Abstract
The stress response is highly variable among individuals, but the causes of this variation remain largely unknown. In response to stressors, vertebrates secrete elevated levels of glucocorticoids which enhance survival, but concurrently interfere with reproduction. We tested the hypothesis that individuals flexibly modulate their stress response with respect to the reproductive value of their brood in free-living house sparrows (Passer domesticus). We experimentally increased or decreased clutch size during the nestling period and found that parents tending enlarged clutches responded less strongly to a stressor than those tending reduced clutches. In addition, we examined whether individuals responded less strongly to a stressor as the breeding season progressed and future reproductive opportunities declined. We found that the stress response decreased with breeding date during the birds' first breeding attempt, but it remained constant during their second breeding attempt. Within-individual variability in the stress response was related to the brood size manipulations the birds received in their two consecutive breeding attempts. These results provide the first experimental support for the hypothesis that individuals actively modulate their stress response with respect to the value of current reproduction.
Keywords: stress-response modulation, corticosterone, life history, parental care, brood size manipulation, Passer domesticus
1. Introduction
A key concept in life-history theory is that energy allocated to the current reproductive event can only be increased at the expense of survival and hence future reproduction (Stearns 1992). This trade-off reflects the ‘cost of reproduction’ (Williams 1966) which is a fundamental component determining the level of ‘parental investment’ (Trivers 1972). However, parental investment depends not only on its costs, but also on the benefits from the current reproduction, e.g. the value of the offspring. Although these factors have been shown to shape the animals' reproductive decisions, our knowledge is still scarce about the actual mechanisms by which these costs and benefits are assessed and integrated within the animals.
Recent theoretical models of resource allocation have shown that the life-history decisions may be influenced by the physiological state of individuals (McNamara & Houston 1996; Houston & McNamara 1999). In that respect, some hormones play a major role in the mediation of such life-history decisions (Sinervo & Svensson 1998). In vertebrates, stressors such as food limitation, predators and inclement weather induce a rapid elevation of circulating glucocorticoids, which promotes survival by the mobilization of energy resources and the occurrence of those behavioural forms that enhance the immediate survival, but may also compromise reproductive and parental behaviour in a longer term. This adrenocortical response to stress is thought to be adaptive, because it may interrupt the current reproductive episode of an individual and promote its survival for future reproduction (Wingfield & Sapolsky 2003). The glucocorticoid levels therefore seem to have a role in mediating this fundamental life-history trade-off.
According to life-history theory, when the value of the current reproduction is high relative to the value of future reproduction and survival, the stress response should be attenuated to ensure that reproduction is not inhibited. The stress response has been shown to be suppressed during the stage of the breeding cycle when adults are providing the most care for young (also referred to as the ‘parental care hypothesis’; Wingfield et al. 1995) in the sex that provides more care for offspring (O'Reilly & Wingfield 2001), and when breeding opportunities are limited and the probability of rebreeding is low (also referred to as the ‘short season hypothesis’; Wingfield et al. 1995; Silverin & Wingfield 1998; Holberton & Wingfield 2003). These studies and recent empirical data (e.g. Heidinger et al. 2006) are consistent with the hypothesis that individuals suppress the stress response when the value of current reproduction is high. However, these studies did not use experimental manipulations, but compared different populations or individuals in different breeding stages, which may have confounded the results and therefore left the topic open for debate. Moreover, the basic yet untested underlying assumption of these predictions is that the same individuals may give different stress responses according to the value of their current reproduction, i.e. that individuals are able to actively modulate their hormone levels.
In this study, we simultaneously tested multiple predictions of the ‘value of reproduction’ hypothesis in breeding house sparrows (Passer domesticus). First, to test experimentally whether the value of the current brood influences the parents' stress response, we manipulated the brood size of parents by adding or subtracting two chicks and compared the response to capture/restraint stress between the experimental groups. We predicted that the rise in corticosterone levels in response to stress would be weaker in birds with increased broods than in birds with reduced broods. Second, in the study population, there is a considerable individual variation in the onset of breeding: some birds start breeding early in the season and may have up to three subsequent clutches, whereas an important proportion of the population start the reproduction later and have only one breeding attempt (Chastel et al. 2003). As the probability of renesting decreases over the breeding season, we used the date of breeding as a surrogate measure to test whether birds with different future breeding opportunities differ in their stress response. To control for the effects of breeding stages, in the present study, we used only parents tending large chicks, i.e. a period when the brood were close to fledging and parents soon either to renest or finish their reproduction for the season. Specifically, we predicted that individuals breeding at the beginning of the season (high renesting probability) would have a stronger adrenocortical response to capture/restraint stress than those that bred later (low renesting probability). Finally, since several birds were sampled both in their first and second breeding attempts, we tested whether the experimental manipulations explained within-individual variance in stress response between the birds' two consecutive breeding attempts, i.e. whether individuals modulated their hormonal stress response according to the actual breeding circumstances.
