Abstract
Neuropsychiatric systemic lupus erythematosus, which often entails cognitive disturbances and memory loss, has become a major complication for lupus patients. Previously, we developed a murine model of neuropsychiatric lupus based on Abs that cross-react with dsDNA and the NMDA receptor (NMDAR). We showed that these murine Abs impair cognition when they access the CNS through a breach in the blood–brain barrier (BBB) triggered by lipopolysaccharide. Because studies show that lupus patients possess anti-NMDAR Abs in their serum and cerebrospinal fluid, we decided to investigate whether these human Abs contribute to cognitive dysfunction. Here, we show that serum with reactivity to DNA and NMDAR extracted from lupus patients elicited cognitive impairment in mice receiving the serum intravenously and given lipopolysaccharide to compromise the BBB integrity. Brain histopathology showed hippocampal neuron damage, and behavioral testing revealed hippocampus-dependent memory impairment. To determine whether anti-NMDAR Abs exist in the brains of systemic lupus erythematosus patients, we eluted IgG from a patient's brain. The IgG bound DNA and NMDAR and caused neuronal apoptosis when injected into mouse brains. We examined four more brains of patients with neuropsychiatric lupus and found that they displayed endogenous IgG colocalizing with anti-NMDAR Abs. Our results indicate that lupus patients have circulating anti-NMDAR Abs capable of causing neuronal damage and memory deficit, if they breach the BBB, and that the Abs exist within patients' brains. Which aspects of neuropsychiatric lupus may be mediated by anti-NMDAR Abs, how often, and in which patients are now important clinical questions.
Keywords: brain-derived antibodies, neuropsychiatric systemic lupus erythematosus, neurotoxic antibodies
Neuropsychiatric lupus has become a prominent problem in patients with systemic lupus erythematosus (SLE) because they live longer due to improved therapy. Neuropsychiatric lupus is a complex set of syndromes, but cognitive impairment, manifested as a memory deficit, represents one of the most common symptoms (1–5). Certainly, multiple pathogenetic mechanisms underlie cognitive dysfunction in neuropsychiatric lupus, including medication, infarction, hypertension, and accelerated atherosclerosis. Because autoAbs clearly contribute to other organ injuries in SLE, we have been interested in their potential contribution to neuropsychiatric lupus. We have demonstrated that a subset of anti-DNA Abs binds a pentapeptide consensus sequence (D/E W E/D Y S/G, or DWEYS for short) present in the NR2A and NR2B subunits of the NMDA receptor (NMDAR) (6–8) but not in NR2C and NR2D. These Abs cross-react with both murine and human NMDAR, and they mediate neuronal death in vitro when added to cultures of fetal brain cells and in vivo when directly injected into a mouse brain (9). Additionally, NMDAR antagonists can protect neurons from Ab-mediated injury, confirming that the Abs function as receptor agonists. Fab′2 fragments of a monoclonal cross-reactive anti-DNA, anti-NMDAR Ab also mediate neuronal death when injected into a mouse brain; thus, there is no absolute requirement for complement activation or for the engagement of Fc receptors on Fc receptor-bearing cells in the brain (9).
Mice immunized with a multimeric form of the DWEYS consensus sequence produce anti-DNA, anti-NMDAR Abs. The mice exhibit no neuronal damage while the blood–brain barrier (BBB) is intact; however, the Abs mediate neuronal death following a breach in the BBB. When the breach is induced by systemic exposure to bacterial LPS, there is preferential death of hippocampal neurons and significant deficit in memory tasks (10). When the BBB is breached by systemic exposure to epinephrine, targeted death of neurons in the basolateral amygdala and disturbances in fear-conditioning tasks results (11). Both brain regions exhibit high-density expression of NMDARs containing NR2A and NR2B subunits, and we have proposed that each agent causes regionally specific vascular permeability leading to regionally specific neuronal damage. We cannot formally exclude a synergy between LPS and Abs in the hippocampus and epinephrine and Abs in the amygdala, but we doubt the possibility of a synergy because Abs directly injected into the brain in the absence of either LPS or epinephrine cause neuronal death. These studies provide a model for aspects of neuropsychiatric lupus and suggest that Ab-mediated neuronal damage in SLE requires both appropriate anti-neuronal Abs and a transient breach in the BBB. Given that animal models of disease represent, at best, approximations to human conditions, we wished to move beyond our original study and test whether human lupus Abs, when present in the circulation of a mouse, can mediate brain damage if permitted access to brain tissue. We also tested whether anti-DNA, anti-NMDAR Abs with pathogenic potential could be identified and isolated from the brains of SLE patients.
Results
Mice with Human SLE Serum Containing Anti-DNA, Anti-NMDAR Abs Display Neuronal Damage in the Hippocampus When Given LPS.
