Abstract
Fish larvae are the world's smallest vertebrates, and their high rates of mortality may be partially owing to a very limited aerobic scope. Unfortunately, however, no complete empirical dataset exists on the relationship between minimal and maximal metabolism (and thus aerobic scope) for any fish species throughout ontogeny, and thus such an association is hard to delineate. We measured standard and maximal metabolism in three marine fish species over their entire life history, and show that while aerobic scope depends greatly on body size and developmental trajectory, it is extremely small during the early life stages (factorial aerobic scope≤1.5). Our findings strongly suggest that limited scope for aerobic activity early in life is likely to constrain physiological function and ultimately impact behaviour and possibly survival. Furthermore, our results have important implications for ecological models that incorporate metabolic scaling, and provide additional evidence against the existence of ‘universal’ scaling exponents.
Keywords: scaling, metabolism, fishes, development, aerobic scope
1. Introduction
The metabolic rate of organisms increases with body mass according to the equation
| (1.1) |
where Y can be basal or maximal metabolic rate, a is the species-specific scaling constant, M is body mass and b is the scaling exponent (bb and bm, for the scaling of basal and maximal metabolic rates, respectively). This relationship forms the basis of a widely accepted tenet in biology, known as ‘Kleiber's law’, which states that the minimal metabolic rate of organisms scales to the three-quarter power of body mass (bb=0.75). However, after a century of research, there remain a number of unresolved issues regarding the effects of body size on metabolic rate. For example, significant controversy still surrounds the precise value of bb (specifically, is bb=0.75 or 0.67? Dodds et al. 2001; White & Seymour 2003, 2005; Savage et al. 2004), and whether or not this value is ‘universal’ across all taxa (Bokma 2004; Glazier 2005). Furthermore, there are sparse data on maximal metabolic rate (MMR) in ectotherms, and it is unclear whether the variation present in the allometry of MMR in endotherms also exists in other animal groups (e.g. in birds and mammals, bm is elevated in ‘athletic’ species; Bishop 1999; Glazier 2005). Understanding the allometry of MMR, in combination with basal metabolic rate (BMR), provides insight into the aerobic scope of organisms at various sizes or stages of development (e.g. factorial aerobic scope; FAS=MMR/BMR), and is critical since aerobic scope reflects the capacity to perform all oxygen-consuming functions above minimal metabolic requirements, and potentially, the ability to respond to environmental extremes or other challenges (Djawdan et al. 1997; Bochdansky et al. 2005).
Fishes are an integral part of aquatic ecosystems, yet when compared with mammals and birds, relatively little is known about the scaling of their basal and active metabolism. Further investigation is warranted, however, since the limited data available for fishes (e.g. Post & Lee 1996; Clark & Johnston 1999; Bokma 2004) suggest that the scaling of standard metabolic rate (SMR; analogous to BMR in endotherms; referred to as ‘standard’ because it is specific for a given temperature) in teleosts differs from either the 0.67 or 0.75 ‘scaling laws’ that have been proposed for endotherms. Furthermore, fishes are the only vertebrate group in which an individual's life history may span a range of wet mass of up to eight orders of magnitude, and larval fishes are believed to have a reduced aerobic scope when compared with adults (Weiser 1995; Glazier 2005). However, it has been difficult to grasp the magnitude and importance of mass-dependent changes in metabolism in this group, because the few studies in this area vary greatly in their methodology and this makes it hard to compare larval data with that obtained for juveniles and adults. A diminished aerobic scope could be an important constraint for larval fishes, as this would limit the energy available for important behaviours and physiological functions. Ten years ago, Post & Lee (1996) emphasized the need for a comprehensive examination of SMR and MMR spanning the complete ontogeny of any teleost species, yet to date, no single study has addressed this issue. As a result, we currently have a limited understanding of how aerobic scope in teleost fishes changes throughout ontogeny.
In this study, we measured SMR and MMR in three species of marine teleost fishes (ocean pout, Macrozoarces americanus; lumpfish, Cyclopterus lumpus; and shorthorn sculpin, Myoxocephalus scorpius) over their entire life history using similar methodologies for all developmental periods. Our goal was to produce accurate intraspecific estimates of bs and bm for these species over several orders of magnitude of body mass, and to calculate the changes in aerobic scope over the life history of these species. The species used in this study are unique, because unlike most fishes, they show no activity in respirometers while at rest, and therefore measures of routine metabolic rate (RMR) closely approximate SMR (i.e. they are directly comparable to measures of BMR in endotherms). This largely eliminates some of the criticisms of earlier studies on metabolic scaling in teleosts, where baseline metabolic measurements were more characteristic of RMR than SMR, and thus measures of aerobic scope were likely to be underestimates (Post & Lee 1996). In addition, these species show different patterns of development during their early life stages, and we hoped comparisons between these species would allow a better understanding of the effects of developmental trajectory on aerobic scope during ontogeny.
