Abstract
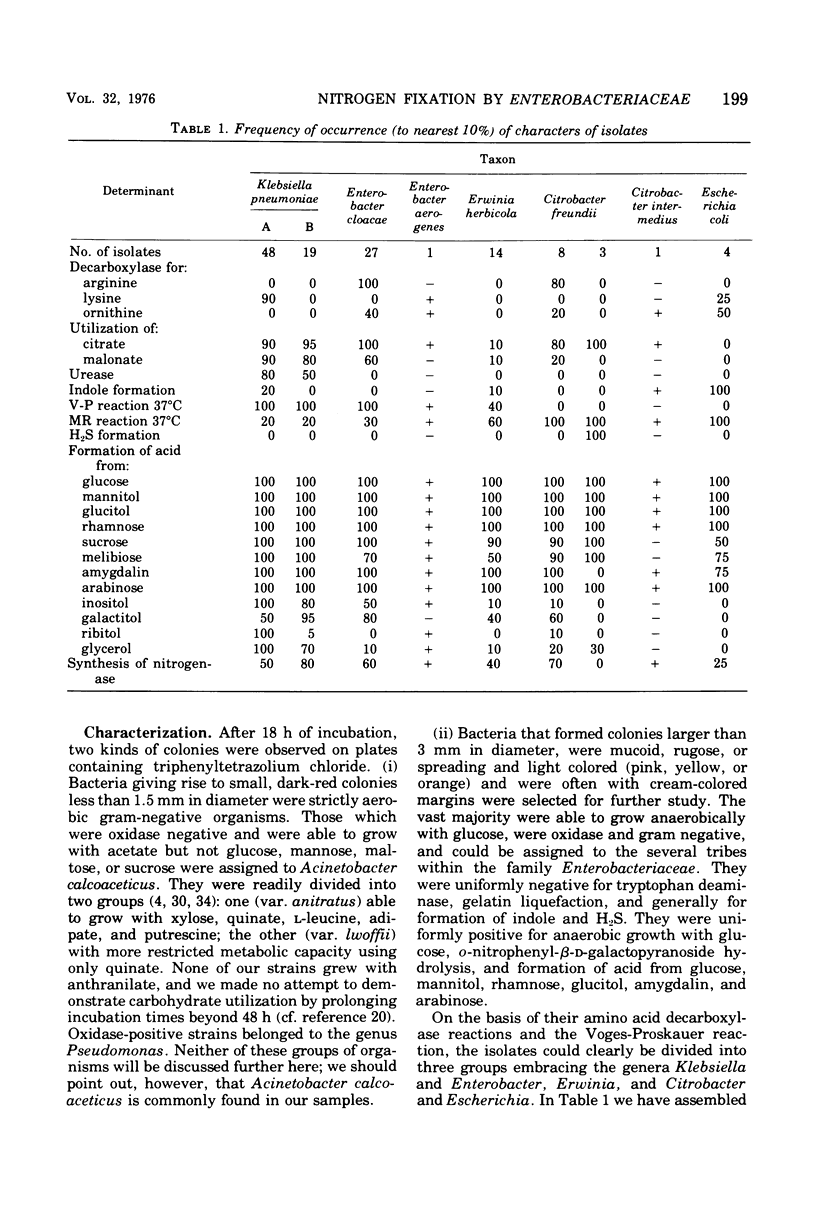
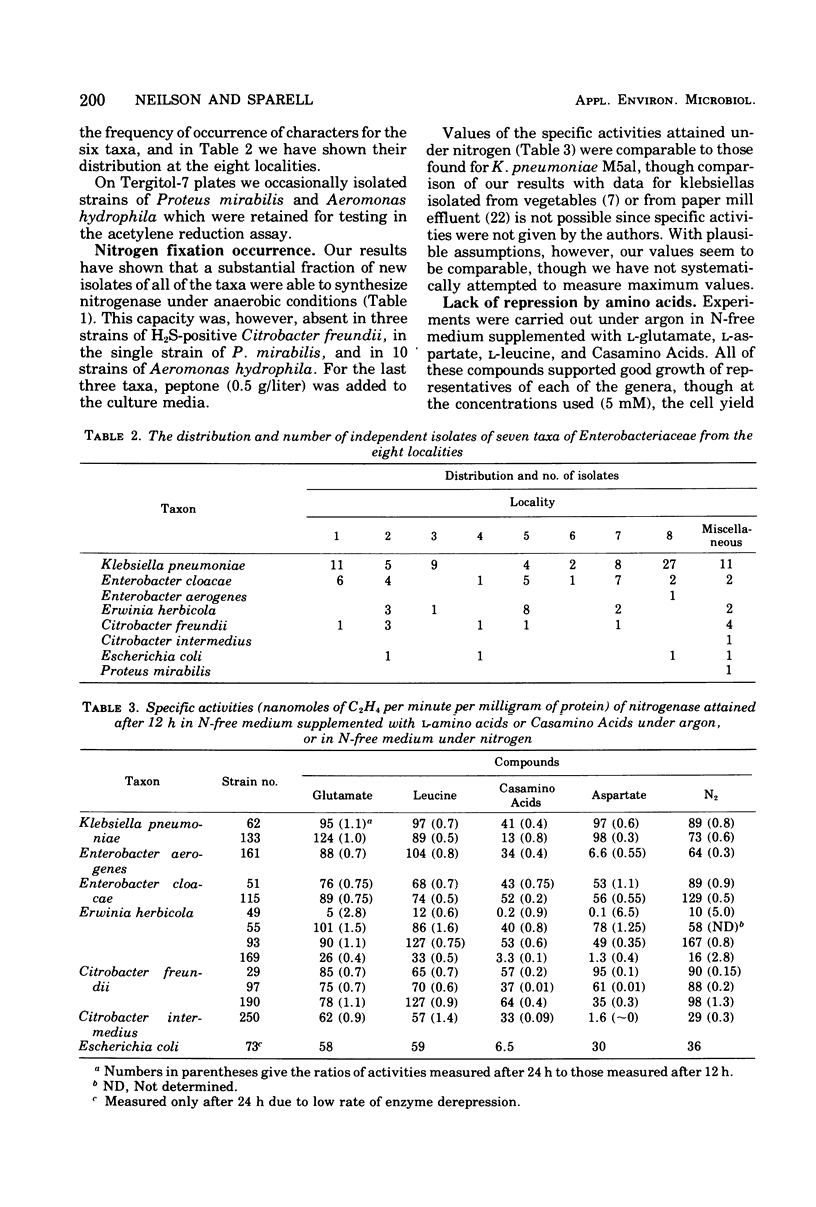
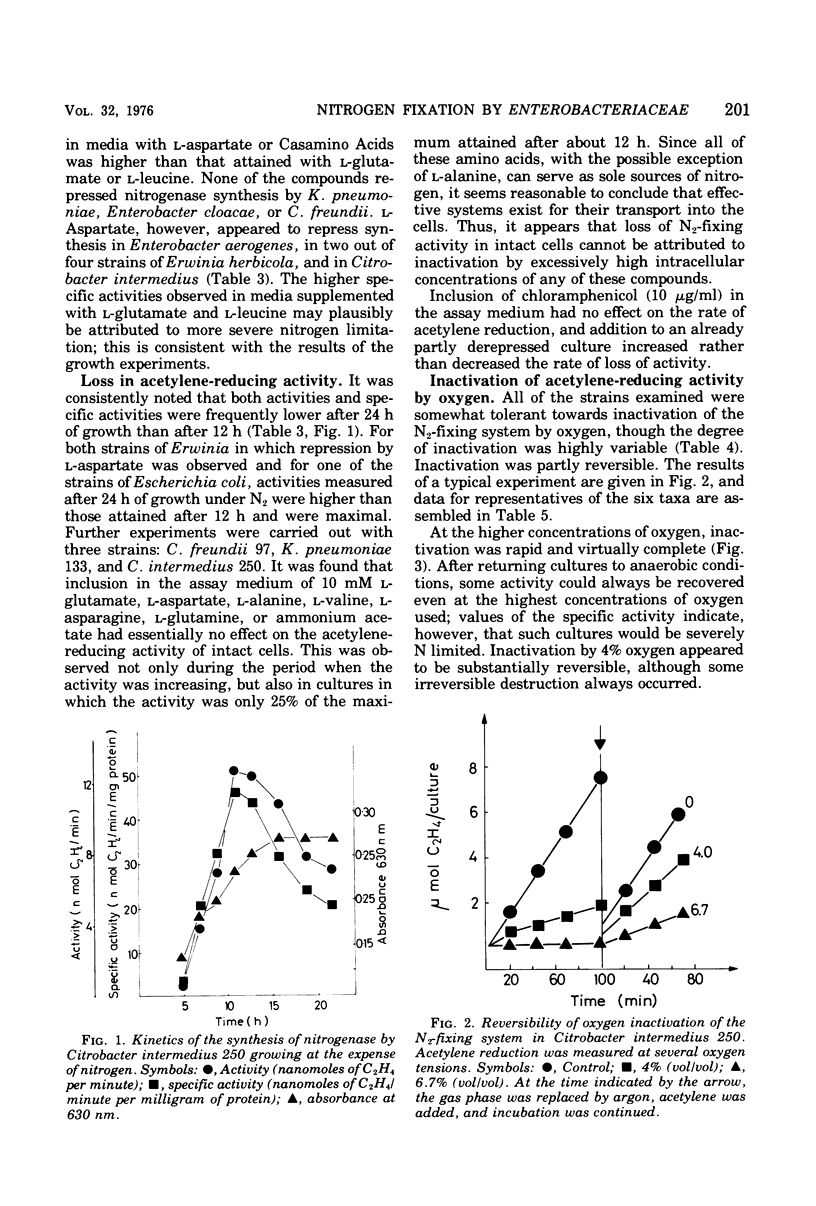
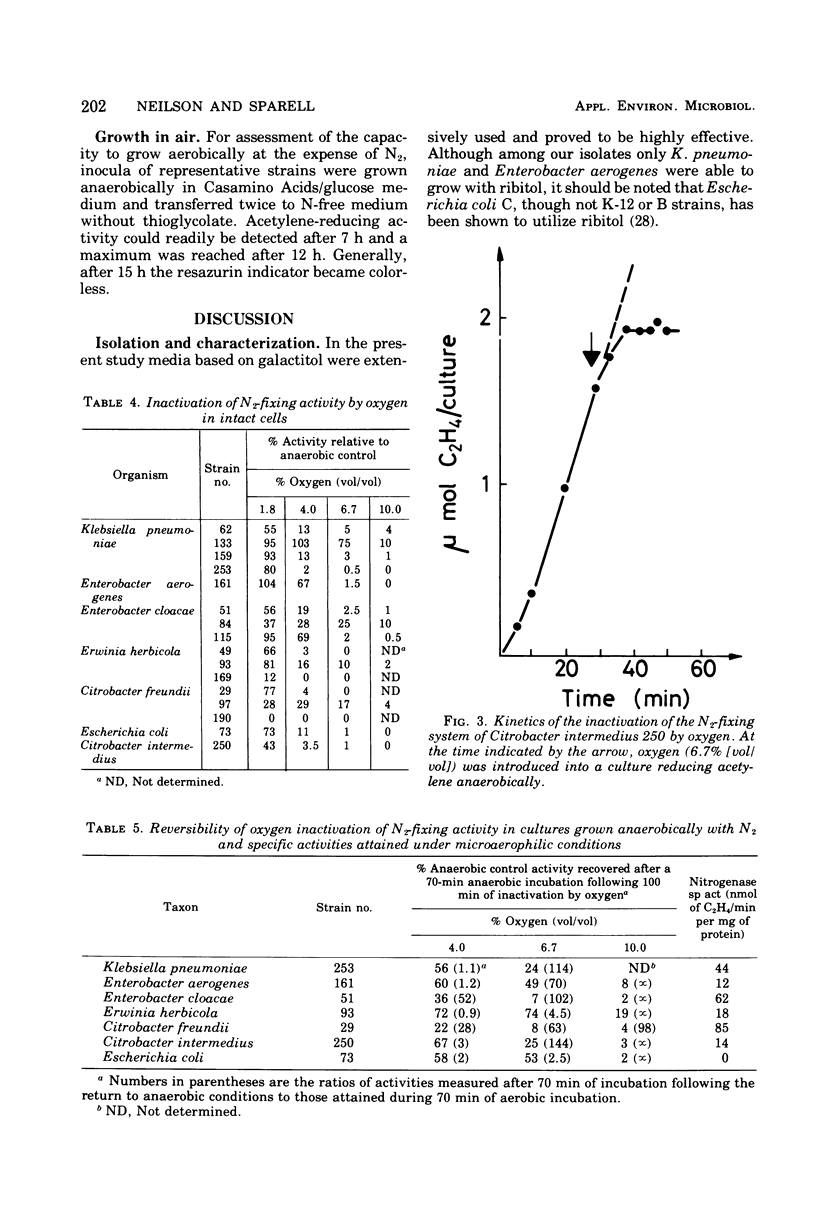
Using selective media containing galactitol, over 130 Enterobacteriaceae have been isolated from paper mill process waters collected from different localities. These bacteria were extensively characterized and tested for acetylene-reducing (nitrogen-fixing) activity under anaerobic conditions. High activity was found in representatives of Klebsiella pneumoniae, Enterobacter aerogenes, Enterobacter cloacae, Erwinia herbicola, Citrobacter freundii, Citrobacter intermedius, and Escherichia coli. Under argon, nitrogenase synthesis was generally not repressed by 5 mM l-glutamate, l-aspartate, l-leucine or Casamino Acids (0.5 g/liter). In many strains, both the specific activities (nanomoles of C2H4 per minute per milligram of protein) and the activities (nanomoles of C2H4 per minute) had considerably declined after 24 h. In three selected strains, activity in intact cells grown under nitrogen was unaffected by the presence during assay of 10 mM l-amino acids or ammonium acetate. All of the strains examined were tolerant towards inactivation of nitrogen-fixing activity by 1.8% (vol/vol) oxygen during assay, and inactivation by up to 10% oxygen was partly reversible. Representatives of the six taxa synthesized nitrogenase in stirred aerobic cultures, though