Abstract
Behavioral studies with amnesic patients and imaging studies with healthy adults have suggested that medial temporal lobe (MTL) structures known to be essential for long-term declarative memory (LTM) may also be involved in the maintenance of information in working memory (WM). To examine whether MTL structures are involved in WM maintenance for faces, and the nature of that involvement, WM and LTM for faces were examined in normal participants via functional magnetic resonance imaging (fMRI) and in amnesic patients behaviorally. In Experiment 1, participants were scanned while performing a WM task in which they determined if two novel faces, presented 7 s apart, were the same or different. Later, participants’ LTM for the faces they saw during the WM task was measured in an unexpected recognition test. During WM maintenance, the hippocampus was activated bilaterally, and there was greater activation during maintenance for faces that were later remembered than faces later forgotten. A conjunction analysis revealed overlap in hippocampal activations across WM maintenance and LTM contrasts, which suggested that the same regions were recruited for WM maintenance and LTM encoding. In Experiment 2, amnesic and control participants were tested on similar WM and LTM tasks. Amnesic patients, as a group, had intact performance with a 1-s maintenance period, but were impaired after a 7-s WM maintenance period and on the LTM task. Thus, parallel neuroimaging and lesion designs suggest that the same hippocampal processes support WM maintenance, for intervals as short as 7 s, and LTM for faces.
Keywords: hippocampus, neuroimaging, lesion, subsequent memory, Korsakoff’s syndrome
INTRODUCTION
A fundamental distinction within declarative or explicit memory is between working memory (WM) and long-term memory (LTM). LTM, measured by delayed recall or recognition tasks, depends on medial temporal lobe (MTL) and diencephalic structures that, when injured, result in global amnesia (Scoville and Milner, 1957; Squire, 1992). Declarative LTM is likely to be stored in neocortical networks (Fuster, 1995), but acquisition of new knowledge in LTM requires the integrity of MTL structures. WM is a process by which goal-relevant information is maintained or manipulated over brief periods of such short duration that it is not typically conceived of as LTM. WM is believed to be dependent on frontal cortex (Goldman-Rakic, 1996; D’Esposito et al., 1998), but it is less clear if and when MTL structures contribute to performance on WM tasks. WM is a term used to describe many processes, including short-term memory for verbal and visual information and also executive or control processes (Baddeley and Hitch, 1974; Miyake and Shah, 1999). Here, we refer specifically to the brief maintenance of information in WM (Goldman-Rakic, 1996; D’Esposito et al., 1998).
Amnesic patients are impaired on LTM tasks, but their performance is more variable on WM tasks. Amnesic patients perform normally on WM verbal tasks without interference between study and test (Baddeley and Warrington, 1970; Cermak et al., 1977), but they sometimes perform poorly on WM tasks that involve maintaining nonverbal information over even brief periods. For example, the amnesic patient H.M. was impaired at remembering ellipses (but not letter strings) over an uninterrupted 5-s period (Sidman et al., 1968). Similarly, amnesic patients have shown WM impairments for other nonverbalizeable materials such as faces, patterns, and motor movements with delays as short as 6–10 s (Warrington and Taylor, 1973; Cermak and Uhly, 1975; Haxby et al., 1983; Buffalo et al., 1998).
Findings from research with nonhuman primates have also linked the MTL with WM. Primates with MTL lesions have shown impairments in maintaining visual information over the period of a few seconds (Zola-Morgan et al., 1989; Baxter and Murray, 2001), and single-unit recordings in primates have shown that MTL cells are active across brief memory delays (Watanabe and Niki, 1985; Nakamura and Kubota, 1995). These studies, together with studies on humans, indicate that WM maintenance for visual, nonverbalizeable information may depend on MTL structures.
Neuroimaging studies have provided convergent evidence that the MTL is recruited during the maintenance portion of WM tasks using nonverbal material (Ranganath and D’Esposito, 2001; Schon et al., 2004; Ranganath et al., 2005). It is unknown, however, whether these MTL activations reflect processes that are important for WM maintenance per se or other processes that operate in parallel. For example, MTL activations may reflect LTM encoding of faces that is irrelevant for WM maintenance. The goal of the present study was to examine the same WM task in normal participants via fMRI and in amnesic patients behaviorally to gain convergent evidence about MTL involvement in WM maintenance.
In Experiments 1 and 2, participants saw a sample face, maintained the face in WM for 7 s, and then judged whether a test face matched the sample face. In Experiment 1, we collected fMRI data on healthy participants to examine MTL activation during face WM maintenance and incidental LTM encoding. A conjunction analysis was done on the WM maintenance and LTM contrasts to determine the extent of the overlap of activation in the MTL. In Experiment 2, we examined amnesic patients on a similar task to test the hypothesis that the MTL is important for maintaining facial WM information over a short delay. Finding that the MTL activation during WM maintenance relates to LTM formation and that amnesic patients are impaired at face WM would support the view that operations supported by the MTL are important for WM maintenance for faces at delays as short as 7 s. By examining similar tasks in normal participants via fMRI and behaviorally in amnesic patients, we are able to test both which regions of the MTL contribute to WM performance and if the MTL is necessary for normal performance on a face WM task. Thus, the imaging data provide anatomical specificity that is unavailable in amnesic patients with variable lesions that affect a variety of structures, and the patient data provide evidence about the necessity of the memory operations supported by those structures. The imaging and lesion evidence, therefore, each addresses the limitations of the other experimental method.
EXPERIMENT 1: fMRI STUDY
Materials and Methods
Participants
Sixteen, right-handed, healthy volunteers (eight females, age range 18–25 yr, mean 21 yr) participated and received payment for their participation. All participants reported having no previous neurological or psychological disorders. In addition to these 16 participants, 10 additional participants were excluded from analysis because of either excessive movement inside the scanner (3 participants) or poor WM or LTM performance (7 participants) that prohibited complete analysis of the data. The Stanford IRB approved this study and all participants provided informed consent.
Task procedure
Participants completed a WM task while undergoing fMRI scanning. For each WM trial, a sample face was presented for 2 s, followed by a 7-s maintenance period, followed by a test face for 2 s, and a 7-s intertrial interval (ITI) (Fig. 1A). A red fixation was presented for 1 s at the beginning of each trial to alert participants that a new trial was beginning. Participants were instructed to maintain the sample face over the delay and then determine if the sample and test faces were the same (35% of trials) or different (65% of trials). A greater proportion of different trials were used relative to same trials because the critical WM and LTM comparisons were restricted to different trials (see WM Maintenance Analysis section). All participants completed 5 WM runs with each run consisting of 37 trials. All stimuli were novel, trial-unique, young, white, male faces on a color noise background, so the task could not be solved using easily verbalizeable features such as age, race, or gender. A total of 305 stimuli were presented (240 in different trials and 65 in same trials) and were counterbalanced along the dimension of order of presentation and face position (sample or test).
FIGURE 1.
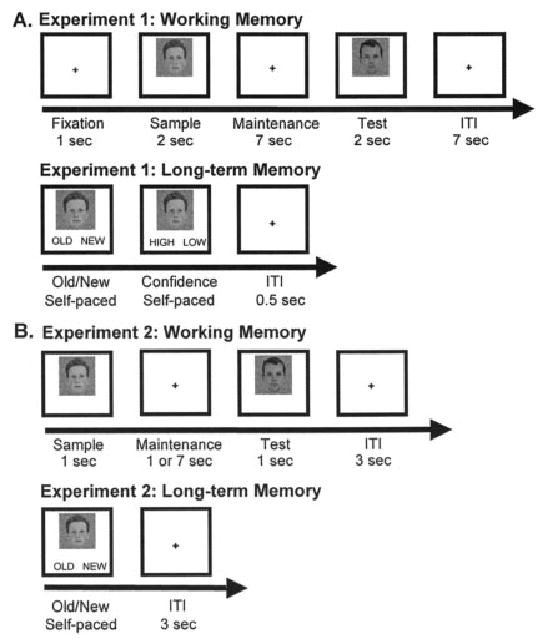
Task design for WM and LTM tasks for (A) Experiment 1 and (B) Experiment 2.
