Abstract
Recognition is considered a critical basis for discriminatory behaviours in animals. Theoretically, recognition and discrimination of parasitic chicks are not predicted to evolve in hosts of brood parasitic birds that evict nest-mates. Yet, an earlier study showed that host reed warblers (Acrocephalus scirpaceus) of an evicting parasite, the common cuckoo (Cuculus canorus), can avoid the costs of prolonged care for unrelated young by deserting the cuckoo chick before it fledges. Desertion was not based on specific recognition of the parasite because hosts accept any chick cross-fostered into their nests. Thus, the mechanism of this adaptive host response remains enigmatic. Here, I show experimentally that the cue triggering this ‘discrimination without recognition’ behaviour is the duration of parental care. Neither the intensity of brood care nor the presence of a single-chick in the nest could explain desertions. Hosts responded similarly to foreign chicks, whether heterospecific or experimental conspecifics. The proposed mechanism of discrimination strikingly differs from those found in other parasite–host systems because hosts do not need an internal recognition template of the parasite's appearance to effectively discriminate. Thus, host defences against parasitic chicks may be based upon mechanisms qualitatively different from those operating against parasitic eggs. I also demonstrate that this discriminatory mechanism is non-costly in terms of recognition errors. Comparative data strongly suggest that parasites cannot counter-evolve any adaptation to mitigate effects of this host defence. These findings have crucial implications for the process and end-result of host–parasite arms races and our understanding of the cognitive basis of discriminatory mechanisms in general.
Keywords: brood parasitism, coevolution, discrimination, mechanism, recognition
1. Introduction
Recognition is a critical underlying mechanism of any discriminatory behavioural pattern, from associating with kin or selecting suitable food types to habitat choice and recognition of enemies (Hauber & Sherman 2001). Not surprisingly, both direct (e.g. innate) and indirect (e.g. learned in a context-dependent manner) recognition are central to many questions in behavioural ecology (Sherman et al. 1997). One of the most thoroughly studied systems for recognition is brood parasitism (Davies 2000). Recognition and discrimination of parasitic eggs has received major scientific and popular attention (Davies & Brooke 1989; Lotem et al. 1995; for review, see Davies 2000), while discrimination of alien chicks has so far been rarely tested experimentally (Davies & Brooke 1989; Soler et al. 1995; Lichtenstein 2001; Payne et al. 2001; Schuetz 2005; for reviews, see Redondo 1993 and Grim 2006a). Several examples of well-developed cases of chick mimicry suggest that host discrimination between own and foreign chicks has arisen during the coevolution between a few hosts and parasites (Redondo 1993; Grim 2005, 2006a). In general, hosts can reject natural or experimental parasitic chicks by nest desertion (Grim et al. 2003; Langmore et al. 2003), refusal to feed them (Lichtenstein 2001; Payne et al. 2001) or by directly attacking, killing and/or ejecting them from the nest (Redondo 1993; Soler et al. 1995). However, the proximate mechanisms of host's differential responses towards foreign chicks under natural conditions are generally unknown. The only thoroughly studied case is the discrimination based on the structure of begging calls in parasitic bronze-cuckoos (Chrysococcyx spp.) by superb fairy-wrens (Malurus cyaneus) in Australia (Langmore et al. 2003).
Studies of brood parasitism in general focused on host responses to eggs, mainly on egg rejection—especially by ejection—based on recognition of own versus alien eggs (Davies 2000). The notion of a rarity of chick discrimination and mimicry (Davies 2000) might be attributed to the assumption that hosts would use similar behavioural (e.g. ejection) and cognitive (e.g. recognition) mechanisms when defending against parasitic chicks and eggs (Redondo 1993). But this need not be so because hosts facing parasitic eggs and chicks are confronted with very different constraints and trade-offs, as elaborated by Redondo (1993). Therefore, it is important to explore other behavioural and cognitive mechanisms when studying chick discrimination by hosts of brood parasitic birds. I have attempted this using a host–parasite system, where discrimination was hypothesized not to be based on recognition (Grim et al. 2003).
A theoretical model (Lotem 1993) predicted the absence of chick discrimination in hosts of evicting parasites. Surprisingly, parasitic chick discrimination has now been reported for one of the best-known evicting brood parasites, the common cuckoo (Cuculus canorus, hereafter, cuckoo). Approximately 15% of the cuckoo chicks were reported to be deserted by host reed warblers (Acrocephalus scirpaceus; Grim et al. 2003). The fact that reed warblers are victimized by an evicting parasite need not be in discrepancy with Lotem's (1993) model. While the model assumed learned chick recognition, Grim et al. (2003) predicted that adaptive host responses to parasitic chicks need not be based on learning or recognition using an internal template (Hauber & Sherman 2001). Accordingly, parent reed warblers may, in principle, discriminate against cuckoo chicks by refusing to provide care for longer periods and/or with increased investments than that needed for successfully fledging their own chicks (see also Holen et al. 2001). My previous study (Grim et al. 2003) provided preliminary, correlative evidence in favour of this ‘discrimination without recognition’ hypothesis. Therefore, I tested the idea experimentally in the same population where parasitic chick desertion had been originally observed. If warblers' responses to parasitic chicks were not based on learning or the recognition of the parasite, then they should work against any parasite-like brood, i.e. any individual(s) requiring longer and/or higher parental care than hosts' own chicks under normal conditions. I used a cross-fostering experiment to alter those normal rearing conditions.
I manipulated brood sizes to create single- and four-chick broods and used cross-fostering of different aged broods to force parents to care for nestlings for prolonged or shortened periods than normal with varying amounts of parental investment (for details on experimental procedure and definition of terms, see §2b below). Under the ‘discrimination without recognition’ scenario, reed warblers could use two types of information for the decision to desert a parasitic cuckoo chick (Grim et al. 2003). According to the ‘parental fatigue’ hypothesis, parents can respond to the amount of parental care elicited by the brood and desert a cuckoo chick when the investment into it is significantly higher than that required for raising a typical host brood. Physiological exhaustion could work as a proximate cue triggering the desertion. I manipulated parental investment by creating single- and four-chick brood nests to vary offspring need for parental care. Under the ‘time limit’ hypothesis, parents can respond to the parental period length irrespective of the level of parental investment delivered to a brood. The parental fatigue hypothesis predicts that desertion rate and chick mortality should be higher and nestling period should be shorter in prolonged four-chick broods than in all other treatment groups (i.e. shortened and control groups) owing to increased costs of parental care in prolonged four-chick broods. In contrast, the time limit hypothesis predicts increased mortality, desertion rate and shorter nestling periods in both four- and single-chick broods in the prolonged treatment group in comparison to all other groups, but no differences between four- and single-chick broods. The parental decisions to care for chicks for a relatively fixed period could also result in longer nestling periods at shortened parental care nests. Here, chicks would not be ‘under parental pressure’ to leave a nest. In shortened nests also, no starvation or desertion was expected.