2. Material and methods
(a) Study species and population
The study was carried out between March and July 2005 on a free-living population of house sparrows that breed in nest boxes in Chizé, France (46°09′ N, 0°24′ W; Chastel et al. 2003). A large proportion of the adults used in this study were first captured during the pre-breeding period using mist nets and marked with a unique metal ring and colour combination. The nest boxes were monitored daily to determine laying dates, clutch sizes, hatching dates and the number of hatchlings.
(b) Brood size manipulation
To experimentally manipulate the reproductive value of the brood, the clutch sizes were either increased or decreased as follows. Two days after hatching, two nestlings were randomly chosen and transferred between two synchronous broods, resulting in enlarged and reduced clutches (Curlee & Bessinger 1995; Chastel & Kersten 2002). Owing to the moderate number of available clutches, we did not use a control group to maximize the sample sizes in the manipulated broods and to maximize the difference in brood sizes between the experimental groups. If no synchronous broods were available to exchange nestlings between nests at exactly the same age (2 days old), or if the original brood was too small (two or three chicks) to be reduced or too large (six chicks) to be enlarged, it was not manipulated. Experimental brood sizes were within the natural variation for this population and ranged between two to four nestlings (reduced broods) and five to seven nestlings (enlarged broods). House sparrows readily accept manipulated broods and adjust their level of parental care accordingly (Seel 1969; Hegner & Wingfield 1987; Chastel & Kersten 2002; Bonneaud et al. 2003).
(c) Measuring the stress response
To measure the stress response, we used a standard capture–handling–restraint protocol that has been successfully used in numerous field studies (Wingfield 1994). Breeding sparrows were captured in their nest box using a wire trap, when nestlings were 10–12 days old. Immediately after capture, a small blood sample (50–100 μl) was collected from the brachial vein and the time required to collect the sample was recorded. Mean handling time was 2:37±0:02 (s.e.m.) min: sec, maximum 3:09 min: sec. Corticosterone levels measured at the initial bleeding were not related to handling time (r=0.206, p=0.112, N=61). Therefore, we assume that the corticosterone levels measured at capture were representative of the circulating baseline hormone levels prior to the capture. In addition, we found no significant effect of the time of capture on corticosterone.
Following the collection of initial blood samples, the birds were placed in cloth bags and subsequent samples were collected after 30 min. We chose 30 min as the time for the second sample because previous studies on this species have shown that it is a good proxy for the maximum corticosterone levels (Breuner & Orchinik 2001; Lindström et al. 2005). After the second blood sample was collected, we measured tarsus length (±0.1 mm) and body mass (±0.1 g) before releasing the birds. The blood samples were kept on ice and centrifuged (5000 rpm (1396 g), 6 min) as soon as possible. Plasma was separated and stored at −20°C for further analyses.
(d) Corticosterone assay
Plasma corticosterone levels were determined by radioimmunoassay at the Centre d'Etudes Biologiques de Chizé following the procedure detailed in Lormée et al. (2003). Corticosterone was extracted from a 20 μl plasma sample and determined in duplicates run in one assay (coefficient of intra-assay variation was 4.3%, N=6 duplicates).
(e) Data processing and analysis
To determine whether the capture–restraint protocol elevated the corticosterone levels, we used repeated measures ANOVA. The magnitude of stress response was expressed as the maximum levels of corticosterone. We also calculated the rate of increase from baseline to maximum corticosterone levels per minute (ng ml−1 min−1; Silverin et al. 1997; Lindström et al. 2005). The latter measure of stress response was highly correlated with the maximum levels of corticosterone (r=0.920, p<0.0001), and the statistical analyses of these variables gave nearly identical results, hence we report only the results for maximum corticosterone levels to facilitate the comparison with other published results. To analyse seasonal changes in the baseline and stress-induced hormone levels, we used the date of blood sampling as the independent variable in the models.
