Abstract
Recombinogenic engineering or recombineering is a powerful new method to engineer DNA without the need for restriction enzymes or ligases. We report here a general method for using recombineering to combine overlapping bacterial artificial chromosomes (BACs) to build larger, unified BACs. In order to test the feasibility of using recombineering to combine two large DNA fragments (>20 kb), we constructed a unified BAC containing the full-length tyrosinase-related protein-1 (Tyrp-1) gene from two library-derived BACs, one containing the 5′ regulatory elements and the other containing the 3′ coding exons. This was achieved using a two-step homologous recombination method enabled by the bacteriophage λ Red proteins. In the first step, retrieval, a large DNA fragment (∼22 kb) was retrieved from one of the original BACs. In the second step, recombination, the retrieved DNA fragment was inserted into the second original BAC to form the unified BAC containing all the desired Tyrp-1 sequence. To further demonstrate the general applicability of our approach, an additional DNA fragment (∼20 kb) was inserted into the unified BAC downstream of the coding region. This method should prove very useful for enabling BAC manipulation in a variety of scenarios.
INTRODUCTION
The ability to manipulate large DNA fragments is very important to contemporary biology. Although recombinant techniques were developed >25 years ago, improved methodologies remain highly prized, especially to aid functional genomic studies (1,2). Conventional approaches for the manipulation of DNA are multi-step and time consuming, involving the digestion of DNA by appropriate restriction enzymes followed by ligation into a suitable cloning vector. A critical factor in the success of traditional DNA manipulation is the length of the DNA to be engineered. Due to the demands of restriction specificity, it is very difficult to manipulate DNA molecules of >20 000 bp using traditional restriction–ligation methods (3). This is of particular concern to bacterial artificial chromosome (BAC) engineering, where there is often a need to engineer DNA fragments large enough to contain the appropriate regulatory elements for a certain gene’s expression (4). BACs have become powerful tools in functional genomic studies (1), so better methods for precisely manipulating BAC DNA are required.
Recently, scientists have developed a new method, known as recombineering, to manipulate large DNA fragments (5–10), and have overcome the difficulties in traditional BAC DNA engineering. These methods have been based on either the RecET proteins or the λ-recombination proteins. The RecET system is based on homologous recombination mediated by the RecE and RecT proteins. The Red system is based on homologous recombination mediated by three λ-recombination proteins, exo (α), bet (β) and gam (γ), collectively known as the Red proteins. The Red system is similar to the RecET system, but has been shown to be 50–100 times more efficient in Escherichia coli (6). A modified DH10B strain, called DY380, harboring a defective λ-prophage carrying the red genes under the tight control of the temperature-sensitive λ-cI857 repressor, has been created (10). Incubation of DY380 cells at 42°C results in the inactivation of the temperature-sensitive λ repressor, and hence the production of the exo (α), bet (β) and gam (γ) proteins, enabling recombination.
In our case, the tyrosinase-related protein-1 (Tyrp-1) gene (11) has been previously isolated from BAC libraries in two BACs, one comprising eight coding exons without upstream regulatory elements (named Tyrp-1 BAC1) and the other comprising the first two exons together with the gene’s 5′-upstream regulatory elements (named Tyrp-1 BAC2). It is a particularly challenging task to combine these original BACs into a single BAC comprising both the upstream regulatory elements and the coding exons. In this work, we used a two-step approach based on Red-mediated recombination to achieve this task: in the first step, retrieval, a large DNA fragment (22 463 bp) was retrieved from one of the original BACs. In the second step, recombination, the large DNA fragment was inserted into the second original BAC to form the unified BAC containing all the desired sequence. We then inserted yet more sequence downstream of the coding region into the unified BAC to generate a final Tyrp-1 BAC 31 160 bp larger than the original Tyrp-1 BAC2. This task would be extremely difficult by conventional means.
