Abstract
Stimulus fading in the form of gradually increased exposure to a fear-evoking stimulus, often combined with differential reinforcement, has been used to treat phobias in children who are otherwise normal and in children with autism. In this investigation, we applied stimulus fading plus differential reinforcement with an adolescent with autism and diabetes whose needle phobia had prevented medical monitoring of his blood glucose levels for over 2 years. Results showed that the treatment was successful in obtaining daily blood samples for measuring glucose levels.
Keywords: autism, diabetes, fading, medical non-compliance, needle phobia, systematic desensitization
The serious and sometimes deadly complications of diabetes (e.g., kidney failure, blindness) can often be mitigated through appropriate diet in combination with insulin therapy, which requires regular blood drawings to monitor glucose levels (Davidson, 2004). Medical treatments that require regular blood drawings are compromised when a child presents with needle phobia (or phlebophobia; Zambanini & Feher, 1997) and can be compromised further when the child also has a developmental disability like autism (Love, Matson, & West, 1990). In fact, when individuals with autism show fear or avoidance reactions to medical procedures (e.g., dental examination), medical professionals have sometimes found it necessary to employ restrictive procedures such as sedation or general anesthesia (e.g., Braff & Nealon, 1979), which pose additional risks to the patient (cf. McDowell, Scher, & Barst, 1995).
Behavioral treatment of childhood phobias often involves stimulus fading in the form of gradually increased exposure to a fear-evoking stimulus (designed to produce extinction of the fear response), as well as differential reinforcement of (a) approach responses (DRA) or (b) the absence of an avoidance response (DRO). Treating needle phobia with behavioral techniques may have the advantage of not only reducing the child's fear but also of facilitating treatment of the child's medical condition without major side effects. As a somewhat related example, Jones and Friman (1999) showed that math performance was impaired in the presence of bugs for a child with insect phobia and that math performance improved following graduated exposure plus differential reinforcement. We attempted to extend these prior studies by treating needle phobia in a youth with autism using stimulus fading plus DRO to allow appropriate monitoring of his blood glucose levels.
Method
Participant, Setting, and Materials
Oliver was a large (height, 6 ft 1 in.; weight, 280 lb) 18-year-old boy who had been diagnosed with autism, mental retardation, and Type 2 diabetes. He attended an outpatient clinic 4 days per week for treatment of noncompliance with medical procedures related to his diabetes. Specifically, he had not allowed medical professionals to draw blood in over 2 years. Previous attempts at drawing blood resulted in responses indicative of distress and avoidance (two essential components of phobias) that ranged from whimpering and crying to screaming, elopement, self-injury, and aggression. Oliver could follow simple instructions (e.g., “sit down and place your hand on the table”); however, he had no vocal speech and communicated through a few idiosyncratic manual signs. Approximately six to nine 10-trial sessions or probes were conducted during each 2-hr outpatient visit. There were a total of nine visits that occurred four to five times per week over the course of 2 weeks. Sessions were conducted in a treatment room (3 m by 3 m) that contained tables, chairs, and assorted reinforcers (e.g., cookies). Generalization sessions were conducted in a nurse's station. Blood samples were drawn using an Accu-Chek Softclix® lancet device, and blood glucose levels were measured using an Accu-Chek Advantage® monitor. Inserting a test strip with a sample of blood operated the monitor. After insertion, the monitor provided a reading of blood glucose levels within 3 to 5 s.
Response Measurement and Interobserver Agreement
The primary dependent measure was the percentage of successful trials, defined as Oliver moving his arm no more than 3 cm during a 10-s trial. He was taught to place his hand and arm between an outline of his hand and arm drawn on posterboard that was attached to the top of the table. If he moved his arm more than 3 cm from the outline in any direction, the trial was immediately terminated and scored as unsuccessful. Trial-by-trial interobserver agreement was assessed during 27% of sessions and was always 100%.
Preference Assessments
Prior to each session, potential food reinforcers were identified using a multiple-stimulus-without-replacement preference assessment (DeLeon & Iwata, 1996). Cookies, potato chips, popcorn, and soda were the most frequently chosen foods.
Experimental Design and Procedure
A variation of an ABAB reversal design was used. During all conditions, the experimenter sat approximately 0.5 m from Oliver and positioned the lancet in front of him at previously determined distances. The horizontal distance from the tip of the lancet to the tip of Oliver's index finger varied based on condition, and the vertical distance (i.e., how far above Oliver's finger) from the tip of the lancet to the tip of Oliver's finger remained consistent at approximately 8 cm to 10 cm. The starting distance during baseline trials remained constant at 61 cm, whereas the starting distances during fading trials were determined prior to each trial and ranged from 1 to 61 cm. The initial distance of 61 cm was selected because Oliver neither withdrew his hand nor showed signs of distress (e.g., crying) when the lancet was at least 61 cm away.
Baseline
Oliver was given a verbal and gentle physical prompt to place his left hand and arm between the outline drawn on the posterboard at the start of each trial. The therapist then slowly moved the lancet toward Oliver's index finger. Immediately upon initiation of movement toward his arm (approximately 60 cm from his arm), he began to pull away. Baseline trials were terminated if Oliver pulled his arm away or if a blood draw was successfully completed. Baseline trials consistently lasted 10 s or less.
Stimulus fading plus DRO
During the first fading step of this condition (F1), the lancet was horizontally positioned approximately 61 cm from Oliver's index finger for 10 s. If Oliver kept his hand and arm between the outline on the posterboard for the entire 10-s interval, he immediately received access to the food item identified during the presession preference assessment. If he moved his arm more than 3 cm from the outline in any direction, the trial was immediately terminated, all the materials were removed, and the experimenter turned away for 10 s. Distances from the tip of his index finger were delineated on the posterboard on which he laid his hand and arm. We progressed from one fading step to the next after the percentage of successful trials was 100% for two or three consecutive sessions.