2. Material and methods
(a) Animals
Fertilized eggs of M. americanus, C. lumpus and M. scorpius were collected by SCUBA and maintained in the laboratory in aerated incubators supplied with a constant flow of seawater. After hatching, larvae were maintained on a diet of enriched Artemia nauplii (1000–1500 Artemia l−1; 3–4 times per day), and holding temperatures (3°C for M. scorpius, 8°C for M. americanus and 11°C for C. lumpus) were chosen to approximate those experienced by the early life stages of each species in the wild (these temperatures were also used when measuring the metabolic rate of each species and were consistent across all life stages). Juveniles were a combination of wild fishes captured by SCUBA and individuals that had been raised in the laboratory after hatching from eggs obtained from the wild. All adult fishes were collected from the wild by SCUBA.
(b) Respirometers
Three separate Blazka-style respirometers were used: a 57 ml respirometer was used for fishes less than 1 g; a 6.8 l respirometer was used for individuals 15–80 g; and an 81 l respirometer was used for individuals greater than 120 g. The methods for measuring the water oxygen concentration in the 6.8 and 81 l Blazka-style respirometers have been described previously (Rodnick et al. 2004). Briefly, oxygen concentration and temperature were measured within each respirometer by pumping water through an external circuit containing a custom flow chamber and a galvanic oxygen electrode equipped with a thermal sensor (Model CellOx 325; WTW, Inc., Welheim, Germany). This oxygen electrode was connected to an oxygen meter (Model Oxi 340; WTW, Inc.) equipped with automatic temperature compensation. Measurements of oxygen consumption were initiated after the flow of water from an external reservoir of aerated, temperature-controlled water was stopped and the respirometer sealed.
To measure the oxygen consumption of fishes in the 57 ml respirometer, we used a fibre-optic flow-through oxygen sensor (Presens, Germany), as this type of sensor does not consume oxygen. Data obtained using this sensor were recorded directly to a computer using the accompanying software (Oxyview v. 4.16). Water was moved from the respirometer and past the sensor via a short external circuit using a peristaltic pump (Masterflex; Cole-Parmer Instrument Co.) and tubing with low oxygen permeability (Masterflex Tygon Food for the majority of the circuit and a small section of Masterflex Tygon LFL at the pump-head). Preliminary blank experiments using hypoxic seawater (30–40% oxygen saturation) confirmed that this system did not gain oxygen after being closed for 1.5 h of monitoring (note: measurements of oxygen consumption in this study lasted for a maximum of 40 min, see below). To maintain the temperature of the water in the respirometer and oxygen concentrations in the water following transfer of the larvae to the respirometer, a second external circuit supplied aerated seawater from a reservoir in a water bath set at the appropriate water temperature for each species. This circuit was closed when measurements of oxygen consumption were made, and therefore to maintain water temperature within the respirometer, the entire set-up was located in a cold-room set at the desired experimental temperature. To reduce background bacterial contamination, seawater used for the 57 ml respirometer was sterilized with ultraviolet radiation and the system was cleansed daily with absolute ethanol. In addition, blank measurements were performed after each trial to quantify any possible bacterial oxygen consumption. In the vast majority of cases (over 85% of trials), there was negligible background oxygen consumption. However, in cases where a background rate of oxygen consumption was observed, this value was subtracted from the experimental data.
(c) Determination of standard metabolic rate
Fishes were fasted (48 h for juveniles and adults, overnight for larvae), and then carefully placed into the respirometers and allowed an appropriate acclimatization period (24 h for juveniles and adults, 4 h for larvae; determined in preliminary experiments). To reduce light levels and external disturbance, a sheet of black plastic was wrapped around the respirometers during the acclimatization period and left in place during measurements of SMR. The behaviour of the fishes was monitored during measurements via a mirror either below (in the case of the two larger respirometers) or behind the respirometers (in the case of the 57 ml respirometer). Owing to the inactive nature of the species used in this study (during all life stages), we were easily able to measure the oxygen consumption of fishes under conditions of zero swimming activity, thus approximating SMR. An exception was larval M. scorpius, which swim constantly under conditions of even low lighting. For this reason, measures of SMR in M. scorpius larvae were performed in complete darkness (larval M. scorpius stop swimming after acclimatization to darkness and rest motionless in the respirometer). For larval M. scorpius and C. lumpus, multiple individuals were placed into the respirometer simultaneously so that a measurable decrease in oxygen could be obtained. The number of individuals varied depending on the size of the larvae, but was enough to comprise at least 70 mg of biomass. Resting measurements of oxygen consumption were made over the course of 20 min for larval fishes, and 20–40 min for juveniles and adults. Water oxygen content was measured once every minute when using the 57 ml respirometer, and once every 2 min for the two larger respirometers. After the measurements of oxygen consumption were completed (either SMR alone, or SMR and MMR—see below), the juvenile and adult fishes were removed from the respirometer and weighed using an electronic scale accurate to 0.1 g sensitivity. Larval fishes were quickly rinsed with freshwater (to remove external salts), blotted with a paper towel and weighed using a microbalance (APX-60; Denver Instrument Co.). In cases where multiple larvae were used for one trial, their total wet mass was divided by the number of individuals to obtain an average value (this value was subsequently used on the log-plots of oxygen consumption versus wet mass—see §2e).
