Abstract
Animal personalities are common across taxa and have important evolutionary and ecological implications. Such consistent individual differences correlate with important life-history traits such as dispersal. Indeed, some environmental conditions are supposed to determine dispersers with a specific personality. For example, an increased density should promote the departure of individuals with less social tolerance. Therefore, we hypothesized that dispersers from high-density populations should primarily be asocial individuals, whereas dispersers from low-density populations should be social individuals. In the common lizard (Lacerta vivipara), we measured attraction towards the odour of conspecifics on juveniles at birth as a metric of social tolerance. We then released these juveniles into populations of different densities and measured dispersal and settlement behaviours with regard to social tolerance. One year later, we again measured the social tolerance of surviving individuals. The social tolerance is constant across time and strongly reflects the individual's dispersal and settlement patterns with respect to population density. These results strongly suggest that social personalities exist and influence dispersal decisions. Further studies will help to elucidate the proximate and ultimate determinants of social personalities.
Keywords: behavioural differences, personality, dispersal, sociability, habitat selection, common lizard
1. Introduction
The ecology of personality is a fast-growing field in animal behaviour (Dall et al. 2004; Sih et al. 2004). For several decades, psychologists have explored the considerable range of human and non-human personalities (reviewed in Gosling & John 1999; Gosling 2001), primarily in reference to a deviation from a norm. While such studies aimed to find a common origin of personalities to resolve human psychological problems (Gosling 2001), behavioural ecologists propose an adaptive framework to these presumed non-adaptive individual differences (Dall et al. 2004; Sih et al. 2004). ‘Personality differences’, defined as consistent individual differences across time and contexts, have been observed in numerous behaviours (Clobert et al. 1994; Verbeek et al. 1994; Marchetti & Drent 2000; Dingemanse et al. 2003b, 2004; Sih et al. 2004; Dall et al. 2004; Aragon et al. 2006). Personality differences have been found in exploration, aggressiveness, reactivity and boldness, and are observed across taxa and in both vertebrates and invertebrates (Dingemanse et al. 2003b; Sinn & Moltschaniwskyj 2005). While in many species some individuals avoid social interactions and others search for conspecifics (Gosling & John 1999), social personality, or sociability, has been rarely studied in non-human species and particularly with an ecological perspective (Gosling & John 1999).
Differences in personalities have been found to correlate with important life-history traits such as reproduction and dispersal (Fraser et al. 2001; Dingemanse et al. 2003a; Both et al. 2005). For instance, recent studies on Trinidad killifish (Rivulus hartii) and great tits (Parus major) revealed that natal dispersal distance is positively correlated to boldness (Fraser et al. 2001; Dingemanse et al. 2003a). Dispersal is a typical response to locally degrading conditions (Clobert et al. 2001). However, not all individuals disperse with respect to the same environmental factor (Clobert et al. 2001). For example, competition among conspecifics or among kin can lead to the departure of particular phenotypes (Léna et al. 1998; Le Galliard et al. 2003; Moore et al. 2006), and dispersal has been shown to correlate with social behaviours such as cooperation (Sinervo & Clobert 2003; Le Galliard et al. 2005b). Recent theoretical and empirical work on the evolution of altruism, sociality and dispersal suggest links between dispersal and sociality (Sinervo & Clobert 2003; Le Galliard et al. 2005b; Sinervo et al. 2006a). For a long time, dispersal has been seen as a means to avoid negative effects of intraspecific competition (Lambin et al. 2001; Clobert et al. 2004). At the same time, behavioural ecologists were demonstrating that settlement (i.e. habitat selection) probability was increased in the presence of conspecifics (Crespi & Taylor 1990; Lambin et al. 2001; Stamps 2001; Doligez et al. 2004). Two opposite responses to increasing density have currently been documented: a higher dispersal rate (reviewed in Lambin et al. 2001) and a higher settlement rate (Stamps 1991; Lambin 1994; Denno & Peterson 1995; Le Galliard et al. 2003). Various interpretations have been given to explain such varying responses of dispersal to density (reviewed in Lambin et al. 2001), but none have attempted to integrate findings from ecologists and behaviouralists. If density is informative about both crowding and habitat suitability, then the sign of the relationship between dispersal and density is likely to be a function of the balance between increased competition and conspecific attraction (Clobert et al. 2004). As dispersal has either a genetic or a strong maternal determinism (Sinervo et al. 2006a), the above hypothesis militates for the existence of an individual variability in the responses to density, for example, along a trade-off between sociability (attraction towards conspecific) and asociability (sensitivity to crowding). Several recent empirical findings militate for such a scenario. Individuals leaving a high-density population have been found to be phenotypically different from those leaving a low-density population (Léna et al. 1998). In a population density manipulation experiment, dispersers were found to have long-lasting behavioural differences to residents (Aragon et al. 2006). Similarly, in a colonization experiment, individuals colonizing empty habitats were found to display different phenotypes to those in adjacent occupied habitats (Le Galliard et al. 2005a). Therefore, we hypothesized that the phenotypic differences between resident and dispersing individuals might be associated with differences in social personalities and that dispersers from high-density populations should primarily be asocial individuals, whereas dispersers from low-density populations should be social individuals.