MATERIALS AND METHODS
Enzymes, media and bacterial strains
Restriction enzymes were purchased from New England Biolabs unless indicated otherwise. T4 DNA ligase was from Gibco. Chloramphenicol (Cm), kanamycin (Kan) and ampicillin (Amp) were from Sigma. Escherichia coli transformants were selected on tryptone–yeast extract agar medium containing the appropriate antibiotic at 12.5, 15 and 50 µg/ml, respectively. Primers were from Gensetoligo (Singapore). Plasmids were purified using a Qia Prep mini-prep kit (Qiagen). PCR products and restriction enzyme digest products were recovered with GeneElut™ agarose spin columns (Sigma). The genotype of DY380 (a gift from D. Court) is DH10B [λcl857 (cro-bioA) <> tet]; it harbors a defective λ-prophage carrying the red genes, but tet replaces the segment between cro and bioA in the DY380 strain. The genotype of 294-Flp was generated by integrating 705-Flp into the lacZ locus of E.coli strain MM294 (12) (a gift from A. F. Stewart).
Construction of plasmids
Two adjacent DNA fragments (70 and 500 bp) from the 3′ portion of Tyrp-1 BAC1 were amplified by PCR and cloned into the SalI and SacI sites of the pIGCN21 plasmid containing a frt–KanR–frt cassette (10) to generate the pIGCN21-Tyrp1 plasmid. The 70 and 500 bp flanked frt–KanR–frt cassette was then amplified from the pIGCN21-Tyrp1 plasmid with PCR primers (5′-TTTGTCGACGCTGTTCGAAGCCTTCACAACC-3′ and 5′-TTTGAGCTCCATGTGTGGCAAGGACTGTGAC-3′) and used to tag the Tyrp-1 BAC1.
The pBRBAC was constructed by subcloning a 870 bp EcoRI-digested fragment from pBeloBAC11 (4) into the EcoRI site of pBR322. The pBRBAC-AB retrieval vector was generated by inserting a 216 bp NdeI–EcoRV-homologous fragment (termed ArmA) and a 330 bp EcoRV–SacI-digested homologous fragment (termed ArmB) into the NdeI–SacI sites of pBRBAC. ArmA and ArmB were PCR amplified from Tyrp-1 BAC1 with primers F3, 5′-TTCATATGGCAAAATCTCTTCAGCGTC-3′ (italics indicating the NdeI site); R3, 5′-TTGATATCGAAGAGATTTTCTGCCAGAC-3′ (italics indicating the EcoRV site); F5, 5′- TGATATCTCATTTCATGCCAGTGCCAC-3′ (italics indicating the EcoRV site); and R5, 5′-GAGCTCAGAACAAATAAAACC-3′ (italics indicating the SacI site). The ArmA-Amp-Ori-Sp6-ArmB targeting cassette used for retrieving the 22.5 kb of the Tyrp-1 gene was amplified from pBRBAC-AB retrieval vector with primers R3 and F5 (see above). PCR products were purified using a QIA quick PCR Purification Kit (Qiagen) and digested with DpnI to remove DNA template. The linearized ArmA-Tyrp-1-ArmB-Sp6 targeting cassette used for targeting into the 5′ end of the Tyrp-1 gene in Tyrp-1 BAC2 was generated by digestion with NotI.
Preparation of the competent cells DY380 and generation of recombinants
For BAC modification, DY380 cells were transformed with Tyrp-1 BAC1 and Tyrp-1 BAC2 separately. A single colony was cultured in LB (+Cm) at 32°C for 16 h, then diluted 50-fold in LB medium (+Cm) and grown to an OD600 = 0.5–0.7. Cultures (10 ml) were exposed to 42°C for 15 min to induce recombination activity, then chilled on ice for 20 min. Cells were then centrifuged for 8 min, 5500 g, at 4°C and washed with 1 ml of ice-cold sterile 10% glycerol three times. Cells were resuspended in 80 µl of ice-cold sterile 10% glycerol containing 100 ng of linear DNA and electroporated. A 1 ml aliquot of LB medium was added after electroporation. Cells were incubated at 32°C for 1.5 h with shaking, then spread on appropriate selective agar media.