(d) Determination of maximal metabolic rate
For juvenile and adult fishes, MMR was determined after individuals were exhaustively exercised using a burst-swimming protocol similar to that described by Reidy et al. (1995). Briefly, the water current speed was gradually increased until the fishes began to perform burst-type swimming and the fishes were then allowed to swim at this speed until exhaustion (as indicated by an inability to maintain swim speed and a tendency to rest against the bottom or back of the respirometer; usually achieved after approximately 3–5 min of burst swimming). This protocol was initiated immediately following the measurement of SMR. The measurements of MMR began immediately after the fishes reached exhaustion and continued for the first 10 min of recovery. During this time, a constant decrease in water oxygen content was observed. This protocol estimates MMR based on excess post-exercise oxygen consumption (EPOC; Lee et al. 2003), and was used instead of a critical swimming speed (Ucrit) protocol because some of our study species were behaviourally averse to sustained swimming in the respirometers. An exception to this protocol were some adult M. scorpius that would not swim at all in the respirometer. These fishes were exercised to exhaustion by manual chasing and immediately placed in the respirometer. Measures of MMR collected via this method were within the range obtained for adult M. scorpius whose MMR was measured using the burst-swimming protocol. For juvenile and adult C. lumpus, we were able to measure MMR under conditions of both sustained maximal swimming and after exhaustion via burst swimming (only data obtained after burst swimming are reported in this study). Importantly, there was no significant difference in the values for MMR obtained via either method for C. lumpus, and so we believe our protocol was a valid method for estimating MMR in M. americanus and M. scorpius as well.
It should be noted that C. lumpus possesses a ventral adhesive disk, to which a thin film of Parafilm (American National Can) was carefully glued under MS-222 anaesthesia, to prevent individuals from adhering to the inside of the respirometers. For juveniles less than 0.5 g, only a thin film of adhesive (VetBond 3M Tissue Adhesive) was applied to the disk (without the addition of a plastic layer) with the aid of a dissecting microscope. The resulting layer of dried adhesive served the same function as the Parafilm that was applied in the case of larger fishes.
For larval M. scorpius, current speeds in the respirometer were gradually increased until larvae began to swim vigorously and rely on burst-type swimming to maintain their position (approx. 5 body lengths s−1). The larvae were allowed to swim in this manner for approximately 2 min, after which time the flow speeds were decreased. After a rest period of approximately 30 s, the current speed was again increased to a level that would force burst-type swimming. This protocol was repeated for a 12-min period, over which time water oxygen content was measured every minute. This protocol was used because some previous studies have found that larval fishes have a low anaerobic capacity (Forstner et al. 1983), and so it was not certain if the measurements of EPOC would be possible for the larval fishes in our study. This method was not possible for larval C. lumpus, however, since they would adhere to the inside of the respirometer using their ventral adhesive disk (unlike juvenile and adult C. lumpus, the larvae of this species are too small to have their ventral disks covered in order to prevent adhesion). Instead, larval C. lumpus were manually exercised to exhaustion prior to placement in the respirometer. Measurements of oxygen consumption were initiated after quickly placing the larvae into the respirometer and continued for 10 min. Using this protocol, larval C. lumpus showed large increases in oxygen consumption post-exercise that were comparable in magnitude to those observed for larval M. scorpius. Therefore, we believe that our measures represent an accurate estimate of MMR in larval C. lumpus.
(e) Data and statistical analysis
Data analyses were performed using Minitab statistical software (v. 13.0; Minitab, Inc.). Rates of oxygen consumption (mg O2 h−1) were calculated for each trial using linear regression. Measures of oxygen consumption for each species were then plotted on log plots against wet mass, and scaling exponents were estimated by applying power curves to the data. FAS throughout ontogeny was calculated as the ratio of MMR to SMR (MMR : SMR) for each species, using the equations for the power curves for standard and maximal metabolism. We chose to examine factorial scope instead of absolute aerobic scope because: (i) absolute aerobic scope expressed on a non-mass-specific basis (e.g. mg O2 h−1; Weiser 1985) provides little insight into comparisons across large size ranges, because small fishes will have comparatively minute increases in O2 consumption with increased aerobic activity simply because they have less biomass, and (ii) absolute aerobic scope expressed on a mass-specific basis (in units of mg O2 kg−1 h−1) is a misleading measure, because small animals require more energy (per unit mass) to perform aerobic functions other than those contributing to SMR. For example, the minimum cost of transport (COT; mg O2 kg−1 km−1) is known to be much higher for smaller animals (Tucker 1975), and net COT is 5–10-fold higher for juvenile C. lumpus (approx. 600 mg) than for adult individuals (>500 g) at swimming speeds appropriate for both life stages (S. S. Killen & A. K. Gamperl 2006, unpublished data). For these reasons, we strongly feel that factorial metabolic scope, where maximum metabolism is expressed relative to the fish's standard metabolic costs, is a more suitable measure for comparing the aerobic capacity of animals across large size ranges.
