Abstract
DNA binding by the ternary complex factor (TCF) subfamily of ETS-domain transcription factors is tightly regulated by intramolecular and intermolecular interactions. The helix–loop–helix (HLH)-containing Id proteins are trans-acting negative regulators of DNA binding by the TCFs. In the TCF, SAP-2/Net/ERP, intramolecular inhibition of DNA binding is promoted by the cis-acting NID region that also contains an HLH-like motif. The NID also acts as a transcriptional repression domain. Here, we have studied the role of HLH motifs in regulating DNA binding and transcription by the TCF protein SAP-1 and how Cdk-mediated phosphorylation affects the inhibitory activity of the Id proteins towards the TCFs. We demonstrate that the NID region of SAP-1 is an autoinhibitory motif that acts to inhibit DNA binding and also functions as a transcription repression domain. This region can be functionally replaced by fusion of Id proteins to SAP-1, whereby the Id moiety then acts to repress DNA binding in cis. Phosphorylation of the Ids by cyclin–Cdk complexes results in reduction in protein–protein interactions between the Ids and TCFs and relief of their DNA-binding inhibitory activity. In revealing distinct mechanisms through which HLH motifs modulate the activity of TCFs, our results therefore provide further insight into the role of HLH motifs in regulating TCF function and how the inhibitory properties of the trans-acting Id HLH proteins are themselves regulated by phosphorylation.
INTRODUCTION
Elk-1, SAP-1 and SAP-2/Net/ERP comprise the ternary complex factor (TCF) subfamily of ETS-domain transcription factors [reviewed in Treisman (1) and Sharrocks (2)]. These proteins all exhibit the common property of being able to form ternary complexes with the serum response factor (SRF) on serum response elements (SREs). In addition, they all share four regions of sequence similarity, the ETS DNA-binding domain, the B-box SRF interaction motif, a mitogen-activated protein (MAP) kinase docking site (D-domain) and a C-terminal transcription activation domain which is the target for MAP kinase-mediated phosphorylation. In addition to ternary complex formation with SRF, the TCFs also exhibit autonomous, SRF-independent DNA-binding activity to high affinity binding motifs. DNA binding by the TCFs is regulated at many levels. DNA binding is enhanced following phosphorylation by MAP kinases (3–5), and interaction with SRF also promotes DNA binding (6–9). Both of these regulatory modes act to overcome autoinhibitory mechanisms that exist to inhibit DNA binding (6,10,11). DNA binding by SAP-2/Net/ERP is autoinhibited by the NID (Net inhibitory domain) region (12). The NID was also shown to act as a transcriptional repression domain, further emphasising its role as a negatively acting element (12). Sequence comparisons and mutational analysis suggest that this region adopts a helix–loop–helix (HLH)-like structure (12). The NID is conserved in SAP-1 but is absent in Elk-1.
In addition to cis-acting mechanisms, DNA binding by the TCFs can also be inhibited in trans by interactions with members of the Id family of HLH proteins (13). Inhibition is mediated by direct interactions of the Ids with the ETS DNA-binding domain of the TCFs. The HLH motif in the Id proteins is crucial for these interactions (13) and exhibits significant sequence similarity with the HLH motif found in the SAP-1 NID (see Fig. 4A). Id proteins are well known for their ability to interact with, and to inhibit the DNA-binding activity of a number of other transcription factor families, of which the basic HLH (bHLH) proteins are best characterised (14–17). The inhibitory properties of the Id proteins on bHLH proteins are regulated through phosphorylation by cyclinA/E–cyclin-dependent kinase (Cdk) complexes. Both Id2 and Id3 can be phosphorylated at Ser5, and this abrogates their ability to inhibit DNA binding by class A bHLH E proteins (18,19).
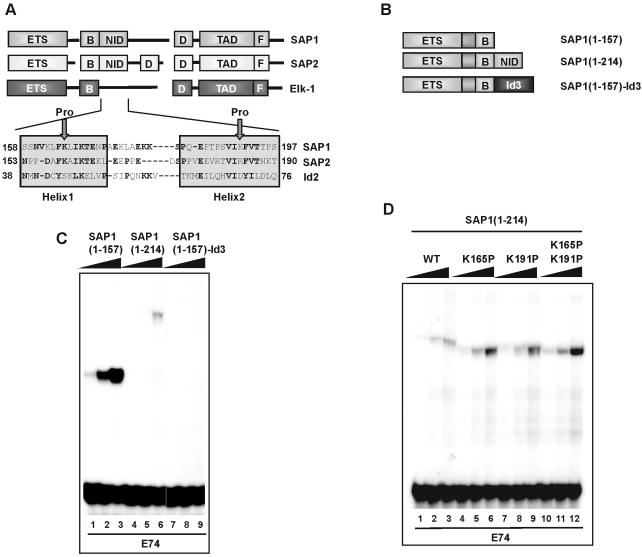
Figure 4.
Id proteins can functionally replace the NID. (A) Alignment of the sequences of the SAP-1 and SAP-2 NID domains, and the HLH domain of Id2. The N- and C-terminal amino acid residues with respect to full-length protein are indicated. Arrows indicate the positions of insertion of proline residues in the SAP-1(1–214) (K165P) and (K191P) mutants. (B) Schematic diagram of chimeric SAP-1 constructs used in (C) and (D). (C and D) Gel retardation analysis of the indicated SAP-1 fusion proteins on the E74-binding site. In vitro translated proteins were normalised to equal molar concentrations, and added in increasing relative amounts (1, 2.5 and 6) (C) and (1, 2 and 4) (D) (indicated schematically above each set of lanes by a triangle).
Here we have investigated how HLH motifs act in cis and in trans to regulate the activity of the TCFs. In common with SAP-2/Net/ERP, the NID region of SAP-1 inhibits DNA binding and also acts as a transcriptional repression domain. Fusion of the Id proteins to SAP-1 functionally replaces the NID and acts to repress DNA binding in cis. Phosphorylation of the Ids results in a reduction in protein–protein interactions between the Ids and TCFs and concomitant relief of their DNA-binding inhibitory activity. Together, our results therefore provide further insights into the role of HLH motifs in modifying the activities of the TCFs and how the inhibitory properties of the trans-acting Id proteins are regulated.
MATERIALS AND METHODS
Plasmid constructs
The following plasmids were used to express GST fusion proteins in Escherichia coli. pAS58 encodes coreSRF (amino acids 132–222) fused to the C-terminus of GST. pAS919 [encoding Id2 (amino acids 1–134) fused to the N-terminus of GST] was constructed by ligating an NcoI–SacI PCR fragment encoding Id2 into the same sites in pETGEXCT (20). pAS1562 and pAS1563 encode equivalent GST fusion proteins with the Id2 mutants Ser5Ala and Ser5Asp, respectively, and were constructed by PCR amplification from pCDNA3Id2Ala and pCDNA3Id2Asp (19), respectively, using oligonucleotide primer pair ADS851–ADS632, and ligated as NcoI–SacI fragments into the same sites of pETGEXCT.
The following plasmids were used for expressing C-terminal hexahistidine-Flag-tagged proteins in E.coli. pAS278 encodes full-length Elk-1 (amino acids 1–428) (21). pAS323 (constructed by Paul Shore) encodes the Elk-1 ETS-domain (amino acids 1–93) and was constructed by ligating an NcoI–XhoI PCR fragment into the same sites of pET-Hnef-PFH (22). pAS1598 and pAS1600 encode SAP-1 (amino acids 1–157) fused to Id2 (amino acids 1–134) and Id3 (amino acids 1–119), respectively. To create these plasmids, a fragment of SAP-1 (amino acids 1–157) was amplified by PCR with the primer pair ADS167–ADS850 on template pT7.SAP-1 (encoding full-length SAP-1; amino acids 1–431) (6) and cloned as an NcoI–XbaI fragment into pAS37 (23) to create pAS1561; Id2 and Id3 were amplified by PCR from templates pAS919 and pCDNA3Id3 (encoding full-length Id3; amino acids 1–119) (24) using primer pairs ADS967–ADS968 and ADS849–ADS969, respectively, followed by ligation as NdeI–XbaI fragments into the same sites of pAS1561 to create pAS1597 and pAS1599, respectively. SAP-1–Id2 or SAP-1–Id3 fusions were excised from pAS1597 and pAS1599 with NcoI–XhoI and ligated into the same sites of pET-Hnef-PFH to create pAS1598 and pAS1600, respectively. pAS1918 and pAS1919 encode Elk-1 ETS-domain and SAP-1 ETS-domain, respectively, with C-terminal hexahistidine flag tags, on a polycistronic plasmid with Id2. First, Id2 was amplified from pAS919 with primer pair ADS967–ADS970, cloned as an NdeI–BamHI fragment into pET3aTr (25), and transferred as an XbaI–BamHI fragment into pST39 (25) to create pAS1917. Next, the SAP-1 ETS-domain (amino acids 1–92) was amplified from pT7.SAP-1 with primer pair ADS972–ADS974 and cloned into NcoI–XhoI sites of pET-Hnef-PFH to create pAS1905. Similarly, the Elk-1 ETS-domain (amino acids 1–93) was amplified from pAS278 using primer pair ADS973–ADS975 and cloned as an NdeI–XhoI fragment into pAS1905 to create pAS1904. The SAP-1 and Elk-1 ETS-domains with C-terminal hexahistidine flag tags were excised from pAS1904 and pAS1905 with NdeI–BamHI and cloned into the same sites of pET3aTr to create pAS1907 and pAS1906; then cloned as SacI–KpnI fragments from pAS1907 and pAS1906 into pAS1917 (pET3aTr containing Id2) to create pAS1919 and pAS1918, respectively.