To test this hypothesis, we used the common lizard as our model system. This species has a density-dependent dispersal probability, which can be either positive or negative (Massot et al. 1992; Le Galliard et al. 2003). Dispersers have been shown to have particular phenotypes different from non-dispersers in terms of morphology (e.g. body size and body condition), physiology (stress response) and reaction to olfactory cues (Clobert et al. 1994; Léna et al. 1998; de Fraipont et al. 2000; Le Galliard et al. 2003; Meylan & Clobert 2004; Meylan et al. 2004). These particular phenotypes could be associated with documented variability in the sensitivity of individuals to changes in social contexts such as density of conspecifics (Aragon et al. 2006). Thus, the presence of both positive and negative reactions to these social contexts might reflect different social personalities. The results from these previous studies allow us to predict the existence of social personalities associated with dispersal decisions in the common lizard (Lacerta vivipara). To characterize social personalities, we measured the attraction of individuals towards the odour of conspecifics at birth, a metric of social tolerance. We then released these individuals as juveniles into semi-natural populations of different densities and measured juvenile dispersal attempts and the settlement success of these individuals during the following year. Finally, the social tolerance of surviving individuals was again measured after 1 year to estimate the stability of the social personality type across time and contexts.
2. Material and methods
(a) Species, study site and rearing conditions
The common lizard (Lacerta vivipara; Jacquin 1787) is a small lacertid (adult snout–vent length: males, 40–60 mm; females, 45–75 mm) inhabiting humid habitats in Eurasia (Avery 1962). Lizards become active between late March and the beginning of April (Massot et al. 1992) and begin hibernation in late September. Females produce offspring once a year and laying occurs in June–July. Juveniles are independent of their mother immediately after birth and disperse after 10 days of age on average (Massot et al. 2002).
This experiment was conducted using lizards which had been living for the previous 3 years in semi-natural populations at the Ecological Research Station of Foljuif (Seine-et-Marne, 48°17′ N, 2°41′ E; Le Galliard et al. 2003). These semi-natural populations occur in enclosures (10 m×10 m) protected from avian and mammalian predation, which are connected to a one-way 20 m dispersal corridor. The enclosure size is equivalent to the individual's core home range size and the length of the corridor corresponds to the minimal dispersal distance observed in natural populations (Boudjemadi et al. 1999). A trap is located at the extremity of each corridor to catch dispersing lizards. The lizards caught in the corridor trap while leaving their enclosure were considered ‘dispersers’.
In June 2004, we captured all lizards maintained in the enclosures. Males were released a few days after the capture, whereas females were maintained in laboratory until they gave birth. To provide each lizard with the same standardized environment (e.g. food, water, heat, social interactions), pregnant females were individually housed in plastic terrariums (25×15.5×15 cm, containing a 3 cm of soil; Le Galliard et al. 2003). In one corner of the terrarium, a bulb provided heat for thermoregulation and light from 9.00 to 12.00 and 13.00 to 17.00 h. A piece of cardboard and a plastic tube were provided to allow the lizards to hide. Female lizards gave birth in the terrariums and all offspring were thereafter released into semi-natural populations as described in §2c. On the day of birth, all offspring were measured for body length (nearest millimetre), tail length (nearest millimetre) and body mass (nearest milligram), and their sex was determined by counting ventral scales (Lecomte et al. 1992). For later identification, juveniles were individually marked by toe-clipping.
(b) Reaction to conspecific odours
Adult males were selected as the source of odour for two reasons. Previous studies showed that juvenile philopatry was increased in the presence of an adult male odour while it was reduced in the presence of adult female odour (Léna et al. 1998), suggesting that adult male odours are socially attractive to juveniles. Second, while juveniles are sensitive to the odour of adult males when selecting a shelter (i.e. juveniles prefer a shelter with or without an odour depending on their origin and morphology), they are indifferent to the odour of other categories of individual (Aragon et al. in press); thus, the important social context again appears to be based on male odour. To obtain olfactory cues, six different pairs of adult males were maintained in the same terrarium for the whole laying period. We collected odours on a piece of absorbent paper placed on the floor of the terrarium. We also created six other terrariums structurally similar to the previous ones and submitted to the same conditions as inhabited terrariums, but they were vacant. This method allows us to obtain pieces of paper differing only in the presence of olfactory cues. We changed the pieces of paper after utilization to avoid a second utilization. The odour was thus collected during 6 days which is sufficient to obtain olfactory cues (Aragon et al. in press). All neonates were tested the day after their birth. Behavioural measurements were performed in plastic terrariums of the same dimensions as maternal terrariums. A piece of egg box (shelter) was added to the centre of the terrarium allowing the lizards to hide and a bulb provided heat for thermoregulation. Each lizard was tested separately in a cleaned terrarium, beginning either with the paper with the odour or with the paper without the odour. This allows us to completely separate the effect of olfactory cues from all other potential effects of experimental procedure. We placed the odorized paper under the shelter. The lizard could choose between staying under the shelter with a conspecific's odour or leaving the shelter and being exposed. We quantified the time spent under the shelter when faced with a conspecific's odour as a metric of social tolerance. The same observer performed all the tests. Each lizard was introduced into a terrarium with the piece of absorbent paper and left for 5 min to acclimate. Then, the time spent hidden under the shelter was measured during 10 min with the software ‘The Observer’. After these measurements, the piece of paper was changed, but lizards were not moved. After 5 min, we again measured the time spent hidden under the shelter during 10 min. The lizard was then removed and placed in the terrarium of its mother. We reiterated the same procedure for all offspring. When it was impossible to test all neonates born within the same day, we randomly selected at least two juveniles per family (one male and one female) for testing. The tests done within the same day were temporally homogeneous for individual characteristics and subsequent density treatment (p>0.2).
