Abstract
Rpb4–Rpb7, a dissociable subcomplex of RNA polymerase II (pol II), is required for transcription initiation. To understand the role of Rpb7 in transcription initiation or other processes in transcription, we carried out a two-hybrid screen for proteins that interact with Rpb7 of the fission yeast Schizosaccharomyces pombe. The screen identified the S.pombe homolog of the Saccharomyces cerevisiae Nrd1, an RNA-binding protein implicated in 3′ end formation of small nucleolar and small nuclear RNAs transcribed by pol II. The S.pombe protein, named Seb1 for seven binding, was essential for cell viability, and bound directly to Rpb7 in vitro. Saccharomyces cerevisiae Rpb7 also interacted with Nrd1, indicating that the interaction is conserved in evolution. Glu166 and/or Asp167 of S.pombe Rpb7, residues near the C-terminus of the 172 amino acid protein, were found to be important for its interaction with Seb1. Our results suggest that Rpb7 may function to anchor a processing factor to the pol II apparatus, thereby coupling RNA processing to transcription. The role for Rpb7 is consistent with its location in the pol II complex determined by recent structural studies.
INTRODUCTION
Eukaryotic RNA polymerase II (pol II), the enzyme responsible for synthesis of all mRNAs and some small nuclear and small nucleolar RNAs, comprises 12 subunits designated Rpb1 to Rpb12. These subunits are conserved in sequence and function from yeast to human (1,2). Rpb1, 2, 3 and 11 subunits are related to the subunits of the bacterial RNA polymerase core enzyme, and subunits Rpb5, 6, 8, 10 and 12 are shared among eukaryotic RNA polymerases I, II and III.
Rpb4 and Rpb7, subunits specific to pol II, form a heterodimer dissociable from the pol II complex in vitro. The Rpb4–Rpb7 complex is dispensable for RNA synthesis but is required for transcription initiation from a promoter (3). Although phenotypes associated with a null mutation in the Saccharomyces cerevisiae RPB4 gene implicated Rpb4–Rpb7 in stress responses (4), recent studies have shown that Δrpb4 causes a global defect in transcription (5,6). The Δrpb4 phenotypes can be suppressed by overexpression of Rpb7, and the high level of Rpb7 allows its interaction with pol II in the absence of Rpb4 (7,8), suggesting that Rpb7 is the critical component of the Rpb4–Rpb7 complex and the role of Rpb4 is to stabilize the complex.
Insights into the function of Rpb4–Rpb7 have also come from structural studies. Comparison of 12-subunit pol II at low resolution with 10-subunit pol II lacking Rpb4–Rpb7 has suggested that Rpb4–Rpb7 is located at the base of the clamp, close to the exit of nascent RNA transcript (9–11). In addition, the structure of an archaeal homolog of the Rpb4–Rpb7 complex has revealed that Rpb7 contains two potential RNA-binding domains (12), which is consistent with the observation that Rpb4–Rpb7 exhibits RNA-binding activity (13). Based on the location in the pol II complex and the presence of the RNA-binding domains, it has been proposed that Rpb7 may interact with nascent RNA transcript (12). Recently, the high resolution structure of 12-subunit pol II has been reported (14,15), confirming the location of Rpb4–Rpb7. However, the details of the function of Rpb7 remain unclear.
The fission yeast Schizosaccharomyces pombe, which is only distantly related to S.cerevisiae, has been used for studies on pol II. The Rpb4 subunit of S.pombe pol II differs from its S.cerevisiae counterpart in some respects (1). First, S.pombe Rpb4 (135 amino acid residues) is more similar in sequence to the human and Arabidopsis counterparts (142 and 138 residues, respectively) than is S.cerevisiae Rpb4 (221 residues). Secondly, S.pombe Rpb4 is essential for cell viability, whereas S.cerevisiae Rpb4 is dispensable under optimal growth conditions (16). Schizosaccharomyces pombe would thus serve as a good model organism for the understanding of the function of Rpb4–Rpb7 in higher eukaryotes.
As an attempt to understand the function of Rpb7, we screened for Rpb7-interacting proteins using the yeast two-hybrid system. Identification of the S.pombe homolog of a S.cerevisiae RNA-binding protein involved in processing of pol II transcripts suggests a role for Rpb7 in coupling of RNA processing to transcription.
MATERIALS AND METHODS
Yeast general methods
Standard methods were used for molecular genetic analysis of S.pombe (17,18) and S.cerevisiae (19,20).
Overproduction and depletion of Rpb7
For expression of Rpb7 in S.pombe cells, rpb7 cDNA was amplified by PCR from pGEM7 (21), ligated between the BamHI and SalI sites of pREP2, and then the rpb7 fragment was transferred to pREP1, pREP41 and pREP81, which contain thiamine-repressible nmt1 promoters of varying strengths (22), to create pREP1(rpb7cDNA), pREP41(rpb7cDNA) and pREP81(rpb7cDNA), respectively. For depletion of Rpb7, pUC18(rpb7::ura4) was constructed from pUC18 containing a 2.6-kb rpb7 genomic DNA fragment by the method used for the disruption of rpb4 (1) and taf73 (23). A Δrpb7::ura4+ fragment was amplified from the plasmid and used to transform the haploid S.pombe strain JY741 (23) carrying pREP81(rpb7cDNA) to yield a strain in which the chromosomal rpb7 gene was disrupted and a plasmid-borne rpb7 gene was present instead.
