Abstract
Estimates of variability in pinniped survival rates are generally based on observations at single sites, so it is not certain whether observed rates represent the whole population. Here, we provide a comprehensive analysis of spatio-temporal variation in age-specific survival rates for endangered Hawaiian monk seals (Monachus schauinslandi) based on capture–recapture analyses of more than 85% of the pups weaned in this population over the last two decades. Uniquely, these data have been collected from six subpopulations, encompassing all major breeding sites across its 1800 km long core range. Analyses of individual subpopulations revealed similar patterns in age-specific survival, characterized by the relatively low survival rates from weaning to 2 years of age, intermediate rates to 4 years of age, and then by relatively high ‘mature’ survival rates until 17 years of age, after which a senescent decline was observed. Juvenile, subadult and adult survival rates all varied significantly over time. Trends in survival among subpopulations were coherent with their relative geographical positions, suggesting regional structuring and connectedness within the archipelago. Survival rates for different age classes tended to be positively correlated, suggesting that similar factors may influence the survival for seals of all ages.
Keywords: Hawaiian monk seal, life history, survival, senescence, spatial variability, temporal variability
1. Introduction
Understanding variability in survival patterns is fundamental to life-history theory, wildlife management and conservation biology (Caughley 1966; Stearns 1976, Eberhardt 1985). In mammals, survival theoretically follows a pattern of relatively low juvenile rates, improved survival in adulthood, followed by a senescent decline (Caughley 1966). However, field studies that confirm these patterns, especially in long-lived species, are rare. In particular, evidence for senescence in mammals (e.g. Promislow 1991) has been criticized, because it relies heavily on cross-sectional age-structure data and associated assumptions of stable age distribution and representative sampling (Gaillard et al. 1994). Long-term studies of marked individuals are required to better characterize variability in survival across time and space.
Pinnipeds are well suited for individual-based longitudinal studies. They are long-lived, can be marked relatively easily and aggregate at terrestrial sites to which they show a high fidelity, thereby allowing survival to be estimated using capture–recapture analyses (Lebreton et al. 1992). However, previous studies of age-specific survival in pinnipeds have generally been based on longitudinal studies at isolated breeding colonies (e.g. Testa & Siniff 1987; Hindell 1991; Boyd et al. 1995; Pistorius & Bester 2002; Cameron & Siniff 2004), which represent just a small portion of the population. Inferences from these studies are constrained for three important reasons. First, it is frequently difficult to discriminate between mortality and emigration to alternative sites. Second, failure to detect senescence may result when the range of sampled ages does not encompass animals old enough to exhibit senescence. Third, these species often inhabit large geographical areas, and it is unclear to what extent the findings from smaller-scale studies represent larger-scale patterns across the species range.
The Hawaiian monk seal (Monachus schauinslandi) presents a rare opportunity to characterize spatio-temporal variation in survival. Currently, the world population of only approximately 1300 Hawaiian monk seals is declining (Antonelis et al. 2006). Poor juvenile survival has been suggested as the primary proximate cause for this downward trend (Craig & Ragen 1999). A better understanding of the recent trends in survival is therefore crucial to support present efforts to conserve this critically endangered species.
The monk seal's endangered status has meant that the species has been consistently monitored throughout nearly its entire range for over the last 20 years. Since the early 1980s, most individuals in the population have been marked in their birth year and resighted throughout their lives, overcoming concerns about whether the marked animals represent the species at large. Resighting surveys have been conducted within each of the six main subpopulations across the species' core range, i.e. the 1800 km wide Northwestern Hawaiian Islands, such that migration has a negligible potential to confound survival estimates. In this paper, we exploit this unique set of circumstances to characterize spatial and temporal variability in age- and gender-specific survival of this long-lived mammal throughout its principal range.
2. Material and methods
(a) Data collection
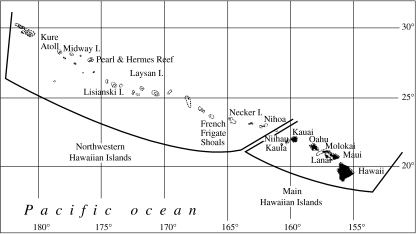
Monk seals occur throughout the lands and waters of the Hawaiian Archipelago (figure 1), although some 80–90% of them inhabit six main subpopulations that are scattered throughout the 1800 km span of the Northwestern Hawaiian Islands (NWHI), including French Frigate Shoals, Laysan Island, Lisianski Island, Pearl and Hermes Reef, Midway Atoll, and Kure Atoll (Antonelis et al. 2006; Stewart et al. 2006). A relatively small number of seals reside at Necker and Nihoa Islands (which have limited landing area for seals) and in the main Hawaiian Islands; only two of the more than 4200 individuals tagged in the NWHI since 1981 have been sighted in the main Hawaiian Islands (Baker & Johanos 2004). This study was conducted at all the six primary NWHI subpopulations, thereby encompassing nearly the entire species range and nearly all individuals born into the world population over the last 20 years.
Figure 1.
The Hawaiian Archipelago, indicating the primary Northwestern Hawaiian Islands subpopulations of monk seals at French Frigate Shoals, Laysan Island, Lisianski Island, Pearl and Hermes Reef, Midway Atoll and Kure Atoll.