The single-chick broods allow the testing of a third hypothesis. Under the ‘single chick’ hypothesis, parents could desert the cuckoo chick because it is alone in the nest (Langmore et al. 2003). According to this hypothesis, the mortality and desertion rate should be higher in single-chick broods, which should also fledge earlier.
Under all hypotheses, I expected that only a portion of tested host pairs would respond to prolonged or shortened nestling periods because not all host pairs desert cuckoo chicks in the study area (Grim et al. 2003).
2. Material and methods
(a) Study area and organism
The study was conducted in 2002–2006 on the Luzice fish pond system in the southeastern part of the Czech Republic (47°40′ N, 16°48′ E). A detailed description of the study area and standard field procedures are presented elsewhere (Grim & Honza 1997, 2001). The study host species, reed warbler, has an average nestling period of 11–12 days, while the duration of parental care for the nests with successfully hatched cuckoos lasts 18 days (range=17–21 days; Grim 2006b). The reed warbler shows weak hatching asynchrony, but it is not a brood reducer under normal conditions (Cramp 1992). Host adults were not individually marked and I assumed that birds regularly visiting a particular nest and its vicinity are its owners, especially when these birds responded by alarm calls to a human intruder (see Grim et al. 2003).
All time variables (nestling periods, etc.) were measured in hours, but results are presented as days for the readers' convenience. When the data fitted normal distributions, I used parametric tests (the use of non-parametric tests gave qualitatively the same results). All the values shown are means±s.e.
(b) Experimental manipulations of nestling periods
The parental care period was defined as a time from the hour of hatching (of the first chick in four-chick broods) to the hour of fledging (of the last chick in four-chick broods) of the brood. In experimental nests, hatching refers to the original brood while fledging refers to the new cross-fostered brood. Thus, the term parental care period refers to nests (i.e. the time the parents at the particular nests spent caring for own chicks followed by chicks cross-fostered to them), while the term nestling period refers to broods (i.e. the time the chick(s) spent in their natal nest followed by the new nest where they were cross-fostered).
First, I manipulated brood sizes for obtaining four- and single-chick broods (see also Grim & Honza 2001) to vary the level of parental expenditure to raise chicks (average brood size of warblers in the study area is 3.2; Grim & Honza 1997).
Second, for both four- and single-chick broods, I established four treatment groups (see also Nilsson & Svensson 1993; Johnsen et al. 1994): (i) unmanipulated control (nests were subjected to the same procedures as all other nests except for cross-fostering), (ii) manipulated control (brood exchanged for the same age brood from another nest at the age of 3 days posthatch), (iii) shortened brood/nest (an older brood that was moved to a nest originally containing a younger one), and (iv) prolonged brood/nest (a younger brood that was cross-fostered to a nest originally containing an older one). I found no differences between unmanipulated and manipulated four-chick control broods in the nestling period length (U19,5=0.60, p=0.55), the length of parental care at the nest (U19,5=0.96, p=0.34) and number of fledged chicks (U19,5=0.54, p=0.59). Therefore, I pooled the data (hereafter control group).
Manipulated four-chick control nests did not differ in the ages of chicks (age difference in days=0.5±0.2, range=0.3–0.8, n=8 nests matched in 4 nest pairs). Matched pairs of prolonged–shortened broods differed 4–5 days on an average in their ages. Both four-chick (age difference=4.7±0.4, range=1.7–7.4, n=16 nest pairs) and single-chick broods (age difference=4.6±0.2, range=3.5–6.8, n=15 nest pairs) were subject to similar experimental changes in brood ages (t31=0.14, p=0.88). I varied the age differences between matched pairs to test the prediction that the longer the prolongation of the care at the nest, the higher the probability of desertion or chick mortality would be.
Shortened broods were transferred to nests where parents were ‘programmed’ to care for chick(s) for a longer time than their new chicks needed, as their original chick(s) were younger. In contrast, prolonged broods were moved to nests where parents were programmed to care for chick(s) for a shorter time than their new chicks needed, as their original chick(s) were closer to fledging.
The interaction between brood size and the change in the length of care required by a particular nest allowed differetiation of the possible effects of: (i) the length of care and (ii) the amount of care which would be impossible to achieve with naturally parasitized nests only. Prolonged four- and single-chick broods greatly (approx. fourfold) differ in the amount of care (e.g. mass of food) needed, but not in the length of care needed. Prolonged four-chick broods need more care than a normal four-chick brood. However, the prolonged single-chick brood obviously needs a much lower amount of food (approx. less than half) than the average host brood under normal conditions. This simultaneous increase in the length of care and decrease in the amount of care provides a strong test of my alternative hypotheses.
At the time when hatching of a clutch or fledging was expected, the nest was checked three times per day. According to my previous experience (1994–2001), reed warbler chicks may sometimes fledge prematurely when handled or when the nest is touched at chick ages of 9 days or more. In contrast, chicks did not leave the nest when they were observed from a distance of 1 m or more. This suggested that the cue for the premature nest leaving was mechanical (the touching of nest and the handling of chicks) rather than visual (seeing an observer close to the nest). Therefore, I avoided mechanical contact with chicks/nests when checking for fledging time and checked nests as quickly as possible and from a distance of at least 1 m or more. Despite regular nest checks most nestlings hatched when an observer was not present. The exact hour of hatching (necessary to calculate the exact fledging age) for these individual chicks was calculated from a standard growth curve based on mass measurements. Chick mass was measured with a portable electronic balance to the nearest 0.01 g. The curve was based on mass growth of nestlings that were directly observed during hatching and weighted immediately thereafter (1.25±0.02 g; range, 1.18–1.31 g, n=5 chicks from 5 different nests). I did not measure chicks until fledging (the last measurement was on day 8 to avoid premature fledging). Therefore, the logistic growth curve could not be fitted to these data as these most likely did not include asymptotic mass (cf Grim 2006b). Thus, the curve was calculated as the third order polynomial regression of chick mass in grams against chick age in hours which best fitted the data (R2=0.99, F3,26=642.59, p<0.0001). The mass of some other nestlings that weighted 1.35–1.60 g (n=12 chicks from 12 different nests) when first measured suggested that they were freshly hatched or only several hours old. The real measured masses of these nestlings during measurements at older ages were well predicted by the standard growth curve (measured mass vs. predicted mass at a particular age: Wilcoxon-paired tests, all p>0.25). This indicates that hatching time estimates are robust. Further, hatching times estimated by the growth curve were in concordance with data on hatching times obtained by direct nest checks. Growth curve estimates of hatching hour were done identically across all treatment-groups.
