Abstract
Hematopoietic progenitors are cells, which under challenging experimental conditions can develop unusual phenotypic properties, rather distant from their original mesodermal origin. As previously reported, cells derived from human umbilical cord blood (HUCB) or human bone marrow (BM) under certain in vivo or in vitro conditions can manifest neural features that resemble features of neural-derived cells, immunocytochemically and in some instances also morphologically. The present study explored how hematopoietic-derived cells would respond to neurogenic signals from the subventricular zone (SVZ) of adult and aged (6 and 16 months old) rats. The mononuclear fraction of HUCB cells was transplanted into the SVZ of immunosuppressed (single cyclosporin or three-drug treatment) animals. The triple-suppression paradigm allowed us to protect transplanted human cells within the brain and to explore further their phenotypic and migratory properties. One week after implantation, many surviving HUCB cells were located within the SVZ and the vertical limb of the rostral migratory stream (RMS). The migration of HUCB cells was restricted exclusively to the pathway leading to the olfactory bulb. In younger animals, grafted cells navigated almost halfway through the vertical limb, whereas, in the older animals, the migration was less pronounced. The overall cell survival was greater in younger animals than in older ones. Immunocytochemistry for surface CD antigen expression showed that many HUCB cells, either cultured or within the brain parenchyma, retained their hematopoietic identity. A few cells, identified by using human-specific antibodies (anti-human nuclei, or mitochondria) expressed nestin and doublecortin, markers of endogenous neural progenitors. Therefore, it is believed that the environment of the neurogenic SVZ, even in aged animals, was able to support survival, “neuralization,” and migratory features of HUCB-derived cells.
Keywords: aging, hematopoietic progenitors, immunosuppression, migration, neural antigens, transplantation
Advances in stem cell research have opened promising avenues for treatment of multiple disorders. Cord blood cell transplantation was already been applied in hematology (Gluckman et al., 1989) and cancer therapy (Wagner et al., 1995). Research on cell-based therapies for diseases of the nervous system has focused on stem/progenitor cells for replenishment of missing cellular components or metabolic products (Sanchez-Pernaute et al., 2001). Embryonic stem cells, currently considered one of the most promising sources of potentially therapeutic cells, are plagued with numerous ethical and technical concerns. Therefore, scientists are looking for alternative cell sources as effective as embryonic stem cells but more readily available and less controversial.
Currently there is an explosion of knowledge on the pluripotency and plasticity of several nonembryonic cell types, including bone marrow (Azizi et al., 1998; Sanchez-Ramos et al., 2000), skin (Toma et al., 2001), umbilical cord blood (Ha et al., 2001; Buzanska et al., 2002; Zigova et al., 2002), fetal liver (Hao et al., 2003), umbilical cord Wharton’s jelly (Mitchell et al., 2003), and peripheral blood (Zhao et al., 2003). The results have not been fully repeatable and frequently raise doubts about whether transdifferentiation actually occurs (Morshead et al., 2002; Wagers et al., 2002; Wells, 2002). Some researchers claim that the explanation for a broader differentiation repertoire of postembryonic progenitor cells is a result of cell fusion (Ying et al., 2002; Terada et al., 2002). On the other hand, there is evidence suggesting the existence of circulating (Labat et al., 2000) or scattered (Young et al., 2001) primitive cells in organs. These cells do not seem to be committed to any phenotype but acquire a phenotype according to the environment in which they reside. Under normal circumstances, the differentiation repertoire of tissue-specific stem cells may be limited by anatomical boundaries, but experimental manipulations (placing the progenitors in ectopic locations) may expand their differentiation potential over conventional anatomical limits.
Human umbilical cord progenitors are an example of potentially pluripotent cells. These noncontroversial, immunologically immature cells are easily obtained, carry a low risk of blood-borne pathogen transmission, and are rich in progenitor cells. Under various experimental conditions, they may acquire the phenotype of hepatocytes (Newsome et al., 2003), myofibroblasts (Kadner et al., 2002), osteoblasts (Rosada et al., 2003), and all neural lines (Sanchez-Ramos et al., 2001; Bicknese et al., 2002; Buzanska et al., 2002; Zigova et al., 2002). Human umbilical cord blood (HUCB) transplantation has been successfully used (without causing tumors) as a therapeutic method for bone marrow (BM) repopulation (Lu et al., 1996). As the extensive collection of histocompatibility-typed HUCB cells in blood banks grows, the matching of cord blood for specific patients may become as simple as the matching required for a blood transfusion.
The rodent brain is one of the most extensively used tools to study therapeutic approaches for neurodegenerative diseases. Among all parts of the brain, only the hippocampus and the subventricular zone (SVZ) have the unique ability to generate neurons throughout life, even in adulthood and senescence (Cameron and McKay, 1999). We have chosen SVZ of aged rat brain as a model with which to study pluripotent properties of hematopoietic progenitors, because it still contains developmental signals for generation, migration, and differentiation of neural cells. In many ways, some neurodegenerative disorders develop a microenvironment similar to that of the aged brain. Therefore, this neurogenic environment is ideal for testing the capacity of progenitor cells for neural differentiation. In addition, the RMS, a natural extension of the SVZ, serves as a pathway for endogenous neuroblasts to reach their final destination in the olfactory bulb and possibly provides instructive signals for HUCB cells, which may mimic the migratory behavior of their endogenous SVZ-derived counterparts.
In the current study, we used the mononuclear fraction of HUCB cells to characterize phenotypic properties and migratory behavior within adult and aged brain. Our study provides insights into the possible “neuralization” of hematopoietic progenitors and addresses difficulties of xenografting.
MATERIALS AND METHODS
HUCB Cells
Cryopreserved samples of the mononuclear fraction of HUCB cells from anonymous donors were obtained from Cambrex Poietics, Inc. According to company specification, cord blood was collected by puncturing the umbilical cord stub, and the mononuclear fraction was isolated on a Ficoll/Hypaque density gradient within 24 hr after collection. A mixture of dimethyl sulfoxide and RPMI media was used for cryopreservation. Upon arrival, the samples were thawed into 10 ml of Dulbecco’s minimal essential medium (DMEM; Gibco, Grand Island, NY) with 10% fetal bovine serum and 0.1% Gentamicin (Sigma, St. Louis, MO), spun down (400 g/7 min), and resuspended in 1 ml of the same media to determine the viability of the cells in each sample. The viability of cells was determined using either trypan blue staining or the fluorescent fluorescein diacetate/propidium iodide method (Zigova et al., 1996). Both methods showed initial viability ranging from 70% to 95%, which usually dropped no more than 5% after grafting.
