Abstract
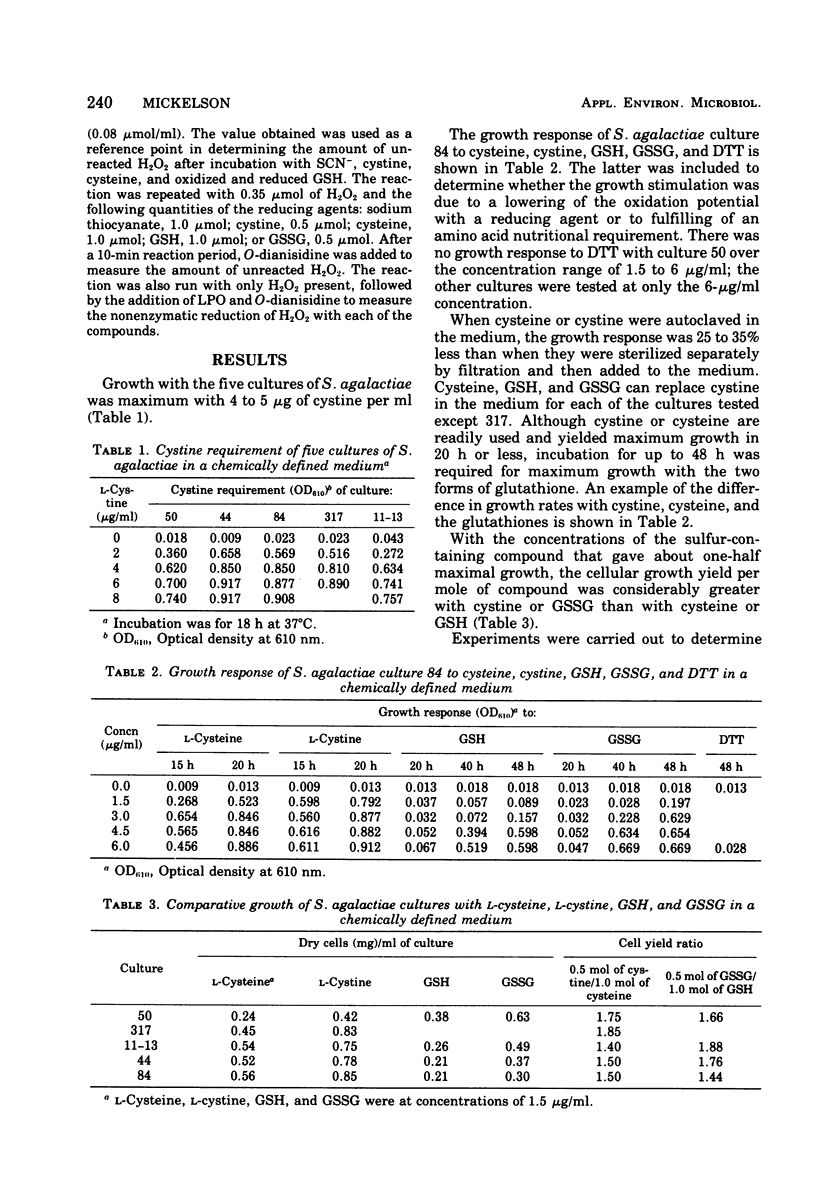
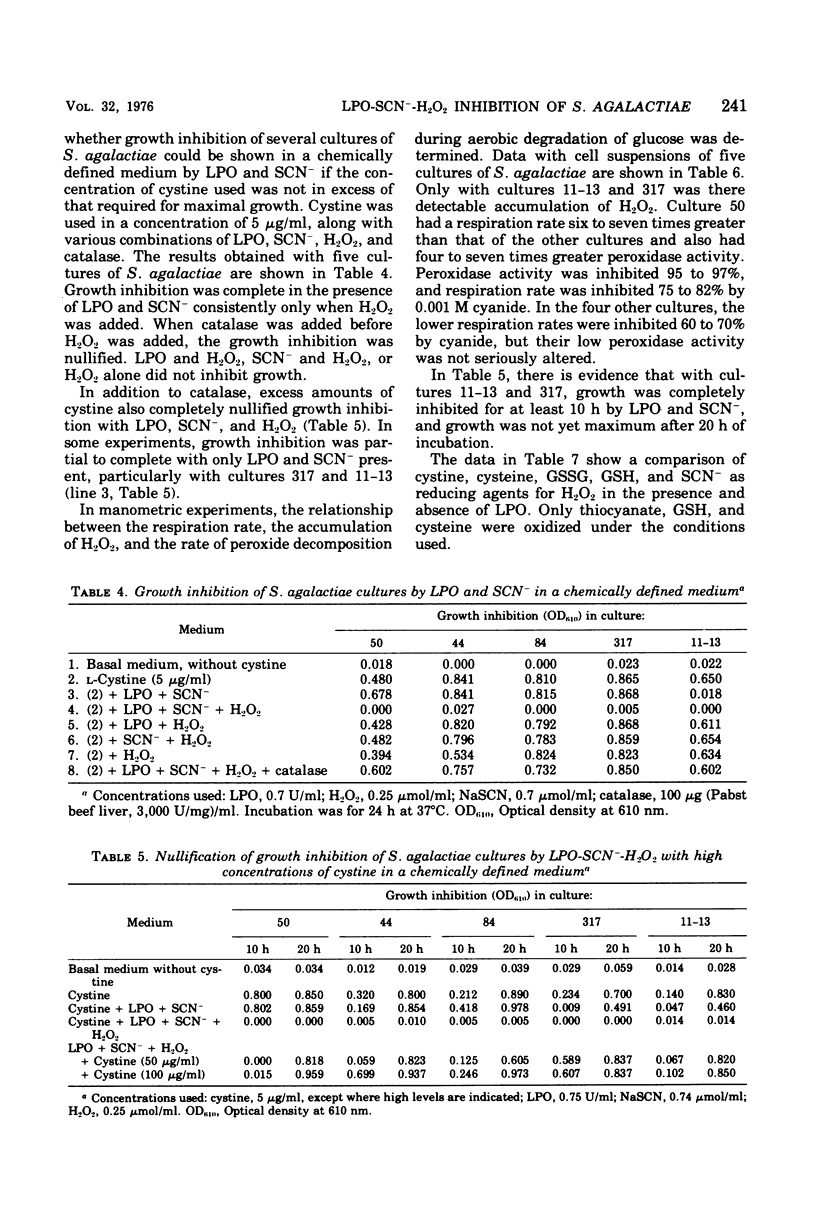
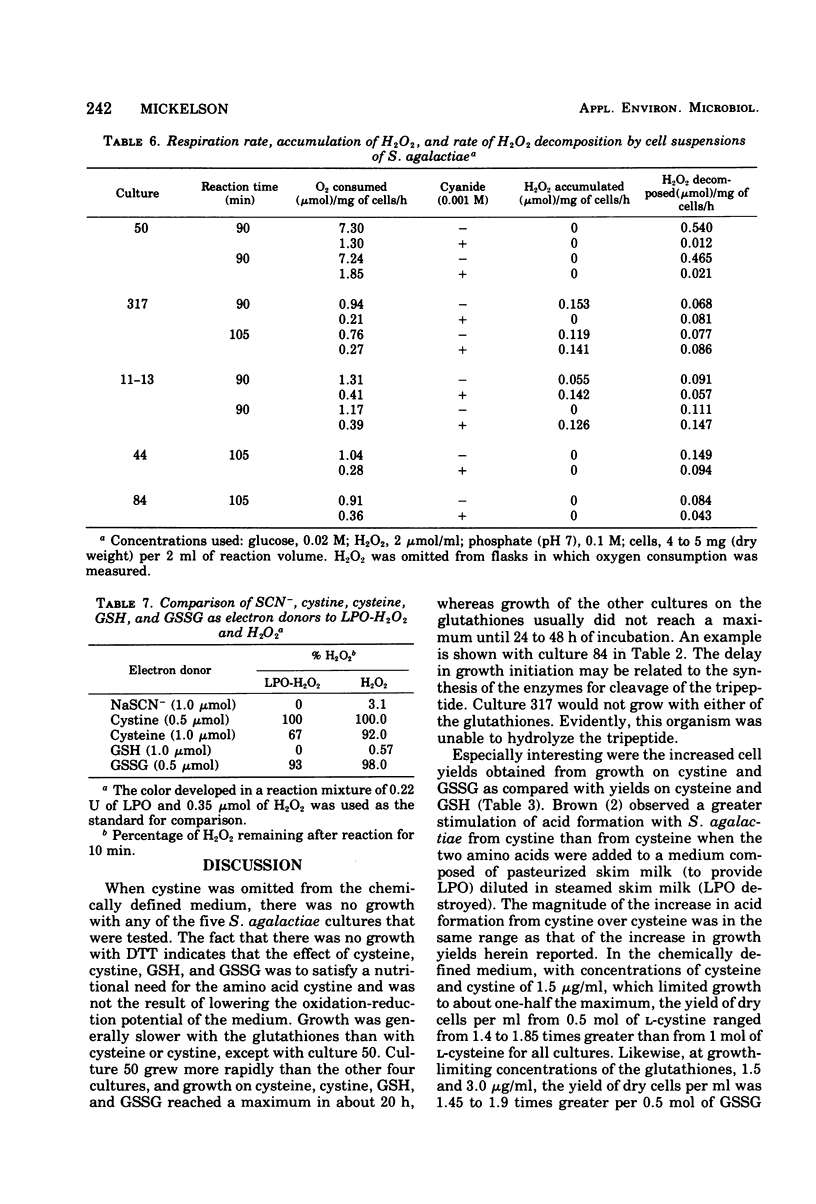
Five cultures of Streptococcus agalactiae have an absolute requirement for L-cystine to grow in a chemically defined medium. The L-cystine could be replaced with cysteine, glutathione, or the disulfide form of glutathione. Dithiothreitol could not substitute for the sulfur-containing amino acids of glutathione; hence, the growth requirement appears to be truly nutritional. Growth was maximum with 4 to 5 mug of L-cystine per ml. If the concentration of L-cystine was no greater than 4 to 5 mug/ml, complete growth inhibition could be obtained by the addition of lactoperoxidase, thiocyanate, and H2O2. The growth inhibition, however, was nullified by additions of L-cystine 10-fold or more in excess of the concentration needed for maximum growth. During the aerobic degradation of glucose by cell suspensions, H2O2 accumulation could be shown with cultures 317 and 11-13, the only cultures the growth of which was inhibited without addition of exogenous H2O2. All of the cultures had varying degrees of peroxidase activity. The balance between H2O2 generation and peroxidase activity of the culture evidently determined