Two-hybrid screen
A two-hybrid screen using a Matchmaker Gal4-based two-hybrid system and the Matchmaker S.pombe cDNA library (Clontech/BD Biosciences) was carried out as described (24). The S.pombe rpb7 cDNA corresponding to amino acids 3–172 was amplified by PCR, ligated between the EcoRI and BamHI sites of pGBKT7 to create pGBKT7(RPB7F), and used to transform the S.cerevisiae strain AH109. Expression of Rpb7 fused to the Gal4 DNA-binding domain (Gal4BD) was verified by immunoblotting. The AH109 strain carrying the bait plasmid was then transformed with the S.pombe cDNA library. Gal4 activation domain (Gal4AD) plasmids were isolated from His+ Ade+ Mel+ transformants and sequenced. For a two-hybrid assay, the S.cerevisiae RPB7 gene corresponding to amino acids 3–171 and the 3′ portion of the S.cerevisiae NRD1 gene corresponding to amino acids 397–575 were amplified from genomic DNA by PCR and cloned into Gal4BD and Gal4AD vectors to create pGBKT7(scRPB7) and pGADT7(scNRD1C), respectively.
Expression of recombinant proteins and in vitro interaction assays
The 3′ portion of the S.pombe seb1 gene corresponding to amino acids 466–620 was amplified by PCR, ligated between the EcoRI and SalI sites of pGEX-5X-1 (Amersham Pharmacia Biotech), and used to transform the Escherichia coli strain DH5α to produce a C-terminal region of Seb1 fused to GST. For production of recombinant Rpb7 protein, pET-Rpb7CH (1) was used to transform E.coli BL21(DE3). The denaturation/renaturation of the recombinant proteins and the following GST pull-down assays were carried out as described (24).
Disruption of the seb1 gene
The seb1 gene was disrupted by a PCR-based method originally developed for S.cerevisiae (25). A ura4+ fragment flanked by 80-bp seb1 sequences was amplified by PCR with ExTaq (TaKaRa) and used to transform the diploid S.pombe strain HMP10 (24), which would result in the replacement of seb1 sequence from +129 to +2064 relative to the start codon with the ura4+ fragment. Ura+ transformants capable of sporulation were tested by PCR for correct disruption. Of 10 transformants examined, five contained the disruption.
Site-directed mutagenesis
Alanine substitutions of Glu166 and Asp167 in S.pombe Rpb7 were introduced into pGBKT7(RPB7F) using the QuikChange XL site-directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions to create pGBKT7(RPB7QC2). Correct substitutions were confirmed by sequencing.
RESULTS
Effects of depletion and overproduction of S.pombe Rpb7
The S.pombe rpb7 gene was previously isolated (21) and an antibody raised against recombinant Rpb7 protein was used to identify Rpb7 in purified S.pombe pol II (1). However, the function of S.pombe Rpb7 had not been analyzed in vivo. As a first step to understand the in vivo function of Rpb7, we examined the effects of depletion and overproduction of Rpb7 on cell growth. A Δrpb7 strain was constructed that carried a plasmid-borne rpb7 gene under the control of a thiamine-repressible promoter. The cells ceased growing upon transfer to medium containing thiamine (Fig. 1A), demonstrating that S.pombe Rpb7 is essential for growth as in the case of S.cerevisiae Rpb7 (26). High expression of Rpb7 with pREP1(rpb7cDNA) resulted in slow growth (Fig. 1B), indicating that a large amount of Rpb7 is inhibitory to cell growth.
Figure 1.
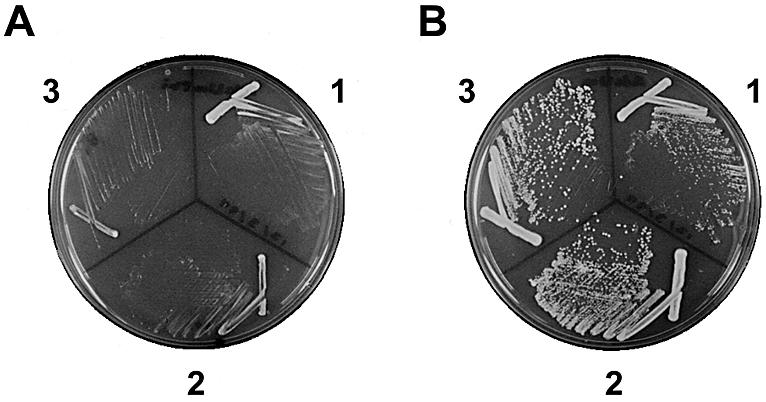
Depletion and overproduction of S.pombe Rpb7 protein. Cells of Δrpb7::ura4+ strains carrying pREP1(rpb7cDNA) (1), pREP41(rpb7cDNA) (2) or pREP81(rpb7cDNA) (3) were streaked onto EMM + AdeUra containing 2 µM thiamine (A) and EMM + AdeUra (B) plates.