Green Fluorescent Protein BM Cells
Adult transgenic green fluorescent protein (GFP) mice (C57BL/6-Tg-N) from Jackson Laboratories were used as donors for BM and SVZ cells. According to the procedure previously described (Song and Sanchez-Ramos, 2002), marrow was flushed with phosphate-buffered salt solution (PBS; pH 7.2) supplemented with 0.5% bovine serum albumin, from femurs and tibiae of anesthetized animals. Cells were disaggregated by frequent trituration and then filtered through 30-μm nylon mesh to remove lipid and tissue fragments, washed by centrifugation, and resuspended in 800 μl of PBS buffer for each sample containing 108 cells. A small aliquot of suspension was dropped on a slide and checked under the fluorescent light for green-fluorescing cells. The viability of the sample was determined in a manner similar to that used for cord blood (see above).
Preparation of Cell Culture
The HUCB cells were plated in DMEM, supplemented with fetal bovine serum and gentamicin on poly-L-lysine-coated eight-well dishes (Nunc, Napperville, IL), according to procedures previously described (Zigova et al., 2002), at a seeding density of 100,000 cells/cm2, and placed into a water-jacketed incubator (95% O2, 5% CO2) for 7 days, with one exchange of media on day 4. The cells were washed with 0.1 M PBS for 5 min, fixed with cold 4% paraformaldehyde in 0.1 M PBS for 10 min, and washed thrice in cold PBS. Soon after fixation, the slides were used for immunocytochemical detection of cell-type-specific antigen expression. In some instances, we used flasks to prelabel the HUCB cells with cholera toxin subunit b (CTb) conjugated to fluorescein isothiocyanate (FITC; Molecular Probes, Eugene, OR), modifying the staining procedure described by Willing et al. (2002). Briefly, 1 day prior to transplantation, lyophilized CTb was mixed with culture media to a final concentration of 2 μg/ml, and cells were incubated in the presence of CTb for 12–24 hr, then harvested, washed three times, and suspended in Hank’s balanced salt solution (Gibco) at a concentration of 50,000 cells/μl. Cells with viability no less than 70% were used for transplantation. A similar procedure for preparation of cultures was employed with GFP-BM cells from mouse donors.
Animals
Transplantation experiments were performed on adult 6 (n = 25)- and 16 (n = 25)-month-old Fisher 344 male rats (NIA, Bethesda, MD) weighing 350 – 450 g, housed two per cage. Animals were maintained on a 12-hr light/12-hr dark cycle, with a continuous supply of food and water. All investigations described in this report were performed in accordance with the National Institute of Health guidelines for the care and use of laboratory animals and were approved by the University of South Florida Institutional Animal Care and Use Committee.
Transplantation
The immunosuppression treatment was started 2 days prior to transplantation either with daily injections of cyclosporin A (10 mg/kg, i.p., n = 40) or with a three-drug therapy (n = 10): cyclosporin A (10 mg/kg, i.p.), azathioprine (5 mg/kg, i.p.), and methylprednisolone (1.5 mg/kg, i.m.; Pedersen et al., 1997). All animals were immunosuppressed for the entire survival period.
Cells (either HUCB or murine GFP-BM), were resuspended in HBSS and diluted into a concentration of 50,000 cells/μl. Animals received a unilateral injection of cells (total 100,000 cells in 2 μl) into the anterior part of the SVZ [level skull (AP) = 1.6, (ML) = 1.5, (DV)= – 4.2, as measured from bregma and dura (Paxinos and Watson, 1986)]. By using a 10-μl Flexifill microsyringe (170 μm OD; World Precision Instruments), cells were introduced slowly (at a rate of 0.5 μl/min) into the brain. The needle was left in place for 5 min and then withdrawn slowly. The incision was closed with surgical glue (Vetbond; Vet Products Labs). After surgery, animals were placed individually into cages and allowed to survive for 1 week.
Tissue Processing
One week after transplantation, animals were anesthetized with pentobarbital (150 mg/kg) and transcardially perfused with 200 ml of 0.1 M PBS, followed by 250 ml of 4% paraformaldehyde in the same buffer. The brains were postfixed for 24 hr in 4% paraformaldehyde and cryopreserved in 30% sucrose. Twenty-micrometer-thick cryostat sagittal sections (from both hemispheres) were thaw mounted on charged slides and completely dried before storing at –20°C. When ready to use, sections were adjusted to room temperature. Every sixth section per brain was stained with cresyl violet, dehydrated, and sealed with Permount.
Immunohistochemistry
After confirmation of graft localization with cresyl violet staining, every other section from the region containing graft was processed for human-specific antigens (human nuclei or mitochondria; 1:50; Chemicon, Temecula, CA). Cell-type-specific antibodies [nestin; 1:500 (BD, San Jose, CA); doublecortin; 1:2,000 (Chemicon); TuJ1; 1:2,000 (Covance); GFAP; 1:500 (Dako, Carpenteria, CA); CD4, CD8, CD45; 1:50 (Chemicon); CD133; 1:50 (Miltenyi Biotech); OX-42; 1:50 (Serotec, Bicester, United Kingdom)] were employed to identify expression of hematopoietic and neural antigens and response of the host to grafted cells. Antibody against caspase-3 (1:1,000; R&D Systems, Minneapolis, MN) was used to detect cells undergoing apoptosis. Sections were incubated with primary antibodies diluted in 0.1 M PBS containing 10% normal goat serum overnight at 4°C and on the next day treated with the appropriate secondary antibodies. Negative controls were prepared using identical procedures, with omission of the primary antibody. For nuclear counterstain, we used a mounting Vectashield medium with DAPI (Vector, Burlingame, CA). Immunostained brain sections were analyzed using the Olympus BX60 and IX71 epifluorescence microscopes with Olympus DP-70 digital acquisition system. Images were obtained using × 20 and × 40 objectives and processed with Photoshop software.
Quantitative Estimation of Surviving HUCB Cells Within the Brain
To determine the number of surviving cells within the SVZ, one week after transplantation, the counts were collected only from triply immunosuppressed animals (n = 10). The data were collected from three younger (6 months) and three older (16 months) animals. This selection was based on the number of human cells found per section (minimum 50 cells). The remaining four animals were excluded from the quantitative analysis. Only those cells were counted that colocalized nuclear DAPI and cytoplasmic mitochondrial marker. Because we only used counts from every sixth brain section, the total count was an extrapolation for the remaining sections with graft. An estimate of the number of surviving cells in these sections was obtained using Abercrombie’s (1946) formula. While this semiquantitative method is biased, it is sufficient for the purpose of our study, which is the optimization of this short-term xenografting model. To determine the dispersion of grafted cells from the needle track and injection area, we counted the number of sections containing HUCB cells. Migratory behavior of HUCB cells within the RMS was estimated by measuring the distance from the center of the injection site within the SVZ.