Seventy per cent of the individuals included in this study were captured only during their first or second breeding attempt, hence the two attempts were analysed separately. No parents tending their third broods were included in this study. The inter-individual differences in the effects of manipulations were analysed only in the individuals captured in manipulated broods.
We also made two comparisons between breeding attempts. First, we compared the stress response of ‘early’ and ‘late’ breeders. Late breeders were defined as the individuals starting their first reproductive attempt when others (early breeders) were already tending their second broods (Chastel et al. 2003). Therefore, early and late breeders were compared in a time span where their breeding activity overlapped, i.e. from the earliest second clutch initiation until the last initiation of a first clutch (from 15 May to 13 June, figure 1). Second, for those individuals that were sampled in both their first and the second brood, we also performed within-individual comparisons to investigate whether the variation in the stress response was a function of the differences between the manipulations they received in their first and the second breeding attempts. We created three categories where: (i) the brood size manipulation induced greater brood value in the first brood compared with the second brood (i.e. the first brood was enlarged and the second was either unmanipulated or reduced), (ii) the individual either received the same type of manipulation or was left unmanipulated in both the breeding attempts, and (iii) the brood size manipulation induced lesser brood value in the first brood compared with the second brood (i.e. the first brood was reduced and the second was either unmanipulated or enlarged).
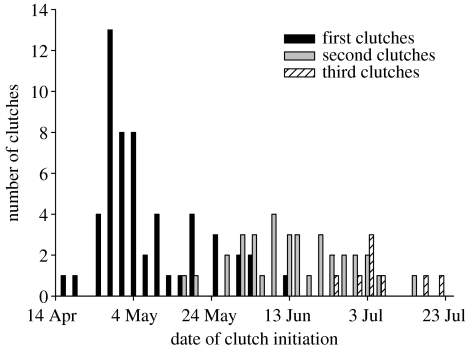
Figure 1.
Number of clutches initiated by house sparrows in 2005 in 3-day periods during the breeding season.
In the nests where both the male and female parent were captured, we found no relationship between the baseline corticosterone levels or the rate of increase in corticosterone levels of the two parents (all p>0.1), indicating that these variables reflect individual variation in the physiological response to the manipulation. Hence, we used males and females as independent data points in our analyses.
To analyse body condition, we used body mass as the dependent variable in a general linear model (GLM) controlling for the tarsus length in the model as a covariate. Dates are presented as the number of days elapsed from the initiation of the first clutch in the population (15 April). Two-tailed probabilities and mean±s.e.m. values are given throughout the paper. We used SPSS v. 11.0 for statistical analyses.
3. Results
(a) The study population
During the breeding season, sparrows initiated nests in three overlapping waves (figure 1). The first wave of clutches were initiated in mid-April, whereas the second wave of clutches were initiated in mid-May at the same time as some individuals which had begun breeding in April were already initiating their second clutches. Most of the individuals in the study population were colour-ringed, hence regular observations of marked individuals and checking of nest boxes confirmed that birds initiating their first clutch had not bred elsewhere. After the first clutch, 46% of the individuals laid a second clutch. The probability of starting a second clutch decreased significantly with the date of the first clutch initiation (binary logistic regression, B=−0.044, p=0.017). From the end of June, 15% of the birds that had a second clutch laid a third clutch, while most birds in the population had finished reproduction. The probability of having a third clutch depended also on the date of starting the second clutch (binary logistic regression, B=−0.210, p=0.006).