After completing the WM task, participants were removed from the scanner and were given an unexpected LTM test of recognition 15 min later. For each LTM trial, a face was presented with the words “OLD” and “NEW” written underneath and participants were instructed to determine if the face had been presented in the WM experiment (old, 65% of trials) or not (new, 35% of trials). After participants responded, the face remained on the screen, but the words “OLD” and “NEW” were replaced with the words “HIGH” and “LOW.” Participants were instructed to determine if they had high confidence about their old/new decision or low confidence. The next trial began 1 s after participants’ confidence response and all participants completed 370 LTM trials. A total of 370 stimuli were used in the LTM task: 120 sample faces from different trials, 120 test faces from different trials, and 130 new stimuli. Because participants saw each stimulus in WM same trials twice (as both the sample and test face), it would not be certain whether LTM encoding was driven by the first exposure, the second exposure, or both. To avoid the confound of number of stimulus presentations in the LTM analysis, only faces that had been presented in WM different trials (i.e., faces seen only once as either the sample or test stimulus) were used as stimuli in the LTM task, and, of those, only sample stimuli were considered in the subsequent LTM analysis.
For both the WM and LTM task, stimuli were presented and responses and reaction times were recorded with the Psyscope stimulus-presentation program (Cohen et al., 1993). Responses during the WM and LTM task were made using a scanner-compatible button-box and a Mac Powerbook laptop, respectively.
Behavioral analysis
For the WM data, paired t-tests were run on percent correct (PC) and median reaction time (RT) data to determine the effect of WM trial type (same trial or different trial). For LTM data, PC and median RT measures were entered into paired t-tests to determine the effect of LTM trial type (old or new), and into ANOVAs to determine the effect of face position (sample or test) and confidence level (high or low).
fMRI acquisition
Magnetic resonance imaging was performed using a 3T GE Signa scanner. Before functional imaging, a spin echo T2-weighted anatomic image was acquired [30 coronal slices; slice thickness 6 mm; minimum echo time (TE) = 85 μs; repetition time (TR) = 4,500 μs; field of view (FOV) 240 × 240 cm2]. A high-order shim procedure was employed to improve B0 magnetic field homogeneity. Functional images were then obtained in the same slice location as the anatomic images, using a T2* weighted 2D gradient-echo spiral in/out sequence (slice thickness 6 mm; no skip; TE = 30 μs; TR =2,000 μs; flip angle = 75°; FOV = 24 × 24 cm2; resulting in 3.75 × 3.75 × 6 mm voxels). The task was performed over 5, 12-min scanning sessions. A total of 1,805 functional volumes were obtained with 361 volumes for each session. A high-resolution T1-weighted multislice anatomical image was acquired axially using a 3D spoiled GRASS (SPGR) pulse sequence (124 slices; slice thickness 1.5 mm; minimum TE; FOV = 24 × 24 cm2; flip angle 15°). To minimize head movement during scanning, a bite bar was fitted with each participant’s dental impressions.
fMRI analysis
Data preprocessing
Data were processed using SPM99 (Wellcome Dept. of Cognitive Neurology, London). A slice-timing correction was used to correct for the different sampling times of the different slices, resampling all slices in time relative to the fifteenth (middle) slice using sine interpolation. The data were corrected for motion and realigned. The T2 anatomical image was coregistered to the mean functional image that was created during motion correction. The anatomical image was then segmented into gray matter, white matter, and cerebral spinal fluid. Anatomical and functional images were spatially normalized based on parameters determined by normalizing the segmented gray matter image to a gray matter template from the MNI series using a 12-parameter affine transformation, and functional data were spatially smoothed with an 8-mm FWHM isotropic Gaussian kernel. At normalization, voxels were resampled to 3.75 × 3.75 × 6 mm3, which was the size of the voxels during data acquisition, not to a smaller size as is commonly done. Thus, 1 voxel in this study is comparable in size to three 3 × 3 × 3 mm3 voxels.
For the analyses, volumes were modeled with a fixed hemodynamic response function using the onset times for the conditions of interest. The data were then passed through a temporal high-pass filter at 0.0156 Hz. The intensity threshold for significance was set at P < 0.001 uncorrected for multiple comparisons for the WM task. Following Strange et al. (2002), the significance threshold was lowered to P < 0.005 uncorrected for multiple comparisons when exploring MTL activations related to subsequent memory, given that this region often has a lower signal-to-noise (S/N) ratio than many cortical areas (Ojemann et al., 1997). Further, the power for the LTM contrast was weaker than for the WM contrast, because the LTM contrast involved subsets of the WM trials. All reported clusters consisted of more than three significant voxels.
WM maintenance analysis
To assess regions that were active during WM maintenance, activation during the maintenance period was compared to the ITI. To minimize the activation during the maintenance period that was residual from the face presentation, only the last 5 s of the 7-s maintenance and the ITI were modeled. Further, because both the WM maintenance and ITI phases followed the presentation of a face, activation related to the perceptual presentation of the faces should have been similar and cancelled out, leaving differences that reflect goal-oriented maintenance rather than perceptual face encoding. Trials with the same faces presented at sample and test (“same trials”) were omitted from analysis to eliminate the concern that hippocampal activation would reflect a greater response for novel than repeated stimuli (i.e., on different trials both the sample and test faces were novel; Ranganath and D’Esposito, 2001; Stern et al., 2001). All incorrect WM trials were excluded from analysis.
LTM analysis
For the LTM analysis, WM maintenance imaging data were back-sorted on a trial-by-trial basis by LTM outcome (remembered or forgotten) and confidence level (high or low). Because there was an average of 18 faces that were forgotten with high confidence, which is fewer than the minimum number of trials needed for the estimate of the hemodynamic response to be stable (Huettel and McCarthy, 2001), we collapsed across confidence levels for forgotten faces. To maximize our sensitivity for detecting subsequent LTM effects, faces remembered with low confidence were excluded from our analysis because some of these trials were likely guesses (Brewer et al., 1998; Wagner et al., 1998; Otten et al., 2001). Additionally, to allow for comparison of the regions that support LTM processes during the perceptual presentation of faces and during the maintenance of faces, imaging data from the presentation of sample faces were subjected to the same LTM analysis. Data from test faces and the ITI phase and all incorrect WM trials were excluded from LTM analysis. Trials with same faces presented at sample and test “same-trials” were omitted from analysis because any subsequent memory for the face could have resulted from either or both of the presentations of a repeated face. This also made the WM and LTM analyses directly comparable because they were performed on the identical subset of trial type.
Conjunction analysis
To examine regions that were activated during both the WM and LTM contrasts, a logical AND conjunction analysis examined regions that were activated in both the WM maintenance and LTM (during maintenance) analyses, using individual thresholds of P < 0.032, and a combined threshold of P < 0.001 (Nichols et al., 2005).
Time-course analysis
Time-courses were extracted from the functional region of interest (ROI) from the left hippocampus in the LTM contrast, which included the peak voxel. Percent signal change was measured for each condition as the deviation from the average signal collapsed across all conditions in this ROI.
EXPERIMENT 1 RESULTS
Behavioral Results
For the WM portion of the experiment, participants performed near ceiling, averaging 95 ± 6% correct with a median RT of 824 ± 157 ms for correct trials. Performance on WM same- and different-trials did not differ significantly in terms of accuracy or latency.
