Abstract
Wild chimpanzees (Pan troglodytes) have a rich cultural repertoire—traditions common in some communities are not present in others. The majority of reports describe functional, material traditions, such as tool use. Arbitrary conventions have received far less attention. In the same way that observations of material culture in wild apes led to experiments to confirm social transmission and identify underlying learning mechanisms, experiments investigating how arbitrary habits or conventions arise and spread within a group are also required. The few relevant experimental studies reported thus far have relied on cross-species (i.e. human–ape) interaction offering limited ecological validity, and no study has successfully generated a tradition not involving tool use in an established group. We seeded one of two rewarded alternative endpoints to a complex sequence of behaviour in each of two chimpanzee groups. Each sequence spread in the group in which it was seeded, with many individuals unambiguously adopting the sequence demonstrated by a group member. In one group, the alternative sequence was discovered by a low ranking female, but was not learned by others. Since the action-sequences lacked meaning before the experiment and had no logical connection with reward, chimpanzees must have extracted both the form and benefits of these sequences through observation of others.
Keywords: social learning, tradition, culture, primates
1. Introduction
The social transmission of interaction patterns not involving a tool or foraging technique among chimpanzees (Pan troglodytes) was first documented by McGrew & Tutin (1978), who observed a customary hand-clasp grooming posture in one wild community that was absent in another 160 km away. When the same grooming posture originated spontaneously in a captive chimpanzee group, it was found to spread gradually along affiliative lines, consistent with transmission through social learning (de Waal & Seres 1997; Bonnie & de Waal 2006).
Social learning may also explain why communication signals appear to carry different meanings in different communities. We now know that variation in handclasp grooming goes beyond the mere presence or absence of this posture: neighbouring communities sometimes vary in the details of the posture (Nakamura 2002). Chimpanzees are also known to orally tear apart dry leaves, a noise-making action that serves as a solicitation for play in one community yet as sexual courtship in another (Nishida 1980; Boesch 1996). The actions themselves are arbitrary; the significance of the behaviour is defined only by the individuals within the group to create a convention. In each community, members know the signal's local meaning, reacting to it in a unique but predictable manner. Tool use traditions are quite different, because they are characterized by a functional, goal-oriented task with foreseeable outcomes; for example, nuts must be cracked to be eaten.
Compared to studies of tool use and foraging traditions, arbitrary conventions such as leaf clipping have received far less attention (de Waal 2001) despite growing evidence for non-subsistence behavioural variants in both monkeys and apes (Huffman 1984; de Waal 1989; Hirata et al. 1998; Nakamura et al. 2000; Whiten et al. 2001; Perry et al. 2003a,b). Sapolsky & Share (2004) even report an entire pacific social culture in wild baboons (Papio anubis). In the same way that observations of material culture in wild apes (e.g. Whiten et al. 1999; van Schaik et al. 2003; Lonsdorf et al. 2004) led to experimental approaches in captivity to confirm the putative social transmission and identify underlying learning mechanisms (e.g. Whiten et al. 1996; Hirata & Morimura 2000; Celli et al. 2001; Horner & Whiten 2005; Whiten et al. 2005; Horner et al. 2006), experiments are also required to determine how habits, arbitrary conventions or signals develop and are learned within a group. Thus far, such experiments have included the transmission of conflict resolution (de Waal & Johanowicz 1993) as well as human-induced mimicking of arbitrary gestures, body movements and actions on objects (Tomasello et al. 1993; Custance et al. 1995; Myowa-Yamakoshi & Matsuzawa 1999; Call 2001; Bjorklund et al. 2002). However, all of these studies have relied on cross-species interaction, making for limited relevance to conventions within primate communities.
In the only previous study of its kind, when one chimpanzee was trained to employ arbitrary gestures to gain food from a human, the behaviour failed to spread to other group members (Tomasello et al. 1997). This was a relatively short, single attempt, but the authors took their negative finding to mean that non-human species probably lack the capacity to observationally learn the significance of arbitrary actions, a capacity considered fundamental to human culture.
