Abstract
P/CAF is a histone acetyltransferase enzyme which was originally identified as a CBP/p300-binding protein. In this manuscript we report that human P/CAF is acetylated in vivo. We find that P/CAF is acetylated by itself and by p300 but not by CBP. P/CAF acetylation can be an intra- or intermolecular event. The intermolecular acetylation requires the N-terminal domain of P/CAF. The intramolecular acetylation targets five lysines (416–442) at the P/CAF C-terminus, which are in the nuclear localisation signal (NLS). Finally, we show that acetylation of P/CAF leads to an increment of its histone acetyltransferase (HAT) activity. These findings identify a new post-translation modification on P/CAF which may regulate its function.
INTRODUCTION
The packaging of the eukaryotic genome into chromatin provides the means for compaction of the entire genome inside the nucleus, restricting the access of many regulatory proteins required for essential biological processes such as replication, transcription, DNA repair and recombination (1). To counterbalance this repressive nature of chromatin, several factors are able to destabilise the nucleosome to facilitate the interaction of proteins with the DNA. Recent studies have identified two types of protein complexes capable of altering chromatin structure. One of them utilises the energy coming from the ATP hydrolysis to alter the structure of nucleosomes (2). The second one includes complexes which modify covalently the histone tails (3,4), by acetylation, methylation, phosphorylation, etc.
It has been known for many years that acetylation of the N-terminal tails of histones correlates with transcription activation (5,6). However, the underlying mechanisms and biological significance are just beginning to be revealed thanks to the identification of the first nuclear histone acetyltransferase (HAT), GCN5. Subsequently, many other transcription coactivators have been shown to be capable of acetylating nucleosomal histones: CBP/p300 (7), P/CAF (8), ACTR/SRC (9) TAFII250 (10), etc.
P/CAF was originally identified as a CBP/p300-binding protein due to its sequence similarity to the yeast GCN5 (yGCN5) (8). P/CAF and GCN5 show sequence conservation in the regions responsible for the HAT activity and the binding to yADA2 co-factor (11,12). In vitro, yGCN5 efficiently acetylates free histones in solution, but does not recognise nucleosomal histones as a substrate (13). However, in vivo, yGCN5 is found in a large complex containing yADA2 and yADA3 (14). In this complex, yGCN5 is the HAT enzymatic component and is responsible for the acetylation of nucleosomal histones (15). In contrast, P/CAF has an intrinsic ability to acetylate nucleosomal histones in vitro.
PCAF is also found in a complex consisting of more than 20 distinct polypeptides (16). Some of them are identical to TBP-associated factors (TAFs) and others are histone fold-containing factors. P/CAF is capable of activating transcription through interaction with CBP (17), and independently from CBP but depending on P/CAF’s intrinsic HAT activity (18). P/CAF is essential for myogenesis (19) and is capable of acetylating and coactivating MyoD transcription factor.
P/CAF and GCN5 are very unstable enzymes that rapidly and irreversibly lose histone acetyltransferase activity. In vitro, P/CAF is stabilised by the continuous presence of acetyl CoA and autoacetylation (20). Here, we demonstrate that P/CAF is autoacetylated in vivo and is a target for p300 acetylation, but not for CBP. We also find that the P/CAF acetylation can occur in cis and in trans. Finally, we show that acetylation of P/CAF leads to an increment of its HAT activity.
MATERIALS AND METHODS
Cell culture and transfections
U2Os and C2C12 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum (FCS) and grown at 37°C, 5% CO2. Cells were transfected by standard calcium phosphate coprecipitation with 10 µg of either expression vector. Transfected cells were washed after 14 h and harvested 36 h after transfection. Finally, they were washed once in phosphate-buffered saline (PBS). C2C12 differentiation to myotubes was induced by changing the 10% FCS by 5% horse serum for 4–10 days.
Plasmids and recombinant proteins
PGEX-P/CAF (FL: full length), pGEX-P/CAF (352–832), pGEX-P/CAF (352–658) and pGEX-P/CAF (352–658 mutated in the HAT domain) have been described elsewhere (18). Mutations in the P/CAF cDNA (pGEX-P/CAF amino acids 352–658) were introduced at lysines 416, 428, 430, 441, 442 or different combinations of them, using the QuikChange mutagenesis kit (Stratagene) following the manufacturer’s instructions and verified by DNA sequencing. GST and GST fusion proteins were expressed in Escherichia coli XA90 using the pGEX (Pharmacia) vector system, for 4 h after 0.1 mM IPTG addition at 30°C. Purification from crude bacterial lysates was performed as in Bannister and Kouzarides (7). The pCX-P/CAF-Flag (FL) was a gift from J. Sinclair. pCX-P/CAFΔHAT-Flag: the pCX-P/CAF-Flag plasmid was digested with BbrpI PvuII. An 84 bp fragment was released and the plasmid religated. This cloning strategy recovers the P/CAF frame leaving a deletion of 28 amino acids in the HAT domain. PCX-GFP-PCAF(FL)-Flag: GFP was inserted in-frame as a SmaI fragment into pCX-P/CAF(FL)-Flag SmaI site. PCX-GFP-PCAFΔHAT-Flag: GFP was inserted in-frame as a SmaI fragment into pCX-P/CAF(FL)-Flag SmaI site. PCX-GFP-P/CAF(K-R)-Flag: the 527 bp BlgII PvuII fragment of P/CAF(FL) was replaced by that containing the K-R mutations. PCX-GFP-P/CAF(K-R/ΔHAT)-Flag: pCX-GFP-P/CAF(K-R)-Flag plasmid was digested with BbrpI PvuII and religated. PGEX-HDAC1, pCDNA3-HDAC1-Flag and pCDNA3-p300-HA have been described elsewhere (7,18,21).