(b) Effects of season and manipulation on baseline corticosterone
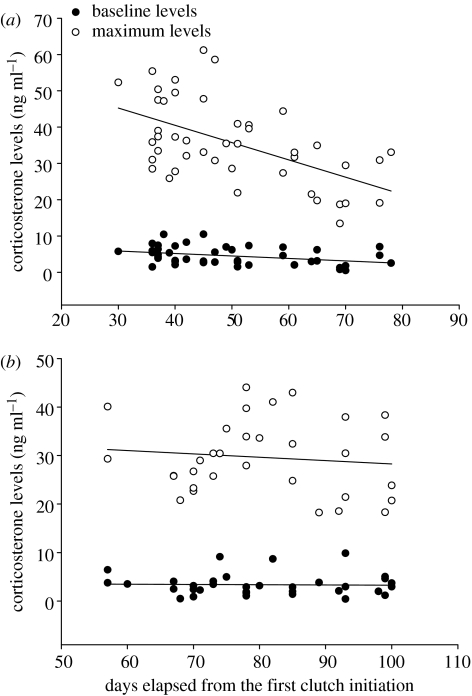
The baseline corticosterone levels decreased during the season in the first, but not in the second breeding attempt (first breeding attempt, r=−0.361, p=0.016, N=44; second breeding attempt, r=−0.028, p=0.877, N=34; figure 2). Manipulation groups did not differ in their baseline corticosterone levels, even after controlling for the seasonal effects (first breeding attempt, F1,28=0.500, p=0.485; second breeding attempt, manipulation: F1,21=1.519, p=0.231). Body condition (body mass controlling for tarsus length) did not differ between the manipulation groups and the sexes either in the first or in the second breeding attempt (first breeding attempt, manipulation: F1,25=1.181, p=0.287, sex: F1,25=0.353, p=0.558, interaction: F1,25=1.712, p=0.203; second breeding attempt, manipulation: F1,18=2.049, p=0.169, sex: F1,18=0.354, p=0.559, interaction: F1,18=4.165, p=0.056). Body condition was positively related to baseline corticosterone levels in the first breeding attempt, whereas this relationship tended to be negative in the second breeding attempt (first breeding attempt, F1,39=10.329, p=0.003, β=0.285; second breeding attempt, manipulation: F1,39=3.304, p=0.079, β=−0.141).
Figure 2.
Seasonal change in the baseline and maximum corticosterone levels in the (a) first breeding attempt and (b) second breeding attempt.
(c) The effects of season and the manipulation on the adrenocortical response to stress
Circulating plasma levels of corticosterone increased markedly following capture, handling and restraint (GLM repeated measures, first breeding attempt, F1,43=346.475, p<0.001; second breeding attempt, F1,31=378.408, p<0.001). The maximum corticosterone levels were not dependent on the baseline corticosterone levels (first breeding attempt, r=0.228, p=0.136, N=44; second breeding attempt, r=0.136, p=0.459, N=32) or the body condition (first breeding attempt, F1,39=0.008, p=0.930; second breeding attempt, F1,29=0.022, p=0.883). The maximum level of corticosterone was not different between the sexes (first breeding attempt, F1,42=0.094, p=0.761; second breeding attempt, F1,30=0.480, p=0.494).
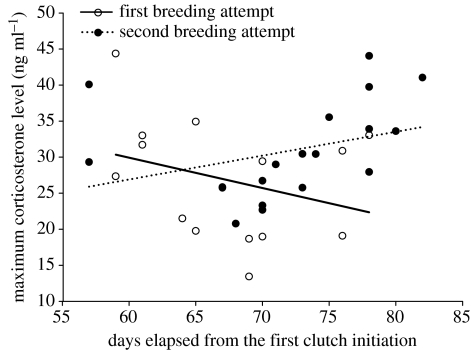
During the first breeding attempt, the magnitude of the stress response decreased over the season and this decrease was also significant after controlling for the baseline corticosterone levels (season, F1,41=15.392, p<0.001; baseline corticosterone, F1,41=3.094, p=0.086; figure 2a). In the second breeding attempt, the maximum level of corticosterone was also independent from baseline corticosterone levels, but did not change over the season (season, F1,29=0.393, p=0.536; baseline corticosterone, F1,29=0. 551, p=0. 464; figure 2b).
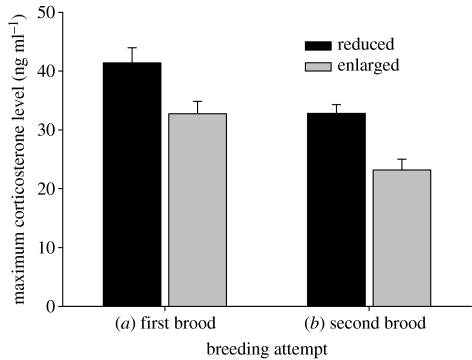
In both the breeding attempts, individuals tending reduced broods had a higher stress response than individuals tending enlarged broods, and this difference remained significant after statistically removing the effect of the season (first breeding attempt, F1,29=6.784, p=0.014; second breeding attempt, F1,20=16.443, p=0.001; figure 3). We found no interaction between the brood size manipulation and the season in either breeding attempt (first breeding attempt, F1,28=1.251, p=0.273; second breeding attempt, F1,19=0.789, p=0.385).