We investigated whether human serum harboring anti-DNA, anti-NMDAR Abs was capable of causing hippocampal dysfunction in mice under physiologic conditions in which the Abs in the serum can penetrate the brain. We chose LPS administration to breach the BBB because, in our strictly murine model, this treatment preferentially caused hippocampal damage and memory loss, a condition that occurs frequently in SLE patients. In addition, damage in the hippocampus lends itself to histological and functional analysis. If hippocampal damage was mediated by human SLE serum, it would imply that the serum is toxic without the need to concentrate the anti-NMDAR-specific Abs, isolate them from other serum IgG, or dissociate them from preexisting immune complexes.
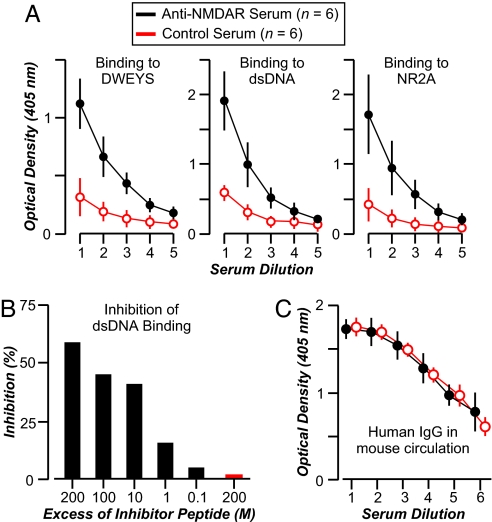
We transferred lupus serum samples (100 μl i.v.) with anti-DNA, anti-NMDAR reactivity to BALB/c mice (n = 5, for each of two sera). Both sera bound DNA, DWEYS, and the extracellular domain of human NR2A (Fig. 1A). The DNA reactivity was blocked by soluble DWEYS (60–70% inhibition) present at 200-fold molar excess to IgG (Fig. 1B), confirming the cross-reactivity of the Abs. These results were consistent with our previous studies showing that DNA binding in SLE sera was blocked by DWEYS (15–90% inhibition) (12). An irrelevant peptide, composed of negatively charged amino acids similar to DWEYS, failed to inhibit DNA binding (Fig. 1B). As control experiments, the human sera from a nonautoimmune individual and from an SLE patient lacking reactivity to DNA, DWEYS, and NR2A were given to mice (n = 5; Fig. 1A). All mice were given LPS to breach the BBB. We obtained sera from mice (n = 2 from each group of five) after LPS administration; the sera displayed equivalent concentrations of human IgG (≈400 μg/ml), demonstrating a similar half-life of human IgG in all mice, those receiving serum with specific Abs and those lacking specific Abs (Fig. 1C).
Fig. 1.
Anti-DNA-, anti-DWEYS-, anti-NMDAR-specific Abs in human SLE serum. (A) Serum with anti-NMDAR reactivity displays strong binding to dsDNA, DWEYS, and NR2A, whereas control serum without specific reactivity does not bind. The graphs represent titrations of reactive serum (●; two samples assayed in triplicate) and control serum (○; two samples assayed in triplicate) in serial dilutions, starting at 1:125 (dilution 1) and decreasing 2-fold each. (B) The graph shows that dsDNA binding by serum cross-reactive with DWEYS is inhibited by the soluble DWEYSNVWLSN peptide (black bars), assayed from 200 M to 0.02 M excess to Ab, but not by the control DYENLREHRR peptide (red bar) at 200 M excess. (C) Mice injected with anti-NMDAR serum (●; n = 4) show levels of human IgG in their circulation similar to mice injected with control serum lacking anti-NMDA Ab (○; n = 4). IgG concentration was measured 48 h after LPS injection, before brain perfusion, with an ELISA. Serial dilutions of mouse serum were assayed, starting from 1:1,000 (dilution 1) and decreasing 2-fold each. All values represent the mean ± SD.
We and others have shown that LPS administration to mice (by i.p. injection) allows serum IgG to enter the brain but does not itself cause a lasting inflammation within the brain (13–19). It has been reported that there is an activation of resident inflammatory cells after LPS injection directly into the brain that peaks at 12 h after LPS administration and diminishes thereafter (20). We have not detected any activation of resident inflammatory cells in the brain or infiltration of blood-borne inflammatory cells at 24 h after systemic (i.p.) LPS treatment.
