Abstract
Theoreticians predict that animal ‘personality’ traits may be maladaptive if fixed throughout different contexts, so the present study aimed to test whether these traits are fixed or plastic. Rainbow trout (Onchorhyncus mykiss) were given emboldening or negative experiences in the forms of watching bold or shy individuals responding to novelty or winning or losing fights to examine whether prior experience affected boldness. Bold individuals that lost fights or watched shy demonstrators became more shy by increasing their latency to approach a novel object, whereas shy observers that watched bold demonstrators remained cautious and did not modify their responses to novelty. Shy winners became bolder and decreased their latency to approach a novel object, but shy losers also displayed this shift. In comparison, control groups showed no change in behaviour. Bold fishes given negative experiences reduced their boldness which may be an adaptive response; however, shy fishes may base their strategic decisions upon self-assessment of their relative competitive ability and increase their boldness in situations where getting to resources more quickly ensures they outcompete better competitors.
Keywords: individuality, social status, novelty, prior experience, Salmonidae
1. Introduction
‘Personality type’ is thought to be a major factor influencing an individual's behavioural variation and has been observed in many species including humans (Wilson et al. 1994), mammals (Spoolder et al. 1996; Armitage & Van Vuren 2003; Svartberg et al. 2005), birds (Carere et al. 2005), reptiles (Lopez et al. 2005) and fishes (Sneddon 2003; Bell 2005; Yoshida et al. 2005). Individual differences, often referred to as the bold–shy continuum or degree of boldness, can have a profound effect upon the decisions made by animals in unpredictable environments. Therefore, ‘personality type’ may be a strong driving force in the evolution of populations because individual variation may have a bearing upon survival of a given individual and ultimately its fitness (Sgoifo et al. 2005). Studies have shown that the degree of boldness displayed by an individual is consistently expressed across a range of behavioural tests with bold individuals being more active, taking more risks, learning more quickly (Sneddon 2003), and higher aggression compared with shy individuals performing who show relatively little activity, take few risks, learn at a slower rate and are usually socially subordinate to bold individuals (Wilson et al. 1993; Koolhaas et al. 1999; Carere et al. 2005). Such consistent differences in personality traits must both have an equal pay-off to persist in natural populations.
However, while one trait may be adaptive in one context, it may be non-adaptive in another. Theoreticians have suggested that behavioural output should be dynamic conditional upon context- and state-dependent differences (Dall et al. 2004). For example, when predation risk is high, it would pay for an animal to reduce foraging rates; however, if energy reserves are low, it may be worth the risk of obtaining food which has increased value in the animal's low-energy state. If these personalities are fixed, then the higher exploratory nature of bold individuals would be adaptive in a low-risk environment, but maladaptive in a high-risk situation. This phenotypic plasticity may be adaptive within populations (Ernande & Dieckmann 2004) and theoreticians are demanding more empirical evidence to improve predictions about how individual variation within populations affects their ecology and evolution (Dall et al. 2004; Neff & Sherman 2004; Sih et al. 2004). Therefore, it is crucial to know whether these behavioural traits are fixed or dynamic and vary according to local circumstances or experience.
Social learning or eavesdropping is well documented within the animal kingdom, whereby individuals gain important information about their environment by observing the behaviour of conspecifics (Katz & Lachlan 2003; Stoinski & Whiten 2003; Schuster et al. 2006). Social transmission of information is important for individuals' fitness within populations as by acquiring beneficial information from simply observing a conspecific reduces the costs paid by learning first hand (Brown & Laland 2003). Studies on Arctic charr, Salvelinus alpinus, demonstrated that individuals with no prior experience of a predator displayed few anti-predator responses; however, when placed in a group with predator-experienced demonstrators, naive individuals displayed more anti-predator responses (Vilhunen et al. 2005). Further studies using conditioning have revealed that bolder individuals are faster learners than their shy conspecifics, but it is not known whether observing personality types has an affect on the subsequent degree of boldness of the observer (Sneddon 2003).