PCR and sequence analysis
BAC DNAs were purified with QiagenR Plasmid Purification Maxi kits. Tyrp-1 BAC was sequenced using an ABI 310 Sequencer with primers (F1, R1, F2, R2, F4, F5, R5 and Sp6) to confirm the position and orientation of the 22.5 kb fragment inserted into Tyrp-1 BAC2. Primers were F1, 5′-TCTAGACTTTTCTGTTTAATGTT-3′; R1, 5′-TAAGTAGGCTTCAGTGACTAGATTC-3′; F2, 5′-GCCTCACGATAAC AATTCCCTCTAC-3′; R2, 5′-GGCCAATGTCACACTTGTATTTTCTG-3′; F4, 5′-CAGGCAACCTCGGGAGGTAG-3′; and Sp6, 5′-ATTTAGGTGACACTATAG-3′. Primers used for checking the second round recombination events were F6, 5′-ATACAACATGGTGCCATTCTG-3′; R6, 5′-CTGGACTGGTGTGAGGCAGGTG-3′; F7, 5′-ACACTC GCCAGACATAAAATC-3′; and BAC-Sp6-R, 5′-ACCGTT CAGCTGGATATTACGGC-3′.
Further details on the cloning of the constructs are available from the authors on request.
RESULTS
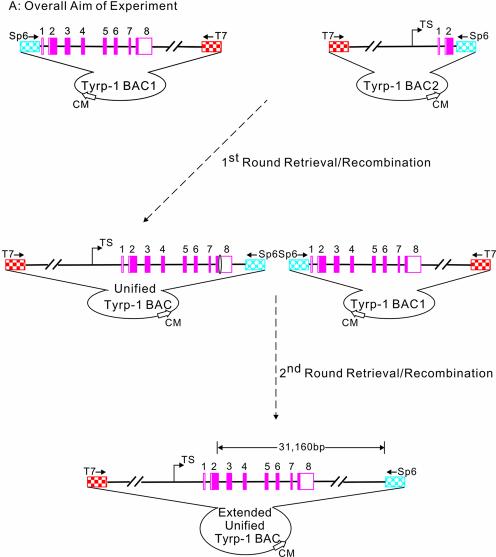
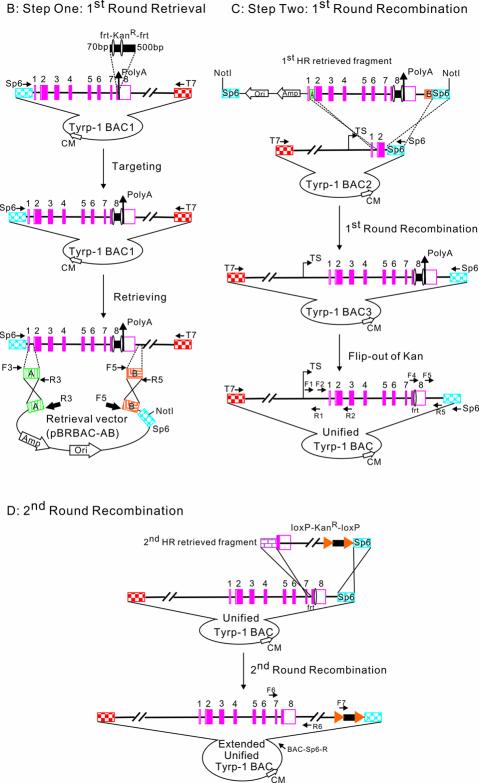
The general strategy for generating the full-length Tyrp-1 gene is shown in Figure 1. Our starting materials were two different BACs, both >100 kb in length. Tyrp-1 BAC1 contained exons 1–8 of the Tyrp-1 gene, whilst Tyrp-1 BAC2 contained the upstream regulatory regions together with exons 1 and 2 of the Tyrp-1 gene. The BACs were sequenced using T7 and Sp6 universal primers to confirm the orientation and breakpoint of the Tyrp-1 gene. The method for generating the unified BAC containing the full-length Tyrp-1 gene is best described by splitting it into two steps: retrieval and recombination. Retrieval (first round) consists of tagging the Tyrp-1 BAC1 with a selectable marker (frt–KanR–frt) and cloning of the KanR-tagged 22 463 bp DNA sequence into a retrieval vector, pBRBAC-AB. Recombination (first round) consists of the site-specific insertion of this 22 463 bp DNA fragment (3749 bp frt–KanR–frt cassette + 18 714 bp Tyrp-1 genomic DNA containing a 2950 bp overlap with Tyrp-1 BAC2) into Tyrp-1 BAC2 followed by the removal of the KanR gene to generate the unified Tyrp-1 BAC. The retrieval– recombination process was then repeated to insert a 19 891 bp DNA fragment (1896 bp loxP–KanR–loxP cassette + 17 995 bp genomic DNA containing 3320 bp overlap with the unified Tyrp-1 BAC) further downstream from the previous one. This fragment was retrieved and site-specifically inserted into the unified Tyrp-1 BAC to create the final product, the extended unified Tyrp-1 BAC that has 31 160 bp more Tyrp-1 genomic DNA than the original Tyrp-1 BAC2.
Figure 1.