The following plasmids were used for in vitro transcription/translation purposes. pAS136 encoding SAP-1(1–92) and pAS168 encoding SAP-1(1–157) have been described previously (26). pAS1552, pAS1589, pAS1590 and pAS1591 encode SAP-1 truncations (amino acids 1–214, 1–197, 1–181 and 1–172, respectively). pAS1552 was constructed by inserting an NcoI–SalI-cleaved PCR product (primers; ADS167–ADS655 on template pT7.SAP-1) into the NcoI–XhoI sites of pAS728 (encoding full-length Elk-1; amino acids 1–428) (27). pAS1589, pAS1590 and pAS1591 were constructed by ligating NcoI–XbaI-cleaved PCR-derived fragments (primer pairs ADS167–ADS934, ADS167–ADS935 and ADS167–ADS933, respectively, on pAS1552 template) into the same sites of pAS37. pAS1571 (encoding Elk-1; amino acids 1–225) was constructed by ligating NcoI–XbaI-cleaved PCR products (primers ADS106–ADS900 and pAS278 template) into the same sites of pAS37. pAS1584 and pAS1583 encode Elk-1(1–168)–SAP-1(158–214) and Elk-1(1–168)–SAP-2(153–209) hybrids, respectively. Elk-1 (amino acids 1–168) was amplified from pAS278 with primer pair ADS106–ADS898, cleaved with NcoI–XbaI, and ligated into the same sites of pAS37 to create pAS1572. SAP-1 amino acids 158–214 and SAP-2 amino acids 153–209 were amplified by PCR [primers ADS901–ADS830 on template pT7.SAP-1, and primers ADS902–ADS903 on template pT7.SAP-2 (encoding full-length SAP-2; amino acids 1–407) (28), respectively], and the resulting fragments were cleaved with NdeI–XbaI and cloned into the same sites of pAS1572 to create pAS1584 and pAS1583, respectively. pAS2007 encodes SAP-1(158–214), and was constructed by inserting a HindIII–XbaI-cut PCR fragment (primers ADS847–ADS830 on pT7.SAP-1 template) into the same sites of pAS37. pAS1859 [encoding SAP-1(1–214)(K191P)], pAS1861 [encoding SAP-1(1–214)(K165P)] and pAS1862 [encoding SAP-1(1–214)(K165P/K191P)] were constructed by two-step PCR [flanking REV and FOR and mutagenic ADS1104, ADS1114 and ADS1114 primers, respectively, on templates pAS1552 (to create K165P and K191P mutants) and pAS1859 (to create K165P and K191P mutants) followed by cleavage with NcoI–XbaI and insertion into the same sites of pAS37]. pAS1560 encodes full-length Id2 (amino acids 1–134), and was constructed by inserting an NcoI–SacI-cleaved PCR fragment (primers ADS633–ADS846 on template pAS919) into the same sites of pAS37. pAS1565 encodes SAP-1(1–157)–Id3 hybrid, and was constructed by insertion of an NdeI–XbaI-cleaved PCR product encoding full-length Id3 (amino acids 1–119) (primers ADS849–ADS848 on template pCDNA3Id3), and ligation into the same sites of pAS1561 (containing SAP-1 amino acids 1–157). pcDNA3-Id3Ala and pCDNA3-Id3Asp contain full-length Id3 (amino acids 1–119) with Ser5Ala and Ser5Asp mutations, respectively, and have previously been described (19).
The following plasmids were used in mammalian cell transfections. pG5tkluc (pAS1567) contains five GAL4 DNA-binding sites cloned upstream of a minimal TK promoter element and the luciferase reporter (29). The L8G5E1a-Luc and LexA-VP16 constructs were provided by C. Lemercier (30). pSRE-luc (13) and pRSV-ElkVP16 (28) have been described previously. pAS571 (pCMV-GAL) has been described previously (29). pAS1901 (constructed by Shen-Hsi Yang) encodes SAP-1 (amino acids 1–157) fused to the GAL4 DNA-binding domain under the control of a cytomegalovirus (CMV) promoter, and was constructed by ligating a SalI–XbaI PCR fragment into the same sites of pAS571. pAS1555 and pAS1554 encode SAP-1 (amino acids 158–214 and 215–316, respectively) fused to the GAL4 DNA-binding domain under the control of a CMV promoter; they were constructed by ligating a SalI–XbaI-cleaved PCR product (primers ADS829–ADS830 and ADS816–ADS817, respectively, on pT7.SAP-1 template) into the same sites of pAS1079 (newpCMV5-GAL4). pAS1079 was created by cloning the HindIII–XbaI fragment from pAS1068 (31) into the same sites of pCMV5. pAS383 (CMV-driven Elk-1 amino acids 1–428 and C-terminal Flag tag) (21), pcDNA3Id2 (24), pCDNA3Id2Ala and pCDNA3Id2Asp have previously been described (19).
All PCR-derived constructs were verified by automated dideoxy sequencing.
Protein expression
The synthesis of proteins by in vitro transcription and translation was carried out with the TNT-coupled reticulocyte lysate system (Promega) according to the manufacturer’s recommendations. Newly synthesised 35S-labelled proteins were analysed by SDS–PAGE followed by visualisation and quantification by phosphoimager analysis (Bio-Rad molecular imager®FX and Quantity One software). GST fusion proteins (32) and hexahistidine-tagged proteins (21) were expressed in E.coli BL21(DE3)(pLysS) and purified as described previously. The purity and concentration of proteins were determined by SDS–PAGE next to standards. To purify Id–TCF complexes, Id2 and TCF ETS-domain (HisFlag-tagged) were co-expressed in E.coli BL21(DE3)(pLysS) transformed with a single plasmid (pAS1918 and pAS1919 encode Elk-1 ETS-domain and SAP-1 ETS-domain with C-terminal hexahistidine flag tag, respectively, on a polycistronic plasmid with Id2). Cells were grown in 0.5 l of LB/Amp medium at 37°C to mid-log phase, then induced with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.1 mM at 30°C for 2 h. Cells were harvested, resuspended in 5 ml of buffer A [50 mM HEPES pH 7.9, 150 mM NaCl, 4 mM phenylmethylsulfonyl fluoride (PMSF), 1.4 mM β-mercaptoethanol, 10% glycerol] and sonicated for 4 × 8 s with 20 s intervals on ice. The supernatant was cleared by centrifugation (10 000 g, 10 min) and applied to 300 µl of Ni2+-NTA–agarose (Qiagen) equilibriated in buffer A. Binding was carried out at 4°C for 1.5 h. Beads were then washed three times with buffer B (buffer A plus 15 mM imidazole). Protein was eluted with 300 µl of buffer C (buffer A plus 250 mM imidazole), and subsequently dialysed overnight into buffer A.
In vitro protein–protein interaction assays
Interactions between GST- or His-tagged fusion proteins and in vitro-translated or His-tagged proteins were investigated using pull-down assays as described previously (33).
Gel retardation assays
Gel retardation assays were performed with 32P-labelled probes as described previously (34). The binding sites include the c-fos SRE and the E74 site (33). DNA–protein complexes were formed at room temperature for 15 min. Proteins used included bacterially expressed coreSRF and bacterially expressed or in vitro-translated TCF and Id derivatives. Reactions containing in vitro translated proteins were normalised for reticulocyte lysate content. Complexes were analysed on non-denaturing 5% polyacrylamide gels cast in 0.5× Tris–borate–EDTA and visualised by phosphoimager analysis.