The outcome of an interaction with a conspecific, i.e. the winning or losing of a fight, is known to have differential physiological consequences (Schuett et al. 1996; Hsu & Wolf 1999) and elicits behavioural changes such that winners of a contest usually go on winning and losers go on losing, i.e. the winner and loser effect (Dugatkin & Druen 2004; Dugatkin & Earley 2004; Oyegbile & Marler 2005; Hsu et al. 2006). Individuals are also shown to base their strategic decisions on previous experience (Hsu & Wolf 1999), thus showing that individuals retain information about past interactions which affects their current behaviour. Two possible mechanisms have been suggested for using past experience: the social-cue hypothesis whereby a cue is used to assess opponents and the self-assessment hypothesis where individuals assess their own ability compared to that of a conspecific (Rutte et al. 2006). Previous research has looked into the effects boldness has had on fighting outcomes (Brick & Jakobsson 2002); however, whether winning or losing affects boldness remains to be tested.
Therefore, we examined plasticity in boldness and how it is affected by immediate prior experience to demonstrate if these traits are dynamic in two different contexts. The first context involved bold and shy subjects observing a conspecific, either bold or shy, responding to both a novel foraging event and a potentially fearful event. Social and observational learning occurs in a wide range of species and important information regarding the current environmental conditions and status of individuals can be obtained by observing the behaviour of conspecifics (Caldwell & Whiten 2004; Ottoni et al. 2005; Sargeant et al. 2005; Worden & Papaj 2005). This will provide information on amount of risk present in an environment, and so it would be adaptive to alter their behaviour accordingly, i.e. display flexibility in boldness. The second set of experiments allowed bold and shy subjects a period of interaction with a conspecific producing either a positive (winning fights) or a negative (losing fights) experience, to determine whether prior experience influences behavioural performance such that individuals experiencing emboldening events become bolder and those subjected to negative experiences more fearful. This dual context paradigm allowed us to assess whether information obtained by observing had an impact upon subsequent behaviour or whether the experience of success and failure directly affected the consistency of these personality types.
2. Material and methods
Juvenile rainbow trout, Oncorhynchus mykiss, (mean 95±0.5 g weight; n=200) were obtained from a commercial fish supplier and transferred to aquaria in Liverpool. Fishes were housed in 2×2×0.5 m stock tanks for a period of two weeks allowing recovery from the stress of transport. Fishes were held in tanks with a constant flow of freshwater at 12±2°C, fully aerated, and under a 10 h : 14 h light : dark regime similar to ambient light levels. Feed consisted of commercial trout pellets given daily ad libitum. Fishes were caught at random and transferred into individual tanks (90×50×45 cm) screened from visual disturbance in another experimental room. The tanks had Perspex lids with an opaque covering providing half the tank with cover, as well as opaque screens between tanks ensuring subject isolation. Opaque vertical, plastic dividers were present in all tanks and approximately halved the tanks. These were held in place using runners and could be raised via a pulley system. These were kept in a raised position (8 cm from substrate) unless stated otherwise, so the fish had access to the whole observation tank. Fishes were left to adjust to their new environment for 7 days with temperature, lighting and feeding regimes as described.
(a) Bold–shy assessment
Low light level cameras were set up outside the screening and observations were recorded through small openings in front of and at the side of each experimental tank to give three-dimensional positioning. The cameras were relayed through a picture in picture unit, so that both images could be viewed at the same time on a single monitor positioned away from the experimental arena. During recording and post-recording video analyses, behaviours were scored on a PC using custom-designed behavioural software. This provides objective measures of strictly defined behaviours in areas such as position, movement and activity, environmental and social interaction. Initially, measuring rulers were arranged both vertically and horizontally along the sides of the tank, so distance from a novel object could be accurately recorded to 5 mm. Behaviour was recorded for 10 min, after which a novel object was dropped approximately 10 cm from the front of the fish. The subject's behaviour was recorded for a further 10 min. The novel object test is a standard paradigm used to assess boldness in humans, primates and fishes (Wilson et al. 1993). Subjects were assessed for their location along the bold–shy continuum via their willingness to come within 5 cm of a novel object. Novel objects consisted of Lego bricks constructed to produce objects of various colours, sizes and dimensions to a maximum of 10×7×4 cm, as used by Sneddon et al. (2003). Fishes that came within 5 cm were deemed bold; those that did not, shy; and those which ventured within 10 cm but not 5 cm, intermediate. Our interest remained in fishes at the extreme ends of this continuum, thus intermediate subjects were withdrawn at this point and excluded from further analysis. Out of the 110 subjects, 26 were intermediate showing that intermediate individuals correspond to approximately a quarter of the study population and hence we used the majority of fishes that we tested, which is thus a valid representation of this population.