Field studies typically ranging from two to five months duration per year were conducted in the NWHI. The six subpopulations are located at either relatively large single islands (Laysan, 4.1 km2; Lisianski, 1.5 km2) or atolls that are each made up of two to nine permanent islets and ephemeral sand spits. The total land area of these atolls ranges from 0.2 to 1 km2 at French Frigate Shoals, Pearl and Hermes Reef, and Kure Atoll, up to 6.4 km2 at Midway Atoll (Juvik & Juvik 1998). Subpopulation abundance has varied dramatically over the last 20 years and presently ranges from less than 100 to approximately 300 at the various sites (Baker 2004; Antonelis et al. 2006).
Female monk seals give birth to single pups and nurse them for five to six weeks during a protracted reproductive season, with most births occurring during March to August (Johanos et al. 1994). In this study, pups were double-tagged on their rear flippers with unique plastic tags (and, since 1990, also marked with injected passive integrated transponders—PIT tags; Wright et al. 1998) soon after weaning. Individual identities were maintained long term by the periodic retagging of individuals to replace lost or broken flipper tags. Also, an extensive photographic (and, previously, hand-drawn) identification system documenting scars, pelage marks and other distinct natural features was maintained annually, so that they could still be identified if they were to lose all flipper and PIT tags (Harting et al. 2004). Finally, temporary pelage bleach marks were applied to as many seals as possible to facilitate within-season and sometimes between-year resighting.
Regular surveys were conducted in each of the six subpopulations to resight individual seals. All land areas used by seals were searched, and seal identity, gender, size, class (pup, juvenile, subadult and adult size; Stone 1984) and location were recorded. Data recorded in the field were entered into a computerized database, and automated error-checking routines that compared tag numbers, other identifiers, gender and size class were used to further validate identifications. Only absolutely certain resightings were used in survival analyses. The duration of field seasons and the intensity of surveys varied between sites and years, but they typically occurred during the main period of pupping and mating. Additional site-specific details of the field studies are provided by Baker (2004).
(b) Analysis
Individual sighting histories were constructed from annual tagging and resight data. Animals were first marked at weaning, thus allowing annual survival estimates to be generated from weaning onwards. Animals observed at any time during a calendar year were considered to have survived the age transition from the previous to the present year.
Maximum-likelihood estimates (MLE) of survival rates and capture probabilities were obtained using the ‘recaptures only’ analysis implemented in Program MARK (White & Burnham 1999). This extended the basic Cormack–Jolly-Seber fully time-dependent model to evaluate age dependence, as well as the influence of categorical factors (e.g. gender and subpopulation; Lebreton et al. 1992). We fit a variety of models, described below, which were ranked and evaluated using the small-sample Akaike's information criterion (AICc, see Anderson et al. 2000).
Goodness of fit was evaluated by using estimates of the variance inflation factor , a measure of overdispersion. We used the median- approach (Cooch & White 2005) to evaluate goodness of fit for the models selected as best (based on AICc) for each subpopulation and the combined subpopulation analysis. Following Lebreton et al. (1992), we considered values of less than 3 as indicative that model structure provided an adequate fit to the data.
Assumptions underlying capture–recapture analysis of this dataset are discussed fully by Baker (2004), with respect to abundance estimation, and by Craig & Ragen (1999) in relation to Jolly-Seber estimates of juvenile survival. Here, we assume that, once marked, individual identities are maintained throughout the study. Craig & Ragen (1999) estimated monk seal survival to 2 years of age and found minimal tag loss occurred. As we evaluate survival to more than 20 years, the potential for tag loss to bias estimates is greater if tags were the only method used for identification. However, flipper tags were periodically replaced when worn or lost, and the use of PIT tags, additional temporary pelage bleach marks and extensive photographic identification effort minimized the risk of mark loss.
Following Craig & Ragen (1999), heterogeneity in capture probability was reduced by collapsing all sighting efforts within a field season to a single event (seen or not seen). Capture heterogeneity was further addressed by explicitly modelling the differences in sightability among groups (classified by age, gender, subpopulation and year), so that constant capture probability need only be assumed within each group (Lebreton et al. 1992).
The following notations were used to describe models and parameters throughout this paper. Survival is denoted with Φ and capture probability is denoted with p. Subscripts denote age-specific survival rates; thus, Φ1 is survival from weaning to 1 year of age, Φ2 is survival from 1 to 2 years of age, etc.
The purpose of our analyses was to characterize lifetime survival patterns, including temporal and spatial variability. As a result, models were fitted to evaluate the influences of age, gender, year and subpopulation. Likewise, these same variables might influence capture probabilities. However, a global model incorporating all these effects would be greatly over-parameterized. With over 20 age classes and resight years, six subpopulations and two sexes, the total number of parameters in this global model would approach 5000. Clearly, some simplification was required, so a separate analysis was conducted at each location.