Identification of a putative RNA processing factor as Rpb7-interacting protein
To gain insight into the function of Rpb7 in transcription initiation or other processes in transcription, we screened for proteins that interact with S.pombe Rpb7 using the yeast two-hybrid system. A Gal4AD-fused S.pombe cDNA library was used to transform a S.cerevisiae strain carrying Gal4BD-Rpb7 plasmid (Fig. 2A). Sequencing of Gal4AD plasmids in transformants showing simultaneous expression of reporter genes revealed that one clone, pFb49 (Fig. 2A), encodes an in-frame fusion of Gal4AD to the C-terminal 155 residues of the SPAC222.09 gene product, a 620-amino acid protein predicted from the S.pombe genome sequence (27; http://www.genedb.org/pombe/). This protein was found to be 28% identical to S.cerevisiae Nrd1, which is a 575-amino acid RNA-binding protein implicated in 3′ end formation of small nucleolar and small nuclear RNAs transcribed by pol II (28). We will refer to the SPAC222.09 gene as seb1 (for seven binding) and its product as Seb1. It should be noted that this gene is different from the previously characterized S.pombe gene named nrd1 (29). Figure 2B shows the interaction between Gal4BD-Rpb7 and Gal4AD-Seb1. Neither Gal4BD-Rpb7 nor Gal4AD-Seb1 by itself activated the reporter genes, indicating the interaction between Rpb7 and Seb1.
Figure 2.
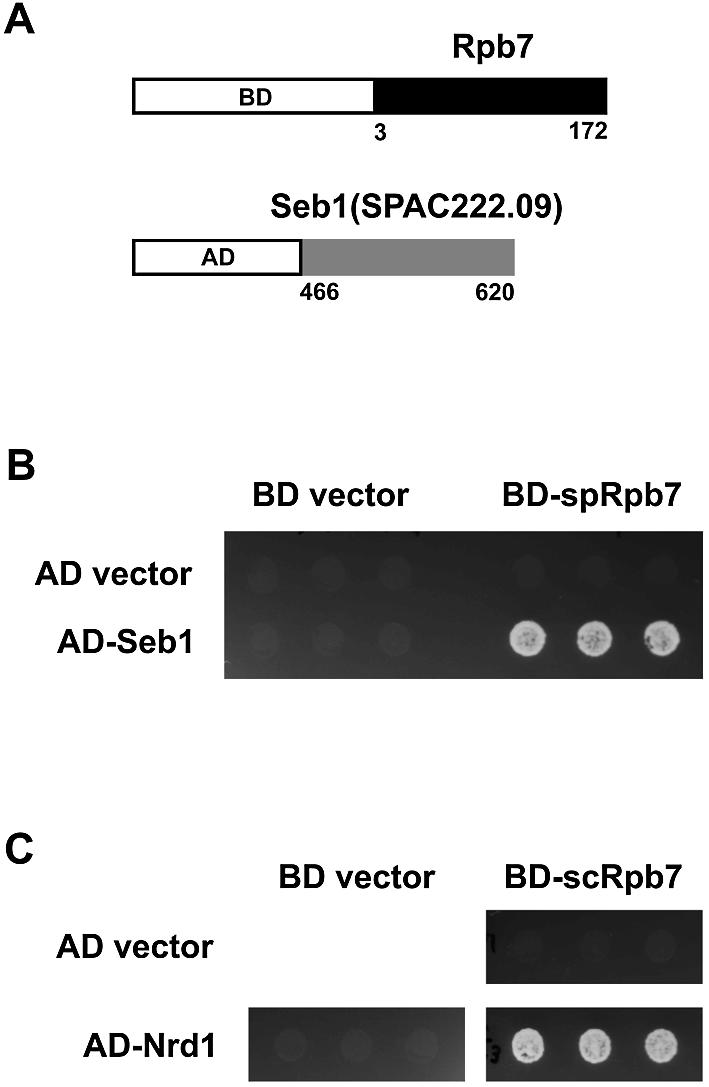
Two-hybrid screen for Rpb7-interacting protein. (A) The bait plasmid pGBKT7(RPB7F) (upper) encodes a Gal4BD fusion of the S.pombe Rpb7. One of the isolated clones, pFb49 (lower), encodes a Gal4AD fusion of the C-terminal quarter of a protein predicted from SPAC222.09, which we will refer to as Seb1. (B) Two-hybrid interaction between S.pombe Rpb7 and Seb1. Cell suspensions of the S.cerevisiae strain AH109 carrying the indicated BD and AD plasmids were spotted onto synthetic complete medium lacking tryptophan, leucine, histidine and adenine, and allowed to grow at 30°C for 3 days. BD plasmid is pGBKT7 (BD vector) or pGBKT7(RPB7F) (BD-spRpb7), and AD plasmid is pGADT7 (AD vector) or pFb49 (AD-Seb1). Since the expression of the reporter genes would result in a His+Ade+ phenotype, growth on this medium implies interaction. Three transformants are shown for each pair. (C) Two-hybrid interaction between S.cerevisiae Rpb7 and Nrd1. BD plasmid is pGBKT7 (BD vector) or pGBKT7(scRPB7) (BD-scRpb7), and AD plasmid is pGADT7 (AD vector) or pGADT7(scNRD1C) (AD-Nrd1).