(b) Control experiment
Bold and shy fishes (n=6 each) were held under standard tank conditions and given bold–shy assessments after the 7-day settling period. They were assessed again after a 7-day break, since the experimental treatments were conducted over a similar time period. A t-test showed no significant change in all subjects' latency to come within 5 cm of the novel object, with shy fish remaining shy (all failed to come within 10 cm) and bold remaining bold (t=0.211, p=0.85, d.f.=11) in the final assessments. Behavioural analysis included duration of activity (B: t=1.33, p=0.31; S: t=−1.28, p=0.33), frequency of entering 5 cm zone around the object (B: t=1.86, p=0.20; S: t=−1.64, p=0.89), duration within this zone (B: t=6.43, p=0.10; S: t=0.94, p=0.44), and duration of cover use (B: t=0.41, p=0.72; S: t=−0.90, p=0.46), demonstrating that all behaviours measured remained consistent over time. Therefore, this proves the consistency of boldness over the experimental period and acted as a negative control for the following experiments. Principal component analysis showed that latency to approach the novel object was the most important behavioural measure in assessing boldness (PCA: eigenvalue=2.5; KMO=0.937; p<0.001; d.f.=10) and as this is a standard paradigm used in humans, mammals and fishes (Wilson et al. 1993, 1994; Wilson 1998; Sneddon et al. 2003; Schjolden et al. 2005), the data on 5 cm latency is used as the primary indicator of boldness in the following results.
(c) Experiment 1: observing bold and shy individuals
A separate set of fishes was assessed for boldness or shyness and individuals assigned to one of four treatment groups (n=6 each): bold subjects observed bold (BB) and shy observed shy (SS) that acted as a positive control for the groups where bold observed shy (BS) and shy observed bold (SB). Tanks were divided in half using one-way mirrors slotted neatly into the middle of the tank, with the dark, covered side holding the observer which could watch the demonstrator fish in the light, uncovered side without the demonstrator being able to see the observer, and verified using video recording. Demonstrator fishes were subjected to two different novel stimuli once a day for 4 days. The novel stimuli were either novel objects presented AM or novel prey items, five red mosquito larvae presented PM. After 4 days, the demonstrator was removed and the observer was tested as follows for 3 days as this was the maximum time taken to eat novel prey for the demonstrators and observers, respectively. AM, five red mosquito larvae were dropped approximately 5 cm in front of the observer and latency to eat was determined as the first prey tasted/eaten until a maximum of 15 min. Thus, fishes which failed to taste the novel prey were given a score of 901 s, since it is possible that these prey were eaten after the recording period as uneaten food was not removed to prevent excessive disturbance. PM, fishes were given a novel object that was dropped 10 cm from the front of their nose. The novel object, as described previously, was different for each presentation to prevent habituation, since it has been shown that using novel objects of different colours and shapes continues to elicit a fear response in rainbow trout when presented consecutively (Sneddon et al. 2003). Time taken to eat the novel prey and latency to approach the novel object were recorded.
(d) Experiment 2: success and failure in fights
Another set of fishes was assessed for boldness as described previously, and individuals were then assigned to one of the four treatment groups; bold fish to win (BW), bold to lose (BL), shy to win (SW) and shy to lose (SL). Either a much larger or much smaller opponent was presented to ensure losing and winning, respectively. The tank was divided in half with an opaque divider and the opponents were placed in the opposite side; therefore, fishes were in visual and physical isolation. Fishes were given 24 h to adjust to the new conditions and then allowed to interact for 10 min daily for 7 days, by lifting the divider just above the water level using a pulley system. Rainbow trout are very aggressive and so dominance is achieved within short time periods (Winberg et al. 2001; Lepage et al. 2005); therefore, an interaction of 10 min was sufficient to obtain meaningful results and for dominance to be established. Longer time periods could result in unnecessary harm or stress. As dominant–subordinate relationships were formed quickly and owing to the ‘winner and loser effect’, 7 days of short interactions were sufficient to produce an emboldening or negative effect on the subjects to allow any behavioural change to be measured (Winberg et al. 2001; Lepage et al. 2005). Aggressive interactions were recorded to determine the winners and losers of these interactions. Individuals fought with varying levels of aggression; however, winners were those who gave a sufficiently greater number of attacks than they received and whose attacks elicited retreats over the total 7-day period. An attack was defined as a rapid charge or bite to an opponent which is displaced and a retreat was a movement more than one body length away in response to an attacking individual. Opponent fishes were removed after the seventh interaction. On the following day, bold–shy assessments were performed using an unfamiliar novel object to re-assess the degree of boldness. Latency to approach the novel object was recorded.