Intraspecific estimates of bs or bb are advantageous because they do not need to consider the phylogeny of species or the evolution of metabolic rates (Bokma 2004; Martin et al. 2005). However, there is some concern that early development could affect the pattern of intraspecific metabolic scaling in fishes. For example, there is evidence that the SMR of larval fish scales differently from that of juveniles and adults, possibly scaling isometrically or with positive allometry (b>1), and that MMR in fishes may also scale in a biphasic manner (Post & Lee 1996). To account for this possibility, we used two separate methods to estimate both bs and bm. In the first analysis, we simply determined bs and bm over the entire life history of each species. In the second, we accounted for early ontogenetic effects on metabolic scaling by performing a biphasic analysis. In this analysis, the two scaling phases were separated in M. scorpius and C. lumpus using a quantifiable, biological criterion—the end of metamorphosis (defined by the appearance of juvenile pigmentation, fully developed fins and fin rays, and flexion of the notochord; occurring at approximately 25 mg wet mass for M. scorpius and 150 mg for C. lumpus). We did not conduct a biphasic analysis on M. americanus because individuals of this species are large at hatching (3–4 cm total length, 100–200 mg), and are considered to be fully formed juveniles in terms of behaviour and morphology (Methven & Brown 1991). Power curves were then fitted to the data to obtain the scaling exponents. Estimates of b are presented ±s.e.m. and with 95% confidence limits.
3. Results
(a) Standard metabolism
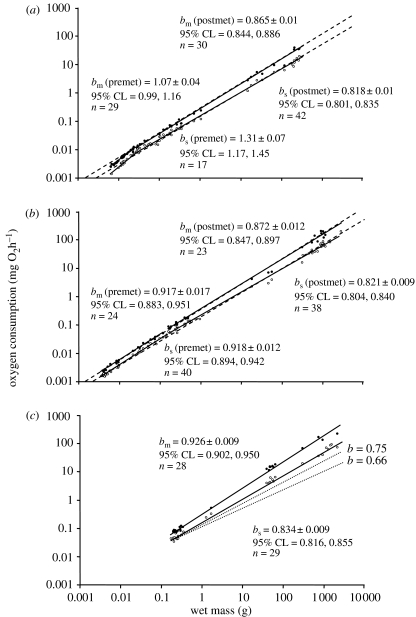
Estimates of bs ranged from 0.82 to 0.84 when calculated across the entire life history of each species (figure 1). When post-metamorphic scaling patterns were analysed separately from the larval stage (using the biphasic analysis), post-metamorphic estimates of bs in C. lumpus and M. scorpius (species with a larval stage) decreased slightly, but were still within this general range. Pre-metamorphosis, bs during the larval stage for C. lumpus and M. scorpius was greatly elevated when compared with post-metamorphosis. This was especially true for larval M. scorpius, which showed positive allometry for SMR during this life stage (pre-metamorphic bs=1.34).
Figure 1.
Oxygen consumption versus body mass for three species of teleost fishes. (a) Myoxocephalus scorpius; (b) Cyclopterus lumpus and (c) Macrozoarces americanus. For M. scorpius and C. lumpus, a biphasic analysis was performed to obtain estimates of both standard and maximal metabolic rates (MMRs; to account for the potential effects of early ontogeny on metabolic scaling), consisting of separate regressions before (‘premet’) and after (‘postmet’) the end of the metamorphic period (see §2 for description). A biphasic analysis was not performed for M. americanus because this species does not possess a larval period. Dashed lines represent estimates of scaling for standard and MMRs in M. scorpius and C. lumpus without attempting to account for ontogenetic effects (no biphasic analysis). Estimated in this manner, bs in M. scorpius is 0.833 (s.e.m.=0.007; 95% confidence limits=0.818, 0.842), while bm is 0.878 (s.e.m.=0.007; 95% CL=0.865, 0.891). Similarly, the combined estimate of bs in C. lumpus is 0.844 (s.e.m.=0.005; 95% CL=0.837, 0.852), while bm is 0.883 (s.e.m.=0.006; 95% CL=0.871, 0.894). For reference, theoretical representations of b=0.75 and 0.67 are also shown on the panel for (c) M. americanus. The filled circles represent maximal metabolic rate (MMR) and the open circles represent standard metabolic rate (SMR).
(b) Maximal metabolism
Estimates of bm were generally higher than for bs, and ranged from 0.88 to 0.93 when calculated across the entire life history of each species (figure 1). Similar to the results for SMR, post-metamorphic estimates of bm in C. lumpus and M. scorpius (species with a larval stage) were slightly decreased when compared with the values obtained without the biphasic analysis, but were not significantly different (95% confidence limits showed overlap). Pre-metamorphosis, bm during the larval stage for C. lumpus and M. scorpius was higher than post-metamorphosis. Once again, this trend was especially pronounced for M. scorpius larvae, which showed pre-metamorphic scaling that was close to isometric (pre-metamorphic bm=1.07).
(c) Aerobic scope
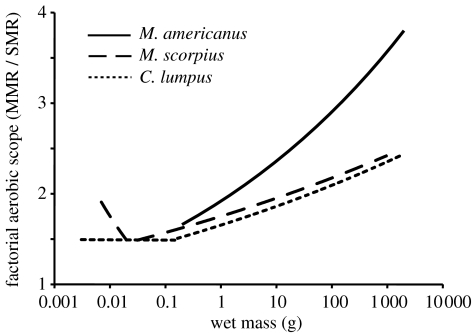
In general, the FAS for each species was low early in life, gradually increased throughout ontogeny and was highest during the adult life stage (figure 2). For example, adult C. lumpus showed a FAS that was 79% higher than at the lowest point during the larval stage, the FAS of adult M. scorpius increased 72%, and FAS in M. americanus increased 2.7-fold between the larval and adult life stages.