Cell culture transfection and reporter gene assays
293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL). Duplicate transfection experiments were done in 6-well plates using polyfect reagent according to the manufacturer’s recommendations (Qiagen). For reporter gene assays, SRE and GAL4 promoter-driven reporters were co-transfected alongside vectors encoding Elk-VP16 and GAL4-SAP-1 fusions, respectively. DNA concentrations were normalised with empty pCMV5 vector. Cell extracts were prepared, and luciferase and β-galactosidase assays were carried out as described previously (21).
Protein phosphorylation
Kinase reactions were carried out as described previously; Elk-1 was phosphorylated by ERK (21); Id was phosphorylated by cyclinA–Cdk2 (19). Mock reactions were done as per phosphorylation reactions, with the omission of kinase.
Immunoprecipitations and western blotting
For in vitro immunoprecipitations, Id2 and Elk-1(1–93)-HisFlag were co-expressed in bacteria and purified on Ni2+-NTA–agarose. Eluted, dialysed protein (1 µg) was used in 20 µl reactions containing 1 mM DTT, 0.05 mM ATP, 0.1 mM Na3VO4, plus or minus 0.5 µl of cyclinA–Cdk2 kinase, and incubated at 37°C for 30 min. Protein G beads (20 µl) were equilibriated in buffer A, then coupled to 0.5 µl of Id2 antibody for 1 h at room temperature. Beads were washed three times in buffer A to remove non-associated antibody. Following phosphorylation or mock treatment, Id2 and Elk-1(1–93) complexes were then added to beads coupled to antibody, and left to bind at room temperature for 1.5 h. Non-specific interactions were subsequently removed by washing three times with buffer A. Bound Elk-93 was detected by western blotting with an anti-Flag antibody.
For in vivo immunoprecipitations, a total of 15 µg of DNA [e.g. 7.5 µg of pAS383 (Flag-tagged Elk-1) and 7.5 µg of pcDNA3-Id2 (WT)] was transfected into 293T cells in 6-well plates using the standard calcium phosphate precipitation method. Cells were harvested 24 h later, rinsed in phosphate-buffered saline (PBS) and a cell lysate was prepared by the two-step lysis method in a total volume of 0.5 ml, essentially as described previously (35). Immunoprecipitations were carried out in the presence of 2 µg of Flag M2 antibody (Sigma) and 10 µl of protein G–Sepharose (Amersham-Pharmacia) for 12 h at 4°C with gentle mixing. Immune complexes were washed three times in lysis buffer [20 mM Tris–HCl pH 7.4, 420 mM NaCl, 10 mM MgCl2, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 25 mM β-glycerophosphate, 10 mM NaF, 1 mM DTT and complete™ protease inhibitors (Roche)], then eluted by boiling in SDS-sample buffer and subjected to 15 or 10% SDS–PAGE for the detection of Id2 or Elk-1 (Flag), respectively. Proteins were blotted onto nitrocellulose membranes and incubated with Id2 antibody (Santa Cruz, 1:100 in 3% skimmed milk/TBS) or Flag M2 antibody (1:2000) and then horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (DAKO, 1:2000) or anti-mouse IgG (Sigma: 1:2000), and visualised by ECL (Amersham-Pharmacia). Equal loading of lysate was confirmed by Coomassie brilliant blue staining (data not shown).
Id3 and GAL4 antibodies for western blotting were obtained from Santa Cruz.
Figure generation
Figures were generated from images generated by Molecular Imager®FX, Fluor-S™ MultiImager and Quantity One software (Bio-Rad). Final figures were assembled using PowerPoint 7.0 software (Microsoft).
RESULTS
The NID of SAP-1 represents a DNA-binding inhibitory element and transcriptional repression domain
Studies on SAP-2/Net/ERP have demonstrated that a region downstream from the B-box region, the NID, acts to inhibit DNA binding in vitro and also to repress transcription in vivo (12). This region exhibits significant sequence conservation in SAP-1 (see Fig. 4A); therefore, we tested whether it was also functionally conserved in this protein.
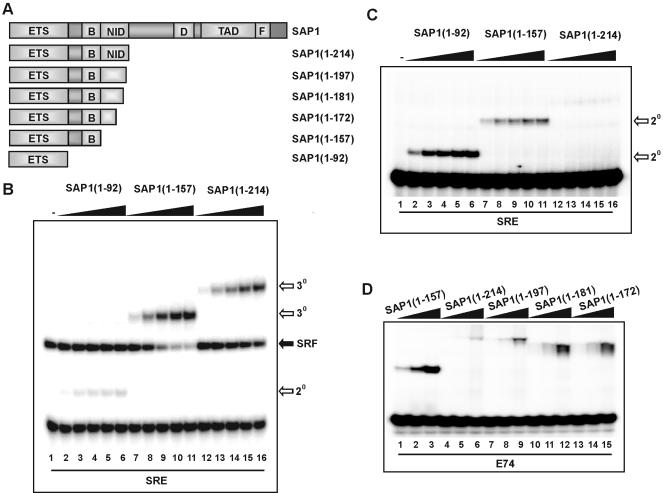
First we created a series of C-terminally truncated SAP-1 derivatives and assayed their DNA-binding characteristics. Ternary complexes were observed on the SRE in combination with SRF for both SAP-1(1–157) and SAP-1(1–214) (Fig. 1B, lanes 7–16). However, binding by SAP-1(1–214) (that contains an intact NID) was weaker than for SAP-1(1–157) (that lacks the NID). As expected, weak SRF-independent binding of SAP-1 was observed upon truncation of the B-box region (Fig. 1B, lanes 2–6) (6). The ability of the same proteins to bind the SRE in the absence of SRF was also tested (Fig. 1C). In comparison with SAP-1(1–92), SAP-1(1–157) bound with a reduced affinity, due to the presence of the B-box (Fig. 1C) (6). However, binding was barely detectable for SAP-1(1–214), demonstrating an important role for the NID region in inhibiting DNA binding (Fig. 1C, lanes 12–16). To further investigate the requirements for autoinhibition of DNA binding, further C-terminal deletions were tested on the high affinity E74 site (Fig. 1D). These deletion endpoints fall within putative structural features found within the NID (Fig. 4A). Upon deletion of the C-terminal residues of the NID, enhanced DNA binding was observed. Sequential deletion to amino acid 197 (to the end of putative helix 2) and 181 (that lacks part of this helix) led to a stepwise enhancement of DNA binding (Fig. 1D, lanes 4–12). This DNA binding was not increased further upon truncation to amino acid 172 (that lacks all helix 2; Fig. 1D, lanes 13–15). Collectively, these data point to an important role for the NID in inhibiting DNA binding by SAP-1 that is particularly noticeable at sites where binding occurs in an SRF- independent manner. Disruption of the structural integrity of the putative HLH motif in the NID abrogates this inhibitory function.
Figure 1.
The SAP-1 NID has a DNA-binding autoinhibitory function. (A) Schematic diagram of SAP-1 domains illustrating regions contained in truncated SAP-1 proteins. Numbers in brackets indicate N- and C-terminal amino acid residues of truncations, with respect to full-length protein. (B) Gel retardation analysis of binary (2o) and ternary (3o) complex formation by SAP-1(1–92) (lanes 2–6), SAP-1(1–157) (lanes 7–11) or SAP-1(1–214) (lanes 12–16) with coreSRF and c-fos SRE. The locations of binary SRF–DNA complexes are indicated. In vitro translated SAP-1 truncations were diluted to equal molar concentrations, and increasing volumes were added (0.2, 0.6, 1, 1.4 and 1.8 µl). (C) Binary (2o) complex formation of SAP-1 truncations on c-fos SRE as in (B) except SRF was omitted. (D) Gel retardation analysis of binary complexes on the E74-binding site by SAP-1 truncations: SAP-1(1–157) (lanes 1–3), SAP-1(1–214) (lanes 4–6), SAP-1(1–197) (lanes 7–9), SAP-1(1–181) (lanes 10–12) and SAP-1(1–172) (lanes 13–15). SAP-1 proteins were diluted to equal molar concentrations, and 0.25 µl was used in lanes 1, 4, 7, 10 and 13; 0.75 µl in lanes 2, 5, 8, 11 and 14; and 2.25 µl in lanes 3, 6, 9, 12 and 15.