For the LTM recognition test, participants had an average hit rate of 54 ± 11%, which was significantly higher than their false alarm rate of 36 ± 13%, [t (15) = 19.06, P < 0.001]. Of correct old responses, 56 ± 12% were remembered with high confidence. Participants were more accurate and faster for faces remembered with high confidence (PC = 63% ± 6%; RT = 1,620 ±521 ms) relative to low confidence (PC = 46% ± 5%; RT =1948 ± 675 ms) responses [PC: F (1, 15) = 29.05, P < 0.001; RT: F (1, 15) = 13.38, P < 0.005]. The accuracy level for high confidence responses provided enough items that were remembered and forgotten to support a subsequent memory analysis (Huettel and McCarthy, 2001). Participants remembered more sample faces (58 ± 12%) than test faces (51 ± 13%; F (1, 15) = 5.142, P < 0.05]. There was an interaction of confidence level and face position, such that for high confidence trials participants remembered more sample faces (69 ± 10%) than test faces (57 ± 18%), but for low confidence trials a similar number of sample (46 ± 16%) and test faces (45 ± 13%) were remembered [F (1, 15) = 8.173, P < 0.05]. At the end of the experiment, participants were asked if they thought there would be a long-term memory task and all participants responded “no.”
fMRI Results
Multiple regions showed greater activation during face WM maintenance than during the ITI (Table 1), including the bilateral hippocampus (Fig. 2A). The time-course of activation in the left hippocampus (Fig. 2B) shows that the difference in activation grew across the maintenance period until the response. WM maintenance also activated several regions outside of the MTL including the bilateral ventrolateral prefrontal cortex (BA 45/47), right dorsolateral prefrontal cortex (BA 46), bilateral insula (BA 13/45), and left anterior cingulate cortex (BA 32).
TABLE 1.
Significant Activations Associated With Working Memory Maintenance (WM Maintenance Period Versus ITI)
| Talairach coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Z-score (voxel) | Cluster size |
| L. insula | 13/45 | −30 | 17 | −4 | 5.11 | 426 |
| L. superior frontal gryus | 6 | −7 | 14 | 51 | 5.03 | |
| L. medial frontal gryus | 32 | −11 | 14 | 48 | 4.82 | |
| L. inferior frontal gryus | 47 | −33 | 29 | 5 | 4.88 | |
| L. precentral gryus | 44/4 | −48 | 18 | 6 | 4.25 | |
| L. postcentral Gryus | 3 | −41 | −21 | 49 | 4.40 | |
| L. thalamus | −11 | −23 | 1 | 4.21 | 53 | |
| R. inferior frontal gryus | 47 | 37 | 29 | −11 | 4.19 | 43 |
| R. middle frontal gryus | 46 | 45 | 24 | 20 | 3.93 | 7 |
| R. inferior frontal gryus | 45 | 48 | 24 | 20 | 3.56 | |
| L. insula | 13 | −41 | −28 | 22 | 3.86 | 46 |
| R. hippocampus | 26 | −18 | −15 | 3.88 | 60 | |
| R. superior temporal gryus | 41 | 37 | −29 | 5 | 3.75 | |
| R. caudate tail | 37 | −30 | −8 | 4.05 | ||
| L. hippocampus | −30 | −18 | −12 | 3.93 | 50 | |
| L. superior temporal gryus | 22 | −41 | −24 | −8 | 3.58 | |
| L. caudate tail | −37 | −24 | −8 | 4.00 | ||
| R. insula | 13 | 33 | −28 | 22 | 3.86 | 17 |
| L. putamen/globus pallidus | −15 | 6 | 3 | 3.69 | 16 | |
| R. cerebellum | 0 | −53 | −16 | 4.41 | 20 | |
Coordinates of local maxima (P < 0.001 uncorrected) are listed according to the Talairach coordinate system. All voxels are 3.75 × 3.75 × 6 mm3. BA, Brodman area; L, left; R, right.
FIGURE 2.
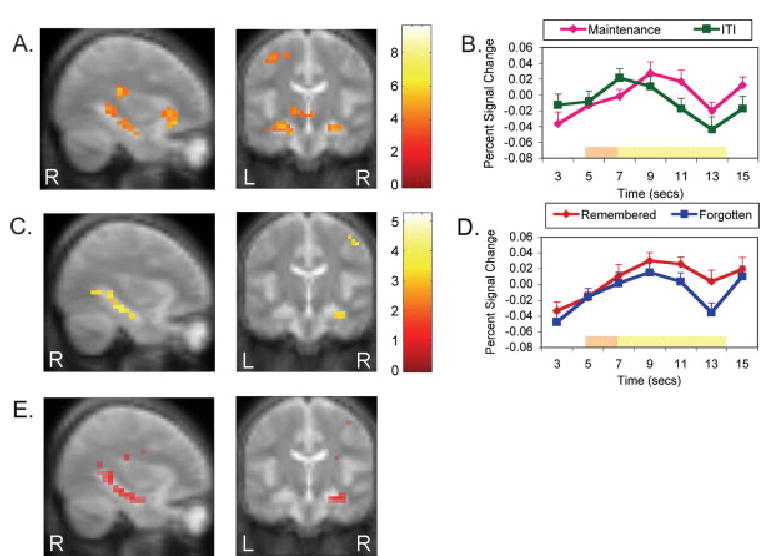
Activation maps displayed on the mean of all participants’ anatomy image and time course data. Activation data is displayed at MNI slices x = 33 and y = −19. Both time-courses were extracted from the functional ROI of the left hippocampus in the LTM contrast, which included the peak voxel, and are measured as percent signal change from the average signal of all trial types in this region. (A) Working memory maintenance greater than ITI voxels surviving a threshold of P < 0.001, unconnected. (B) Working memory maintenance and ITI activation across time. The bars at the bottom depict when the responses related to the presentation of the sample and test face (orange) and the maintenance and ITI (yellow) would be expected to peak, assuming a 5 s peak latency (Eldridge et al., 2000; Kirchhoff et al., 2000). (C) Long-term memory remembered greater than forgotten voxels surviving a threshold of P < 0.005, unconnected. (D) Activation across time for trials where faces were remembered and forgotten. The bars at the bottom depict when the responses related to the presentation of the sample face (orange) and the maintenance (yellow) would be expected to peak, assuming a 5 s peak latency (Eldridge et al., 2000; Kirchhoff et al., 2000). (E) Conjunction analysis of all regions that were activated in both the WM maintenance and LTM analysis, with individual thresholds of P < 0.032, and a combined threshold of P < 0.001. R, Right. L, Left.
To determine whether hippocampal activation during WM maintenance was functionally related to LTM encoding processes, WM maintenance of faces that were later remembered with high confidence were contrasted with those later forgotten (only for sample faces from different trials). This LTM contrast revealed bilateral hippocampal activation that was greater during the maintenance of faces that were subsequently remembered than subsequently forgotten (Table 2 and Fig. 2C). Time-course data from the ROI in the left hippocampus reveled that the activation related to remembered faces was consistently greater across the delay than the activation related to forgotten faces (Fig. 2D). Indeed, the difference between remembered and forgotten faces grew greater across the maintenance period, indicating that the subsequent memory effect was more related to the maintenance period than the face presentation period. Activations were also seen in the left middle frontal gryus (BA 8) and bilateral medial frontal gryus (left, BA 11; right, BA 25). Next, to determine which regions were involved in LTM processes during the perceptual presentation of faces, sample faces that were later remembered with high confidence were compared to those later forgotten. The bilateral fusiform/middle temporal gyrus (BA 37) was more active during the perceptual presentation of faces that were later remembered; however, no other regions were activated in this contrast.
TABLE 2.