In April 2005, 32 one-year old lizards (i.e. two lizards per enclosure) were captured during a 4-day session. Lizards were individually maintained as described previously. Two days after capture, we measured the reaction to conspecific odour of all these lizards. The same protocol used with neonates was applied to measure the reaction as well as to obtain olfactory cues. For 17 of these 32 one-year old lizards, the reactions to conspecific odour were also tested at birth. This allowed us to test for the stability of the behaviours during the first year of life.
(c) Field study
All males and females came from populations kept in semi-natural conditions in Foljuif (see §2a). In June 2004, 96 adult males and 176 adult females were captured. We then created 16 semi-natural populations with two density levels. Population densities were either high (adults: 8 males, 12 females; yearlings: 10 males, 10 females and 34 juveniles) or low (adults: 4 males, 6 females; yearlings: 5 males, 5 females and 17 juveniles) with each density level being applied to eight populations (Le Galliard et al. 2003). All 16 populations had age and sex structures similar to natural populations (Massot et al. 1992) and individual characteristics (i.e. body size, body mass and date of birth for juveniles) were not different between levels of density treatment for all age and sex classes (p>0.5 for all). Males and all yearlings were released 7 days after their initial capture, whereas juveniles and their mothers were released 2 days after laying. The release of juveniles and females (just after females' laying) began three weeks after capture and spread over three weeks. However, 85% of the juveniles were released within two weeks after the laying started. We maintained the ratio between density treatments (1 : 2) during the whole laying period by releasing juveniles accordingly, such that densities were different during the whole releasing period. Moreover, no dispersal event was observed before all juveniles were released. Dispersal was monitored daily from release of the first family through hibernation. Dispersers were measured (body size and weight) and released in another population the same day. We released dispersers randomly in a high- or low-density population. High- and low-density populations received exactly the same number of juveniles, but juveniles were randomly assigned to a population. Therefore, on average, juveniles released in high- and low-density populations did not react differently to odour at birth (density in arrival population: F1,11=0.23, p=0.64; density in arrival population×density in initial population: F1,8=0.45, p=0.52). This procedure allowed us to measure a second dispersal attempt, and thus settlement decision depending on the densities in the destination and initial populations. After release in their destination population, dispersers had the possibility to disperse again in the same manner as the first dispersal. If a disperser again left its new population, we removed it from the experiment and we released it in a non-experimental enclosure. When a juvenile dispersed from a population, an immigrant was released in the following days. It allowed us to maintain the density constant, and thus to avoid changes in the intensity of density treatments.
(d) Statistical analyses
Our analyses aimed to determine whether the dispersal status of lizards depended on the reaction to conspecific odours and to the density of their population. The dispersal status was analysed using the GLIMMIX procedure in SAS v. 8.02 (Littell et al. 1996) with a logit link function and a binomial error term. The fixed effects were the density of the population, the odour-dependent time spent hidden (time spent hidden when the piece of paper contained odours minus time spent hidden when the piece of paper contained no odour) and their interaction. We also added individual covariates (body length and body mass) and sex as additional factors. The random effects were populations nested within the density treatment and family nested within the population. The probability of a second dispersal event was analysed on all the juveniles released (juveniles tested for the reaction to odour and juveniles not tested) using the GENMOD procedure in SAS v. 8.02 with a logit link function and a binomial error term. The fixed effects were the density of the ‘natal’ population, the density of the arrival population, sex and all interaction terms. Dispersers were released equally in all populations and there were no family biases. Independent contrasts were performed for the first and second dispersal attempts using the CONTRAST option of each statistical procedure used to analyse dispersal probability. The correlation between reactions to conspecific odour at birth and after 1 year was tested using the MIXED procedure in SAS v. 8.02. This allowed us to add a population effect as a random factor and the density treatment as a fixed effect. We could thus test for environmental dependence of behavioural reaction. The assumptions of these models were verified on residuals. Likelihood ratios were used to test the significance of each factor or interaction term. Simplification of all these models was made using backward elimination of the non-significant terms. Significance level was set at p=0.05.
3. Results
(a) Reaction to conspecific odour throughout the life
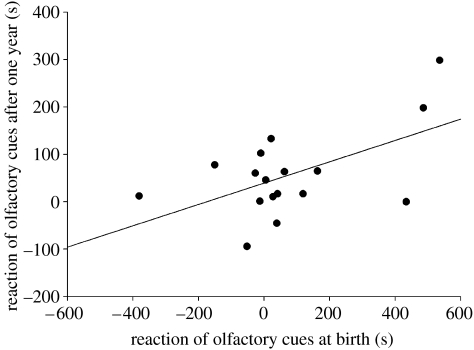
The reaction towards conspecific odour measured in April 2005 (1 year after the beginning of the experiment) was positively correlated with the reaction measured at birth (n=17, F1,7=13.17, p=0.0084, estimate 0.25±0.07, R2=0.31, figure 1). The density of the population did not affect the reaction after 1 year nor the correlation between the reactions throughout the life (density: F1,6=0.66, p=0.45; reaction measured at birth×density: F1,5=0.01, p=0.92). One year later, the reaction to odour did not also depend on the population of the juvenile (Wald z-tests–population: z=0.77, p=0.22; reaction measured at birth×population: z=0.51, p=0.30).
