Abstract
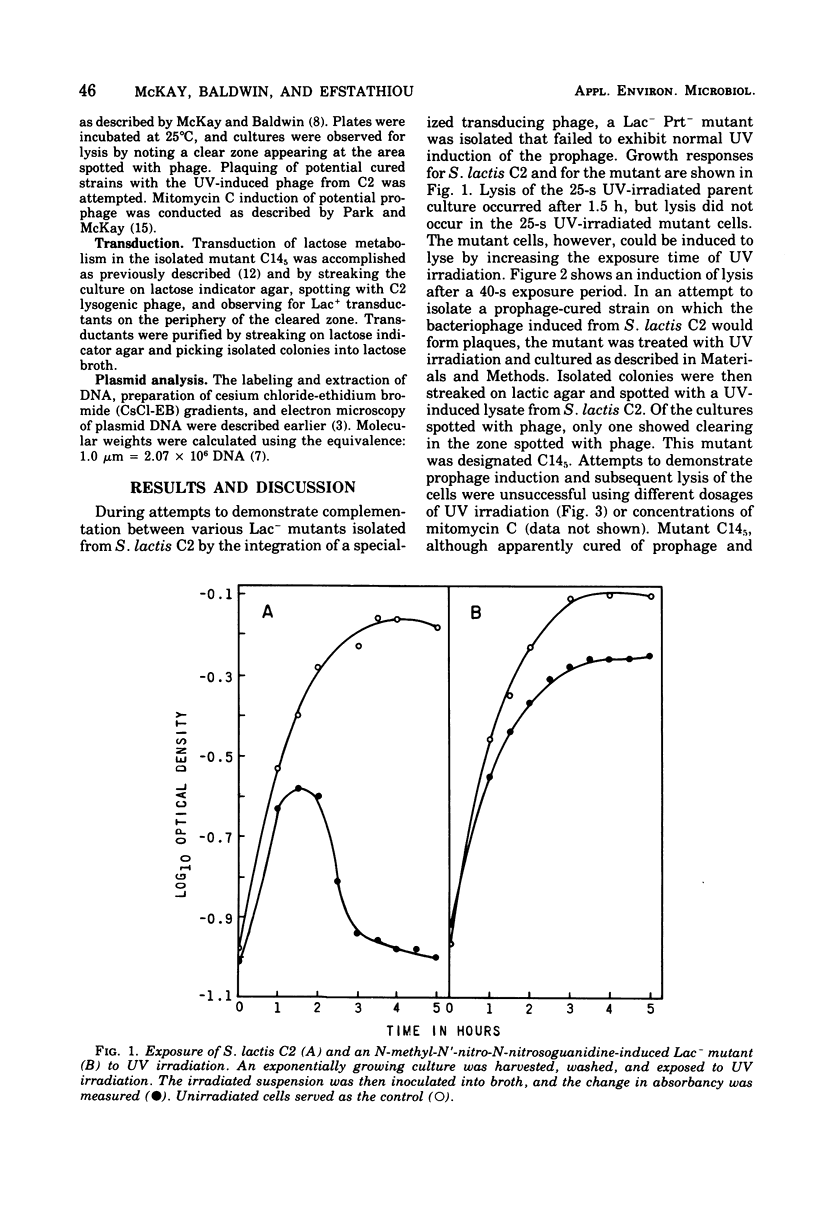
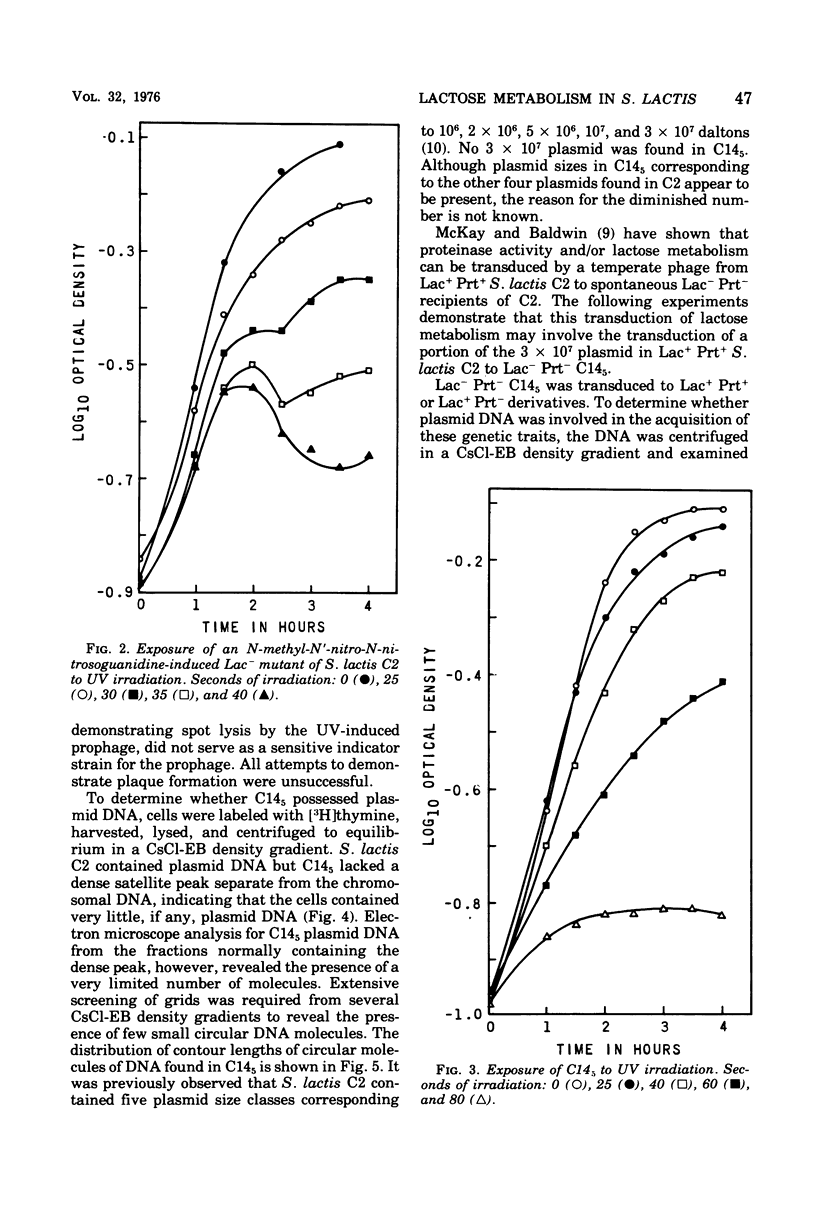
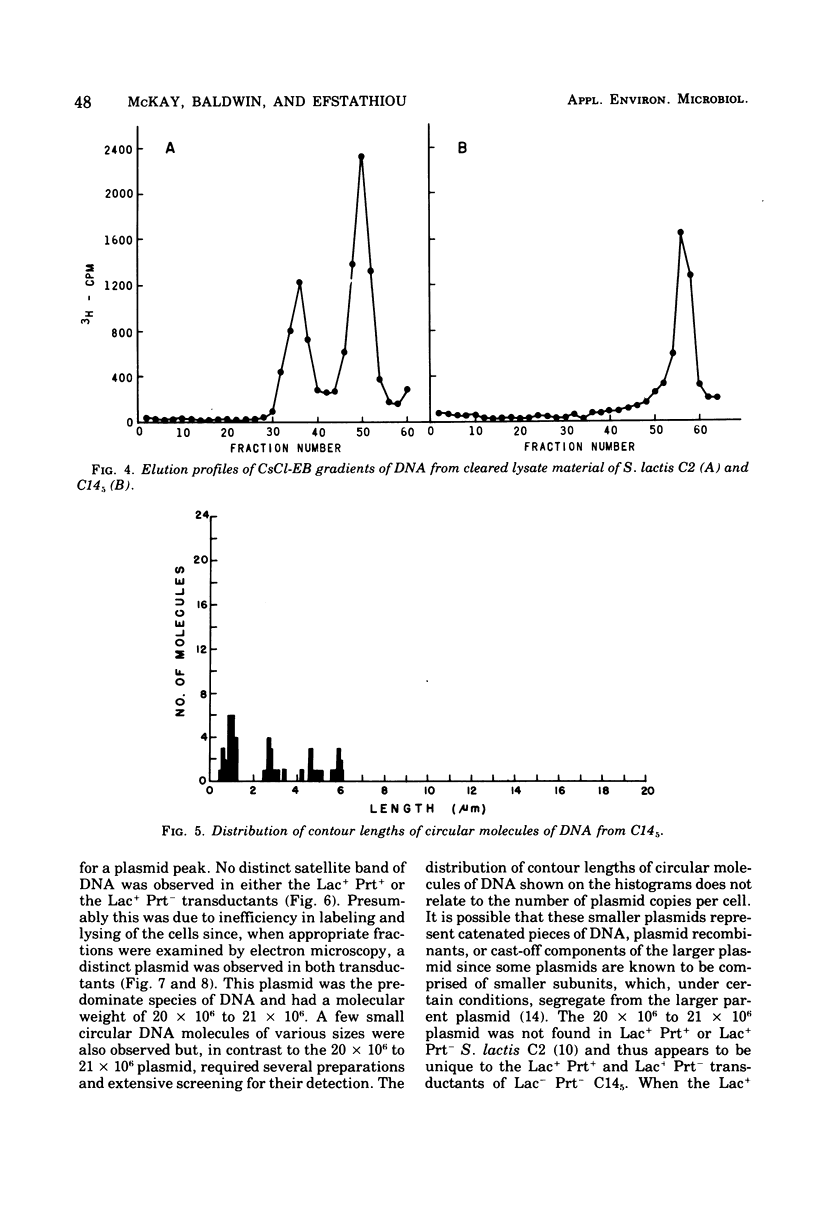
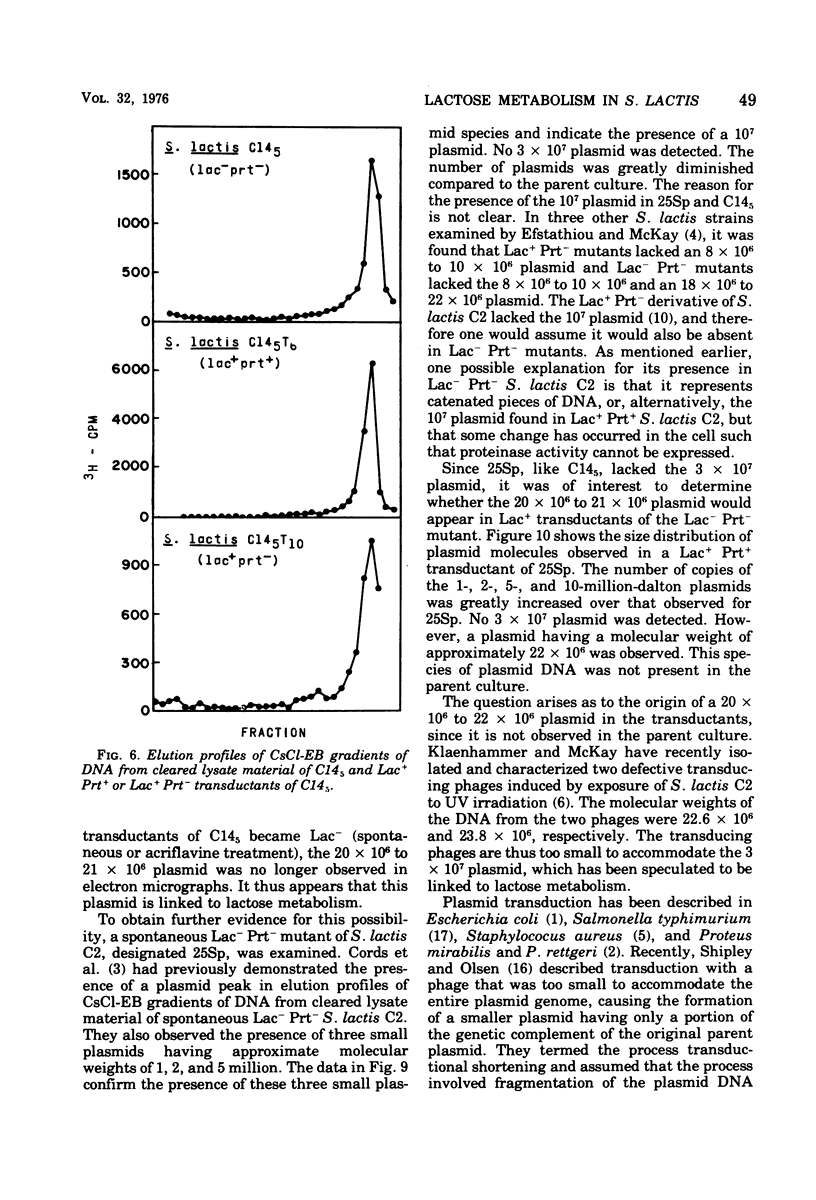
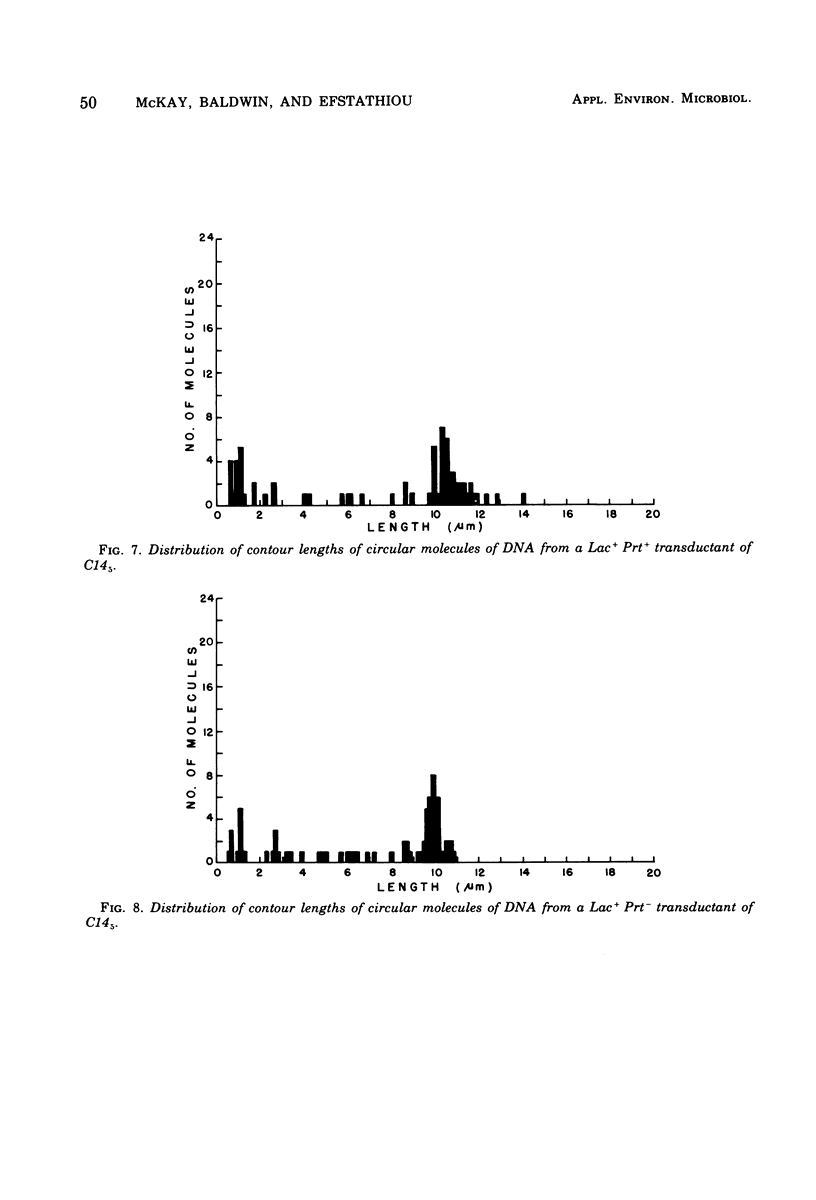
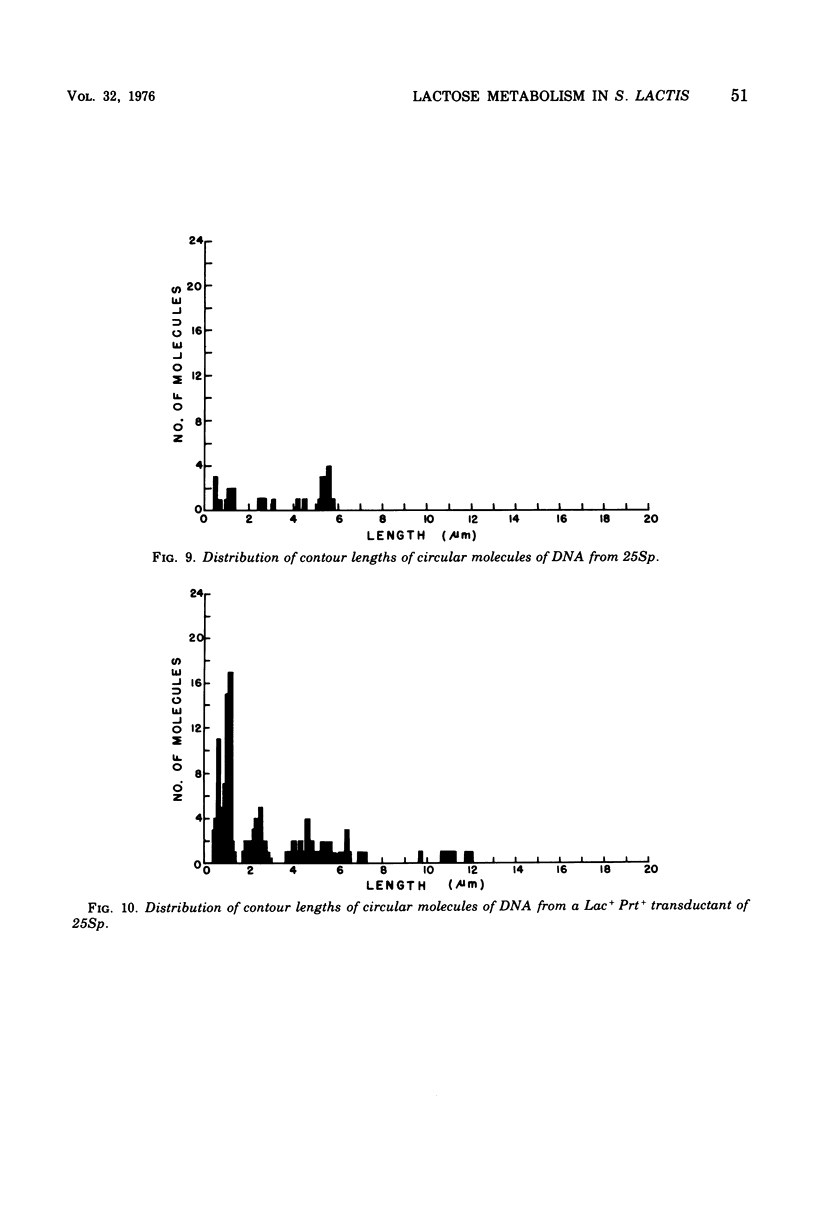
A lactose-negative (Lac-), proteinase-negative (Prt-) mutant, designated C145 was isolated from Streptococcus lactis C2 after treatment with nitrosoguanidine and ultraviolet irradiation. The mutant appeared to be cured of the prophage(s) present in S. lactis C2 based on non-inducibility by ultraviolet irradiation or mitomycin C. When cleared lysate material from C145 was subjected, to cesium chloride-ethidum bromide (EB) density gradient centrifugation, no