RESULTS
Morphology and Immunophenotype of Cultured HUCB Cells
Mononuclear HUCB cells were plated in cell culture dishes to provide an in vitro comparison with cells grafted into the brain parenchyma. Twenty-four hours after plating, the cells were quite uniform in their size and shape. Over time in vitro, a wide range of distinct morphologies became evident, including round cells, spindle-shaped or fibroblast-like cells, and the occasional neuron-like polar cells. In these short-term monolayer cultures, single cells or smaller clumps were observed, whereas formation of clusters was quite rare. Independently of their morphological appearance, the majority of HUCB cells retained their hematopoietic phenotype and expressed the pan-leukocyte marker CD45 (Fig. 1A, parts A, B). The remaining cell population (~10%) did not reveal CD45 antigen, indicating that these cells were either hematopoietic progenitor cells (possibly expressing CD34 antigen) or even more immature, perhaps pluripotent cells (Chen et al., 2003).
Fig. 1.
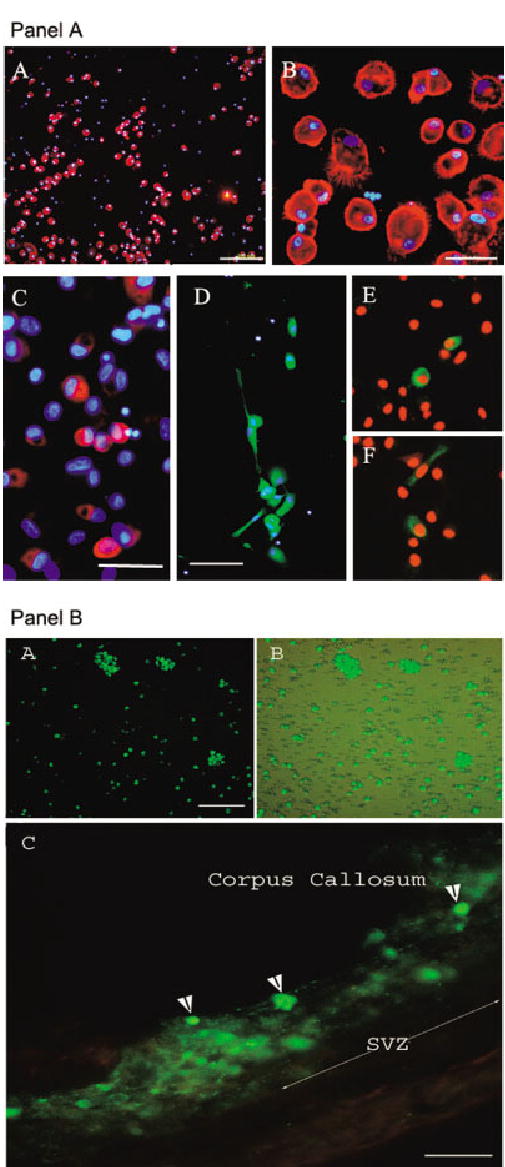
Immunophenotype of cultured HUCB cells. A: Part A: Most cells at this time point were positive for the panleukocyte marker CD45 (red). DAPI counterstaining (blue) helped us to visualize all nucleated cells in culture. Note that approximately 10% of the cells were not CD45 immunopositive. Part B: Higher magnification from A depicts in detail the morphology of cultured CD45-positive cells. Part C: Few HUCB cells expressed nestin (red), an early neural marker. DAPI counterstain (blue). Part D: Some human cord blood-derived cells were positive for GFAP (green), a marker of mature glia, and a few cells expressed TuJ1 (βIII-tubulin), an early neuronal marker (parts E,F). B: Photomicrographs showing fluorescing HUCB cells prela-beled with cholera toxin subunit b (CTb) conjugated to FITC. Part A: Freshly thawed HUCB cells were cultured for 24 hr and then incubated with cholera toxin (green) for 12 hr prior to transplantation. Part B: The same culture visualized through a combined fluorescent and brightfield microscope showing that not every cell was labeled with this dye. Part C: CTb-labeled green fluorescing HUCB cells within the subventricular zone of a 16-month-old Fisher 344 rat. One week after grafting, we found numerous single cells or clumps of cells intensely labeled with the dye (arrowheads). In some instances, the blurriness surrounding the graft in the brain parenchyma may indicate possible dye leakage. Scale bars = 200 μm in A(A); 30 μm in A(B); 100 μm in A(C,D); B(A,B); 50 μm in A(C), B(C).
The cultures were immunolabeled for several neural antigens, including nestin, glial fibrillary acidic protein (GFAP), and βIII-tubulin (TuJ1). Only a subpopulation (~10%) of the small, round cells expressed nestin (Fig. 1A, part C). Among the morphologically diversified cells with larger bodies and prominent processes, 35% were GFAP immunoreactive (Fig. 1A, part D). At this time point, only a few (>0.5%) round and occasionally polarized cells with neuron-like morphology expressed the early neuronal marker βIII-tubulin (Fig. 1A, parts E, F).
Prelabeling of HUCB Cells With the Fluorescent Tag
To identify HUCB cells within the brain parenchyma, the cultures were incubated with CTb conjugated to the fluorescent FITC probe prior to surgery. After 12 hr of incubation with CTb, only approximately 40% of mononuclear HUCB cells were labeled (Fig. 1B, parts A, B). Even increasing the incubation time up to 3 days failed to improve labeling efficiency, suggesting that unlabeled cells presumably do not have the VAMP receptor responsible for internalization of the dye (McIntosh and Schnitzer, 1999). In one set of experiments, CTb-prelabeled HUCB cells were cultured for an extended period (14 –21 days) to determine whether the dye affects normal cellular development. In those cells, which were originally labeled, the intensity of signal was very strong in the perikarya and diminished in newly growing processes (not shown). On the other hand, prelabeled cells transplanted into the SVZ were usually surrounded by blurry brain tissue, indicating possible dye release. This, however, suggests that some of the transplanted labeled cells could have been damaged; this could lead to dye release and possible transfer to endogenous cells (Fig. 1B, part C; Fig. 2A, part D).
Fig. 2.