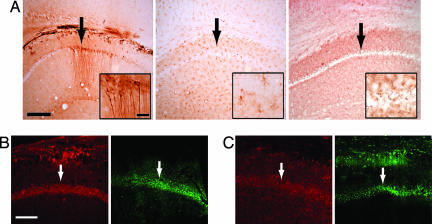
To determine whether human lupus Abs could damage hippocampal neurons, mice harboring the human serum described in Fig. 1 were given LPS (two i.p. injections) to allow human IgG access to brain tissue. LPS given in this way induces a 3- to 4-fold increase in IgG in a mouse brain (C.K. and B.D., unpublished observation). The brains of all animals showed diffuse staining for human IgG, but brains of mice given sera with high-titered reactivity to DNA and NMDAR had selective binding of human IgG to hippocampal neurons (Fig. 2A). There was evidence of neuronal damage assessed by the presence of activated caspase-3, an indicator of preapoptotic cells, and by fluorojade reactivity, an indicator of stressed neurons (Fig. 2B). Thus, there was sufficient titer of anti-NMDAR Abs in the lupus serum to mediate hippocampal neuron damage, even when diluted ≈20- to 30-fold (100 μl into 2–3 ml of blood volume in the mouse). To confirm that IgG was the neurotoxic substance in the SLE serum with high-titered reactivity against DNA, DWEYS, and NMDAR, we purified the IgG fraction and injected the IgG into mice (i.v., 2 mg in 100 μl of saline, followed by LPS). The IgG bound hippocampal neurons and caused neuronal damage (Fig. 2C), confirming that Abs were a causative agent for the neurotoxicity of SLE serum. Finally, to demonstrate that the neurotoxic Abs were those reactive to DWEYS, the toxic serum was depleted of DWEYS reactivity on a peptide affinity column. This serum failed to cause neuronal damage (Fig. 2A). Thus, only the anti-NMDAR Ab was responsible for hippocampal neuron death, and serum lacking anti-DWEYS reactivity had no detectable neurotoxicity.
Fig. 2.
Neurotoxicity of serum containing anti-DNA, anti-DWEYS Ab. (A) (Left) Upon accessing the mouse brain after LPS treatment, we found that serum from an SLE patient with high-titered Abs to DNA and DWEYS bound CA1 neurons in the hippocampus, as revealed by anti-IgG staining. (Center and Right) Control serum lacking anti-NMDAR Ab (Center) and neurotoxic serum depleted of DWEYS reactivity (Right) were diffusely present but did not bind to CA1 neurons. (Scale bars: 800 μm; Inset, 100 μm.) (B) Neurons from mice given serum with anti-DWEYS reactivity and LPS treatment showed activated caspase-3 (red signal, Left) and fluorojade reactivity (green signal, Right). (Scale bar: 600 μm.) (C) Injection of purified IgG (2 mg in 100 μl of saline) from serum with high-titered anti-DNA anti-DWEYS Ab followed by LPS treatment reveals that the IgG bound hippocampal neurons and caused neuronal damage, as shown by activated caspase-3 (Left) and fluorojade (Right). Arrows identify the CA1 region of the hippocampus.
Mice with Human SLE Serum Containing Anti-DNA, Anti-NMDAR Abs Display Flexible Memory Deficit.
Mice were given SLE serum or nonautoimmune serum followed by LPS, and they were subjected to behavioral testing 1 month after LPS administration (21–27). We divided the animals into a first set that was injected with serum with high-titered anti-NMDAR Abs (n = 6; three mice with each of two sera), and a second set with serum lacking anti-NMDAR Abs (n = 6; three mice with serum from normal individuals and three mice with SLE serum lacking anti-NMDAR reactivity). Both groups had comparable weights; fed, groomed, explored the cage environment normally; and had similar reflexes, strength, basal muscle tone, sensorimotor skills, and anxiety (data not shown) (23, 25). Based on our previous results (10, 27, 28), we expected that mice with high-titered anti-NMDAR serum would be deficient in hippocampus-dependent tasks. To examine this hypothesis, the mice were subjected to a series of cognitive tests: a response learning task that depends on the striatum, a place learning task, and a reference memory task that are mildly dependent on hippocampal and cortical function, and a flexible memory task that is highly dependent on the hippocampus (10, 22).