We anticipated that the duration of field seasons would have the largest impact on p. Thus, within each subpopulation-specific analysis, we fitted an age-dependent Φ and time-dependent p model. We then reduced the number of ps by assigning a single parameter to years when estimated ps did not differ. Next, we explored whether age and gender significantly influenced p. Once the best-fitting and most parsimonious model with respect to p was found, we turned to analysing Φ.
According to our a priori expectation, age-specific survival would start relatively low, rise to an asymptotically ‘mature’ rate and possibly exhibit senescence among older animals. However, we had no expectation of the ages at which these transitions would occur. We therefore examined age-specific estimates of Φ, gradually combining consecutive ages (i.e. constraining them to share the same parameters) and evaluating the support for the reduced models with AICc. After determining which age groups' Φs differed, we tested whether those age groups' survival rates varied over time. If time dependence significantly improved models, we re-evaluated the support for age group break points with time dependence in the model. Finally, the effect of gender on survival was evaluated as both a simple additive factor (i.e. a single adjustment for all ages and years) and an interactive effect with time and age. In this way, we derived a best model for each subpopulation.
To evaluate spatial patterns in survival, we combined sighting histories from all six subpopulations. To minimize the potential for confounding location and time effects, we limited this analysis to years for which data were available at all sites (1984–2004). The exception was Midway Atoll, which was included even though only a few pups were born there until the mid-1990s. Significant differences in ps found in the subpopulation-specific models were duplicated in the combined analysis. For this analysis, we set identical age groupings among sites to avoid confounding age and subpopulation effects. We began by fitting distinct, time-dependent survival rates for each age group at each subpopulation, and then sequentially fitted models with multiple combinations of subpopulations to determine where differences between subpopulations occurred.
3. Results
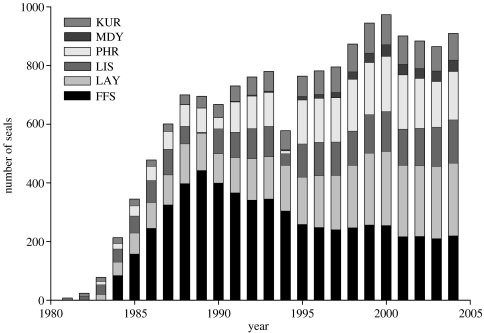
Marking weaned monk seals and subsequent resighting efforts began at a few sites in the early 1980s and at all the primary subpopulations sites by 1984. Effort at Midway began later because few animals were sighted at the location in earlier years. A total of 3421 pups were marked, representing more than 85% of pups weaned during the study. The number of animals marked or resighted for each year is shown in figure 2. Intensity of effort varied considerably over time, but became more consistent after the mid-1990s, with field seasons typically ranging from approximately 50 to 200 days depending on the site.
Figure 2.
Number of known-aged Hawaiian monk seals identified (marked or resighted) at six Northwestern Hawaiian Islands subpopulations during 1981–2004. Abbreviations for subpopulations are as follows: FFS, French Frigate Shoals; LAY, Laysan Island; LIS, Lisianski Island; PHR, Pearl and Hermes Reef; MDY, Midway Atoll; KUR, Kure Atoll.
Values of ranged from 1.01 to 1.33 for the best subpopulation model and the combined subpopulation analysis, indicating only modest overdispersion and adequate model fit.
(a) Individual subpopulations
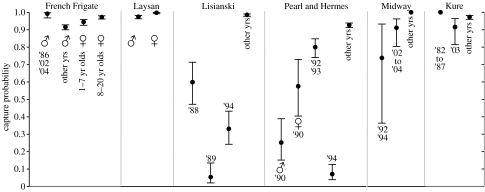
Capture probabilities (p) were typically high at most sites, often approaching 1. Notable exceptions corresponded to years and locations where field effort was low (figure 3). In a few cases, model fits were significantly improved by taking into account gender differences in capture probabilities. At French Frigate Shoals, males generally had lower capture probabilities than females, and females aged 1–7 years were less likely to be seen than older females. At Laysan Island, males also had a slightly lower capture probability, and this pattern was seen at Pearl and Hermes Reef in 1 year (1990).
Figure 3.
Estimated capture probabilities (p; with 95% confidence intervals) of Hawaiian monk seals at the six main subpopulations in the Northwestern Hawaiian Islands. Text and symbols indicate years and gender to which distinct estimates apply.
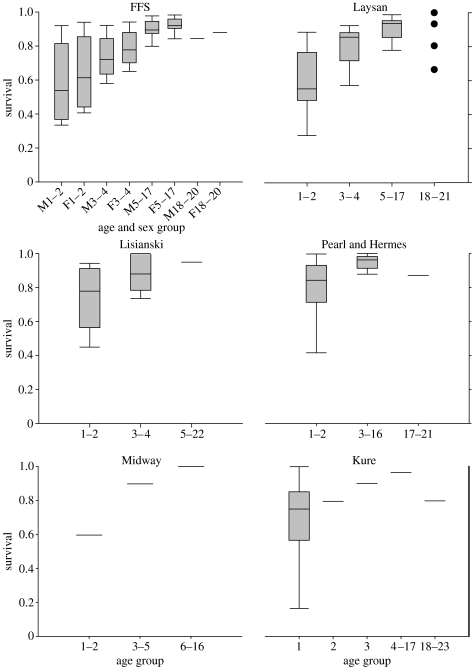
Independent analyses of survival rates from the six subpopulations revealed remarkably similar patterns (table 1, figure 4). In particular, age-specific differences in survival were consistent among sites. In five of the six subpopulations, survival to 1 and 2 years was the same, but significantly lower than older animals. There was some variation in the next older, ‘subadult’ group among subpopulations, but a ‘mature’ rate was consistently achieved between the 4th and the 6th year and maintained for many years. Finally, a senescent drop in survival was detected at four of the six sites, beginning in the 18th year at three sites, and just 1 year earlier at another. Senescence was not detectable at either Lisianski nor Midway Atoll.