Cell extract preparation
Total cell extracts were prepared in RIPA (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA) buffer by keeping the cells on ice for 20 min followed by centrifugation at 13 000 r.p.m. for 10 min at 4°C. All buffers contained protease inhibitors (Boehringer).
HAT and immunoprecipitation (IP) HAT assay
The in vitro acetylation assay IP-HAT assays were performed as described elsewhere (7). Basically, extracts from transfected U2OS cells were made using 500 µl of RIPA buffer. The lysis mixture was incubated on ice for 20 min and cleared by centrifugation at 12 000 g for 10 min at 4°C. Two micrograms of HA antibody was used to precipitate for 3 h at 4°C with rotation. Then 15 µl of protein A/protein G-Sepharose beads (a 50:50 mix) were added and incubated overnight at 4°C with rotation. The immune complexes were pelleted by gentle centrifugation and washed three times with 1 ml of RIPA. After the final wash, the buffer was aspirated down to 30 µl and the liquid HAT assay was done as described (7). Purified chicken erythrocyte histones (Sigma) were used as substrate.
In vitro translation and pull-down assay
In vitro translations and GST pull-downs were performed essentially as described previously (18). The buffer used for the pull-downs was a variation of Z′ (25 mM HEPES pH 7.5, 12.5 mM MgCl2, 20% glycerol, 0.1% NP40, 250 mM KCl).
Immunoprecipitations and western analysis
Immunoprecipitations and western analysis were performed as described elsewhere (22), with modifications; 10-cm dishes of U2Os were transfected with 10 µg of each expression vector and harvested 36 h post-transfection. Cells were lysed in 1 ml of RIPA lysis buffer, and debris were removed by centrifugation. The cleared lysate was subjected to immunoprecipitation with 2 µg of the antibody for 3 h at 4°C with rotation. Then 15 µl of protein A/protein G-Sepharose beads (a 50:50 mix) was added and incubated overnight at 4°C with rotation. The immune complexes were pelleted by gentle centrifugation and washed three times with 1 ml of RIPA buffer and resuspended in loading buffer for SDS–PAGE. All buffers contained protease inhibitors (Boehringer). Western blotting was performed using standard procedures and visualised using an ECL kit (Amersham). The antibodies used were whole antisera anti-acetyl-Lys ab193 (abcam) at 1:1000 dilution, whole antisera anti P/CAF at 1:500 dilution, affinity purified anti-Flag M2 (Kodak) at 1:1100 dilution and affinity purified anti HA (Santa Cruz) at 1:1000 dilution.
RESULTS
P/CAF is acetylated in vivo
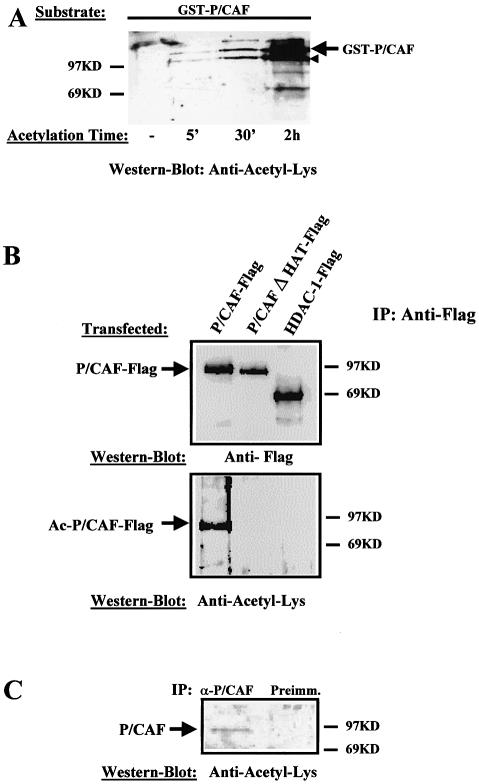
Previous work by Herrera et al. (20) has shown that P/CAF is autoacetylated in vitro. However, it is not yet known whether P/CAF acetylation occurs in vivo. To investigate this possibility, we have used an antibody raised against chemically acetylated histone H4, which recognises many acetylated proteins such as E2F, CBP, etc. (23) (anti-acetyl-Lys; ab-193 from abcam). First, we tested the antibody specificity for P/CAF acetylation. To that end, FL recombinant GST-P/CAF was incubated with acetyl-CoA and the acetylation status of GST-P/CAF was analysed by western blotting using the antibody anti-acetyl-Lys. The result (Fig. 1A) demonstrates that the antibody recognises specifically acetylated P/CAF in vitro, in an incubation-time dependent manner. The bottom band in the gel (indicated with a small arrow) corresponds to a GST-P/CAF degradation product.
Figure 1.
P/CAF is an acetyl-protein in vivo. (A) The antibody against Acetyl-Lys recognises acetylated P/CAF; 0.5 µg of recombinant GST-P/CAF was incubated for 0–2 h with acetyl-CoA, and the acetylated products were analysed by western blotting using an antibody that recognises acetylated lysines (anti-acetyl-Lys). The bottom band in the gel (indicated by a small arrow) corresponds to a GST-P/CAF degradation product. (B) P/CAF is acetylated in vivo. U2Os cells were transfected with 10 µg of Pcx-P/CAF-Flag, Pcx-P/CAFΔHAT-Flag and pCDNA3-HDAC1-Flag plasmids. Whole cell extracts from transfected cells were prepared, and Flag-proteins from them were immunoprecipitated using an anti-Flag antibody. The immunocomplexes were tested for P/CAF acetylation status by western blotting, using the antibody, which recognises acetylated lysines (anti-acetyl- Lys) (bottom part), and an antibody against the Flag-tag (top part). (C) Endogenous P/CAF is acetylated in vivo. P/CAF from C2C12 cells was immunoprecipitated using an anti P/CAF antibody or the preimmune serum (as a control), and the immunocomplexes were analysed for their acetylation level by western blotting, using the anti-acetyl-Lys antibody.
