Abstract
The extent of our reliance on animal pollination for world crop production for human food has not previously been evaluated and the previous estimates for countries or continents have seldom used primary data. In this review, we expand the previous estimates using novel primary data from 200 countries and found that fruit, vegetable or seed production from 87 of the leading global food crops is dependent upon animal pollination, while 28 crops do not rely upon animal pollination. However, global production volumes give a contrasting perspective, since 60% of global production comes from crops that do not depend on animal pollination, 35% from crops that depend on pollinators, and 5% are unevaluated. Using all crops traded on the world market and setting aside crops that are solely passively self-pollinated, wind-pollinated or parthenocarpic, we then evaluated the level of dependence on animal-mediated pollination for crops that are directly consumed by humans. We found that pollinators are essential for 13 crops, production is highly pollinator dependent for 30, moderately for 27, slightly for 21, unimportant for 7, and is of unknown significance for the remaining 9. We further evaluated whether local and landscape-wide management for natural pollination services could help to sustain crop diversity and production. Case studies for nine crops on four continents revealed that agricultural intensification jeopardizes wild bee communities and their stabilizing effect on pollination services at the landscape scale.
Keywords: agriculture, conservation, pollination, biodiversity, spatial ecology, wild bees
1. Introduction
Ecosystem services, defined as the benefits to human welfare provided by organisms interacting in ecosystems, are considered to be at risk (Daily 1997; Palmer et al. 2004). Pollination by wild animals is a key ecosystem service. Although crop pollination is commonly cited as an example of an endangered ecosystem service (Corbet 1991; Williams 1994; Ingram et al. 1996; Matheson et al. 1996; Allen-Wardell et al. 1998; Kearns et al. 1998; Kevan & Phillips 2001; Steffan-Dewenter et al. 2005, but see Ghazoul 2005), detailed studies of the crop pollination systems are incomplete or out of date. Animal pollination is important to the sexual reproduction of many crops (McGregor 1976; Crane & Walker 1984; Free 1993; Williams 1994; Nabhan & Buchmann 1997; Westerkamp & Gottsberger 2000) and the majority of wild plants (Burd 1994; Kearns et al. 1998; Larson & Barrett 2000; Ashman et al. 2004), which can also be important for providing calories and micronutrients for humans (Sundriyal & Sundriyal 2004). Furthermore, the decline of pollinating species can lead to a parallel decline of plant species (Biesmeijer et al. 2006).
For tropical crops, Roubik (1995) provided a detailed list for 1330 species and compiled a list of potential breeding systems and pollinating taxa. From this list, ca 70% of tropical crops seem to have at least one variety for which production is improved by animal pollination.
For European crops, Williams (1994) assessed the pollinator needs for 264 crop species and concluded that the production of 84% of these depends at least to some extent upon animal pollination. Previous estimates have used mostly secondary data and relied on crude guesses of the proportional contribution of pollinators to crop production. These rough estimates can be deceptive as they often neither consider variation in the level of dependence on animal pollination nor take into account the importance of the crop to consumers. The major caloric inputs in the human diet come from a few staple foods with large world production for which animal pollination is irrelevant (Richards 2001; Ghazoul 2005), or come indirectly via animals fed with these same staple crops. Some authors provide coefficients of dependence on animal-mediated pollination for several crops (Borneck & Merle 1989; Robinson et al. 1989a,b; Morse & Calderone 2000), but despite their continuing acceptance, most of these reports do not cite data sources, and so it is impossible to assess the reported level of dependence. Williams (1994) provided coefficients for the dependence of European crops on animal pollination and estimated the proportion of insect pollinators that are honeybees, using information from Crane & Walker (1984) and Free (1993). Both studies are less relevant today, because many new crop varieties and pollination studies are available. To adequately evaluate the importance of animal pollination for plant products in our food supply, and for economic analyses of crop pollination by animals, we need a global review of crops considering their breeding systems, their flower-visiting fauna and the level of production increase resulting from animal visitation and pollination, as supported by experimental evidence (Kevan & Phillips 2001).
Honeybees, mainly Apis mellifera, remain the most economically valuable pollinators of crop monocultures worldwide (McGregor 1976; Watanabe 1994; also shown for several single crops, e.g. Roubik 2002 for coffee in Panama) and yields of some fruit, seed and nut crops decrease by more than 90% without these pollinators (Southwick & Southwick 1992). When wild bees do not visit agricultural fields, managed honeybee hives are often the only solution for farmers to ensure crop pollination. Compared with the management of several wild bees, honeybees are versatile, cheap and convenient, but for some crops they are not the most effective pollinators on a per flower basis (reviewed in Parker et al. (1987), Torchio (1990), Richards (1996), Cane (1997a) and Westerkamp & Gottsberger (2000); see also Bosch & Blas (1994) for almond; Cane (1997b) and Javorek et al. (2002) for blueberry; Kremen et al. (2002, 2004) for watermelon; Klein et al. (2003a,b) for highland and lowland coffee; Cane (2005) for raspberry and blackberry; Greenleaf & Kremen (in press) for field tomatoes; Bosch et al. (2006) for cherry). Other crops await similar comparative pollinator study. The numbers of managed honeybee colonies are declining in some parts of the world (Williams et al. 1991; Matheson et al. 1996; Delaplane & Mayer 2000; Anonymous 2005) largely owing to: (i) the spread of pests like parasitic mites (Varroa jacobsoni, V. destructor and Acarapis woodi; Downey & Winston 2001; Chen et al. 2004), the small hive beetle (Aethina tumida; Evans et al. 2003) and the microsporidian parasite Nosema ceranae (Higes et al. 2006), (ii) improper pesticide and herbicide use (Ingram et al. 1996), (iii) ageing of the beekeeper population in Europe and North America, and (iv) lower market prices for their products and services. Indeed, declining honeybee availability led to recent concern over pollination shortfalls such as those seen for almonds in California (www.almondboard.com). This situation also highlights the potential risk of our sole reliance on honeybees for agricultural pollination.
Fragmentation and degradation of near- and semi-natural habitats can be detrimental to bee communities (Rathcke & Jules 1994; Kremen et al. 2002, 2004; Steffan-Dewenter et al. 2002, 2006; Larsen et al. 2005; Cane et al. 2006). The main causal factor is loss or dissociation of important resources for food and nesting (Hines & Hendrix 2005; Potts et al. 2005). Conservation of natural- and semi-natural habitats in agricultural landscapes to increase and protect bee's resources may be useful to improve pollination services. While landscape effects are known to affect communities of herbivorous and predatory/parasitic insects in agro-ecosystems (reviewed in Cronin & Reeve 2005; Tscharntke et al. 2005; Bianchi et al. 2006), a similar evaluation of landscape impact on crop pollination is lacking.
In this review, we summarize and evaluate information on three issues:
the identification of leading global crops that depend on animal pollination for their production and their level of dependence on pollinators,
the influence of land-use changes at both local and landscape scales for pollinator communities and their services, and
future options for landscape and agricultural management to enhance wild pollinators and ensure pollination services for crop production.