Here, we describe a new approach that demonstrates this capacity experimentally in chimpanzees. We achieved this by seeding in two ape groups two different conventions concerning a sequence of actions using exactly the same objects, making any spread of such actions clear and unambiguous. The alternative sequences involved collecting, transporting and depositing plastic tokens into either a bucket receptacle or a pipe receptacle in order to gain a food reward from a separate, unrelated location. Since the objects involved in the action sequence (tokens and receptacles) had no logical, causal connection to a food reward, this experiment addresses the question of whether chimpanzees can socially learn arbitrary, non-tool conventions. Furthermore, including two alternative endpoints (bucket versus pipe) to the same procedure within a group diffusion paradigm (Whiten et al. 2005) allowed us to control for individual learning, because both groups had the opportunity to discover both action sequences and would be duly rewarded for either. Any behavioural differences could thus be ascribed only to ape-to-ape transmission.
2. Material and methods
(a) Subjects
Subjects were two groups of chimpanzees (FS1 and FS2) housed at the field station of the Yerkes National Primate Research Center, Atlanta, Georgia. The FS1 group comprised 15 individuals (two adult males), aged from 8 to 41 years. The FS2 group comprised 14 individuals (three adult males), aged from 7 to 38 years. Additional information about the subjects in each group can be found in electronic supplementary material. Each group has access to indoor areas and large outdoor compounds (700 and 520 m2, respectively). Subjects have ad libitum access to chow and water, and are provided with fresh fruits and vegetables twice daily.
A pilot investigation conducted 1 year prior to the present study showed that FS1 chimpanzees could learn to throw a plastic toy in the air, once the significance of this arbitrary behaviour was demonstrated by a juvenile. The present study employed a more systematic methodology and used high ranking adult females as models. These models were chosen based on their willingness to cooperate with experimenters and participation as models in a previous study (Whiten et al. 2005).
(b) Materials
We provided each group with ‘tokens’ constructed from PVC tube (length=10 cm; diameter=4 cm) painted orange. Approximately 25 tokens were available to the chimpanzees for each session.
Two receptacles, a ‘bucket’ and ‘pipe’, were available to both groups throughout testing. The bucket (20×34×61 cm) was a plastic bin modified for the testing purposes. A diamond shaped hole (7.5×7.5 cm) was cut into the side of the bucket at a height of 45 cm. The bucket's lid and an opaque barrier inside the bucket (added during the baseline phase) prevented subjects from seeing any objects within. The bucket sat on the ground and was secured to the mesh wall of the subjects' outdoor compound. The pipe was a larger PVC pipe (length=85 cm; diameter=7.5 cm) angled downward from 90 cm high into a covered opaque container. The pipe was secured to the mesh, approximately 60 cm from the bucket. Both receptacles were available to subjects only during the testing (see figure 1 for an overview of the experimental set-up).
Figure 1.
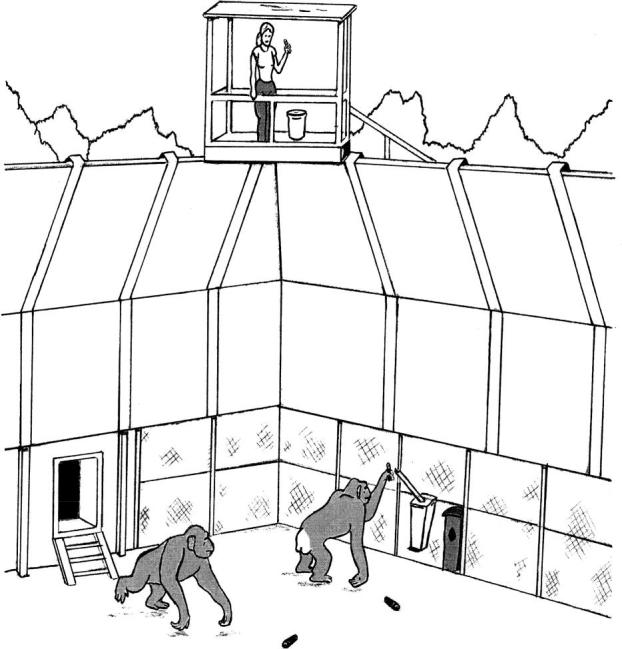
Illustration of the experimental set-up in the chimpanzee compounds at the field station of the Yerkes National Primate Research Center. Chimpanzees are rewarded from the observation tower for depositing tokens into either of two receptacles.