Figure 1.
Reactions to olfactory cues through life. Relationship between reaction to olfactory cues at birth and after 1 year. Linear regression is shown.
(b) Reaction to olfactory cues and dispersal decision
The probability of dispersing did not depend on the density of the population nor on the reaction to odour alone (table 1). However, the interaction between the density and the reaction to odour was significant (table 1). Independent contrasts revealed that the dispersal probability from low-density populations is positively related to the odour-dependent time spent hidden (i.e. difference in time spent hidden; F1,145=3.84, p=0.05) or a high attraction for conspecific odour. In contrast, this relationship tended to be negative in high-density populations (F1,145=2.39, p=0.12). A high dispersal probability from low-density populations was thus correlated to a high attraction for conspecific odour.
Table 1.
Natal dispersal probability depends on the reaction to conspecific odour at birth and the density in the population. (Dispersal probability was modelled with mixed effects logistic regressions using the GLIMMIX macro in SAS. The initial model included the density of the population, the odour-dependent time spent hidden (time spent hidden when the piece of paper contained odours minus time spent hidden when the piece of paper contained no odour), sex and body size at birth as fixed factors as well as the random effects of enclosure nested within the origin of immigrants and family nested within enclosure. The final model was obtained by backward elimination, dropping in a stepwise process all non-significant effects. Statistical tests were type III F-tests for fixed effects.)
| natal dispersal probability | |
|---|---|
| density | F1,14=2.10, p=0.17 |
| reaction to conspecific odour at birth | F1,145=1.45, p=0.23 |
| density×reaction to conspecific odour at birth | F1,145=5.79, p=0.0174 |
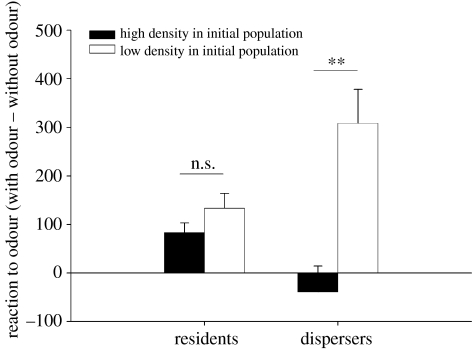
Non-dispersing juveniles of the two density treatments did not differ in the time spent with the male odour (residents: F1,145=0.95, p=0.33; figure 2). Among dispersers, those from low population density spent more time with male odour than those from high population density treatments (contrasts, dispersers: F1,145=8.58, p=0.004; figure 2). Sex, body length and date of birth had no significant effect on the probability of dispersal (sex: F1,143=0.04, p=0.83; body length: F1,143=0.36, p=0.55; date of birth: F1,143=0.57, p=0.44) but lizards in better condition at birth dispersed more (body condition: F1,145=4.95, p=0.03).
Figure 2.
Reaction to olfactory cues at birth depending on densities in initial populations and dispersal status. Differences of time spent hidden with the olfactory cues minus the time spent hidden without the olfactory cues. Mean difference (seconds±s.e.) in relation to density treatments and dispersal status is shown (n.s. p>0.05; **p<0.01).
(c) Settlement of dispersers
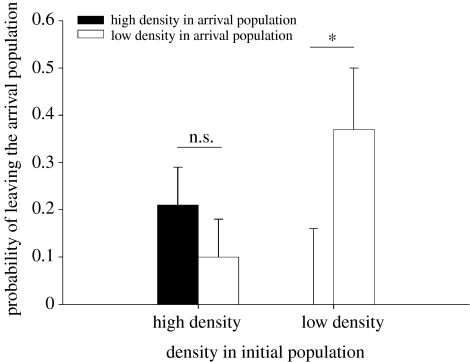
Dispersers from low- and high-density populations (i.e. 52 juveniles) were introduced randomly in high- and low-density populations. All of the 16 populations received dispersers and we homogenized the number of dispersers across populations. Among all dispersers, 17% of dispersers left their arrival populations. The probability of leaving their arrival population was dependant on the interaction between density of the arrival populations and density of the initial populations (initial density×arrival density: χ1=6.33, p=0.0119; figure 3). Independent contrasts showed that dispersers from low-density populations settled more in high-density populations than in low-density populations (χ1=5.21, p=0.02) and the opposite pattern was observed, but was not significant for dispersers from high-density populations (χ1=1.25, p=0.2641). Interestingly, no dispersers coming from low-density populations and introduced into high-density populations left their arrival populations. Female dispersers left their arrival populations more often than male dispersers (χ1=4.31, p=0.0379). All these effects remained significant when we added date of dispersal in the model and there was no relationship between date at dispersal and the probability of settlement (χ1=1.32, p=0.25).
Figure 3.