Significant Activations Associated With Subsequent Long-Term Memory (Remembered with High Confidence Versus Forgotten)
| Talairach coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Z-score (voxel) | Cluster size |
| L. hippocampus | −33 | −35 | −8 | 3.90 | 37 | |
| L. fusiform gyrus | 37 | −33 | −36 | −11 | 3.01 | |
| L. middle frontal gryus | 8 | −19 | 37 | 43 | 3.47 | 37 |
| L. medial frontal gryus | 11 | 0 | 52 | −15 | 3.41 | 3 |
| R. hippocampus | 37 | −35 | −8 | 3.29 | 39 | |
| R. postcentral gryus | 3 | 45 | −20 | 56 | 3.16 | 7 |
| R. medial frontal gryus | 25 | 4 | 22 | −17 | 2.76 | 3 |
| R. caudate | 19 | 24 | 9 | 3.26 | 3 | |
Coordinates of local maxima (P < 0.005 uncorrected) are listed according to the Talairach coordinate system. All voxels are 3.75 × 3.75 × 6 mm3. BA, Brodman area; L, left; R, right.
Within the MTL for the maintenance period, the WM contrast yielded activations bilaterally in the anterior hippocampus and on the right in the posterior hippocampus. The LTM contrast produced activations in the right anterior hippocampus and bilaterally in the posterior hippocampus. The conjunction analysis of the WM and LTM contrast revealed a high degree of overlap within the MTL (Fig. 2E). More specifically, the conjunction analysis revealed bilateral hippocampal activation that was more extensive on the right side than on the left, and, in general, was similar to the pattern of activation produced by the LTM contrast. To summarize, there was bilateral hippocampal activation in all analyses, except for absences of significant activation in the left posterior hippocampus in the WM analysis, the left anterior hippocampus in the LTM analysis, and the left anterior hippocampus in the conjunction analysis. We conducted further post hoc analyses on the regions in the left hippocampus that were not activated in the WM and LTM contrasts. The absence of activation in the left posterior hippocampus in the WM contrast appears to be the consequence of the more conservative threshold used in the WM analysis. When the threshold of the WM contrast was lowered to P < 0.005, there was activation in the bilateral anterior and posterior hippocampi. Thus, it appears that there was bilateral activation of the anterior and posterior hippocampi for WM maintenance, with the left posterior hippocampal activation being slightly less robust. On the other hand, the lack of activation in the LTM analysis in the left anterior hippocampus does not appear to be a consequence of thresholding. Even when the threshold of the LTM contrast was lowered to the very liberal value of P < 0.05, there was no activation in the left anterior hippocampus. Thus, there appears to be a genuine asymmetry of LTM activation in anterior hippocampus, with right-sided activation, but not left-sided activation, associated with successful LTM encoding of faces.
EXPERIMENT 2: AMNESIA STUDY
Materials and Methods
Participants
Nineteen amnesic patients and 20 controls from the Boston area participated in Experiment 2. The amnesia was the consequence of a variety of etiologies and patients were grouped according to the locus of their documented or presumed damage. Eleven of the amnesic patients (3 females and 8 males) had etiologies that suggested bilateral medial temporal lobe damage (“MTL patients”) and 8 of the amnesic patients (1 female and 7 males) had etiologies that suggested diencephalic damage (“DNC patients”) (Table 3). Among the MTL patients, 8 became amnesic following anoxia, and 3 following encephalitis. Among the DNC patients, 7 had alcoholic Korsakoff’s syndrome, and 1 had bilateral thalamic damage secondary to stroke. All of the MTL patients and the DNC patient with the bithalamic stroke had radiologically verified lesions. For the patients with alcoholic Korsakoff’s syndrome, DNC damage was presumed on the basis of previous reports (Victor et al., 1989; Jernigan et al., 1991).
TABLE 3.
Summary of Neuropsychological Characteristics of Amnesic Patients and Control Participants
| WAIS-III |
WMS-III |
|||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Etiology | Age (yr) | Ed. | VIQ | GM | AD | VD | WM |
| MTL01 | Anoxia | 52 | 12 | 83 | 52 | 55 | 56 | 91 |
| MTL02 | Anoxia | 24 | 10 | 92 | 45 | 55 | 50 | 81 |
| MTL03 | Anoxia | 65 | 20 | 111 | 52 | 64 | 56 | 83 |
| MTL04 | Encephalitis | 74 | 18 | 135 | 45 | 58 | 53 | 141 |
| MTL05 | Encephalitis | 47 | 14 | 92 | 45 | 55 | 56 | 85 |
| MTL06 | Encephalitis | 59 | 12 | 106 | 69 | 77 | 68 | 111 |
| MTL07 | Anoxia | 39 | 16 | 86 | 49 | 52 | 53 | 93 |
| MTL08 | Anoxia | 46 | 14 | 111 | 59 | 52 | 72 | 96 |
| MTL09 | Anoxia | 44 | 14 | 90 | 45 | 52 | 53 | 93 |
| MTL10 | Anoxia | 19 | 10 | 91 | 45 | 46 | 56 | 79 |
| MTL11 | Anoxia | 73 | 18 | 113 | 75 | 80 | 72 | 102 |
| DNC01 | Korsakoff | 57 | 12 | 97 | 66 | 74 | 62 | 108 |
| DNC02 | Korsakoff | 84 | 9 | 100 | 72 | 74 | 75 | 91 |
| DNC03 | Korsakoff | 61 | 16 | 92 | 47 | 58 | 56 | 85 |
| DNC04 | Korsakoff | 78 | 14 | 103 | 72 | 71 | 68 | 115 |
| DNC05 | Korsakoff | 75 | 14 | 99 | 59 | 58 | 65 | 115 |
| DNC06 | Korsakoff | 53 | 18 | 111 | 69 | 64 | 72 | 81 |
| DNC07 | Korsakoff | 82 | 14 | 105 | 66 | 64 | 62 | 121 |
| DNC08 | Bithalamic stroke | 61 | 12 | 84 | 73 | 67 | 84 | 99 |
| MTL patients | (n = 11) | 50 | 14 | 101 | ||||
| DNC patients | (n = 8) | 69 | 14 | 99 | ||||
| MTL controls | (n = 11) | 52 | 15 | 108 | ||||
| DNC controls | (n = 9) | 68 | 14 | 104 | ||||
WAIS-III, Wechsler Adult Intelligence Scale-third edition (Wechsler, 1997a); WMS-III, Wechsler Memory Scale-third edition (Wechsler, 1997b); Ed., education, in years; VIQ, verbal IQ; GM, general memory index; AD, auditory delay index; VD, verbal delay index; WM, working memory index; MTL, damage to the medial temporal lobe; DNC, damage to the diencephalic regions. Numbers in italic are the means of each group.
Eleven normal control participants without a history of alcoholism (3 females and 8 males) served as control participants for the MTL group (“MTL controls”). Nine participants (1 female and 8 male) served as controls for the DNC patients, 8 with a history of alcoholism and 1 without (“DNC controls”). Participants with a history of alcoholism had abstained from alcohol for at least 1 month prior to the experiment. Both control groups were matched to their respective patient group in terms of age, education, and verbal IQ, as measured by the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler, 1997a), and there were no differences between the patients and their control groups in any of these measures. The MTL group was significantly younger than the DNC group (51 ± 18 and 68 ± 9 yr, respectively, P < 0.001) but the groups did not differ in education or verbal IQ (P’s > 0.30). The MTL and DNC patients did not differ on any tested clinical measures of WM and STM, including controlled oral word association (Benton and Hamsher, 1989), Trail Making Test part B (Spreen and Strauss, 1991), immediate recall on Rey’s Auditory-Verbal Learning Test (Rey, 1964), and backward digit span (WAIS-III; Wechsler, 1997a). However, MTL and DNC patients did differ on delayed recall on Rey’s Auditory-Verbal Learning Test (Rey, 1964) and on delayed visual memory of WMS-III (Wechsler, 1997b). The DNC patients performed worse than the MTL patients on delayed recall of Rey’s Auditory-Verbal Learning Test (z scores of 2.02 ± 0.83 and 3.59 ± 1.90 yr, respectively, P < 0.05), but performed better than the MTL patients on delayed visual memory of WMS-III (68 ± 9 and 59 ± 8, respectively, P < 0.05).