(c) Procedure
Testing involved four phases—habituation, baseline, model training and transmission. With the exception of the training phase in which the model was temporarily isolated from her group, all subjects could freely access both the indoor and outdoor areas of their enclosures. To avoid biasing the attention of subjects towards the apparatus, the experimenter observed the group from an observation tower at one corner of the compound (figure 1).
(i) Habituation
Chimpanzees often show neophobic responses to novel objects, so subjects were exposed to the apparatus prior to starting the experiment. PVC tubes are commonly given as enrichment, so only the bucket and the pipe were included in this phase. For 72 consecutive hours, the receptacles were secured to the mesh and the chimpanzees allowed to interact with them freely. Chimpanzees exhibited no adverse responses to the apparatus during informal observations conducted in this phase.
(ii) Baseline phase
Baseline sessions were conducted to check if chimpanzees would spontaneously deposit tokens in the receptacles prior to observing a model demonstrate the task, and to gather baseline data on the chimpanzees' interactions with the tokens and apparatus. Six sessions were conducted in each social group.
Just prior to each session, the bucket and pipe were secured to the mesh of the compound. Each session lasted for 30 min and began when tokens were thrown from the observation tower into the compound. At the end of the session, the receptacles were removed. We made no attempt to collect tokens not deposited in a receptacle, but many were collected by care-staff during regular husbandry procedures, and put aside until the next session took place. Collected tokens were thrown back into the compound at the start of each session, ensuring that 25 tokens were always available.
(iii) Model training
A high-ranking adult female from each group was selected as a model. Models were rewarded for depositing tokens into one of the receptacles (FS1: GG—bucket, FS2: ER—pipe). Training took place in the indoor area, where the model could be temporarily isolated from her group. Training lasted several days and continued until models would walk approximately 10 m to find tokens and deposit them in only one receptacle when both were available to them.
To check that the model would continue to perform the trained behaviour while in the compound, we released her while the other chimpanzees in her group were kept indoors. This proved to be an uneasy situation for both the models, so we allowed one additional individual (GG's daughter LZ in FS1 and ER's daughter JA in FS2) outdoors. LZ and JA were distracted by a second experimenter and did not interact with the apparatus during this phase. Within two training sessions of this type, both GG and ER deposited multiple tokens as trained.
(iv) Transmission phase
In the transmission phase (20 sessions), subjects were able to observe the model being rewarded for depositing tokens into a receptacle. All subjects, including the model, were rewarded for depositing tokens in either receptacle, such that both alternatives were equally profitable. As with the baseline phase, each session lasted 30 min (except for the first session, which was 60 min) and began when tokens were thrown by an experimenter into the outdoor compound.
To obtain a reward, chimpanzees needed to complete the following behavioural sequence: (i) look for a token similar to that handled by the model (i.e. requiring generalization from the original token to all tokens), (ii) collect the token, (iii) transport the token towards the containers (usually 10 m or more), (iv) deposit the token into a container, and (v) turn and look up at the tower to catch the reward, which the experimenter would throw into the actor's hands or close by.
Whenever a token was deposited, one-sixth apple or one-quarter banana was thrown to the appropriate chimpanzee. If the chimpanzee was not able to keep the reward (as a result of a poor throw or theft by another chimpanzee), this was noted, and a second reward was thrown. At the end of each session, the receptacles were removed.