2. Material and methods
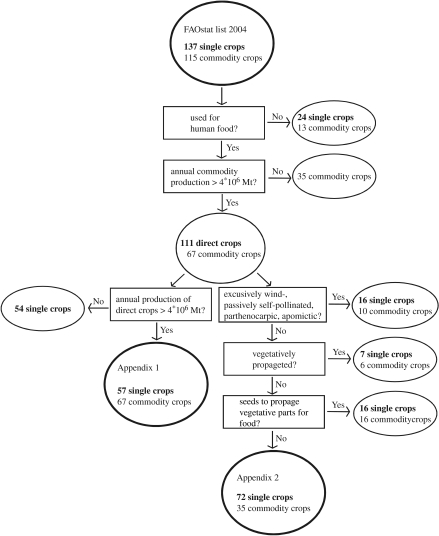
We first estimated the proportion of crop production depending on animal pollination. We selected the leading global crops on the world market out of the FAO crop production list for the year 2004 (FAOSTAT 2005), such that the aggregate represented 99% of total global food production (figure 1). We chose single crops and commodities used for human food with an annual production of at least 4 000 000 Metric tonnes (Mt). Production values are listed individually for the single crops. Production of the commodity crops is pooled in not elsewhere specified (NES) commodities. A commodity is an aggregation of different crops (e.g. fresh vegetables NES includes 21 crops). Commodity compilation is based on a questionnaire that countries fill out to include important crops for the world market which are not listed as a single crop by the FAO. Fifty-seven leading single crops and five commodities (including 67 commodity crops) represented 99% (94.5 and 4.5%, respectively) of the total global food production.
Figure 1.
Crop selection pathway to estimate the annual world production that is influenced by animal pollination (electronic supplementary material 1; lower left side) and to evaluate the levels of dependence on animal pollination for crops important in the global market (electronic supplementary material 2; right side). Single crops are crops directly listed with their production by the FAO and commodity crops are combined to a commodity with an aggregated production value.
Although production quantities for each commodity group are known, there is no breakdown for each commodity crop within these five groups, so we classified the annual production of the commodities with respect to its pollinator dependence as ‘unknown’. We individually classified each of the resulting 124 crops (57 leading single and 67 leading commodity crops) into four categories of pollinator dependence:
production increase with pollinators for plant parts that we consume (we define production as increased fruit set, fruit weight and/or quality, and seed number and/or quality, when pollinators have access to the flowers in contrast to pollinator exclusion experiments),
increase in seed production with pollinators to produce the vegetative parts that we consume,
increase in seed production with pollinators for breeding alone, as the plants reproduce vegetatively and we consume the vegetative parts, and
no production increase with pollinators.
We next assessed the level to which animal pollination matters to global crops directly used by humans. For this approach, we expanded our list using all the crops listed to be important on the world market, not restricted to the leading crops, as was the case for electronic supplementary material 1. We started with the same list used for electronic supplementary material 1, the complete set of 137 single crops and 5 commodities (93 commodity crops) listed by the FAO for the year 2004. We then reduced this list to 74 single crops and 33 commodity crops, a total of 107, following the pathway illustrated in figure 1.
Free (1993) summarized the key references for pollination requirements for 75 out of the 107 crops. We extended and updated his review, including both more recent literature and earlier studies not cited in Free (1993). For each listed crop, we provide the following information:
Flower morphology and breeding system.
Capacity of the crop to produce fruit and/or seeds without pollinators.
Animal groups or species known to be important flower visitors or pollinators; the primary pollinating species are identified if there is a species for which at least 80% of their single flower visits result in a fruit (Klein et al. 2003a,b) or species that improve fruit and seed quality and quantity when abundant as compared with the level when all flower visitors are excluded.
Magnitude of the improvement in production and quality when pollinated by animals. We scored the degree of production dependence into five classes: (i) essential (production reduction by 90% or more without flower visitors), meaning that production requires animal pollination, (ii) high (40 to less than 90% reduction), (iii) modest (10 to less than 40%), (iv) little (greater than 0 to less than 10%), (v) no reduction, and (vi) unknown, meaning that no literature was available to adequately review the breeding systems or draw conclusions about pollinator dependence.
3. Results and discussion
(a) Importance of animal pollination for global crop production
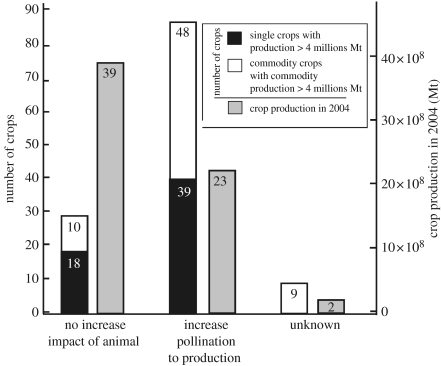
Production of 39 of the leading 57 single crops increases with pollinating animals (figure 2). In aggregate, these crops account for 35% (23×108 Mt) of global food production (figure 2), but because most of these crops are not entirely dependent on animal pollination, the amount of production directly attributable to animals is lower than this value. In addition, production of 48 of the 67 crops of the five leading global commodities increases with pollinating animals (figure 1). Only insects are demonstrated pollinators of the single crops, while vertebrates pollinate very few commodity crops (e.g. feijoa is pollinated by birds and durian seems to be pollinated by bats, electronic supplementary material 2). Among the 57 single crops that show increased production, 26 (55% with 12×108 Mt or 19% of global food) increase seed production with animal pollination to produce vegetative parts for human food, while an additional seven crops (8×108 Mt, 36%) show increased seed production for breeding alone, as the plants reproduce vegetatively and only vegetative parts are consumed (e.g. potatoes, sweet potatoes and manioc, electronic supplementary material 1). The production increase with pollinators for seeds of vegetatively propagated crops permits breeding progress and hybridization for the development of new varieties.
Figure 2.
Relative importance of animal pollination for the leading global crops and commodities used for human food and selected by their annual production in 2004. We considered crops and commodities with an annual production greater than 4 000 000 Metric tonnes (Mt) as these comprise 99% of the 2004 total crop production listed for human food. The number of crops and the production are listed according to their production increase with pollinators (see electronic supplementary material 1 for details). Single crops and commodity crops in NES* commodities are separated. The category ‘unknown’ includes only commodity crops for the number of crops while the ‘unknown’ production is the production of the leading commodities, as the production value of each commodity crop is not known. Crops in the ‘increase’ category could be classified into three sub-categories with the following number of species and total production figure for the individual crops: production increase with pollinators for plant parts that we consume (fruits and/or seeds: 26 crops with 12 108 Mt=55%); increase in seed production with pollinators to produce the vegetative parts that we consume (six crops with 2108 Mt=9%); and increase in seed production with animals for breeding alone, as the plants reproduce vegetatively and we consume the vegetative parts (seven crops with 8108 Mt=36%). NES* is an abbreviation for not elsewhere specified; leading commodities are fresh vegetables NES, fresh fruits NES, fresh tropical fruits NES, roots and tubers NES and pulses NES. Commodity crops are included based on a questionnaire that countries fill out to include important crops for the world market which are not listed as single crops.
Animal pollination is irrelevant to 18 of the leading single crops (comprising 60% or 39×108 Mt of the world production) and 10 of the leading commodity crops. These are wind- or passively self-pollinated grasses (cereals and sugarcane), dominating the leading global crop list (electronic supplementary material 1; figure 2).
Twenty per cent of the overall crop production comes from crops that increase fruit and vegetable production with animal pollination, and ca 15% comes from crops that increase seed production with animal pollination. Our results further show that a majority of global crops could experience production loss owing to pollinator limitation (39 single crops increase fruit, vegetable or seed production with pollinators compared with 18 that do not, and 87 of the commodity crops increase production compared with 28 that do not; figure 2). Included are many fruit crops that provide essential macro- and micronutrients contributing to a healthy diet. These results support the contention of Richards (2001) and Ghazoul (2005) that primary food production, and especially our staple foods, is independent of insect pollination. Thinking beyond caloric intake, however, our results support the opinion of Steffan-Dewenter et al. (2005) that our diet would be greatly impoverished, both nutritionally and culturally, if pollination services further decline.