whether growth could be inhibited with lactoperoxidase and thiocyanate without H2O2 addition. The growth yeilds per 0.5 mol of the disulfide forms (cystine and oxidized glutathione) were 1.5 and 1.9 times greater than that per 1 mol of the sulfhydryl forms (cysteine and glutathione).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björck L., Rosén C., Marshall V., Reiter B. Antibacterial activity of the lactoperoxidase system in milk against pseudomonads and other gram-negative bacteria. Appl Microbiol. 1975 Aug;30(2):199–204. doi: 10.1128/am.30.2.199-204.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. W. Compounds affecting Streptococcus agalactiae growth in milk. J Dairy Sci. 1974 Jul;57(7):797–802. doi: 10.3168/jds.S0022-0302(74)84967-2. [DOI] [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- Mickelson M. N. Effect of lactoperoxidase and thiocyanate on the growth of Streptococcus pyogenes and Streptococcus agalactiae in a chemically defined culture medium. J Gen Microbiol. 1966 Apr;43(1):31–43. doi: 10.1099/00221287-43-1-31. [DOI] [PubMed] [Google Scholar]
- Mickelson M. N. Glucose degradation, molar growth yields, and evidence for oxidative phosphorylation in Streptococcus agalactiae. J Bacteriol. 1972 Jan;109(1):96–105. doi: 10.1128/jb.109.1.96-105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Phosphorylation and the reduced nicotinamide adenine dinucleotide oxidase reaction in Streptococcus agalactiae. J Bacteriol. 1969 Nov;100(2):895–901. doi: 10.1128/jb.100.2.895-901.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem J. 1966 Aug;100(2):373–381. doi: 10.1042/bj1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem. 1969 Jan 10;244(1):9–16. [PubMed] [Google Scholar]
- Thompson G. A., Meister A. Utilization of L-cystine by the gamma-glutamyl transpeptidase-gamma-glutamyl cyclotransferase pathway. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1985–1988. doi: 10.1073/pnas.72.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON A. T., ROSENBLUM H. The antistreptococcal property of milk. II. The effects of anaerobiosis, reducing agents, thiamine, and other chemicals on lactenin action. J Exp Med. 1952 Jan;95(1):39–50. doi: 10.1084/jem.95.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett N. P., Morse G. E. Long-chain fatty acid inhibition of growth of Streptococcus agalactiae in a chemically defined medium. J Bacteriol. 1966 Jun;91(6):2245–2250. doi: 10.1128/jb.91.6.2245-2250.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