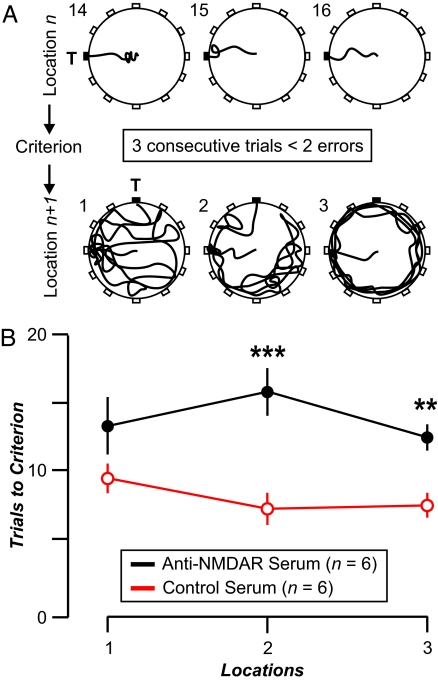
Mice given high-titered anti-NMDAR serum were impaired in the flexible memory task (22, 24, 26) (anti-NMDAR serum, 13.9 ± 4.1; control serum, 8.1 ± 2.5 trials-to-criterion; mean ± SEM; P = 0.001) (Fig. 3). Conversely, mice given high-titered anti-NMDAR serum reached similar performance levels as the control mice in the response learning task, the place learning task, and the reference memory task (results not shown). Thus, we have demonstrated that human SLE Abs, when present in the systemic circulation of mice and accorded access to brain tissue by systemic LPS treatment, can cause a selective cognitive impairment. These results confirmed observations obtained in mice immunized to produce murine anti-NMDAR Ab (10). We cannot eliminate the possibility that LPS sensitized neurons to Ab-mediated injury, but clearly LPS together with IgG that lacks specificity for the NMDAR failed to cause neuronal damage.
Fig. 3.
Memory impairment in mice injected with serum containing anti-NMDAR Abs and given LPS. The animals show a deficit in the flexible memory task that requires them to find sequential escape targets in the wet maze. Each target is in a fixed position until the mouse finds it with less than two errors in each trial for three consecutive trials, then a new target position is selected. (A) Each circle depicts a top view of the paddling maze with water added to a shallow depth (2 cm). Holes are located in the perimeter, but only the target (T) offers escape. In each diagram, the navigation paths (thick lines) correspond to a mouse (with serum containing anti-NMDAR Ab) moving from the start (center of maze) to the target. (Upper) Trials 14, 15, and 16 (numbers at top left) with the target at 9:00; the mouse finds it with no errors in each trial, thus reaching criterion. (Lower) Trials 1, 2, and 3 with the target in a new location (12:00). It is clear that the errors increase dramatically and that the animal makes numerous visits to the old target, which is now incorrect. (B) Spatial flexibility, measured as trials to criterion, for mice in locations 1 (P = 0.108), 2 (P = 0.004), and 3 (P = 0.01). ∗∗, P < 0.01; ∗∗∗, P < 0.001; Mann–Whitney U tests. Overall effect from all locations, P = 0.001.
Anti-DNA, Anti-NMDAR Abs Exist Inside the Brains of SLE Patients.
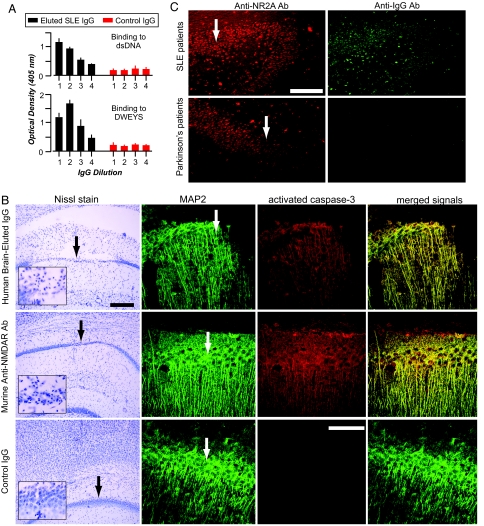
The brain from a lupus patient who had experienced progressive and profound cognitive impairment was obtained as frozen tissue, permitting us to elute the IgG present within the parenchyma and purify it on a Protein G Sepharose column. We obtained IgG (≈270 μg) from 2 g of brain tissue. Although the brain was not perfused before IgG elution, the IgG/albumin ratio in the eluate was >15:1, indicating that the eluted IgG was derived from IgG already in the brain parenchyma premortum and was not the result of postmortem contamination by serum IgG. Starting at a concentration of 5 μg/ml and serially diluted to 0.67 μg/ml, the eluted IgG bound DNA and DWEYS (Fig. 4A). Crucially, this Ab mediated neuronal damage when injected directly into a mouse brain (10 μg in 2 μl) (Fig. 4B). Thus, Ab that binds NMDAR is present in the brains of SLE patients and is capable of mediating neuronal damage. To examine whether anti-NMDAR Abs were present in brains of other SLE patients, we obtained four brains postmortem from individuals who exhibited various neuropsychiatric manifestations. Three brains had cognitive impairment and one had depression. It is important to note, however, that each patient had multiple potential causes for neuropsychiatric lupus. For example, two of the patients had seizures, one had malignant cerebral edema, and one had cerebral infarcts. In all four specimens, human IgG was bound to neuronal cell bodies and the IgG colocalized with rabbit Ab to NR2A and NR2B (Fig. 4C). The presence of endogenous IgG Ab was not detected in brains of patients with Parkinson's disease (n = 2) (Fig. 4C).