Table 1.
Summary of separate subpopulation analyses of Hawaiian monk seal survival rates, indicating where significant differences in age-specific survival, temporal variability and gender differences in survival were found.
| subpopulation | years | age groups (years) | time variation | gender |
|---|---|---|---|---|
| French Frigate Shoals | 1984–2004 | 1–2, 3–4, 5–17, 18–20a | all but 18–20 years | all agesb |
| Laysan Island | 1983–2004 | 1–2, 3–4, 5–17, 18–21a | all age groups | no |
| Lisianski Island | 1982–2004 | 1–2, 3–4, 5–22 | 1–2 and 3–4 years | no |
| Pearl and Hermes | 1983–2004 | 1–2, 3–16, 17–21a | all but 17–20 years | no |
| Midway | 1988–2004 | 1–2, 3–5, 6–16 | no | no |
| Kure | 1981–2004 | 1, 2, 3, 4–17, 18–23a | only 1 year | no |
Oldest age group had reduced survival.
Females had higher survival than males.
Figure 4.
Results of separate subpopulation analyses of Hawaiian monk seal survival rates. Age groups found to have significantly different survival rates are plotted separately, showing an increase in survival with age until senescence is expressed. Gender differences in survival were found only at French Frigate Shoals. Box plots show the distribution of point estimates of survival rates over time. The lower boundary of the box indicates the 25th percentile, a line within the box marks the median and the upper boundary of the box indicates the 75th percentile. Error bars indicate the 90th and 10th percentiles. Horizontal lines alone indicate single-point estimates where time variation was not detected. At Laysan Island, significant temporal variation was detected in the age group of 18–21 years and the four available estimates are shown in lieu of a box plot.
Similarly, some time variation in survival rates was detected at all sites, except Midway Atoll (where the sample size was smallest). At Lisianski Island and Kure Atoll (with intermediate samples sizes), time variation was detected only in the younger age groups. However, at French Frigate Shoals, Laysan Island, and Pearl and Hermes Reef (largest sample sizes), significant variation in adult survival was evident (figure 4).
Gender appeared to have little influence on survival. The exception was French Frigate Shoals, where females were found to have significantly higher survival rates than males and was most apparent among the 1–2 year-old age group. However, including gender as an additive factor across all ages at this site was a significant improvement over a model with gender differences only in the youngest age group (ΔAICc=6.9), and far better than a model with no gender effect (ΔAICc=13.3). At most other subpopulations, models involving gender differences showed marginally worse fits than those with an equal male and female survival (ΔAICc=1.0–2.0). We selected the latter models for their parsimony and slightly better fits. Moreover, the models with gender differences indicated opposing trends among sites—slightly higher female survival at Midway Atoll and slightly higher male survival at Lisianski and Kure. At Pearl and Hermes Reef, there was no support for gender differences (ΔAICc=16.5, no gender difference versus additive gender effect).
(b) Combined subpopulations
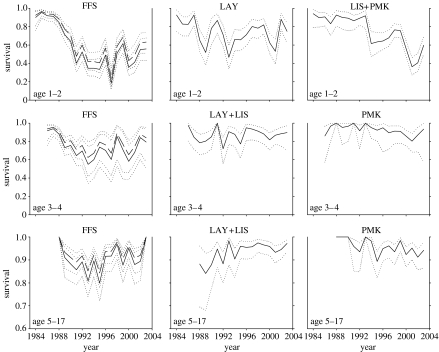
In the analysis of all subpopulations combined, the best model had subpopulations that were grouped in a manner coherent with their geographical distribution (figure 5). Survival rate estimates from this model, their standard errors and confidence intervals can be found in Appendix A (electronic supplementary material). At one end of the Hawaiian chain, survival rates at French Frigate Shoals were decoupled from other sites for all except the oldest senescent age group. At the opposite end of the island chain, Pearl and Hermes, Midway, and Kure survival rates were indistinguishable from each other at all ages. Finally, at the central sites, most survival rates at Laysan and Lisianski Islands did not significantly differ from each other. The exception was the youngest animals' survival (Φ1–2) at Lisianski, which was more similar to rates at Pearl and Hermes, Midway, and Kure. For the oldest age group (Φ18–20), no detectable temporal or spatial variability was observed, but sample sizes were small. Within the subpopulation groups found to have similar survival rate trends, pairwise correlations of estimated survival rates among age groups were positive in all cases and statistically significant in 50% of the cases (table 2).