The fledging time was calculated as a midpoint hour between two nest checks when chick(s) left the nest. For some nests (n=10) the exact time of fledging was obtained from time-laps video recordings. Nestlings in four-chick broods were marked with a small colour dot on the nape with non-toxic colours for individual recognition when they were 8 days old. This enabled the checking of nests with minimal disturbance and without the need to handle chicks in the period shortly before fledging.
(c) Methodological aspects of cross-fostering
I followed the experimental procedure of Nilsson & Svensson (1993) and Johnsen et al. (1994) as described earlier. It could be argued that this cross-fostering method is not ideal as parents perhaps expect to see their chicks develop and the prolonged treatment would then be an unnatural situation for them as the chick suddenly appears developmentally ‘retarded’ after cross-fostering. Thus, parents were suddenly faced with a younger brood with a lower reproductive value, which could itself influence their level of parental care (e.g. Redondo & Carranza 1989). To avoid this, an alternative way of manipulation of nestling periods would be to establish a ‘slow-growing’ brood. Continual replacing, each day, of a brood of chicks with another brood of the same age, would keep the brood age the same in the long term. The disadvantage of such method, however, would be a high level of disturbance at ‘slow-growing’ nests owing to a very high number of visits and chick exchanges. Moreover, the application of the method would be technically impossible in my study area owing to high predation rates. Despite high breeding densities of reed warblers, it was very hard to find any reasonably synchronized brood for experiments and many broods were left unmanipulated as there were simply no other broods for cross-fostering. Moreover, a large part of available nests had to be used as a ‘home’ for chicks that were removed from other nests to create single-chick broods. A telling fact is that it took five breeding seasons to reach the sample sizes given in the present study. In addition to this logistic constraint, there is also evidence suggesting that the design of this study did not bias results owing to the sudden change in chick appearance and/or the change in the reproductive value of the brood, as follows. (i) Latency from cross-fostering to desertion at deserted nests was typically 3–6 days, which strongly indicates that desertion occurred not as a response to the sudden change of chick appearance. (ii) Virtually the same extent of change in chick appearance at shortened nests did not elicit any desertion. (iii) Reed warblers are ready to accept developed warbler chicks (approx. 5 days and older) even when these are moved to nests at incubation stage (own observations) or of different species (Davies et al. 1998). (iv) Desertions could have not been caused by the chicks' apparent lack of development immediately after cross-fostering rather than by the length of care as all chicks normally developed after cross-fostering at least for several days. Additionally, in other study areas, reed warblers rapidly adapt to changes in nest content (Davies & Brooke 1989). (v) Although little is known about parent–offspring recognition in reed warblers, one could reasonably expect that the mechanism is the same as in other birds, i.e. parents learn to recognize nestlings only shortly before fledging (e.g. Beecher et al. 1981; Lessells et al. 1991; Soler et al. 1995), a relatively long time after cross-fostering manipulations in this study.
(d) Data analyses
In the main analysis, I fitted regression models with experimental treatment (shortened, control, prolonged), brood size (four, one) and their interaction as effect variables and the parental care period or nestling period as a response. I repeated this critical analysis with individual nestlings as units of analysis. I fitted general linear mixed model (PROC MIXED in SAS; normal error distribution, parameters estimated by REML and degrees of freedom calculated using Kenward–Roger method) with the same predictors and the brood identity as a random effect. In all these analyses, I also included the effects of year, the number of visits to the brood during the nestling period, video-recording status during fledging (the fledging recorded by time-laps video: yes/no) and the date of hatching of the brood (centred by median hatching date within a year). All these terms were non-significant in all analyses and were sequentially removed from the final models.
When I repeated all analyses presented in the results separately for the four- and single-chick brood datasets, I obtained qualitatively identical results. Therefore, I pooled the data when testing the effect of age differences in matched-pair broods on nestling period lengths and fledging success.
Sample sizes for fledging success (total n=105 nests) and nestling period lengths (total n=88 nests) differ between some groups as at some nests, the exact nestling period could not be reliably estimated.
(e) Ethical note
The aim of this study was to study brood desertion experimentally. This inevitably led to suffering (starving and death) of some nestlings. To alleviate these issues, I obtained the lowest sample sizes that allowed for meaningful comparisons among experimental treatments with the application of robust statistical tests. I did not continue to increase the sample sizes after six desertions were observed which conforms to the previously mentioned rule. At one of the deserted four-chick nests, one chick was still alive and begging and I transferred it to another nest with similar aged brood, where the chick was accepted by fosterers and survived; this recipient nest was excluded from all analyses. The experiments were done under licence from The Central Commission for Animal Welfare of the Czech Republic (no. 065/2002-V2) and in accordance with the laws and ethical guidelines of the Czech Republic.
3. Results
(a) The parental care and nestling periods
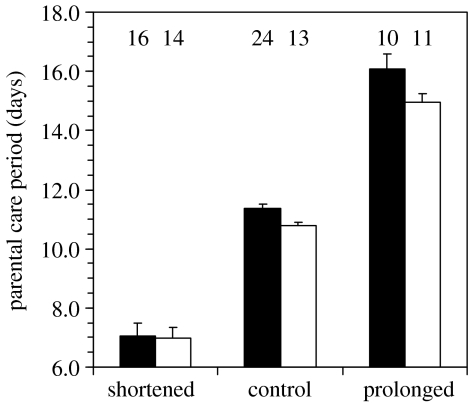
Parents from shortened groups cared for nests for a shorter time, whereas those from prolonged groups cared for a longer time, than those from control groups (figure 1; F2,85=320.92, p<0.0001). Four-chick nests were, on average, attended slightly longer than single-chick nests (F1,86=4.09, p=0.046). No interaction between experimental treatments and brood sizes was observed (F2,85=1.23, p=0.30) and this interaction was eliminated from the final model.
