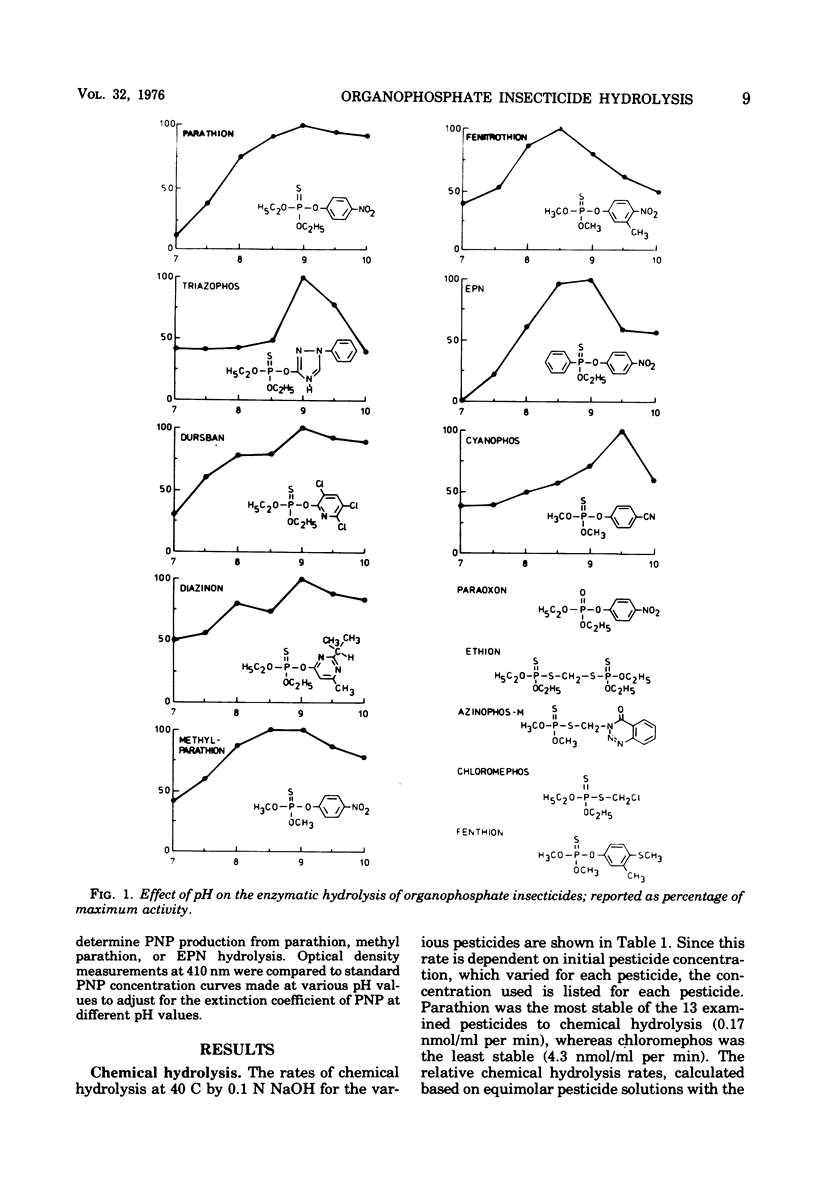
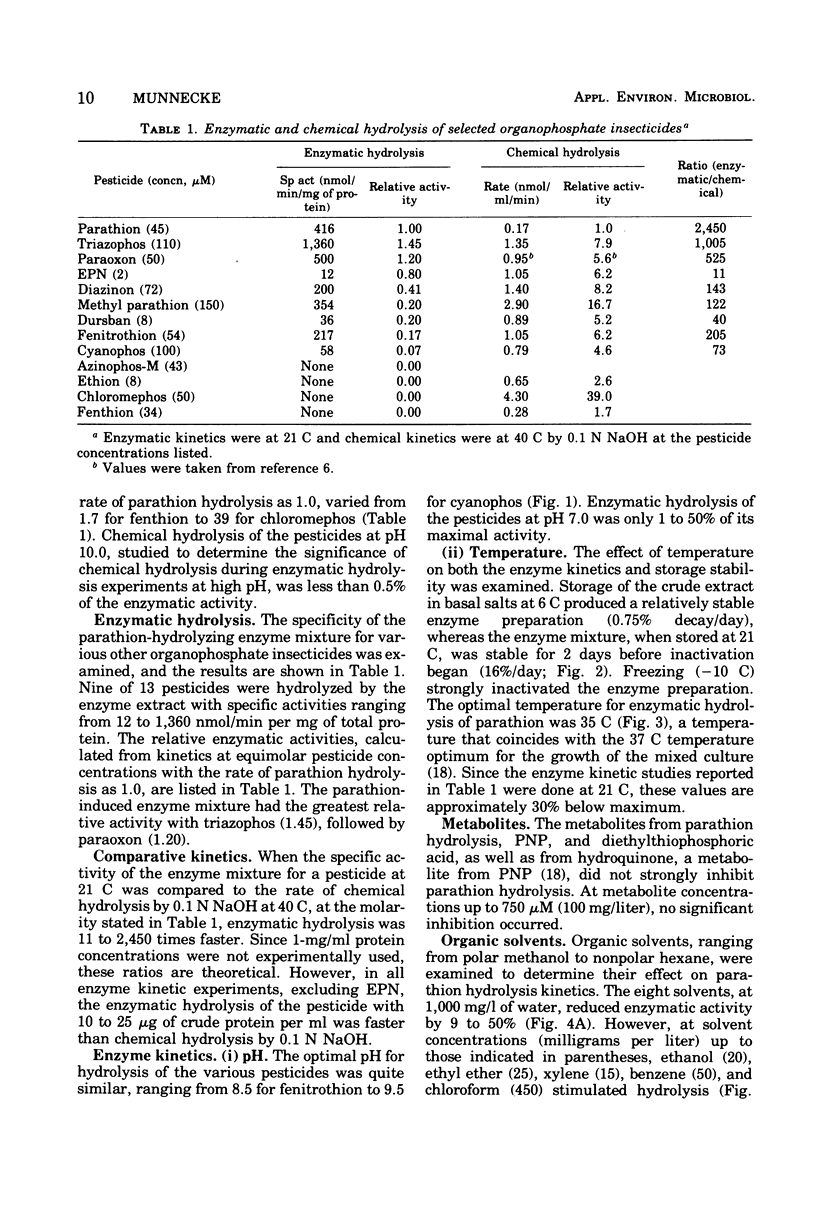
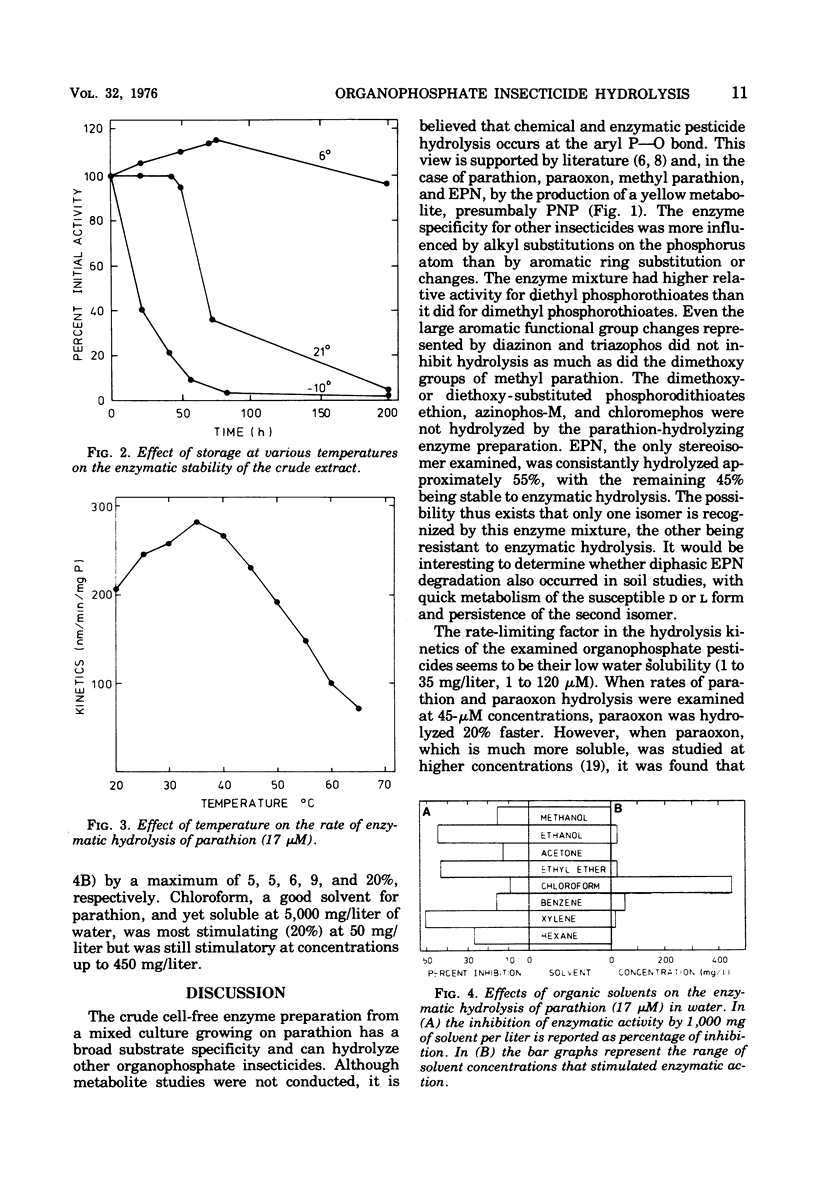
Abstract
A crude cell extract from a mixed bacterial culture growing on parathion, an organophosphate insecticide, hydrolyzed parathion (21 C) at a rate of 416 nmol/min per mg of protein. This rate of enzymatic hydrolysis, when compared with chemical hydrolysis by 0.1 N sodium hydroxide at 40 C, was 2, 450 times faster. Eight of 12 commonly used organophosphate insecticides were enzymatically hydrolyzed with this enzyme preparation at rates ranging from 12 to 1,360 nmol/min per mg of protein. Seven pesticides were hydrolyzed at rates significantly higher (40 to 1,005 times faster) than chemical hydrolysis. The pH optimum for enzymatic hydrolysis of the eight pesticides ranged from 8.5 to 9.5, with less than 50% of maximal activity expressed at pH 7.0. Maximal enzyme activity occurred at 35 C. The crude extract lost its activity at the rate of only 0.75%/day when stored at 6 C. Eight organic solvents, ranging from methanol to hexane, at low concentrations stimulated enzymatic hydrolysis by 3 to 20%, whereas at higher