Abstract
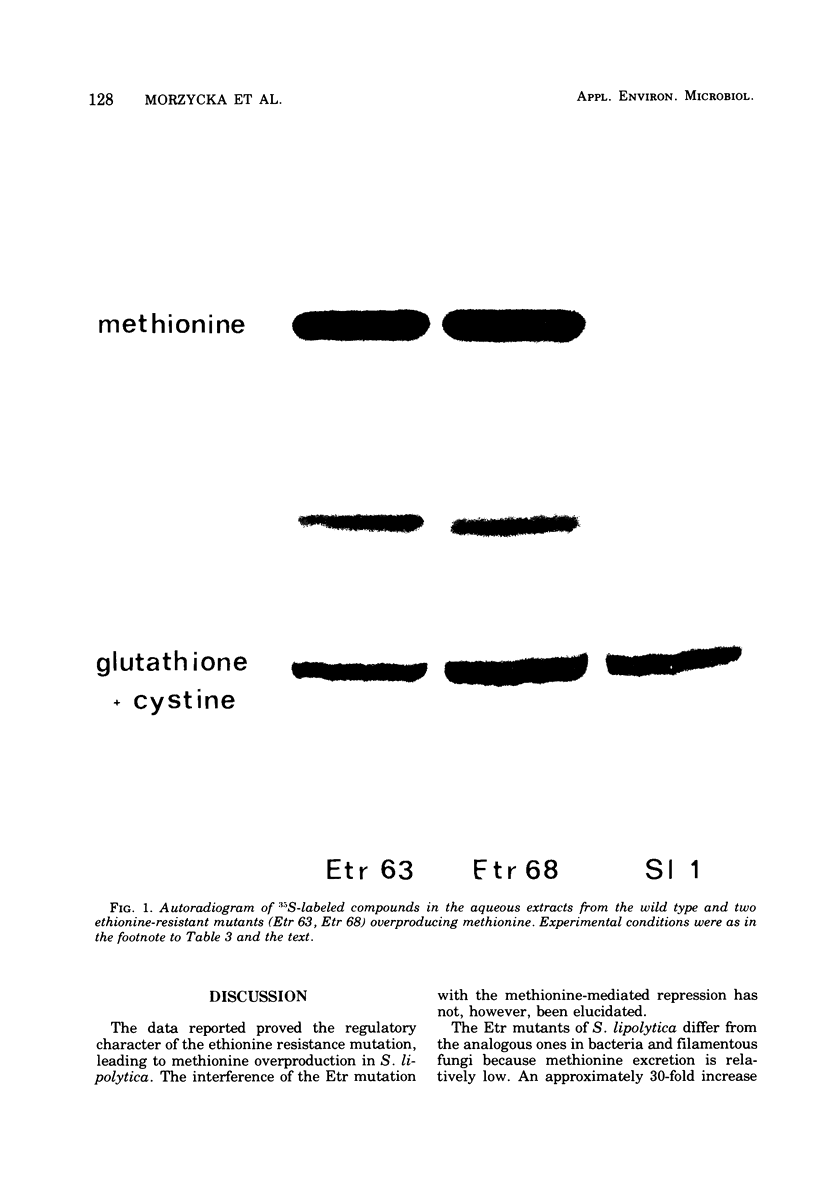
Six ethionine-resistant (Etr) regulatory mutants of Saccharomycopsis lipolytica Sl/1 overproducing methionine have been isolated. Five of them are also resistant to seleno-methionine. The activity of homocysteine synthase (O-acetyl-L-hormoserine-acetate lyase, adding hydrogen sulfide) is derepressed in these mutants and is not susceptible to the methionine-mediated repression. The pool of free methionine in Etr mutants is enhanced 1.5 to 18 times, and incorporation of 35S into methionine is 1.5 to 50 times higher than that in the wild strain. Neither accumulation of endogenous free methionine in Etr mutants nor the uptake of exogenous methionine is accompanied by an increase in the S-adenosylmethionine pool. This implies compartmentation of methionine metabolism in S. lipolytica.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassel J., Mortimer R. Genetic analysis of mating type and alkane utilization in Saccharomycopsis lipolytica. J Bacteriol. 1973 May;114(2):894–896. doi: 10.1128/jb.114.2.894-896.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALZY P., SLONIMSKI P. P. Variations physiologiques de la levure au cours de la croissance sur l'acide lactique comme seule source de carbone. C R Hebd Seances Acad Sci. 1957 Dec 16;245(25):2423–2426. [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- Masselot M., de Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae: mutations at the regulatory locus ETH2. II. Physiological and biochemical data. Mol Gen Genet. 1974 Apr 3;129(4):349–361. doi: 10.1007/BF00265698. [DOI] [PubMed] [Google Scholar]
- Michalik J., Raczyńska-Bojanowska K. The methionine pool and lincomycin biosynthesis in Streptomyces lincolnensis. Bull Acad Pol Sci Biol. 1974;22(7-8):461–464. [PubMed] [Google Scholar]
- Nurse P., Wiemken A. Amino acid pools and metabolism during the cell division cycle of arginine-grown Candida utilis. J Bacteriol. 1974 Mar;117(3):1108–1116. doi: 10.1128/jb.117.3.1108-1116.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszewski A., Grabski J. Regulation of S-amino acids biosynthesis in Aspergillus nidulans. Role of cysteine and-or homocysteine as regulatory effectors. Mol Gen Genet. 1974;132(4):307–320. doi: 10.1007/BF00268571. [DOI] [PubMed] [Google Scholar]
- Pieniazek N., Stepień P. P., Paszewski A. An Aspergillus nidulans mutant lacking cystathionine -synthase: identity of L-serine sulfhydrylase with cystathionine -synthase and its distinctness from O-acetyl-L-serine sulfhydrylase. Biochim Biophys Acta. 1973 Jan 24;297(1):37–47. doi: 10.1016/0304-4165(73)90047-0. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Yarrow D. Four new combinations in yeasts. Antonie Van Leeuwenhoek. 1972;38(3):357–360. doi: 10.1007/BF02328105. [DOI] [PubMed] [Google Scholar]