Abstract
Leaf-cutting ants (Atta spp.) are known for their extensive defoliation in neo-tropical forests and savannahs. Debate about the costs and benefits of their activities has been largely dominated by their detrimental effects on agriculture and agroforestry. However, the large accumulation of nutrients and changes in soil properties near their nests might benefit plants growing near them. Here, we test whether trees use nutrients that accumulate in debris piles near, or refuse chambers within, leaf-cutting ant nests. At two tropical sites (a moist tropical forest site in Panama and a savannah site in Brazil), we fed leaves labelled with the stable isotope 15N to two species of leaf-cutting ants (Atta colombica and Atta laevigata) and traced the stable isotope label in plants surrounding the two nests. Thus, we show that plants in both sites access resources associated with Atta nests. In addition, leaf tissue of trees near the nests labelled with 15N had significantly higher calcium concentrations than those of distal, unlabelled conspecifics. It has been documented that calcium is a limiting macronutrient in tropical forests and savannahs. Atta may thus play an important ecological role through their long-distance transport, redistribution and concentration of critical macronutrients.
Keywords: leaf-cutting ants, Atta, nitrogen isotopes, nutrients, tropical forests, savannahs
1. Introduction
Leaf-cutting ants of the genus Atta contribute greatly to defoliation in neo-tropical forests and savannahs (Cherrett 1986; Hölldobler & Wilson 1990; Wirth et al. 2003). Nonetheless, the net costs and benefits of their impact have been debated (Moutinho et al. 2003). On the one hand, the effect of their defoliation can reduce fitness or even kill harvested plants (Wirth et al. 2003). On the other hand, Atta colonies cut prodigious quantities of nutrient-rich leaf material from plants growing within hundreds of square meters of the nests. The ants then concentrate this material by transporting it to the nest where it is used to feed fungus gardens in underground chambers (Hölldobler & Wilson 1990). The fungi grow on this resource and parts of the fungal colonies are then fed to the developing ant brood.
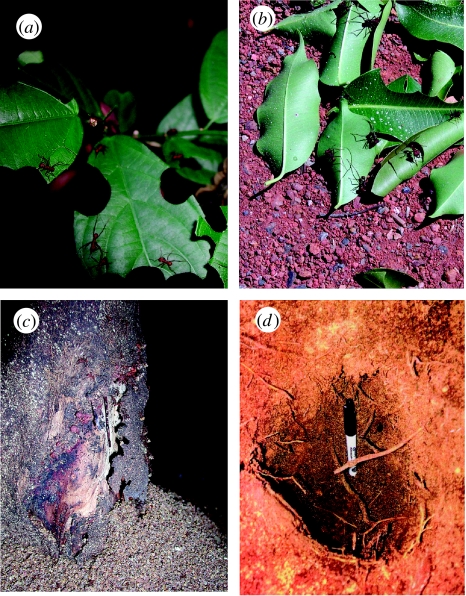
Most leaf-cutting Atta species create underground chambers to deposit nest refuse composed of dead ants, old fungi and other plant detritus (figure 1). However, Atta colombica, one of the species studied here, is an exception in that it deposits nest refuse in piles external to the nest (figure 1; Wirth et al. 2003). Analyses of the nest refuse and soils associated with leaf-cutting ant nests showed a substantially greater concentration of macronutrients and soil penetrability relative to non-nest soils (Haines 1975, 1983; Farji-Brener & Silva 1995; Farji-Brener & Ghermandi 2000; Moutinho et al. 2003). Excavations in neo-tropical forests show that there is an abundance of plant roots in abandoned subterranean leaf-cutting ant nest trails and pits (Haines 1978; Carvalheiro & Nepstad 1996; Farji-Brener & Medina 2000; Moutinho et al. 2003), which may have an important effect on the overall distribution of roots in the soil profile (Sternberg et al. 1998). Even in active nests, coarse and fine roots exploit deeper layers of the soil profile (greater than 6.0 m) and are more prolific in nest-associated soils (Moutinho et al. 2003). However, the direct utilization of nest nutrient resources has never been shown. Here, we use a stable isotope of nitrogen (15N) as a tracer to test whether plants surrounding leaf-cutting ant nests directly use accumulated nest nutrients.
Figure 1.
