B.F., Johnson,D.G., Gorelick,R.J., Henderson,L.E. and Levin,J.G. (2000) Zinc finger structures in the human immunodeficiency virus type 1 nucleocapsid protein facilitate efficient minus- and plus-strand transfer. J. Virol., 74, 8980–8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J.K., Palaniappan,C., Wu,W., Fay,P.J. and Bambara,R.A. (1997) Evidence for a unique mechanism of strand transfer from the transactivation response region of HIV-1. J. Biol. Chem., 272, 16769–16777. [DOI] [PubMed] [Google Scholar]
- 18.Lapadat-Tapolsky M., Gabus,C., Rau,M. and Darlix,J.L. (1997) Possible roles of HIV-1 nucleocapsid protein in the specificity of proviral DNA synthesis and in its variability. J. Mol. Biol., 268, 250–260. [DOI] [PubMed] [Google Scholar]
- 19.Driscoll M.D. and Hughes,S.H. (2000) Human immunodeficiency virus type 1 nucleocapsid protein can prevent self-priming of minus-strand strong stop DNA by promoting the annealing of short oligonucleotides to hairpin sequences. J. Virol., 74, 8785–8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Déméné H., Dong,C.Z., Ottmann,M., Rouyez,M.C., Jullian,N., Morellet,N., Mély,Y., Darlix,J.L., Fournié-Zaluski,M.C., Saragosti,S. et al. (1994) 1H NMR structure and biological studies of the His23→Cys mutant nucleocapsid protein of HIV-1 indicate that the conformation of the first zinc finger is critical for virus infectivity. Biochemistry, 33, 11707–11716. [DOI] [PubMed] [Google Scholar]
- 21.Guo J., Wu,T., Kane,B.F., Johnson,D.G., Henderson,L.E., Gorelick,R.J. and Levin,J.G. (2002) Subtle alterations of the native zinc finger structures have dramatic effects on the nucleic acid chaperone activity of human immunodeficiency virus type 1 nucleocapsid protein. J. Virol. 76, 4370–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams M.C., Rouzina,I., Wenner,J.R., Gorelick,R.J., Musier-Forsyth,K. and Bloomfield,V.A. (2001) Mechanism for nucleic acid chaperone activity of HIV-1 nucleocapsid protein revealed by single molecule stretching. Proc. Natl Acad. Sci. USA, 98, 6121–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams M.C., Gorelick,R.J. and Musier-Forsyth,K. (2002) Specific zinc-finger architecture required for HIV-1 nucleocapsid protein’s nucleic acid chaperone function. Proc. Natl Acad. Sci. USA, 99, 8614–8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan J.F. and Uhlenbeck,O.C. (1989) Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol., 180, 51–62. [DOI] [PubMed] [Google Scholar]
- 25.Lee B.M., De Guzman,R.N., Turner,B.G., Tjandra,N. and Summers,M.F. (1998) Dynamical behavior of the HIV-1 nucleocapsid protein. J. Mol. Biol., 279, 633–649. [DOI] [PubMed] [Google Scholar]
- 26.Carteau S., Gorelick,R.J. and Bushman,F.D. (1999) Coupled integration of human immunodeficiency virus type 1 cDNA ends by purified integrase in vitro: stimulation by the viral nucleocapsid protein. J. Virol. 73, 6670–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chertova E.N., Kane,B.P., McGrath,C., Johnson,D.G., Sowder,R.C.,II, Arthur,L.O. and Henderson,L.E. (1998) Probing the topography of HIV-1 nucleocapsid protein with the alkylating agent N-ethylmaleimide. Biochemistry, 37, 17890–17897. [DOI] [PubMed] [Google Scholar]
- 28.Hampel K.J., Walter,N.G. and Burke,J.M. (1998) The solvent-protected core of the hairpin ribozyme-substrate complex. Biochemistry, 37, 14672–14682. [DOI] [PubMed] [Google Scholar]
- 29.Lakowicz J.R. (1999) Principles of Fluorescence Spectroscopy. Plenum Publishers, New York, NY. [Google Scholar]
- 30.Lundblad J.R., Laurance,M. and Goodman,R.H. (1996) Fluorescence polarization analysis of protein-DNA and protein-protein interactions. Mol. Endocrinol., 10, 607–612. [DOI] [PubMed] [Google Scholar]
- 31.Zuker M. (2000) Calculating nucleic acid secondary structure. Curr. Opin. Struct. Biol., 10, 303–310. [DOI] [PubMed] [Google Scholar]
- 32.Latham J.A. and Cech,T.R. (1989) Defining the inside and outside of a catalytic RNA molecule. Science, 245, 276–282. [DOI] [PubMed] [Google Scholar]
- 33.Celander D.W. and Cech,T.R. (1991) Visualizing the higher order folding of a catalytic RNA molecule. Science, 251, 401–407. [DOI] [PubMed] [Google Scholar]
- 34.Cate J.H., Gooding,A.R., Podell,E., Zhou,K., Golden,B.L., Kundrot,C.E., Cech,T.R. and Doudna,J.A. (1996) Crystal structure of a group I ribozyme domain: principles of RNA packing. Science, 273, 1678–1685. [DOI] [PubMed] [Google Scholar]