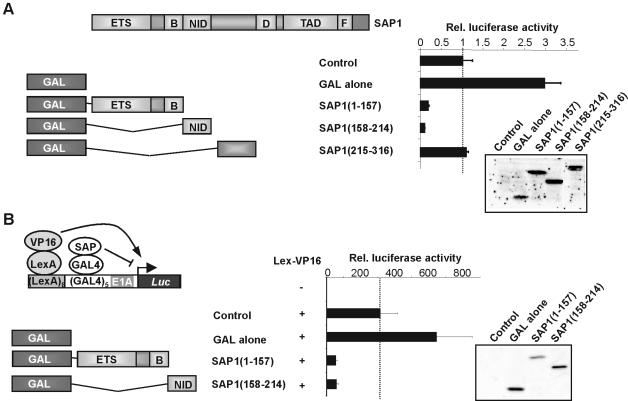
A series of GAL4 fusion proteins with portions of SAP-1 were created to assess the potential role of the NID as a transcriptional repression domain (Fig. 2). GAL fusions to both SAP-1(1–157) and SAP-1(158–214) exhibited strong repressive activity on a TK-luc reporter construct in comparison with SAP-1(215–316) (Fig. 2A). The level of reporter activity in the presence of GAL-SAP(158–214) was well below that observed with the reporter alone, demonstrating that the GAL fusion protein must be tethered to the DNA and therefore that the NID does not inhibit DNA binding in this context. Western blotting demonstrates that all constructs were expressed to similar levels. SAP-1(1–157) contains the ETS DNA-binding domain which has previously been shown to act as a potent repression domain in the related protein, Elk-1 (29), whereas SAP-1(158–214) just contains the NID. To establish whether the SAP-1 NID could repress highly active transcription, we analysed the ability of GAL-SAP-1 fusion proteins to repress a luciferase reporter gene driven by composite LexA- and GAL-binding sites (Fig. 2B) (30). Constructs encoding LexA-VP16 fusion proteins were transfected to activate the reporter gene, and GAL fusion proteins were co-transfected to assess transcriptional repressive properties. The GAL4 DNA-binding domain alone cooperated with Lex-VP16 to give enhanced reporter activity. However, GAL fusions with SAP-1(1–157) and SAP-1(158–214) both resulted in substantial repression of reporter activity. Thus, the SAP-1 NID appears to be a potent transcriptional repression domain that can also act in trans to inhibit transcription.
Figure 2.
The SAP-1 NID is a transcriptional repression domain. (A) The GAL4 DNA-binding domain (amino acids 1–147) was fused to a series of truncated SAP-1 proteins (amino acids are shown in brackets). Cells were co-transfected with 1 µg of pG5tkluc reporter vector, 0.5 µg of pCH110 and 0.1 µg of either pCMV alone (lane 1), pCMVGAL (lane 2), pCMVGAL-SAP-1(1–157) (lane 3), pCMVGAL-SAP-1(158–214) (lane 4), or pCMVGAL-SAP-1(215–316) (lane 5). Data presented are normalised with respect to β-gal activity, and values are given relative to control plasmid (taken as 1). Protein expression levels in 30 µl whole-cell extracts of transfected cells were determined by western analysis, using GAL4-specific antibodies. (B) GAL–SAP-1 truncations were tested for repression function using a LexA-VP16-activated LexA-GAL4-luc reporter. 293 cells were transfected with 1 µg of L8G5E1aluc reporter, 0.5 µg of pCH110 and either 0.2 µg of LexA-VP16 (lanes 2–5) or empty plasmid (lane 1) in addition to 0.1 µg of either pCMVGAL (lane 3), pCMVGAL-SAP-1(1–157) (lane 4) or pCMVGAL-SAP-1(158–214) (lane 5). Data presented are normalised with respect to β-galactosidase activity, and values are given relative to control plasmid (taken as 1). Protein expression levels were determined by western analysis of 10 µl of protein extract using GAL4-specific antibodies.
In summary, the NID in SAP-1 appears to act in an analogous manner to the NID in SAP-2/Net/ERP, to cause inhibition of DNA binding and provide transcriptional repression activities.
The SAP-1 NID represses DNA binding in trans and in heterologous contexts
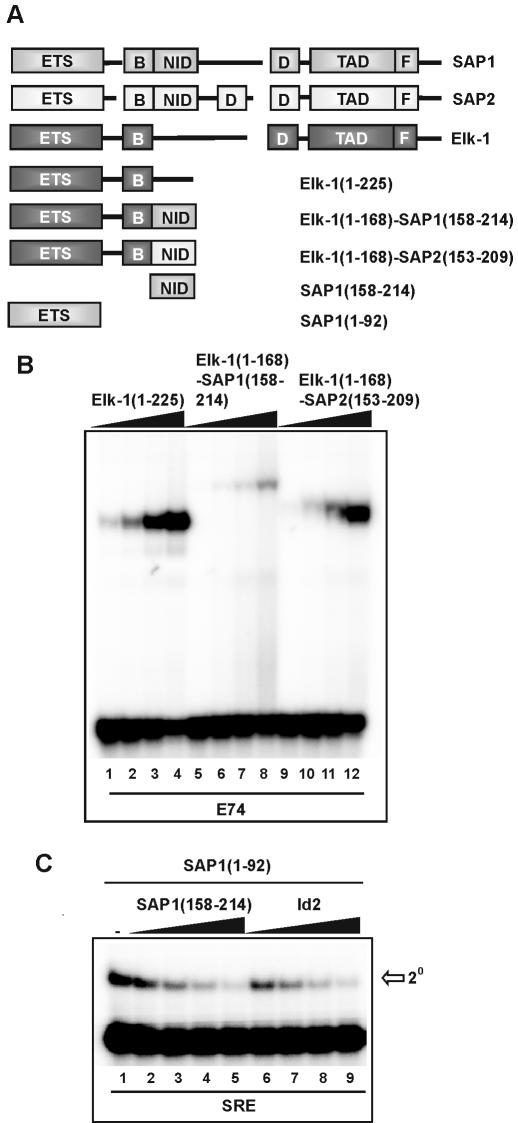
Deletion analysis indicated that the SAP-1 NID plays an important role in regulating its DNA-binding activity. To investigate whether the NID was sufficient for inhibiting DNA binding and whether the effect was context dependent, we first tested the role of the NID in inhibiting DNA binding when fused to Elk-1 (Fig. 3A). The ability of Elk-1 derivatives to bind DNA in the absence or presence of fusion to the NIDs from SAP-1 and SAP-2 was compared (Fig. 3B). Fusion of the NID from SAP-2 had a moderate effect in inhibiting DNA binding by Elk-1 that is most apparent at lower protein concentrations (Fig. 3B; compare lanes 2 and 10). This is consistent with previous results with similar chimeric Elk-1–SAP-2 proteins (12). However, fusion of the NID from SAP-1 resulted in potent inhibition of DNA binding (Fig. 3B, lanes 5–8). Similar effects are seen on the binding of Elk-1 in ternary complexes with SRF and the SRE (data not shown).
Figure 3.
The NID inhibits DNA binding in trans and in a heterologous context. (A) Schematic diagram of SAP-1 domains illustrating regions contained in full-length Elk-1, SAP-1 and SAP-2 proteins. Gaps are introduced to permit alignment of the conserved regions. The structures of the isolated domains used and chimeric Elk-1–NID constructs are indicated below (numbers in brackets indicate N- and C-terminal amino acid residues of truncations, with respect to full-length proteins). (B) Gel retardation analysis of the indicated wild-type and chimeric Elk-1 proteins on the E74-binding site. In vitro translated proteins were diluted to equal molar concentrations, and added in increasing amounts (0.2, 0.6, 1.8 and 5.4 µl) (indicated schematically above each set of lanes by a triangle). (C) SAP-1 NID inhibits binding of the SAP-1 ETS domain to the c-fos SRE in trans. In vitro translated SAP-1 NID and Id2 were diluted to equal molar amounts, and 0.5, 1, 2 and 4 µl (indicated schematically above each set of lanes by a triangle) were added in the presence of a constant amount of SAP-1(1–92). Total amounts of reticulocyte lysate were equalised by adding unprogrammed lysate.
The Id proteins contain a helix–turn–helix motif that is related to the NID sequence (Fig. 4A) and inhibits DNA binding by TCF family members when added in trans (13; Fig. 3C, lanes 6–9). This effect is elicited through the ETS DNA-binding domain. We therefore tested whether the NID could act in an analogous manner when added in trans. Addition of increasing concentrations of the SAP-1 NID [SAP-1(158–214)] led to a dose-dependent decrease in DNA binding by the SAP-1 ETS-domain [SAP-1(1–92)] (Fig. 3C lanes 2–5). Thus, the SAP-1 NID can act both in trans and in cis to inhibit DNA binding through the SAP-1 ETS-domain.