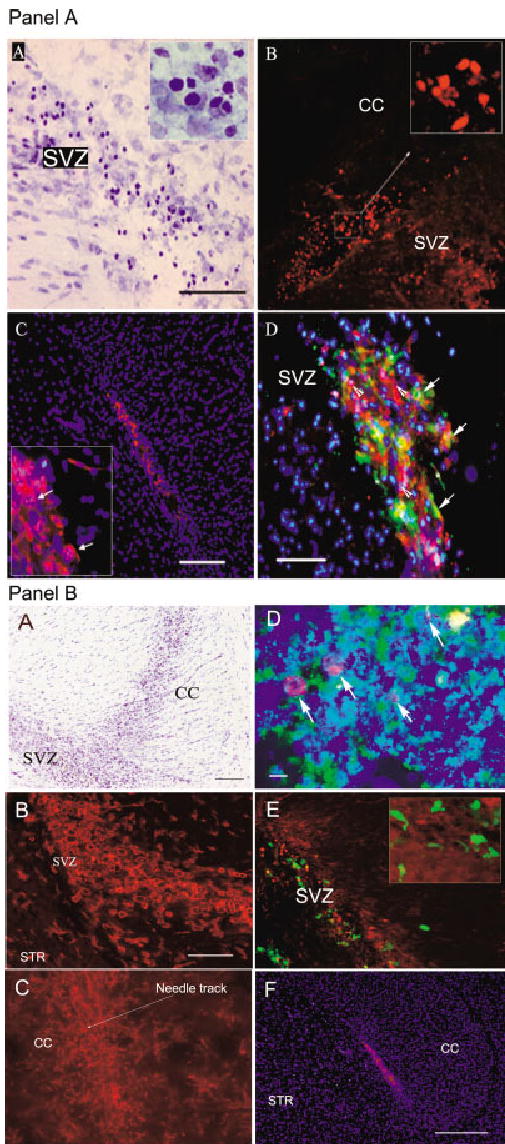
Identification of HUCB cells within the subventricular zone of adult rat brain 1 week after transplantation. These animals were subjected to triple immunosuppression. A: Part A: Bright field photomicrograph of cresyl violet-stained sagittal brain section of a 6-month-old rat showing morphology and distribution of grafted HUCB cells. Easily identifiable darkly stained grafted cells (see also inset) are evenly distributed throughout the SVZ. Part B: Human nuclei-specific immunostaining (red) identifies HUCB-derived cells injected into the SVZ. The inset shows the pattern of nuclear labeling at higher magnification. Part C: To detect transplanted cells, we also used human-specific antibody against mitochondria (red). Blue DAPI counterstaining reveals the main anatomical structures and allows quantitative analysis of the graft. Inset clearly shows several double-labeled (human mitochondria-positive HUCB cells with DAPI-positive nuclei) cells with punctate pattern of cytoplasmic labeling (arrows). Part D: Combined double immunocytochemistry for human mitochondria (red), DAPI (blue), and CTb/FITC-labeled (green) grafted cells. We found numerous triple-labeled cells (arrows) and also single cells that were positive either for human mitochondrial marker (arrowheads) or for CTb. This confirms our previous findings (see Fig. 1) that CTb labels only a subpopulation of cells and, therefore, many of those that “escaped” labeling can be tracked by human-specific immunocytochemical markers. The cells that reveal CTb only are presumably host cells that incorporated the dye passively. B: Optimization of immunosuppression for HUCB xenografting. Part A: Brightfield photomicrograph showing a sagittal section through the rat brain, immunosuppressed with cyclosporin A only, 1 week after grafting. Note the prominent infiltration of the graft area in the corpus callosum (CC) and subventricular zone (SVZ). Part B: We found that a large fraction of cells observed on cresyl violet stained sections was immunoreactive for OX-42, a marker of microglia and macrophages (red). Numerous round cells, typical of activated microglia, were detected inside and around the area of the graft within the SVZ. Staining for human-specific mitochondria did not confirm the presence of grafted HUCB cells. Part C: OX-42 immunostaining in 6-month-old rat brain after triple immunosuppression. One week after transplantation, there was significantly lower microglial activation (red). Part D: Human mitochondria stained grafted cells in SVZ (green). Only few cells were positive for apoptotic marker caspase-3 (red; arrows). Blue DAPI as counterstain. Part E: For comparison, we transplanted murine bone marrow cells isolated from transgenic GFP mice. We noticed that cyclosporin A immunosuppression was sufficient to protect the grafted cells (green) and that OX-42 immunopositivity (red) was considerably milder, suggesting that, in rat brain, murine cells did not trigger an immune response as human cells did. Green fluorescence-labeled bone marrow cells were able to survive, disperse, and acquire morphological diversity (see inset). Part F: We found healthy HUCB cells within the SVZ, under similar immunosuppressive treatment. The cells were identified by antibody against human mitochondria (red). DAPI counterstain (blue) was used to show anatomical structures of the brain. This animal was 16 months old. STR, striatum. Scale bars = 100 μm in A(A) for A(A,B), B(A); 200 μm in A(C), B(F); 50 μm in A(D), B(B) for B(B,C,E); 10 μm in B(D).
Identification of Grafted Cells Within the Brain
On cresyl violet-stained sections, darkly labeled, round HUCB cells were easily discerned from surrounding endogenous SVZ cells (Fig. 2A, part A). In triply immunosuppressed animals, grafted cells were randomly dispersed within the SVZ and adjacent areas, without a restraining layer of glia or macrophage infiltration. Immunocytochemistry against human antigens was a specific and reliable method for tracking grafted cells. The two human-specific markers (human nuclei, Fig. 2A, part B; mitochondria, Fig. 2A, part C) detected the HUCB cells with over 95% efficiency. The mitochondria had a prominent granular pattern, indirectly suggesting the metabolic activity of the HUCB mitochondrial pool.
Immunological/Inflammatory Response
One week after grafting of HUCB cells to the SVZ of cyclosporin A-immunosuppressed Fisher 344 rats (6 or 16 months old), we noticed signs of graft rejection. Cresyl violet staining showed densely packed, small cells infiltrating the area of the graft (Fig. 2B, part A). To identify the origin of these cells, we performed immunostaining for human-specific markers, but few labeled cells were found. To determine the phenotype of infiltrating cells, a series of immunohistochemical reactions against the host cells, including lymphocytes, astroglia, and microglia/macrophages, was performed. T lymphocytes expressing CD4 or CD8 antigens represented an insignificant fraction of the infiltrate; most of the cells were expressing OX-42, a marker of macrophages and microglia (Fig. 2B, part B). The round, unbranched morphology of OX-42 cells, known to be the main effectors of acute inflammatory/immunological response within the brain, is characteristic of activated microglia of the host. At the same time, an unusually strong intensity of immunolabeling for fibrillary acidic protein (GFAP) was detected in the proximity of the graft (data not shown). Therefore, to improve the graft survival, a three-drug treatment (see Materials and Methods) was substituted for the cyclosporin-only immunosuppression paradigm. This resulted in significantly reduced microglia/macrophages infiltration in both age groups (Fig. 2B, part C), and numerous easily identifiable HUCB cells survived within the SVZ (Fig. 2B, part F) and RMS (Fig. 3A, part F). After stronger immunosuppression, we found approximately 7% of surviving cells in 6-month-old animals and 3% cells in 16-month-old animals. Immunocytochemistry for caspase-3 antigen identified only single grafted cells positive for this apoptotic marker (Fig. 2B, part D).