Fig. 4.
IgG with NR2A reactivity in postmortem brains of SLE patients. (A) The eluted IgG from lupus brain exhibits anti-DNA, anti-DWEYS reactivity. The graphs represent titrations of IgG binding by serial dilutions, starting at 5 μg/ml (dilution 1) and decreasing 2-fold each. (B) Eluted anti-DNA, anti-DWEYS Ab kills neurons. (Top and Middle) IgG (5 μg/2 μl) eluted from the brain of an SLE patient provokes widespread neuronal death when injected into the CA1 region of a mouse hippocampus (Top), similar to that of R4A, a murine monoclonal Ab that binds DNA and DWEYS (Middle). (Bottom) Injection of normal IgG causes no CA1 damage. (Scale bars: Top, 300 μm; Bottom, 150 μm.) MAP2 (green signal) labels neurons, and activated caspase-3 (red signal) identifies neurons destined to die. (C) Neurons in the hippocampus of SLE patients (n = 4) demonstrate colocalized immunoreactivity with Abs against the NR2A subunit (red signal) and human IgG (green signal). Hippocampal neurons from Parkinson's disease patients (n = 3) demonstrate immunoreactivity for the NR2A subunit but lack colocalizing endogenous Ab. Arrows identify the CA1 region of the hippocampus. (Scale bar, 300 μm.)
Discussion
There are many potential causes of cognitive impairment in SLE. Although anti-phospholipid Abs can cause cerebral infarcts, studies attempting to correlate cognitive impairment and anti-phospholipid Abs have yielded inconsistent results. Clearly, Abs of this specificity are not present in all SLE patients who experience a cognitive decline (29–34). Abs to ribosomal P-protein appear to correlate with lupus psychosis in some studies (35–38) but not in others (39). There is literature suggesting that inflammatory cytokines are neurotoxic in vitro, although no correlation between cognitive loss and cerebrospinal fluid cytokines has been demonstrated in SLE (40, 41). Glucocorticoids may be neurotoxic, especially to hippocampal neurons, and the cytotoxic drugs used to treat lupus flares cause impaired cognition when used at higher doses, such as in cancer chemotherapy (3, 5, 42–45). Yet many SLE patients experience cognitive decline that is not associated with cytotoxic therapy or the use of high doses of corticosteroids. This impairment occurs in the absence of anti-phospholipid Abs or any evidence of inflammation in the CNS.
We previously established a mouse model in which murine anti-DNA, anti-NMDAR cross-reactive Abs were shown to cause neuronal death and consequent cognitive dysfunction (9, 10, 28). CNS dysfunction required specific Abs and an LPS-induced breach in the BBB. In contrast, epinephrine administration in mice harboring murine anti-DNA, anti-NMDAR Abs led to the death of amygdala neurons and a behavioral deficit that did not include memory dysfunction (11). Presumably, this reflects the fact that epinephrine causes a preferential increase in blood flow to the amygdala (46–48). Thus, the neuropsychiatric dysfunction mediated by anti-DNA, anti-NMDAR Abs in mice depended on the agent used to compromise the BBB and the regional specificity of the neuropathological damage.
The current study clearly shows that human anti-DNA, anti-NMDAR Ab concentrations present in the serum of SLE patients are capable of damaging neurons. Our results, however, do not reveal the frequency with which anti-DNA, anti-NMDAR Abs might penetrate the BBB in SLE patients or how often they bind hippocampal neurons and mediate memory impairment or contribute to other manifestations of neuropsychiatric lupus. Moreover, our current study shows that anti-NMDAR Abs are found in lupus brain, implying that breaches of the BBB occur in SLE patients, even in those without a history of inflammatory events in the CNS. Our previous studies suggest that neuronal death can occur with no contribution of the complement cascade or Fc receptor engagement. We do not yet know whether in this physiologic model those pathways might contribute to neuronal damage.
Many factors can disrupt the BBB, such as infection (as modeled in this study), stress, hypertension, or nicotine exposure (49–52). Our current results highlight the importance of maintaining the integrity of the BBB through, perhaps, such approaches as strict control of blood pressure, smoking cessation, and stress management. Our work also suggests that the BBB may be a crucial therapeutic target for preventing CNS damage from autoAbs.