General experimental strategy for BAC engineering. The light blue diamond hatched pattern represents the Sp6 fragment of BAC. The red square checked pattern represents the T7 fragment of BAC. The green vertically striped pattern represents ArmA at the breakpoint of the Tyrp-1 gene of Tyrp-1 BAC1. The orange mosaic pattern represents ArmB at the end of the Tyrp-1 gene of BAC1. The pink solid rectangles represent exons of the Tyrp-1 gene. Double backslash lines represent disproportional genomic DNA. Amp is the ampicillin resistance gene. Ori refers to the origin of replication. The jointed arrow represents the transcriptional site; the vertical arrow represents the poly(A) site; the solid square with an oval on each side represents the 3749 bp frt–KanR–frt cassette (the oval represents the frt site); the solid rectangle with a triangle on each side represents 1896 bp of the loxP–KanR–loxP cassette. (A) Overall aim of the experiment. Top left: Tyrp-1 BAC1 containing Tyrp-1 exons 1–8 and the 3′ downstream region. Top right: Tyrp-1 BAC2 containing the Tyrp-1 5′ region and exons 1 and 2. Bottom: the final extended unified Tyrp-1 BAC containing the Tyrp-1 5′ region, all the exons and a 16.5 kb 3′ region. 31 160 bp is the distance between the 3′ breakpoint of Tyrp-1 BAC2 and the last base pair in the extended unified Tyrp-1 BAC. (B) Step one: first round retrieval (upper left). A kanamycin gene is first inserted into Tyrp-1 BAC1. The Tyrp-1 genomic DNA tagged by the kanamycin gene is then cloned into the retrieval vector (pBRBAC-AB). The positions of the primers which were used to amplify two homology arms are indicated by F3 and R3, and F5 and R5. In the retrieval vector, the ampicillin gene and origin of replication site are indicated by open arrows; the location of primers used to amplify the pBRBAC-AB retrieve vector are shown as thick closed arrows (R3 and F5). Homologous recombination events are denoted by crosses. NotI cleavage sites are also shown. (C) Step two: first round recombination (upper right). Linearized Tyrp-1 genomic fragment containing Tyrp-1 exons 1–8 and the 4 kb 3′ region was recombined with Tyrp-1 BAC2 through the homologous ArmA and Sp6 DNA regions. The resulting Tyrp-1 BAC3 and the unified Tyrp-1 BAC contained the 5′ regulatory region, all the Tyrp-1 exons and a 4 kb 3′ downstream region. The position and orientation of primers (F1, R1, F2, R2, F4, F5, Sp6 and R5) that were used to detect and sequence the BACs are shown. (D) Second round recombination (lower). A second round of retrieval–recombination was carried out with the unified Tyrp-1 BAC and a 19 891 bp fragment downstream of exon 8 to generate an extended unified Tyrp-1 BAC. The purple brick pattern represents a long homologous arm at Tyrp-1 intron 7 upstream of the frt site in the unified Tyrp-1 BAC. The positions and orientations of the primers (F6, F7, R6 and BAC-Sp6-R) that were used to detect the extended unified Tyrp-1 BAC are shown.