Figure 5.
Spatial and temporal variation in estimated Hawaiian monk seal survival rates from combined analysis of six subpopulations. Solid lines (and dashed lines, French Frigate Shoals females), survival estimates; dotted lines, 95% confidence intervals. Survival to 1–2 years of age is presented in the top row of graphs, 3–4 years of age in the middle row and 5–17 years of age in the bottom row. Columns of graphs separate subpopulation groups that had significantly different survival rate trends over time. FFS, French Frigate Shoals; LAY, Laysan Island; LIS, Lisianski Island; PMK, Pearl and Hermes Reef, Midway Atoll, and Kure Atoll. Gender differences in survival were only detected at FFS. Note that the y-axis scale in the bottom row differs from the two rows above.
Table 2.
Correlation of survival rates among age groups (1–2, 3–4, 5–17 years) within subpopulations with indistinct survival rate trends. (Subpopulations are abbreviated as follows: FFS, French Frigate Shoals; LAY, Laysan Island; LIS, Lisianski Island; PMK, Pearl and Hermes Reef, Midway Atoll, and Kure Atoll. Correlation coefficients (r) are shown and statistical significance is indicated. *p<0.05; **p<0.01.)
| FFS 3–4 | FFS 5–17 | LAY+LIS 3–4 | LAY+LIS 5–17 | PMK 3–4 | PMK 5–17 | |
|---|---|---|---|---|---|---|
| FFS 1–2 | 0.73** | 0.42 | — | — | — | — |
| FFS 3–4 | — | 0.47 | — | — | — | — |
| LAY 1–2 | — | — | 0.38 | 0.22 | — | — |
| LAY+LIS 3–4 | — | — | — | 0.55* | — | — |
| LIS+PMK 1–2 | — | — | — | — | 0.80** | 0.61* |
| PMK 3–4 | — | — | — | — | — | 0.55* |
4. Discussion
Age-specific survival trends in Hawaiian monk seals accord well with the generalized pattern proposed by Caughley (1966). At most of the six subpopulations, the survival rate was lower for the first 2 years, then somewhat higher for 2 more years before reaching a ‘mature’ level maintained until the 18th year of life. We expected that the survival rate from weaning to 1 year of age would be significantly lower than the subsequent year, as weaned pups must learn to feed independently. Post-weaning survival rates may in fact be lowest, but this difference could be obscured, because we pooled all sightings over several-months-long field seasons to reduce capture heterogeneity. Consequently, a seal initially marked late 1 year and resighted early the next year, but which dies soon thereafter, will be credited with having survived the full first year, even though it may have died a few months earlier. As a result, the estimated survival rate for that year will be biased upward and the subsequent year's estimate will be biased downward. Few dead animals are observed during field seasons, making the magnitude of this error source difficult to assess. However, it will mostly affect juvenile survival estimates, as mortality is highest for young animals; therefore, they are most likely to die after being observed within a field season than adults. This may blur the distinction between consecutive age-specific survival rates of juveniles.
(a) Senescence
The senescence we detected in Hawaiian monk seals is consistent with declining reproductive rates among older females reported by Harting et al. (submitted). They found that the proportion of females giving birth began to decline between 15 and 18 years of age, slightly earlier than the decline in survival reported here.
Detection of senescence in marine mammals is the exception rather than the rule. Cameron & Siniff (2004) reported no evidence of senescence in Weddell seals (Leptonychotes weddellii), at least up to 17 years old. Pistorius & Bester (2002) analysed survival rates in southern elephant seals (Mirounga leonina) at Marion Island and likewise found no increased mortality up to 17 years of age. However, they suggested that the high adult mortality rates in this population may mean individuals did not live sufficiently long for senescence to occur. Boyd et al. (1995) did not detect a statistically significant drop in survival among Antarctic fur seals (Arctocephalus gazella) up to 18 years old, although point estimates of survival declined after 13 years of age. Beauplet et al. (2006) found both decreased survival and fecundity of subantarctic fur seals (Arctocephalus tropicalis) after 13 years of age. Failure to detect senescence in some populations may not necessarily mean that it does not exist. Rather, the lack of a statistically significant senescence may be due to small sample sizes (or lumping ages) among the oldest animals.
(b) Gender differences in survival
In mammals, females frequently have higher survival rates than males, and this pattern has been largely attributed to the costs of sexual selection for males in polygynous systems (Ralls et al. 1980; Clutton-Brock et al. 1985; Promislow 1992; but see Loison et al. 1999; Owen-Smith & Mason 2005). Some gender-specific survival rate estimates are available from longitudinal studies of marine mammals. Hindell (1991) found female southern elephant seals exhibited a higher survival rate in this highly dimorphic, polygynous species. The Weddell seal, another polygynous pinniped, also has a higher female survival rate (Hastings et al. 1999; Cameron & Siniff 2004). The polygynous grey seal (Halichoenus grypus) exhibits higher first-year female survival (Hall et al. 2001). Conversely, survival does not vary with gender in Florida manatees (Trichechus manatus latirostris), a species whose females are slightly larger and whose levels of male–male competition are low (Langtimm et al. 1998).