Figure 1.
Effects of experimental treatments on parental care periods at nests that successfully fledged. Mean total lengths of time (+s.e.) that the four-chick nests (filled bars) or single-chick nests (open bars) were attended by parents and fosterers. Inset numbers show sample sizes.
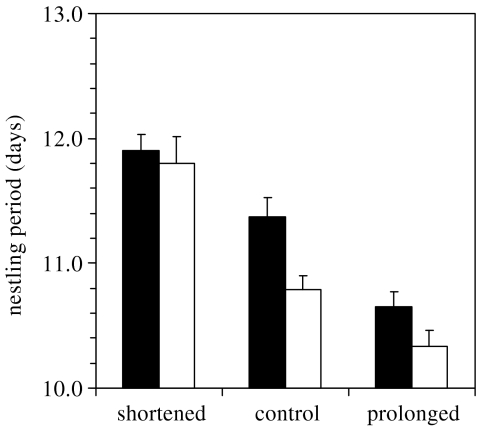
As with parental care periods, the variation in nestling periods was best explained using the same predictors of both treatment and brood size and the interaction between treatments and brood sizes was not significant (F2,85=1.21, p=0.30). Nestlings from shortened broods spent more time, whereas nestlings from prolonged broods spent less time, in the nest than those from control group (figure 2; F2,85=30.81, p<0.0001). The nestling period was longer in four-chick broods than in single-chick broods across treatments (F2,85=6.82, p=0.01).
Figure 2.
Effect of experimental treatments on nestling periods in broods that successfully fledged. Mean total lengths of time (+s.e.) that the four-chick broods (filled bars) or single-chick broods (open bars) spent in their original and recipient nests. Sample sizes are the same as in figure 1.
Analyses of nestling periods at the level of individual chicks (with brood identity as a random effect) gave qualitatively similar results. Nestling periods significantly decreased from shortened through control to prolonged broods (general linear mixed model F2,128.2=20.62, p<0.0001) and the brood size effect was also significant (F1,121=11.17, p=0.001). The interaction between treatments and brood sizes was significant (F2,128.2=6.12, p=0.003) because in shortened broods, there was no significant difference between single- and four-chick broods, whereas nestling periods of individual chicks in prolonged and control broods were longer in the single-chick broods than in the four-chick broods (Tukey–Kramer HSD, p<0.05). This test at the level of individual chicks is conservative as it artificially decreases the length of nestling periods in four-chick broods, but not in single-chick broods, in comparison with the total nestling period from hatching of the first to fledging of the last chick within a brood. Indeed, the average length (10.6±0.1 days) of nestling period in four chick broods was significantly shorter than the total length (11.4±0.1 days; paired t-test, t49=−8.68, p<0.0001).
The length of the period that nests were prolonged (+days) or shortened (−days) significantly and negatively correlated with the nestling period length (r=−0.65, n=88, p<0.0001). However, when only prolonged nests were analysed, the relationship disappeared (rs=−0.12, n=21, p=0.60). This means that not all individuals in the population follow the same decision rules when forced to care for nest for prolonged periods. This suggests that there is a high inter-individual variability in host responses to ‘parasitic’ chicks in the study population corresponding to similarly high intrapopulation variability in responses to parasitic eggs in hosts of parasitic birds in general (Davies 2000).
(b) Brood desertions
Desertions occurred solely in prolonged broods at a rate of 22.2% (n=27). Both parents were observed in the close vicinity of deserted nests, but they did not feed nestlings. Thus, the nestlings' death did not result from insufficient provisioning by a widowed member of parental pair or predation—the nestlings were without any bites or peck marks (see also Grim et al. 2003).
Contrary to the parental fatigue hypothesis, the desertion rate was not higher in prolonged four-chick broods (3 out of 13) than single-chick broods (3 out of 14; one-tailed Fisher's exact test, p=0.71). Deserted single- and four-chick broods did not significantly differ in any of their breeding parameters (table 1).
Table 1.
Breeding parameters of deserted prolonged single- and four-chick broods. (Mean±s.e. and p-values for Mann–Whitney tests are shown.)
| breeding characteristic | single-chick broods (n=3) | four-chick broods (n=3) | p |
|---|---|---|---|
| hatching date centred within year | 3.0±5.5 | 3.0±3.6 | 0.99 |
| the age of brood at the time of cross-fostering | 2.4±0.1 | 2.9±1.1 | 0.66 |
| the age of the recipient nest at the time of cross-fostering | 6.9±0.1 | 8.6±0.3 | 0.08 |
| latency from cross-fostering to desertion | 5.6±0.4 | 3.1±0.2 | 0.08 |
| the length of parental care at the deserted nests | 12.5±0.3 | 11.7±0.5 | 0.38 |
| the age of chicks when deserted | 7.9±0.4 | 6.0±1.0 | 0.38 |
Successfully fledged prolonged broods were similar to deserted prolonged broods in most of their breeding characteristics (table 2). The total length of parental care for deserted nests was lower than that for prolonged, but successful, nests (table 2). Desertions occurred significantly later (12.1±0.3 days) than was the normal parental care period of 11.2±0.1 days at control nests (U6,37=2.47, p=0.01). The probability of brood desertion significantly increased with increasing prolongation of parental care when all data were included in the analyses (nominal logistic regression: χ2=16.50, n=105, p<0.0001).
Table 2.