the protein concentrations attained were lower than under anaerobic conditions. It seems reasonable to suggest that under natural conditions, nitrogen fixation is able to contribute significantly to the nitrogen economy of the cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascomb S., Lapage S. P., Curtis M. A., Willcox W. R. Identification of bacteria by computer: identification of reference strains. J Gen Microbiol. 1973 Aug;77(2):291–315. doi: 10.1099/00221287-77-2-291. [DOI] [PubMed] [Google Scholar]
- Bascomb S., Lapage S. P., Willcox W. R., Curtis M. A. Numerical classification of the tribe Klebsielleae. J Gen Microbiol. 1971 Jun;66(3):279–295. doi: 10.1099/00221287-66-3-279. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen F. J., Hipsley E. H. The presence of N2-fixing bacteria in the intestines of man and animals. J Gen Microbiol. 1970 Jan;60(1):61–65. doi: 10.1099/00221287-60-1-61. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Skerman F. J., Falkow S. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J Bacteriol. 1972 Mar;109(3):953–965. doi: 10.1128/jb.109.3.953-965.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., Seidler R. J. Potential pathogens in the environment: Klebsiella pneumoniae, a taxonomic and ecological enigma. Appl Microbiol. 1973 Jun;25(6):900–904. doi: 10.1128/am.25.6.900-904.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris R. H. Nitrogen fixation--assay methods and techniques. Methods Enzymol. 