Settlement in arrival populations and interaction between densities in initial and in arrival populations. Differences between density treatments in the probability that a disperser leaves his arrival population. Mean probability (±s.e.) in relation to density treatments is shown (n.s. p>0.05, *p<0.05).
4. Discussion
(a) Consistent social personalities
The reaction towards the odour of a pair of males can be interpreted either as juvenile sensitivity to interactions among males and/or to their simple presence. During another experiment, we quantified some characteristics of the six pairs of males used to collect odour (e.g. males' body size, body condition, coloration, age, bites). Juveniles did not show any reaction towards the variation in males' characteristics within the pair (p>0.5 for effects of males' body size, body condition, coloration, age and bites on reaction towards odour). If juvenile sensitivity was linked to adult male interactions, pairs of males displaying strong interactions should not induce the same reaction as pairs of males displaying weak interactions (e.g. no bites). Therefore, male presence alone is a more probable explanation for variation in sensitivity, which suggests that the juvenile reaction towards male odour measures social tolerance or phobia. In this study, we used the odour only of adult males, but juvenile reactions could be related to the sex of the signaller. As stated in §2, adult females seem to be perceived as important competitors by juveniles since they promote juvenile dispersal (Léna et al. 1998), and experimental manipulation of density has indeed shown that females are closer competitors of juveniles (yearlings) than adult males (Massot et al. 1992; Lecomte et al. 1994). Furthermore, the sensitivity of juveniles was not dependent on their gender, and we should not have observed such a result if the signal was indicating the gender of the adult. These points strongly suggest that males are an indicator of something other than their gender alone. One year later, the same individuals displayed a similar behaviour towards the presence of male odours as they did at birth and this reaction did not depend on the environmental conditions (i.e. density and population) undergone during juvenile growth. This demonstrates that the individual's response to a same social context is constant through time and context, a trait that is a characteristic of personalities. The above two results strongly suggest the existence of social personalities in this lizard species. Social personalities, here measured by the reaction to odour, might be part of a more general behavioural pattern. Indeed, personality traits, such as boldness, aggressiveness and exploration, are correlated types of behaviour (Verbeek et al. 1996; Marchetti & Drent 2000; Sih et al. 2004) which might correspond to a suit of behavioural traits defining some individual strategies. Some individuals therefore exhibit a set of personality traits likely to constitute behavioural syndromes (Sih et al. 2004), which can be genetically and/or developmentally controlled (Dufty et al. 2002; Sih et al. 2004). Our study develops the idea that behavioural syndromes may also exhibit a social component, which might be seen as complementary to other personality traits such as boldness, aggressiveness and exploration. For example, we might predict that, according to the pattern observed in other species, and particularly in humans, ‘asocial’ lizards will also be shyer and less aggressive individuals.
(b) Social personalities, dispersal and habitat selection
Individuals who dispersed from low- and high-density populations had different social phenotypes. Dispersers from low-density populations were attracted to the odour of males, while dispersers from high-density populations tended to be repulsed by male odour. Indeed, only dispersers from high-density populations reacted negatively to male odour. These results indicate that juveniles have different social personalities, which affect their reaction to social context (i.e. density). Some juveniles leave to search for more socially attractive or dense environments when faced with reduced social interactions. These individuals display social attraction to odour at birth. In contrast, other juveniles prefer to leave environments with too much social interaction. These individuals are repulsed by the presence of conspecifics at birth.
If the above scenario is true, we should expect dispersers from low-density populations to settle more often when released in high-density destinations than when released in low-density destinations. This is indeed what we found. This result reinforces the idea that dispersers from low- and high-density populations exhibit different social personalities and search for different social habitats. All dispersers from low-density populations settled when they were released in a high-density population. Even if the number of dispersers was low (i.e. 52 dispersers), this dependence of settlement on the density in natal and arrival populations is completely concordant with the other results and strongly supports the hypothesis. The lack of secondary attempts for the dispersers originating from low-density populations and released in high ones is not the result from these individuals dying before being able to move again, because they were found to survive for at least a month after release. Furthermore, all secondary dispersal attempts were completed within five days of the release in their novel populations.
Population density is known to affect dispersal in numerous ways. Here, we showed that the perception of density varies among individuals, which results in strong variability of which individuals disperse with respect to natal density. Here, the age and context invariance of the behavioural response to adult male odour strongly suggests the existence of fixed social personalities (Aragon et al. in press; Meylan & Clobert submitted), which is subsequently associated with dispersal decisions. Low densities repulse some individuals while attract others. Our results contradict the assumption that every disperser prefers the same habitat. While several theoretical reviews have recently developed this idea (Stamps 2001; Clobert et al. 2004), experimental tests are still quite scarce. To explain such differences, both proximate and ultimate causes of dispersal should be considered as well as their associated costs and benefits (Massot et al. 2002). For example, costly kin interactions are selecting for the departure of pioneer individuals which are not sensitive to density (and might display their own particular behavioural syndrome; Cote et al. submitted; Léna et al. 1998; Le Galliard et al. 2003) while, even within the same species, beneficial kin-like interactions will promote philopatry with individuals displaying mutualistic or even altruistic behaviours. In the latter case, such behaviours have been proved to be adaptive (Sinervo & Clobert 2003; Sinervo et al. 2006a,b).