(e) Statistical analysis
Data were tested for normality and parametric statistics were used. In experiment 1, a two-way ANOVA was used to compare whether demonstrators and observers differed in their latency to ingest novel prey and whether latency to approach the novel object was affected over time. This test was also used to assess whether bold and shy observers differed in their latency to eat novel prey and whether the demonstrator's latency had an impact on when they commenced eating. Since in all observers, the latency to approach the novel object remained consistent over the 3-day experimental period for both the bold and the shy groups (F(1,11)=0.27, p=0.56 and F(1,11)=1.77, p=0.09, respectively), paired t-tests examined whether latency to approach differed between the boldness assessments performed before and after (mean of three values) the demonstrations within each of the four treatment groups. In experiment 2, the frequency of aggressive attacks was compared using a two-way ANOVA to test whether the four groups differed in the frequency of aggression performed and whether there was a decline in aggression over the experimental treatment. This approach was also used to test whether the factors of treatment group and pre- and post-boldness assessments affected the latency to approach a novel object. All the statistical analyses were conducted using SPSS software and two-tailed tests were used throughout.
3. Results
(a) Experiment 1: observing bold and shy individuals
There was no difference between demonstrators and observers in their latency to taste novel prey (F1,72=0.01, p=0.92), but there was a significant decrease in latency to taste over the 3 days (F2,72=4.92, p=0.01) with no interaction between these factors (F2,72=0.42, p=0.66). Bold observers were quicker to taste the novel prey than shy observers (F1,11=15.41, p<0.001), and there was an effect of whether the demonstrator ate on the first day with these observers eating the prey more quickly (F3,11=10.02, p<0.001) resulting in a significant interaction (F1,11=6.95, p=0.039) between boldness and demonstrator efficiency.
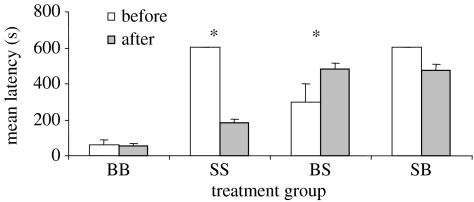
Bold individuals that observed bold (BB) remained constant in their latency to approach a novel object (t=−0.656, p=0.54; n=6); however, shy that observed shy (SS) showed a significant decrease in their latency to approach (t=3.214, p=0.03; n=6; figure 1). Previously classed bold subjects observing shy (BS) showed an increase in their latency to approach (t=−2.27, p=0.04; n=6). Shy observers watching bold (SB) demonstrators showed no change in their latency to approach (t=1.58, p=0.18; n=6; figure 1).
Figure 1.
Mean (±s.e.) latency (s) to come within 5 cm of the novel object for bold individuals observing bold demonstrators (BB), shy observing shy (SS), bold observing shy (BS) and shy observing bold (SB) before and after observing demonstrators (n=6 per group; *p<0.05).
(b) Experiment 2: success and failure in fights
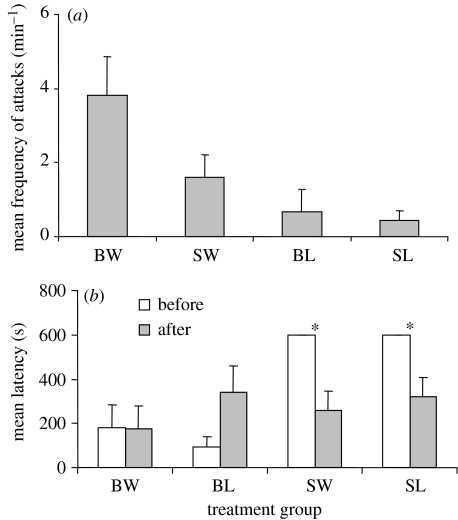
Bold winners (BW) were the most aggressive followed by shy winners (SW) with both groups of losers (BL and SL; figure 2a) performing the least aggressive attacks (F3,21=5.74, p<0.01). There was a significant decrease in frequency of attacks over the experimental period (F6,126=2.55, p=0.02) resulting in a significant interaction, where all groups declined in the amount of aggression performed over time (F18,126=2.45, p<0.01).