After 12 sessions per group, it became apparent that two individuals in each group, the original models plus two others (PE and ST), were able to monopolize the majority of the tokens or the receptacles. It seemed that these two individuals might be preventing others from collecting and depositing tokens. For the next three sessions (13–15), these individuals (and PE's dependant daughter AZ) were kept indoors and could not participate. This manipulation did not leave either group without a model, as by this point at least three other individuals had learned the task. During sessions 16–18, all subjects were able to participate. For sessions 19 and 20, these same individuals plus two others in each group (LZ, SK, KE and AM) were again kept indoors.
(d) Data collection and analyses
Scan sampling was used to record the location of each subject (indoors or outdoors) and token handling at 3 min intervals throughout sessions. In addition, all token deposits were recorded. Data were collected via an audiocassette recorder by an experimenter located in the observation tower at each compound. A video camera located on the ground recorded activity around the receptacles, and was used to confirm the occurrence of token deposits and the identity of the chimpanzee involved. The video thus ensured reliability in data collection.
We used non-parametric statistics to compare the two groups in terms of the total of bucket and pipe users in each, using a two-tailed Fisher's exact test.
3. Results
(a) Baseline phase
Prior to training a model, we conducted a baseline phase in which both the groups were provided with approximately 25 tokens and both receptacles, and their activities were then monitored over six sessions for a total of 3 h. No rewards were given during this phase. Every 3 min, the location of each individual was recorded, along with any occurrences of token interactions (pick-up, deposit, etc.). Thirteen out of 15 individuals in the FS1 group and 10 out of 14 individual in the FS2 group were observed interacting with a token at some point, but the overall level of token interactions was low and declined across sessions.
During the first baseline session in the FS2 group, KE, a young adult female, deposited a token into the bucket. Later, during the same session, VR and DA also deposited tokens into the bucket after first looking inside, hence seeing the original token deposited by KE. An opaque barrier was then added to the receptacles so that tokens disappeared from view as soon as they were deposited. In subsequent baseline sessions, we observed no further interest in the receptacles, and no further depositions of tokens.
Data from the baseline phase provided evidence that, with the exception of KE, VR and DA, the tokens and receptacles were not combined in a meaningful way. Thus, we proceeded to determine if, after observing a group member obtain a reward by depositing a token into one of the receptacles, others would learn to do so. Because KE, VR and DA deposited tokens in the bucket, the model in their group was trained to use the alternative sequence; deposit tokens in the pipe.
(b) Transmission phase
In 20 sessions (a total of 10.5 h), nine chimpanzees in the FS1 group and six in the FS2 group deposited at least one token in a receptacle and were rewarded for doing so (figure 2). Both models deposited tokens only in the trained receptacle. In the FS1 group, tokens were deposited exclusively in the bucket, the receptacle used by the model in this group, yet never observed during baseline. In the FS2 group, all but one chimpanzee (DA) deposited tokens in the pipe (figure 2; Fisher's exact test comparing both groups, two-tailed, p=0.002). Of those individuals not observed to deposit a token in either the bucket or the pipe (5 in FS1 and 7 in FS2), all but two had been seen holding a token at least once during the transmission phase. Moreover, failure to learn the task was not owing to lack of opportunity for observation, as there were no significant differences in either group in the proportion of total scan samples in which individuals were observed outdoors between token users and non-learners (two-tailed Mann–Whitney U-test, NS).
Figure 2.
Differential spread of the bucket and pipe conventions in two groups of chimpanzees. (a) FS1 group and (b) FS2 group. Grey circles indicate≥1 deposit to bucket and black circles indicate≥1 deposit to pipe. Numbers within the circle indicate tokens deposited. Horizontal bars indicate that no deposit was made during that session; X indicates that the individual was kept indoors. Individuals are listed, from bottom to top, in the order of first deposit. Individuals GG and ER were the trained models.