We then wanted to establish whether P/CAF is also acetylated in vivo. To that end, U2OS cells were transfected with P/CAF-Flag, P/CAF-Flag mutated in the HAT domain (P/CAFΔHAT-Flag) which has lost the ability to acetylate histones (data not shown), and HDAC1-Flag as a control for the Flag epitope. Flag proteins were immunoprecipitated using the anti-Flag antibody and their acetylation level was determined by western blotting using the anti-acetyl-Lys antibody. We found (Fig. 1B, bottom part) that P/CAF-Flag is clearly recognised by the anti-acetyl-Lys antibody. Neither P/CAF mutated in the HAT domain (P/CAFΔHAT-Flag) nor HDAC1-Flag were recognised by the anti-acetyl-Lys antibody. This suggests that P/CAF is acetylated in vivo. To confirm this hypothesis, we analysed the acetylation status of the endogenous P/CAF from C2C12 cells. To do that, we immunoprecipitated P/CAF from C2C12 cells using a specific anti-P/CAF antibody and tested its acetylation level by western blotting, using the anti-acetyl-Lys antibody. The results in Figure 1C indicate that endogenous P/CAF is also acetylated in vivo.
P/CAF N-terminal domain is required for P/CAF trans acetylation in vivo
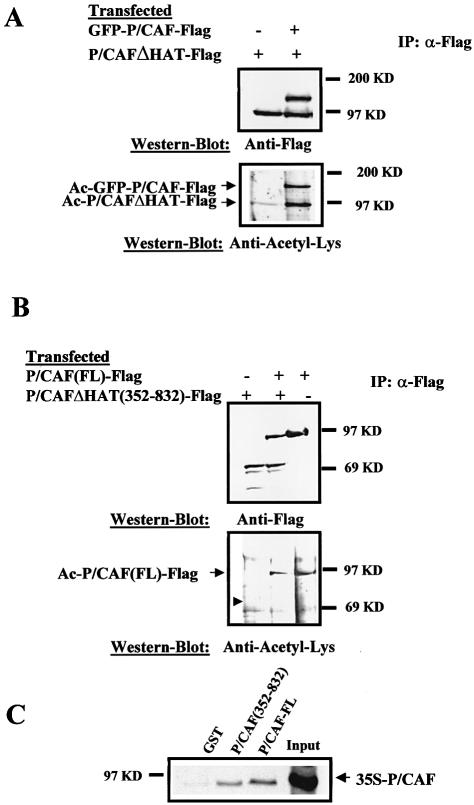
Having established that P/CAF is acetylated in vivo, we sought to investigate whether P/CAF is acetylated by an intramolecular (cis) or intermolecular (trans) event. To do so, we should be able to follow P/CAF (HAT-active protein) and P/CAFΔHAT (HAT-inactive protein) after co-transfection. Since both proteins co-migrate in an SDS–PAGE gel, we made a fusion of GFP to P/CAF-Flag to get GFP-P/CAF-Flag, which can be easily distinguished from P/CAF-Flag in an SDS–PAGE gel and maintains HAT activity (data not shown). Then, we transfected U2OS cells with combinations of vectors expressing active (GFP-P/CAF-Flag) and inactive (P/CAFΔHAT-Flag) proteins; immunoprecipitated them by using the anti-Flag antibody, and finally analysed their expression level and acetylation status by western blotting using the anti-Flag and anti-acetyl-Lys antibodies, respectively. The results in Figure 2A (bottom panel, lane 2) show that inactive P/CAF (P/CAFΔHAT-Flag) is acetylated in the presence of active P/CAF (GFP-P/CAF-Flag). Both proteins were expressed in U2OS to similar levels (top panel). The weak acetylation band of P/CAFΔHAT-Flag that we detect in the absence of GFP-P/CAF-Flag (bottom panel, lane 1) could be due to the activity of endogenous P/CAF enzyme. The results in Figure 2A suggest that acetylation of P/CAF(FL) can occur in vivo by an intermolecular reaction (in trans) in vivo.
Figure 2.
P/CAF is acetylated in cis and in trans in vivo. (A) P/CAF is acetylated in trans. U2Os cells were transfected with 10 µg of pCX-GFP-P/CAF-Flag and pCX-P/CAFΔHAT-Flag plasmids. Whole cell extracts from transfected cells were prepared and Flag-proteins from them were immunoprecipitated using an anti-Flag antibody. The immunocomplexes were tested for P/CAF acetylation status by western blotting, using the anti-acetyl-Lys antibody (bottom part of the figure) and an antibody against the Flag tag (top part). Positions of the acetylated GFP-P/CAF-Flag and acetylated P/CAFΔHAT-Flag are indicated by arrows. (B) P/CAF N-terminal domain is required for P/CAF trans acetylation in vivo. U2OS cells were transfected with 10 µg of pCX-P/CAF(FL)-Flag and pCX-P/CAFΔHAT (352–832)-Flag plasmids. Whole cell extracts were prepared, Flag proteins were immunoprecipitated by using the Flag antibody and P/CAF acetylation was analysed by western blotting using the anti-acetyl-Lys antibody (bottom part) and the anti-Flag antibody as a control (top part). The small arrow indicates where acetylated P/CAFΔHAT (352–832)-Flag should be found. (C) Deletion of the N-terminal domain does not affect P/CAF-P/CAF interaction. GST, GST-P/CAF and GST-P/CAF (352–832) fusion proteins were expressed and the binding to P/CAF FL in vitro translated and radiolabelled was determined by an in vitro pull down experiment (see Materials and Methods). Input represents 25% of total P/CAF input. The arrow indicates the P/CAF position in the gel.