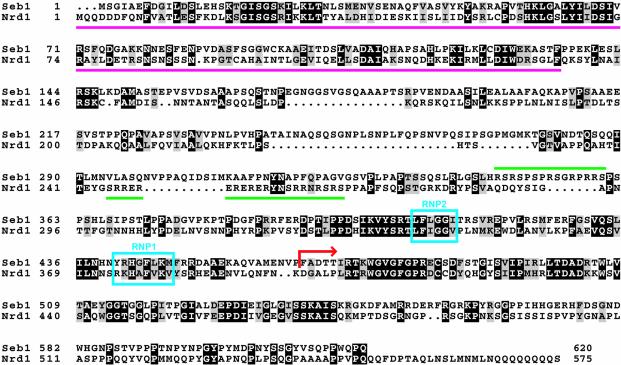
The N-terminal region of S.cerevisiae Nrd1 binds to the C-terminal domain (CTD) of Rpb1, the largest subunit of pol II (30), while its C-terminal half, which contains an RNA-binding domain known as the RNP or RRM domain (31), binds to RNA (32). As shown in Figure 3, the N-terminal region of Seb1 shows homology to the CTD-binding domain of Nrd1, and the two RNP motifs in the RNP domain of Nrd1 are conserved in Seb1, suggesting that Seb1 binds to both the CTD and RNA as does Nrd1. Between the CTD-binding domain and the RNA-binding domain, Seb1 and Nrd1 both contain a region rich in arginine–serine dipeptides (RS domain), which is found in SR proteins involved in pre-mRNA splicing (33). The RS domain of Nrd1 contains arginine–glutamate dipepetides in addition to arginine–serine dipeptides, whereas that of Seb1 contains only arginine–serine dipeptides. Taken together, the conservation of the functional domains between Seb1 and Nrd1 suggests that S.pombe Seb1 is involved in processing of pol II transcripts as in the case of S.cerevisiae Nrd1. Consistent with the possible involvement of Seb1 in RNA processing, it has been reported that a portion of Seb1 (residues 418–604) fused to green fluorescent protein, which is contained in a random genomic DNA fusion library, is localized in the nucleus (34; accession no. AB027948).
Figure 3.
Amino acid sequence alignment of S.pombe Seb1 and S.cerevisiae Nrd1. Identical residues are shaded in black and similar residues are shaded in gray. Magenta lines indicate the CTD-binding domain of Nrd1. Green lines indicate regions rich in arginine–serine or arginine–glutamate dipeptides. The RNP motifs are boxed in blue. A red arrow indicates the C-terminal region of Seb1 found to be fused to Gal4AD in pFb49.
Interaction between the S.cerevisiae counterparts
Identification of the interaction between Rpb7 and Seb1 of S.pombe prompted us to determine whether their S.cerevisiae counterparts also interact with each other. The C-terminal region of Nrd1 corresponding to that of Seb1 identified in the two-hybrid screen was fused to Gal4AD and tested for interaction with S.cerevisiae Rpb7. As shown in Figure 2C, two-hybrid interaction was observed between the S.cerevisiae counterparts, suggesting that the interaction is conserved in evolution and is of biological relevance. The result also confirms that S.pombe Seb1 is the functional homolog of S.cerevisiae Nrd1.
Direct interaction of Rpb7 with Seb1
To test whether Rpb7 directly interacts with Seb1 in vitro, we produced recombinant Rpb7 and GST–Seb1 proteins in E.coli and carried out GST pull-down assays. The proteins in inclusion bodies were solubilized with 6 M urea and renatured by dialysis against urea-free buffer prior to incubation with glutathione–agarose beads. As shown in Figure 4, Rpb7 bound to GST–Seb1 (lane 6) but not to GST alone (lane 3), indicating that Rpb7 specifically interacts with the Seb1 moiety of GST–Seb1.
Figure 4.
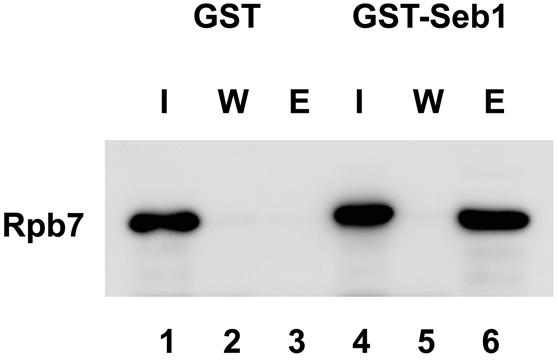
Direct interaction of Rpb7 with Seb1. Rpb7 was mixed with GST or GST–Seb1 and incubated with glutathione–agarose beads. One-tenth of input (I), final wash fraction of the same volume with elution (W) and elution fraction with glutathione (E) were separated by 12.5% SDS–PAGE, transferred to a PVDF membrane, and probed with anti-Rpb7 antibody. Immunoblotting with anti-GST antibody confirmed the presence of GST and GST–Seb1 (data not shown).