4. Discussion
This study demonstrates that chimpanzees are capable of duplicating a modelled sequence of initially arbitrary actions with familiar objects with sufficient fidelity to sustain different conventions. The two-option design of the experiment allowed us to control for individual learning, because both groups had the opportunity to learn both sequences and were rewarded for performing either. This design makes it unlikely that all individuals in a single group would discover one receptacle, but not the other. Moreover, data collected during the baseline phase show that chimpanzees were unlikely to develop the differences in behaviour observed between the two groups through individual experience. Any behavioural differences can thus be ascribed to ape-to-ape transmission, probably based on an evaluation of the significance of the sequence of actions by successful performers, generalization from tokens used by them to similar tokens, and stimulus enhancement, such as being drawn to the receptacle favoured by others.
Our results lend support to the idea that the significance of certain object-directed behaviours in wild chimpanzees is socially learned (Boesch 1996, 2003). The result contrasts with an earlier failure of chimpanzees to learn arbitrary gestures from each other (Tomasello et al. 1997). Differences in experimental design between the present study and that by Tomasello et al., including the behaviours rewarded and the location of the experimenter during data collection, may have contributed to the contrasting findings.
Our study further shows unprecedented fidelity in experimentally seeded alternative traditions in a non-human primate. Although one female in FS2 (DA) consistently deposited tokens in the ‘wrong’ receptacle, the bucket, and was rewarded for doing so, no other member of her group adopted this particular sequence. This provides further evidence consistent with the conformity bias documented among the same chimpanzees in a previous study on foraging techniques (Whiten et al. 2005). However, it is also consistent with theories that high-status individuals are preferred as a source of information (Henrich & Gil-White 2001; Laland 2004) because DA is among the lowest ranking females in FS2, whereas the group's model, ER, is its alpha female.
Although the majority of individuals in each group interacted with tokens during the baseline phase, and were in a position to observe one or more group members being rewarded for depositing a token in a receptacle during the transmission phase, a number of individuals (n=5 and 7 in FS1 and FS2, respectively) did not learn either tradition. It is possible that social factors, such as dominance, may explain these differences. Future research could be directed at the social factors, which support or inhibit acquisition of traditions within a group.
Acknowledgments
This project was conducted at the Yerkes National Primate Research Center's field station, supported by the National Institutes of Health (RR-00165) and the Living Links Center of Emory University. Additional support was provided by the National Science Foundation (K.B.), BBSRC (A.W., V.H.) and the Leverhulme Trust (A.W.). We are grateful to Devyn Carter, Marietta Dindo and Deborah Moore for logistical support, and to Anna Garr and Jennifer Rybak for contributions to the preliminary study. Two anonymous reviewers provided helpful comments on a previous draft. Figure 1 was drawn by Devyn Carter. Yerkes is fully accredited by the American Association for Accreditation for Laboratory Animal Care.
Supplementary Material
Table showing the identification code, sex and age (in 2005) of chimpanzees in two social groups at the Field Station of the Yerkes National Primate Research Center, Atlanta, GA.