It was difficult to anticipate whether Hawaiian monk seals might exhibit gender differences in survival. The mating system of monk seals is poorly understood, but they are not sexually dimorphic, and it is likely to be a promiscuous species (Stirling 1983). Observed levels of male–male aggression are lower than that seen in polygynous, territorial pinnipeds. Given the uncertainty about the role of male competition in the mating system, the finding that gender did not strongly influence survival in Hawaiian monk seals is not surprising. However, the fact that female-biased survival was detected at French Frigate Shoals, and only there, is perplexing. Insufficient sample sizes did not prohibit detecting the trend elsewhere. In the individual subpopulation analyses at other locations, fitted survival rates were not consistently higher for females, regardless of the lack of statistical significance. Further, the analysis of all subpopulations combined bore the highest statistical power to detect gender differences had they existed, but instead confirmed that female survival rates exceeded that of males only at French Frigate Shoals. The unique finding of gender differences in survival at French Frigates Shoals therefore remains unexplained.
(c) Temporal and spatial variance in survival
Juvenile survival of monk seals has been highly variable over the last two decades (figure 5). The rates generally declined after the late 1980s, leading to an overall decline in total abundance (Craig & Ragen 1999; Ragen & Lavigne 1999; Harting 2002). We found that adult and subadult monk seal survival rates also varied significantly over time. Though the magnitude of adult survival variability was much lower than for juveniles, the population growth rate is more sensitive to the former, such that adult survival has the potential to greatly influence population trends (Goodman 1981). Furthermore, the positive correlation of survival rates among age groups (table 2) suggests that similar factors influence all age groups. This covariation of survival among age groups will tend to amplify their influence on the population growth rate (Coulson et al. 2005).
Spatial patterns in monk seal survival suggest that the NWHI archipelago is not a homogenous habitat, but neither is each monk seal subpopulation subject to unique environmental influences. Rather, the survival prospects of seals at some subpopulations appear to be in synchrony, while others tend to be independent. The fact that these groups are consistent with the spatial distribution of different breeding sites suggests regional structuring and connectedness (see also Schmelzer 2000; Parrish 2004).
The spatial and temporal patterns we found in monk seal survival demonstrate two important points. First, sampling a single localized population of a widely distributed species may poorly represent the dynamics of the species at large, especially where habitat is heterogeneous. Conversely, broad sampling throughout a species range can be a powerful tool for elucidating biogeographic structure.
Spatial links in survival rates among subpopulations presumably result from individuals at different sites experiencing similar conditions. This can occur because either environmental conditions span more than one site or animals from different sites move sufficiently that their ranges overlap. Seals were assigned to a subpopulation at birth, and this assignment was not altered if they were subsequently seen at other locations. Overall, the probability of seals being seen away from their initial capture sites was low (less than 10%), but these rates were higher among sites with similar survival trends (e.g. Pearl and Hermes, Midway and Kure) than that with dissimilar trends (e.g. Laysan and French Frigate Shoals; Harting 2002). Stewart et al. (2006) also found that seals tracked from different subpopulations showed similar patterns in overlap of presumed foraging areas. Thus, spatial patterns in monk seal survival probably result from regional structuring of the ecosystem coupled with animal movements.
Acknowledgments
We are greatly indebted to Thea Johanos for her role in designing and maintaining the integrity of the Hawaiian monk seal demographic database for over two decades. Dozens of field researchers collected the mark and resighting data used in this study. Jeff Laake provided invaluable consultation on statistical analysis. We thank Bert Harting and Thea Johanos for their thoughtful reviews of the manuscript. Field research was conducted in the Hawaiian Islands and Midway Atoll National Wildlife Refuges (managed by the US Fish and Wildlife Service) and the State of Hawaii wildlife preserve at Kure Atoll. We thank the officers and crew of the NOAA ships Townsend Cromwell and Oscar Elton Sette for transport to and from the Northwestern Hawaiian Islands.
Supplementary Material
Model estimates of Hawaiian monk seal survival rates. The selected model had four age groups, an additive gender effect at one site (French Frigate Shoals), annual variability for most age groups, and three subpopulation groupings. “Year” indicates survival year such that 1984 refers to survival from 1984 to 1985. The age groups are 1–2 yr, 3–4 yr, 5–17 yr, and 18–20 yr. By convention, 1–2 yr survival indicates the annual survival of animals from weaning to age 1 yr and from age 1 yr to age 2 yr, and so on. Survival rates, their standard errors (s.e.), lower (LCL) and upper (UCL) 95% confidence limits are presented. Separate tables are provided for subpopulation groups with distinct survival patterns (and gender differences in the case of French Frigate Shoals). Note that Lisianski age 1–2 yr survival was combined with Pearl and Hermes Reef, Midway Atoll and Kure Atoll, whereas older seals at Lisianski Island had rates in synchrony with seals at Laysan Island. For the oldest seals (18–20 yr), no spatial or temporal variation was detected. Modeling was conducted using Program MARK.