Id proteins can inhibit DNA binding in cis
The Id proteins exhibit significant sequence similarity to the NID found in SAP-1 and SAP-2 (Fig. 4A), and the NID can act in trans to inhibit DNA binding by the TCFs in an analogous manner to the Id proteins. We therefore tested whether Id proteins could functionally replace the NID in SAP-1 and inhibit DNA binding in cis when fused to SAP-1 (Fig. 4B). In the presence of the SAP-1 NID [SAP-1(1–214)], DNA binding was severely inhibited (Fig. 4C, lanes 4–6). Fusion of Id3 to the C-terminus of SAP-1(1–157) also resulted in inhibition of DNA binding. This inhibition was even more pronounced than that observed in SAP-1(1–214) (Fig. 4C, lanes 7–9). Similarly, the fusion of Id2 to SAP-1 in the SAP-1(1–157)–Id2 protein results in inhibition of ternary complex formation by SAP-1 and SRF on the SRE (data not shown). Deletions into the C-terminus of the Id3 HLH motif, which are predicted to affect its structural integrity, result in reduced DNA binding inhibition (data not shown).
Our results suggest that the SAP-1 NID is structurally and functionally related to the HLH motif (Figs 1, 3 and 4C). To confirm this conclusion, we introduced helix-breaking proline residues into the putative helices in the context of the intact NID (Fig. 4A). We then tested the resulting mutant proteins [SAP-1(1–214)(K191P), SAP-1(1–214)(K165P) and SAP-1(1–214)(K165P/K191P)] in comparison with their wild-type counterparts for binding to the E74 site (Fig. 4D). In comparison with the wild-type protein, enhanced DNA binding was observed for SAP-1(1–214)(K165P) and SAP-1(1–214)(K191P) (Fig. 4D, lanes 4–9). This binding was further enhanced upon simultaneous introduction of the two mutations in SAP-1(1–214)(K165P/K191P) (Fig. 4D, lanes 10–12). Thus autoinhibition of DNA binding was lost in these mutants. A difference in complex mobility was also observed in these mutants, which is consistent with the expected conformational change upon insertion of proline residues. These data are consistent with the effects seen upon introduction of a proline residue into the related NID motif in SAP-2/Ner/Erp (12).
Thus, our data further support the conclusion that the NID is structurally related to the HLH motif and that the NID and Id proteins can act both in trans and in cis to inhibit DNA binding by SAP-1.
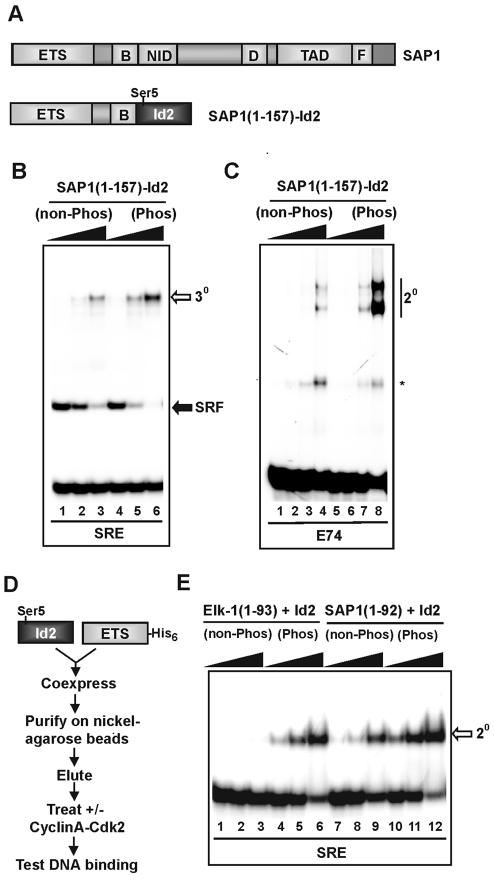
Control of Id-regulated DNA binding inhibition by Cdk-mediated phosphorylation
The Id2 and Id3 proteins are phosphorylated in vivo by cyclin–Cdk2 complexes in a cell cycle-dependent manner. This phosphorylation event occurs exclusively at a conserved Ser5 residue in the Id2/Id3 proteins and regulates their ability to interact with bHLH transcription factors (18,19). We therefore tested whether phosphorylation of Id2/3 proteins by cyclinA–Cdk2 complexes affected their ability to inhibit DNA binding by TCF ETS-domain transcription factors.
First, we tested the effect of phosphorylation on the inhibitory actions of Id proteins in the context of SAP-1(1–157)–Id2 fusion proteins. Phosphorylation of SAP-1(1–157)–Id2 by cyclinA–cdk2 complexes led to an increase in DNA binding by SAP-1(1–157)–Id2 in the context of ternary complexes containing SRF (Fig. 5B) and also when binding in the absence of SRF (Fig. 5C). Similar results were seen with fusions with Id3 (data not shown).
Figure 5.
Phosphorylation of Id reduces its DNA-binding inhibitory properties. (A) Schematic diagram of the chimeric SAP-1–Id2 construct. The location of the cyclinA–Cdk2 phosphorylation site (Ser5) is indicated. (B) Gel retardation analysis of SAP-1–Id2 chimeras in the absence and presence of prior phosphorylation by cyclinA–Cdk2 complexes. The SAP-1–Id2 chimeric protein was expressed in bacteria and purified. SAP-1–Id2 was either left unphosphorylated or was phosphorylated by cyclinA–Cdk2. Increasing amounts (24, 48 and 96 ng) were tested in a gel retardation assay for effects on ternary (3o) complex formation with coreSRF and c-fos SRE. (C) As in (B), except SRF was omitted from the reaction, the E74-binding site was used, and binary (2o) complexes detected. SAP-1–Id2 was added at 12, 24, 48 and 96 ng (indicated schematically above each set of lanes by a triangle). The asterisk represents a complex formed from truncated SAP-1–Id protein. (D) Methodology for co-expressing Id2 and the ETS DNA- binding domain of TCFs. Id2 and either the Elk-1 or SAP-1 ETS-domain were co-expressed in E.coli from a single plasmid. Induced protein complexes were purified, and used in subsequent experiments. (E) Complexes containing Id2 and the ETS-domain of either Elk-1 or SAP-1 were either left unphosphorylated or phosphorylated with cyclinA–Cdk2, and tested for their ability to bind to the c-fos SRE in gel retardation analysis. Aliquots of 9.5 (lanes 1 and 4), 19 (lanes 2 and 5) and 57 ng (lanes 3 and 6) of Elk-1(1–93)–Id2 complexes, and 15 (lanes 7 and 10), 30 (lanes 8 and 11) and 90 ng (lanes 9 and 12) of SAP-1(1–157)–Id2 complexes were added.
Next we examined whether phosphorylation of Ids also affected their ability to inhibit DNA binding in trans. Polyhistidine-tagged ETS DNA-binding domains of Elk-1 [Elk-1(1–93)] or SAP-1 [SAP-1(1–92)] were co-expressed in bacteria with Id2 to permit the formation of complexes upon folding (25). Resulting ETS-domain–Id complexes were then purified by nickel affinity chromatography, eluted and tested for their DNA-binding activity either in the absence of phosphorylation or following cyclinA–Cdk2-mediated phosphorylation. If phosphorylation leads to the disruption of the inhibitory properties of the Id proteins, it would be predicted that enhanced DNA binding by the ETS-domain should be observed. In the absence of phosphorylation of the Elk-1(1–93)–Id2 complex, very low levels of DNA binding by Elk-1(1–93) are observed (Fig. 5E, lanes 1–3). However, prior phosphorylation through cyclinA–Cdk2 results in enhanced DNA binding by Elk-1(1–93) (Fig. 5E, lanes 4–6). Similarly, treatment of SAP-1(1–92)–Id2 complexes with cyclinA–Cdk2 results in enhanced DNA binding by SAP-1(1–92) (Fig. 5E, lanes 7–12).
Collectively, these data demonstrate that phosphorylation of Id proteins by cyclinA–Cdk2 complexes results in reduction of the DNA-binding inhibitory capacity of the Ids when acting either in cis or in trans.