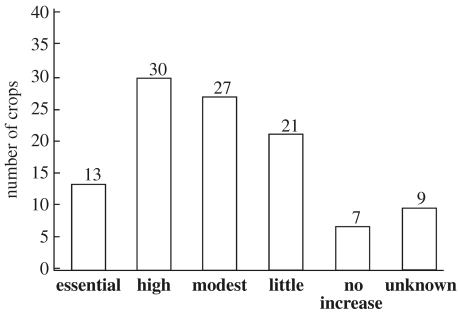
In a second list (electronic supplementary material 2), we quantified the level of dependence on animal pollination. We found empirical evidence for increased production with pollinators in 92 out of 108 selected crops (figure 3). Among these 92 crops, for the majority (82 crops), data were available from experiments comparing measures of pollination (e.g. fruit set, number of seeds, fruit or seed weight, or pollen deposition) at the level of flowers, inflorescences or whole plants, with and without access to pollinators. For 10 crops, we classified the evidence for increased production with pollinators as ‘indirect evidence’, because experiments with pollinator exclusion were lacking, but the experiments demonstrated, for example, self-incompatibility and a need for cross pollination that could not be achieved by wind (electronic supplementary material 2; figure 3). Animal pollination was found to be essential for most varieties of the following 13 crops: atemoya, Brazil nut, cantaloupe, cocoa, kiwi, macadamia nut, passion fruit, pawpaw (Indian banana), rowanbarry, sapodilla, squashes and pumpkins, vanilla and watermelon. An additional 30 crops showed increased fruit and/or seed production for most species and varieties with animal pollination. Twenty-seven crops show a modest increase in production, and for 21 crops, production of some species or varieties increase little, others not at all. For seven crops, production did not increase in the studies available: chick pea, garden and field peas and lentil, which are passively self-pollinated, and olive, pepper, quinoa and grapes, which rely on passive self- and wind-pollination. Pollination needs of nine crops remain unknown (figure 3; electronic supplementary material 2).
Figure 3.
Level of dependence on animal-mediated pollination. The selected crops are those included directly in the production list published by the FAO for 2004 (FAOSTAT 2005). We further included commodity crops for which the production was pooled in commodities with an annual 2004 commodity production greater than 4 000 000 Metric tonnes (Mt). Only crops that produce fruits or seeds for direct human use as food were considered. We did not include: (i) crops for which seeds are only used for breeding or to grow vegetable parts for direct human use or for forage, and (ii) crops known to be only wind-pollinated, passively self-pollinated or reproduced vegetatively. Essential, pollinators essential for most varieties (production reduction by 90% more, comparing experiments with and without animal pollinators); high, animal pollinators are extreme (40 to less than 90% reduction); modest, animal pollinators are clearly beneficial (10 to less than 40% reduction); little, some evidence suggests that animal pollinators are beneficial (greater than 0 to less than 10% reduction); no increase, no production increase with animal-mediated pollination; unknown, empirical studies are missing.
Gaps in our knowledge of pollination requirements are illustrated by the example of highland coffee, one of the better studied crops. Although the breeding systems are well studied and pollinators have been identified in different coffee production regions, few varieties have been studied, and production of some varieties may not increase with animal pollination as much as those studied to date (A.-M. Klein, unpublished data). The need to consider different genetic materials is also highlighted by the fact that varieties of many crops, such as citrus, blueberries, most stone fruit crops, and almonds, show great production variation with animal pollination (see Ortega et al. 2002 for almond). We also do not know much about the mechanisms of pollination provided by most pollinator species (Klein et al. 2003a), and flower-visiting insect communities of different production regions across the world can differ greatly. For example, the flower visitors to coffee in Ecuador with more than 95% social and less than 5% solitary bees (Veddeler et al. 2006) are very different from flower-visiting communities in Indonesia with 70% social and ca 30% solitary bees (Klein et al. 2003a,b). Such differences may lead to differences in pollination success.
(b) Consequences of agricultural management at local and landscape scales for wild versus managed pollinators
Wild bees and other insects can pollinate many crops, but their value for crop pollination has been overlooked for centuries. As their services are increasingly being recognized for agriculture (e.g. O'Toole 1993; Cane 1997b; Kevan & Phillips 2001; Klein et al. 2003a; Slaa et al. 2006), the adequate management of local agro-ecosystems and the conservation of suitable natural or semi-natural pollinator habitats in the surrounding landscapes are receiving more attention. Little information exists on the ways in which local management influences agricultural pollination (Richards 2001). Considering the 107 crops listed in electronic supplementary material 2, we found increased production with animal pollination of at least 10% or higher (categories essential, great and modest) for 63 crops, when considering only the crops for which field experiments were available (N=93). Therefore, we suggest that pollination of at least these 63 crops should be vulnerable to agricultural intensification that may reduce the diversity and abundance of pollinators (e.g. Kremen et al. 2002; Klein et al. 2003a,b). Among the 63 crops, the production of 13 crops that are entirely dependent on pollinators to set fruits might be severely impacted by pollinator loss through agricultural intensification. This risk is the greatest for crops that rely on a narrow range of pollinating species, such as passion fruit and vanilla.
We found 16 studies on the effects of agricultural intensification on pollination at local or landscape scale of nine crops on four continents (table 1). All of these studies show negative consequences of local and/or regional agricultural intensification for pollination. For watermelon and coffee, higher variation in pollination success was found in sites of intensified agriculture isolated from natural or semi-natural habitats (Kremen et al. 2004; Steffan-Dewenter et al. 2006).
Table 1.
Pollinator and pollination limitation in crop plants in response to land-use and landscape changes. (Significance *p<0.05; **p<0.01; ***p<0.001.)