Leaf-cutting ants and their refuse deposits. (a) Atta colombica and (b) Atta laevigata in the process of cutting leaf fragments. Note that the 15N-labelled potassium nitrate crystals can be seen on the surface of the leaves being cut by (b) A. laevigata. Also shown is (c) the nest refuse being deposited on an outside pile by A. colombica and (d) a typical underground refuse chamber of Atta sexdens which is very similar to that observed for A. laevigata.
2. Material and methods
(a) Experimental sites
Experiments were carried out at Barro Colorado Island (BCI, Smithsonian Tropical Research Institute, Republic of Panama; 9°09′ N, 79°5′ W) and at the Reserva Ecologica do Roncador of the Instituto Brasileiro de Geografia e Estatística (RECOR; 15°56′ S, 47°53′ W) located approximately 35 km south of Brasilia (Brazil). The vegetation at BCI is characterized as semi-deciduous tropical moist forest with an average canopy height between 30 and 40 m. Yearly average rainfall at this site approaches 2700 mm with a pronounced dry season between January and March. The vegetation at RECOR is characterized as savannah (known locally as ‘cerrado’) having various densities ranging from clear grassy fields with occasional shrubs or small trees (‘campo sujo’) to gallery forest (‘floresta ciliar’; Ratter 1992; Ribeiro & Walter 1998). Yearly rainfall averages 1500 mm and is highly seasonal with a pronounced dry season occurring approximately from April to the end of August, during which less than 100 mm of rainfall occurs.
(b) Introduction of 15N label into ant nests
We identified two leaf-cutting ant nests of A. colombica in a tropical forest site for isotopic labelling on 13 February 2004. For each nest, we located one or two trees approximately 60 m (nest 1) and 30 m (nest 2) away from the nest entrance where ants were actively harvesting leaves and forming an above-ground trail from the canopy, down the tree trunk and on the ground to the nest. We sprayed ants and leaves with a fine mist of potassium nitrate solution (5 g per 100 ml, 98% 15N; Icon Services, Summit, NJ, USA) as they descended the trunk of the tree that was harvested for a period of approximately 6 h on each of 2 days, which was equivalent to 1 l of the above solution. We do not present data for nest 2 because this nest gradually lost activity, ants acquired only one-quarter of the label found in those of nest 1 and none of the plants around this nest were labelled.
At the savannah site, one ant nest of the species Atta laevigata was located in the cerrado, sensu stricto as previously defined (Ratter 1992; Ribeiro & Walter 1998), for isotopic labelling on 4 July 2003. We located an active entrance 30 m from the nest where ants were transporting leaf-cuttings from a cultivated tree species (Eugenia jambolana Lamark). Branches of the Eugenia were cut and sprayed with a fine mist with a total of 1 l of 5% labelled potassium nitrate solution and placed at the entrance for a period of 3 days. Ants were observed to be cutting leaves and transporting the cut pieces into the nest until stems were denuded of leaves (figure 1). A total of approximately 750 g of leaf biomass was consumed by these ants during labelling.
(c) Sampling of ants and trees neighbouring the nests
At the tropical forest site, we chose all trees within 11 m from the nest (ranging from 1.6 to 10.6 m away; see table 1 in the electronic supplementary material) having a trunk diameter at breast height of 3 cm or greater and leaves in reach for harvesting. We identified these trees by species and tagged them for future sampling. There was one individual tree for which the species could not be exactly determined. For every tree chosen in the vicinity of the A. colombica nest, we chose an individual of the same species in a control area located far from (greater than 100 m) any leaf-cutting ant nest. Leaves were collected bimonthly, starting one month after the label introduction. We collected nine individual ants from both the treated nest and a control nest at the same time as leaf samples were being collected. Leaf and ant samples were dried at 60°C in an oven and taken to the laboratory for isotopic analysis.
At the savannah site, we chose only trees and large shrubs at various distances from the ant nest (ranging from 2.5 to 30.5 m away; see table 2 in the electronic supplementary material) and selected all trees and shrubs within 5 m of the nest for sampling. At distances greater than 5 m from the nest, we selected only trees of the same species as those chosen for the control. We collected leaf samples monthly, starting one month after the label introduction. As a control, we sampled leaves from 8 to 10 trees (Byrsonima crassifolia, Caryocar brasiliense, Kielmeyera coriacea and Sclerolobium paniculatum) far from (greater than 100 m) any leaf-cutting ant nest. Five to 10 ants from both the treated nest and a control nest were collected each month, pooled as individual treatment and control samples, and preserved for isotopic analysis as in the tropical forest site.