Figure 2.
(a) Mean (±s.e.) frequency of aggression performed by bold winners (BW), shy winners (SW), bold losers (BL) and shy losers (SL) (n=24; *p<0.05). (b) The mean (±s.e.) latency to approach a novel object before and after fight interactions for each treatment group (n=24; *p<0.05).
There was a significant difference between bold and shy latencies to approach a novel object (F3,21=5.05, p=0.01) as well as a difference between pre- and post-assessments (F1,21=6.60, p=0.02), resulting in a highly significant interaction between the group and the assessment (F3,21=9.69, p<0.001). This analysis demonstrates that both shy winners (SW) and shy losers (SL) showed a significant decrease in their latency to approach within 5 cm, whereas bold losers (BL) showed a slight increase (figure 2b). Bold winners (BW) displayed no change in their latency to come within 5 cm of the novel object.
4. Discussion
The degree of boldness in our model species, rainbow trout, was consistent over time when fishes were left isolated and unmanipulated in our control tests, yet our dual context paradigm demonstrated that previous experience had a significant impact upon boldness that differed according to the specific context. Observing other individuals has had a measurable effect upon animal behaviour and physiology in other studies (McGregor et al. 2001; Oliveira et al. 2001). Here, bold fishes observing bold demonstrators remained bold in their responsiveness to novel stimuli, whereas bold individuals observing shy became more cautious in their reaction to novelty. This provides further evidence that social learning occurs in rainbow trout with decisions being based upon conspecifics' behaviour. If such effects operate in natural populations, this plasticity seen in bold individuals may allow them to adjust their behaviour, such that they show an adaptive response when local conditions change; however, this will depend upon how others respond to such change. In contrast, shy individuals observing bold demonstrators showed no change in their sensitivity to novelty and remained cautious. The shyer nature of these individuals may mean that they continue to behave in a timid manner or that they may perceive the bold demonstrator as a much better competitor and as such do not increase their boldness against an individual they are unlikely to outcompete. This is confirmed by the fact that shy subjects who observed shy became bolder by decreasing their latency to approach a novel object. In this case, the shy observer may perceive their competitive ability as being similar; therefore, if they decrease the time they take to approach stimuli, they may outcompete shy conspecifics by simply getting there first. Theories regarding intraspecific competition do predict that an animal's strategic decision should be based upon their relative competitive ability compared with that of an opponent (Maynard Smith & Parker 1976). Thus, in the context of observing individuals with opposing ‘personality’ traits, bold individuals appear to be more flexible adjusting their behaviour according to how their shy demonstrators behave whereas shy fishes do not show this plasticity and do not become more bold perhaps showing that the shy ‘personality’ is more ‘fixed’ and less dependent upon conspecific behaviour. In the context of observing others, bold individuals are more plastic in their responsiveness than shy individuals and thus they may be more adaptable to changing conditions and fitter in natural populations. These results are in contrast to many other studies which demonstrate that bold or proactive individuals are relatively ‘fixed’ in their behaviour compared with shy or reactive individuals which display greater flexibility (Benus et al. 1991; Koolhaas et al. 1999; Sih et al. 2004).
Fishes were deemed either bold or shy via their latency to come within close proximity of a novel object. This potentially fearful event does not correlate with the boldness displayed by individuals when presented with a novel prey item. Comparing the latency to taste novel prey on day 1 revealed a difference between bold and shy fishes with bold individuals tasting more quickly. Similar results have been found in other species such as perch (Perca fluviatilis Magnhagen & Staffan 2003), pumpkinseed sunfish (Lepomis gibbosus Coleman & Wilson 1998) and great tits (Parus major van Oers et al. 2005), where bold individuals responded to novelty more quickly. This finding in trout can be attributed to bold observers that had bold demonstrators which ate on the first day of demonstrations. Of course, in the present study, the fish would already associate the addition of items to the tank with feeding. However, bold subjects did have shorter latencies to taste the novel prey item after observing individuals ate on the first day of demonstration, indicating that bold subjects may be quicker to recognize that the new prey is edible. This may show that bold rainbow trout may be more adaptable in the face of biotic (prey type) change, since they are faster learners (Sneddon 2003) and this has also been demonstrated in great tits (Marchetti & Drent 2000).