| species name (common crop name) | land-use and landscape variable | pollination variable and significance level of reduction | reference |
|---|---|---|---|
| Annona squamosa × A. cherimola (sugar apple) | comparison of sites near and far from forest fragments | pollinator diversity*** (fruit set reduction with pollinator exclusion***) | Blanche & Cunningham (2005) |
| Brassica napus and B. rapa (turnip rape, canola and oilseed rape) | comparison of organic, conventional and genetically modified (GM) fields | number of seeds per silique from a flower sample*** | Morandin & Winston (2005) |
| proportional area of uncultivated land around fields within a 750 m radius | number of seeds per silique from a flower sample* | Morandin & Winston (2006) | |
| Citrullus lanatus (watermelon) | comparison of organic versus conventional fields | number of pollen grains/stigma, n.s. | Kremen et al. (2002, 2004) |
| proportional area of oak woodland and chaparral habitat | number of pollen grains/stigma*** | Kremen et al. (2002, 2004) | |
| Citrus paradisi (grapefruit) | distance from forest | number of pollen grains/stigma* number of pollen tubes/stigma* | Chacoff (2006) and Chacoff & Aizen (2006) |
| Coffea arabica (coffee) | coffee plants near, intermediate and far from forest fragments | number of pollen grains/stigma***, fruit set*, seed mass** | Ricketts (2004) and Ricketts et al. (2004) |
| distance from forest | fruit set** | Klein et al. (2003a) | |
| plant diversity | fruit set** | Klein et al. (2003a) | |
| coffee monocultures versus agroforestry | fruit set* | De Marco & Coelho (2004) | |
| comparison sites near and far from forest fragments | fruit set* | De Marco & Coelho (2004) | |
| Coffea canephora (coffee) | distance from forest | fruit set** | Klein et al. (2003b) |
| Dimocarpus longan (longan fruit) | comparison sites near and far from forest fragments | number of fruits per centimetre panicle* | Blanche et al. (in press) |
| Helianthus annuus (sunflower) | proportional area of natural habitat | wild bee diversity and abundance*** (estimated increase in seed set via single visit studies) | Greenleaf & Kremen (2006) |
| organic versus conventional farm management | wild bee diversity and abundance, n.s. | Greenleaf & Kremen (2006) | |
| Lycopersicon esculentum (tomato) | distance to natural habitat | Bombus vosnesenskii abundance***; Anthophora urbana abundance, n.s. (fruit set and fruit weight reduction with pollinator exclusion for variety with exserted stigma) | Greenleaf & Kremen (in press) |
| Macadamia integrifolia (macadamia nut) | percentage of eucalyptus forest surrounding orchards | Trigona abundance (seed set reduction with pollinator exclusion* and only Trigona pollinated*) | Heard (1994) and Heard & Exley (1994) |
| comparison of sites near and far from forest fragments | number of fruits/raceme* | Blanche et al. (in press) |
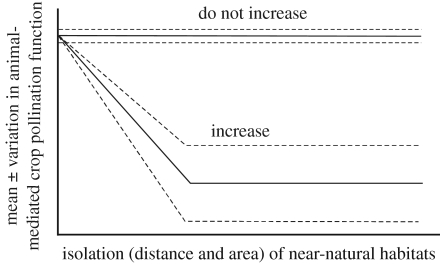
The existing studies suggest that crops having a production increase with pollinators of at least 10% might show reduced fruit set and increased variance in fruit set at locations increasingly isolated from near-natural habitats (figure 4). The impact of landscape context on visitation rates and fruit set of crops has been assessed as the proportion of near-natural habitats in the surrounding landscape (e.g. Kremen et al. 2004; Morandin & Winston 2006) or as the linear isolation distance from near-natural habitat (e.g. Klein et al. 2003a,b; Chacoff & Aizen 2006). We found a linear positive relationship between fruit set stability and isolation to the rainforest margin for lowland and highland coffee (Klein et al. 2003a,b), whereas a log-linear relationship was found for watermelons (Kremen et al. 2004). Agro-ecosystems with more semi-natural habitats are often more pollinator-species rich (Steffan-Dewenter et al. 2002; Kremen & Chaplin 2006; Steffan-Dewenter et al. 2006). There might be a threshold level of diversity necessary to maintain lower variation or higher stability in pollination. The exact shape of the function will depend on the biology of crop, crop variety, pattern of the landscape and regional pollinator community, but the available data indicate that pollination stability will increase in landscapes with a diverse and abundant pollinator community. The positive pollination effect on crop yield can however be reduced or hidden when other factors affecting crop yield, such as soil nutrients, micro-climate, water, pest or disease status are suboptimal. Further, agricultural land use is not always expected to reduce pollination services. Some wild bees may benefit from agriculture, such as ground-nesting bees that use disturbed areas for nesting, or pollinators may benefit from pollen-rich crop fields, such as oilseed rape (Westphal et al. 2003), or from ecosystems in which agricultural areas provide a greater diversity, continuity or abundance of floral resources than original habitat types (e.g. Winfree et al. in press). Therefore, knowledge of the pollinator's resources and life-history traits is required to correctly predict the likely pollination responses (Cane et al. 2006). Failure of wild pollinators can be overcome by the provision of commercially managed bees, where they are effective and manageable pollinators available (Kremen et al. 2002), but this service generally comes at a cost. Finally, crops with little or no dependence on animal pollination will exhibit no relationship between pollination rates and isolation (figure 4).
Figure 4.
Expected relationship between the loss of animal-mediated crop pollination function (pollination variable usually measured as fruit or seed set in pollination studies and the variation usually measured as the coefficient of variation in the number or yield of fruits indicating crop production stability) and the effect of isolation from near-natural habitats (which means the area and distance of the main nesting and foraging habitats for the pollinators). Expected relationships in the absence of pollinator introduction are given for crops which are independent of animal pollination and for crops depending on animal pollination. Mean, solid line; variation, dashed line.
Unfortunately, none of the landscape studies have been conducted over enough years to reliably estimate temporal variability in pollination. In some studies, samples were taken in two consecutive seasons (Kremen et al. 2002, 2004; Ricketts 2004; Ricketts et al. 2004), but a majority were carried out over only one season.
Studies that compare fruit or seed set of flowers in treatments with and without access by wild-pollinating species or with additional hand-pollination provide important data to identify key pollinating species (Canto-Aguilar & Parra-Tabla 2000; Javorek et al. 2002; Cane & Schiffhauer 2003; Klein et al. 2003a,b; Greenleaf & Kremen 2006, in press; Blanche et al. in press), but few such studies are yet available. In spite of this information shortage, many reviews mention the neglected potential of wild bee species for crop pollination (O'Toole 1993; Corbet 1996; Williams 1996; Westerkamp & Gottsberger 2000; Goulson 2003). Buchmann & Nabhan (1996) suggested that ca 80% of the 100 most important staple crops (Prescott-Allen & Prescott-Allen 1990) are pollinated by wild insects. We found evidence for only 24 out of the 57 leading crops (42%) being pollinated by at least one wild bee species. We identified 57 species (mainly bees and only two vertebrate species) as not only flower visitors, but also true pollinators for the 107 global crops for direct human use (electronic supplementary material 2; table 2). Considering these 107 crops, empirical evidence with direct testing revealed that both honeybees (which can be managed or feral) and wild pollinators are valuable pollinators for 35 crops. For 12 crops, empirical studies provided evidence only for honeybees contributing to successful pollination, with wild pollinators mentioned as pollinators for 10 of these 12 crops, but without empirical data. For those cases where there was evidence for honeybees but not wild bees, the problem was generally a shortage of evidence, rather than evidence that wild bees were in fact poor pollinators. For nine crops, empirical studies showed evidence that wild pollinators contributed to successful pollination without similar evidence for honeybees, and for six (atemoya, cocoa, fig, passion fruit, oil palm and sapodilla) of these nine crops honeybees were not mentioned as pollinators. These nine crops depend strictly on, or production increased greatly with, wild pollinators, and interestingly, three of these crops—atemoya, passion fruit and vanilla—are produced by hand-pollination in many parts of the world, showing the severe lack of wild pollinators.
Table 2.
Species list of known pollinators for global crops that are grown for direct human consumption.