References
- Anderson D.R, Burnham K.P, Thompson W.L. Null hypothesis testing: problems, prevalence, and an alternative. J. Wildlife Manag. 2000;64:912–923. [Google Scholar]
- Antonelis G.A, Baker J.D, Johanos T.C, Braun R.C, Harting A.L. Hawaiian monk seal (Monachus schauinslandi): status and conservation issues. Atoll Res. Bull. 2006;543:75–101. [Google Scholar]
- Baker J.D. Evaluation of closed capture–recapture methods to estimate abundance of Hawaiian monk seals, Monachus schauinslandi. Ecol. Appl. 2004;14:987–998. [Google Scholar]
- Baker J.D, Johanos T.C. Abundance of the Hawaiian monk seal in the main Hawaiian Islands. Biol. Conserv. 2004;116:103–110. doi:10.1016/S0006-3207(03)00181-2 [Google Scholar]
- Beauplet G, Barbraud C, Dabin W, Küssener C, Guinet C. Age-specific survival and reproductive performances in fur seals: evidence of senescence and individual quality. Oikos. 2006;112:430–441. doi:10.1111/j.0030-1299.2006.14412.x [Google Scholar]
- Boyd I.L, Croxall J.P, Lunn N.J, Reid K. Population demography of Antarctic fur seals: the costs of reproduction and implications for life-histories. J. Anim. Ecol. 1995;64:505–518. doi:10.2307/5653 [Google Scholar]
- Cameron M.F, Siniff D.B. Age-specific survival, abundance, and immigration rates of a Weddell seal (Leptonychotes weddellii) population in McMurdo Sound, Antarctica. Can. J. Zool. 2004;82:601–615. doi:10.1139/z04-025 [Google Scholar]
- Caughley G. Mortality patterns in mammals. Ecology. 1966;47:906–918. doi:10.2307/1935638 [Google Scholar]
- Clutton-Brock T.H, Albon S.D, Guinness F.E. Parental investment and sex differences in juvenile mortality in birds and mammals. Nature. 1985;313:131–133. doi:10.1038/313131a0 [Google Scholar]
- Cooch, E. & White, G. 2005 Program MARK: a gentle introduction, 4th edn.
- Coulson T, Gaillard J.M, Festa-Bianchet M. Decomposing the variation in population growth into contributions from multiple demographic rates. J. Anim. Ecol. 2005;74:789–801. doi:10.1111/j.1365-2656.2005.00975.x [Google Scholar]
- Craig M.P, Ragen T.J. Body size, survival, and decline of juvenile Hawaiian monk seals, Monachus schauinslandi. Mar. Mamm. Sci. 1999;15:786–809. doi:10.1111/j.1748-7692.1999.tb00843.x [Google Scholar]
- Eberhardt L.L. Assessing the dynamics of wild populations. J. Wildlife Manag. 1985;49:997–1012. [Google Scholar]
- Gaillard J.-M, Allaine D, Pontier D, Yoccoz N.G, Promislow E.L. Senescence in natural populations of mammals: a reanalysis. Evolution. 1994;48:509–516. doi: 10.1111/j.1558-5646.1994.tb01329.x. doi:10.2307/2410110 [DOI] [PubMed] [Google Scholar]
- Goodman D. Life history analysis of large mammals. In: Fowler C.W, Smith T.D, editors. Dynamics of large mammal populations. Wiley; New York, NY: 1981. pp. 415–436. [Google Scholar]
- Hall A.J, McConnell B.J, Barker R.J. Factors affecting first-year survival in grey seals and their implications for life history strategy. J. Anim. Ecol. 2001;70:138–149. doi:10.1046/j.1365-2656.2001.00468.x [Google Scholar]
- Harting, A. L. 2002 Stochastic simulation model for the Hawaiian monk seal Ph.D. thesis, Montana State University.
- Harting A.L, Baker J.D, Becker B.L. Nonmetrical digital photo identification system for the Hawaiian monk seal. Mar. Mamm. Sci. 2004;20:886–895. doi:10.1111/j.1748-7692.2004.tb01200.x [Google Scholar]
- Harting, A. L., Baker, J. D. & Johanos, T. C. Submitted Reproductive patterns of the Hawaiian monk seal.
- Hastings K.K, Testa J.W, Rexstad E.A. Interannual variation in survival of juvenile Weddell seals (Leptonychotes weddellii) from McMurdo Sound, Antarctica: effects of cohort, sex and age. J. Zool. Lond. 1999;248:307–323. [Google Scholar]
- Hindell M.A. Some life-history parameters of a declining population of southern elephant seals, Mirounga leonina. J. Anim. Ecol. 1991;60:119–134. doi:10.2307/5449 [Google Scholar]
- Johanos T.C, Becker B.L, Ragen T.J. Annual reproductive cycle of the female Hawaiian monk seal (Monachus schauinslandi) Mar. Mamm. Sci. 1994;10:13–30. doi:10.1111/j.1748-7692.1994.tb00386.x [Google Scholar]
- Juvik S.P, Juvik J.O. University of Hawaii Press; Honolulu, HI: 1998. Atlas of Hawaii. [Google Scholar]
- Langtimm C.A, O'shea T.J, Pradel R, Beck C.A. Estimates of annual survival probabilities for Florida manatees (Trichechus manatus latirostris) Ecology. 1998;79:981–997. doi:10.2307/176594 [Google Scholar]
- Lebreton J.D, Burnham K.P, Clobert J, Anderson D.R. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 1992;62:67–118. doi:10.2307/2937171 [Google Scholar]
- Loison A, Festa-Bianchet M, Gaillard J.M, Jorgenson J.T, Jullien J.M. Age-specific survival in five populations of ungulates: evidence of senescence. Ecology. 1999;80:2539–2554. doi:10.2307/177239 [Google Scholar]
- Owen-Smith N, Mason D.R. Comparative changes in adult vs. juvenile survival affecting population trends of African ungulates. J. Anim. Ecol. 2005;74:762–773. doi:10.1111/j.1365-2656.2005.00973.x [Google Scholar]
- Parrish, F. A. 2004 Foraging landscape of the Hawaiian monk seal Ph.D. thesis, University of Hawaii, Honolulu.
