Abstract
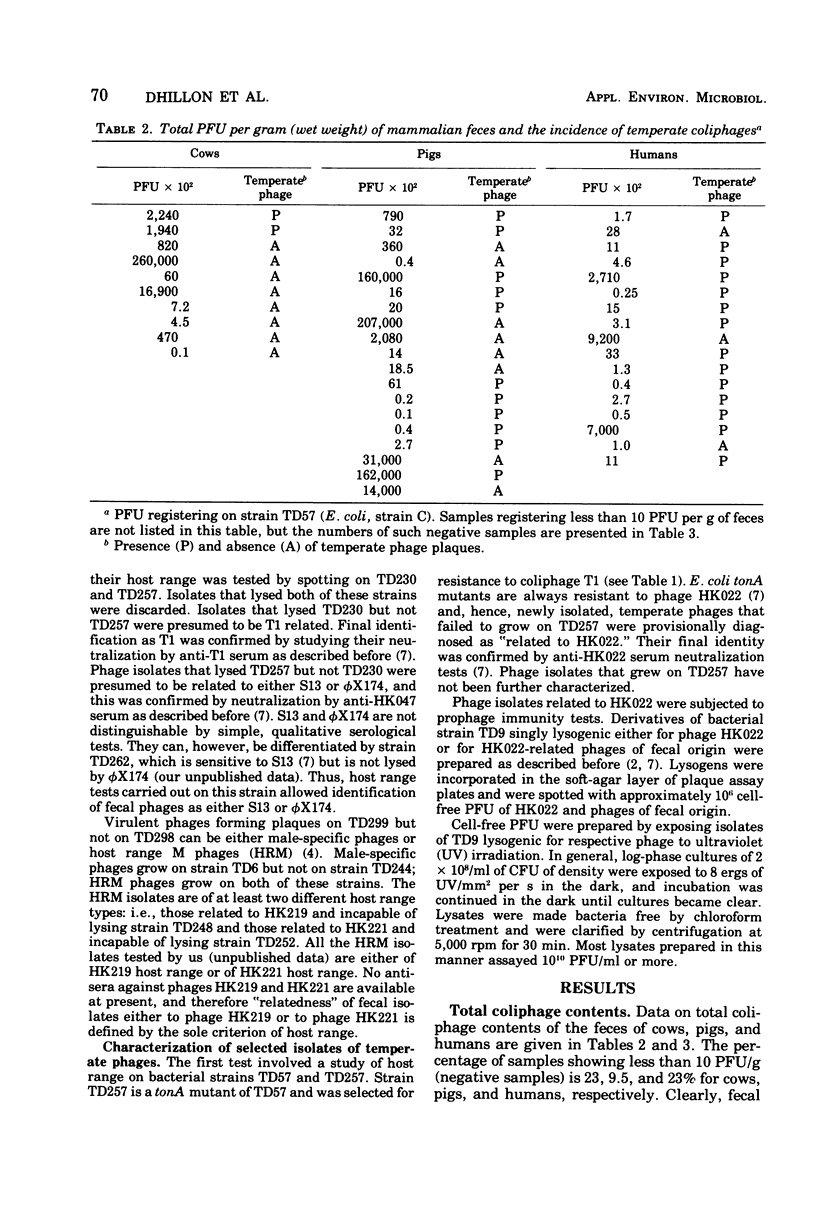
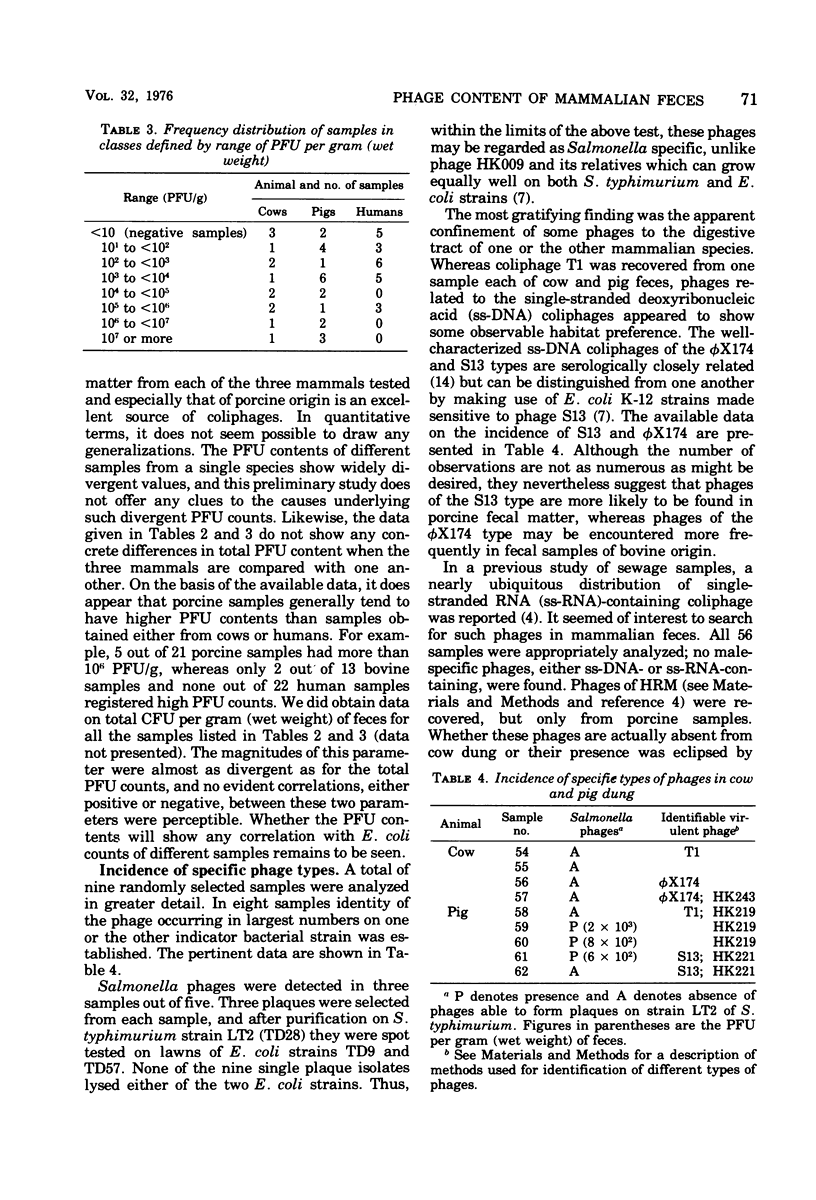
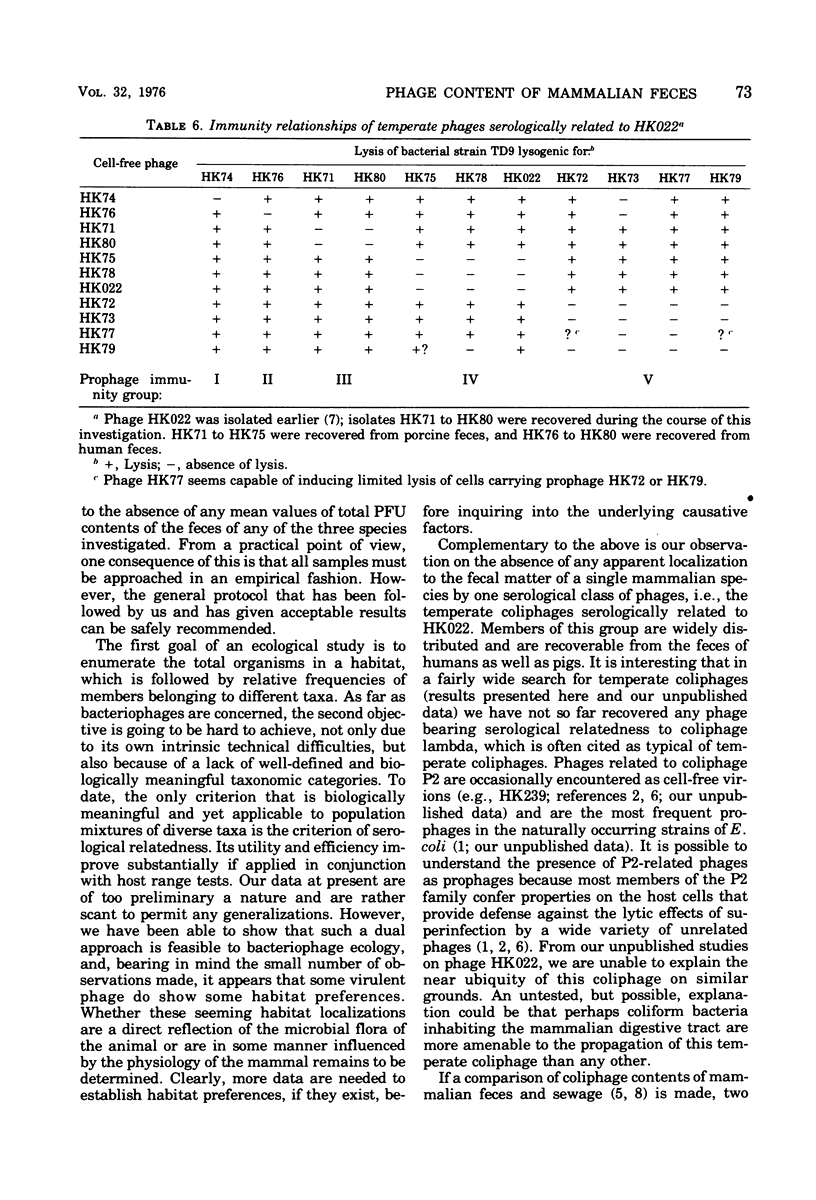
Freshly voided samples of the feces of cows, pigs, and humans were analyzed for the enumeration of cell-free plaque-forming units (PFU) of coliphages and Salmonella phages. Coliphage PFU counts per gram (wet weight) of feces were found to range from less than 10(1) to greater than 10(7). Salmonella phages were found in three out of five porcine samples, but none were found in the four bovine samples analyzed. Virulent coliphages related to the phiX174/S13 serological group showed some "habitat preference" in that the S13 type of phages was found only in pig feces, whereas the phiX174 type of phages was found only in cow dung. Temperate coliphages were detectable in a majority of samples of both human and porcine origin but were infrequently found in bovine samples. About 80% of the temperate coliphages of fecal origin have been found to be serologically related to phage HK022 (Dhillon and Dhillon, 1973), and all are efficiently