Figure 3.
Maximum corticosterone levels as a function of the brood size manipulation in the (a) first and (b) second breeding attempt.
(d) Within-individual comparisons
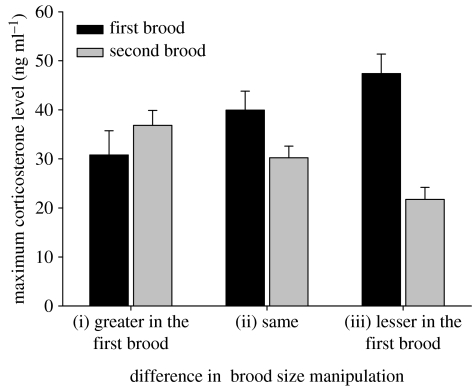
We compared the stress response in the first and second breeding attempts for those individuals that were sampled on both occasions. We investigated whether the difference in the stress response in the first and the second breeding attempt was related to the difference in the brood size manipulation that individuals received for their first and second broods. A GLM with repeated measures showed that there was a significant change in the stress response of individuals between their first and second breeding attempts (F1,12=34.532, p<0.001), and this change was related to the differences in manipulation (F2,12=15.945, p<0.001; figure 4). In this model, we also controlled for the date of the first breeding (F1,12=5.062, p=0.044) and the number of days elapsed between the two captures (F1,12=0.955, p=0.348).
Figure 4.
Within-individual comparisons of the adrenocortical response to stress as a function of the difference between the brood size manipulations received in the first and in the second breeding attempts. The brood value induced by the manipulation was either (i) ‘greater in the first brood’ if the first brood was enlarged and the second was either unmanipulated or reduced, (ii) ‘same’ if the individual either received the same type of manipulation or was left unmanipulated in both breeding attempts; and (iii) ‘lesser in the first brood’ if the first brood was reduced and the second was either unmanipulated or enlarged.
(e) Comparison of early and late breeders
To distinguish between the seasonal effects and the individual differences in the adrenocortical response to stress, we compared the stress response of the early breeders tending their second brood and the late breeders tending their first brood, in the same time span. During this limited time span, the effects of season and manipulation on the response to stress were still significant (table 1). The magnitude of the stress response did not differ between early and late breeders; however, it decreased over the season in the late breeders, while it increased slightly in early breeders resulting in a significant interaction between breeding attempt and the date of capture (table 1, figure 5).
Table 1.
Comparison of the stress responses of individuals during their first or second breeding attempt in 2005. (The table shows the results of a GLM model with the maximum level of corticosterone (ng ml−1) as the dependent variable. Significant effects are shown in bold.)
| source of variation | F1,16 | p |
|---|---|---|
| season | 8.806 | 0.009 |
| manipulation | 7.886 | 0.013 |
| breeding attempt | 0.398 | 0.537 |
| breeding attempt*manipulation | 0.515 | 0.483 |
| breeding attempt*season | 5.635 | 0.030 |
Figure 5.
Comparison of the adrenocortical response to stress between individuals reproducing for the first or the second time in 2005 during the time span when they bred simultaneously. The stress response is expressed as the maximum corticosterone levels.
4. Discussion
In this study, we found that adults with experimentally enhanced brood sizes responded less strongly to a stressor than adults with experimentally reduced brood sizes. In addition, we found that the stress response declined with increasing breeding date during the first breeding attempt. To the best of our knowledge, this study is the first experimental test of the hypothesis that individuals flexibly modulate their stress response with respect to the reproductive value of their brood. Our results lend robust support for this hypothesis and also corroborate its essential, yet untested underlying assumption, namely that the same individuals may react with a different physiological response to stress according to the actual costs and benefits of their reproductive status, and therefore are able to flexibly modulate their hormonal response to stress.
First, we found that in both breeding attempts, birds tending enlarged broods raised their circulating corticosterone levels to a smaller degree than those that tended reduced broods. Second, we found that individuals responded in different ways to a standardized stress protocol in their two consecutive breeding attempts, and this difference was explained by the different types of manipulation they received. In short, the rise in corticosterone was weaker in the breeding attempt in which their reproductive value was experimentally increased compared with the other breeding attempt.