Then we tried to identify the P/CAF region that is acetylated in trans. To that end, U2OS cells were transfected with P/CAF (FL)-Flag and P/CAFΔHAT (352–832)-Flag (which lacks the first 352 amino acids) expression vectors. Flag proteins were immunoprecipitated using the anti-Flag antibody and their expression level and acetylation status were determined by western blotting using the anti-Flag antibody (Fig. 2B, top panel) and anti-acetyl-Lys antibody (Fig. 2B, bottom panel), respectively. The result confirms our previous findings, showing that P/CAF FL is acetylated in vivo (lanes 2 and 3), and also indicates that P/CAFΔHAT (352–832)-Flag cannot be acetylated by P/CAF(FL)-Flag (lane 3, arrow). The lack of acetylation of P/CAFΔHAT (352–832)-Flag could be due to the deletion of the P/CAF-P/CAF interaction domain and not the acetylation sites per se. To rule out that possibility, we analysed the P/CAF-P/CAF interaction by in vitro pull down experiments. More precisely, radiolabelled P/CAF was pulled down with GST-P/CAF(FL) and GST-P/CAFΔHAT (352–832) and the bound protein was detected by radiography. Figure 2C shows that P/CAF is capable of interacting weakly with itself in vitro and this interaction is not affected when the N-terminal domain is deleted. We conclude that deletion of the N-terminal domain abrogates the P/CAF acetylation in trans in vivo.
P/CAF is acetylated in cis at its C-terminal domain
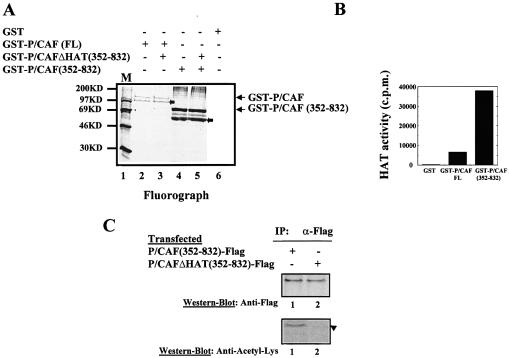
Once established that the P/CAF N-terminal domain is the major acetylation site in trans in vivo, we sought to determine whether P/CAF could also be acetylated in cis, as an intramolecular event. To ensure that we were monitoring a trans-independent event, we have used recombinant GST-P/CAF (352–832) (which cannot be acetylated in trans by P/CAF(FL) in vivo, Fig. 2B) and GST-P/CAFΔHAT (352–832) in an in vitro acetylation assay. Both recombinant proteins were incubated with 14C-acetyl-CoA, in presence or absence of GST-P/CAF(FL), and their acetylation levels were analysed by fluorography. Figure 3A shows that GST-P/CAF (352–832) is autoacetylated in vitro (lanes 4 and 5) while confirming that P/CAF(FL) cannot acetylate P/CAFΔHAT (352–832) (lane 3). We conclude from this experiment that P/CAF can be acetylated in cis, by an intramolecular event at the C-terminal domain.
Figure 3.
P/CAF is acetylated in cis at the C-terminus. (A) Recombinant GST-P/CAF(FL), GST-P/CAF (352–832) and GST-P/CAFΔHAT (352–832) were expressed in E.coli and purified. Fusion proteins were used in an in vitro acetylation assay. Acetylated proteins were visualised by fluorography. (B) HAT assay of GST-P/CAF(FL) and GST-P/CAF (352–832) recombinant proteins. (C) U2Os cells were transfected with 10 µg of pCX-P/CAF (352–832)-Flag and pCX-P/CAFΔHAT (352–832)-Flag plasmids. Whole cell extracts were prepared, Flag proteins were immunoprecipitated by using the Flag antibody and the immunocomplexes were tested for P/CAF acetylation status by western blotting, using the antibody which recognises acetylated lysines (anti-acetyl-Lys) (bottom part) and an antibody against the Flag-tag (top part). The position of the inactive protein is indicated by an arrow.
The bands of autoacetylated GST-P/CAF(FL) are weaker than autoacetylated GST-P/CAF (352–832); this is probably due to the fact that GST-P/CAF (352–832) has a higher intrinsic HAT activity than GST-P/CAF(FL) in vitro, as shown in Figure 3B, and as described previously (18).
Once it was established that P/CAF (352–832) is acetylated in vitro as an intramolecular event, we sought to determine whether it is also acetylated in vivo. To do that, we transfected U2OS cells with P/CAF (352–832)-Flag and P/CAFΔHAT (352–832)-Flag expression vectors. The Flag proteins were immunoprecipitated using the anti-Flag antibody and analysed by western blotting, using the anti-acetyl-Lys and anti-Flag antibodies. Figure 3C, shows that P/CAF (352–832)-Flag is acetylated (lane 1) in vivo. This autoacetylation is likely to occur as an intramolecular event because P/CAF lacking the N-terminal domain (1–352) cannot be acetylated in trans, either in vivo (Fig. 2B) or in vitro (Fig. 3A), by P/CAF(FL).
We conclude that P/CAF can be autoacetylated in vivo, both in trans and in cis at the C-terminal domain (amino acids 352–832).