inducible by ultraviolet light irradiation. However, considerable diversity with the group was found when the prophage immunity pattern of 10 randomly selected isolates was examined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertani L. E., Bertani G. Genetics of P2 and related phages. Adv Genet. 1971;16:199–237. doi: 10.1016/s0065-2660(08)60359-4. [DOI] [PubMed] [Google Scholar]
- Dhillon E. K., Dhillon T. S. HK239: a P2 related temperate phage which excludes rII mutants of T4. Virology. 1973 Sep;55(1):136–142. doi: 10.1016/s0042-6822(73)81015-3. [DOI] [PubMed] [Google Scholar]
- Dhillon E. K., Dhillon T. S. N-methyl-N'-nitro-N-nitrosoguanidine and hydroxylamine induced mutants of the rII region of phage T4. Mutat Res. 1974 Mar;22(3):223–233. doi: 10.1016/0027-5107(74)90023-2. [DOI] [PubMed] [Google Scholar]
- Dhillon E. K., Dhillon T. S. Synthesis of indicator strains and density of ribonucleic acid-containing coliphages in sewage. Appl Microbiol. 1974 Apr;27(4):640–647. doi: 10.1128/am.27.4.640-647.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon T. S., Chan Y. S., Sun S. M., Chau W. S. Distribution of coliphages in Hong Kong sewage. Appl Microbiol. 1970 Aug;20(2):187–191. doi: 10.1128/am.20.2.187-191.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon T. S., Dhillon E. K. Mutants of phage HK239 defective in excluding phages lambda T4rII, P1, and P2. Mol Gen Genet. 1973 Dec 31;127(3):249–254. doi: 10.1007/BF00333764. [DOI] [PubMed] [Google Scholar]
- Dhillon T. S., Dhillon E. K. Studies on bacteriophage distribution. II. Isolation and host rage based classification of phages active on three species of Enterobacteriaceae. Jpn J Microbiol. 1972 Jul;16(4):297–306. doi: 10.1111/j.1348-0421.1972.tb00662.x. [DOI] [PubMed] [Google Scholar]
- HAYES W. The mechanism of genetic recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1953;18:75–93. doi: 10.1101/sqb.1953.018.01.016. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- ZINDER N. D., LEDERBERG J. Genetic exchange in Salmonella. J Bacteriol. 1952 Nov;64(5):679–699. doi: 10.1128/jb.64.5.679-699.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]