Task procedure
Participants completed a WM and LTM task similar to the task used in Experiment 1, with the addition of an immediate memory condition with a 1-s maintenance period (Fig. 1B). For the WM task, a sample face appeared for 1 s, followed by a maintenance period of either 1 s (50% of trials) or 7 s (50% of trials), followed by a test face for 1 s. Participants were instructed to determine if the sample and test faces were the same (50% of trials) or different (50% of trials). All stimuli were novel, trial-unique, young, white, male faces, so the task could not be solved using easily verbalizeable features such as age, race, or gender. To eliminate the difficulty of maintaining the appropriate stimulus-response mappings that are required for key press responses, participants were asked to respond aloud. Stimuli were counterbalanced across maintenance duration (1 or 7 s) and WM trial type (same trial or different trial), and participants completed 32 WM trials.
Approximately 15 min later, participants were given an unexpected LTM task of recognition memory during which a face was presented and they determined if the face had been presented during the WM task (“old,” 50% of trials) or not (“new,” 50% of trials). Of the old trials, all faces had been presented as sample faces in the WM task in either same trials (50% of old stimuli) or different trials (50% of old stimuli). Participants completed 64 trials.
Behavioral analysis
Data were subjected to ANOVAs with two between-participants factors, health (amnesic or control) and region (MTL or DNC). WM and LTM percent correct measures were entered into separate repeated measures ANOVAs to determine the influence of health, region, maintenance length (1 or 7 s), and WM trial type (same trial or different trial). An additional repeated measures analysis of variance (ANOVA) was run on LTM percent correct data to determine the effect of health, region, and LTM trial type (old or new). Incorrect WM trials were excluded from LTM analysis. Post hoc two-tailed t-tests were run on the WM 7 s data and LTM data separately for MTL and DNC groups. Statistics were corrected using the Greenhouse-Geisser method when necessary.
Forced entry stepwise regressions were performed to determine whether several clinical measures predicted task performance of the patients. Accuracy measures from the 1-s WM maintenance, the 7-s WM maintenance, and the LTM task were entered as dependent variables into separate stepwise regressions. The independent variables in the regressions were age, education, WAIS-III verbal IQ and backward digit span (Wechsler, 1997a), immediate and delayed recall on Rey’s Auditory-Verbal Learning Test (Rey, 1964), the WMS-III (general memory, delayed auditory memory, delayed visual memory, and working memory; Wechsler, 1997b), Warrington Recognition Memory Test (faces and words; Warrington, 1984), and an executive function composite score. The executive function composite score was obtained by calculating each patient’s mean rank on four scores from three tests known to be sensitive to frontal-lobe lesions: Trail Making Test part B (reaction time; Spreen and Strauss, 1991), the controlled oral word association test (total number of appropriate responses; Benton and Hamsher, 1989), and Wisconsin card sorting task (number of categories and percent perseverative errors; Grant and Berg, 1948). Additionally, accuracy measures from the conditions other than the condition yielding the dependent variable were included in each regression. For example, the regression for 1-s maintenance included 7-s maintenance and LTM accuracy as independent variables.
The WM and LTM accuracy is reported as percent correct. A similar pattern of results were obtained for the WM and LTM analyses when d’ measures, rather than percent correct, were analyzed.
EXPERIMENT 2 RESULTS
Working Memory Results
Collapsing across all groups, participants were more accurate at the 1-s maintenance period (99 ± 3%) than the 7-s maintenance period [94 ± 11%; F (1, 35) = 10.11, P < 0.005]. Control participants were, overall, more accurate than amnesic patients [99 ± 3% and 94 ± 7%, respectively; F (1, 35) = 8.07, P < 0.01]. Importantly, there was a significant interaction of health X maintenance length, because at the 1-s maintenance period amnesic (99 ± 3%) and control (99 ± 3%) groups performed identically, but at the 7-s maintenance period the amnesic group (89 ± 13%) was less accurate than the control group [98 ± 5%; Fig. 3A; F (1, 35) = 6.62, P < 0.05]. The amnesic patients performed worse than the control participants after the 7-s maintenance [t (1, 37) = 2.89, P < 0.005, one-tailed]. Separate comparisons were made between the two amnesic groups and their respective control groups for performance after the 7-s maintenance. The MTL patients were significantly impaired relative to MTL controls [86 ± 15% and 97 ± 6%, respectively; t (1, 13) = 2.28, P < 0.05, corrected for unequal variances]. DNC patients tended to perform worse than their control group, but this difference was not reliable [93 ± 11% and 100 ± 0%, respectively; t (1, 7) = 1.84, P = 0.11, corrected for unequal variances]. Despite these suggestive differences, there were no significant interactions of health X region.
FIGURE 3.
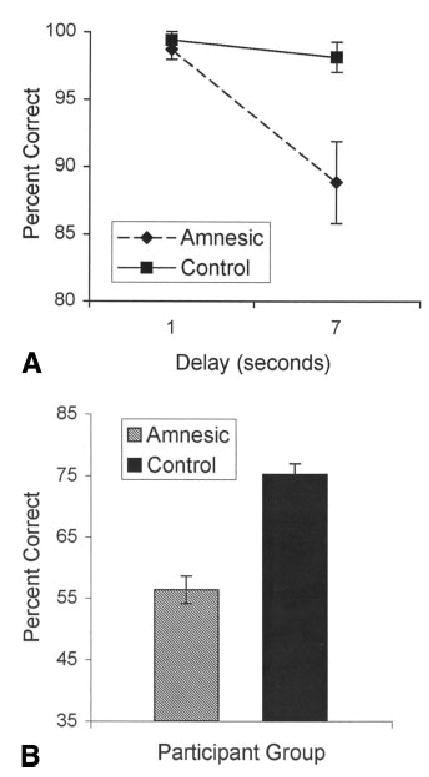
Performance of amnesic patients and controls (collapsed across DNC and MTL groups) in Experiment 2 for the (A) working memory task and (B) long-term memory task.
Inspection of individual results for 7-s maintenance revealed that four patients (MTL01, MTL03, MTL10, and DNC05) performed more than four standard deviations below the mean of the other patients and additionally had executive composite scores that were below the mean. To ensure that these outliers were not driving the impairment at 7 s, WM data were analyzed after excluding these four patients. This analysis revealed that at 7 s, the amnesic patients still performed significantly worse than the controls [t (1, 33) = 1.91, P < 0.05, one-tailed].
Long-Term Memory Results
Owing to a software error, data from one MTL patient (MTL05) could not be analyzed. For the long-term memory task, participants (collapsed across all groups) were more accurate on trials with new faces (71 ± 17%), than old faces (62 ± 24%), although this difference did not reach significance (P < 0.08). Overall, more faces were remembered in the LTM task when they were presented in trials with a 7-s maintenance period (65 ± 26%) than with a 1-s maintenance period (58 ± 25%), suggesting that LTM encoding is occurring during WM maintenance [F (1, 34) = 5.84, P < 0.05]. More faces from WM same-trials were remembered on the LTM task (66 ± 26%) than from different-trials (58 ± 25%), as was expected given that the faces from same-trials were presented twice [F (1, 34) = 10.39, P < 0.005]. Amnesic patients were significantly less accurate in the LTM task than were the controls [Fig. 3B; F (1, 34) = 27.94, P < 0.001]. There was an interaction between health and LTM trial type because control participants performed equally well on new (73 ± 13%) and old faces (78 ± 13%), but amnesic patients were more accurate on new faces (69 ± 20%) than old faces [46 ± 23%; F (1, 34) = 8.71, P < 0.001].