P/CAF is acetylated by p300 in vivo
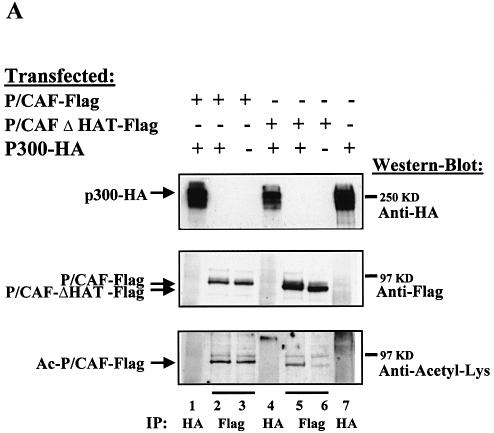
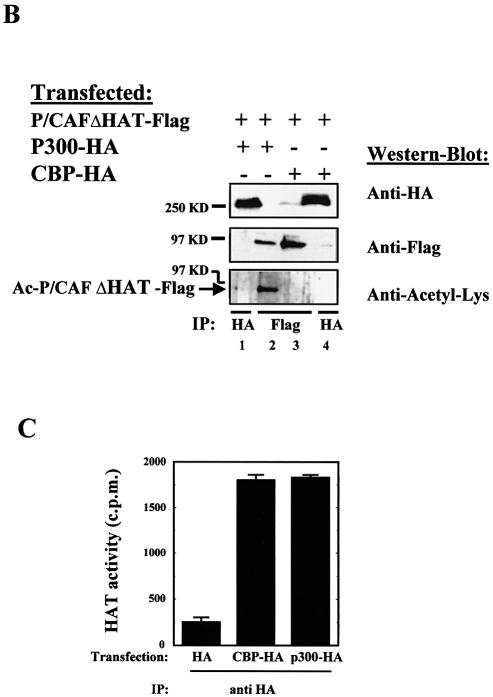
P/CAF was originally identified as a p300/CBP-interacting protein (8). CBP and p300 have HAT activity and are able to acetylate other factors different from histones (p53, GATA, 1, ELKF1, E2F1, TCF, HMGi(Y), MyoD, TFIIE, F, etc.) (24–31). Therefore, we sought to determine whether either CBP or p300 could acetylate P/CAF in vivo. To that end, we transfected P/CAF-Flag and P/CAFΔHAT-Flag in the presence or absence of p300-HA. Flag proteins were purified by immunoprecipitation using the anti-Flag antibody (Fig. 4A, lanes 2, 3, 5 and 6) and their acetylation levels were determined by western blotting, using the anti-acetyl-Lys antibody. The amount of p300-HA expressed in each transfection was monitored by IP with anti-HA antibody (lanes 1, 4 and 7). Our results show that p300 is able to acetylate P/CAF in vivo (lane 5). The weak acetylation band of P/CAFΔHAT-Flag that we detect in absence of P/CAF-Flag (lane 6) could be due to the HAT activity of endogenous p300 or P/CAF enzymes.
Figure 4.
P/CAF is acetylated by p300 in vivo. (A) PCX-P/CAF-Flag, PCX-P/CAFΔHAT-Flag and pCDNA3-p300-HA plasmids were transfected into U2OS cells. Proteins were immunoprecipitated using antibodies against HA (lanes 1, 4 and 7) and Flag (lanes 2, 3, 5 and 6) tags. The acetylation level of P/CAF was analysed by western blotting using the anti-acetyl-Lys antibody (bottom panel). (B) PCX-P/CAFΔHAT-Flag, pCDNA3-p300-HA and pCDNA3-CBP-HA plasmids were transfected into U2OS cells. The proteins were immunoprecipitated using antibodies against HA and Flag tags and the acetylated status of P/CAF was analysed by western blotting using the anti-acetyl-Lys antibody. (C) IP-HAT assay. U2OS cells were transfected with pCDNA3-p300-HA, pCDNA3-CBP-HA or the empty vector. CBP and p300 were immunoprecipitated using antibodies against HA and the HAT activity associated with the immunocomplexes was determined by liquid HAT assay.
We did not detect any increase in acetylation by p300 over the autoacetylation levels on active P/CAF (lanes 2 and 3). The reasons may be that acetylation by p300 could be masked by the strong autoacetylation signal due to P/CAF itself or that acetylation sites in P/CAF are redundant.
We also wanted to determine whether CBP could acetylate P/CAF. To do that, P/CAFΔHAT-Flag and CBP-HA plasmids were transfected into U2OS cells, Flag proteins were purified by immunoprecipitation using the anti-Flag antibody (Fig. 4B, lanes 2 and 3) and their acetylation levels were determined by western blotting using the anti-acetyl-Lys antibody. The expression levels of P300-HA and CBP-HA in each transfection were monitored by IP with anti-HA antibody (lanes 1 and 4). Figure 4B shows that p300 (lane 2) but not CBP (lane 3) is capable of acetylating P/CAF in vivo. The presence of HA tag on CBP is not affecting its HAT activity, because when we analysed HAT activity of HA-CBP and HA-p300 by IP-HAT assay, both proteins showed similar HAT activity on histones (Fig. 4C).