Intraspecific competition is likely to act in a different way. Indeed, high-density populations can indicate either a good quality habitat (conspecific attraction) or costs linked to conspecific competition. Both have been documented in our species (Massot et al. 1992; Léna et al. 1998; Le Galliard et al. 2003). First, conspecific attraction would be beneficial if the fitness of juveniles is increased in high-density populations (Shields et al. 1988; Stamps 2001). Proximate mechanisms of conspecific attraction have been shown to be reduced predation rate, reduced costs of settlement and increased future reproductive success (Shields et al. 1988; Stamps 2001). Conversely, conspecific repulsion might be explained by high levels of competition and aggressiveness (Shields et al. 1988; Stamps 2001). The latter causes should mostly affect lizards with low competitive abilities and low aggressive level, which might correspond to ‘asocial’ individuals. However, one might ask why/how such different personalities (syndromes) are maintained within a population. Recently, theoretical models have shown that the polymorphism of dispersal strategies can be generated and maintained whenever the direction of selection was different within the different patches within a metapopulation (Doebeli & Ruxton 1997). If variations in densities are sufficient to generate such differences in selective pressure within a metapopulation, one can then predict the existence of different dispersal strategies, i.e. different dispersal-based personalities or syndromes. The widespread existence of density-dependent dispersal strategies is however likely to depend on the possibility that a species has to acquire some knowledge about the density in its own and in surrounding populations. Some recent findings militate for such a possibility. First, common lizards have recently been demonstrated to acquire environmental information during social interactions (Aragon et al. in press; Boudjemadi et al. 1999) either through private or public (socially acquired) information. Second, there is evidence that they can recognize the dispersal status of conspecifics (Aragon et al. 2006, in press). In this way, social personalities might reflect variable abilities in acquiring public information (Marchetti & Drent 2000) or variable balance between private and public information-based decision.
The existence of such social personality polymorphism might have a considerable impact on understanding the evolution of condition-dependent dispersal strategies. In our case, for example, the relationship between density and dispersal can then be either positive or negative depending on the frequency of each type of individual, and it offers an alternative explanation to the recently reported variations in the direction of the correlation between density and dispersal (Lambin et al. 2001; Le Galliard et al. 2003; Clobert et al. 2004). More generally, dispersers with different personalities (or behavioural syndromes) might be a good research avenue for understanding the multiple causes of dispersal evolution (Gandon & Michalakis 2001; Clobert et al. 2004) and the way they interact. Recent studies indeed propose that different selective pressures induce the evolution of dispersers with specific phenotypes in terms of morphology, physiology and also behaviour (Léna et al. 1998; Le Galliard et al. 2003; Meylan et al. 2004; Moore et al. 2006), leading to complex and variable associations among these phenotypic variables. Different selective pressures resulted in specific traits associations or ‘phenotypic syndromes’ conferred to philopatric or dispersing individuals such that it minimizes the cost associated with each strategy. For example, the common lizard seems to exhibit three dispersing phenotypic syndromes: (i) colonizers (i.e. dispersers with high success in empty habitat (Cote et al. submitted), mainly promoted by kin competition), (ii) dispersers attracted by low-density populations, and (iii) dispersers attracted by high-density populations. Such variations are also to be expected in philopatric strategies (i.e. sneaking, mutualistic or altruistic behaviour). These three types of dispersers should affect the composition of a metapopulation, both at a spatial and at a temporal scale. Indeed, an empty habitat should be colonized by the first type of dispersers, then dispersers attracted by low densities should reinforce the population, and finally dispersers of the third type should be attracted by high densities.
Our results should therefore have important implications in the understanding of metapopulation dynamics and dispersal evolution with respect to crowding. Particularly, it proposes an alternative explanation to opposite dispersal density-dependant responses. More generally, it might strongly change our views on metapopulation functioning and resilience. However, more research will be needed to better understand how and under which conditions these social personalities are produced, as well as their impact on metapopulation functioning and evolution.
Acknowledgments
The authors are grateful to Alexis Chaine, Joël White, Sandrine Meylan, David Laloi and three anonymous referees for their comments on this article. Claire Lamotte, Simon Boudsocq and Laurent Carlier kindly assisted during the experiment. The authors acknowledge the Ecole Normale Supérieure for technical support at the Biological Station of Foljuif. The work was financially supported by the Observatoire de Recherche en Environnement no. 53 of the Ministère de la Recherche française.
References
- Aragon P, Clobert J, Massot M. Individual dispersal status influences spacing behavior of conspecific residents. Behav. Ecol. Sociobiol. 2006;60:430–438. [Google Scholar]
- Aragon, P., Massot, M., Clobert, J. & Gasparini, J. In press. Socially acquired information through chemical cues in the common lizard. Anim. Behav.