Separate comparisons were made between the two amnesic groups and their respective control groups for LTM performance. MTL patients were significantly impaired relative to MTL controls [54 ± 7% and 76 ± 8%, respectively; t (1, 19) = 7.32, P < 0.001], and DNC patients were significantly impaired relative to DNC controls [62 ± 8% and 74 ± 8%, respectively; t (1, 15) = 3.03, P < 0.05]. Further, the MTL patients performed significantly worse than the DNC patients [t (1, 16) = 2.42, P < 0.05]. However, no effects involving region of damage were significant.
Regression Results
Forced-entry stepwise regressions were computed on the patient data to determine which of several clinical and behavioral measures could predict performance on the WM and LTM tasks (see Behavioral Analysis section for all measures entered into regression). No model could correctly predict accuracy for the 1-s WM task. For the 7-s WM task, performance on the Warrington Recognition Memory Test for faces and for words and the executive composite score were included in the model (R2 = 0.742, P < 0.001), with Warrington Recognition Memory Test for faces accounting for the most variance, followed by the executive composite score (Warrington faces (β = 1.06, P < 0.005; executive composite (β = 0.483, P < 0.01; Warrington words (β = −0.69, P < 0.05). Lastly, stepwise regressions on LTM accuracy produced a model that included only performance on WMS-III delayed visual memory (R2 = 0.237, P < 0.05).
Because the four lowest performers on the WM 7-s task also had low executive function composite scores, further post hoc analyses were completed without these patients to see if their scores were driving the regression results. With these four patients removed from the analysis, the correlation between executive function scores and WM-7 accuracy was no longer significant [with subset of patients: R2 = 0.138, P = 0.12; with all patients: R2 = 0.398, P < 0.005]. This suggests that these four patients caused the executive composite score to be included in the stepwise regression for WM 7-s accuracy. Additional stepwise regressions were run after excluding the data from the four lowest performers to see which variables best accounted for WM 7-s accuracy for the remaining patients. These regressions revealed that WM 7-s accuracy was best predicted by a model that included only LTM task performance (R2 = 0.381, P < 0.05).
DISCUSSION
We examined the same WM maintenance task in normal participants via fMRI and in amnesic patients behaviorally to gain convergent evidence about whether MTL structures are both activated by and important for face WM maintenance over the period of a few seconds. In Experiment 1, the hippocampus was activated during face WM maintenance and hippocampal activation during WM maintenance was predictive of subsequent LTM performance. The hippocampal regions activated for WM maintenance and for LTM formation were strongly overlapping, suggesting that the same encoding processes mediated by the hippocampus are supporting these processes. The fMRI study demonstrated MTL activation during WM maintenance; however, it could not indicate whether the MTL activation reflected processes that were necessary for or incidental to WM processes. To determine this, in Experiment 2 we tested amnesic patients on similar WM and LTM tasks. Amnesic patients were impaired at maintaining a face for 7 s, but not for 1 s. This deficit indicates that the MTL functions reflected by activations in Experiment 1 are necessary for WM for faces. This is the first study to address whether the hippocampus is essential for WM maintenance using parallel neuroimaging and neuropsychological designs that assess a similar task. Taken together, these data suggest that MTL regions activated during WM maintenance of faces are supporting LTM encoding processes, and that these regions are important for maintaining faces for 7-s within WM.
Imaging Findings
Our imaging results add to the accumulating evidence that MTL regions are active during WM maintenance of nonverbal material (Ranganath and D’Esposito, 2001; Schon et al., 2004; Ranganath et al., 2005). Our study found MTL activation related to the maintenance of faces that was restricted to the bilateral hippocampus proper. Previous fMRI studies have reported activations related to WM maintenance in bilateral anterior hippocampus for faces (Ranganath and D’Esposito, 2001), bilateral midfusiform and parahippocampal gryus for scenes (Schon et al., 2004), and bilateral perirhinal cortex and right hippocampus for nonverbalizeable line drawings (Ranganath et al., 2005). The different MTL activations across studies suggest that MTL contributions to WM maintenance vary topographically as a consequence of the material being maintained.
The hippocampal activation observed in this experiment during the WM maintenance of faces might be related to the novelty of the stimuli. Studies looking at the maintenance of familiar faces have revealed persistent fusiform face activation, but did not show hippocampal activation (Ranganath and D’Esposito, 2001). On the other hand, studies examining the maintenance of novel faces or other complex information have revealed persistent activation in the MTL (Ranganath and D’Esposito, 2001; Schon et al., 2004; Ranganath et al., 2005). This suggests that the hippocampus is important for the maintenance of items lacking prior representations in the brain.
The strong overlap in activations independently defined for WM maintenance and LTM encoding success in Experiment 1 suggests that the same hippocampal-dependent processes are recruited for both. Similar overlaps between WM maintenance and MTL activations have been reported within the left anterior hippocampus for novel line drawings (Ranganath et al., 2005), the right parahippocampal gryus for scenes (Schon et al., 2004), and the right posterior and left anterior hippocampus for words studied with relational processing (Davachi and Wagner, 2002). In the Schon et al. study (2004), the right parahippocampal regions activated during WM maintenance and LTM subsequent memory for scenes were similar, but the LTM peak activation was more posterior than the WM maintenance peak. Our study goes beyond the above imaging studies by quantitatively examining the relation between WM and LTM activations in the MTL via a conjunction analysis, and demonstrating statistically a strong overlap of MTL activation for WM maintenance and LTM formation. There was only one notable difference between WM and LTM activation in the hippocampus. WM maintenance for faces activated anterior and posterior hippocampus bilaterally, as did LTM activation in posterior hippocampus. There was, however, an asymmetry in LTM activation in the anterior hippocampus—activation in right anterior hippocampus was associated with LTM encoding of faces, but there was an absence of such activation in left anterior hippocampus (even at a liberal threshold). The right-lateralization of anterior hippocampal activation is consistent with neuropsychological evidence for right-hemisphere dominance for memory for faces (Milner, 1968; Crane and Milner, 2002).
For the fMRI experiment, we were interested in looking at the maintenance portion of WM, as opposed to encoding of the sample face or retrieval during responding to the test face in a trial. However, because the three events (sample, maintenance, and test) always appeared in the same order, they were not independent of each other, and our design permits only a tentative separation of the presumed processing stages based on differential activation patterns. Several factors, however, indicate that the hippocampal activation of interest was occurring during the maintenance phase. First, the time-course data show sustained activation during the maintenance period that is not simply a response to the perceptual encoding period. Importantly, the largest activation difference between subsequently remembered and forgotten faces occurred late in the maintenance phase, when the influence of the sample face presentation was least. Second, an analysis restricted to the presentation of the sample face showed greater activation for remembered than forgotten faces in fusiform cortex, but not in the hippocampus. The absence of hippocampal activation in this analysis points also to the importance of the maintenance phase in LTM formation. Third, our results are similar to those of the other studies that employed multiple regression to address this issue (Ranganath and D’Esposito, 2001; Ranganath et al., 2005). Thus, it is likely that we were indeed observing maintenance-period activations in the hippocampus that were associated with LTM.
Amnesia Findings
The amnesic patients’ deficit after merely 7 s of uninterrupted maintenance is striking, and can be interpreted in the context of other aspects of the findings. The deficit cannot be attributed to impaired face processing, because amnesic patients were unimpaired for 1-s maintenance. Near perfect performance by both groups at the 1-s delay and by the control group at the 7-s delay, however, raises issues of interpretation. Perfect performance by both groups could mask a patient deficit at the 1-s delay, a conceptual issue raised previously in primate research (Ringo, 1991, 1993). At the 7-s delay, the amnesic patients’ deficit was small in magnitude, but the patient deficit could be underestimated due to the control groups’ ceiling performance. The intact performance after 1 s and the impaired performance after 7 s suggest that the time-dependent impairment is just becoming apparent around the 7-s delay.