1972;24:415–431. doi: 10.1016/0076-6879(72)24088-5. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Gibbins L. N. Induction of nonpigmented variants of Erwinia herbicola by incubation at supraoptimal temperatures. J Bacteriol. 1971 Jan;105(1):107–112. doi: 10.1128/jb.105.1.107-112.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Steigerwalt A. G., Fanning G. R., Brenner D. J. Polynucleotide sequence divergence in the genus Citrobacter. J Gen Microbiol. 1974 Aug;83(2):271–282. doi: 10.1099/00221287-83-2-271. [DOI] [PubMed] [Google Scholar]
- Duncan D. W., Razzell W. E. Klebsiella biotypes among coliforms isolated from forest environments and farm produce. Appl Microbiol. 1972 Dec;24(6):933–938. doi: 10.1128/am.24.6.933-938.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller C., Edwards F. F. Nitrogen-deficient medium in the differential isolation of Klebsiella and Enterobacter from feces. Appl Microbiol. 1968 Jun;16(6):896–899. doi: 10.1128/am.16.6.896-899.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Campbell N. E., Hill S. Asymbiotic nitrogen-fixing bacteria from the surfaces of nodules and roots of legumes. Can J Microbiol. 1972 Jan;18(1):13–21. doi: 10.1139/m72-003. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Pirt S. J. The influence of dissolved oxygen concentration on the respiration and glucose metabolism of Klebsiella aerogenes during growth. J Gen Microbiol. 1967 Feb;46(2):193–211. doi: 10.1099/00221287-46-2-193. [DOI] [PubMed] [Google Scholar]