References
- Bjorklund D.F, Yunger J.L, Bering J.M, Ragan P. The generalization of deferred imitation in encultured chimpanzees (Pan troglodytes) Anim. Cogn. 2002;5:49–58. doi: 10.1007/s10071-001-0124-5. [DOI] [PubMed] [Google Scholar]
- Boesch C. Three approaches for assessing chimpanzee culture. In: Russon A.E, Bard K.A, Parker S.T, editors. Reaching into thought: the minds of the great apes. Cambridge University Press; Cambridge, UK: 1996. pp. 404–429. [Google Scholar]
- Boesch C. Is culture a golden barrier between human and chimpanzee? Evol. Anthropol. 2003;12:82–91. doi:10.1002/evan.10106 [Google Scholar]
- Bonnie K.E, de Waal F.B.M. Affiliation promotes the transmission of a social custom: handclasp grooming among captive chimpanzees. Primates. 2006;47:27–34. doi: 10.1007/s10329-005-0141-0. doi:10.1007/s10329-005-0141-0 [DOI] [PubMed] [Google Scholar]
- Call J. Body imitation in an enculturated orangutan (Pongo pygmaeus) Cybern. Syst. 2001;32:97–119. doi:10.1080/019697201300001821 [Google Scholar]
- Celli M.L, Tomonaga M, Udono T, Teramoto M, Nagano K. Learning processes in the acquisition of a tool using task by captive chimpanzees. Psychologia. 2001;44:70–81. [Google Scholar]
- Custance D.A, Whiten A, Bard K.A. Can young chimpanzees imitate arbitrary actions? Hayes and Hayes (1952) revisited. Behaviour. 1995;132:839–858. [Google Scholar]
- de Waal F.B.M. Harvard University Press; Cambridge, MA: 1989. Peacemaking among primates. [Google Scholar]
- de Waal F.B.M. Basic Books; New York, NY: 2001. The ape and the sushi master. [Google Scholar]
- de Waal F.B.M, Johanowicz D.L. Modification of reconciliation behavior through social experience: an experiment with two macaque species. Child Dev. 1993;64:897–908. [PubMed] [Google Scholar]
- de Waal F.B.M, Seres M. Propagation of handclasp grooming among captive chimpanzees. Am. J. Primatol. 1997;43:339–346. doi: 10.1002/(SICI)1098-2345(1997)43:4<339::AID-AJP5>3.0.CO;2-Y. doi:10.1002/(SICI)1098-2345(1997)43:4<339::AID-AJP5>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- Henrich J, Gil-White F.J. The evolution of prestige: freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evol. Hum. Behav. 2001;22:165–196. doi: 10.1016/s1090-5138(00)00071-4. doi:10.1016/S1090-5138(00)00071-4 [DOI] [PubMed] [Google Scholar]
- Hirata S, Morimura N. Naive chimpanzees' (Pan troglodytes) observation of experienced conspecifics in a tool-using task. J. Comp. Psychol. 2000;114:291–296. doi: 10.1037/0735-7036.114.3.291. doi:10.1037/0735-7036.114.3.291 [DOI] [PubMed] [Google Scholar]
- Hirata S, Myowa M, Matsuzawa T. Use of leaves as cushions to sit on wet ground by wild chimpanzees. Am. J. Primatol. 1998;44:215–220. doi: 10.1002/(SICI)1098-2345(1998)44:3<215::AID-AJP4>3.0.CO;2-Z. doi:10.1002/(SICI)1098-2345(1998)44:3<215::AID-AJP4>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Horner V, Whiten A. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens) Anim. Cogn. 2005;8:164–181. doi: 10.1007/s10071-004-0239-6. doi:10.1007/s10071-004-0239-6 [DOI] [PubMed] [Google Scholar]
- Horner V, Whiten A, Flynn E, de Waal F.B.M. Faithful copying of foraging techniques along cultural transmission chains by chimpanzees and children. Proc. Natl Acad. Sci. USA. 2006;103:13 878–13 883. doi: 10.1073/pnas.0606015103. doi:10.1073/pnas.0606015103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman M.A. Stone-play of Macaca fuscata in Arashiyama B troop: transmission of a non-adaptive behavior. J. Hum. Evol. 1984;13:725–735. doi:10.1016/S0047-2484(84)80022-6 [Google Scholar]