The patient regression analyses further indicate that performance at the 7-s delay was functionally related to LTM processes. Performance at the 7-s delay was consistently related to LTM abilities as measured by standardized measures of LTM. Executive functions may also play a role in performance after a 7-s delay, because patients with poor executive functions performed below the group mean at the 7-s delay and also introduced an influence of executive function scores on 7-s delay performance. These findings are consistent with the idea that WM capacity and executive functions are closely related constructs (Miyake et al., 2001). When the four patients with especially low executive functions were excluded, only LTM measures predicted 7-s delay WM performance. This is consistent with the view that LTM processes supported by MTL structures are playing an important role maintaining a face in WM for 7 s.
The MTL and DNC patients exhibited similar memory deficits, despite differing etiologies of amnesia and presumed loci of primary injury. Some studies report that DNC patients do not have MTL damage per se (Squire et al., 1990). Thus, the impaired performance of the DNC patients may have reflected injury to non-MTL diencephalic structures that may also play a critical role in LTM and certain kinds of WM. Other studies have found evidence of MTL injury in DNC patients. For example, DNC patients have hippocampal volume deficits that are negatively correlated with declarative memory performance (Sullivan and Marsh, 2003), and have functional hypoactivity in MTL cortex (Fazio et al., 1992; Heiss et al., 1992; Reed et al., 2003; Caulo et al., 2005 but see Paller, 1997). These studies suggest that DNC patients have MTL injury, or that injury to diencephalic structures compromises MTL function. Although the interpretation of DNC amnesia in relation to MTL structure and function is uncertain, the separate analysis of the MTL patients supports directly the importance of MTL structures in LTM and in WM for a single face after a 7-s unfilled interval.
CONCLUSION
A fundamental assumption about the brain organization of human memory has been the dissociation between intact WM and impaired LTM in global amnesia. This dissociation has supported the view that WM processes are mediated by neural systems that operate independently from the MTL system that is essential for LTM. Indeed, the literature is consistent in the finding that amnesic patients have intact WM when WM is measured by verbal digit span (Haxby et al., 1983; Cave and Squire, 1992). Intact WM in amnesia also extends to verbal information maintained over a delay (Cermak et al., 1977), and to visuospatial information tested immediately (Haxby et al., 1983; Cave and Squire, 1992). Further, the intact performance of the amnesic patients on the 1-s delay face-matching task may reflect another example of intact WM in amnesia. In contrast, patients with focal cortical lesions have exhibited modality-specific WM deficits on digit span (Ghent et al., 1962) or block span (De Renzi and Nichelli, 1975; De Renzi et al., 1977) tasks, sometimes exhibiting spared LTM (Shallice and Warrington, 1970). In combination, the above findings have supported the idea of a double dissociation between WM, mediated by specialized cortical systems, and LTM, which is dependent upon MTL integrity. It has been noted, however, that these studies do not hold information content and encoding and retrieval conditions constant (see Ranganath and Blumenfeld, 2005 for a review).
The dissociation between WM and LTM in amnesia does not always hold, however, as amnesic patients do not always exhibit intact WM performance. Our study adds a new insight to the evidence that amnesic patients are impaired on nonverbal WM tasks (Sidman et al., 1968; Warrington and Taylor, 1973; Cermak and Uhly 1975; Haxby et al., 1983; Buffalo et al., 1998). With the exception of the Sidman study of a single amnesic patient, all previous studies used either filled delays or required that multiple items be remembered. Experiment 2 is the first study to show that a group of amnesic patients is impaired on a WM task requiring only that a single stimulus (face) be kept in mind for a few seconds without interference. These results suggest that WM impairments in amnesic patients are coupled with both a delay and fine-grained visual or spatial representations that cannot be well supported by verbal rehearsal.
One possibility is that WM can be fractionated into two classes of operation. One class reflects specialized cortical circuits that support WM for verbal and certain kinds of visuospatial information. These WM circuits are spared in amnesia, but can be injured by cortical lesions. The other WM class involves other kinds of information that lack a dedicated cortical system, and rely on MTL-dependent LTM processes immediately or within seconds. Thus, an amnesic patient would be impaired on WM tasks that tap the second class of WM processes. Faces in particular may lack a specialized WM circuit: Warrington and Taylor (1973) showed that WM forgetting rates of faces looked comparable to LTM forgetting rates and not typical WM forgetting rates. This interpretation is similar to ideas from Cermak (1977), who noted that amnesic patients are impaired at recirculating visual information, which causes gradual forgetting over unfilled delays.
From this perspective, it could be argued that maintenance of a face for 7 s exceeds the temporal storage capacity of neocortical WM mechanisms and therefore requires hippocampal-dependent LTM in both healthy and amnesic individuals. This is the most parsimonious interpretation of our fMRI and patient studies on WM, and is in accord with a review from Ranganath and Blumenfeld (2005) that challenged the double dissociation between the neural substrates of WM (or short-term memory) and LTM. The maintenance of a face for 1-s may not exceed this capacity limit and may be dependent on cortical regions, or this task may also rely on LTM processes but the patients do not show a deficit because the task remains relatively easy (i.e., the controls are at ceiling). There are now many well-documented examples of both intact and impaired WM in amnesia, and further research will be needed to discover the principles that underlie whether a particular form of WM is LTM and MTL-dependent. It appears that specific forms of WM maintenance do or do not depend upon the MTL, and the forms that do depend on the MTL are LTM-dependent within seconds.
Alternatively, it could be the case that the MTL is important for both WM maintenance and LTM encoding but that these processes remain distinct in the hippocampus. If there were adjacent but distinct hippocampal circuits supporting WM maintenance and LTM encoding, both circuits would be injured in amnesia and may not be separable given the limited spatial resolution of fMRI. This possibility cannot be ruled out, but both the imaging and patient experiments support the association of WM and LTM for faces rather than dissociation between WM and LTM for faces. In Experiment 1, there were overlapping activations between WM and LTM contrasts, and this suggests that the same hippocampal processes are mediating WM and LTM on this task. In Experiment 2, LTM performance by the amnesic patients was the best predictor for WM performance after 7 s. These findings are most consistent with the view that WM for faces by 7 s relies on MTL-dependent LTM or, stated most strongly, that by 7 s only LTM processes are relevant for remembering a face in detail.
Because there are limitations with both imaging and patient study methods, stronger conclusions about brain functions can be reached by employing convergent methods with the same task. One limitation of imaging data is that, used alone, it cannot establish the necessity of a brain structure for a mental operation (Gabrieli, 1998). For example, MTL activation has been reported for delay eye-blink conditioning in healthy people (Blaxton et al., 1996), despite normal acquisition of delay eye-blink conditioning in amnesic patients with MTL lesions (Gabrieli et al., 1995). Thus, MTL activation for such conditioning must reflect a learning-correlated process that does not underlie the learning itself. On the other hand, patient studies have several limitations, including variation in lesion size and location that can be difficult to confirm radiologically. Small lesions rarely injure an entire structure in the MTL, whereas large lesions compromise multiple MTL structures. Further, static lesions prevent study of dynamic processes, such as hippocampal activation across the maintenance period. However, by examining the same WM task via imaging and behaviorally in amnesic patients, we were able to compensate for these limitations to a degree. The impaired performance of the amnesic patients for 7-s WM maintenance in Experiment 2 suggests that the MTL activation observed during WM maintenance in Experiment 1 reflects an operation that supports task performance. The imaging data from Experiment 1 complements the patient data by localizing activity in the WM task to the hippocampus proper and suggests that the patient results are not solely due to neural damage outside of the MTL. Furthermore, Experiment 1 showed that faces can be actively maintained in WM as shown by the persistent hippocampal activation across WM maintenance. Thus, the combination of imaging and lesion evidence supports the involvement of the hippocampus during WM maintenance of faces for a period as short as 7 s. These findings, in conjunction with past research, indicate that dissociations and associations between the functional neural architectures of WM and LTM vary as a consequence of the nature of the information being maintained in WM.