We analysed leaf macronutrients from labelled individual plants near the nest as well as those from the respective conspecific individual in the control area in the tropical forest site and five replicates of conspecific individuals in the control area in the savannah site. The nutrient concentration of some trees near the nest in each site could not be compared with controls. In the forest site, one tree could not be identified by species, and another did not have sufficient leaf material for analysis. In the savannah site, there was one tree near the nest for which we could not find a tree of the corresponding species in the control area for comparison.
(d) Isotopic and nutrient analyses
All leaf and ant samples were ground to a fine powder before isotopic and nutrient analyses. Stable isotope analyses were performed at the Centro de Energia Nuclear na Agricultura (CENA, University of São Paulo, Piracicaba, São Paulo, Brazil) for the savannah samples and at The Laboratory of Stable Isotope Ecology of Tropical Ecosystems for the tropical forest samples (LSIETE, University of Miami, Coral Gables, FL, USA). A Carlo-Erba coupled with Delta-Plus isotope mass spectrometer (CENA) or a Eurovector coupled with a GV Isoprime (LSIETE) was used for nitrogen isotope analyses. Nitrogen isotope ratios are expressed as
in which Rsample is the 15N/14N ratio of the sample and Rstandard is the 15N/14N ratio of atmospheric nitrogen as a universal standard. The precision of analysis is ±0.1‰.
Ground samples were sent to Cornell Nutrient Analysis Laboratory (Ithaca, NY, USA) or the Agricultural Analytical Services Laboratory (Pennsylvania State University, University Park, PA, USA) for analyses of the following macronutrients: calcium, magnesium, potassium and phosphorus. Nitrogen was analysed at LSIETE with a Carlo-Erba CN analyser (ThermoQuest Italia, Milan, Italy).
(e) Statistical tests
We determined whether labelling of ants in the treated nest was significantly higher relative to a control nest with a two-way ANOVA (time and nest) with replication for the tropical forest site and without replication for the savannah site. We determined whether the 15N label was incorporated into plants neighbouring the labelled nests with a two-way ANOVA (time and location relative to the treated nest). We used only data from plants that were sampled for leaves throughout the whole monitoring period within an 11 m radius of the nest refuse pile for the tropical forest site and within 5 m for the savannah site as well as those of corresponding control trees. Some individuals, owing to their deciduous nature, temporarily lost their leaves during the monitoring period and were not included in the statistical analysis. For the tropical forest site, there were two species (Virola sebifera and Hirtella triandra) which had outlying δ15N-values in the control population according to Dixon's outlier test (Sokal & Rohlf 1995) and were eliminated from the sample set. We expanded the sampling radius to 11 m at the tropical forest site because individuals were more sparsely distributed at this site.
To compare whether a leaf sample from a single individual was significantly labelled relative to background levels, we applied the method of Sokal & Rohlf (1995) for comparing a single individual with a population mean. We compared the δ15N-value of each individual near the nest in the month with the highest average δ15N-value after label introduction (four months for the tropical forest site and six months for the savannah site) with the average of the control population. We tested whether any of the macronutrients measured above occurred at a higher concentration in labelled individuals near the nests relative to those in the control areas by using Wilcoxon signed-rank test (Sokal & Rohlf 1995) between the macronutrient concentrations in each individual near the nest compared with that in an individual of the same species (tropical forest site) or the average of five replicates of the same species (savannah site) in the control area. Samples from both sites were pooled to increase sample number. Probabilities that indicated a significant difference were adjusted for the experiment-wise error rate according to the Dunn–Šidák method (Sokal & Rohlf 1995).