Figure 2.
Factorial aerobic scope (FAS) in three species of teleost fishes over their complete life history. Values were calculated from the fitted curves in figure 1 (FAS=MMR/SMR).
Within this general trend of increasing factorial scope throughout ontogeny, there were differences between species with regard to changes in FAS during early development. For C. lumpus, FAS was relatively constant pre-metamorphosis (approx. 1.49), while it decreased throughout the larval stage for M. scorpius and reached its lowest point at the end of metamorphosis (approx. 1.41).
4. Discussion
(a) Standard metabolism
To our knowledge, the present analysis is the only single-study dataset for SMR over the complete life history of any teleost species. Our results clearly show that the intraspecific scaling of SMR in teleost fishes differs significantly from either the 0.67 or the 0.75 scaling exponents that are commonly discussed with regard to endotherms. Instead, using both types of analyses (i.e. examining bs over the entire life history of each species, or examining the pre- and post-metamorphic periods separately), estimates of post-larval bs were between 0.82 and 0.84 in the three species examined. Thus, even when the potential biases of early developmental effects on SMR are eliminated, bs in teleosts is substantially different from bb in endotherms. Although our results may not be directly comparable to previous fish studies where RMR was measured, when combined, the data strongly suggest that the scaling exponent for SMR in teleost fishes is 0.80–0.85. For example, Bokma (2004) performed an intraspecific analysis of teleost RMR by compiling data from various life stages for 113 species. In that analysis, the largest dataset compiled was for the sea trout (Salmo trutta trutta; 0.1–600 g), and b for that species was approximately 0.86. In the only three species for which Post & Lee (1996) found sufficient data to conduct an analysis of RMR over full life histories (the common carp, Cyprinus carpio; rainbow trout, Oncorhynchus mykiss; and sea bream, Pagrus major), b-values of the second scaling phase were all between 0.82 and 0.84. Clark & Johnston (1999) performed an interspecific analysis of RMR in post-larval teleosts and found that b was approximately 0.80. Most recently, White et al. (2006) surveyed 82 fish species and found an overall bs value of approximately 0.88.
These findings for bs in fishes may have important implications with respect to the recent attempts made to extrapolate the metabolic rate of individuals to broad-scale ecosystem effects (Enquist et al. 2003; Ernest et al. 2003; Brown et al. 2004; West & Brown 2005; Woodward et al. 2005). The application of metabolism to the study of ecology has great potential, and in their ‘Metabolic Theory of Ecology’ (MTE), Brown et al. (2004) state that BMR can be used to predict diverse ecological phenomena including rates of predation, patterns of species diversity and rates of biomass production. Their theory also uses the 0.75 scaling exponent to correct for the effects of body mass on BMR, and thus isolate the effects of temperature on ecosystems. However, if the scaling exponent for teleost fishes is closer to 0.85, the role of body size in determining the effects that fishes exert on ecosystems may differ from that observed for endothermic species. Clearly, the MTE should be used in a manner that accounts for potential differences in the scaling of minimal metabolic rate between taxa, as opposed to a steadfast reliance on the 0.75 scaling exponent.
Early development had an effect on standard metabolic scaling in C. lumpus and M. scorpius, as both species showed elevated values for bs during the larval stage as compared with the juvenile and adult stages. This biphasic scaling pattern supports the general model for RMR throughout ontogeny in teleosts theorized by Post & Lee (1996). However, although both C. lumpus and M. scorpius showed elevated values for bs during the larval stage, the exact value for bs was very different between the two species. Specifically, M. scorpius showed strong positive allometry during the larval stage (bs=1.34), while bs for C. lumpus was much lower (bs=0.92). This difference in scaling between the two species could be attributable to differences in the developmental trajectory. C. lumpus larvae have a gradual progression to the juvenile stage, while larval M. scorpius display a relatively abrupt metamorphosis (characterized by rapid changes in morphology and behaviour). Elevated scaling exponents for RMR have previously been observed during the larval stage of fishes (Post & Lee 1996; Giguere et al. 1988), and have generally been attributed to either: (i) increased rates of growth or protein turnover during the larval stage (Weiser 1991, 1995; Glazier 2005), (ii) the differential emergence of metabolically active tissues and organs during larval development (Oikawa et al. 1991), or (iii) increases in respiratory surface area as larvae switch from cutaneous respiration to the use of gills (Kamler 1992; Post & Lee 1996). A more abrupt change in any (or all) of these factors in M. scorpius as compared with C. lumpus during development could cause a proportionally greater increase in absolute metabolic rate beyond that which would be predicted based on body size alone. Although further research is required to determine the role that the above factors play in elevating the scaling exponent for RMR in larval fishes, our results suggest that differing patterns of development during metamorphosis can cause variation in larval metabolic scaling between species.