- Avery R.A. Notes on the biology of Lacerta vivipara L. Brit. J. Herpetol. 1962;3:165–170. [Google Scholar]
- Both C, Dingemanse N.J, Drent P.J, Tinbregen J.M. Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 2005;74:667–674. doi:10.1111/j.1365-2656.2005.00962.x [Google Scholar]
- Boudjemadi K, Lecomte J, Clobert J. Influence of connectivity on demography and dispersal in two contrasting habitats: an experimental approach. J. Anim. Ecol. 1999;68:1207–1224. doi:10.1046/j.1365-2656.1999.00363.x [Google Scholar]
- Clobert J, Massot M, Lecomte J, Sorci G, de Fraipont M, Barbault R. Determinants of dispersal behavior: the common lizard as a case of study. In: Vitt J, Pianka E.R, editors. Lizard ecology: the third generation. Princeton University Press; Princeton, NJ: 1994. pp. 183–206. [Google Scholar]
- Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; New York, NY: 2001. [Google Scholar]
- Clobert J, Ims R.A, Rousset F. Causes, mechanisms and consequences of dispersal. In: Hanski I, Gaggiotti O.E, editors. Ecology, genetics and evolution of metapopulations. Elsevier Academic Press; Amsterdam, The Netherlands: 2004. pp. 307–336. [Google Scholar]
- Cote, J., Clobert, J. & Fitze, P. S. Submitted. Kin competition promotes colonisation success. Proc. Natl Acad. Sci. USA [DOI] [PMC free article] [PubMed]
- Crespi B.J, Taylor P.D. Dispersal rates under variable patch density. Am. Nat. 1990;135:48–62. doi:10.1086/285031 [Google Scholar]
- Dall S.R.X, Houston A.I, McNamara J. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 2004;7:734–739. doi:10.1111/j.1461-0248.2004.00618.x [Google Scholar]
- de Fraipont M, Clobert J, John-Adler H, Meylan S. Increased pre-natal maternal corticosterone promotes philopatry of offspring in common lizards Lacerta vivipara. J. Anim. Ecol. 2000;69:404–413. doi:10.1046/j.1365-2656.2000.00405.x [Google Scholar]
- Denno R.F, Peterson M.A. Density-sependant dispersal and its consequences for population dynamics. In: Cappuccino N, Price P.W, editors. Population dynamics: new approaches and synthesis. Academic Press; San Diego, CA: 1995. pp. 113–130. [Google Scholar]
- Dingemanse N.J, Both C, van Noordwijk A.J, Rutten A.L, Drent P.J. Natal dispersal and personalities in great tits (Parus major) Proc. R. Soc. B. 2003a;270:741–747. doi: 10.1098/rspb.2002.2300. doi:10.1098/rspb.2002.2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N.J, Both C, van Noordwijk A.J, Rutten A.L, Drent P.J. Natal personalities in great tits (Parus major) Proc. R. Soc. B. 2003b;270:741–747. doi: 10.1098/rspb.2002.2300. doi:10.1098/rspb.2002.2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N.J, Both C, Drent P.J, Tinbregen J.M. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. B. 2004;271:847–852. doi: 10.1098/rspb.2004.2680. doi:10.1098/rspb.2004.2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebeli M, Ruxton G.D. Evolution of dispersal rates in metapopulation models: branching and cyclic dynamics in phenotype space. Evolution. 1997;51:1730–1741. doi: 10.1111/j.1558-5646.1997.tb05097.x. doi:10.2307/2410996 [DOI] [PubMed] [Google Scholar]
- Doligez B, Pärt T, Danchin E, Clobert J, Gustafsson L. Availability and use of public information and conspecific density for settlement decisions in the collared flycatcher. J. Anim. Ecol. 2004;73:75–87. doi:10.1111/j.1365-2656.2004.00782.x [Google Scholar]
- Dufty A.M, Clobert J, Moller A.P. Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 2002;17:190–196. doi:10.1016/S0169-5347(02)02498-9 [Google Scholar]
- Fraser D.F, Gilliam J.F, Daley M.J, Le A.N, Skalski G.T. Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 2001;158:124–135. doi: 10.1086/321307. doi:10.1086/321307 [DOI] [PubMed] [Google Scholar]
- Gandon S, Michalakis Y. Multiple causes of the evolution of dispersal. In: Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; Oxford, UK: 2001. pp. 155–167. [Google Scholar]
- Gosling S.D. From mice to men: what can we learn about personality from animal research? Psychol. Bull. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. doi:10.1037/0033-2909.127.1.45 [DOI] [PubMed] [Google Scholar]
- Gosling S.D, John O.P. Personality dimensions in nonhuman animals: a cross-species review. Curr. Dir. Psychol. Sci. 1999;8:69–75. doi:10.1111/1467-8721.00017 [Google Scholar]
- Lambin X. Nata philopatry, competition for resources, and inbreeding avoidance in Townsend's voles (Microtus townsendii) Ecology. 1994;75:224–235. doi:10.2307/1939396 [Google Scholar]
- Lambin X, Aars J, Piertney S.B. Dispersal, intraspecific competition, kin competition and kin facilitation: a review of the emperical evidence. In: Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; New York, NY: 2001. pp. 110–122. [Google Scholar]
- Lecomte J, Clobert J, Massot M. Sex identification in juveniles of Lacerta vivipara. Amphibia–Reptilia. 1992;13:21–25. [Google Scholar]