plasmid peak was observed, suggesting that C145 was cured of plasmid deoxyribonucleic and (DNA). A histogram showing distribution of contour lengths of circular molecules of DNA from C145, however, revealed the presence of a greatly diminished number of DNA molecules as compared with the parent culture and indicated the absence of the 30 x 10(6) plasmid. Cesium chloride-ethidium bromide gradient profiles from Lac+, Prt- and Lac+ Prt+ transductants of C145 revealed no plasmid peak, but electron microscopy of the fractions normally possessing the satellite band of DNA showed the presence of a new plasmid species having a molecular weight from 20 x 10(6) to 22 x 10(6). This plasmid was lost when the transductants became Lac-. Examination of a plasmid histogram from a spontaneous Lac- Prt- mutants of S. lactis C2 resembled that of C145, with the absence of the 30 x 10(6) plasmid and the presence of the 22 x 10(6) plasmid in Lac+ Prt+ transductants. The results suggest that lactose metabolism is mediated through the 30 x 10(6) plasmid in S. lactis C2 and that the transducing bacteriophage, which is too small to accommodate the entire plasmid, is transferring about two-thirds of the original plasmid through a process termed transductional shortening.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Natkin E. Transduction of resistance determinants and R factors of the transfer systems by phage Plkc. Mol Gen Genet. 