3. Results and discussion
(a) Labelling of ants in nests
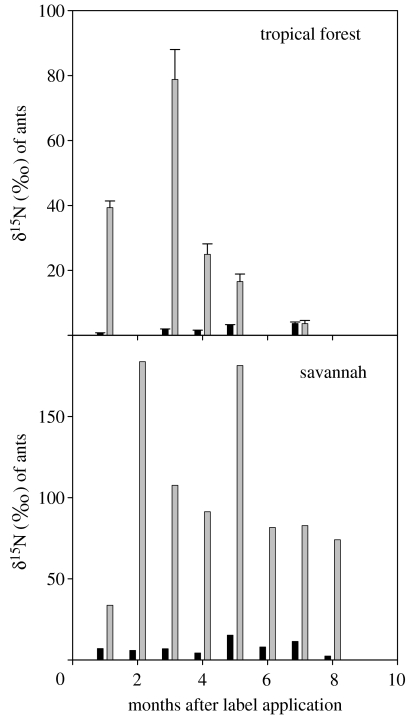
The δ15N-values measured for ants in the tropical forest nest were significantly higher than those measured for the control nest (F=184.7, d.f.=1, p<0.001) reaching the highest average value of 78.8±3.2‰ (±s.e.m., n=9), which was far above the background levels averaging (1.7±0.1‰, n=9). A significant time effect was also observed (F=30.8, d.f.=4, p<0.001) with peak values occurring approximately three months after label application and decreasing thereafter (figure 2). Ants from the treated nest in the savannah site had significantly higher δ15N than those of the control nest (F=28.9, d.f.=1, p<0.01) with an average value during the monitoring period of 104.5±18.6‰ (n=8) compared with the average value of the control nest of 7.6±1.5‰. (n=8). We did not observe a significant time effect in the δ15N of the savannah ants throughout the monitoring period (F=1.1, d.f.=7, p=0.44). Ants remained labelled for at least seven months after the label introduction, having above-background δ15N-values (greater than 50‰) compared with ants from a control nest (figure 2).
Figure 2.
δ15N-values of ants periodically collected near the treated and control nests in the tropical forest and the savannah sites (single value from merged samples). Light grey bars represent δ15N-values of ants from the treated nest, and black bars represent the average values of ants collected in the control nest. The bars shown for the tropical forest site represent the average±s.e.m. The bars shown for the savannah site represent a single value from merged samples.
The two- to three-month delay for the highest δ15N-value in ants from both nest sites reflects the time it takes for the isotopically enriched leaves to be processed into fungal growth, consumed by developing ant larvae, pupation and final maturation of the ants. We observed, however, that 15N label appeared in the ant colony as early as one month after label introduction, which probably reflects dissipation of the labelling substance to other workers by social activity and possible contamination of the nest walls and floor. The seven-month period of sustained high label in ants from the savannah site compared with that observed in ants from the tropical forest site (figure 2) might indicate that the nests of these two leaf-cutting ant species have very different turnover rates.
(b) Trees acquire 15N transported by the ants
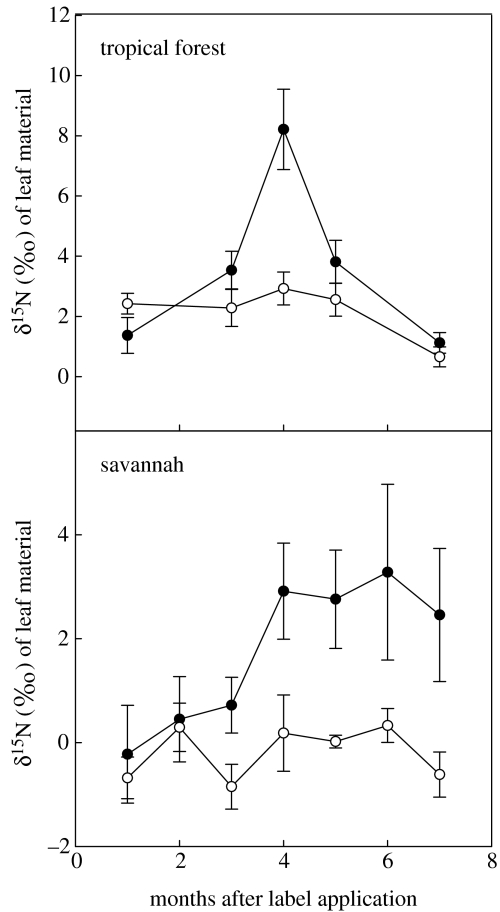
There were significant differences between the nitrogen isotope ratios of leaves from nest and non-nest trees in the tropical forest site (F=8.65, d.f.=1, p<0.01), with the group of trees within 11 m of the nest having significantly greater δ15N-values than trees in the control area. There was also a significant time effect observed (F=13.70, d.f.=4, p<0.001), with a rapid increase in the δ15N-values of leaves four months after the label introduction and a subsequent sharp decline (figure 3). Likewise, the δ15N-value of leaves from monitored plants in the savannah site differed significantly between nest and non-nest trees (F=24.80, d.f.=1, p<0.001), with trees within 5 m of the nest having foliar δ15N greater than those in the control area. A time effect was also observed at this site (F=2.68, d.f.=6, p<0.05). Foliar δ15N-values increased sharply 3–4 months after label application and higher-than-background levels were sustained over the seven-month period of observation (figure 3).