Breeding parameters of prolonged broods compared between successfully fledged and deserted broods. (Single- and four-chick broods pooled in both datasets; mean ±s.e. and p-values for Mann–Whitney tests are shown.)
| breeding characteristic | fledged (n=21) | deserted (n=6) | p |
|---|---|---|---|
| hatching date centred within year | 3.7±1.5 | 3.0±2.9 | 0.98 |
| the age of the brood at the time of cross-fostering | 3.2±0.2 | 2.6±0.5 | 0.11 |
| the age of the recipient nest at the time of cross-fostering | 7.9±0.2 | 7.8±0.4 | 0.54 |
| the length of parental care for the nest | 15.5±0.3 | 12.1±0.3 | 0.0004 |
The desertion of some broods and the shorter nestling periods in prolonged nests are unlikely to be explained by visitation rates of particular nests. In general, observer visitation rates could have little effect on both desertion rates and nestling period lengths as: (i) reed warblers are tolerant to the presence of humans in our study area (for supporting data see Honza et al. 2004), (ii) despite much more frequent visits to nests in earlier studies, no adverse effect on chicks' growth and no desertions were detected in those studies (Grim & Honza 1997, 2001; Grim 2006b), and (iii) length of the nestling period did not differ between nests checked personally (11.4±0.1) and nests checked by long-term monitoring with time-laps video (11.7±0.2), which were not visited for 2 days before fledging (U56,10=0.98, p=0.33). Specifically, the number of visits throughout the nestling period only slightly differed between the three groups of nests (ANOVA: R2=0.14, F2,91=7.51, p=0.001). In addition, shortened broods were visited more frequently (7.5±0.3) than both prolonged (6.4±0.3) and control broods (6.1±0.2) (Tukey–Kramer HSD, p=0.03), while the last two groups did not differ. This was because shortened broods fledged at higher nestling ages and thus these nests had to be checked more times than prolonged broods that fledged earlier. Most importantly, deserted prolonged broods were visited significantly fewer times (4.7±0.6) than successful prolonged broods (6.9±0.3; U6,21=2.86, p=0.0043).
(c) Fledging success and comparison with cuckoos
Additionally, there was some chick mortality within broods in prolonged and control four-chick broods. The number of fledged chicks differed significantly among the three four-chick brood treatments (ANOVA: R2=0.30, F2,51=10.93, p<0.0001). The shortened and control groups did not differ from each other (4.0±0.0 versus 3.9±0.1 fledglings per brood; Tukey–Kramer HSD, p>0.05), but prolonged broods showed significantly lower fledging success (2.7±0.5) than both control and shortened groups (Tukey–Kramer HSD, p=0.0004). The difference remained significant even when the three deserted nests were excluded from the analyses (ANOVA: R2=0.18, F2,48=5.40, p=0.008). Again the prolonged group (3.5±0.2) differed significantly from the other two four-chick groups (Tukey–Kramer HSD, p=0.03), which in turn did not differ from each other.
Furthermore, the single-chick brood treatment had a significant effect on fledging success (ANOVA: R2=0.17, F2,48=4.75, p=0.01). The shortened and control single-chick groups did not differ from each other (1.0±0.0 versus 1.0±0.0 fledglings per brood, Tukey–Kramer HSD, p>0.05), but prolonged broods showed significantly lower fledging success (0.8±0.1) than both control and shortened groups (Tukey–Kramer HSD, p=0.04). I did not repeat the test excluding deserted nests because there can be no partial mortality in single-chick broods and thus the fledging success across treatments, in principle, cannot vary.
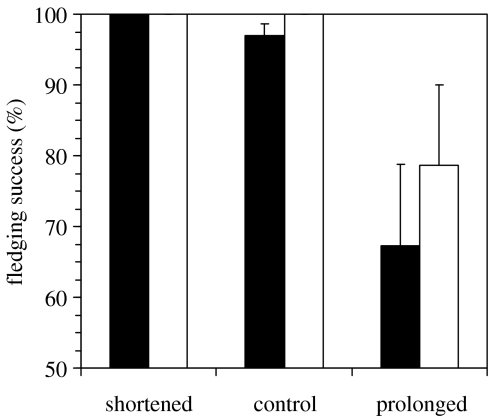
Owing to desertions and partial brood mortality, the fledging success (percentage of hatched chicks that fledged, predation excluded) was significantly lower in prolonged broods in comparison with both control and shortened broods in the pooled four- and single-chick broods dataset (figure 3; ANOVA, R2=0.22, F2,102=14.74, p<0.0001; Tukey–Kramer HSD, p<0.0001). The control and shortened broods did not differ from each other (Tukey–Kramer HSD, p>0.05). Excluding deserted nests did not change the results qualitatively (ANOVA, R2=0.08, F2,96=4.07, p=0.02; prolonged broods versus control and shortened broods, Tukey–Kramer HSD, p=0.046). Fledging success significantly decreased with increasing prolongation of the care at the nest (rs=−0.40, n=105, p<0.0001). The effect was significant even when excluding deserted nests (rs=−0.23, n=99, p=0.02).
Figure 3.
Fledging success (percentage of fledged chicks) in the four-chick broods (filled bars) or single-chick broods (open bars) (excluding predated nests) in relation to experimental treatments. Mean+s.e. shown for four- and single-chick shortened (n=16 and 14), control (n=25 and 23) and prolonged (n=13 and 14) broods.
Reed warblers made no rejection errors as they did not desert any of shortened (n=30) or control (n=48) nests. The total desertion rate of prolonged warbler broods (22.2%, n=27) did not differ significantly from that of naturally parasitized broods with single cuckoo chicks in the same study area (15.8%, n=57; χ2=0.50, p=0.48). However, warbler broods were deserted at younger ages (12.1±0.3 days) than cuckoo chicks (14.6±0.3 days; U6,9=−3.04, p=0.002).
4. Discussion
I experimentally tested the predictions of three hypotheses that were specifically designed to untangle the proximate mechanisms of chick desertion behaviour in a common cuckoo host, reed warbler. I observed that prolonged broods suffered from higher rates of breeding failure than control and shortened broods. Latencies from cross-fostering to desertion at deserted prolonged nests were 3–6 days, implying that the desertions occurred not as a response to a sudden change of chick appearance. Moreover, virtually the same level of change in chick appearance at shortened nests did not elicit any desertions (see also Davies & Brooke 1989). Chicks in prolonged broods also fledged at an earlier age. Experimentally induced changes in fledging success and nestling periods did not differ between four- and single-chick broods. These results provide strong support for the time limit hypothesis. The temporal pattern of desertion of cuckoo chicks also supports the time limit hypothesis (see §4a). In contrast, I found no support for the two alternative hypotheses. Specifically, the slightly longer care observed in four-chick nests than single-chick nests is contrary to what was predicted by the parental fatigue hypothesis and is in agreement with the time limit hypothesis. Zero desertion rates in control single-chick broods are also contrary to the single chick hypothesis and imply that brood size of one is not the cue triggering desertion of cuckoo chicks (see also Grim & Honza 2001).