- Pistorius P.A, Bester M.N. A longitudinal study of senescence in a pinniped. Can. J. Zool. 2002;80:395–401. doi:10.1139/z02-017 [Google Scholar]
- Promislow D.E.L. Senescence in natural populations of mammals: a comparative study. Evolution. 1991;45:1869–1887. doi: 10.1111/j.1558-5646.1991.tb02693.x. doi:10.2307/2409837 [DOI] [PubMed] [Google Scholar]
- Promislow D.E.L. Costs of sexual selection in natural populations of mammals. Proc. R. Soc. B. 1992;247:203–210. [Google Scholar]
- Ragen T.J, Lavigne D.M. The Hawaiian monk seal: biology of an endangered species. In: Twiss J.R Jr, Reeves R.R, editors. Conservation and management of marine mammals. Smithsonian Institution Press; Washington, DC: 1999. pp. 224–245. [Google Scholar]
- Ralls K, Brownell R.L, Jr, Ballou J. Differential mortality by sex and age in mammals, with specific reference to the sperm whale. Rep. Int. Whaling Comm. Spec. Issue. 1980;2:233–243. [Google Scholar]
- Schmelzer I. Seals and seascapes: covariation in Hawaiian monk seal subpopulations and the oceanic landscape of the Hawaiian Archipelago. J. Biogeogr. 2000;27:901–914. doi:10.1046/j.1365-2699.2000.00451.x [Google Scholar]
- Stearns S.C. Life-history tactics: a review of the ideas. Q. Rev. Biol. 1976;51:3–47. doi: 10.1086/409052. doi:10.1086/409052 [DOI] [PubMed] [Google Scholar]
- Stewart B.A, Antonelis G.A, Baker J.D, Yochem P.Y. Foraging biogeography of the Hawaiian monk seal in the Northwestern Hawaiian Islands. Atoll Res. Bull. 2006;543:131–145. [Google Scholar]
- Stirling I. The evolution of mating systems in pinnipeds. In: Eisenberg J.F, Kleiman D.G, editors. Advances in the study of mammalian behavior. Special publication. vol. 7. American society of Mammalogists; Washington, DC: 1983. pp. 489–527. [Google Scholar]
- Stone, H. S. 1984 Hawaiian monk seal population research, Lisianski Island, 1982. U.S. Dep. Commer., NOAA Tech. Memo. NOAA-TM-NMFS_SWFSC_47, 33 p.
- Testa J.W, Siniff D.B. Population dynamics of Weddell Seals (Leptonychotes Weddelli) in McMurdo Sound, Antarctica. Ecol. Monogr. 1987;57:149–165. doi:10.2307/1942622 [Google Scholar]
- White G.C, Burnham K.P. Program MARK: survival estimation from populations of marked animals. Bird Study. 1999;46(Supp.1):120–139. [Google Scholar]
- Wright I.E, Wright S.D, Sweat J.M. Use of passive integrated transponder (PIT) tags to identify manatees (Trichechus manatus latirostris) Mar. Mamm. Sci. 1998;14:641–645. doi:10.1111/j.1748-7692.1998.tb00752.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model estimates of Hawaiian monk seal survival rates. The selected model had four age groups, an additive gender effect at one site (French Frigate Shoals), annual variability for most age groups, and three subpopulation groupings. “Year” indicates survival year such that 1984 refers to survival from 1984 to 1985. The age groups are 1–2 yr, 3–4 yr, 5–17 yr, and 18–20 yr. By convention, 1–2 yr survival indicates the annual survival of animals from weaning to age 1 yr and from age 1 yr to age 2 yr, and so on. Survival rates, their standard errors (s.e.), lower (LCL) and upper (UCL) 95% confidence limits are presented. Separate tables are provided for subpopulation groups with distinct survival patterns (and gender differences in the case of French Frigate Shoals). Note that Lisianski age 1–2 yr survival was combined with Pearl and Hermes Reef, Midway Atoll and Kure Atoll, whereas older seals at Lisianski Island had rates in synchrony with seals at Laysan Island. For the oldest seals (18–20 yr), no spatial or temporal variation was detected. Modeling was conducted using Program MARK.