Seb1 is essential for cell viability
We next determined whether the S.pombe seb1 gene is essential for cell viability. A diploid S.pombe strain was constructed in which one copy of seb1 was disrupted. The seb1+/Δseb1::ura4+ cells were sporulated and subjected to tetrad analysis. The tetrads showed a 2+:2– segregation for spore viability, and no tetrads with more than two viable spores were observed (Fig. 5A). Importantly, all the viable spores were Ura– and thus presumed to be seb1+, indicating that Δseb1::ura4+ spores are inviable. These results indicate that seb1, like its S.cerevisiae counterpart NRD1 (35), is an essential gene.
Figure 5.
Disruption of the seb1 gene. (A) The seb1+/Δseb1::ura4+ cells were sporulated on malt extract medium at 27°C for 2 days. Tetrads were dissected on yeast extract medium, and spores were grown at 30°C for 3 days. Four spores from each tetrad are aligned vertically; 13 tetrads are shown. Of 27 tetrads dissected, 24 showed a 2+:2– segregation for spore viability. (B) Morphology of a representative of the Δseb1 spores at 30°C for 3 days. Of 24 Δseb1 spores observed, 20 showed this morphology and the others had divided once to give rise to two cells.
Microscopic observation revealed that the Δseb1::ura4+ spores germinated but did not divide (Fig. 5B). No spores gave rise to more than two cells. To our knowledge, this is not a typical phenotype observed when an essential gene of S.pombe is disrupted; for example, Δrpb4 (1), Δtaf73 (23) and Δtaf50 (24) spores divide several times before they cease growing.
Residues of Rpb7 that are important for the interaction with Seb1
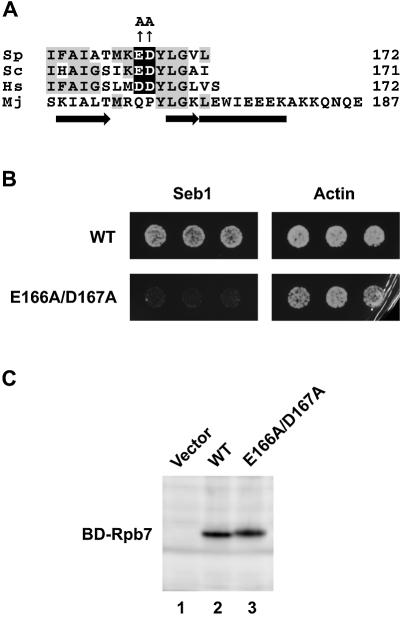
Clustered charged-to-alanine mutagenesis has been used to systematically analyze the function of residues on the surface of proteins [for example Wertman et al. (36)]. This method is based on the assumption that clusters of charged residues are unlikely to be accommodated in buried structure. To identify amino acid residues of Rpb7 involved in the interaction with Seb1, we developed a variant of the method, named ‘conserved’ clustered charged-to-alanine mutagenesis, in which clustered charged residues that are conserved evolutionarily are changed to alanine. Six clusters were found in Rpb7 by using the criteria that two or more charged residues in a stretch of three residues are conserved among S.pombe, S.cerevisiae and human, and one of the clusters is Glu166/Asp167 (Fig. 6A).
Figure 6.
Rpb7 defective in the interaction with Seb1. (A) Alignment of the C-terminal regions of the Rpb7 homologs. Sp, S.pombe; Sc, S.cerevisiae; Hs, Homo sapiens; Mj, Methanococcus jannaschii. Identical residues are shaded in gray and conserved clustered charged residues are shaded in black. Alanine substitutions of Glu (E)166 and Asp (D)167 are indicated. The secondary structure elements in the M.jannaschii E subunit (12) are shown below the sequence. An α-helix is shown as a rectangle and β-strands are shown as arrows. (B) AH109 strains carrying BD and AD plasmids were tested for two-hybrid interaction as described in the legend to Figure 2. BD plasmid is pGBKT7(RPB7F) (WT) or pGBKT7(RPB7QC2) (E166A/D167A), and AD plasmid is pFb49 (Seb1) or pFb210 (Actin), which encodes a C-terminal portion (amino acids 255–375) of S.pombe actin fused to Gal4AD. Note that E166A/D167A cells showed residual growth, indicating that the alanine substitutions does not completely disrupt the interaction. (C) AH109 strains carrying pFb49 and either pGBKT7 (Vector), pGBKT7(RPB7F) (WT) or pGBKT7(RPB7QC2) (E166A/D167A) were grown in synthetic complete medium lacking tryptophan and leucine to an OD600 of ∼0.5. Extracts from the equal amount of cells (0.4 OD600 unit) were separated by 10% SDS–PAGE, transferred to a PVDF membrane, and probed with anti-Myc antibody (BAbCO/Covance Research Products) to detect Gal4BD-Rpb7 proteins, which have a Myc epitope between Gal4 BD and Rpb7. Note that E166A/D167A migrated slightly more slowly than wild type.