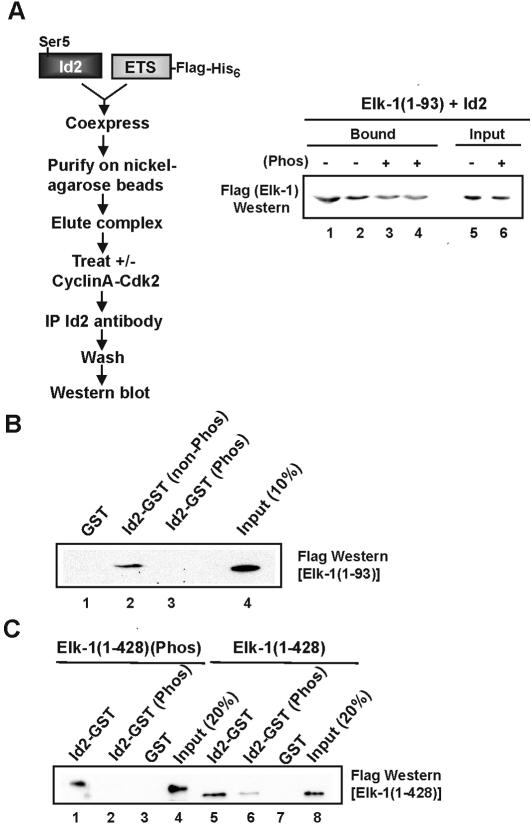
Id phosphorylation inhibits interactions with TCFs in trans
The loss of Id-mediated DNA binding inhibition caused by phosphorylation probably reflects a change in the interactions between the Id proteins and TCFs. To establish whether this is the case, we investigated the effect of phosphorylation of Id proteins on interactions with the TCFs. First, we probed the ability of cyclinA–Cdk2 to promote dissociation of pre-formed complexes between the ETS-domain of Elk-1 and Id2. Id2–Elk-1(1–93) complexes were purified, treated with cyclinA–Cdk2 and the resulting Id2-associated complexes re-precipitated using Id2-specific antibodies (Fig. 6A). In comparison with the non-phosphorylated samples, reduced binding of Elk-1 was observed following cyclinA–Cdk2-mediated phosphorylation of Id2–Elk-1(1–93) complexes (Fig. 6A, lanes 3 and 4). Similar results were obtained by carrying out reciprocal immunoprecipitations with anti-Flag antibody, followed by detection of Id2 in the resulting complexes (data not shown). These results are consistent with the enhanced DNA-binding activity observed following cyclinA–Cdk2-mediated phosphorylation of Id2/Elk-1(1–93) complexes (Fig. 5E).
Figure 6.
Effect of phosphorylation on Id2 interactions with Elk-1 in vitro. (A) A flow diagram depicting steps used to test binding of the Elk-1 ETS-domain and Id2 is shown on the left. Id2 and the Elk-1 ETS-domain were co-expressed in E.coli from a single plasmid. Induced protein complexes were purified and used in subsequent experiments. Purified complexes were either phosphorylated with cyclinA–Cdk2 or mock treated and immunoprecipitated with Id2 antibody coupled to agarose beads (∼1 µg of total purified protein complex was used). After washing, the remaining Elk-1 in the complex was detected by western blot using Flag antibody. Duplicate samples are shown in lanes 1 and 2, and 3 and 4. Ten percent of the input protein is shown. (B) GST pull-down analysis of the ETS-domain (amino acids 1–93) of Elk-1 (1 µg) with 1.5 µg of GST (lane 1) or 1.5 µg of Id2–GST fusion protein, with or without prior phosphorylation by cyclinA–Cdk2 (lanes 3 and 2, respectively). Lane 4 shows 10% of input protein. (C) GST pull-down analysis of full-length Elk-1 (amino acids 1–428) with Id2–GST. Elk-1 (220 ng) was left unphosphorylated (lanes 5–8) or phosphorylated with Erk (lanes 1–4). Id2–GST (1.5 µg) was either non-phosphorylated (lanes 1 and 5) or phosphorylated with cyclinA–Cdk2 (lanes 2 and 6). GST (1.5 µg) was used as a control (lanes 3 and 7). Lanes 4 and 8 show 20% of input. Precipitated Elk-1 was detected using anti-Flag antibody.
Interactions between Id2 and Elk-1 were also investigated using GST pull-down analysis. Firstly, Id2–GST fusion proteins were tested for interactions with Elk-1(1–93). Id2–GST–Elk-1(1–93) complexes were detectable in the absence of prior Id2 phosphorylation (Fig. 6B, lane 2). However, phosphorylation of Id2–GST by cyclinA–Cdk2 led to a large decrease in complex formation with Elk-1(1–93) (Fig. 6B, lane 3). Next, we compared the interaction of full-length Elk-1 with Id2–GST. In this case, we also investigated the role of Elk-1 phosphorylation by Erk2 in regulating these interactions. Phosphorylation of Id2–GST led to decreases in interactions with both phosphorylated and non-phosphorylated Elk-1 (Fig. 6C, compare lanes 1 and 5 with 2 and 6). However, residual binding to non-phosphorylated Elk-1 was observed that is further reduced upon Elk-1 phosphorylation, adding a further tier of regulation on these interactions (Fig. 6C, lanes 2 and 6).
In summary, interactions between Id proteins and Elk-1 are reduced following phosphorylation of Ids with cyclinA–Cdk2. This reduction in protein–protein interactions is consistent with the reductions observed in the inhibitory activity of the Ids towards the TCFs.
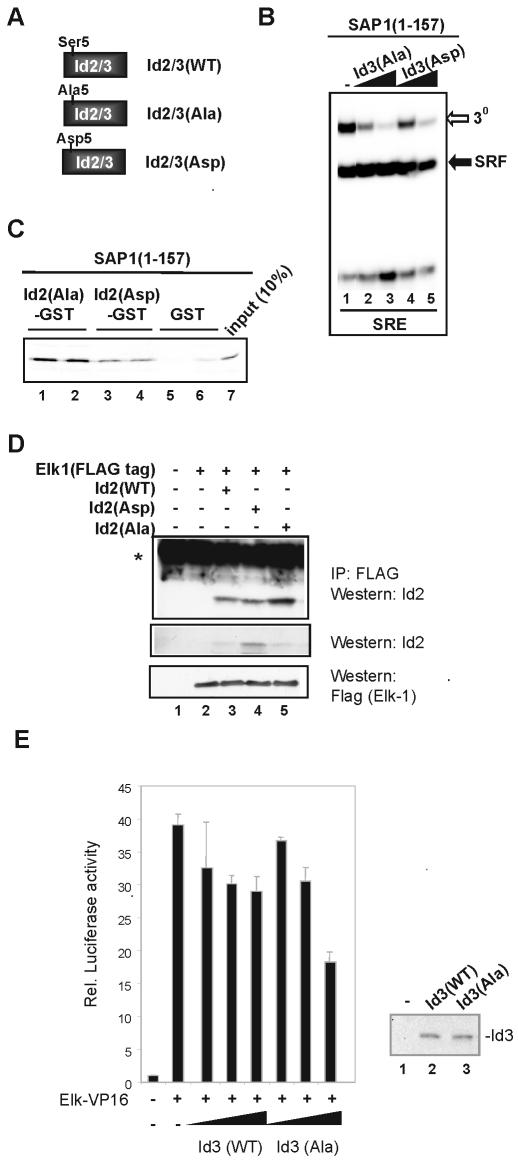
Phosphomimetic residues mimic the effect of phosphorylation on Id–TCF interactions in vitro and in vivo
To further establish the importance of Ser5 in Id proteins for regulating interactions with TCFs, we analysed the effect of introducing alanine (to block phosphorylation) or aspartate (to mimic phosphorylation) residues at this position (Fig. 7A). Proteins harbouring these mutations have previously been shown to be refractory to phosphorylation by cyclin A–cdk2 and to affect interactions with bHLH proteins (19).
Figure 7.