Figure 3.
Average δ15N-values of leaf tissue collected periodically from trees and/or shrubs in the vicinity of the tropical forest and savannah ant nests. Samples were collected within 11 m in the tropical forest site and within 5 m in the savannah site. Filled circles represent the δ15N isotope ratios of leaves from plants near the treated nest in either the forest or savannah site. Open circles represent δ15N isotope ratios of leaves collected in the control areas. Error bars represent ±s.e.m.
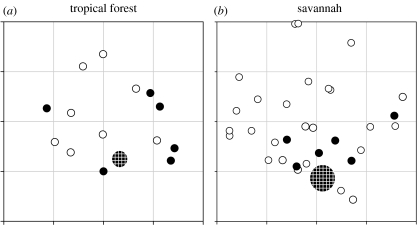
The four-month period required for the appearance of the label in plants neighbouring the nest (figure 3) almost certainly corresponds to turnover of the material in the fungal gardens, followed by ejection into disposal pits or external piles. In general, trees nearer the nest were more likely to accumulate 15N label; however, several trees very close to the nest did not (figure 4). Furthermore, trees as far as approximately 9 m from the refuse pile in the tropical site and as far as approximately 11 m from the nest mound in the savannah site were significantly labelled.
Figure 4.
Mapping of trees near treated nests in the tropical forest and savannah sites. Filled circles represent trees that had leaves with significantly higher δ15N-values compared with the average of the control trees, and open circles represent trees with leaf δ15N-values similar to those of the control plants. The large cross-hatched circle in each panel represents either the external refuse pile in the tropical forest site or the nest mound in the savannah site. Distance between grid lines for both sites represents 5 m. Only trees that were tagged and sampled are shown in the figure, whereas trees farther than 15 m in the savannah site are not shown.
These results conclusively show the uptake of resources stocked in ant nests by plants neighbouring leaf-cutting ant nests. Label uptake in the tropical forest site most probably occurred through fine surface roots found abundantly in the refuse pile and in the upper 10 cm of the surface of the tropical forest floor (Haines 1978; Farji-Brener & Medina 2000). In the savannah site, uptake probably occurred through root growth in refuse chambers (3–4 m deep in the soil profile), but not in fungal chambers or tunnel soils, because Atta will cut away any plant root growing in tunnels or fungal chambers.
The sustained label in the trees neighbouring the savannah nest (A. laevigata), in contrast to that observed by the tropical forest nest (A. colombica), is consistent with the long-term retention of the 15N label in the ants in the respective nests. This phenomenon may also be related to other factors operating in the exterior refuse piles of A. colombica (Wirth et al. 2003; figure 1) versus underground refuse chambers of A. laevigata. For example, the rapid decay of the isotopic signal in the tropical forest trees compared with that in the savannah site may be due to the fast leaching of soluble nutrients from the refuse pile by surface runoff or by the presumably higher nitrogen volatilization rates. In addition, leaf turnover rates might be higher in the tropical forest compared with the savannah site.