This study represents an example of Ab-mediated cognitive change in a noninflamed brain. Abs specific for DNA and NMDAR have been identified in the serum of 33–50% of SLE patients (12, 53–55). Two reports have recently asked whether their presence in serum correlates with cognitive impairment. In one study, there was a significant correlation of anti-NMDAR Ab memory loss and depression (54), whereas in the other study there was little evidence of cognitive impairment but there was a correlation with depression (53). It is not, however, our hypothesis that there will be a correlation between serum Abs and CNS symptoms. To the contrary, in addition to serum Ab titers, there needs to be an insult that compromises the BBB, allowing the Ab access to the brain. Interestingly, anti-DNA, anti-NMDAR Abs have been found in the cerebrospinal fluid of SLE patients (9). In a recent study, their presence in the cerebrospinal fluid correlated with manifestations of neuropsychiatric lupus. Patients with the highest titer of Ab in the cerebrospinal fluid had the most severe manifestations (55). Additionally, successful therapy led to a decrease of Ab titer in the cerebrospinal fluid (55). This latter observation is of particular interest because neuropsychiatric symptoms in SLE can be progressive or can improve. In our studies, the anti-DNA, anti-NMDAR Abs clearly cause neuronal death. We do not yet know whether these Abs can sometimes cause nonlethal, reversible damage to neurons and, if so, under what conditions. In addition, we do not know the potential role of neurogenesis recently found in the hippocampus of adult rodents and humans (56–58).
It is a virtual certainty that there are other autoAbs capable of functioning as neuronal receptor agonists or antagonists once they gain access to brain tissue. These autoAbs may be important in many clinical situations. In SLE patients with Abs cross-reactive with DNA and the NMDAR, the Abs may contribute to neuropsychiatric symptoms. It will be crucial to determine how frequently this occurs and what constitutes the major insults to the integrity of the BBB in lupus patients to permit these Abs access to neurons.
Materials and Methods
Animals.
Six- to 8-week-old BALB/c female mice from The Jackson Laboratory (Bar Harbor, ME) were used in all experiments and were treated in accordance with institutional animal care and use committees.
Purification of Serum IgG.
The IgG fraction of serum was purified by the use of a Protein G column (Amersham Biosciences, Uppsala, Sweden). Elution was performed by using 0.1 M glycine (pH 2.5) and 0.15 M sodium chloride. Fractions were neutralized with 2 M Tris·HCl (pH 9.0), tested for IgG concentration and for reactivity to dsDNA and to DWEYS by ELISA.
Purification of IgG from Frozen Brain Tissue.
Brain tissue was homogenized in sucrose lysis buffer as described (59). IgG from the soluble fraction was purified on a Protein G column and extensively dialyzed against normal saline. For direct injection to the brain, the final concentration of IgG fraction was 5 mg/ml. For comparison to IgG eluted from brain, normal serum IgG was subjected to the same elution buffers as brain IgG.
Absorption of Serum.
Serum with high-titered, anti-NMDAR activity was passaged through a peptide affinity column (9); specific Ab depletion was confirmed by ELISA with DWEYS.
ELISAs.
Assays were performed as described (10). Antigens were adsorbed onto Costar plates (no. 3690; Costar, Corning, NY) in 0.1 M NaHCO3 (pH 8.6) overnight at 4°C for DWEYS and NR2A (both at 15 μg/ml) or overnight at 37°C for calf thymus dsDNA (100 μg/ml). Serum was assayed beginning at a 1:125 dilution. NR2A was the recombinant human subunit (which included only the 550 aa comprising the extracellular domain of the protein). The inhibition ELISA was performed as described above on serum diluted 1:250 and was preincubated with dilutions of specific peptide (DWEYSVWLSN) or irrelevant peptide (DYENLREHRR) starting at 72 μg in 25 μl (a 200-fold M excess of inhibitor to IgG).
Animal Treatment.
Human serum (100 μl), purified IgG fraction (2 mg in 100 μl of saline), or serum depleted of DWEYS reactivity (100 μl) was injected (i.v.) into mice; 15 min later, LPS was injected (i.p., 3 mg/kg). The LPS treatment was performed twice, 48 h apart. IgG purified from human brain was injected stereotaxically into mouse hippocampus (5 μg/2 μl) according to previous protocols (9).
Immunohistology.
Mouse brains were obtained after perfusion of the mice 48 h after the second LPS injection (9, 10). To assess IgG deposition, sections were incubated in biotinylated horse anti-human IgG at a 1:200 dilution in 0.1 M PBS (pH 7.4) for 1 h (Vector Laboratories, Burlingame, CA) and avidin–biotin horseradish peroxidase complex at a 1:100 dilution for 1 h. Fluorojade-B and activated caspase-3 staining was performed as described (9–11). Human brains were fixed in 10% neutral-buffered formalin. Sections were prepared from specific brain regions.
Behavioral Assays.