Retrieval
DNA engineering often requires the introduction of a selectable marker into a targeted gene in order to select the targeted candidate conveniently either in E.coli or in mammalian cells (13). Our initial task was to introduce a selectable marker into the targeted gene to facilitate later candidate identification. This would facilitate selection during both retrieval and later recombination into Tyrp-1 BAC2. As the BACs originally encoded chloramphenicol resistance, a frt–KanR–frt cassette was introduced between the eighth exon and polyadenylation site. Red-mediated homologous recombination was facilitated by two regions (70 and 500 bp) of homology flanking the frt–KanR–frt cassette (Fig. 1B). Approximately 500 KanR colonies were obtained from 42°C treated cells, compared with 10 colonies from uninduced cells (Table 1).
Table 1. Efficiency of homologous recombination.
| Step | No. of colonies obtaineda | No. of colonies examined | No. of correct colonies | Efficiency (%)b | Length of homology arms (bp) | |
|---|---|---|---|---|---|---|
| Non-induced | Induced | examined | colonies | |||
| Insertion of Kan | 10 | 500 | 22 | 14 | 64 | 70, 500 |
| Retrieval of the 22.5 kb DNA | 38 | 3600 | 28 | 26 | 93 | 216, 330 |
| BAC recombination | 0 | 1500 | 11 | 10 | 90 | 216, 303 |
| Reported retrieval (10) | – | 555 | 12 | 8 | 67 | 53, 51 |
aInduced DY380 competent cells carrying the modified BAC1 and BAC2 were electroporated with different linear DNAs. The total number of colonies obtained is shown.
bEfficiency is defined as the number of correct recombinants as a percentage of the number of examined colonies.
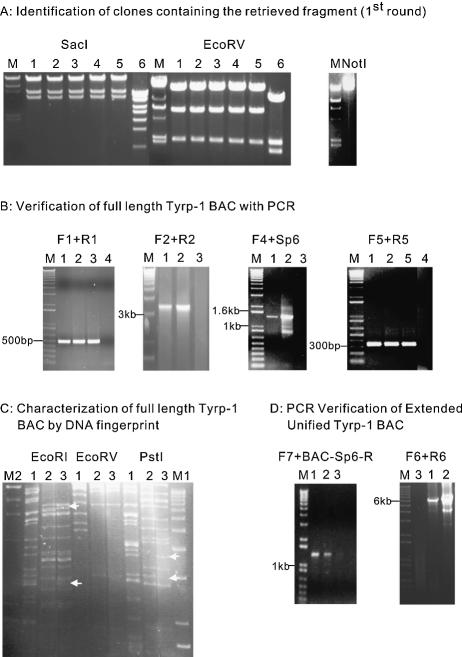
Homologous recombination was then used to retrieve the 22.5 kb sequence of interest from Tyrp-1 BAC1 containing the newly inserted frt–KanR–frt cassette, and then to clone this fragment into a retrieval vector. pBR322 was used as the backbone of the retrieval vector due to its low copy number which had low toxicity to the bacterial strain when carrying a very large fragment (10). The retrieval vector contained an ampicillin resistance gene, an origin of replication, an Sp6 fragment to facilitate sequencing, and two homologous end sequences, one from the breakpoint of Tyrp-1 BAC1 and one from the region 4 kb downstream from the stop codon (encoding 216 and 330 bp of homology, respectively). A linearized vector was generated by PCR amplification using the R3 and F5 primers. This linearized retrieval vector was transformed into DY380 competent cells carrying Tyrp-1 BAC1; the transformants were then selected on LB medium plates containing both kanamycin and ampicillin. Homologous recombination generated a circular plasmid consisting of the 22.5 kb Tyrp-1 DNA cloned into pBRBAC-AB (Fig. 1B). In this step, some 3600 KanR colonies were obtained from red-induced cells compared with 38 colonies from uninduced cells (Table 