(c) Foliar macronutrient concentrations
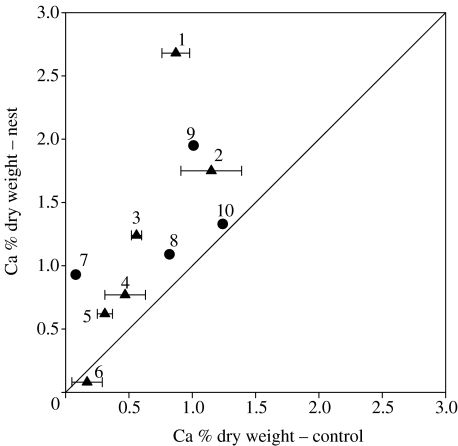
Although we followed only the direct path of 15N incorporation in ants and nearby plants, plants that accessed the isotopic label in the nest almost certainly accessed other nutrients found at high concentrations in the refuse chambers or piles, some of which might even be more critical than nitrogen for plant fitness. Yet, no significant differences in foliar phosphorus (Ts=20, n=10, p>0.05), potassium (Ts=17, n=10, p>0.05), magnesium (Ts=23, n=10, p>0.05) or nitrogen (Ts=12, n=8, p>0.05) concentrations were observed between labelled individuals near the nests at both sites and the corresponding species far from the nests. Only foliar calcium concentrations (Ts=2, n=10, p<0.05) were greater in individuals near the nest relative to the conspecifics in the control area (figure 5). Individuals close to the nest in the tropical forest site that were not labelled did not show a significantly higher calcium concentration than their conspecifics in the control population (Ts=5, n=5, p>0.05). We therefore argue that leaf-cutting ants transport and concentrate calcium into their nest soil and refuse rather than simply choose to build nests in micro-sites that have high calcium concentrations. It is probable that labelled plants neighbouring the nests also use P, K, Mg and N, but the extra nutrients absorbed by these plants are diluted into new tissue. Since calcium is a relatively immobile element in the plant, its foliar concentration is only a function of root uptake and foliar leaching (McLaughlin & Wimmer 1999), whereas P, K, Mg and N are all mobile and their foliar concentrations are a function of foliar reabsorption in addition to the above processes. Therefore, foliar calcium concentration should more closely reflect its greater availability in ant nest refuse piles or chambers. Calcium is one of the macronutrients critical for establishment of above-ground woody biomass in neo-tropical savannahs and forests. Wood harvesting of forests, for example, can severely impact the calcium availability that is critical to woody growth (Nykvist 2000). Central Brazilian savannahs, known as ‘cerrado’, can cover a spectrum of vegetation densities having different above-ground biomass. The ‘cerradão’ is the densest vegetation that contains large trees up to approximately 20 m high, whereas the ‘campo sujo’ represents the least dense vegetation type (Ribeiro & Walter 1998). One of the major nutrients modulating the vegetation gradient from cerrado, cerradão and deciduous forest is the amount of calcium present in the soil and biomass (Haridasan 2000, 2001). Indeed, there are several reports of leaf-cutting ant nests aiding in the establishment of woody vegetation in cleared areas (Farji-Brener & Illes 2000).
Figure 5.
Calcium concentration (% dry weight) of leaves from individuals close to the ant nests showing significant 15N enrichment versus the calcium concentration of leaves from conspecific individuals in a control area far from the treated nest. Triangles represent the following species in the savannah site: 1, Rapanea guianensis; 2, Aspidosperma macrocarpon; 3, Caryocar brasiliense; 4, Roupala montana; 5, Kielmeyera coriacea and 6, Sclerolobium paniculatum. Circles represent the following species in the tropical forest site: 7, Chrysophyllum argenteum; 8, Tocoyena pittieri; 9, Quararibea asterolepis and 10, Faramea occidentalis. Horizontal error bars for the savannah site represent ±s.e.m.
(d) Broader implications
Previous reports and the present study consistently indicate higher nutrient availability in ant nest refuse chambers (Haines 1975, 1983; Farji-Brener & Silva 1995; Moutinho et al. 2003), direct nutrient uptake by plants neighbouring ant nests (this study), an abundance of leaf-cutting nests in disturbed areas (Jaffe & Vilela 1989; Vasconcelos & Cherrett 1995; Carvalheiro & Nepstad 1996; Farji-Brener 2001; Wirth et al. 2003) and a long range of ant foraging activities (Wirth et al. 2003). Together, these observations lead us to the conclusion that leaf-cutting ants concentrate and supply critical macronutrients and may serve an important role in the re-establishment and maintenance of forest trees in cleared areas. This benefit, however, must be weighed against leaf-cutting ant herbivory.