- Hill S. Acetylene reduction by Klebsiella neumoniae in air related to colony dimorphism on low fixed nitrogen. J Gen Microbiol. 1975 Nov;91(1):207–209. doi: 10.1099/00221287-91-1-207. [DOI] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. Nitrogen fixation by Klebsiella grown in the presence of oxygen. Can J Microbiol. 1972 Dec;18(12):1845–1850. doi: 10.1139/m72-288. [DOI] [PubMed] [Google Scholar]
- Knowles R., Neufeld R., Simpson S. Acetylene reduction (nitrogen fixation) by pulp and paper mill effluents and by Klebsiella isolated from effluents and environmental situations. Appl Microbiol. 1974 Oct;28(4):608–613. doi: 10.1128/am.28.4.608-613.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Nunez W. J., Colmer A. R. Differentiation of Aerobacter-Klebsiella isolated from sugarcane. Appl Microbiol. 1968 Dec;16(12):1875–1878. doi: 10.1128/am.16.12.1875-1878.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju P. N., Evans H. J., Seidler R. J. An asymbiotic nitrogen-fixing bacterium from the root environment of corn. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3474–3478. doi: 10.1073/pnas.69.11.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Genes for ribitol and D-arabitol catabolism in Escherichia coli: their loci in C strains and absence in K-12 and B strains. J Bacteriol. 