Regardless of the exact scaling pattern during early development, metabolic scaling throughout ontogeny causes mass-specific metabolic demand to be much greater early in the life of fishes (with metamorphosis being a particularly demanding period for some species). Considering that energy is the main ‘currency’ used in most foraging models, it seems likely that these increased requirements could not only increase the likelihood of starvation during the early life stages, but also affect the behavioural ecology of larval and juvenile teleosts. For example, it is known that for adult fishes, increases in energetic demand (due to factors such as parasitic infection) can increase feeding motivation and cause individuals to be more ‘risky’ when foraging under predation threat (Godin & Sproul 1988; Godin & Crossman 1994). Conversely, although young M. americanus are known to reduce foraging in the presence of a predator (Killen & Brown 2006), their high mass-specific metabolism suggests that such foraging interruptions are energetically very costly. Clearly, additional research should examine how the increased mass-specific metabolic requirements of young fishes impact their foraging decisions and survivability.
(b) Maximal metabolism
With regard to our analysis of MMR, we found that the post-metamorphic bm for all three species ranged from 0.87 to 0.93. This is an interesting result, as it has traditionally been accepted that MMR in teleost fish scales isometrically when examined intraspecifically (Brett & Glass 1973; Weiser 1985; Goolish 1991; Blier et al. 1997). However, this result is not surprising, since previous studies on the scaling of MMR in fishes have concentrated on relatively athletic salmonid species, whereas none of the species in the present study can be considered athletic (M. americanus and M. scorpius are benthic while C. lumpus are semi-pelagic). Previous research on birds and mammals has shown that bm is consistently higher than bb and varies substantially between species (Bishop 1999; Glazier 2005). Furthermore, Weibel et al. (2004) state that MMR should show high interspecies variability owing to its dependence on features such as mitochondrial and capillary volumes (factors that vary with a species’ athleticism but may not be directly related to body size) and report that the bm for ‘athletic’ mammalian species was about 0.942, while the bm of ‘normal’ species was lower at around 0.849. Our results, when compared with the data available for salmonids (Brett & Glass 1973; Weiser 1985), support the findings of Weibel et al. (2004) and provide further evidence that the only quality that is ‘universal’ with respect to MMR allometry is that bm tends to be higher than bs, although the degree to which it is elevated is probably dependent on a species’ lifestyle (‘athletic’ versus ‘sedentary’).
(c) Aerobic scope
The ratio of MMR to minimal metabolic rate (SMR or BMR) is an individual's FAS. This measure represents the factor by which an organism can increase its metabolic activity above maintenance levels, and is thus an animal's capacity to support various oxygen-consuming physiological functions (e.g. activity, digestion, response to stressors, etc.; Jobling 1983; Priede 1985; Bishop 1999). The differential effects of scaling on MMR and SMR in the three species examined results in an overall increase in FAS throughout ontogeny. In endotherms, it has also been noted that MMR is less dependent on body mass than SMR, and thus that larger animals have a greater factorial scope as compared with smaller animals (Bishop 1999; Weibel et al. 2004). For fishes, a limited aerobic capacity could be an important constraint during the early life stages, especially since many fish species possess a larval stage of exceedingly small body size as compared with other vertebrates. Our results confirm that the FAS of fishes is extremely limited early in life (figure 2). Furthermore, our results demonstrate that the early relationship between FAS and body mass can differ in species with a distinct larval period (i.e. C. lumpus and M. scorpius), as compared with species that are well developed at hatch (M. americanus). For example, the positive allometry displayed for SMR in larval M. scorpius results in a decline in FAS during this life stage, while M. americanus simply display a gradual increase in FAS throughout their life history.
Such a limited FAS early in life could affect the ability of young fishes to ‘multitask’ physiologically demanding processes. For example, the energetic demands of rapid growth, when combined with the high energetic cost of locomotion needed for foraging and predator evasion (i.e. large increases in aerobic metabolism occur during recovering from burst-type anaerobic activity like that utilized while escaping predators), may leave little room for homeostatic maintenance during times of environmental or nutritional stress. This probably contributes to the enormous rates of mortality (often greater than 90%) often observed for larval marine fishes (Bailey & Houde 1989), and suggests why even minor environmental fluctuations can affect recruitment to the juvenile and adult life stages. While it is clear that the life-history strategies utilized by fish species with exceedingly small larvae are indeed successful at producing adult individuals, the metabolic challenges imposed by scaling results in certain physiological tradeoffs early in life that may be important for the ecology of these animals. For example, it is often assumed that increased SMR in larval fishes is advantageous since it is associated with rapid growth and thus decreased size-dependent mortality owing to predation (Glazier 2005), but recent work with fish larvae has shown that there can be selection against individuals with high SMR when food supply is limited or variable (which is commonly the case for larval fishes; Bochdansky et al. 2005). Interestingly, lowered SMR in fishes is correlated with an increased FAS (Cutts et al. 2002), and therefore a reduced SMR could be beneficial for larvae since it lowers the ‘floor’ on the bounds of aerobic metabolism and allows greater room for the simultaneous performance of important physiological functions. However, additional work is required to specifically examine individual variability in the aerobic scope of young fishes, and whether a decreased SMR and/or increased metabolic scope are critical factors determining which young fishes are among the small number that reach adulthood.