An experimental group (n = 6, high-titered anti-DWEYS human serum, LPS-treated) and a control group (n = 6, SLE serum without anti-DWEYS activity or normal serum, LPS-treated) were studied. Mice underwent the following tests in sequential order: behavioral screen, response task, place task, reference memory task, and flexible memory task (10, 11). The apparatus for memory testing consisted of a circular paddling maze filled with water (20°C) to 2-cm depth, sufficient to wet the underside of the belly of mice (24). For reference memory, we sealed 11 escape holes, leaving one hole that led to the exit pipe (“the target”). The time to find the target and the number of visits to decoys were recorded. An error was defined as passing within half a body length from any decoy hole. A mouse was given 60 sec to find the exit. Mice received five trials per day with an interval of ≈10 min between trials, for a total of 20 trials. For the hippocampus-dependent flexible memory task, a mouse was required to find the target in a fixed location. However, mice needed to find three consecutive locations in the maze. Each animal was trained for up to eight trials per day to a performance criterion of three successive trials with one or no errors before transfer to the next location on the next day (maximum number of trials per location was 32).
Acknowledgments
We thank the families of lupus patients who donated the brain tissue used in this study. This work was supported by the National Institutes of Health, the Lupus Research Institute, and the Burke Medical Research Institute.
Abbreviations
- BBB
blood–brain barrier
- NMDAR
N-methyl-d-aspartate receptor
- SLE
systemic lupus erythematosus.
Footnotes
The authors declare no conflict of interest.
References
- 1.The American College of Rheumatology. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, Valdez CR, Escalante A, del Rincon I, Gronseth G, et al. Neurology. 2002;58:1214–1220. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 3.Hanly JG, Fisk JD, Sherwood G, Jones E, Jones JV, Eastwood B. J Rheumatol. 1992;19:562–567. [PubMed] [Google Scholar]
- 4.Jennekens FG, Kater L. Rheumatology (Oxford) 2002;41:619–630. doi: 10.1093/rheumatology/41.6.619. [DOI] [PubMed] [Google Scholar]
- 5.Kozora E, Thompson LL, West SG, Kotzin BL. Arthritis Rheum. 1996;39:2035–2045. doi: 10.1002/art.1780391213. [DOI] [PubMed] [Google Scholar]
- 6.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Proc Natl Acad Sci USA. 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz JB, Limpanasithikul W, Diamond B. J Exp Med. 1994;180:925–932. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. J Exp Med. 1991;173:287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 10.Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, Volpe BT. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Huerta PT, Kowal C, Degiorgio LA, Volpe BT, Diamond B. Proc Natl Acad Sci USA. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A, Isenberg D, Diamond B. Rheumatology (Oxford) 2003;42:453–463. doi: 10.1093/rheumatology/keg161. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA, Kastin AJ, Brennan JM, Vallance KL. Exp Neurol. 1999;156:165–171. doi: 10.1006/exnr.1998.7011. [DOI] [PubMed] [Google Scholar]
- 14.Nonaka N, Hileman SM, Shioda S, Vo TQ, Banks WA. Brain Res. 2004;1016:58–65. doi: 10.1016/j.brainres.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 15.Nonaka N, Shioda S, Banks WA. Exp Neurol. 2005;191:137–144. doi: 10.1016/j.expneurol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Xaio H, Banks WA, Niehoff ML, Morley JE. Brain Res. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- 17.Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Peptides. 2002;23:2223–2226. doi: 10.1016/s0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 18.Ivey NS, Martin EN, Jr, Scheld WM, Nathan BR. J Neurosci Methods. 2005;142:91–95. doi: 10.1016/j.jneumeth.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. J Clin Invest. 1988;82:1339–1346. doi: 10.1172/JCI113736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadeau S, Rivest S. J Immunol. 2002;169:3370–3381. doi: 10.4049/jimmunol.169.6.3370. [DOI] [PubMed] [Google Scholar]
- 21.Chang Q, Gold PE. Behav Brain Res. 2003;144:19–24. doi: 10.1016/s0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 23.Crawley JN. What's Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. New York: Wiley; 2000. [Google Scholar]
- 24.Deacon RM, Rawlins JN. Behav Neurosci. 2002;116:472–478. doi: 10.1037//0735-7044.116.3.472. [DOI] [PubMed] [Google Scholar]