P/CAF is autoacetylated in its nuclear localisation signal (NLS)
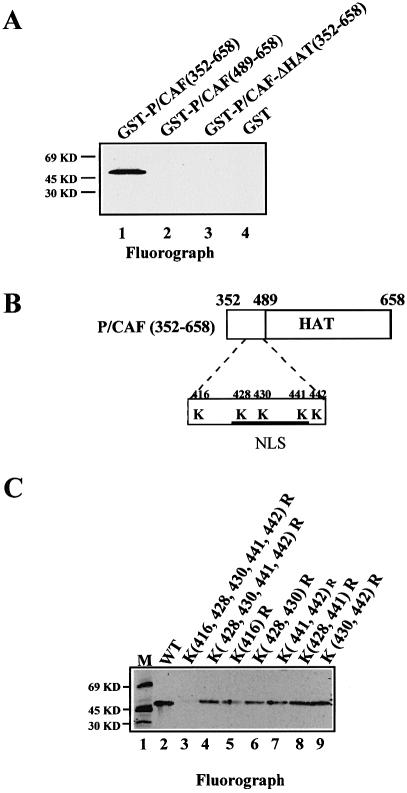
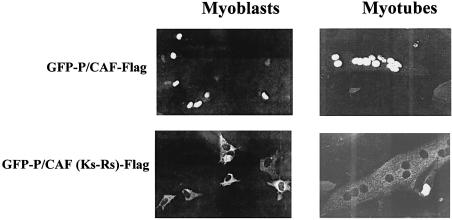
To try to establish the function of P/CAF autoacetylation, we decided to identify the lysines on P/CAF, which are acetylated in cis. To this end, we expressed various domains of P/CAF as GST-fusion proteins and tested their autoacetylation by an in vitro acetylation assay. Figure 5A clearly shows that GST-P/CAF (352–658) (which contains the HAT domain) is autoacetylated in vitro. The GST fusion [GST-P/CAF (489–658)], which does not include the region between 352 and 489 (but still maintains HAT activity, data not shown) lost the ability to be acetylated (Fig. 5A, lane 2). To analyse this region (amino acids 352–489) more closely, we mutated the lysines (Ks) present between positions 352 and 658 in the GST-P/CAF (352–658) construct (Fig. 5B) and analysed the autoacetylation activities of these mutants in an in vitro assay (Fig. 5C). (All the mutants were active in a HAT activity assay, data not shown.) The mutant that has lost the five Ks (416, 428, 430, 441 and 442) is not autoacetylated in vitro (Fig. 5C, lane 3). After examination of the 352–444 region, we realised that this region contains a putative NLS consensus sequence, between amino acids 425 and 445 (Fig. 5B). Therefore, we wanted to investigate whether P/CAF contains a functional NLS between these amino acids and whether P/CAF autoacetylation in cis could affect the NLS function. To investigate these possibilities, we constructed a GFP-P/CAF-Flag in which the five lysines at the putative NLS (428, 430, 441 and 442) were mutated to arginines (which maintain the positive charge of the NLS) [GFP-PCAF (Ks-Rs)]. C2C12 cells were transfected with GFP-P/CAF and GFP-PCAF(Ks-Rs) and we determined their cellular localisation by fluorescence. The results in Figure 6 show that GFP-P/CAF is nuclear before C2C12 differentiation (myoblast) and after differentiation to myotubes (top panels), and that the lysines between 425 and 445 are essential for localising the protein in the nucleus (bottom panels) defining the unique nuclear localisation signal within P/CAF between 425 and 445 amino acids. On the other hand, the acetylation of the NLS on P/CAF opened the possibility of regulating intracellular localisation by acetylation. However, GFP-P/CAF shows exclusively intranuclear localisation on different cell types (293-T, U2OS, C2C12; Fig. 6 and data not shown) and does not change localisation upon differentiation (Fig. 6), high confluence or growth in minimal medium (data not shown).
Figure 5.
Mapping of P/CAF cis-acetylation sites. (A) Recombinant GST-P/CAF(352–658) (lane 1), GST-P/CAF(489–658) (lane 2), GST-P/CAFΔHAT(352–658) (lane 3) and GST (lane 4) were tested for autoacetylation in an in vitro acetylation assay. The acetylated proteins were visualised by fluorography. (B) Schematic representation of the P/CAF (352–658) region (acetylated lysines are indicated). (C) Fluorograph showing P/CAF acetylation of the GST-P/CAF (352–658) wt (lane 2) and punctual mutants (lanes 3–9) indicated on the top of the figure. Lane 1 corresponds to the protein molecular weight marker.
Figure 6.
The acetylated lysines in cis are essential for the P/CAF NLS function. C2C12 cells were transfected with pCX-GFP-P/CAF(FL) and pCX-GFP-P/CAF(FL)(Ks-Rs) in which lysines 428, 430, 441 and 442 have been mutated to arginines. Localisation of the expressed proteins was detected before and after differentiation of C2C12 cells by fluorescence.
P/CAF autoacetylation and HAT activity
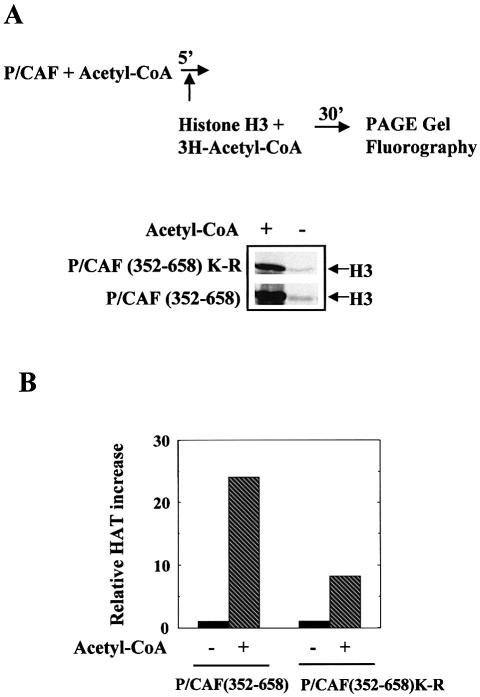
It has been proposed that P/CAF autoacetylation could play a role in regulating its HAT enzymatic activity (20). So we sought to determine whether autoacetylation in cis could affect the acetyltransferase activity. To do that, we incubated first either GST-P/CAF (352–658) or GST-P/CAF (352–658) mutated at lysines 416, 428, 430, 441 and 442 (P/CAF non-acetylable mutant) in the presence and in the absence of cold acetyl-CoA and then P/CAF HAT activity on identical amounts of histone H3 was analysed in an in vitro acetylation assay by fluorography (see diagram in Fig. 7A). The results in Figure 7A and their quantification in Figure 7B show that after incubation with acetyl-CoA, P/CAF is more active to acetylate histone H3. This effect is probably due to two independent processes: first, P/CAF stabilisation by acetyl-CoA as has been previously described by Herrera et al. (20), which probably affects to the same extent P/CAF and the non-acetylable P/CAF mutant; and secondly, P/CAF intra-acetylation, which increases 2- to 3-fold P/CAF HAT activity on histone H3.