1972;114(3):261–265. doi: 10.1007/BF01788895. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W., Appelbaum P. C. Transduction of R factors in Proteus mirabilis and P. rettgeri. J Gen Microbiol. 1973 Jun;76(2):355–368. doi: 10.1099/00221287-76-2-355. [DOI] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Plasmids in Streptococcus lactis: evidence that lactose metabolism and proteinase activity are plasmid linked. Appl Environ Microbiol. 1976 Jul;32(1):38–44. doi: 10.1128/aem.32.1.38-44.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Wüst J., Corrodi P. Transduction and elimination of resistance determinants in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Sep;2(3):217–223. doi: 10.1128/aac.2.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L. Isolation and examination of transducing bacteriophage particles from Streptococcus lactis C2. J Dairy Sci. 1976 Mar;59(3):396–404. doi: 10.3168/jds.s0022-0302(76)84219-1. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Induction of prophage in Streptococcus lactis C2 by ultraviolet irradiation. Appl Microbiol. 1973 Apr;25(4):682–684. doi: 10.1128/am.25.4.682-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Plasmid distribution and evidence for a proteinase plasmid in Streptococcus lactis C2-1. Appl Microbiol. 1975 Apr;29(4):546–548. doi: 10.1128/am.29.4.546-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Simultaneous loss of proteinase- and lactose-utilizing enzyme activities in Streptococcus lactis and reversal of loss by transduction. Appl Microbiol. 1974 Sep;28(3):342–346. doi: 10.1128/am.28.3.342-346.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley P. L., Olsen R. H. Isolation of a nontransmissible antibiotic resistance plasmid by transductional shortening of R factor RP1. J Bacteriol. 1975 Jul;123(1):20–27. doi: 10.1128/jb.123.1.20-27.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Ogata Y. Abortive transduction of resistance factor by bacteriophage P22 in Salmonella typhimurium. J Bacteriol. 1970 May;102(2):596–597. doi: 10.1128/jb.102.2.596-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