| pollinator group | species |
|---|---|
| honey bees | Apis cerana Fabr., A. dorsata Fabr., A. florea Fabr. and A. mellifera L. |
| stingless bees | Melipona favosa Fabr., M. subnitida Ducke, M. quadrifasciata Lepeletier, Nanotrigona perilampoides Cresson, N. testaceicornis Lepeletier, Trigona cupira Sm., T. iridipennis Smith, T. (Lepidotrigona) terminata Smith, T. (Tetragonoula) minangkabau Sakagami, T. toracica Smith and Scaptotrigona depilis Moure |
| bumble bees | Bombus affinis Cresson, B. californicus F. Smith, B. hortorum L., B. hypnorum L., B. impatiens Cresson, B. lapidarius L., B. (Thoracobombus) pascuorum Scop., B. sonorus L., B. terrestris L. and B. vosnesenskii Radoszkowski |
| solitary bees | Amegilla chlorocyanea Cockerell, A. (Zonamegilla) holmesi Rayment, Andrena ilerda Cam., Anthophora pilipes Fabr., Centris tarsata Smith, Creightonella frontalis Fabr., Habropoda laboriosa Fabr., Halictus tripartitus Cockerell, Megachile (Delomegachile) addenda Cresson, M. rotundata Fabr., Osmia aglaia Sandhouse, O. cornifrons Radoszkowski, O. cornuta Latreille, O. lignaria lignaria Say, O. lignaria propinqua Cresson, O. ribifloris Cockerell, Peponapis limitaris Cockerell, P. pruinosa Say, Pithitis smaragdula Fabr., Xylocopa (Zonohirsuta) dejeanii Lepeletier, Xylocopa frontalis Oliver and Xylocopa suspecta Moure |
| wasps | Blastophaga psenes L. |
| hover flies and other flies | Eristalis cerealis Fabr., E. tenax L. and Trichometallea pollinosa Townsend |
| beetles | Carpophilus hemipterus L. and Carpophilus mutilatus Erichson |
| thrips | Thrips hawaiiensis Morgan and Haplothrips (Haplothrips) tenuipennis Bagnall |
| birds | Turdus merula L. and Acridotheres tristis L. |
In most environments, both wild pollinators and honeybees will exploit flowers of crop species. For example, males of wild bees searching for mates disturbed honeybees during foraging, so that honeybees switched more often between lines of hybrid sunflower, and carried more pollen, thereby increasing the overall pollination service (Degrandi-Hoffmann & Watkins 2000; Greenleaf & Kremen 2006). Strawberry flowers visited by both wild and honeybees are more likely to be completely developed in contrast to flowers that are visited by only honeybees or only wild bees that tended to have misshapen fruits (Chagnon et al. 1993). Effects such as this have rarely been looked for, but may prove to be widespread.
4. Management conclusions and future directions
(a) Pollinator management
Populations of wild pollinators can enhance production of some crops and are, in this way, an important natural resource; but populations of wild pollinators are frequently too sparse to adequately pollinate crops in agriculturally intensive environments (table 1). The landscape studies summarized in this review were all published during the last 5 years. Although more research is needed on a landscape scale, we are in a much better position today than we have been in the past to recommend landscape management practices to enhance wild pollinators. We need landscape management practices that boost native pollinator densities by increasing habitat-carrying capacity. We suggest integrating the following general practices into management plans: (i) increase nesting opportunities with the particular nesting needs of different pollinating species in mind and these may include gaps in surface vegetation or modifying cultivation practices (Shuler et al. 2005), retaining neighbouring forest nesting sites for ground-nesting bees (Cane 1997a,b) or leaving dead wood providing holes for cavity-nesting bees (Westrich 1996), (ii) increase forage by providing suitable diverse floral resources in the local area and the broader landscape during the season of pollinator activity (Kevan et al. 1990; Banaszak 1992; Westrich 1996; Goulson 2003; Ghazoul 2006). Crop rotation using these flowering plants should be especially applied in intensified uniform agricultural landscapes and may also help to enhance other ecosystem services such as soil improvement, pest management by breaking cycles of damaging pests or erosion control, (iii) enhance opportunities for colonization by connecting habitats with flowering strips and hedgerows around arable fields, small forest patches or even single trees as ‘stepping stones’ (Steffan-Dewenter et al. 2002, 2006; Pywell et al. 2006), and (iv) reduce the risk of population crashes in the field and the surrounding habitats by foregoing use of broad-spectrum insecticides during bloom, especially those with systemic or micro-encapsulated formulations that can contaminate nectar and pollen (Kevan 1975; Wood 1979; Delaplane & Mayer 2000). Financial burdens of these recommendations could be ameliorated through agro-environmental schemes, such as those in Europe and the United States, which compensate farmers who apply management strategies to conserve biodiversity.
(b) Research needs
In this review, we found that inadequate information is available on the pollination biology and pollinator requirements of many crops, especially when considering differences among modern varieties and the contribution to pollination services by different pollinator species.
We need to assess the potential impact of pollinator loss for a given crop in a given production area. For this, we need to collect the following data: experimental fruit and seed set from flowers visited by animal pollinators versus unvisited flowers and those receiving airborne pollen flow or any passive self-pollination. As plants are often resource limited, treatments should ideally be applied to entire plants and not just a few flowers or a single branch, otherwise, extrapolation can overestimate pollen limitation (Ashman et al. 2004; Knight et al. 2006). Multi-year data are valuable as periodic weather perturbations are the norm and perennial plants tend towards alternate year of fruit and seed production (e.g. Herrera et al. 1998; Pías & Guitián 2006). Studies over multiple seasons are also necessary to truly understand the stability of the pollination service, because insect communities often show high temporal variation (Cane & Payne 1993; Roubik 2001) and habitat-specific temporal species turnover (Williams et al. 2001; Cane et al. 2005; Tylianakis et al. 2005).
Studies for only three crops (watermelon, highland- and lowland coffee) are available to address the links between a landscape variable and the stability of crop pollination. More research of this kind is needed. The list of pollinators known to be important for global crops was only 57 species, mainly bees. We found only one study showing birds to be effective pollinators on feijoa (Stewart 1989). We still need experiments to determine to what extent non-insects (birds, bats and other vertebrates) contribute to crop production. In addition, to adequately judge the value of conserving and managing for wild pollinators, key pollinators in the main producing areas must be identified, their habitat requirements studied and the economic benefit of their presence estimated (e.g. Cane 1997b; Larsen et al. 2005). Today, only few areas and crops have all the necessary data elements to access the impact of pollinator loss.
Our four general recommendations for landscape management (nesting opportunities, floral resources, habitat connectivity and reduction of pesticides) can be applied to all crops dependent on animal pollination in all production areas. For further specific recommendations, we emphasize the need to monitor the effects of applied management practices on crop production and stability in restoration programmes (e.g. Pywell et al. (2006) for pollinator foraging resources and Albrecht et al. in press for the pollination of three herb species). We also emphasize the collection of data for understanding the effects of spatial and temporal pollinator resource availability and for interaction effects between honeybees and other bee species for crop pollination to recommend future management applications.
Therefore, we urgently need more research in crop pollination along with better coordination of the research efforts at the community level in different producing areas to help sustain production of the diverse crops that nourish humanity.
Acknowledgments
We thank Nora Hornsdorf for helping to collect literature, Sarah Greenleaf and Barbara Gemmill for help with the crop selection and two anonymous referees for helpful comments on the manuscript. This work was partly conducted as a part of the Restoring Pollination Services Working Group supported by the National Center for Ecological Analysis and Synthesis, a Center funded by NSF (grant no. DEB-00-72909), the University of California at Santa Barbara, and the State of California, and with funding by the Sixth European Union Framework programme—Assessing Large-scale Environmental Risks to Biodiversity with Tested Methods (Project ALARM (GOCE-CT-2003-506675); www.alarmproject.net).
Supplementary Material
Crop selection pathway to estimate the annual world production that is influenced by animal pollination
Crop selection pathway to evaluate the levels of dependence on animal pollination for crops important in the global market
References
- Albrecht, M., Duelli, P., Müller, C. B., Kleijn, D. & Schmid, B. In press. Swiss agri-environment scheme enhances pollinator diversity and plant reproductive success in nearby intensively managed farmland. J. Appl. Ecol.