(a) Response to cuckoos versus prolonged warbler broods
Experimental findings from this study support the earlier hypothesis that reed warblers may discriminate against parasitic cuckoo chicks by restricting the parental care period to a time needed for successful fledging of their own young. The parental care period at successfully fledged control nests was always less than 13 days and average nestling period was always less than 12 days, which is in line with earlier observations (Grim et al. 2003). In contrast, cuckoo chicks probably cannot fledge when younger than 16–17 days owing to weak motor abilities—their feet are so feeble that chicks cannot grasp reed stems and move anywhere from the nest (own observations; see also Grim et al. 2003). If parents would not care for a chick in the nest for much longer than 12–13 days, they would effectively discriminate against cuckoos. This prediction was in line with the observations that deserted cuckoo chicks died in reed warbler nests when 14–15 days old (Grim et al. 2003).
Surprisingly, warbler chicks were deserted ca 2 days earlier than cuckoo chicks. As cuckoo chicks provide their warbler hosts with a supernormal stimulus (Grim & Honza 2001), they could perhaps delay the desertion response of their fosterers as predicted by the parental manipulation hypothesis (Redondo 1993). This seems to be a suitable explanation as other cuckoo species chicks can even save themselves from host physical aggression with intense begging (Redondo 1993; Grim 2006a). Alternatively or additionally, around the time of desertion cuckoo chicks are much bigger than warbler chicks (approx. 50 g versus less than approx. 10 g). Thus, they have more resources and better thermoregulation abilities (Hund & Prinzinger 1980) than small warbler chicks. This might help them to survive for longer after being deserted.
The longer latencies until desertion at nests parasitized by cuckoos than at nests experimentally prolonged through conspecific ‘parasitism’ also support the time limit hypothesis, but not the parental fatigue or single chick brood hypotheses. If foster parents used the amount of parental expenditure as a cue for desertion (the parental fatigue hypothesis), then they would have to desert cuckoo chicks much earlier than they actually did. In fact, they should desert parasitic chicks earlier than they deserted prolonged warbler broods, as a cuckoo chick overgrows average host brood before the fledging period of warblers, at the age of 8–9 days (Grim 2006b). Already, Grim et al. (2003) provided evidence against parental fatigue hypothesis (‘cuckoo chicks … require more food than an average sized host brood when 8 days old or older’, p. 74), although they did not interpret the data in that way. The parental fatigue hypothesis cannot be reconciled with the long delay between the 8-day size threshold and the desertion of the cuckoo chick 6–7 days later. This is because during this period, the cuckoo chick receives approximately the same amount of food as the average host brood from hatching to fledging (Grim & Honza 2001).
The proximate mechanism underlying the temporary restrictive parental care in reed warblers in the study population could be the hormonal control of the duration of parental care delivered to parasitized versus non-parasitized broods. Silverin & Goldsmith (1984) provided experimental evidence for the partly endogenous programmed period of high-prolactin plasma concentrations in pied flycatchers (Ficedula hypoleuca). Prolactin remained high for a fixed period (16–17 days) after the onset of incubation, but fell sharply in females incubating eggs in experimentally prolonged nests before the eggs hatched (Silverin & Goldsmith 1984).
(b) Discrimination without recognition and cuckoo survival in the study population
The desertion rate of cuckoo chicks (Grim et al. 2003) and prolonged warbler broods (this study) did not differ statistically. This indicates that reed warbler parents used similar decision rules when faced with prolonged warbler broods and those parasitized by cuckoos. The probability of being deserted strongly increased with the increasing prolongation of nestling period in both single- and four-chick broods. However, within prolonged nests, the deserted chicks were deserted prior to the end of the parental care period of successful prolonged nests. Still, the majority of prolonged broods were fledged successfully. Coupled with shorter nestling periods in prolonged broods, this suggests that most individuals in the study population are restrictive as for length of care at the nest which they are ready to provide. However, only some of them desert the nest if chicks do not fledge ‘in time’. These deserters are also more restrictive as regards the length of parental care at the nest.
The shortening of nestling periods in the majority of prolonged nests raises a question of how cuckoo chicks can survive in such a population. Yet, there was a high variation in host responses to prolonged care at the nest: from the ability to care for cross-fostered chicks according to their demands, through forcing them to fledge prematurely, to deserting them if they did not fledge in time (the strategy was independent of the length of prolongation period). This variability in host responses to own chicks may explain the relatively low desertion rate of alien cuckoo chicks. Some cuckoo chicks ‘hit upon’ hosts that vary in their tolerance to prolonged care and fledge at varying fledging ages (17–21 days; Grim 2006b), while other chicks find themselves in nests of deserters and are not able to fledge at all (Grim et al. 2003). Additionally, my study population has probably been parasitized only for a historically short time which is in line with relatively low egg rejection rates (Honza et al. 2004), low parasitic chick rejection rates (Grim et al. 2003; this study) and low adult enemy recognition ability shown by reed warblers in my study area (Honza et al. 2004). Finally, chick desertion is more costly to fitness than egg desertion both with respect to time and energy, which may slow down the spread of chick discrimination through evolutionary time (Grim 2006a).
Observations of longer nestling periods in shortened rather than in control nests (figure 2) indicate that chicks fledge earlier during normal, compared with less-restrictive, experimental conditions. The absence of evidence for similarly restrictive parental behaviour in other reed warbler populations may be owing to methodological reasons because other authors rarely studied older cuckoo chicks (see discussions in Redondo 1993; Grim et al. 2003; Grim 2006a) and, to my knowledge, there are no published experimental cross-fostering studies of determination of nestling period length in open nesting passerines. In addition, high predation rates in the study population (M. Honza, B. Matysioková, T. Grim, unpublished data) may increase the benefits of forcing the brood to fledge as soon as possible. The extent of experimental manipulation (approx. 5 days difference in ages of matched-pair broods) was much larger than observed differences in nestling periods between prolonged and shortened warbler broods (approx. 1 day). This suggests that there are limits to parental manipulation resulting probably from ontogenetic constraints on chick growth (Starck & Ricklefs 1998).