Acknowledgments
The authors thank Ben Hutchinson for his help with data analysis in Experiments 1 and 2, Dara Ghahremani, Jeffrey Cooper, and Susan Gabrieli for their help with methodological issues in Experiment 1, and Frances Orlando for testing participants in Experiment 2. Additionally, we thank two anonymous reviewers for their helpful suggestions on prior versions of this manuscript.
Footnotes
Grant sponsor: National Institute of Mental Health; Grant number: MH59940 and MH57681; Grant sponsor: National Institute on Aging; Grant number: AG12995; Grant sponsor: National Institute of Neurological Disorders and Stroke; Grant number: NS 26985; Grant sponsor: Medical Research Service of the Department of Veterans Affairs.
References
- Baddeley AD, Hitch GJ. Working memory. In: Bower G, editor. Recent Advances in Learning and Motivation. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baddeley AD, Warrington EK. Amnesia and the distinction between long- and short-term memory. J Verbal Learn Verbal Behav. 1970;9:176–189. [Google Scholar]
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KD. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1989. [Google Scholar]
- Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, Disterhoft JE. Functional mapping of human learning: A positron emission tomography activation study of eye-blink conditioning. J Neurosci. 1996;16:4032–4040. doi: 10.1523/JNEUROSCI.16-12-04032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8:330–339. doi: 10.1002/(SICI)1098-1063(1998)8:4<330::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, Toma L, Ferretti A, Tartaro A, Colosimo C, Romani GL, Uncini A. Functional MRI study of diencephalic amnesia in Wernicke-Korsakoff syndrome. Brain. 2005;128 (Part 7):1584–1594. doi: 10.1093/brain/awh496. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2:151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Uhly B. Short-term motor memory in Korsakoff patients. Percept Mot Skills. 1975;40:275–281. doi: 10.2466/pms.1975.40.1.275. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Reale L, De Luca D. Korsakoff patients’ nonverbal vs verbal memory: Effects of interference and mediation on rate of information loss. Neuropsychologia. 1977;15:303–310. doi: 10.1016/0028-3932(77)90039-2. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- Crane J, Milner B. Do I know you? Face perception and memory in patients with selective amygdalo-hippocampectomy. Neuropsychologia. 2002;40:530–538. doi: 10.1016/s0028-3932(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Nichelli P. Verbal and non-verbal short-term memory impairment following hemispheric damage. Cortex. 1975;11:341–354. doi: 10.1016/s0010-9452(75)80026-8. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Faglioni P, Previdi P. Spatial memory and hemispheric locus of lesion. Cortex. 1977;13:424–433. doi: 10.1016/s0010-9452(77)80022-1. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fazio F, Perani D, Gilardi MC, Colombo F, Cappa SF, Vallar G, Bettinardi V, Paulesu E, Alberoni M, Bressi S, Franceshci M, Lenzi GL. Metabolic impairment in human amnesia: A PET study of memory networks. J Cereb Blood Flow Metab. 1992;12:353–358. doi: 10.1038/jcbfm.1992.52. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Memory in the Cerebral Cortex: An Empirical Approach to Neural Networks in the Human and Nonhuman Primate. Cambridge: MIT Press; 1995. pp. 1–372. [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, McGlinchey-Berroth R, Carrillo MC, Gluck MA, Cermak LS, Disterhoft JE. Intact delay-eyeblink classical conditioning in amnesia. Behav Neurosci. 1995;109:819–827. doi: 10.1037//0735-7044.109.5.819. [DOI] [PubMed] [Google Scholar]
- Ghent L, Mishkin M, Teuber HL. Short-term memory after frontal-lobe injury in man. J Comp Physiol Psychol. 1962;55:705–709. doi: 10.1037/h0047520. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Grant DA, Berg EA. A behavioral analysis of the degree of reinforcement and ease of shifting to new responses in a Weigl-type card sorting problem. J Exp Psychol Gen. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Lundgren SL, Morley GK. Short-term retention of verbal, visual shape and visuospatial location information in normal and amnesic subjects. Neuropsychologia. 1983;21:25–33. doi: 10.1016/0028-3932(83)90097-0. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Pawlik G, Holthoff V, Kessler J, Szelies B. PET correlates of normal and impaired memory functions. Cerebrovasc Brain Metab Rev. 1992;4:1–27. [PubMed] [Google Scholar]
- Huettel SA, McCarthy G. The effects of single-trial averaging upon the spatial extent of fMRI activation. Neuroreport. 2001;12:2411–2416. doi: 10.1097/00001756-200108080-00025. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Schafer K, Butters N, Cermak LS. Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology. 1991;4:175–186. [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Visual recognition and recall after right temporal-lobe excision in man. Neuropsychologia. 1968;6:191–209. doi: 10.1016/j.yebeh.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Miyake A, Shah P. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. J Exp Psychol Gen. 2001;130:621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. Mnemonic firing of neurons in the monkey temporal pole during a visual recognition memory task. J Neurophysiol. 1995;74:162–178. doi: 10.1152/jn.1995.74.1.162. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: Relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Paller KA. Consolidating dispersed neocortical memories: The missing link in amnesia. Memory. 1997;5:73–88. doi: 10.1080/741941150. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci. 2005;9:374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen M, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: Neural and behavioral evidence. J Cogn Neurosci. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Lasserson D, Marsden P, Stanhope N, Stevens T, Bello F, Kingsley D, Colchester A, Kopelman MD. FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex. 2003;39:1027–1045. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen Clinique en Psychologic. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Ringo JL. Memory decays at the same rate in macaques with and without brain lesions when expressed in d’ or arcsine terms. Behav Brain Res. 1991;42:123–134. doi: 10.1016/s0166-4328(05)80003-8. [DOI] [PubMed] [Google Scholar]
- Ringo JL. Spared short-term memory in monkeys following medial temporal lobe lesions is not yet established: A reply to Alvarez-Royo, Zola-Morgan and Squire. Behav Brain Res. 1993;59:65–72. doi: 10.1016/0166-4328(93)90152-g. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: A functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurochem. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Warrington EK. Independent functioning of verbal memory stores: A neuropsychological study. Q J Exp Psychol. 1970;22:261–273. doi: 10.1080/00335557043000203. [DOI] [PubMed] [Google Scholar]
- Sidman M, Stoddard LT, Mohr JP. Some additional quantitative observations of immediate memory in a patient with bilateral hippocampal lesions. Neuropsychologia. 1968;6:245–254. [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1991. [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Amaral DG, Press GA. Magnetic resonance imaging of the hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. J Neurosci. 1990;10:3106–3117. doi: 10.1523/JNEUROSCI.10-09-03106.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11:337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L. Hippocampal volume deficits in alcoholic Korsakoff’s syndrome. Neurology. 2003;61:1716–1719. doi: 10.1212/01.wnl.0000098940.31882.bb. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition. Philadelphia: FA Davis Co; 1989. [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Recognition Memory Test. Windsor, UK: NFER-Nelson; 1984. [Google Scholar]
- Warrington EK, Taylor AM. Immediate memory for faces: Long- or short-term memory? QJ Exp Psychol. 1973;25:316–322. doi: 10.1080/14640747308400352. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Niki H. Hippocampal unit activity and delayed response in the monkey. Brain Res. 1985;325:241–254. doi: 10.1016/0006-8993(85)90320-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. San Antonio: Psychological Corp; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. San Antonio: Psychological Corp; 1997b. [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