But does an individual's flexibility to modulate its stress response work in both directions? With the lack of a control group, we cannot unambiguously determine whether the regulation of the stress response occurred in one or both experimental groups. However, in both breeding attempts, the stress response of birds tending unmanipulated broods was intermediate compared with birds tending enlarged or reduced broods (first breeding attempt, enlarged: 32.742±2.112, unmanipulated: 35.000±2.640, reduced: 41.404±2.556; second breeding attempt, enlarged: 23.182±1.853, unmanipulated: 30.935±2.219, reduced: 32.816±1.486). As the unmanipulated nests were not part of the experimental design (see §2), we did not assign these data to a control group post hoc. However, even if we had done this, the difference between the groups would be significant (p=0.048 and p=0.007 for the first and the second breeding attempt, respectively). To date, most of the studies have suggested that when the value of the brood is high, individuals should suppress their response to stress. However, it seems plausible that individuals experiencing a reduction in the value of the current reproduction may also upregulate their hormonal response to stress. We recommend further research to investigate this issue.
The prediction about the relationship between the parental care and the stress response is based on the idea that individuals actively modulate their response to stress to prevent the risks of nest abandonment when the value of the offspring, and therefore their level of parental care, is high, and considers this reaction as a reproductive tactic to maximize fitness (Jessop 2001). However, one may argue that these results can be interpreted without supposing an active regulation of the hormone levels. From an energetic perspective, the corticosteroid response to stress may be viewed as an energy demanding process to restore homeostasis (Nelson et al. 2002). Individuals should not only allocate energy between survival and reproduction, but should also balance energy between maintaining body condition and combating the stressor. Mounting an intensive response to stress may be costly because it may increase the risk of mortality from impaired body maintenance (McNamara & Buchanan 2005). Therefore, individuals whose energetic resources were mainly devoted to parental duties would show a modest response to stress. Nevertheless, in the present study, the brood size manipulations had no detectable effects on body condition or on baseline corticosterone levels, and the magnitude of the stress response was unrelated to body condition.
Although our measure of body condition is rather a rough approximation of the physiological resources available to the animal, the fact that we found no relationship between body condition and stress response does not support the view that some birds were physiologically limited to produce high corticosterone levels. On the contrary, increased workload has been evoked to explain the stronger stress response of female pied flycatchers (Ficedula hypoleuca) that were not assisted by their male (Silverin & Wingfield 1998). Therefore, there is a need for more studies aiming to sort the effects of the brood value from those of parental workload to determine whether the modulation of stress response is the consequence of the animal's perception of their reproductive status or a result of a differential resource allocation between maintenance and combating the stressor.
Parents are also expected to suppress the stress response during the breeding season as the probability of renesting decreases. In accordance with this idea we found that individuals tending their first brood at the beginning of the reproductive season showed a higher response to capture and handling stress than individuals that started to breed later in the season. Were these late-breeding birds really constrained by time? We found that about half of the individuals had only one breeding attempt; moreover, the probability of starting a second clutch was significantly greater for individuals that began the reproduction early. Accordingly, it seems that the convenient circumstances for breeding are indeed limited for some individuals, and by giving up their young very easily, these individuals would be at risk of producing no offspring in a given year. Missing a year of reproduction may have serious fitness consequences for a short-lived animal such as the house sparrow. In this species, the modal lifespan is only 2 years and the most important component determining an individual's lifetime reproductive success is the number of successful broods produced per year (Jensen et al. 2004). However, long-lived species often maintain robust stress responses during breeding, probably to ensure the opportunity for future reproduction (Chastel et al. 2005).
Contrary to the first breeding attempt, birds tending their second brood showed a similar corticosterone response to stress, irrespective of the laying date. Although the probability of laying a third clutch was also related to the date of the second breeding attempt, only a small proportion (15%) of these birds started a new clutch. Hence, the individual differences in the future breeding opportunities may be smaller in the second breeding attempt compared with the first breeding. Moreover, the third clutch is generally less productive than the second in the house sparrow (e.g. McGillivray 1983). According to the latter two arguments, individuals may base their reproductive decisions on the value of the second brood rather than the possibilities of future breeding, and therefore the timing of breeding may have less influence on the response to stress in the second brood than in the first one.