We found that substitutions of Glu166 and Asp167 by alanine greatly reduced the interaction with Seb1 (Fig. 6B), indicating that Glu166 and/or Asp167 of Rpb7 are important for the interaction with Seb1. Since Glu166 and Asp167 are close to the C-terminus of 172-amino acid Rpb7 protein, it is unlikely that the alanine substitutions affect the overall structure of the protein. Evidence for the integrity of the variant protein also came from the observation that the protein was able to interact with actin (Fig. 6B), another Rpb7-interacting protein identified in our screen (data not shown). In addition, no difference in the expression levels was noticeable between wild-type and the variant (Fig. 6C). These results strongly suggest that the variant protein is specifically defective in the interaction with Seb1. Interestingly, Glu166/Asp167 are not conserved in archaeal homologs; the corresponding residues in the E subunit of archaeal RNA polymerase are glutamine and proline (Fig. 6A).
Recently, backbone models of 12-subunit pol II of S.cerevisiae have been reported (14,15). The models show that the Rpb4–Rpb7 complex protrudes from the base of the clamp, and Rpb7 interacts with the linker to the CTD. The location of the residues corresponding to Glu166 and Asp167 of S.pombe Rpb7 is shown in Figure 7. Based on the structure of the archaeal homolog (12), these residues are exposed and accessible for interactions with proteins (data not shown).
Figure 7.
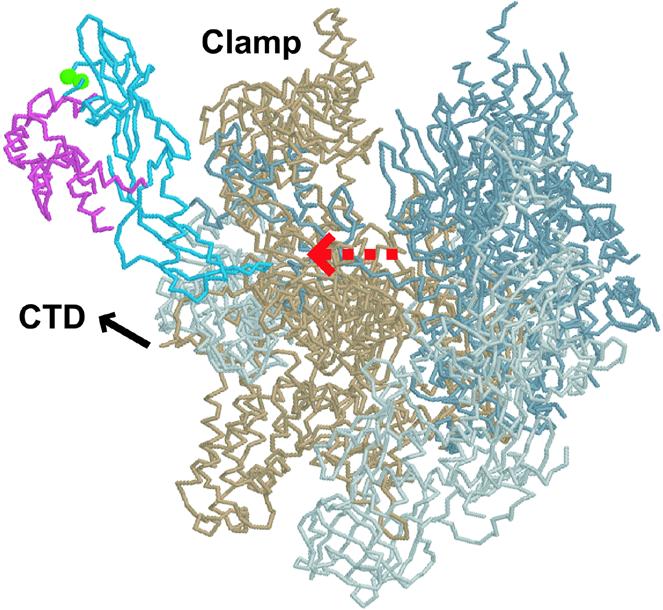
Presumed location of Glu166 and Asp167 of S.pombe Rpb7 in the pol II complex. Shown is a backbone structure [back view as in Cramer et al. (39)] of S.cerevisiae pol II (Protein Data Bank ID 1NT9), in which the residues corresponding to Glu166 and Asp167 of S.pombe Rpb7 are indicated as green spheres. Rpb7 is blue, Rpb4 is pink, Rpb1 is beige, Rpb2 is gray, and the remaining subunits are light gray. A red dashed arrow indicates the presumed RNA exit. The figure was generated with Protein Explorer (41).
DISCUSSION
In this study, we identified Seb1, the S.pombe homolog of S.cerevisiae Nrd1, as a protein that interacts with the Rpb7 subunit of pol II. Interaction of Nrd1 with the pol II transcription apparatus has been reported. Nrd1 interacts with the CTD of the largest subunit (Rpb1) of pol II in a two-hybrid assay (30). In addition, a related mammalian protein, SCAF8, interacts with the CTD in a phosphorylation-dependent manner (37). Our results demonstrate that Nrd1 also interacts with the Rpb7 subunit of pol II, and suggest that Rpb7 may have a role in coordination between transcription and processing.
Capping, splicing and 3′ end formation of pol II transcripts are now known to be tightly linked to transcription. A number of RNA processing factors associate with the CTD, which plays key roles in co-transcriptional RNA processing events (38). X-ray crystallography and NMR spectroscopy have shown that the CTD is disordered (39,40). Because the CTD is likely to be mobile, the interaction with Rpb7 may be required to anchor processing factors to the pol II transcription apparatus, by which processing machinery on the CTD is in close proximity to nascent RNA transcript.
The role of Rpb7 in coupling transcription to processing is consistent with the structural data reported recently (14,15). In the pol II complex, Rpb4–Rpb7 is located in the vicinity of the linker to the CTD (Fig. 7), and Rpb7 may bind and guide RNA as it emerges from the active center of pol II. The position of Rpb7 explains its interaction with a processing factor associated with the CTD.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Makoto Kimura for Rpb7 expression plasmids. We also thank Patrick Cramer for providing information before publication. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, and the Japan Science and Technology Corporation.