An estimate of the costs and the benefits of leaf-cutting ants in neo-tropical ecosystems must include several biotic and abiotic parameters. For example, in addition to their intense herbivory, leaf-cutting ant nests can change the soil texture and penetrability (Moutinho et al. 2003), change the light environment by forming upside-down gaps (Farji-Brener & Illes 2000; Hull-Sanders & Howard 2003), remove competition (Coutinho 1982) and provide high nutrient concentrations in their nest refuse pit or external pile (Haines 1975, 1983; Farji-Brener & Silva 1995; Farji-Brener & Ghermandi 2000; Moutinho et al. 2003). A previous study noted a lack of correlation between proximity to the nest and growth rates measured as tree trunk diameter increment rates (Moutinho et al. 2003), whereas others have observed a proliferation of roots in abandoned nest and nest refuse chambers and piles (Haines 1978; Carvalheiro & Nepstad 1996; Farji-Brener & Medina 2000; Moutinho et al. 2003). These seemingly ambiguous results may be due in part to the observation that proximity to the nest is a poor indicator of nest nutrient resource utilization as evidenced by our study showing several plant individuals close to the nest that did not acquire nest nutrients. The experimental protocol introduced here will allow us to pinpoint the exact individuals proximal to the nest that are utilizing nest nutrient resources. Identification of these individuals can then be followed by measurements of growth rates, seed and fruit production, photosynthesis and several other fitness parameters, in comparison with individuals not utilizing nest resources. The protocol introduced here will also allow us to distinguish between benefits brought by soil texture and/or light availability versus those brought by enriched nutrient availability.
An important question regarding Atta nests in forests and savannahs is whether their impact is sufficient to promote natural selection of particular plant species (Farji-Brener & Illes 2000). Indeed, there are cases of specific genera or species colonizing Atta nests with a greater frequency than others (Jonkman 1978; Coutinho 1982; Farji-Brener & Silva 1996). It is not known, however, whether this greater frequency is caused by factors such as soil texture, lack of spatial competition or the additional nutrients. Although the number of nests studied here is insufficient to address these questions, the experimental protocol introduced here will certainly facilitate the investigation of this long-standing question.
Acknowledgments
We thank Diane Davidson, Brian Fisher, Hubert Herz and Jed Sparks for their assistance. This work was supported by grants from the Guggenheim Foundation and National Science Foundation nos DBI-0420553 and EAR-0322051 (L. da S.L.S.) and the Smithsonian Tropical Research Institute (E.A.H.).
Supplementary Material
Species identification of sampled individuals neighbouring a leaf-cutting ant nest in a tropical forest and a savannah are shown in Table 1 and 2 respectively. Plant distances from the refuse pile (Atta colombica) or the nest mound (A. laevigata) are also shown. Individuals marked with an asterisk were significantly labelled with 15N transported by leaf-cutting ants.
References
- Carvalheiro K.O, Nepstad D.C. Deep soil heterogeneity and fine root distribution in forests and pastures of eastern Amazonia. Plant Soil. 1996;182:279–285. [Google Scholar]
- Cherrett J.M. History of the leaf-cutting ant problem. In: Lofgren C.S, Vander Meer R.K, editors. Fire ants and leaf-cutting ants: biology and management. Westview Press; Boulder, CO: 1986. pp. 10–17. [Google Scholar]
- Coutinho L. Aspectos ecológicos da saúva no cerrado: Os murundus de terra, as caracteristicas psamofíticas das especies de sua vegetação e a sua invasao pelo Capim Gordura. Rev. Brasil Biol. 1982;42:147–153. [Google Scholar]
- Farji-Brener A.G. Why are leaf-cutting ants more common in early secondary forests than in old-growth tropical forests? An evaluation of the palatable forage hypothesis. Oikos. 2001;92:169–177. doi:10.1034/j.1600-0706.2001.920120.x [Google Scholar]
- Farji-Brener A.G, Ghermandi L. The influence of nests of leaf-cutting ants on plant species diversity in road verges of northern Patagonia. J. Veg. Sci. 2000;11:453–460. doi:10.2307/3236638 [Google Scholar]