- Allen-Wardell G, et al. The potential consequences of pollinator declines on the conservation of biodiversity and stability of crop yields. Conserv. Biol. 1998;12:8–17. doi:10.1046/j.1523-1739.1998.97154.x [Google Scholar]
- Anonymous Audit de la filière miel août 2005. Abeille de France. 2005;919:479–496. [Google Scholar]
- Ashman T.L, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Banaszak J. Strategy for conservation of wild bees in an agricultural landscape. Agric. Ecosyst. Environ. 1992;40:179–192. doi:10.1016/0167-8809(92)90091-O [Google Scholar]
- Bianchi F.J.J.A, Booij C.J.H, Tscharntke T. Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B. 2006;273:1715–1727. doi: 10.1098/rspb.2006.3530. doi:10.1098/rspb.2006.3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesmeijer J.C, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. doi:10.1126/science.1127863 [DOI] [PubMed] [Google Scholar]
- Blanche R, Cunningham S.A. Rain forest provides pollinating beetles for atemoya crops. J. Econ. Entomol. 2005;98:1193–1201. doi: 10.1603/0022-0493-98.4.1193. [DOI] [PubMed] [Google Scholar]
- Blanche, K. R., Ludwig, J. A. & Cunningham, S. A. In press. Proximity to rainforest enhances pollination and fruit set in macadamia and longan orchards in north Queensland, Australia. J. Appl. Ecol. (doi:10.1111/j.1365-2664.2006.01234.x)
- Borneck R, Merle B. Essai d'une evaluation de l'incidence economique de l'abeille pollinisatrice dans l'agriculture Europeenne. Apiacta. 1989;XXIV:33–38. [Google Scholar]
- Bosch J, Blas M. Foraging behaviour and pollinating efficiency of Osmia cornuta and Apis mellifera on almond (Hymenoptera, Megachilidae and Apidae) Appl. Entomol. Zool. 1994;29:1–9. [Google Scholar]
- Bosch J, Kemp W.P, Trostle G.E. Bee population returns and cherry yields in an orchard pollinated with Osmia lignaria (Hymenoptera: Megachilidae) J. Econ. Entomol. 2006;99:408–413. doi: 10.1603/0022-0493-99.2.408. [DOI] [PubMed] [Google Scholar]
- Buchmann S.L, Nabhan G.P. Island Press; Washington, DC: 1996. The forgotten pollinators. [Google Scholar]
- Burd M. Bateman's principle and reproduction: the role of pollinator limitation in fruit and seed set. Bot. Rev. 1994;60:83–139. [Google Scholar]
- Cane J.H. Ground-nesting bees: the neglected pollinator resource for agriculture. Acta Hort. 1997;437:309–324. [Google Scholar]
- Cane J.H. Lifetime monetary value of individual pollinators: the bee Habropoda laboriosa at rabbiteye blueberry (Vaccinium ashei Reade) Acta Hort. 1997b;446:67–70. [Google Scholar]
- Cane J.H. Pollination potential of the bee Osmia aglaia for cultivated red raspberries and blackberries (Rubus: Rosaceae) Hortscience. 2005;40:1705–1708. [Google Scholar]
- Cane J.H, Payne J.A. Regional, annual and sesonal-variation in pollinator guids — intriusic traits of bees (Hymenoptera, Apoidea) underlie their patterns of abudance at Vaccinium ashei (Ericaceae) Ann. Entomol. Soc. Am. 1993;86:577–588. [Google Scholar]
- Cane J.H, Schiffhauer D. Dose-response relationships between pollination and fruiting refine pollinator comparisons for cranberry (Vaccinium macrocarpon [Ericaceae]) Am. J. Bot. 2003;90:1425–1432. doi: 10.3732/ajb.90.10.1425. [DOI] [PubMed] [Google Scholar]
- Cane J.H, Minckley R, Kervin L, Roulston T. Temporally persistent patterns of incidence and abundance in a pollinator guild at annual and decadal scales: the bees of Larrea tridentata. Biol. J. Linn. Soc. 2005;85:319–329. doi:10.1111/j.1095-8312.2005.00502.x [Google Scholar]
- Cane J.H, Minckley R, Roulston T, Kervin L, Williams N.M. Multiple response of desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 2006;16:632–644. doi: 10.1890/1051-0761(2006)016[0632:crwadb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Canto-Aguilar A, Parra-Tabla V. Importance of conserving alternative pollinators: assessing the pollination efficiency of the squash bee, Peponapis limitaris in Cucurbita moschata (Cucurbitaceae) J. Ins. Conserv. 2000;4:203–210. [Google Scholar]
- Chacoff, N. P. In press. Los ecosistemas naturales como fuente de polinizadores para cultivos en el pedemonte de las yungas. Ph.D. thesis. Universidad Nacional del Comahue, Argentina.
- Chacoff N.P, Aizen M.A. Edge effects on flower-visiting insects in grapefruit plantations bordering premontane subtropical forest. J. Appl. Ecol. 2006;43:18–27. doi:10.1111/j.1365-2664.2005.01116.x [Google Scholar]
- Chagnon M, Gingras J, De Oliveira D. Complementary aspects of strawberry pollination by honey and indigenous bees (Hymenoptera) J. Econ. Entomol. 1993;86:416–420. [Google Scholar]
- Chen Y, Pettis J.S, Evans J.D, Kramer M, Feldlaufer M.F. Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie. 2004;35:441–448. doi:10.1051/apido:2004031 [Google Scholar]
- Corbet S.A. Bees and the pollination of crops and wild flowers in the European community. Bee World. 1991;72:47–59. [Google Scholar]
- Corbet S.A. Which bees do plants need? In: Matheson A, Buchmann S.L, O'Toole C, Westrich P, Williams J.H, editors. The conservation of bees. Academic Press; London, UK: 1996. pp. 105–114. [Google Scholar]
- Crane E, Walker P. International Bee Research Association; Bucks, UK: 1984. Pollination directory for world crops. [Google Scholar]
- Cronin J.T, Reeve J.D. Host-parasitoid spatial ecology: a plea for a landscape-level synthesis. Proc. R. Soc. B. 2005;272:2225–2235. doi: 10.1098/rspb.2005.3286. doi:10.1089/rspb.2005.3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily G.C. Island Press; Washington, DC: 1997. Nature's services: societal dependence on natural ecosystems. [Google Scholar]
- Degrandi-Hoffmann G, Watkins J.C. The influence that honey bees and wild bees foraging together have on sunflower cross-pollination and seed set. Am. Bee J. 2000;137:565–566. [Google Scholar]
- Delaplane K.S, Mayer D.F. CABI Publishing; New York, NY: 2000. Crop pollination by bees. [Google Scholar]
- De Marco P, Coelho F.M. Services performed by the ecosystem: forest remnants influence agricultural cultures' pollination and production. Biodivers. Conserv. 2004;13:1245–1255. doi:10.1023/B:BIOC.0000019402.51193.e8 [Google Scholar]
- Downey D.L, Winston M.L. Honey bee colony mortality and productivity with single and dual infestations of parasitic mite species. Apidologie. 2001;32:567–575. doi:10.1051/apido:2001144 [Google Scholar]
- Evans J.D, Pettis J.S, Hood W.M, Shimanuki H. Tracking an invasive honey bee pest: mitochondrial DNA variation in Noth American small hive beetles. Apidologie. 2003;34:103–109. doi:10.1051/apido:2003004 [Google Scholar]
- FAOSTAT data 2005 Data available at http://faostat.fao.org; Agricultural data/Agricultural production/Crops primary. Last accessed in July 2006.
- Free J.B. Academic Press; London, UK: 1993. Insect pollination of crops. [Google Scholar]
- Ghazoul J. Buzziness as usual? Questioning the global pollination crisis. Trends Ecol. Evol. 2005;20:367–373. doi: 10.1016/j.tree.2005.04.026. doi:10.1016/j.tree.2005.04.026 [DOI] [PubMed] [Google Scholar]
- Ghazoul J. Floral diversity and the facilitation of pollination. J. Ecol. 2006;94:295–304. [Google Scholar]
- Goulson D. Conserving wild bees for crop pollination. Food Agric. Environ. 2003;1:142–144. [Google Scholar]
- Greenleaf S.A, Kremen C. Wild bees enhance honey bees' pollination of hybrid sunflower. Proc. Natl Acad. Sci. USA. 2006;103:13 890–13 895. doi: 10.1073/pnas.0600929103. doi:10.1073/pnas.0600929103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf, S. A. & Kremen, C. In press. Wild bee species increase tomato production and respond differently to surrounding land use in Northern California. Biol. Conserv. (doi:10.1016/j.biocon.2006.05.025).