REFERENCES
- 1.Sakurai H., Mitsuzawa,H., Kimura,M. and Ishihama,A. (1999) The Rpb4 subunit of fission yeast Schizosaccharomyces pombe RNA polymerase II is essential for cell viability and similar in structure to the corresponding subunits of higher eukaryotes. Mol. Cell. Biol., 19, 7511–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shpakovski G.V., Gadal,O., Labarre-Mariotte,S., Lebedenko,E.N., Miklos,I., Sakurai,H., Proshkin,S.A., Van Mullem,V., Ishihama,A. and Thuriaux,P. (2000) Functional conservation of RNA polymerase II in fission and budding yeasts. J. Mol. Biol., 295, 1119–1127. [DOI] [PubMed] [Google Scholar]
- 3.Edwards A.M., Kane,C.M., Young,R.A. and Kornberg,R.D. (1991) Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J. Biol. Chem., 266, 71–75. [PubMed] [Google Scholar]
- 4.Choder M. and Young,R.A. (1993) A portion of RNA polymerase II molecules has a component essential for stress responses and stress survival. Mol. Cell. Biol., 13, 6984–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maillet I., Buhler,J.M., Sentenac,A. and Labarre,J. (1999) Rpb4p is necessary for RNA polymerase II activity at high temperature. J. Biol. Chem., 274, 22586–22590. [DOI] [PubMed] [Google Scholar]
- 6.Miyao T., Barnett,J.D. and Woychik,N.A. (2001) Deletion of the RNA polymerase subunit RPB4 acts as a global, not stress-specific, shut-off switch for RNA polymerase II transcription at high temperatures. J. Biol. Chem., 276, 46408–46413. [DOI] [PubMed] [Google Scholar]
- 7.Sheffer A., Varon,M. and Choder,M. (1999) Rpb7 can interact with RNA polymerase II and support transcription during some stresses independently of Rpb4. Mol. Cell. Biol., 19, 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Q., Li,X., Sadhale,P.P., Miyao,T. and Woychik,N.A. (2000) Multiple mechanisms of suppression circumvent transcription defects in an RNA polymerase mutant. Mol. Cell. Biol., 20, 8124–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen G.J., Meredith,G., Bushnell,D.A. and Kornberg,R.D. (1998) Structure of wild-type yeast RNA polymerase II and location of Rpb4 and Rpb7. EMBO J., 17, 2353–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramer P., Bushnell,D.A., Fu,J., Gnatt,A.L., Maier-Davis,B., Thompson,N.E., Burgess,R.R., Edwards,A.M., David,P.R. and Kornberg,R.D. (2000) Architecture of RNA polymerase II and implications for the transcription mechanism. Science, 288, 640–649. [DOI] [PubMed] [Google Scholar]
- 11.Craighead J.L., Chang,W. and Asturias,F.J. (2002) Structure of yeast RNA polymerase II in solution: implications for enzyme regulation and interaction with promoter DNA. Structure, 10, 1117–1125. [DOI] [PubMed] [Google Scholar]
- 12.Todone F., Brick,P., Werner,F., Weinzierl,O.J.R. and Onesti,S. (2001) Structure of an archaeal homolog of the eukaryotic RNA polymerase II RPB4/RPB7 complex. Mol. Cell, 8, 1137–1143. [DOI] [PubMed] [Google Scholar]
- 13.Orlicky S.M., Tran,P.T., Sayre,M.H. and Edwards,A.M. (2001) Dissociable Rpb4–Rpb7 subassembly of RNA polymerase II binds to single-strand nucleic acid and mediates a post-recruitment step in transcription initiation. J. Biol. Chem., 276, 10097–10102. [DOI] [PubMed] [Google Scholar]
- 14.Bushnell D.A. and Kornberg,R.D. (2003) Complete, 12-subunit RNA polymerase II at 4.1-Å resolution: implications for the initiation of transcription. Proc. Natl Acad. Sci. USA, 100, 6964–6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armache K.-J., Kettenberger,H. and Cramer,P. (2003) Architecture of initiation-competent 12-subunit RNA polymerase II. Proc. Natl Acad. Sci. USA, 100, 6969–6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woychik N.A. and Young,R.A. (1989) RNA polymerase II subunit RPB4 is essential for high- and low-temperature yeast cell growth. Mol. Cell. Biol., 9, 2854–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 795–823. [DOI] [PubMed] [Google Scholar]
- 18.Alfa C., Fantes,P., Hyams,J., McLeod,M. and Warbrick,E. (1993) Experiments with Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 19.Sherman F. (1991) Getting started with yeast. Methods Enzymol., 194, 3–21. [DOI] [PubMed] [Google Scholar]
- 20.Adams A., Gottschling,D.E., Kaiser,C.A. and Stearns,T. (1997) Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 21.Sakurai H. and Ishihama,A. (1997) Gene organization and protein sequence of the small subunits of Schizosaccharomyces pombe RNA polymerase II. Gene, 196, 165–174. [DOI] [PubMed] [Google Scholar]