With regards to fighting success, the winner and loser effect is commonly observed on a behavioural level (Whitehouse 1997; Rutte et al. 2006), but also the physiology can be significantly altered by an aggressive win or defeat (Yeh et al. 1996; Øverli et al. 1999; Sneddon et al. 2000). In the present study, winners expressed a higher degree of boldness overall by decreasing their latency to approach a novel object, whereas bold losers increased their latency to approach displaying more fearful and shyer behaviour. The positive experience of winning several interactions may increase one's boldness by reinforcing strategic decisions in initiating and winning fights, whereas the negative experience of losing may reduce the degree of boldness expressed by an individual owing to the physiological effects of defeat that impact upon subsequent behavioural output (Whitehouse 1997). This is confirmed by the fact that winners perform more aggressive acts than losers regardless of boldness. Thus, this experimental context had the desired effect of emboldening individuals when they won and negatively affecting bold losers. The degree of aggression expressed by winners decreased over time, and this is not surprising as this decline normally occurs when individuals are shifting from an undecided dominance status to a stable dominant–subordinate relationship (Øverli et al. 1999).
Surprisingly, shy losers expressed a bolder and a more confident behaviour after successive defeats. Shy losers may display a bolder behaviour post-fights owing to these subjects being subordinate. It would pay a subordinate in a competitive situation to increase their willingness to engage in risky behaviour and investigate novel events quickly. Subordinate fishes are known to obtain food sneakily by waiting till the dominant is engaged in aggression (Sneddon & Yerbury 2004). This result was unexpected since observing bold fish in experiment 1 did not affect the strategy shy fish adopted when responding to novelty. Engaging in aggressive interactions may be a stronger cue than simply observing others and fighting does result in major changes in physiology and subsequent behaviour (e.g. Yeh et al. 1996; Øverli et al. 1999; Sneddon et al. 1999, 2000). Therefore, shy losers may become bolder owing to the ‘Desperado effect’ to increase their chances of obtaining resources whereas bold losers may decrease their reactivity, since they are low in status and are maintaining their place in the pecking order. Bold fishes only show a change in behaviour after losing which would be adaptive, since animals should defer to better competitors and not engage in further provocative behaviour with an individual of higher status (Just & Zhu 2004). While becoming bolder when high status is held may be adaptive to ensure that status is not lost, increasing boldness when being of low status could be maladaptive since this may incite more attacks from individuals of higher dominance status.
The more dramatic change witnessed in the shy subjects' behaviour after fighting could also be explained by the evolution of the shy personality trait. It has been observed that shy individuals display a more flexible behaviour enabling them to adapt to a stochastic environment (Benus et al. 1991; Koolhaas et al. 1999). Whereas bold individuals show an ability to form routines more easily (Koolhaas et al. 1999; Sih et al. 2004), benefiting them in a constant environment. Although the outcomes of winning and losing affect shy individuals in separate ways, it pays in terms of costs versus benefits for shy fishes to change their behaviour and increase their level of boldness. This may provide evidence that they use self-assessment of relative competitive ability upon which to base their strategic decisions (in Rutte et al. 2006).
The present study has yielded new data showing that individual traits are dynamic but dependent upon context. Shy individuals appear to alter their behaviour when their relative competitive ability may be similar or less than their conspecifics in both the observation and dominance status contexts. Bold individuals appear to be more flexible and behave as predicted when given negative information or losing fights and reduce their boldness. This suggests that it is bold individuals which show a more adaptive response in this species with shy individuals being less predictable. This will have profound effects upon how individuals within populations respond to abiotic and biotic variation in the natural environment. These context-dependent changes in animal ‘personality’ traits provide novel empirical information that can be used by theoreticians to model how these individual differences may have an impact upon the ecology and evolution of populations (Dall et al. 2004; Neff & Sherman 2004; Sih et al. 2004).
Acknowledgments
LS is funded by a NERC fellowship; AF, PA and AWG were supported by the Nuffield Foundation, ASAB, NERC and BBSRC Research Grants awarded to LS. We are grateful to Gregor Govan for technical support.