- Heard T.A. Behaviour and pollinator efficiency of stingless bees and honey bees on macadamia flowers. J. Apicult. Res. 1994;33:191–198. [Google Scholar]
- Heard T.A, Exley E. Diversity, abundance and distribution of insect visitors to macadamia flowers. Environ. Entomol. 1994;23:91–100. [Google Scholar]
- Herrera C.M, Jordano P, Guitian J, Traverset A. Annual variability in seed production by woody plants and the masting concept: reassessment of principles and relationship to pollination and seed dispersal. Am. Nat. 1998;152:576–594. doi: 10.1086/286191. doi:10.1086/286191 [DOI] [PubMed] [Google Scholar]
- Higes M, Martin R, Meana A. Nosema ceranae, a new microsporidian parasite in honeybees in Euope. J. Inv. Pathol. 2006;92:93–95. doi: 10.1016/j.jip.2006.02.005. doi:10.1016/j.jip.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Hines H.M, Hendrix S.D. Bumble bee (Hymenoptera: Apidae) diversity and abundance in tallgrass prairie patches: effects of local and landscape floral resources. Environ. Entomol. 2005;34:1477–1484. [Google Scholar]
- Ingram M, Nabhan G.C, Buchmann S.L. Impending pollination crisis threatens biodiversity and agriculture. Tropinet. 1996;7:1. [Google Scholar]
- Javorek S.K, Mackenzie K.E, Vander Kloet S.P. Comparative pollination effectiveness among bees (Hymenoptera: Apoidea) at lowbush blueberry (Ericaceae: Vaccinium angustifolium Ait.) Ann. Entomol. Soc. Am. 2002;95:245–351. doi:10.1603/0013-8746(2002)095[0345:CPEABH]2.0.CO;2 [Google Scholar]
- Kearns C.A, Inouye D.W, Waser N. Endangered mutualisms: the conservation of plant–pollinator interactions. Annu. Rev. Ecol. Syst. 1998;29:83–112. doi:10.1146/annurev.ecolsys.29.1.83 [Google Scholar]
- Kevan P.G. Forest application of the insecticide Fenitrothion and its effect on wild bee pollinators (Hymenoptera: Apoidea) of lowbush blueberries (Vaccinium spp.) in Southern New Brunswick, Canada. Biol. Conserv. 1975;7:301–309. doi:10.1016/0006-3207(75)90045-2 [Google Scholar]
- Kevan P.G, Phillips T. The economics of pollinator declines: assessing the consequences. Conserv. Ecol. 2001;5:8. url:http://www.consecol.org/vol5/iss1/art8 [Google Scholar]
- Kevan P.G, Clark E.A, Thomas V.G. Insect pollinators and sustainable agriculture. Am. J. Altern. Agr. 1990;5:12–22. [Google Scholar]
- Klein A.M, Steffan-Dewenter I, Tscharntke T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. B. 2003a;270:955–961. doi: 10.1098/rspb.2002.2306. doi:10.1098/rspb.2002.2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A.M, Steffan-Dewenter I, Tscharntke T. Pollination of Coffea cenephora in relation to local and regional agroforestry management. J. Appl. Ecol. 2003b;40:837–845. doi:10.1046/j.1365-2664.2003.00847.x [Google Scholar]
- Knight T.M, Steets J.A, Ashman T.-L. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am. J. Bot. 2006;93:271–277. doi: 10.3732/ajb.93.2.271. [DOI] [PubMed] [Google Scholar]
- Kremen, C. & Chaplin, R. In press. Insects as providers of ecosystem services: crop pollination and pest control. In Insect conservation biology. Proceedings of the Royal Entomological Society's 23rd Symp. (ed. A. J. A. Stewart, T. R. New, & O. T. Lewis), Wallingford, UK: CABI Publishing.
- Kremen C, Williams N.M, Thorp R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl Acad. Sci. USA. 2002;99:16 812–16 816. doi: 10.1073/pnas.262413599. doi:10.1073/pnas.262413599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C, Williams N.M, Bugg R.L, Fay J.P, Thorp R.W. The area requirements on an ecosystem service: crop pollination by native bee communities in California. Ecol. Lett. 2004;7:1109–1119. doi:10.1111/j.1461-0248.2004.00662.x [Google Scholar]
- Larsen T.H, Williams N, Kremen C. Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol. Lett. 2005;8:538–547. doi: 10.1111/j.1461-0248.2005.00749.x. doi:10.1111/j.1461-0248.2005.00749.x [DOI] [PubMed] [Google Scholar]
- Larson B.M.H, Barrett S.C.H. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc. 2000;69:503–520. doi:10.1006/bijl.1999.0372 [Google Scholar]
- Matheson A, Buchmann S.L, O'Toole C, Westrich P, Williams J.H. Academic Press; London, UK: 1996. The conservation of bees. [Google Scholar]
- McGregor, S. E. 1976 Insect pollination of cultivated crop-plants. U.S.D.A. Agriculture Handbook No. 496, 93–98. Version with some updated information for some crop species available at http://gears.tucson.ars.ag.gov/book/
- Morandin L.A, Winston M.L. Wild bee abundance and seed production in conventional, organic, and genetically modified canola. Ecol. Appl. 2005;15:871–881. [Google Scholar]
- Morandin L.A, Winston M.L. Pollinators provide economic incentive to preserve natural land in agroecosystems. Agric. Ecosyst. Environ. 2006;116:289–292. doi:10.1016/j.agee.2006.02.012 [Google Scholar]
- Morse R, Calderone N.W. The value of honey bees as pollinators of U.S. Crops in 2000. Bee Culture. 2000;128:1–15. [Google Scholar]
- Nabhan G.P, Buchmann S. Services provided by pollinators. In: Daily G.G, editor. Nature's services: societal dependence on natural ecosystems. Island Press; Washington, DC: 1997. pp. 133–150. [Google Scholar]
- Ortega E, Egea J, Cánovas J.A, Dicenta F. Pollen tube dynamics following half- and fully-compatible pollinations in self-compatible almond cultivars. Sex. Plant Reprod. 2002;15:47–51. doi:10.1007/s00497-002-0137-5 [Google Scholar]
- O'Toole C. Diversity of native bees and agroecosystems. In: La Salle J, Gould I.D, editors. Hymenoptera and biodiversity. CAB International; London, UK: 1993. pp. 169–196. [Google Scholar]
- Palmer M, et al. Ecology for a crowded planet. Science. 2004;304:1251–1252. doi: 10.1126/science.1095780. doi:10.1126/science.1095780 [DOI] [PubMed] [Google Scholar]
- Parker F.D, Batra S.W.T, Tependino V.J. New pollinators for our crops. Agri. Zool. Rev. 1987;2:279–304. [Google Scholar]
- Pías B, Guitián P. Breeding system and pollen limitation in the masting tree Sorbus aucuparia L. (Rosaceae) in the NW Iberian Peninsula. Acta Oecol. 2006;29:97–103. doi:10.1016/j.actao.2005.08.005 [Google Scholar]
- Potts S.G, Vulliamy B, Robert S, O'Toole C, Dafni A, Neeman G, Willmer P. Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol. Entomol. 2005;30:78–85. doi:10.1111/j.0307-6946.2005.00662.x [Google Scholar]
- Prescott-Allen R, Prescott-Allen C. How many plants feed the world? Conserv. Biol. 1990;4:366–374. doi:10.1111/j.1523-1739.1990.tb00310.x [Google Scholar]