- 25.Irwin S. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 26.Kesner RP, Rogers J. Neurobiol Learn Mem. 2004;82:199–215. doi: 10.1016/j.nlm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Tsien JZ, Huerta PT, Tonegawa S. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 28.Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. Proc Natl Acad Sci USA. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowther MA, Wisloff F. Thromb Res. 2005;115:3–8. doi: 10.1016/j.thromres.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 30.Hanly JG. Rheum Dis Clin North Am. 2005;31:273–298. doi: 10.1016/j.rdc.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Menon S, Jameson-Shortall E, Newman SP, Hall-Craggs MR, Chinn R, Isenberg DA. Arthritis Rheum. 1999;42:735–741. doi: 10.1002/1529-0131(199904)42:4<735::AID-ANR17>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 32.Sastre-Garriga J, Montalban X. Lupus. 2003;12:877–882. doi: 10.1191/0961203303lu496oa. [DOI] [PubMed] [Google Scholar]
- 33.Scolding NJ, Joseph FG. Neuropathol Appl Neurobiol. 2002;28:173–189. doi: 10.1046/j.1365-2990.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 34.Waterloo K, Omdal R, Husby G, Mellgren SI. Rheumatology (Oxford) 2002;41:411–415. doi: 10.1093/rheumatology/41.4.411. [DOI] [PubMed] [Google Scholar]
- 35.Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, Brot N, Elkon KB. N Engl J Med. 1987;317:265–271. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- 36.Eber T, Chapman J, Shoenfeld Y. Lupus. 2005;14:571–575. doi: 10.1191/0961203305lu2150rr. [DOI] [PubMed] [Google Scholar]
- 37.Reichlin M. Clin Exp Med. 2006;6:49–52. doi: 10.1007/s10238-006-0094-7. [DOI] [PubMed] [Google Scholar]
- 38.Bluestein HG, Zvaifler NJ. J Clin Invest. 1976;57:509–516. doi: 10.1172/JCI108303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karassa FB, Afeltra A, Ambrozic A, Chang DM, De Keyser F, Doria A, Galeazzi M, Hirohata S, Hoffman IE, Inanc M, et al. Arthritis Rheum. 2006;54:312–324. doi: 10.1002/art.21539. [DOI] [PubMed] [Google Scholar]
- 40.Hirohata S, Tanimoto K, Ito K. Clin Immunol Immunopathol. 1993;66:225–229. doi: 10.1006/clin.1993.1029. [DOI] [PubMed] [Google Scholar]
- 41.Svenungsson E, Andersson M, Brundin L, van Vollenhoven R, Khademi M, Tarkowski A, Greitz D, Dahlstrom M, Lundberg I, Klareskog L, Olsson T. Ann Rheum Dis. 2001;60:372–379. doi: 10.1136/ard.60.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginsburg KS, Wright EA, Larson MG, Fossel AH, Albert M, Schur PH, Liang MH. Arthritis Rheum. 1992;35:776–782. doi: 10.1002/art.1780350711. [DOI] [PubMed] [Google Scholar]
- 43.Hay EM, Huddy A, Black D, Mbaya P, Tomenson B, Bernstein RM, Lennox Holt PJ, Creed F. Ann Rheum Dis. 1994;53:298–303. doi: 10.1136/ard.53.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papero PH, Bluestein HG, White P, Lipnick RN. Clin Exp Rheumatol. 1990;8:417–424. [PubMed] [Google Scholar]
- 45.Sapolsky RM. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 46.Johansson BB. In: Implications of the Blood–Brain Barrier and Its Manipulation. Neuwelt EA, editor. Vol 2. New York: Plenum; 1989. pp. 389–410. [Google Scholar]
- 47.Sokrab TE, Johansson BB, Tengvar C, Kalimo H, Olsson Y. Acta Neurol Scand. 1988;77:387–396. doi: 10.1111/j.1600-0404.1988.tb05924.x. [DOI] [PubMed] [Google Scholar]
- 48.Tuor UI, Edvinsson L, McCulloch J. Am J Physiol. 1986;251:H824–H833. doi: 10.1152/ajpheart.1986.251.4.H824. [DOI] [PubMed] [Google Scholar]
- 49.Abdel-Rahman A, Shetty AK, Abou-Donia MB. Neurobiol Dis. 2002;10:306–326. doi: 10.1006/nbdi.2002.0524. [DOI] [PubMed] [Google Scholar]
- 50.Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC. J Pharmacol Exp Ther. 2002;303:1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- 51.Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 52.Hawkins BT, Davis TP. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 53.Lapteva L, Nowak M, Yarboro CH, Takada K, Roebuck T, Weickert T, Bleiberg J, Rosenstein D, Pao M, Volpe B, et al. Arthritis Rheum. 2006;54:2505–2514. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 54.Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R, Mellgren SI. Eur J Neurol. 2005;12:392–398. doi: 10.1111/j.1468-1331.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 55.Yoshio T, Onda K, Nara H, Minota S. Arthritis Rheum. 2006;54:675–678. doi: 10.1002/art.21547. [DOI] [PubMed] [Google Scholar]
- 56.Altman J, Das GD. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 57.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Nat Med. 1998;4:1313–1337. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 58.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owens GP, Burgoon MP, Devlin ME, Gilden DH. J Virol Methods. 1997;68:119–125. doi: 10.1016/s0166-0934(97)00118-3. [DOI] [PubMed] [Google Scholar]