- Lecomte J, Clobert J, Massot M, Barbault R. Spatial and behavioural consequences of a density manipulation in the common lizard. Ecoscience. 1994;1:300–310. [Google Scholar]
- Le Galliard J.F, Ferrière R, Clobert J. Mother–offspring interactions affect natal dispersal in a lizard. Proc. R. Soc. B. 2003;270:1163–1169. doi: 10.1098/rspb.2003.2360. doi:10.1098/rspb.2003.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Galliard J.F, Ferrière R, Clobert J. Effect of patch occupancy on immigration on the common lizard. J. Anim. Ecol. 2005a;74:241–249. doi:10.1111/j.1365-2656.2005.00912.x [Google Scholar]
- Le Galliard J.F, Ferrière R, Dieckmann U. Adaptive evolution of social traits: origin, trajectories, and correlations of altruism and mobility. Am. Nat. 2005b;165:206–224. doi: 10.1086/427090. doi:10.1086/427090 [DOI] [PubMed] [Google Scholar]
- Léna J.-P, Clobert J, de Fraipont M, Lecomte J, Guyot G. The relative influence of density and kinship on dispersal in the common lizard. Behav. Ecol. 1998;9:500–507. [Google Scholar]
- Littell R.C, Miliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute, Inc.; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Marchetti C, Drent P.J. Individual differences in the use of social information in foraging by captive great tits. Anim. Behav. 2000;60:131–140. doi: 10.1006/anbe.2000.1443. doi:10.1006/anbe.2000.1443 [DOI] [PubMed] [Google Scholar]
- Massot M, Clobert J, Pilorge T, Lecomte J, Barbault R. Density dependence in the common lizard: demographic consequences of a density manipulation. Ecology. 1992;73:1742–1756. doi:10.2307/1940026 [Google Scholar]
- Massot M, Clobert J, Lorenzon P, Rossi J.-M. Condition-dependent dispersal and ontogeny of the dispersal behaviour: an experimental approach. J. Anim. Ecol. 2002;71:253–261. doi:10.1046/j.1365-2656.2002.00592.x [Google Scholar]
- Meylan S, Clobert J. Maternal effects on offspring locomotion: influence of density and corticosterone elevation in the lizard Lacerta vivipara. Physiol. Biochem. Zool. 2004;77:450–458. doi: 10.1086/383508. doi:10.1086/383508 [DOI] [PubMed] [Google Scholar]
- Meylan, S. & Clobert, J. Submitted. Are dispersal-dependant personalities produced by phenotypic plasticity? Behav. Ecol. [DOI] [PubMed]
- Meylan S, de Fraipont M, Clobert J. Maternal size, and stress and offspring philopatry: an experimental study in the common lizard (Lacerta vivipara) Ecoscience. 2004;11:123–129. [Google Scholar]
- Moore J.C, Loggenberg A, Greeff J.M. Kin competition promotes dispersal in a male pollinating fig wasp. Biol. Lett. 2006;2:17–19. doi: 10.1098/rsbl.2005.0370. doi:10.1098/rsbl.2005.0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields W.M, Crook J.R, Hebblethwaite M.L, Wiles-Ehmann S.S. Ideal free coloniality in the swallows. In: Slobodchikoff C.N, editor. Ecology of social behavior. Academic Press; New York, NY: 1988. pp. 189–228. [Google Scholar]
- Sih A, Bell A, Johnson J.C. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 2004;19:372–377. doi: 10.1016/j.tree.2004.04.009. doi:10.1016/j.tree.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Sinervo B, Clobert J. Morphs, dispersal behavior, genetic similarity, and the evolution of cooperation. Science. 2003;300:1949–1951. doi: 10.1126/science.1083109. doi:10.1126/science.1083109 [DOI] [PubMed] [Google Scholar]
- Sinervo B, Calsbeek R, Comendant T, Both C, Adamopoulou C, Clobert J. Genetic and maternal determinants of effective dispersal: the effect of sire genotype and size at birth in side-blotched lizards. Am. Nat. 2006;168:88–99. doi: 10.1086/505765. doi:10.1086/505765 [DOI] [PubMed] [Google Scholar]
- Sinervo B, et al. Self-recognition, color signals, and cycles of greenbeard mutualism and altruism. Proc. Natl Acad. Soc. USA. 2006;103:7372–7377. doi: 10.1073/pnas.0510260103. doi:10.1073/pnas.0510260103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn D.L, Moltschaniwskyj N.A. Personality traits in dumpling squid (Euprymna tasmanica): context-specific traits and their correlation with biological characteristics. J. Comp. Phys. 2005;119:99–110. doi: 10.1037/0735-7036.119.1.99. [DOI] [PubMed] [Google Scholar]
- Stamps J.A. The effect of conspecifics on habitat selection in territorial species. Behav. Ecol. Sociobiol. 1991;28:29–36. doi:10.1007/BF00172136 [Google Scholar]
- Stamps J.A. Habitat selection by dispersers: integrating proximate and ultimate approaches. In: Clobert J, Danchin A.A, Nichols J.D, editors. Dispersal. Oxford University Press; New York, NY: 2001. pp. 110–122. [Google Scholar]
- Verbeek M.E.M, Drent P.J, Wiepkema P.R. Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 1994;48:1113–1121. doi:10.1006/anbe.1994.1344 [Google Scholar]
- Verbeek M.E.M, Boon A, Drent P.J. Exploration, aggressive behaviour and dominance in pair-wise confrontations of juvenile male great tits. Behaviour. 1996;133:945–963. [Google Scholar]