Within-individual comparisons also showed that the stress response was higher in the first attempt than in the second attempt. It is possible that this resulted in habituation to capture and handling stress; however, this is unlikely because in the second breeding attempt, the stress response of the birds sampled for the first and the second time was not different (p=0.595 after controlling for the effects of season and the manipulation). Moreover, most birds included in this study were captured at least once earlier in the season before the onset of breeding. Although blood samples were not collected during the early captures, the birds experienced similar capture and handling as during the stress protocol.
We also found similar seasonal patterns in the basal corticosterone levels: they decreased with the date in the first breeding attempt, whereas they were unrelated to the date in the second breeding attempt. These results might be interpreted as a part of an intrinsic seasonal change in both the baseline hormone levels and the endocrine responsiveness to stress, which would be steeper at the beginning of the reproductive season. In fact, several endocrine, metabolic or neural processes undergo changes during the annual cycle (Nelson et al. 2002; Romero 2002). For example, in the house sparrow, corticosterone levels, plasma concentrations of corticosteroid binding globulin (CBG) and corticosteroid receptors in the brain changed between different periods of the year, resulting together in a more pronounced corticosterone response to stress in the nesting than in the post-breeding period (Breuner & Orchinik 2001). To separate the effects of the season per se (i.e. some environmental variable acting as a common stimulus on the physiology of the birds, such as the day length, ambient temperature, etc.) and the differences in reproductive status of individuals (e.g. probabilities of future reproduction), we compared the stress response of the early breeders tending their second brood and the late breeders tending their first brood, in a time span when their breeding activity overlapped. The seasonal pattern of the stress response differed significantly between the two breeding attempts. This result further supports the idea that the responsiveness to the stressors depends not only on the environmental factors, but also on the individual differences, for instance, in the recent breeding history or the prospects for renesting.
Acknowledgments
We are grateful to C. Trouvé and S. Dano for their excellent technical assistance in the hormone assays and to B. Heidinger, Z. Barta and the anonymous referees for their insightful comments on the manuscript. Thanks to C. Loiseau, F. Angelier, C. Clement-Chastel, N. Mignot and N. Guillon for helping us in the field. We also thank B. Kulin for assistance in capturing the birds and developing the trapping method, and the CRBPO for providing the metal rings. Á.Z.L. was supported by a scholarship of the French Government, the Hungarian Scholarship Board (MÖB) and a joint grant from the Hungarian Academy of Sciences and the Centre National de la Recherche Scientifique.
References
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am. Nat. 2003;161:367–379. doi: 10.1086/346134. doi:10.1086/346134 [DOI] [PubMed] [Google Scholar]
- Breuner C.W, Orchinik M. Seasonal regulation of membrane and intracellular corticosteroid receptors in the house sparrow brain. J. Neuroendocrinol. 2001;13:412–420. doi: 10.1046/j.1365-2826.2001.00646.x. doi:10.1046/j.1365-2826.2001.00646.x [DOI] [PubMed] [Google Scholar]
- Chastel O, Kersten M. Brood size and body condition in the house sparrow Passer domesticus: the influence of brooding behaviour. Ibis. 2002;144:284–292. doi:10.1046/j.1474-919X.2002.00062.x [Google Scholar]
- Chastel O, Lacroix A, Kersten M. Pre-breeding energy requirements: thyroid hormone, metabolism and the timing of reproduction in house sparrows Passer domesticus. J. Avian Biol. 2003;34:298–306. doi:10.1034/j.1600-048X.2003.02528.x [Google Scholar]
- Chastel O, Lacroix A, Weimerskirch H, Gabrielsen G.W. Modulation of prolactin but not corticosterone responses to stress in relation to parental effort in a long-lived bird. Horm. Behav. 2005;47:459–466. doi: 10.1016/j.yhbeh.2004.10.009. doi:10.1016/j.yhbeh.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Curlee A.P, Bessinger S.R. Experimental analysis of mass change in female green-rumped parrotlets (Forpus passerinus): the role of male cooperation. Behav. Ecol. 1995;6:192–198. [Google Scholar]