Figure 7.
P/CAF auto-acetylation and HAT activity. (A) GST-P/CAF (352–658) or the non-acetylable P/CAF mutant [GST-P/CAF (352–658)K-R mutated at 416, 428, 430, 441 and 442 lysines] were incubated in the presence or absence of acetyl-CoA for 5 min, then purified H3 was added to the reaction and the P/CAF HAT activity on H3 was analysed by fluorography. (B) The results in (A) were quantified using a Molecular Dynamics machine and normalised relative to the values obtained in absence of acetyl-CoA (value 1).
DISCUSSION
The results presented here show that P/CAF is an acetyl-protein in vivo. P/CAF is acetylated by itself and by p300, but not by CBP in vivo. Acetylation of P/CAF seems to increase P/CAF stability/histone acetyltransferase activity.
In this paper, we give some evidence for the implementation of an acetylation cascade: P/CAF is acetylated in vivo by itself and by another HAT, p300, suggesting that an acetylase modifies the activity of a second acetylase, probably in order to transmit a biological signal. The fact that the bromodomain, which is present in many chromatin-working proteins, recognises acetyl-lysines (32–36) also suggests a cascade mechanism mediated by acetylation. How is the acetylation signal transmitted? We have shown that incubation of P/CAF with acetyl-CoA increases P/CAF HAT activity on histone H3, suggesting that acetylation could be a mechanism to regulate P/CAF HAT activity. On the other hand, our work demonstrates that P/CAF can be acetylated at the N-terminal domain in trans and at the C-terminus by an intramolecular event. This acetylation at different sites could have different in vivo consequences. It is possible that P/CAF is recruited to different promoters by distinct sets of transcription factors and P/CAF acetylation could contribute to this specificity. P/CAF acetylation could be involved in this recruitment by affecting the P/CAF-transcription factor interaction. In fact, HAT-transcription factor interactions have been shown to be regulated by acetylation (31), although we have not detected any difference in binding to CBP2, E1A, E2F and p53 in vitro (data not shown). Another possibility is that acetylation may change P/CAF specificity to acetylate different histones or factors. This may lead to differential activated states necessary for some promoters but not others. We have not observed any change in the P/CAF specificity to acetylate, in vitro, different histones upon autoacetylation (data not shown); however, the pool of P/CAF autoacetylated represents very little of the total amount of enzyme in the reaction so any small change could be masked. Even in this unfavourable condition what we could observe is an increase in the global HAT activity of P/CAF on histone H3.
In summary, we have identified a new post-translational modification of P/CAF in vivo, which leads to an increment of its HAT activity and could be part of a signal transduction cascade leading to regulation of the cellular HAT activities.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Xavier de la Cruz for critical reading of the manuscript. This study was funded by an EU grant to H.S.R., a Cancer Research UK grant to T.K. and by grants (PB-98-0468) and (SAF 2002-00741) from the Spanish Ministerio de Ciencia y Tecnología to M.M.B.
REFERENCES
- 1.Konberg R.D. and Lorch,Y. (1999) Chromatin-modifying and remodelling complexes. Curr. Opin. Genet. Dev., 9, 148–151. [DOI] [PubMed] [Google Scholar]
- 2.Kingston R.E. and Narlikar,G.J. (1999) ATP-dependent remodelling and acetylation as regulators of chromatin fluidity. Genes Dev., 13, 2339–2352. [DOI] [PubMed] [Google Scholar]
- 3.Wu J. and Grunstein,M. (2000) 25 years after the nucleosome model: chromatin modifications. Trends Biochem. Sci., 25, 619–623. [DOI] [PubMed] [Google Scholar]
- 4.Berger S. (2002) Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev., 12, 142–148. [DOI] [PubMed] [Google Scholar]
- 5.Hebbes T.R., Thorne,A.W. and Crane-Robinson,C. (1988) A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J., 7, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner B.M. and O’Neill,L.P. (1995) Histone acetylation on chromatin and chromosomes. Semin. Cell Biol., 6, 229–236. [DOI] [PubMed] [Google Scholar]
- 7.Bannister A.J. and Kouzarides,T. (1996) The CBP co-activator is a histone acetyltransferase. Nature, 384, 641–643. [DOI] [PubMed] [Google Scholar]
- 8.Yang X.-J., Ogryzko,V.V., Nishikawa,J., Howard,B. and Nakatani,Y. (1996) A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature, 382, 319–324. [DOI] [PubMed] [Google Scholar]
- 9.Mizzen C.A., Yang,X.J., Kokubo,T., Brownell,J.E., Bannister,A.J., Owen-Hughes,T., Workman,J., Wang,L., Berger,S.L., Kouzarides,T. et al. (1996) The TAF(II) 250 subunit of TFIID has histone acetyltransferase activity. Cell, 87, 1261–1270. [DOI] [PubMed] [Google Scholar]
- 10.Spencer T.E., Jenster,G., Burcin,M.M., Allis,C.D., Zhou,J., Mizzen,C.A., McKenna,N.J., Onate,S.A., Tsai,S.Y., Tsai,M.J. and O’Malley,B.W. (1997) Steroid receptor coactivator-1 is a histone acetyltransferase. Nature, 389, 194–198. [DOI] [PubMed] [Google Scholar]