Fig. 3.
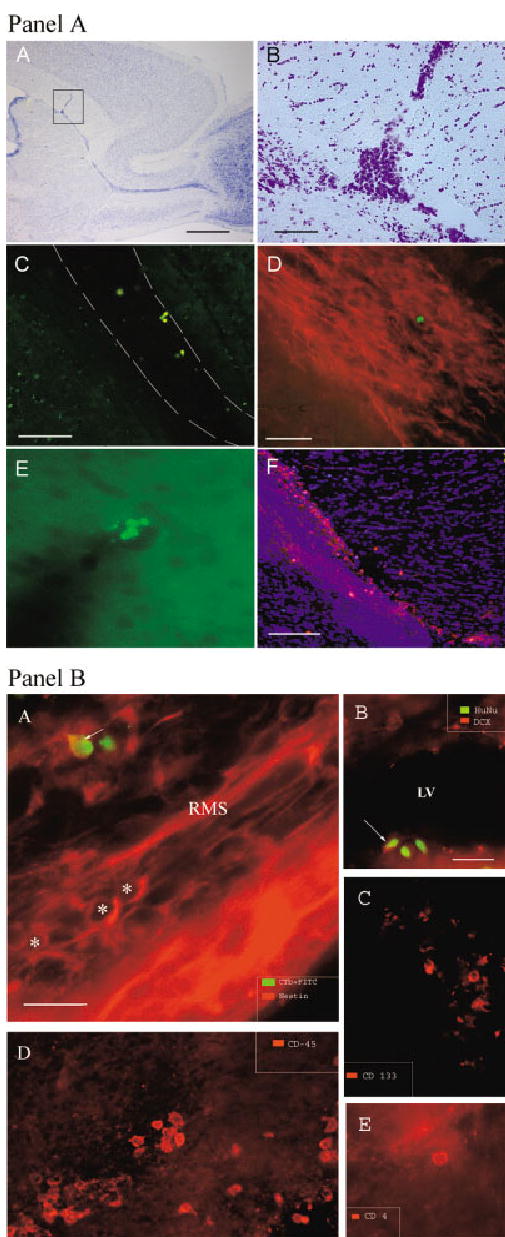
Distribution and migration of grafted HUCB cells. A: Part A: Brightfield photomicrograph of the sagittal section through the hemisphere of a 6-month-old rat (3 hr after grafting) with needle track penetrating corpus callosum and targeting SVZ. Part B: Higher magnification of the same brain area showing a deposit of HUCB cells within the SVZ. Part C: Grafted cells, prelabeled with CTb (green), migrated along the rostral migratory stream (RMS) delineated by dashed lines. Part D: Additionally, we used an antibody against human nuclei (green) to identify cells in the RMS. The migratory pathway is highlighted by immunostaining against doublecortin (red). This marker identifies endogenous migrating neuronal progenitors within the RMS. Part E: Grafted cells, identified with human specific antibody against mitochondria (green), were frequently found inside or in close proximity to cortical vessels surrounding the needle track. Part F: The same human specific antibody conjugated to rhodamine (red) was used to detect cells within the RMS. The slide was counterstained with nuclear DAPI (blue). B: The immunophenotype of HUCB cells within the rat brain one week after grafting. Parts A,B: Neural antigens. Part A: Occasionally, HUCB-derived cells within the migratory pathway co-expressed nestin, an early marker of neural progenitors. Asterisks indicate several endogenous neural progenitors (red) within the pathway; arrow points to CTb-labeled (green), nestin-positive (yellow) HUCB cell. Part B: Some HUCB cells were identified within the SVZ by a dual immunostaining for human nuclei (green) and neuronal marker doublecortin (red). Arrow indicates a cell expressing both markers. LV, lateral ventricle. Parts C–E: Hematopoietic antigens. Part C: Few HUCB cells within the SVZ were positive for human-specific CD133 antigen (red). This marker is common for neural and hematopoietic progenitors. Part D: Expression of CD45, a panleukocyte marker, was detected in some HUCB-derived cells (red). Part E: Human-specific CD4 positivity was found in only a few cells (red), indicating the possibility that the subpopulation of mature lymphocytes, major inducers of rejection, might have been eliminated from the graft and that further strong immunoshielding may not be required. Scale bar = 500 μm in A(A); 50 μm in A(B), in A(D) for A(D,E), and in B(B) for B(B–E); 150 μm in A(C,F); 25 μm in B(A).
Immunogenicity of Mouse BM Cells
GFP bone marrow cells from transgenic mouse were transplanted into the SVZ of adult (6 and 16 months old) cyclosporin A-immunosuppressed rats. The number of activated microglial cells was much lower than in HUCB grafted cells. In addition, GFP bone marrow-derived cells survived significantly better (~10%), dispersed within the brain (Fig. 2B, part C), and attained complex morphology (Hudson et al., this issue).
Distribution/Migration of Grafted Cells
The injection of a cell suspension into the brain parenchyma, using a commercially available Flexifill microsyringe, employs high pressure on the target region and is accompanied by passive dispersion of injected cells. This feature has to be taken into consideration when analyzing active migration of cells. To define the extent of passive dispersion, a transplant experiment was performed in which animals were perfused almost immediately (3 hr) after transplantation (Fig. 3A, part A). Immunohistochemical and histological analysis revealed that needle passage caused a visible track; on fixed sagittal sections, it appeared as an approximately 70-μm-thick gap in tissue. Within the target area, presumably the SVZ (place of cell deposit), there was a round cavity (~150 μm in diameter). This graft site was usually filled with cells; single cells frequently infiltrated the surrounding parenchyma. The largest distance of passive dispersion was ~200 μm from the graft center. One week after grafting, the needle track was filled with moderately packed multiform cells (Fig. 2A, part A and B part A). Immunolabeling defined them either as host micro- and astroglia or as grafted cells. In both age groups, human immunopositive cells were found distant from those cells previously defined as passively migrating cells. Interestingly, a fraction of grafted cells was migrating exclusively along the RMS either toward the olfactory bulb or backward on the dorsal surface of the lateral ventricle. Whereas the tendency to migrate caudally was not very marked and could be misread as passive distribution of cells, the migration in RMS toward olfactory bulb was prominent and supported by cues operating within this pathway. In older animals, grafted cells were found usually in the initial part of RMS (~400 μm from the center of the graft deposit); in younger animals, these cells reached the elbow of the pathway (~1,000 μm from the center of the graft deposit), which is the transition from vertical to horizontal limb (Fig. 3A, part F). In addition to the distance, the number of migrating cells also differed between experimental age groups. In 16-month-old rats, very few cells entered the RMS (Fig. 3A, part D), whereas, in 6-month-old rats, the number of human cells entering the pathway was significantly greater (Fig. 3A, part F). The transvascular distribution of human cells was also evident. Grafted cells were occasionally detected in proximity to or within cortical vessels (Fig. 3A, part E).