(c) Implications of ‘discrimination without recognition’ for coevolutionary processes
Overall, both observational (Grim et al. 2003) and experimental (this study) pieces of evidence suggest that reed warbler desertion behaviour is relatively rare and that the cue for discrimination without recognition is the duration of the parental care period. Although the evolutionary origin of this parental ‘programmed care’ is unclear (discussed earlier), this restrictive provisioning behaviour may still have severe implications for coevolutionary dynamics. Previous studies argued that within the framework of arms race, it is expected that the evolution of host discrimination will be followed by the evolution of chick mimicry (Davies 2000; Stokke et al. 2005; Grim 2006a). Surprisingly, several lines of evidence suggest that this cannot be the case of the common cuckoo in Central Europe. First, the length of nestling period appears to be genetically fixed in the cuckoo, as indicated by the data on growth patterns of cuckoo chicks in various host species (Grim 2006b). This entails cuckoo chicks raised by reed warblers growing at the maximum possible rate allowed by host provisioning capacities (Grim 2006b) and cannot evolve any counter-adaptation against host discrimination without recognition based on the length of care at the nest. Second, this host behaviour, in turn, is probably not costly in terms of recognition errors as reed warblers did not mistakenly desert any of their not-prolonged broods (n=78). Whatever the evolutionary origin of programmed host care, it would be selected positively more strongly in a parasitized rather than in a non-parasitized population. Under normal conditions when a host is not parasitized, there is no need for such restrictive care as chicks are always able to fledge within 13 days (figure 2). Therefore, a desertion response simply makes no sense in a non-parasitized warbler population. Not surprisingly, I did not observe any case of desertions at non-parasitized nests (this study; Grim & Honza 2001; Grim et al. 2003; Grim 2006b). In contrast, the temporally more restricted care leading to desertion would easily spread in the parasitized population, as it would provide effective anti-parasite defence while not being costly when the deserter was not parasitized in a particular breeding attempt. Although at the time of desertion, the host had already provided approximately one-third of parental care needed for raising a parasitic chick to independence, there is still a large benefit to desertion: fosterers that do not desert when the cuckoo chick is ca two weeks old will have to provision for it for another week in the nest and then for 2–3 weeks outside nest (Davies 2000). Under such conditions, ‘deserters’ saving a month of their parental care should have higher fitness than ‘non-deserters’. Then, desertion may be viewed as an anti-parasitic adaptation because it would be selected by parasitism pressure (Servedio & Hauber 2006). This hypothesis requires empirical testing in both parasitized and non-parasitized populations.
The resulting spread of this adaptation in the long term may render such a host an unsuitable fosterer for cuckoos. This option has not previously been considered in debates on host selection by parasites. Only suitable diet composition, large population sizes, short nestling periods (Soler et al. 1999) and presence or absence of host brood reduction strategy (Soler 2002) are thought to be the main factors facilitating parasitism by cuckoos. Thus, the results of the present study have implications not only for coevolution, signalling and causal mechanisms in evolutionary perspective, but also for host selection by brood parasites. The above presented scenario may also prove fruitful for theoretical models of host–parasite coevolution (e.g. Planqué et al. 2002; Britton et al. in press).
5. Conclusions
The results of this study highlight an important point made earlier by Redondo (1993, p. 282): ‘Let us assume that birds can only recognize parasitic eggs and chicks by the same mechanism and we shall conclude that chick-recognition will never evolve but in a few rare cases’. Indeed, general study focus on egg-related adaptations in hosts and brood parasites and extrapolations of our knowledge of egg-related adaptations to potential chick-related adaptations may have obscured our understanding of what happens after the parasite egg hatches in a host nest (Redondo 1993; Grim 2005, 2006a). Hopefully, the present study will induce more research on the poorly explored host responses to parasite chicks.
The findings of the present study strikingly differ from those predicted by other studies on host–parasite discrimination, in that hosts do not need to have an internal representation or recognition template of the parasite's appearance to afford discrimination (Hauber & Sherman 2001). In turn, physiological or temporal decision rules, evolved perhaps even in the absence of parasitism, regarding when to terminate the feeding of a brood, whether conspecific or parasitic, alone are sufficient to implement effective anti-parasite responses.
Acknowledgments
Suggestions by two anonymous referees and the editor substantially improved the paper. This study could not have been carried out without the help of co-workers who assisted with the collection of data: A. Dvorská, M. Honza, B. Matysioková, P. Procházka, P. Samaš and Z. Skoumalová. I am grateful for comments by N. B. Davies, M. E. Hauber, O. Kleven, M. Krist, V. Remeš and E. Tkadlec, and the hospitality of the School of Biological Sciences, University of Auckland. I was supported by grants MSM6198959212 and GACR 206/03/D234.
References
- Beecher M.D, Beecher I.M, Hahn S. Parent-offspring recognition in bank swallows. II. Development and acoustic basis. Anim. Behav. 1981;29:95–101. [Google Scholar]
- Britton, N. F., Planqué, R. & Franks, N. R. In press. Evolution of defence portfolios in exploiter-victim systems. Bull. Math. Biol [DOI] [PubMed]
- Cramp, S. (ed.) 1992 Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic vol. VI, Warblers, p. 728. Oxford, UK: University Press.