- 11.Candau R., Zhou,J.X., Allis,D. and Berger,S.L. (1997) Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J., 16, 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Liu,L. and Berger,S.L. (1998) Critical residues for histone acetylation by Gcn5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev., 12, 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownell J.E., Zhou,J., Ranalli,T., Kobayashi,R., Edmondson,D.G., Roth,S.Y. and Allis,C.D. (1996) Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5 linking histone acetylation to gene activation. Cell, 84, 843–851. [DOI] [PubMed] [Google Scholar]
- 14.Candau R. and Berger,S.L. (1996) Structural and functional analysis of yeast putative adaptors. Evidence for an adaptor complex in vivo. J. Biol. Chem., 271, 5237–5245. [DOI] [PubMed] [Google Scholar]
- 15.Grant P.A., Duggan,L., Cote,J., Roberts,S.M., Brownell,J.E. Candau,R., Ohba,R., Owen-Hughes,T., Allis,C.D., Winston,F. et al. (1997). Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev., 11, 1640–1650. [DOI] [PubMed] [Google Scholar]
- 16.Ogryzko V.V., Schiltz,R.L., Russanova,V., Howars,B.H. and Nakatani,Y. (1996) The transcription coactivators p300 and CBP are histone acetyltransferases. Cell, 87, 953–959. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Balbás M.A., Bannister,A.J., Martin,K., Haus-Seuffert,P., Meisterernst,M. and Kouzarides,T. (1998) The acetyltransferase activity of CBP stimulates transcription. EMBO J., 17, 2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid L.J. Bannister,A.J., Zagerman,P., Martínez-Balbás,M.A. and Kouzarides,T. (1998) E1A directly binds and regulates the P/CAF acetyltransferase. EMBO J., 17, 4469–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puri P.L., Sartorelli,V., Yang,X.J., Hamamori,Y., Ogryzko,V.V., Howard,B.H., Kedes,L., Wang,J.Y., Graessmann,A., Nakatani,Y. and Levrero,M. (1998) Differential roles of p300 and P/CAF acetyltransferases in muscle differentiation. Mol. Cell, 1, 35–45. [DOI] [PubMed] [Google Scholar]
- 20.Herrera J.E., Bergel,M., Yang,X.-J., Nakatani,Y. and Bustin,M. (1997) The histone acetyltransferase activity of human GCN5 and P/CAF is stabilized by coenzymes. J. Biol. Chem., 43, 27253–27258. [DOI] [PubMed] [Google Scholar]
- 21.Brehm A., Miska,E.A., McCance,D.J., Reid,J.L., Bannister,A.J. and Kouzarides,T. (1998) Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature, 391, 597–601. [DOI] [PubMed] [Google Scholar]
- 22.Lavender P., Vandel,L., Bannister,A.J. and Kouzarides,T. (1997) The HMG-box transcription factor HBP1 is targeted by the pocket proteins and E1A. Oncogene, 14, 2721–2728. [DOI] [PubMed] [Google Scholar]
- 23.Hebbes T.R., and Allen,S.C. (2000) Multiple histone acetyltransferases are associated with a chicken erythrocyte chromatin fraction enriched in active genes. J. Biol. Chem. 275, 31347–31352. [DOI] [PubMed] [Google Scholar]
- 24.Gu W. and Roeder,R.G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of p53 C-terminal domain. Cell, 90, 595–606. [DOI] [PubMed] [Google Scholar]
- 25.Boyes J., Byfield,P., Nakatani,Y. and Ogryzko,V. (1998) Regulation of the activity of the transcription factor GATA-1 by acetylation. Nature, 396, 594–598. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Balbás M.A., Bauer,U.M., Nielsen,S.J., Brehm,A. and Kouzarides,T. (2000) Regulation of E2F activity by acetylation. EMBO J., 19, 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sartorelli V., Puri,P.L., Hamamori,Y., Ogryzko,V., Chung,G., Nakatani,Y., Wang,J.Y. and Kedes,L. (1999) Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell, 4, 725–734. [DOI] [PubMed] [Google Scholar]
- 28.Imhof A., Yang,X.J., Ogryzko,V.V., Nakatani,Y., Wolfe,A.P. and Ge,H. (1997). Acetylation of general transcription factors by histone acetyltransferases. Curr. Biol., 7, 689–692. [DOI] [PubMed] [Google Scholar]
- 29.Munshi N., Merika,M., Yie,J., Senger,K., Chen,G. and Thanos,D. (1998) Acetylation of HMG I(Y) by CBP turns off INF beta expression by disrupting the enhanceosome. Mol. Cell, 2, 457–467. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W. and Bieker,J.J. (1998) Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl Acad. Sci. USA, 95, 9855–9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waltzer L. and Bienz,M. (1998) Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature, 395, 521–525. [DOI] [PubMed] [Google Scholar]
- 32.Dhalluin C., Carlson,J.E., Zeng,L., He,C., Aggarwal,A.K. and Zhou,M.M. (1999) Structure and ligand of a histone acetyltransferase bromodomain. Nature, 3, 491–496. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson R.H., Ladurner,A.G., King,D.S. and Tjian,R. (2000) Structure and function of a human TAFII250 double bromodomain module. Science, 288, 1422–1425. [DOI] [PubMed] [Google Scholar]
- 34.Owen D.J., Ornaghi,P., Yang,J.C., Lowe,N., Evans,P.R., Ballario,P., Neuhaus,D., Filetici,P. and Travers,A.A. (2000) The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J., 19, 6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polesskaya A., Naguibneva,I., Duquet,A., Bengal,E., Robin,P. and Harel-Bellan,A. (2001) Interaction between acetylated MyoD and the bromodomain of CBP and/or p300. Mol. Cell. Biol., 21, 5312–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mujtaba S., Zeng,H.Y., Farooq,A., Carlson,J.E., Ott,M., Verdin,E. and Zhou,M.M. (2002) Structural basis of lysine-acetylated HIV-1 Tat recognition by PCAF bromodomain. Mol. Cell, 3, 575–586. [DOI] [PubMed] [Google Scholar]