- 22.Basi G., Schmid,E. and Maundrell,K. (1993) TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene, 123, 131–136. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuzawa H., Seino,H., Yamao,F. and Ishihama,A. (2001) Two WD repeat-containing TATA-binding protein-associated factors in fission yeast that suppress defects in the anaphase-promoting complex. J. Biol. Chem., 276, 17117–17124. [DOI] [PubMed] [Google Scholar]
- 24.Mitsuzawa H. and Ishihama,A. (2002) Identification of histone H4-like TAF in Schizosaccharomyces pombe as a protein that interacts with WD repeat-containing TAF. Nucleic Acids Res., 30, 1952–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baudin A., Ozier-Kalogeropoulos,O., Denouel,A., Lacroute,F. and Cullin,C. (1993) A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res., 21, 3329–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKune K., Richards,K.L., Edwards,A.M., Young,R.A. and Woychik,N.A. (1993) RPB7, one of two dissociable subunits of yeast RNA polymerase II, is essential for cell viability. Yeast, 9, 295–299. [DOI] [PubMed] [Google Scholar]
- 27.Wood V., Gwilliam,R., Rajandream,M.-A., Lyne,M., Lyne,R., Stewart,A., Sgouros,J., Peat,N., Hayles,J., Baker,S. et al. (2002) The genome sequence of Schizosaccharomyces pombe. Nature, 415, 871–880. [DOI] [PubMed] [Google Scholar]
- 28.Steinmetz E.J., Conrad,N.K., Brow,D.A. and Corden,J.L. (2001) RNA-binding protein Nrd1 directs poly(A)-independent 3′-end formation of RNA polymerase II transcripts. Nature, 413, 327–331. [DOI] [PubMed] [Google Scholar]
- 29.Tsukahara K., Yamamoto,H. and Okayama,H. (1998) An RNA binding protein negatively controlling differentiation in fission yeast. Mol. Cell. Biol., 18, 4488–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuryev A., Patturajan,M., Litingtung,Y., Joshi,R.V., Gentile,C., Gebara,M. and Corden,J.L. (1996) The C-terminal domain of the largest subunit of RNA polymerase II interacts with a novel set of serine/arginine-rich proteins. Proc. Natl Acad. Sci. USA, 93, 6975–6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varani G. and Nagai,K. (1998) RNA recognition by RNP proteins during RNA processing. Annu. Rev. Biophys. Biomol. Struct., 27, 407–445. [DOI] [PubMed] [Google Scholar]
- 32.Steinmetz E.J. and Brow,D.A. (1998) Control of pre-mRNA accumulation by the essential yeast protein Nrd1 requires high-affinity transcript binding and a domain implicated in RNA polymerase II association. Proc. Natl Acad. Sci. USA, 95, 6699–6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu X.D. (1995) The superfamily of arginine/serine-rich splicing factors. RNA, 1, 663–680. [PMC free article] [PubMed] [Google Scholar]
- 34.Ding D.-Q., Tomita,Y., Yamamoto,A., Chikashige,Y., Haraguchi,T. and Hiraoka,Y. (2000) Large-scale screening of intracellular protein localization in living fission yeast cells by the use of a GFP-fusion genomic DNA library. Genes Cells, 5, 169–190. [DOI] [PubMed] [Google Scholar]
- 35.Steinmetz E.J. and Brow,D.A. (1996) Repression of gene expression by an exogenous sequence element acting in concert with a heterogeneous nuclear ribonucleoprotein-like protein, Nrd1 and the putative helicase Sen1. Mol. Cell. Biol., 16, 6993–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wertman K.F., Drubin,D.G. and Botstein,D. (1992) Systematic mutational analysis of the yeast ACT1 gene. Genetics, 132, 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patturajan M., Wei,X., Berezney,R. and Corden,J.L. (1998) A nuclear matrix protein interacts with the phosphorylated C-terminal domain of RNA polymerase II. Mol. Cell. Biol., 18, 2406–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proudfoot N.J., Furger,A. and Dye,M.J. (2002) Integrating mRNA processing with transcription. Cell, 108, 501–512. [DOI] [PubMed] [Google Scholar]
- 39.Cramer P., Bushnell,D.A. and Kornberg,R.D. (2001) Structural basis of transcription: RNA polymerase II at 2.8 Ångstrom resolution. Science, 292, 1863–1876. [DOI] [PubMed] [Google Scholar]
- 40.Cagas P.M. and Corden,J.L. (1995) Structural studies of a synthetic peptide derived from the carboxyl-terminal domain of RNA polymerase II. Proteins, 21, 149–160. [DOI] [PubMed] [Google Scholar]
- 41.Martz E. (2002) Protein Explorer: easy yet powerful macromolecular visualization. Trends Biochem. Sci., 27, 107–109. [DOI] [PubMed] [Google Scholar]