Phosphomimetics imitate phosphorylation of Id. (A) Schematic illustration of a series of mutants of Ser5 in Id2 and Id3. (B) Gel retardation analysis of in vitro translated SAP-1 (amino acids 1–157) in a ternary complex (3o) with coreSRF and c-fos SRE. Complexes were challenged with increasing amounts, 0.5 (lanes 2 and 4) and 2.5 µl (lanes 3 and 5), of equimolar stocks of in vitro-translated Id3 Ala5 or Asp5 mutants. (C) GST pull-down analysis of SAP-1(1–157) binding to the indicated mutant GST–Id2 fusions. Lanes 1 and 2 [Id2(Ala)–GST], lanes 3 and 4 [Id2(Asp)–GST] and lanes 5 and 6 (GST) represent duplicate samples; lane 7 shows 10% of input. (D) Co-immunoprecipitation of Id2 mutants with Elk-1 from 293T cells. Cells were either mock transfected with empty vector (lane 1) or transfected with 7.5 µg of Elk-1 (lane 2), or 7.5 µg of Elk-1 and 7.5 µg of either Id2(WT), Id2(Asp) or Id2(Ala) (lanes 3, 4 and 5, respectively). Elk-1 complexes were precipitated using anti-Flag antibody coupled to Sepharose beads, and bound protein was detected by western analysis with Id2 antibody. Westerns with Id2 and Flag antibodies were used to compare relative expression levels of Id2 and Elk-1 in the input samples (bottom two panels). (E) Reporter gene analysis of Id-mediated inhibition of TCF activity. Cells were co-transfected with 100 ng of pSRE-luc reporter vector, 100 ng of pCH110, 10 ng of pRSV-Elk-VP16 (lanes 2–7) and increasing amounts of wild-type Id3 and Id3(Ala) (0.3, 1 and 3 µg) (indicated schematically with a triangle under each set of bars). Data presented are duplicate samples normalised with respect to β-gal activity, and values are given relative to reporter alone (taken as 1). Protein expression levels in whole-cell extracts of transfected cells were determined by western analysis, using Id3-specific antibodies (right hand panel). Extracts were analysed from samples taken from untransfected cells (lane 1) and cells transfected with 3 µg of wild-type Id3 (lane 2) or Id3(Ala) (lane 3).
The ability of Id3(Ala) and Id3(Asp) to regulate DNA binding by SAP(1–157) was compared (Fig. 7B). Both Id proteins caused a decrease in ternary complex formation, but Id3(Ala) was the most potent (Fig. 7B, compare lanes 2 and 4). Consistent with this observation, SAP-1(1–157)–Id3(Asp) fusion proteins also exhibited higher DNA-binding activity than their alanine mutant counterparts (data not shown).
Next we compared the ability of mutant Id2–GST fusion proteins to bind to SAP-1(1–157). Reduced binding of SAP-1(1–157) was observed to Id2(Asp)–GST in comparison with Id2(Ala)–GST (Fig. 7C). Similar results were obtained for interactions with Elk-1(1–168) (data not shown). Finally, we compared the ability of the Id2 mutants to interact with full-length Elk-1 in vivo. Again, more binding of Elk-1 to Id2(Ala) was observed than to Id2(Asp) (Fig. 7D, lanes 4 and 5). This is probably an underestimate of the difference due to the higher expression levels of Id2(Asp) (Fig. 7D, middle panel).
To further confirm the functional importance of Ser5 phosphorylation for Id function towards the TCFs, we carried out transfection experiments in which we analysed the ability of Id3 to inhibit transcription driven by a constitutively active Elk-VP16 fusion protein. Wild-type Id3 was compared with Id3(Ala), in which Ser5 is mutated to alanine and hence can no longer be phosphorylated by cyclin–Cdk complexes (19). Elk-VP16 strongly activated the SRE reporter gene (Fig. 7E). Co-transfection with increasing amounts of wild-type Id3 led to a decrease in transactivation. However, co-transfection with Id3(Ala) led to an even greater inhibition of transactivation which is most apparent at the highest concentrations of expression vector used [20% reduction with wild-type Id3 and >50% reduction with Id3(Ala)] (Fig. 7E). Importantly, the expression levels of the wild-type and mutant Id3 proteins were virtually identical at these concentrations (Fig. 7E, right hand panel). These data therefore support the notion that phosphorylation weakens interactions between Ids and the TCFs and hence reduces their inhibitory activity.
In summary, mutation of Ser5 in the Id proteins leads to effects consistent with the importance of this residue as a phosphorylation-regulated switch that controls the interactions between the Id proteins and TCFs and hence the Id-mediated inhibitory properties.
DISCUSSION
DNA binding by ETS-domain transcription factors is a tightly regulated process that often involves autoinhibitory mechanisms [reviewed in Graves et al. (36)]. This autoinhibition is thought to stop promiscuous binding by these proteins in the absence of appropriate regulatory cues or co-regulatory partner proteins. Indeed, different members of the TCF subfamily are regulated by multiple autoinhibitory mechanisms. For example, DNA binding by Elk-1 is regulated by a combination of the B-box region and its C-terminal region (10,11). DNA binding by SAP-2/Net/ERP is regulated by the NID region (12). Here we show that the analogous NID region of SAP-1 plays a major role in inhibiting its DNA-binding activity (Fig. 1). In addition, the NIDs from both SAP-2/Net/ERP and SAP-1 act as transcriptional repression domains (Fig. 2) (12). These regions therefore are functionally equivalent in addition to exhibiting significant sequence similarity (Fig. 4A). Mutagenic studies on SAP-2/Net/ERP provided evidence to underpin the idea that the NID contains a HLH motif (12). Deletion analysis demonstrated that removal of part of the C-terminal helix in the NID HLH motif abrogated the inhibitory activity of this motif in SAP-1 (Fig. 1). Furthermore, the introduction of helix-breaking proline residues into the putative helices of the HLH motif also abrogated the inhibitory activity of the NID (Fig. 4D), further enhancing our conclusion that as in SAP-2/Net/Erp (12), this region probably represents a HLH motif.
In addition to the NID, the N-terminal domain encompassing the ETS-domain was identified as a transcriptional repression domain in SAP-1 (Fig. 2). This is consistent with the observation that the equivalent region of Elk-1 has repressive activity through recruiting the Sin3A/HDAC complex (29). Elk-1 contains an additional repression domain, the R-motif, that is not conserved in SAP-1 and SAP-2/Net/ERP (37). In addition to the ETS-domain and NID, SAP-2/Net/ERP contains a further repression domain, the CID, which is not conserved in the other TCFs (38). Thus, each of the TCFs appears to have acquired different negatively acting regions that serve to modify their transcriptional activation properties.
The Id proteins act in trans to inhibit DNA binding of TCFs through binding to the ETS DNA-binding domain (13). The Ids exhibit significant sequence similarity to the NID, but this is restricted to the HLH motifs (Fig. 4A). Fusion of the Ids to the TCFs inhibits their DNA-binding capacity (Fig. 4), and the isolated SAP-1 NID acts in trans to inhibit the DNA-binding activity of the TCF ETS-domain (Fig. 3). Furthermore, the target for inhibition for both the NID and the Id proteins is through the ETS-domain. Therefore, in addition to their sequence conservation, the Ids and NID are functionally equivalent in inhibiting DNA binding by TCFs whether analysed in cis or in trans.
Phosphorylation of the Id proteins on Ser5 by cyclinA/E–Cdk2 complexes reduces their ability to inhibit DNA binding by the class A bHLH ‘E’ proteins (18,19). Replacement of this residue by the phosphomimetic aspartate causes a similar effect (19). Here we show that phosphorylation of Id2 and Id3 reduces their potency as inhibitors of TCF DNA binding (Fig. 5). This reduction in DNA-binding activity is mirrored by reductions in protein–protein interactions between the Ids and the TCFs (Fig. 6). Similarly, the replacement of Ser5 with aspartate leads to a loss of inhibitory activity and interactions with TCFs in vitro and in vivo (Fig. 7), but this appears to be a weak phosphomimetic by comparison with the data obtained with cyclinA–Cdk2 phosphorylated proteins. Low level binding of phosphorylated Id2 to non-phosphorylated Elk-1 is still detectable, which is further reduced upon phosphorylation of Elk-1 (Fig. 6). Thus the active phosphorylated form of Elk-1 appears refractory to Id binding and hence to inhibition of its DNA binding. A potential role for the Id proteins is to remove inactive TCFs from the promoter and permit exchange with other TCFs. Indeed, evidence to support this is provided by the observation that Id proteins cause the inhibition of TCF-mediated transactivation (Fig. 7E) (13). The non-phosphorylatable Ser5Ala mutant form of Id3 acts as a more potent inhibitor, consistent with our conclusion that phosphorylation causes disruption of Id–TCF complexes. Phosphorylation of the Id proteins occurs at the G1/S phase boundary, which would cause release of the TCFs to permit recycling of these transcription factors. The observation that phosphorylation of Id proteins causes the disruption of pre-formed Id–TCF complexes and blocks the formation of new complexes (Fig. 6A and B) provides further support for this model.