1975 Aug;123(2):530–536. doi: 10.1128/jb.123.2.530-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Knittel M. D., Brown C. Potential pathogens in the environment: cultural reactions and nucleic acid studies on Klebsiella pneumoniae from clinical and environmental sources. Appl Microbiol. 1975 Jun;29(6):819–825. doi: 10.1128/am.29.6.819-825.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Tomfohrde K. M., Rhoden D. L., Balows A. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl Microbiol. 1972 Sep;24(3):449–452. doi: 10.1128/am.24.3.449-452.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Shah V. K., Brill W. J. Regulation of nitrogenase synthesis by oxygen in Klebsiella pneumoniae. J Bacteriol. 1974 Jul;119(1):266–269. doi: 10.1128/jb.119.1.266-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubb R. S., Postgate J. R. Control of nitrogenase synthesis in Klebsiella pneumoniae. J Gen Microbiol. 1973 Nov;79(1):103–117. doi: 10.1099/00221287-79-1-103. [DOI] [PubMed] [Google Scholar]
- Warskow A. L., Juni E. Nutritional requirements of Acinetobacter strains isolated from soil, water, and sewage. J Bacteriol. 1972 Nov;112(2):1014–1016. doi: 10.1128/jb.112.2.1014-1016.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Pengra R. M. Effect of amino acids on the nitrogenase system of Klebsiella pneumoniae. J Bacteriol. 1966 Sep;92(3):618–622. doi: 10.1128/jb.92.3.618-622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