concentrations (1,000 mg/liter) they inhibited the reaction (9 to 50%). Parathion metabolites p-nitrophenol, hydroquinone, and diethylthiophosphoric acid, at up to 100-mg/liter concentrations, did not significantly influence enzyme activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. P., Lichtenstein E. P. Effects of various soil fungi and insecticides on the capacity of Mucor alternans to degrade DDT. Can J Microbiol. 1972 May;18(5):553–560. doi: 10.1139/m72-088. [DOI] [PubMed] [Google Scholar]
- Coley G., Stutz C. N. Treatment of parathion wastes and other organics. J Water Pollut Control Fed. 1966 Aug;38(8):1345–1349. [PubMed] [Google Scholar]
- Engelhardt G., Wallnöfer P. R., Plapp R. Degradation of linuron and some other herbicides and fungicides by a linuron-inducible enzyme obtained from Bacillus sphaericus. Appl Microbiol. 1971 Sep;22(3):284–288. doi: 10.1128/am.22.3.284-288.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt G., Wallnöfer P. R., Plapp R. Purification and properties of an aryl acylamidase of Bacillus sphaericus, catalyzing the hydrolysis of various phenylamide herbicides and fungicides. Appl Microbiol. 1973 Nov;26(5):709–718. doi: 10.1128/am.26.5.709-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust S. D. Chemical hydrolysis of some organic phosphorus and carbamate pesticides in aquatic environments. Environ Lett. 1972;3(3):171–201. doi: 10.1080/00139307209435465. [DOI] [PubMed] [Google Scholar]
- Lanzilotta R. P., Pramer D. Herbicide transformation. II. Studies with an acylamidase of Fusarium solani. Appl Microbiol. 1970 Feb;19(2):307–313. doi: 10.1128/am.19.2.307-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnecke D. M., Hsieh D. P. Microbial decontamination of parathion and p-nitrophenol in aqueous media. Appl Microbiol. 1974 Aug;28(2):212–217. doi: 10.1128/am.28.2.212-217.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnecke D. M., Hsieh D. P. Microbial metabolism of a parathion-xylene pesticide formulation. Appl Microbiol. 1975 Oct;30(4):575–580. doi: 10.1128/am.30.4.575-580.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnecke D. M., Hsieh D. P. Pathways of microbial metabolism of parathion. Appl Environ Microbiol. 1976 Jan;31(1):63–69. doi: 10.1128/aem.31.1.63-69.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