1). Twenty-six out of 28 clones examined were correct recombinants (93%). Our data indicated that by using homology arms of >200 bp in length, a homologous recombination efficiency >90% could be achieved. This compares with a previously reported 60% efficiency when homology arms of 50 bp in length were used to retrieve a 25 kb fragment from a BAC (10) (Table 1). Representative results are shown in Figure 2A, left panel. Digestion of six retrieved colonies with SacI and EcoRV showed that only one had an abnormal digestion pattern (expected digestion pattern for SacI: 15 189, 587 and 4417 bp; for EcoRV: 16 975, 6248 and 2173 bp).
Figure 2.
(A) Identification of clones containing the retrieved fragment (first round). The left panel shows the digestion pattern of the retrieved BAC fragment. M: λ/HindIII DNA. Different clones labeled 1–6 were digested by the restriction enzymes labeled above. Clones 1–5 show the expected digestion pattern. The right panel shows a 25.4 kb NotI-linearized pBRBAC-AB construct containing the 22.5 kb retrieved fragment. (B) Verification of Tyrp-1 BACs with PCR. A PCR product of 3.3 kb by primer pair (F2 + R2) could only be generated after homologous recombination occurred between the retrieved DNA and Tyrp-1 BAC2 to generate Tyrp-1 BAC3 and Tyrp-1 BAC. The four figures show the PCR verification of the full-length Tyrp-1 BAC3 and Tyrp-1 BAC with different primer pairs (F1 + R1, F2 + R2, F4 + Sp6 and F5 + R5). Each picture shows a band of the expected size. M: 1 kb plus Marker. Template DNAs used are indicated by numbers above each lane. (1) Tyrp-1 BAC3; (2) unified Tyrp-1 BAC; (3) Tyrp-1 BAC2; (4) PCR negative control (water); (5) Tyrp-1 BAC1. (C) Characterization of Tyrp-1 BACs by DNA fingerprint. The figure shows the DNA fingerprint results. The digestion pattern of unified Tyrp-1 BAC (labeled as 3) is very similar to that of the Tyrp-1 BAC2 (labeled as 2), but it also contains fragments unique to Tyrp-1 BAC1 (labeled as 1). The white arrows indicate DNA fragments present in unified Tyrp-1 BAC as well as in either Tyrp-1 BAC2 or Tyrp-1 BAC1, but not both. M1, λ/HindIII DNA; M2, 1 kb plus Marker. The restriction enzymes used are listed at the top of each panel. (D) PCR verification of second round recombination products. The two figures show the PCR verification of the second round recombination products. Two PCR products of 1.38 kb (left panel) and 6.2 kb (right panel) by primer pair (F7 + BAC-Sp6-R) and (F6 + R6), respectively, can only be generated after the second round recombination has occurred between the 19 891 bp retrieved DNA and Tyrp-1 BAC, creating the extended unified Tyrp-1 BAC. Each figure shows bands of the expected size. M: 1 kb plus Marker. Template DNAs used are indicated by numbers above each lane: lane 1, clone 1 of extended unified Tyrp-1 BAC; lane 2, clone 2 of extended unified Tyrp-1 BAC; lane 3, unified Tyrp-1 BAC.