In conclusion, our results provide further evidence that ecologists must consider variations in metabolic scaling across taxa if they are to incorporate aspects of metabolism into their work. Furthermore, since the metabolic capacity of species or individuals probably influences many ecological processes, future research should consider including data on bm or aerobic scope when extrapolating metabolic physiology to broad-scale ecosystem effects. This approach may be especially important for fishes, since the observed scaling patterns for SMR and MMR result in an extremely limited aerobic scope during early life-history stages.
Acknowledgments
We thank Dr David Schneider for his statistical advice, Dr Bill Driedzic, Dr Trish Schulte, Paula Mendonça, Alexandre Garcia, and two anonymous reviewers for comments on earlier versions of this paper, and Memorial University's Technical Services for the construction of equipment. This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery and RTI grants to J.A.B. and A.K.G., a Canada Foundation for Innovation Grant to A.K.G and a NSERC post-graduate scholarship to S.S.K. This study was conducted in accordance with the guidelines of the Canadian Council on Animal Care, and with the approval of the Institutional Animal Care Committee of Memorial University of Newfoundland.
References
- Bailey K.M, Houde E.D. Predation on eggs and larvae of marine fishes and the recruitment problem. Adv. Mar. Biol. 1989;25:1–89. [Google Scholar]
- Bishop C.M. The maximum oxygen consumption and aerobic scope of birds and mammals: getting to the heart of the matter. Proc. R. Soc. B. 1999;266:2275–2281. doi: 10.1098/rspb.1999.0919. doi:10.1098/rspb.1999.0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P.U, Pelletier D, Dutil J.-D. Does aerobic capacity set a limit on fish growth rate? Rev. Fish Sci. 1997;5:323–340. [Google Scholar]
- Bochdansky A.B, Gronkjaer P, Herra T.P, Leggett W.C. Experimental evidence for selection against fish larvae with high metabolic rates in a food limited environment. Mar. Biol. 2005;147:1413–1417. doi:10.1007/s00227-005-0036-z [Google Scholar]
- Bokma F. Evidence against universal metabolic allometry. Funct. Ecol. 2004;18:184–187. doi:10.1111/j.0269-8463.2004.00817.x [Google Scholar]
- Brett J.R, Glass N.R. Metabolic rates and critical swim speeds of sockeye salmon (Oncorhynchus nerka) J. Fish. Res. B. Can. 1973;30:379–387. [Google Scholar]
- Brown J.H, Gillooly J.F, Allen A.P, Savage V.M, West G.B. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- Clark A, Johnston N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999;68:893–905. doi:10.1046/j.1365-2656.1999.00337.x [Google Scholar]
- Cutts C.J, Metcalfe N.B, Taylor A.C. Juvenile Atlantic Salmon (Salmo salar) with relatively high standard metabolic rates have small metabolic scopes. Funct. Ecol. 2002;16:73–78. doi:10.1046/j.0269-8463.2001.00603.x [Google Scholar]
- Djawdan M, Rose M.R, Bradley T.J. Does selection for stress resistance lower metabolic rates? Ecology. 1997;78:828–837. doi:10.2307/2266062 [Google Scholar]
- Dodds P.S, Rothman D.H, Weitz J.S. Re-examination of the ‘3/4’ law of metabolism. J. Theor. Biol. 2001;209:9–27. doi: 10.1006/jtbi.2000.2238. doi:10.1006/jtbi.2000.2238 [DOI] [PubMed] [Google Scholar]
- Ernest S.K.M, et al. Thermodynamic and metabolic effects on the scaling of production and population energy use. Ecol. Lett. 2003;6:990–995. doi:10.1046/j.1461-0248.2003.00526.x [Google Scholar]
- Enquist B.J, Economo E.P, Huxman T.E, Allen A.P, Ignace D.D, Gillooly J.F. Scaling metabolism from organisms to ecosystems. Nature. 2003;423:639–642. doi: 10.1038/nature01671. doi:10.1038/nature01671 [DOI] [PubMed] [Google Scholar]
- Forstner H, Hinterleitner S, Mahr K, Weiser W. Towards a better definition of “metamorphosis” in Coregonus sp.: biochemical, histological, and physiological data. Can. J. Fish. Aquat. Sci. 1983;40:1224–1232. [Google Scholar]
- Giguere L.A, Cote B, St-Pierre J.-F. Metabolic rates scale isometrically in larval fishes. Mar. Ecol. Prog. Ser. 1988;50:13–19. [Google Scholar]
- Glazier D.S. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. 2005;80:611–662. doi: 10.1017/S1464793105006834. doi:10.1017/S1464793105006834 [DOI] [PubMed] [Google Scholar]
- Godin J.-G.J, Crossman S.L. Hunger-dependent predator inspection and foraging behaviors in the threespine stickleback (Gasterosteus aculeatus) under predation risk. Behav. Ecol. Sociobiol. 1994;34:359–366. [Google Scholar]