Phenotype of Transplanted HUCB Cells
Consistently with the in vitro experiment, 1 week after grafting, approximately 90% of transplanted cells expressed the panleukocyte marker CD45 (Fig. 3B, part D), whereas few cells (> 1%) were positive for CD133 (Fig. 3B, part C), a marker common for hematopoietic and neural progenitors (Uchida et al., 2000). Surprisingly, a low number of the grafted cells expressed lymphocytic markers (Fig. 3B, part E), the host immune system response eliminated them. Although the astroglial marker GFAP and the early neuronal marker TuJ1 were observed in culture experiments, these markers were not expressed in grafted cells 1 week after transplantation. In some cases, colocalization of human-specific labeling with markers of migrating SVZ neuroblasts such as nestin (Fig. 3B, part A) and doublecortin (Fig. 3B, part B) was observed in the SVZ or RMS but never in cortical parts or the corpus callosum.
DISCUSSION
In this study, we have begun to define properties of HUCB-derived cells focusing on their differentiation potential and migration within the neurogenic (SVZ) parenchyma of aged rat brain. We have compared the phenotype of transplanted HUCB-derived cells (either hematopoietic or neural) with the antigenic profile of cultured HUCB cells in the same time frame. We also have found that, with aggressive immunosuppression treatment, a subpopulation of mononuclear HUCB cells survived and migrated within the RMS, similarly to endogenous neuroblasts. Under both in vivo and in vitro conditions, the majority of HUCB cells retained their hematopoietic identity, whereas a few expressed markers of neural lineages. The survival and migratory abilities of these cells within the ectopic location were better in younger animals than in older ones. These findings also suggest that neurogenic signals produced by SVZ are weaker with increasing age of the animal.
Why Use the Mononuclear Fraction and Not a Selected Population of HUCB Cells?
Numerous reports have shown potential trophic benefits (Chen et al., 2001; Lu et al., 2002; Garbuzova-Davis et al., 2003), or even replacement of missing enzymes (Sanberg et al., 2003), after systemic administration of the mononuclear cord blood cells. Several groups are exploring the differentiation potential of HUCB-derived progenitors. However, the lack of appropriate markers makes it very difficult to dissect a specific population of pluripotent cells. We can partially rely on hematopoietic CD markers for immature cells, such as CD34, CD133, or CD117, but these also do not guarantee the recognition of pluripotent cells within the HUCB cell population. The full mononuclear fraction is a mixture of cells including the minute subpopulation of presumably “real progenitor cells”; therefore, elimination or selection of cells based on available surface antigens may not give a satisfactory answer. Thus initial characterization of the full mononuclear fraction suggests four possible explanations for the current and previous results: 1) naïve cells differentiate depending on environmental cues, 2) hematopoietic cells transdifferentiate into neural cells, 3) HUCB cells fuse with neural cells to produce a cellular chimera, 4) mature cells dedifferentiate to an earlier stage of cellular development.
Pretransplantation Labeling of Cells
However, fluorescent labeling of cells prior to grafting is a very useful method for further analysis; cholera toxin does not appear to be a sufficient labeling method for HUCB-derived cells. Very low labeling efficiency and evidence of dye transfer to host cells suggest the necessity for utilization of different methods of tagging, such as lenti-GFP transfections (Woods et al., 2001) or iron nanoparticles (Frank et al., 2003). An optimal solution for cell detection would be utilization of hematopoietic progenitors from transgenic GFP mouse. At the same time, this approach would help to minimize the immunosuppressive treatment.
Phenotypic Features of Mononuclear HUCB Cells In Vitro and In Vivo
The mononuclear HUCB fraction consists mostly of committed lymphocytic and monocytic blood lines (~90%) characterized by the common panleukocyte marker CD45. Only a small portion of HUCB cells (1–4%) consists of hematopoietic progenitors identifiable by surface markers such as CD34, CD133, CD90, and CD117. By using existing markers, we can recognize ~95% of the mononuclear population; the remaining ~5% of cells were not characterized by the markers we used, so further, definitive markers have to be employed.
A growing body of evidence indicates that, in every organism, there is a pool of circulating (Labat et al., 2000) and/or tissue residual (Prockop, 1997; Young et al., 2001) pluripotent progenitor cells able to differentiate in vitro into multiple phenotypes. These cells migrate to wherever there is damage, become organ-specific progenitor cells, and repopulate missing phenotypes. Blood is a natural vehicle for these progenitors, especially in the early stages of development, and is an optimal source of expandable progenitors (Kawano et al., 2003) that could be utilized for therapeutic purposes. There are numerous reports showing evidence for differentiation of cord-blood-derived progenitors into neural lineages in vitro (Sanchez-Ramos et al., 2001; Bicknese et al., 2002) and in vivo (Zigova et al., 2002). In hematology, it is relatively easy to determine functional differentiation by testing bone marrow repopulation after lethal irradiation (Hogan et al., 2002). In experiments related to nervous tissue, the task of functional integration is more complicated.
Most studies testing potential neural differentiation are based on the presence of antigens defined as “specific” for neural lineages. However, there are caveats with this approach; the proteins that are considered characteristic for neural cells (and are responsible for sophisticated functions of neurons or glia) could be expressed by cells committed to quite distant nonneural lineages through the experimental manipulations and not necessarily support the complete neural performance of the cell. In our study, we noticed that a surprisingly high number of cells expressed the astroglial protein GFAP (35%), whereas, at the same time, 90% of cells expressed the hematopoietic marker CD45. This duality in the phenotype was observed in many cell types (Davidoff et al., 2002) under normal and experimental conditions. The other two neural markers, nestin and early neuronal βIII-tubulin, are present in our short-term cultures only in very low numbers. Other investigators reported the presence of these antigens or mRNAs in cultured HUCB cells under similar conditions or after exposure to proliferative/differentiating factors (Sanchez-Ramos et al., 2001; Bicknese et al., 2002; Buzanska et al., 2002). However, none of these studies addressed the issues of stability of expression after removal of stimulating factors, or coexistence of hematopoietic and neural proteins in HUCB cells. The present study shows that, even though we see the expression of neural markers in culture, we do not see them in the transplants in a similar time frame. The occasional presence of nestin-positive (Fig. 3B, part A) and CD133-positive (Fig. 3B, part C) HUCB cells is a very promising finding. Based on a recent report by Ha et al. (2003), human cord blood monocytes coexpress these two markers, thus supporting the idea that cord blood cells can adopt a neuronal fate. This, however, has to be addressed in long-term studies, which allow these cells to develop appropriate phenotypes and more complex morphologies. Further experiments in our laboratory will focus on identification of the pluripotent HUCB population and the coexistence of two phenotypes (hematopoietic and neural) in one cell (Chen et al., 2003).