Recombination
After the cloning of the 22.5 kb Tyrp-1 fragment, the pBRBAC-AB vector containing the retrieved fragment was linearized by NotI digestion (right panel, Fig. 2A) and transformed into DY380 cells containing Tyrp-1 BAC2. Homologous recombination occurred between the retrieved Tyrp-1 DNA and Tyrp-1 BAC2 through the 216 bp ArmA and the 303 bp Sp6 fragment to give rise to the Tyrp-1 BAC3 (Fig. 1C). Kanamycin and chloramphenicol double resistant colonies were selected. Over 1500 KmR + CmR colonies were obtained from the induced cells; no colonies were obtained from uninduced cells (Table 1). PCR amplification was used to determine whether the 22.5 kb Tyrp-1 DNA fragment was inserted precisely into Tyrp-1 BAC2. Several pairs of primers at different positions were used. Results from the PCR amplification showed that the recombination occurred as expected in 10 of 11 clones examined. A full-length Tyrp-1 gene was obtained in Tyrp-1 BAC3 (Fig. 2B). Moreover, one of the recombinant BACs was analyzed further by DNA fingerprinting (Fig. 2C) and sequencing (data not shown). The data showed that the retrieved 22.5 kb DNA fragment had been precisely inserted into the Tyrp-1 BAC2 by homologous recombination. These data indicated that this targeting reaction was very efficient due to the longer homologous recombination arms between the targeting fragment and Tyrp-1 BAC2.
Although the selectable marker provides an easy method to select the targeted colonies, it might have interfered with Tyrp-1 gene expression (13) and with the next round of recombination. Selectable markers were therefore removed after targeting to eliminate any undesirable effects. Tyrp-1 BAC3 DNA was introduced into E.coli strain 294-Flp (12), which can express Flp recombinase to excise the kanamycin gene between the two frt sites, leaving only one frt site behind. This resulted in the unified Tyrp-1 BAC containing both the upstream regulatory elements and the eight coding exons.
In order to further validate the approach, a second 19 891 bp DNA fragment was inserted into the unified BAC to precisely add sequence 3′ to the coding region. A loxP–KanR–loxP cassette was inserted into the original Tyrp-1 BAC1 DNA at a position ∼17 kb downstream of the poly(A) site to facilitate the retrieval. A second-round retrieval and recombination was carried out to generate the final product, the extended unified Tyrp-1 BAC (Fig. 1D). The recombination products were verified by PCR (Fig. 2D). Efficiency comparable with the first round of retrieval–recombination was obtained (data not shown).
DISCUSSION
We have generated an unified Tyrp-1 BAC containing an intact gene by a two-step recombinogenic method. Our results show that it is feasible to retrieve large DNA fragments from one BAC and precisely insert them into other BACs using Red-mediated homologous recombination. The design of the retrieval vector was the most important factor for subsequent homologous recombination. Insertion of the Sp6 fragment into pBR322 was vital as it incorporates the rare cutter NotI restriction site, enabling later linearization of the retrieval plasmid, and provides a homologous arm for directional insertion of the retrieved DNA fragment into BACs. Our data also indicate that longer homologous arms can generate more recombinant colonies as well as higher recombination efficiencies.
After removing the selectable marker in the Tyrp-1 BAC3 to generate the unified Tyrp-1 BAC, another round of retrieval–recombination can be carried out. The frt site left in Tyrp-1 BAC can be removed after the second round of recombination facilitated by a long homologous region upstream of the frt site (Fig. 1D). Through two rounds of retrieval–recombination, a total of 31 160 bp of genomic DNA from Tyrp-1 BAC1 was inserted into Tyrp-1 BAC2 in a site-specific manner. Since the loxP–KanR–LoxP sequence can be removed similarly to the frt–KanR–frt sequence, the approach described herein allows multiple rounds of retrieval–recombination, effectively enabling the construction of very large BACs limited only by the nature of the BAC backbone vector. Since retrieval of DNA fragments of >80 kb has been achieved (10), we are currently testing recombination of DNA fragments larger than 20 kb into BACs.