- 35.Rupert P.B. and Ferré-D’Amare,A.R. (2001) Crystal structure of a hairpin ribozyme-inhibitor complex with implications for catalysis. Nature, 410, 780–786. [DOI] [PubMed] [Google Scholar]
- 36.Amarasinghe G.K., de Guzman,R.N., Turner,R.B., Chancellor,K.J., Wu,Z.R. and Summers,M.F. (2000) NMR structure of the HIV-1 nucleocapsid protein bound to stem-loop SL2 of the Ψ-RNA packaging signal. J. Mol. Biol., 301, 491–511. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchihashi Z. and Brown,P.O. (1994) DNA strand exchange and selective DNA annealing promoted by the human immunodeficiency virus type 1 nucleocapsid protein. J. Virol., 68, 5863–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herschlag D. (1995) RNA chaperones and the RNA folding problem. J. Biol. Chem., 270, 20871–20874. [DOI] [PubMed] [Google Scholar]
- 39.Rein A., Henderson,L.E. and Levin,J.G. (1998) Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem. Sci., 23, 297–301. [DOI] [PubMed] [Google Scholar]
- 40.Lorsch J.R. (2002) RNA chaperones exist and DEAD box proteins get a life. Cell, 109, 797–800. [DOI] [PubMed] [Google Scholar]
- 41.Dib-Hajj F., Kahn,R. and Giedroc,D.P. (1993) Retroviral nucleocapsid proteins possess potent nucleic acid strand renaturation activity. Protein Sci., 2, 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorelick R.J., Nigida,S.M.,Jr, Bess,J.W.,Jr, Arthur,L.O., Henderson,L.E. and Rein,A. (1990) Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J. Virol., 64, 3207–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorfman T., Luban,J., Goff,S.P., Haseltine,W.A. and Gottlinger,H.G. (1993) Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J. Virol., 67, 6159–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y. and Barklis,E. (1995) Nucleocapsid protein effects on the specificity of retrovirus RNA encapsidation. J. Virol., 69, 5716–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berkowitz R., Fisher,J. and Goff,S.P. (1996) RNA packaging. Curr. Top. Microbiol. Immunol., 214, 177–218. [DOI] [PubMed] [Google Scholar]
- 46.Tanchou V., Decimo,D., Pechoux,C., Lener,D., Rogemond,V., Berthoux,L., Ottmann,M. and Darlix,J.L. (1998) Role of the N-terminal zinc finger of human immunodeficiency virus type 1 nucleocapsid protein in virus structure and replication. J. Virol., 72, 4442–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorelick R.J., Gagliardi,T.D., Bosche,W.J., Wiltrout,T.A., Coren,L.V., Chabot,D.J., Lifson,J.D., Henderson,L.E. and Arthur,L.O. (1999) Strict conservation of the retroviral nucleocapsid protein zinc finger is strongly influenced by its role in viral infection processes: characterization of HIV-1 particles containing mutant nucleocapsid zinc-coordinating sequences. Virology, 256, 92–104. [DOI] [PubMed] [Google Scholar]
- 48.Derebail S.S., Heath,M.J. and DeStefano,J.J. (2003) Evidence for the differential effects of nucleocapsid protein on strand transfer in various regions of the HIV genome. J. Biol. Chem., 278, 15702–15712. [DOI] [PubMed] [Google Scholar]
- 49.Bernacchi S., Stoylov,S., Piémont,E., Ficheux,D., Roques,B.P., Darlix,J.L. and Mély,Y. (2002) HIV-1 nucleocapsid protein activates transient melting of least stable parts of the secondary structure of TAR and its complementary sequence. J. Mol. Biol., 317, 385–399. [DOI] [PubMed] [Google Scholar]
- 50.Hong M.K., Harbron,E.J., O‘Connor,D.B., Guo,J., Barbara,P.F., Levin,J.G. and Musier-Forsyth,K. (2003) Nucleic acid conformational changes essential for HIV-1 nucleocapsid protein-mediated inhibition of self-priming in minus-strand transfer. J. Mol. Biol., 325, 1–10. [DOI] [PubMed] [Google Scholar]
- 51.Stoylov S.P., Vuilleumier,C., Stoylova,E., de Rocquigny,H., Roques,B.P., Gérard,D. and Mély,Y. (1997) Ordered aggregation of ribonucleic acids by the human immunodeficiency virus type 1 nucleocapsid protein. Biopolymers, 41, 301–312. [DOI] [PubMed] [Google Scholar]
- 52.Le Cam E., Coulaud,D., Delain,E., Petitjean,P., Roques,B.P., Gérard,D., Stoylova,E., Vuilleumier,C., Stoylov,S.P. and Mély,Y. (1998) Properties and growth mechanism of the ordered aggregation of a model RNA by the HIV-1 nucleocapsid protein: an electron microscopy investigation. Biopolymers, 45, 217–229. [DOI] [PubMed] [Google Scholar]