- Godin J.-G.J, Sproul C.D. Risk taking in parasitized sticklebacks under threat of predation: effects of energetic need and food availability. Can. J. Zool. 1988;66:2360–2367. [Google Scholar]
- Goolish E.M. Aerobic and anaerobic scaling in fish. Biol. Rev. 1991;66:33–56. [Google Scholar]
- Jobling M. Towards an explanation of specific dynamic action (SDA) J. Fish Biol. 1983;23:549–555. [Google Scholar]
- Kamler E. Ontogeny of yolk-feeding fish: an ecological perspective. Rev. Fish Biol. Fish. 1992;12:79–103. doi:10.1023/A:1022603204337 [Google Scholar]
- Killen S.S, Brown J.A. Energetic cost of reduced foraging under predation threat in newly hatched ocean pout. Mar. Ecol. Prog. Ser. 2006;321:255–266. [Google Scholar]
- Lee C.G, Farrell A.P, Lotto A, Hinch S.G, Healey M.C. Excess post-exercise oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon following critical speed swimming. J. Exp. Biol. 2003;206:3253–3260. doi: 10.1242/jeb.00548. doi:10.1242/jeb.00548 [DOI] [PubMed] [Google Scholar]
- Martin R.D, Genoud M, Hemelrijk C.K. Problems of allometric scaling analysis: examples from mammalian reproductive biology. J. Exp. Biol. 2005;208:1731–1747. doi: 10.1242/jeb.01566. doi:10.1242/jeb.01566 [DOI] [PubMed] [Google Scholar]
- Methven D.M, Brown J.A. Time of hatching affects development, size, yolk volume, and mortality of newly hatched Macrozoarces americanus (Pisces: Zoarcidae) Can. J. Zool. 1991;69:2161–2167. [Google Scholar]
- Oikawa S, Itazawa Y, Gotoh M. Ontogenetic change in the relationship between metabolic rate and body mass in a sea bream Pagrus major (Temminck & Schlegel) J. Fish Biol. 1991;38:483–496. doi:10.1111/j.1095-8649.1991.tb03136.x [Google Scholar]
- Post J.R, Lee J.A. Metabolic ontogeny of teleost fishes. Can. J. Fish. Aquat. Sci. 1996;53:910–923. doi:10.1139/cjfas-53-4-910 [Google Scholar]
- Priede, I. G. 1985 Metabolic scope in fishes Fish energetics: new perspectives (ed. P. Tytler & P. Calow), pp. 33–64. Baltimore, MD: Johns Hopkins University Press.
- Reidy S.P, Nelson J.A, Tang Y, Kerr S.R. Post-exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J. Fish Biol. 1995;47:377–386. doi:10.1111/j.1095-8649.1995.tb01907.x [Google Scholar]
- Rodnick K.J, Gamperl A.K, Lizars K.R, Bennett M.T, Rausch R.N, Keeley E.R. Thermal tolerance and metabolic physiology among redband trout populations in south-eastern Oregon. J. Fish Biol. 2004;64:310–335. doi:10.1111/j.0022-1112.2004.00292.x [Google Scholar]
- Savage V.M, Gillooly J.F, Woodruff W.H, West G.B, Allen A.P, Enquist B.J, Brown J.H. The predominance of quarter-power scaling in biology. Funct. Ecol. 2004;18:257–282. doi:10.1111/j.0269-8463.2004.00856.x [Google Scholar]
- Tucker V.A. The energetic cost of moving about. Am. Sci. 1975;63:413–419. [PubMed] [Google Scholar]
- Weibel E.R, Bacigalupe L.D, Schmitt B, Hoppeler H. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respir. Physiol. Neurobiol. 2004;140:115–132. doi: 10.1016/j.resp.2004.01.006. doi:10.1016/j.resp.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Weiser W. Developmental and metabolic constraints of the scope for activity in young rainbow trout (Salmo gairdneri) J. Exp. Biol. 1985;118:133–142. [Google Scholar]
- Weiser W. Limitations of energy acquisition and energy use in small poikilotherms: evolutionary implications. Funct. Ecol. 1991;5:234–240. doi:10.2307/2389261 [Google Scholar]
- Weiser W. The energetics of fish larvae, the smallest vertebrates. Acta Physiol. Scan. 1995;154:279–290. doi: 10.1111/j.1748-1716.1995.tb09912.x. [DOI] [PubMed] [Google Scholar]
- West G.B, Brown J.H. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 2005;208:1575–1592. doi: 10.1242/jeb.01589. doi:10.1242/jeb.01589 [DOI] [PubMed] [Google Scholar]
- White C.R, Seymour R.S. Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl Acad. Sci. USA. 2003;100:4046–4049. doi: 10.1073/pnas.0436428100. doi:10.1073/pnas.0436428100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C.R, Seymour R.S. Allometric scaling of mammalian metabolism. J. Exp. Biol. 2005;208:1611–1619. doi: 10.1242/jeb.01501. doi:10.1242/jeb.01501 [DOI] [PubMed] [Google Scholar]
- White C.R, Phillips N.F, Seymour R.S. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2006;2:125–127. doi: 10.1098/rsbl.2005.0378. doi:10.1098/rsbl.2005.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward G, Ebenman B, Emmerson M, Montoya J.M, Olesen J.M, Valido A, Warren P.H. Body size in ecological networks. Trends Ecol. Evol. 2005;20:403–409. doi: 10.1016/j.tree.2005.04.005. doi:10.1016/j.tree.2005.04.005 [DOI] [PubMed] [Google Scholar]