This method is not limited to BAC manipulation; it could be used to engineer any large DNA fragments or to insert large DNA fragments into the E.coli chromosome. With suitable vectors, this method may be used to construct and modify much larger DNA molecules such as human artificial chromosomes (HACs). In this study, the templates for genomic DNA retrieval and recombination were BACs obtained through a library screen. However, it would be possible to avoid the library screen steps and generate these large DNA molecules by long-range PCR or by direct recombinogenic retrieval from a complex genomic DNA mixture (14). Due to its simplicity, speed and precision, the method represents a powerful approach for BAC manipulation to aid in future studies of gene function in the post-genomic era.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank Dr Julian Tanner for reading and commenting on the manuscript and Mr Linyu Lu for help with graphics. This work was supported by grants from the Hong Kong Research Grants Council (HKU 7278/99M and NSFC/HKU 17) to J.D.H. (1999).
REFERENCES
- 1.Copeland N.G., Jenkins,N.A. and Court,D.L. (2001) Recombineering: a powerful new tool for mouse functional genomics. Nature Rev. Genet., 2, 769–779. [DOI] [PubMed] [Google Scholar]
- 2.Muyrers J.P., Zhang,Y. and Stewart,A.F. (2001) Techniques: recombinogenic engineering—new options for cloning and manipulating DNA. Trends Biochem. Sci., 26, 325–331. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S.N., Chang,A.C., Boyer,H.W. and Helling,R.B. (1973) Construction of biologically functional bacterial plasmids in vitro. Proc. Natl Acad. Sci. USA, 70, 3240–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shizuya H., Birren,B., Kim,U.J., Mancino,V., Slepak,T., Tachiiri,Y. and Simon,M. (1992) Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Natl Acad. Sci. USA, 89, 8794–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Buchholz,F., Muyrers,J.P.P. and Stewart,A.F. (1998) A new logic for DNA engineering using recombination in Escherichia coli. Nature Genet., 20, 123–128. [DOI] [PubMed] [Google Scholar]
- 6.Yu D., Ellis,H.M., Lee,E.C., Jenkins,N.A., Copeland,N.G. and Court,D.L. (2000) An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl Acad. Sci. USA, 97, 5978–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datsenko K.A. and Wanner,B.L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA, 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muyrers J.P., Zhang,Y., Benes,V., Testa,G., Ansorge,W. and Stewart,A.F. (2000) Point mutation of bacterial artificial chromosomes by ET recombination. EMBO Rep., 1, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muyrers J.P., Zhang,Y., Testa,G. and Stewart,A.F. (1999) Rapid modification of bacterial artificial chromosomes by ET-recombination. Nucleic Acids Res., 27, 1555–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E.C., Yu,D., Martinez de Velasco,J., Tessarollo,L., Swing,D.A., Court,D.L., Jenkins,N.A. and Copeland,N.G. (2001) A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics, 73, 56–65. [DOI] [PubMed] [Google Scholar]
- 11.Jackson I.J., Chambers,D.M., Budd,P.S. and Johnson,R. (1991) The tyrosinase-related protein-1 gene has a structure and promoter sequence very different from tyrosinase. Nucleic Acids Res., 19, 3799–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchholz F., Angrand,P.O. and Stewart,A.F. (1996) A simple assay to determine the functionality of Cre or FLP recombination targets in genomic manipulation constructs. Nucleic Acids Res., 24, 3118–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewandoski M. (2001) Conditional control of gene expression in the mouse. Nature Rev. Genet., 2, 743–755. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Muyrers,J.P., Testa,G. and Stewart,A.F. (2000) DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol., 18, 1314–1317. [DOI] [PubMed] [Google Scholar]