- Pywell R.F, Warman E.A, Hulmes L, Hulmes S, Nuttall P, Sparks T.H, Critchley C.N.R, Sherwood A. Effectiveness of new agri-environment schemes in providing foraging resources for bumblebees in intensively farmed landscapes. Biol. Conserv. 2006;129:192–206. doi:10.1016/j.biocon.2005.10.034 [Google Scholar]
- Rathcke B.J, Jules E. Habitat fragmentation and plant/pollinator interactions. Curr. Sci. 1994;65:273–278. [Google Scholar]
- Richards K.W. Comparative efficacy of bee species for pollination of legume seed crops. In: Matheson A, Buchmann S.L, O'Toole C, Westrich P, Williams J.H, editors. The conservation of bees. Academic Press; London, UK: 1996. pp. 81–103. [Google Scholar]
- Richards A.J. Does low biodiversity resulting from modern agricultural practice affect crop pollination and yield? Ann. Bot. 2001;88:165–172. doi:10.1006/anbo.2001.1463 [Google Scholar]
- Ricketts T. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv. Biol. 2004;18:1262–1271. doi:10.1111/j.1523-1739.2004.00227.x [Google Scholar]
- Ricketts T, Daily G.C, Ehrlich P.R, Michener C.D. Economic value of tropical forest to coffee production. Proc. Natl Acad. Sci. USA. 2004;101:12 579–12 582. doi: 10.1073/pnas.0405147101. doi:10.1073/pnas.0405147101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W.S, Nowogrodzki R, Morse R.A. Pollination parameters. Glean. Bee Cult. 1989a;117:148–152. [Google Scholar]
- Robinson W.S, Nowogrodzki R, Morse R.A. The value of honey bees as pollinators of U.S. crops. Am. Bee J. 1989b;129:411–423. see also pp. 477–478. [Google Scholar]
- Roubik, D. W. 1995 Pollination of cultivated plants in the tropics. Food and agriculture organization of the United Nations, Rome, Italy. Bull. 118.
- Roubik D.W. Ups and downs in pollinator populations: when is there a decline? Conserv. Ecol. 2001;5:2. http://www.consecol.org/vol5/iss1/art2/ [Google Scholar]
- Roubik D.W. The value of bees to the coffee harvest. Nature. 2002;417:708. doi: 10.1038/417708a. doi:10.1038/417708a [DOI] [PubMed] [Google Scholar]
- Shuler R.E, Roulston T.H, Farris G.E. Farming practices influence wild pollinator populations on squash and pumpkin. J. Econ. Entomol. 2005;98:790–795. doi: 10.1603/0022-0493-98.3.790. [DOI] [PubMed] [Google Scholar]
- Slaa E.J, Sánchez C.L.A, Malagodi-Braga K.S, Hofstede F.E. Stingless bees in applied pollination. Practice and perspectives. Apidologie. 2006;37:293–315. doi:10.1051/apido:2006022 [Google Scholar]
- Southwick E.E, Southwick L., Jr Estimating the economic value of honey bees (Hymenoptera: Apidae) as agricultural pollinators in the United States. J. Econ. Entomol. 1992;85:621–633. [Google Scholar]
- Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T. Scale-dependent effects of landscape structure on three pollinator guilds. Ecology. 2002;83:1421–1432. [Google Scholar]
- Steffan-Dewenter I, Potts S.G, Packer L. Pollinator diversity and crop pollination services are at risk. Trends Ecol. Evol. 2005;20:651–652. doi: 10.1016/j.tree.2005.09.004. doi:10.1016/j.tree.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Steffan-Dewenter I, Klein A.M, Alfert T, Gaebele V, Tscharntke T. Bee diversity and plant–pollinator interactions in fragmented landscapes. In: Waser N.M, Ollerton J, editors. Specialization and generalization in plant–pollinator interactions. Chicago Press; Chicago, IL: 2006. pp. 387–408. [Google Scholar]
- Stewart A.M. Factors affecting pollinator effectiveness in Feijoa sellowiana. New Zeal. J. Crop Hort. 1989;17:145–154. [Google Scholar]
- Sundriyal M, Sundriyal R.C. Wild edible plants of the Sikkim Himalaya: nutritive values of selected species. Econ. Bot. 2004;58:286–299. doi:10.1663/0013-0001(2004)058[0286:WEPOTS]2.0.CO;2 [Google Scholar]
- Torchio P.F. Diversification of pollination strategies for U.S. crops. Environ. Entomol. 1990;19:1649–1656. [Google Scholar]
- Tscharntke T, Klein A.M, Kruess A, Steffan-Dewenter I, Thies C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 2005;8:857–874. doi:10.1111/j.1461-0248.2005.00782.x [Google Scholar]
- Tylianakis J.M, Klein A.M, Tscharntke T. Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology. 2005;86:3296–3302. [Google Scholar]
- Veddeler D, Klein A.M, Tscharntke T. Contrasting responses of bee communities to coffee flowering at different spatial scales. Oikos. 2006;112:594–601. doi:10.1111/j.0030-1299.2006.14111.x [Google Scholar]
- Watanabe M.E. Pollination worries rise as honey bees decline. Science. 1994;265:1170. doi: 10.1126/science.265.5176.1170. [DOI] [PubMed] [Google Scholar]
- Westerkamp C, Gottsberger G. Diversity pays in crop pollination. Crop Sci. 2000;40:1209–1222. [Google Scholar]
- Westphal C, Steffan-Dewenter I, Tscharntke T. Mass-flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003;6:961–965. doi:10.1046/j.1461-0248.2003.00523.x [Google Scholar]
- Westrich P. Habitat requirements of central European bees and the problems of partial habitats. In: Matheson A, Buchmann S.L, O'Toole C, Westrich P, Williams H, editors. The conservation of bees. Linnean Society of London and the International Bee Research Association by Academic Press; London, UK: 1996. pp. 1–16. [Google Scholar]
- Williams I.H. The dependences of crop production within the European Union on pollination by honey bees. Agric. Zool. Rev. 1994;6:229–257. [Google Scholar]
- Williams I.H. Aspects of bee diversity and crop pollination in the European Union. In: Matheson A, Buchmann S.L, O'Toole C, Westrich P, Williams H, editors. The conservation of bees. Linnean Society of London and the International Bee Research Association by Academic Press; London, UK: 1996. pp. 63–80. [Google Scholar]
- Williams I.H, Corbet S.A, Osborne J.L. Beekeeping, wild bees and pollination in the European community. Bee World. 1991;72:170–180. [Google Scholar]
- Williams N.M, Minckley R.L, Silveira F.A. Variation in native bee faunas and its implications for detecting community changes. Conserv. Ecol. 2001;5:7. [Google Scholar]
- Winfree, R., Griswold, T. & Kremen, C. In press. A positive response from bee pollinators to human disturbance in a forested ecosystem. Conserv. Biol. [DOI] [PubMed]
- Wood G.W. Recuperation of native bee populations in blueberry fields exposed to drift of fenitrothion from forest spray operations in New Brunswick. J. Econ. Entomol. 1979;72:36–39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crop selection pathway to estimate the annual world production that is influenced by animal pollination
Crop selection pathway to evaluate the levels of dependence on animal pollination for crops important in the global market