In summary, we have demonstrated the importance of the HLH motifs in regulating the DNA-binding activities of the TCFs both in cis and in trans, and uncovered further complexities in how the inhibitory activities of the Id proteins are regulated by phosphorylation.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank Linda Shore for excellent technical assistance, R. Treisman, S. Tan and S. Kochbin for reagents, Paul Shore and Shen-Hsi Yang for comments on the manuscript, and members of our laboratory for helpful discussions. This work was supported by the Wellcome Trust and Cancer Research UK. A.D.S. was supported as a Research Fellow of the Lister Institute of Preventive Medicine. T.I. was supported in part by a Yamada Science Foundation Fellowship.
REFERENCES
- 1.Treisman R. (1994) Ternary complex factors: growth regulated transcriptional activators. Curr. Opin. Genet. Dev., 4, 96–101. [DOI] [PubMed] [Google Scholar]
- 2.Sharrocks A.D. (2002) Complexities in ETS-domain transcription factor function and regulation; lessons from the TCF subfamily. Biochem. Soc. Trans., 30, 1–9. [DOI] [PubMed] [Google Scholar]
- 3.Gille H., Sharrocks,A.D. and Shaw,P.E. (1992) Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature, 358, 414–417. [DOI] [PubMed] [Google Scholar]
- 4.Gille H., Kortenjann,M., Thomae,O., Moomaw,C., Slaughter,C., Cobb,M.H. and Shaw,P.E. (1995) ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J., 14, 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharrocks A.D. (1995) ERK2/p42 MAP kinase stimulates both autonomous and SRF-dependent DNA binding by Elk-1. FEBS Lett., 368, 77–80. [DOI] [PubMed] [Google Scholar]
- 6.Dalton S. and Treisman,R. (1992) Characterisation of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell, 68, 597–612. [DOI] [PubMed] [Google Scholar]
- 7.Giovane A., Pintzas,A., Maira,S-M., Sobieszcuk,P. and Wasylyk,B. (1994) Net, a new ets transcription factor that is activated by Ras. Genes Dev., 8, 1502–1513. [DOI] [PubMed] [Google Scholar]
- 8.Hipskind R.A., Rao,V.N., Mueller,C.G., Reddy,E.S. and Nordheim,A. (1991) Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature, 354, 531–534. [DOI] [PubMed] [Google Scholar]
- 9.Janknecht R. and Nordheim,A. (1992) Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res., 20, 3317–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janknecht R., Zinck,R., Ernst,W.H. and Nordheim,A. (1994) Functional dissection of the transcription factor Elk-1. Oncogene, 9, 1273–1278. [PubMed] [Google Scholar]
- 11.Yang S.-H., Shore,P., Willingham,N., Lakey,J.H. and Sharrocks,A.D. (1999) Mechanism of phosphorylation-inducible activation of the ETS-domain transcription factor Elk-1. EMBO J., 18, 5666–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maira S.M., Wurtz,J.M. and Wasylyk,B. (1996) Net (ERP/SAP2) one of the Ras-inducible TCFs, has a novel inhibitory domain with resemblance to the helix–loop–helix motif. EMBO J., 15, 5849–5865. [PMC free article] [PubMed] [Google Scholar]
- 13.Yates P.R., Atherton,G., Deed,R.W., Norton,J.D. and Sharrocks,A.D. (1999) Id helix–loop–helix proteins inhibit nucleoprotein complex formation by the TCF ETS-domain transcription factors. EMBO J., 18, 968–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benezra R (2001) Regulation by Id. Oncogene, 20, 8288–8289. [Google Scholar]
- 15.Norton J.D., Deed,R.W., Craggs,G. and Sablitzky,F. (1998) Id helix–loop–helix proteins in cell growth and differentiation. Trends Cell Biol., 8, 58–65. [PubMed] [Google Scholar]
- 16.Norton J.D. (2000) Id helix–loop–helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci., 113, 3897–3905. [DOI] [PubMed] [Google Scholar]
- 17.Yokota Y. and Mori,S. (2002) Role of Id family proteins in growth control. J. Cell. Physiol., 190, 21–28. [DOI] [PubMed] [Google Scholar]
- 18.Hara E., Hall,M. and Peters,G. (1997) Cdk2-dependent phosphorylation of Id2 modulates activity of E2A-related transcription factors. EMBO J., 16, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deed R.W., Hara,E., Atherton,G.T., Peters,G. and Norton,J.D. (1997) Regulation of Id3 cell cycle function by Cdk-2-dependent phosphorylation. Mol. Cell. Biol., 17, 6815–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharrocks A.D. (1994) A T7 expression vector for producing N-terminal fusions with glutathione S-transferase. Gene, 138, 105–108. [DOI] [PubMed] [Google Scholar]
- 21.Yang S.-H., Whitmarsh,A.J., Davis,R.J. and Sharrocks,A.D. (1998) The Elk-1ETS-domain transcription factor contains a MAP kinase targeting motif. Mol. Cell. Biol., 18, 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L.-J. and Narayan,O. (1993) A gene expression vector useful for protein purification and studies of protein–protein interactions. Gene, 137, 345–346. [DOI] [PubMed] [Google Scholar]
- 23.Sharrocks A.D., von Hesler,F. and Shaw,P.E. (1993) The identification of elements determining the different DNA binding specificities of the MADS box proteins p67SRF and RSRFC4. Nucleic Acids Res., 21, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norton J.D. and Atherton,G.T. (1998) Coupling of cell growth control and apoptosis functions of Id proteins. Mol. Cell. Biol., 18, 2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan S. (2001) A modular polycistronic expression system for overexpressing protein complexes in Escherichia coli. Protein Expr. Purif., 21, 224–234. [DOI] [PubMed] [Google Scholar]
- 26.Shore P. and Sharrocks,A.D. (1995) The ETS-domains of the TCFs Elk-1 and SAP-1 exhibit differential DNA binding specificities. Nucleic Acids Res., 23, 4698–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling Y., Lakey,J., Roberts,E.C. and Sharrocks,A.D. (1997) Molecular characterisation of the B-box protein–protein interaction motif of the ETS-domain transcription factors Elk-1. EMBO J., 16, 2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price M.A., Rogers,A.E. and Treisman,R. (1995) Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET). EMBO J., 14, 2589–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S.H., Vickers,E., Brehm,A., Kouzarides,T. and Sharrocks,A.D. (2001) Temporal recruitment of the mSin3A-histone deacetylase corepressor complex to the ETS domain transcription factor Elk-1. Mol. Cell. Biol., 21, 2802–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemercier C., Verdel,A., Galloo,B., Curtet,S., Brocard,M.P. and Khochbin,S. (2000) mHDA1/HDAC5 histone deacetylase interacts with and represses MEF2A transcriptional activity. J. Biol. Chem., 275, 15594–15599. [DOI] [PubMed] [Google Scholar]
- 31.Galanis A., Yang,S.-H. and Sharrocks,A.D. (2001) Targeting of MAP kinase signal transduction pathways to SAP-1. J. Biol. Chem., 276, 965–973. [DOI] [PubMed] [Google Scholar]
- 32.Shore P., Bisset,L., Lakey,J., Waltho,J.P. and Sharrocks,A.D. (1995) Characterization of the Elk-1 ETS DNA-binding domain. J. Biol. Chem., 270, 5805–5811. [DOI] [PubMed] [Google Scholar]
- 33.Shore P. and Sharrocks,A.D. (1994) The transcription factors Elk-1 and serum response factor interact by direct protein–protein contacts mediated by a short region of Elk-1. Mol. Cell. Biol., 14, 3283–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharrocks A.D., Gille,H. and Shaw,P.E. (1993) Identification of the amino acids essential for DNA binding and dimerization in p67SRF: implications for a novel DNA-binding motif. Mol. Cell. Biol., 13, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts E.C., Deed,R.W., Norton,J.D. and Sharrocks,A.D. (2001) Id helix–loop–helix proteins antagonise the activity of Pax transcription factors by inhibiting DNA binding. Mol. Cell. Biol., 21, 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graves B.J., Cowley,D.O., Goetz,T.L., Petersen,J.M., Jonsen,M.D. and Gillespie,M.E. (1998) Autoinhibition as a transcriptional regulatory mechanism. Cold Spring Harbor Symp. Quant Biol., 63, 621–629. [DOI] [PubMed] [Google Scholar]
- 37.Yang S.-H., Bumpass,D.C., Perkins,N.D. and Sharrocks,A.D. (2002) The ETS-domain transcription factor Elk-1 contains a novel class of repression domain. Mol. Cell. Biol., 22, 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Criqui-Filipe P., Ducret,C., Maira,S.M. and Wasylyk,B. (1999) Net, a negative Ras-switchable TCF, contains a second inhibition domain, the CID, that mediates repression through interactions with CtBP and de-acetylation. EMBO J., 18, 3392–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]