Except for Session 21, Steps F2 through F7 differed from Step F1 only in the distance between the lancet and Oliver's index finger; the distances were 46, 31, 15, 8, 5, and 1 cm for Steps F2 through F7, respectively. During Session 21, each trial began with the lancet 8 cm from his finger, and we probed whether he would keep his hand still for a blood draw on each trial.
In Step F8, we conducted 10 trials with the lancet 1 cm above his finger and then attempted to draw blood on the 11th trial. Step F9 was identical to Step F8 except that attempts to draw blood occurred intermittently, sometimes after 10 trials with the lancet held 1 cm above his finger and sometimes after 20 trials.
Results and Discussion
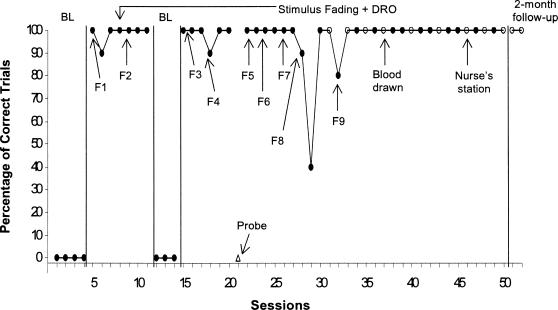
The percentages of successful trials during baseline and treatment are presented in Figure 1. During the initial baseline, Oliver pulled his hand and arm away every time the experimenter attempted to draw blood with the lancet. During F1 (M = 97%) and F2 (M = 100%), Oliver consistently kept his hand and arm within the outline drawn on the posterboard. During a return to baseline, he continued to pull his hand and arm back when the experimenter attempted to draw blood. Fading was reintroduced, and Oliver continued to keep his hand and arm within the posterboard outline. During the probe session (i.e., Session 21), Oliver consistently pulled his hand back. During the remainder of Step F5 and Steps F6 and F7, he continued to keep his hand still. In Step F8, a blood draw attempt was initiated following Session 30 (the first session in Step 8 in which Oliver was successful for 100% of the trials). In Step F9, his hand remained still during 100% of trials for all sessions except the first one (Session 32, 80%). In addition, all attempts to draw blood in Step F9 were successful, and glucose levels were obtained. In addition, one of the blood draws took place in another setting (the nurse's station), and the trials and blood draws conducted at a 2-month follow-up visit were all successful. Oliver's mother also reported that she was able to draw blood and measure glucose levels on a daily basis with no problems.
Figure 1. Percentage of trials in which Oliver laid his hand and arm on a table for 10 s during baseline (BL) and stimulus fading plus DRO.
The arrows and labels F1 through F9 show when fading steps were initiated. Open circles represent sessions in which blood was drawn and glucose levels were measured. The open triangle (Session 21) represents a probe session in which attempts to draw blood occurred during each trial.
Results of this investigation suggest that procedures used to treat phobias in individuals with less severe disabilities may also be effective with individuals diagnosed with autism and mental retardation. In addition, the results are important in addressing the challenges of assessing and treating phobias in individuals who do not speak. One challenge consists of developing a fear hierarchy, which is generally developed using a self-report measure. The current results suggest that fear hierarchies for individuals who do not speak can be based on specific overt escape behaviors.
One limitation of the current investigation is that we did not conduct a component analysis to determine the independent contributions of the stimulus fading and DRO components, which should be addressed in future research. A second limitation is that we included a measure of Oliver's avoidance responses (e.g., withdrawing his hand) but we did not specifically measure his level of distress during each trial. Anecdotally, he showed clear signs of distress (whimpering, crying, and other negative vocalizations) during the baseline phases and at the start of treatment, and these responses were absent at the end of the treatment and during follow-up. Future investigations should include objective measures of both avoidance responses and distress (the two essential components of phobias) when evaluating the effects of behavioral interventions for phobia among children who do not speak.
Acknowledgments
We thank Becky Kelso, April Kisamore, Ashley Glover, and John McCollough for their assistance in data collection. Daniel Shabani is now at the Lovaas Institute.
References
- Braff M, Nealon L. Sedation of autistic dental patient for dental procedures. Journal of Dentistry for Children. 1979;46:404–407. [PubMed] [Google Scholar]
- Davidson M.B. Type-1 diabetes mellitus with insufficient serum immunoreactive insulin elevation after subcutaneous NPH-insulin injection. Diabetes Research and Clinical Practice. 2004;64:229. doi: 10.1016/j.diabres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- DeLeon I.G, Iwata B.A. Evaluation of a multiple-stimulus presentation format for assessing reinforcer preferences. Journal of Applied Behavior Analysis. 1996;29:519–533. doi: 10.1901/jaba.1996.29-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.M, Friman P.C. A case study of behavioral assessment and treatment of insect phobia. Journal of Applied Behavior Analysis. 1999;32:95–98. doi: 10.1901/jaba.1999.32-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S.R, Matson J.L, West D. Mothers as effective therapists for autistic children's phobias. Journal of Applied Behavior Analysis. 1990;23:379–385. doi: 10.1901/jaba.1990.23-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell R.H, Scher C.S, Barst S.M. Total intravenous anesthesia for children undergoing brief diagnostic or therapeutic procedures. Journal of Clinical Anesthesia. 1995;7:273–280. doi: 10.1016/0952-8180(95)00017-c. [DOI] [PubMed] [Google Scholar]
- Zambanini A, Feher M.D. Needle phobia in type 1 diabetes mellitus. Diabetic Medicine. 1997;14:321–323. doi: 10.1002/(SICI)1096-9136(199704)14:4<321::AID-DIA356>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]