- Davies N.B. T & A.D. Poyser; London, UK: 2000. Cuckoos, cowbirds and other cheats. [Google Scholar]
- Davies N.B, Brooke M.L. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 1989;58:225–236. 10.2307/4996 [Google Scholar]
- Davies N.B, Kilner R.M, Noble D.G. Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proc. R. Soc. B. 1998;265:673–678. doi:10.1098/rspb.1998.0346 [Google Scholar]
- Grim T. Mimicry vs. similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol. J. Linn. Soc. 2005;84:69–78. doi:10.1111/j.1095-8312.2005.00414.x [Google Scholar]
- Grim T. The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol. Ecol. Res. 2006a;8:785–802. [Google Scholar]
- Grim T. Cuckoo growth performance in parasitized and unused hosts: not only host size matters. Behav. Ecol. Sociobiol. 2006;60:716–723. doi:10.1007/s00265-006-0215-z [Google Scholar]
- Grim T, Honza M. Differences in parental care of reed warbler (Acrocephalus scirpaceus) to its own nestlings and parasitic cuckoo (Cuculus canorus) chicks. Folia Zool. 1997;46:135–142. [Google Scholar]
- Grim T, Honza M. Does supernormal stimulus influence parental behaviour of the cuckoo's host? Behav. Ecol. Sociobiol. 2001;49:322–329. doi:10.1007/s002650000295 [Google Scholar]
- Grim T, Kleven O, Mikulica O. Nestling discrimination without recognition: a possible defence mechanism for hosts towards cuckoo parasitism? Proc. R. Soc. B. 2003;270:S73–S75. doi: 10.1098/rsbl.2003.0017. doi:10.1098/rsbl.2003.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber M.E, Sherman P.W. Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci. 2001;24:609–616. doi: 10.1016/s0166-2236(00)01916-0. doi:10.1016/S0166-2236(00)01916-0 [DOI] [PubMed] [Google Scholar]
- Holen Ø.H, Saetre G.P, Slagsvold T, Stenseth N.C. Parasites and supernormal manipulation. Proc. R. Soc. B. 2001;268:2551–2558. doi: 10.1098/rspb.2001.1818. doi:10.1098/rspb.2001.1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honza M, Grim T, Capek M, Moksnes A, Røskaft E. Nest defence, enemy recognition and nest inspection behaviour of experimentally parasitized reed warblers Acrocephalus scirpaceus. Bird Study. 2004;51:256–263. [Google Scholar]
- Hund K, Prinzinger R. Zur Jugendentwicklung der Körpertemperature und des Körpergewichtes beim Kuckuck Cuculus canorus. Ökologie der Vogel. 1980;2:130–131. [Google Scholar]
- Johnsen I, Erikstad K.E, Saether B.-E. Regulation of parental investment in a long-lived seabird, the puffin Fratercula arctica—an experiment. Oikos. 1994;71:273–278. [Google Scholar]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 2003;422:157–160. doi: 10.1038/nature01460. doi:10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Lessells C.M, Coulthard N.D, Hodgson P.J, Krebs J.R. Chick recognition in European bee-eaters: acoustic playback experiments. Anim. Behav. 1991;42:1031–1033. [Google Scholar]
- Lichtenstein G. Low success of shiny cowbird chicks parasitizing rufous-bellied thrushes: chick–chick competition or parental discrimination? Anim. Behav. 2001;61:401–413. doi:10.1006/anbe.2000.1595 [Google Scholar]
- Lotem A. Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature. 1993;362:743–745. doi:10.1038/362743a0 [Google Scholar]
- Lotem A, Nakamura H, Zahavi A. Constraints on egg discrimination and cuckoo-host co-evolution. Anim. Behav. 1995;49:1185–1209. doi:10.1006/anbe.1995.0152 [Google Scholar]
- Nilsson J.A, Svensson M. Fledging in altricial birds: parental manipulation or sibling competition? Anim. Behav. 1993;46:379–386. doi:10.1006/anbe.1993.1200 [Google Scholar]
- Payne R.B, Woods J.L, Payne L.L. Parental care in estrildid finches: experimental tests of a model of Vidua brood parasitism. Anim. Behav. 2001;62:473–483. doi:10.1006/anbe.2001.1773 [Google Scholar]
- Planqué R, Britton N.F, Franks N.R, Peletier M.A. The adaptiveness of defence strategies against cuckoo parasitism. Bull. Math. Biol. 2002;64:1045–1068. doi: 10.1006/bulm.2002.0311. doi:10.1006/bulm.2002.0311 [DOI] [PubMed] [Google Scholar]
- Redondo T. Exploitation of host mechanisms for parental care by avian brood parasites. Etología. 1993;3:235–297. [Google Scholar]
- Redondo T, Carranza J. Offspring reproductive value and nest defense in the magpie (Pica pica) Behav. Ecol. Sociobiol. 1989;25:369–378. doi:10.1007/BF00302995 [Google Scholar]
- Schuetz J.G. Reduced growth but not survival of chicks with altered gape patterns: implications for the evolution of nestling similarity in a parasitic finch. Anim. Behav. 2005;70:839–848. doi:10.1016/j.anbehav.2005.01.007 [Google Scholar]
- Servedio M.R, Hauber M.E. To eject or to abandon? Life history traits of hosts and parasites interact to influence fitness payoffs of alternative anti-parasite strategies. J. Evol. Biol. 2006;19:1585–1594. doi: 10.1111/j.1420-9101.2006.01124.x. doi:10.1111/j.1420-9101.2006.01124.x [DOI] [PubMed] [Google Scholar]
- Sherman P.W, Reeve H.K, Pfenning D.W. Recognition systems. In: Krebs J.R, Davies N.B, editors. Behavioural ecology. Blackwell Scientific; Oxford, UK: 1997. pp. 69–96. [Google Scholar]
- Silverin B, Goldsmith A. The effects of modifying incubation on prolactin secretion in free-living pied flycatchers. Gen. Comp. Endocr. 1984;55:239–244. doi: 10.1016/0016-6480(84)90107-2. doi:10.1016/0016-6480(84)90107-2 [DOI] [PubMed] [Google Scholar]
- Soler M. Breeding strategy and begging intensity: influences on food delivery by parents and host selection by parasitic cuckoos. In: Wright J, Leonard M.L, editors. The evolution of begging. Kluwer; Dordrecht, The Netherlands: 2002. pp. 413–427. [Google Scholar]
- Soler M, Soler J.J, Martinez J.G, Møller A.P. Chick recognition and acceptance: a weakness in magpies exploited by the parasitic great spotted cuckoo. Behav. Ecol. Sociobiol. 1995;37:243–248. doi:10.1007/s002650050187 [Google Scholar]
- Soler J.J, Møller A.P, Soler M. A comparative study of host selection in the European cuckoo. Oecologia. 1999;118:265–276. doi: 10.1007/s004420050727. doi:10.1007/s004420050727 [DOI] [PubMed] [Google Scholar]
- Starck J.M, Ricklefs R.E, editors. Avian growth and development. Oxford University Press; New York, NY: 1998. [Google Scholar]
- Stokke B.G, Moksnes A, Røskaft E. The enigma of imperfect adaptations in hosts of avian brood parasites. Ornithol. Sci. 2005;4:17–29. doi:10.2326/osj.4.17 [Google Scholar]