- Laland K.N. Social learning strategies. Learn. Behav. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Lonsdorf E.V, Eberly L.E, Pusey A.E. Sex differences in learning in chimpanzees. Nature. 2004;428:715–716. doi: 10.1038/428715a. doi:10.1038/428715a [DOI] [PubMed] [Google Scholar]
- McGrew W.C, Tutin C.E.G. Evidence for a social custom in wild chimpanzees. Man. 1978;13:234–251. doi:10.2307/2800247 [Google Scholar]
- Myowa-Yamakoshi M, Matsuzawa T. Factors influencing imitation of manipulatory actions in chimpanzees (Pan troglodytes) J. Comp. Psychol. 1999;113:128–136. doi: 10.1037/0735-7036.113.2.128. doi:10.1037/0735-7036.113.2.128 [DOI] [PubMed] [Google Scholar]
- Nakamura M. Grooming-hand-clasp in Mahale M group chimpanzees: implications for culture in social behaviours. In: Boesch C, Hohmann G, Marchant L.F, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge University Press; Cambridge, UK: 2002. pp. 71–83. [Google Scholar]
- Nakamura M, McGrew W.C, Marchant L.F, Nishida T. Social scratch: another custom in wild chimpanzees? Primates. 2000;41:237–248. doi: 10.1007/BF02557594. [DOI] [PubMed] [Google Scholar]
- Nishida T. The leaf-clipping display: a newly discovered expressive gesture in wild chimpanzees. J. Hum. Evol. 1980;9:117–128. doi:10.1016/0047-2484(80)90068-8 [Google Scholar]
- Perry S, et al. Social conventions in wild white-faced capuchin monkeys: evidence for traditions in a neotropical primate. Curr. Anthropol. 2003;44:241–268. doi:10.1086/345825 [Google Scholar]
- Perry S, et al. Traditions in wild white-faced capuchin monkeys. In: Fragaszy D, Perry S, et al., editors. The biology of traditions. Cambridge University Press; Cambridge, UK: 2003b. pp. 391–425. [Google Scholar]
- Sapolsky R.M, Share L.J. A pacific culture among wild baboons: its emergence and transmission. PLoS Biol. 2004;2:534–541. doi: 10.1371/journal.pbio.0020106. doi:10.1371/journal.pbio.0020106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Savage-Rumbaugh S, Kruger A.C. Imitative learning of actions on objects by children, chimpanzees, and enculturated chimpanzees. Child Dev. 1993;64:1688–1705. doi:10.2307/1131463 [PubMed] [Google Scholar]
- Tomasello M, Call J, Warren J, Frost G.T, Carpenter M, Nagell K. The ontogeny of chimpanzee gestural signals: a comparison across groups and generations. Evol. Commun. 1997;1:223–259. [Google Scholar]
- van Schaik C, Ancrenaz M, Borgen G, Galdikas B, Knott C.D, Singleton I, Suzuki A, Utami S.S, Merrill M. Orangutan cultures and the evolution of material cultures. Science. 2003;299:102–105. doi: 10.1126/science.1078004. doi:10.1126/science.1078004 [DOI] [PubMed] [Google Scholar]
- Whiten A, Custance D.A, Gomez J.-C, Texidor P, Bard K.A. Imitative learning of artificial fruit processing in children (Homo sapiens) and chimpanzees (Pan troglodytes) J. Comp. Psychol. 1996;110:3–14. doi: 10.1037/0735-7036.110.1.3. doi:10.1037/0735-7036.110.1.3 [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew W.C, Nishida T, Reynolds V, Sugiyama Y, Tutin C.E.G, Wrangham R.W, Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. doi:10.1038/21415 [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew W.C, Nishida T, Reynolds V, Sugiyama Y, Tutin C.E.G, Wrangham R.W, Boesch C. Charting cultural variation in chimpanzees. Behaviour. 2001;138:1481–1516. doi:10.1163/156853901317367717 [Google Scholar]
- Whiten A, Horner V, de Waal F.B.M. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. doi:10.1038/nature04047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table showing the identification code, sex and age (in 2005) of chimpanzees in two social groups at the Field Station of the Yerkes National Primate Research Center, Atlanta, GA.