- Farji-Brener A.G, Illes A.E. Do leaf-cutting ant nests make “bottom-up” gaps in neotropical rain forests?: a critical review of the evidence. Ecol. Lett. 2000;3:219–227. doi:10.1046/j.1461-0248.2000.00134.x [Google Scholar]
- Farji-Brener A.G, Medina C.A. The importance of where to dump refuse: seed banks and fine roots in nests of the leaf-cutting ants Atta cephalotes and A. colombica. Biotropica. 2000;32:120–126. [Google Scholar]
- Farji-Brener A.G, Silva J.F. Leaf-cutting ant nests and soil fertility in a well-drained savanna in western Venezuela. Biotropica. 1995;27:250–254. doi:10.2307/2389001 [Google Scholar]
- Farji-Brener A.G, Silva J.F. Leaf-cutting ant Atta laevigata aid to the establishment success of Tapirira velutinifiolia (Anacardiaceae) seedlings in a parkland savanna. J. Trop. Ecol. 1996;12:163–168. [Google Scholar]
- Haines B. Impact of leaf-cutting ants on vegetation development at Barro Colorado Island. In: Golley F.G, Medina E, editors. Tropical ecological systems: trends in terrestrial and aquatic research. Springer; New York, NY: 1975. pp. 99–111. [Google Scholar]
- Haines B. Element and energy flows through colonies of the leaf-cutting ant, Atta colombica, in Panama. Biotropica. 1978;10:270–277. doi:10.2307/2387679 [Google Scholar]
- Haines B. Leaf-cutting ants bleed mineral elements out of rainforest in southern Venezuela. Trop. Ecol. 1983;24:85–93. [Google Scholar]
- Haridasan M. Nutrição mineral das plantas nativas do cerrado. Rev. Brasil Fisiol. Veg. 2000;12:54–64. [Google Scholar]
- Haridasan M. Nutrient cycling as a function of landscape and biotic characteristics in the cerrado of central Brazil. In: McClain M.E, Victoria R.L, Richey J.E, editors. Biogeochemistry of the Amazon basin and its role in a changing world. Oxford University Press; New York, NY: 2001. pp. 68–83. [Google Scholar]
- Hölldobler B, Wilson E.O. Belknap; Cambridge, MA: 1990. The ants. [Google Scholar]
- Hull-Sanders H, Howard J.J. Impact of Atta colombica colonies on understory vegetation and light availability in a Neotropical forest. Biotropica. 2003;35:441–445. [Google Scholar]
- Jaffe K, Vilela E. On nest densities of the leaf-cutting ant Atta cephalotes in tropical primary forest. Biotropica. 1989;21:234–236. doi:10.2307/2388649 [Google Scholar]
- Jonkman J. Nests of the leaf-cutting ant Atta vollenweideri as accelerators of succession in pastures. Z. Angew. Entomol. 1978;86:25–34. [Google Scholar]
- McLaughlin S.B, Wimmer R. Tansley review No. 104: calcium physiology and terrestrial ecosystem processes. New Phytol. 1999;142:373–417. doi:10.1046/j.1469-8137.1999.00420.x [Google Scholar]
- Moutinho P, Nepstad D.C, Davidson E.A. Influence of leaf-cutting ant nests on secondary forest growth and soil properties in Amazonia. Ecology. 2003;84:1265–1276. [Google Scholar]
- Nykvist N. Tropical forests can suffer from a serious deficiency of calcium after logging. Ambio. 2000;29:310–313. [Google Scholar]
- Ratter J.A. Transitions between cerrado and forest vegetation in Brazil. In: Furley P.A, Proctor J, Ratter J.A, editors. Nature and dynamics of forest-Savanna boundaries. Chapman Hall; London, UK: 1992. pp. 417–427. [Google Scholar]
- Ribeiro J.F, Walter B.M.T. Fitofisionomias do bioma Cerrado. In: Sano S.M, de Almeida S.P, editors. Cerrado: Ambiente e Flora. EMBRAPA-Cerrados; Planaltina, Brasilia: 1998. pp. 89–166. [Google Scholar]
- Sokal R.R, Rohlf F.J. W.H. Freeman; New York, NY: 1995. Biometry. [Google Scholar]
- Sternberg L, da S L, Green L, Moreira M.Z, Nepstad D.C, Martinelli L.A, Victória R.L. Root distribution in an Amazonian seasonal forest as derived from δ13C profiles. Plant Soil. 1998;205:45–50. doi:10.1023/A:1004361029428 [Google Scholar]
- Vasconcelos H.F, Cherrett J.M. Changes in leaf-cutting ant populations (Formicidae: Attini) after the clearing of mature forest in Brazilian Amazonia. Stud. Neotrop. Fauna Environ. 1995;30:107–113. [Google Scholar]
- Wirth R, Herz H, Ryel R.J, Beyschlag W, Hölldobler B. Springer; Berlin, Germany: 2003. Herbivory of leaf-cutting ants. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Species identification of sampled individuals neighbouring a leaf-cutting ant nest in a tropical forest and a savannah are shown in Table 1 and 2 respectively. Plant distances from the refuse pile (Atta colombica) or the nest mound (A. laevigata) are also shown. Individuals marked with an asterisk were significantly labelled with 15N transported by leaf-cutting ants.