- Hegner R.E, Wingfield J.C. Effects of brood-size manipulations on parental investment, breeding success, and reproductive endocrinology of house sparrows. Auk. 1987;104:470–480. [Google Scholar]
- Heidinger B.J, Nisbet I.C.T, Ketterson E.D. Older parents are less responsive to a stressor in a long-lived seabird: a mechanism for increased reproductive performance with age? Proc. R. Soc. B. 2006;273:2227–2231. doi: 10.1098/rspb.2006.3557. doi:10.1098/rspb.2006.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holberton R.L, Wingfield J.C. Modulating the corticosterone stress response: a mechanism for balancing individual risk and reproductive success in Arctic-breeding sparrows? Auk. 2003;120:1140–1150. doi:10.1642/0004-8038(2003)120[1140:MTCSRA]2.0.CO;2 [Google Scholar]
- Houston A.I, McNamara J.M. Cambridge University Press; Cambridge, UK: 1999. Models of adaptive behaviour: an approach based on state. [Google Scholar]
- Jessop T.S. Modulation of the adrenocortical stress response in marine turtles (Cheloniidae): evidence for a hormonal tactic maximizing maternal reproductive investment. J. Zool. Lond. 2001;254:57–65. [Google Scholar]
- Jensen H, Sæther B.-E, Ringsby T.H, Tufto J, Griffith S.C, Ellegren H. Lifetime reproductive success in relation to morphology in the house sparrow Passer domesticus. J. Anim. Ecol. 2004;73:599–611. doi:10.1111/j.0021-8790.2004.00837.x [Google Scholar]
- Lindström K.M, Hasselquist D, Wikelski M. House sparrows (Passer domesticus) adjust their social status position to their physiological costs. Horm. Behav. 2005;48:311–320. doi: 10.1016/j.yhbeh.2005.04.002. doi:10.1016/j.yhbeh.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Lormée H, Jouventin P, Trouvé C, Chastel O. Sex-specific patterns in baseline corticosterone and body condition changes in breeding red-footed boobies (Sula sula) Ibis. 2003;145:212–219. doi:10.1046/j.1474-919X.2003.00106.x [Google Scholar]
- McGillivray W.B. Intraseasonal reproductive costs for the house sparrow (Passer domesticus) Auk. 1983;100:25–32. [Google Scholar]
- McNamara J.M, Buchanan K.L. Stress, resource allocation, and mortality. Behav. Ecol. 2005;16:1008–1017. doi:10.1093/beheco/ari087 [Google Scholar]
- McNamara J.M, Houston A.I. State-dependent life-histories. Nature. 1996;380:215–221. doi: 10.1038/380215a0. doi:10.1038/380215a0 [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Demas G.E, Klein S.L, Kriegsfeld L.J. Cambridge University Press; Cambridge, UK: 2002. Seasonal patterns of stress, immune function, and disease. [Google Scholar]
- O'Reilly K.M, Wingfield J.C. Ecological factors underlying the adrenocortical response to acute stress in arctic breeding shorebirds. Gen. Comp. Endocrinol. 2001;12:1–11. doi: 10.1006/gcen.2001.7676. doi:10.1006/gcen.2001.7676 [DOI] [PubMed] [Google Scholar]
- Romero L.M. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. doi:10.1016/S0016-6480(02)00064-3 [DOI] [PubMed] [Google Scholar]
- Seel D.C. Food, feeding rates and body temperature in the nestling House Sparrow Passer domesticus at Oxford. Ibis. 1969;111:36–47. [Google Scholar]
- Silverin B, Wingfield J.C. Adrenocortical response to stress in breeding Pied flycatchers, Ficedula hypoleuca: relation to altitude, sex and mating status. J. Avian Biol. 1998;29:228–234. [Google Scholar]
- Silverin B, Arvidsson B, Wingfield J.C. The adrenocortical response to stress in breeding Willow Warblers Phylloscopus trochilus in Sweden: effects of latitude and gender. Funct. Ecol. 1997;11:376–384. doi:10.1046/j.1365-2435.1997.00097.x [Google Scholar]
- Sinervo B, Svensson E. Mechanistic and selective causes of life-history trade-offs and plasticity. Oikos. 1998;83:432–442. [Google Scholar]
- Stearns S.C. Oxford University Press; New York, NY: 1992. The evolution of life histories. [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man 1871–1971. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]
- Wingfield J.C. Modulation of the adrenocortical response to stress in birds. In: Davey K.G, Peter R.E, Tobe S.S, editors. Perspectives in comparative endocrinology. National Research Council of Canada; Ottawa, Canada: 1994. pp. 520–528. [Google Scholar]
- Wingfield J.C, Sapolsky R. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, O'Reilly K.M, Astheimer L.B. Modulation of the adrenocortical response to acute stress in arctic birds: a possible ecological basis. Am. Zool. 1995;35:285–294. [Google Scholar]