Do HUCB Cells Survive in the Rat Brain?
We have demonstrated that the xenotransplantation model is a reliable method for testing biological features of human cells; however, optimization of experimental conditions has to be carefully performed. A critical issue in our current experiment was immunosuppression. We realized that graft rejection was never a problem in studies utilizing neonatal hosts (Zigova et al., 2000, 2002). However, with adult and senescent rats, we observed a remarkable immunological response. Cyclosporin treatment appeared to be insufficient to protect the intraparenchymal graft. This may have happened for several reasons. One reason is the high lipid solubility of the cyclosporin, which may be trapped in the cerebral endothelial cells of the blood– brain barrier. Thus the effectiveness of cyclosporin relies on T-lymphocyte suppression prior to intraparenchymal penetration. In our case, we did not see lymphocytic infiltrate (probably because of cyclosporin treatment or the short survival period) but the immunological/inflammatory rejection was associated with activated microglial cells. Utilization of the three-drug pretreatment (Pedersen et al., 1997) substantially decreased microglial activity and allowed us to detect human cells within the brain parenchyma. However, in comparison with the BM cells from the transgenic GFP mouse, survival was poor. This finding is in agreement with observed deviations from the rule of hyperacute rejection of xenograft (Platt, 1996), suggesting that there are concordant and disconcordant xenografts. In that case, animals may produce natural xenoreactive antibodies against some species (e.g., human) and reject such grafts in hyperacute mechanism (disconcordant system). These animals do not produce xenoreactive antibodies against different species (e.g., mouse), thus rejecting transplant in an allograft-like mechanism (concordant system).
As a result of pretransplantation manipulations, up to 95% of grafted cells undergo cell death (Sortwell et al., 2000; Ahn et al., 2003). To minimize the effect of apoptosis, we cultured cells prior to transplantation for 2 days. Immunostaining against caspase-3 1 week after grafting showed very low number of human cells undergoing apoptosis (Fig. 2B, part D). Relatively low cell survival (3–7%) and marginal apoptosis in transplanted cells may reflect an already stabilized situation, whereas the majority of apoptotically dying cells would probably be detected shortly (1–2 days) after grafting.
Active Migration or Just Passive Traversing Through the RMS?
One of the most interesting findings of this study is the migration of HUCB cells within the well-defined pathway utilized by endogenous cells. It has been previously shown that other neural-derived cells can use signals within this pathway and obey their instructions (Zigova et al., 1996, 2000). In the current study, HUCB cells that are known to move within the blood stream, their natural environment, were also able to understand cues governing migration in this neurogenic region. It is possible that, besides their inherent migratory abilities, these cells may have more features in common with neuroblasts in this region. Therefore, additional information has to be acquired to define/confirm whether these cells have CXCR receptors and possibly other determinants that might be responsible for migration. The fact that migration in older animals is slightly slower also suggests that these cells respond to cues in the pathway, and it is not just a passive diffusion of cells through the RMS. Long-term studies will answer the questions of whether HUCB cells can reach the horizontal limb of the RMS and whether they recognize signals within the olfactory bulb directing them to switch from tangential migration to the radial mode (Kishi, 1987).
Where Do We Go From Here?
This area of research is very young and will need a lot of nurturing before there are conclusive results. Our laboratory is focused on properties of HUCB-derived cells with respect to finding the most suitable population of cells that could be used as a source of multipotent progenitors for various therapeutic applications. Ongoing experiments will answer the question of whether the duality of HUCB cell phenotype is a natural feature or a feature acquired in the process of exposure to serum-containing culture conditions. We also have to examine long-term survival and different routes of cell administration, followed by behavioral testing, to confirm that these cells are good candidates for cell-based strategies designed to slow the aging process and treat age-related neurodegenerative disorders of the human brain. Future experiments should reveal whether immunorejection is manageable and HUCB cells can survive for a long time within the rodent brain. If necessary, we will perform xenotransplantation experiments with neonatal GFP mouse blood-derived cells or allografts with neonatal rat blood-derived cells transfected with GFP.
Acknowledgments
This work is dedicated to the memory of Dr. Tanja Zigova, my irreplaceable mentor, teacher and friend; and in the honor of Dr. David Cahill, Professor and Chair of the Department of Neurosurgery, in gratitude for his kindness, support, and encouragement. We are grateful to Marci McCall for editorial assistance. Human umbilical cord blood cells were obtained from Cambrex, Poietics, Inc. P.R.S. is a cofounder and A.E.W., J.S.R., S.G.D., P.C.B., and T.Z. are consultants of Saneron CCELL Therapeutics, Inc. (Tampa, FL). This study was supported by NIH/NIA grant R01 AG20927-01 to T.Z.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anatomical Record. 1946;94:239 –247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Ahn YH, Emgard M, Brundin P. Ultrastructural characterization of dissociated embryonic ventral mesencephalic tissue treated with neuroprotectants. Cell Transplant. 2003;12:235–241. doi: 10.3727/000000003108746795. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908 –3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknese AR, Goodwin HS, Quinn CO, Henderson VC, Chien SN, Wall DA. Human umbilical cord blood cells can be induced to express markers for neurons and glia. Cell Transplant. 2002;11:261–264. [PubMed] [Google Scholar]
- Buzanska L, Machaj EK, Zablocka B, Pojda Z, Domanska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci. 2002;115:2131–2138. doi: 10.1242/jcs.115.10.2131. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894 –7. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen N, Song S, Hudson JE, Willing AE, Garbuzova-Davis S, Sanchez-Ramos J, Sanberg PR, Zigova T. Neural and hematopoietic antigens in cultured human umbilical cord blood cells. Experimental Neurology. 2003;181:89. [Google Scholar]
- Davidoff MS, Middendorff R, Kofuncu E, Muller D, Jezek K, Holstein AF. Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules. Acta Histochem. 2002;104:39 – 49. doi: 10.1078/0065-1281-00630. [DOI] [PubMed] [Google Scholar]
- Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH, Jr, Bulte JW. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480 – 487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, Hudson JE, Chen N, Davis CD, Sanberg PR. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J Hematother Stem Cell Res. 2003;12:255–270. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174 –1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- Ha Y, Choi JU, Yoon DH, Yeon DS, Lee JJ, Kim HO, Cho YE. Neural phenotype expression of cultured human cord blood cells in vitro. Neuroreport. 2001;12:3523–3527. doi: 10.1097/00001756-200111160-00030. [DOI] [PubMed] [Google Scholar]
- Ha Y, Lee JE, Kim KN, Cho YE, Yoon DH. Intermediate filament nestin expressions in human cord blood monocytes (HCMNCs) Acta Neurochir. 2003;145:483– 487. doi: 10.1007/s00701-003-0023-4. [DOI] [PubMed] [Google Scholar]
- Hao HN, Zhao J, Thomas RL, Parker GC, Lyman WD. Fetal human hematopoietic stem cells can differentiate sequentially into neural stem cells and then astrocytes in vitro. J Hematother Stem Cell Res. 2003;12:23–32. doi: 10.1089/152581603321210109. [DOI] [PubMed] [Google Scholar]
- Hogan CJ, Shpall EJ, Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci USA. 2002;99:413– 418. doi: 10.1073/pnas.012336799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner A, Hoerstrup SP, Tracy J, Breymann C, Maurus CF, Melnitchouk S, Kadner G, Zund G, Turina M. Human umbilical cord cells: a new cell source for cardiovascular tissue engineering. Ann Thorac Surg. 2002;74:S1422–S1428. doi: 10.1016/s0003-4975(02)03910-3. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kobune M, Yamaguchi M, Nakamura K, Ito Y, Sasaki K, Takahashi S, Nakamura T, Chiba H, Sato T, Matsunaga T, Azuma H, Ikebuchi K, Ikeda H, Kato J, Niitsu Y, Hamada H. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood. 2003;101:532–540. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- Kishi K. Golgi studies on the development of granule cells of the rat olfactory bulb with reference to migration in the subependymal layer. J Comp Neurol. 1987;258:112–124. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- Labat ML, Milhaud G, Pouchelet M, Boireau P. On the track of a human circulating mesenchymal stem cell of neural crest origin. Biomed Pharmacother. 2000;54:146 –162. doi: 10.1016/S0753-3322(00)89048-4. [DOI] [PubMed] [Google Scholar]
- Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11:275–281. [PubMed] [Google Scholar]
- Lu L, Shen RN, Broxmeyer HE. Stem cells from bone marrow, umbilical cord blood and peripheral blood for clinical application: current status and future application. Crit Rev Oncol Hematol. 1996;22:61–78. doi: 10.1016/1040-8428(96)88370-3. [DOI] [PubMed] [Google Scholar]
- McIntosh DP, Schnitzer JE. Caveolae require intact VAMP for targeted transport in vascular endothelium. Am J Physiol. 1999;277:H2222–H2232. doi: 10.1152/ajpheart.1999.277.6.H2222. [DOI] [PubMed] [Google Scholar]
- Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50 – 60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Benveniste P, Iscove NN, van der Kooy D. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat Med. 2002;8:268 –273. doi: 10.1038/nm0302-268. [DOI] [PubMed] [Google Scholar]
- Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC, Harrison DJ, Bickmore WA, Plevris JN. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- Pedersen EB, Zimmer J, Finsen B. Triple immunosuppression protects murine intracerebral, hippocampal xenografts in adult rat hosts: effects on cellular infiltration, major histocompatibility complex antigen induction and blood– brain barrier leakage. Neuroscience. 1997;78(3):685–701. doi: 10.1016/s0306-4522(96)00620-3. [DOI] [PubMed] [Google Scholar]
- Platt JL. The immunological barriers to xenotransplantation. Crit Rev Immunol. 1996;16:331–358. [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72:135–142. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Willing AE, Austin LA, Mallery J, Floyd R, Koksen SL, Davis C, Garbuzova-Davis S. Cerebral intraventricular transplantation of human umbilical cord blood cells as a potential treatment of Sanfilippo syndrome type. Experimental Neurology. 2003;181:104 –105. [Google Scholar]
- Sanchez-Pernaute R, Harvey-White J, Cunningham J, Bankiewicz KS. Functional effect of adeno-associated virus mediated gene transfer of aromatic L-amino acid decarboxylase into the striatum of 6-OHDA-lesioned rats. Mol Ther. 2001;4:324 –330. doi: 10.1006/mthe.2001.0466. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos JR, Song S, Kamath SG, Zigova T, Willing A, Cardozo-Pelaez F, Stedeford T, Chopp M, Sanberg PR. Expression of neural markers in human umbilical cord blood. Exp Neurol. 2001;171:109 –115. doi: 10.1006/exnr.2001.7748. [DOI] [PubMed] [Google Scholar]
- Song S, Sanchez-Ramos J. Preparation of neural progenitors from bone marrow and umbilical cord blood. Methods Mol Biol. 2002;198:79 – 88. doi: 10.1385/1-59259-186-8:079. [DOI] [PubMed] [Google Scholar]
- Sortwell CE, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: implications for improving grafted dopamine neuron survival. Exp Neurol. 2000;165:268 –277. doi: 10.1006/exnr.2000.7476. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778 –784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720 –14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256 –2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wagner JE, Kernan NA, Steinbuch M, Broxmeyer HE, Gluckman E. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet. 1995;346:214 –219. doi: 10.1016/s0140-6736(95)91268-1. [DOI] [PubMed] [Google Scholar]
- Wells WA. Is transdifferentiation in trouble? J Cell Biol. 2002;157:15– 8. doi: 10.1083/jcb.200203037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing AE, Garbuzova-Davis S, Sanberg PR, Saporta S. Routes of stem cell administration in the adult rodent. Methods Mol Biol. 2002;198:357–374. doi: 10.1385/1-59259-186-8:357. [DOI] [PubMed] [Google Scholar]
- Woods NB, Mikkola H, Nilsson E, Olsson K, Trono D, Karlsson S. Lentiviral-mediated gene transfer into haematopoietic stem cells. J Intern Med. 2001;249:339 –343. doi: 10.1046/j.1365-2796.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, Duenzl M, Lucas PA, Black AC., Jr Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51– 62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci USA. 2003;100:2426 –2431. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigova T, Betarbet R, Soteres BJ, Brock S, Bakay RA, Luskin MB. A comparison of the patterns of migration and the destinations of homo-topically transplanted neonatal subventricular zone cells and heterotopically transplanted telencephalic ventricular zone cells. Dev Biol. 1996;173:459 –474. doi: 10.1006/dbio.1996.0040. [DOI] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Sanberg PR, Luskin MB. The properties of hNT cells following transplantation into the subventricular zone of the neonatal forebrain. Exp Neurol. 2000;163:31–38. doi: 10.1006/exnr.2000.7344. [DOI] [PubMed] [Google Scholar]
- Zigova T, Song S, Willing AE, Hudson JE, Newman MB, Saporta S, Sanchez-Ramos J, Sanberg PR. Human umbilical cord blood cells express neural antigens after transplantation into the developing rat brain. Cell Transplant. 2002;11:265–274. [PubMed] [Google Scholar]