References
- Armitage K.B, Van Vuren D.H. Individual differences and reproductive success in yellow-bellied marmots. Ethol. Ecol. Evol. 2003;15:207–233. [Google Scholar]
- Bell A.M. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus) J. Evol. Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. doi:10.1111/j.1420-9101.2004.00817.x [DOI] [PubMed] [Google Scholar]
- Benus R.F, Bohus B, Koolhaas J.M, Vanoortmerssen G.A. Heritable variation for aggression as a reflection of individual coping strategies. Experientia. 1991;47:1008–1019. doi: 10.1007/BF01923336. doi:10.1007/BF01923336 [DOI] [PubMed] [Google Scholar]
- Brick O, Jakobsson S. Individual variation in risk taking: the effect of a predatory threat on fighting behavior in Nannacara anomala. Behav. Ecol. 2002;13:439–442. doi:10.1093/beheco/13.4.439 [Google Scholar]
- Brown C, Laland K.N. Social learning in fishes: a review. Fish Fish. 2003;4:280–288. [Google Scholar]
- Caldwell C.A, Whiten A. Testing for social learning and imitation in common marmosets, Callithrix jacchus, using an artificial fruit. Anim. Cogn. 2004;7:77–85. doi: 10.1007/s10071-003-0192-9. doi:10.1007/s10071-003-0192-9 [DOI] [PubMed] [Google Scholar]
- Carere C, Drent P.J, Privitera L, Koolhaas J.M, Groothuis T.G.G. Personalities in great tits, Parus major: stability and consistency. Anim. Behav. 2005;70:795–805. doi:10.1016/j.anbehav.2005.01.003 [Google Scholar]
- Coleman K, Wilson D.S. Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim. Behav. 1998;56:927–936. doi: 10.1006/anbe.1998.0852. doi:10.1006/anbe.1998.0852 [DOI] [PubMed] [Google Scholar]
- Dall S.R.X, Houston A.I, McNamara J.M. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 2004;7:734–739. doi:10.1111/j.1461-0248.2004.00618.x [Google Scholar]
- Dugatkin L.A, Druen M. The social implications of winner and loser effects. Proc. R. Soc. B. 2004;271:S488–S489. doi: 10.1098/rsbl.2004.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugatkin L.A, Earley R.L. Individual recognition, dominance hierarchies and winner and loser effects. Proc. R. Soc. B. 2004;271:1537–1540. doi: 10.1098/rspb.2004.2777. doi:10.1098/rspb.2004.2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernande B, Dieckmann U. The evolution of phenotypic plasticity in spatially structured environments: implications of intraspecific competition, plasticity costs and environmental characteristics. J. Evol. Biol. 2004;17:613–628. doi: 10.1111/j.1420-9101.2004.00691.x. doi:10.1111/j.1420-9101.2004.00691.x [DOI] [PubMed] [Google Scholar]
- Hsu Y.Y, Wolf L.L. The winner and loser effect: integrating multiple experiences. Anim. Behav. 1999;57:903–910. doi: 10.1006/anbe.1998.1049. doi:10.1006/anbe.1998.1049 [DOI] [PubMed] [Google Scholar]
- Hsu Y.Y, Earley R.L, Wolf L.L. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 2006;81:33–74. doi: 10.1017/S146479310500686X. doi:10.1017/S146479310500686X [DOI] [PubMed] [Google Scholar]
- Just W.F, Zhu F. Individual-based simulations of the war of attrition. Behav. Process. 2004;66:53–62. doi: 10.1016/j.beproc.2004.01.005. doi:10.1016/j.beproc.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Katz M, Lachlan R.F. Social learning of food types in zebra finches (Taenopygia guttata) is directed by demonstrator sex and feeding activity. Anim. Cogn. 2003;6:11–16. doi: 10.1007/s10071-003-0158-y. [DOI] [PubMed] [Google Scholar]
- Koolhaas J.M, Korte S.M, De Boer S.F, Van Der Vegt B.J, Van Reenen C.G, Hopster H, De Jong I.C, Ruis M.A.W, Blokhuis H.J. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. doi:10.1016/S0149-7634(99)00026-3 [DOI] [PubMed] [Google Scholar]
- Lepage O, Larson E.T, Mayer I, Winberg S. Serotonin, but not melatonin, plays a role in shaping dorninant–subordinate relationships and aggression in rainbow trout. Horm. Behav. 2005;48:233–242. doi: 10.1016/j.yhbeh.2005.02.012. doi:10.1016/j.yhbeh.2005.02.012 [DOI] [PubMed] [Google Scholar]
- Lopez P, Hawlena D, Polo V, Amo L, Martin J. Sources of individual shy–bold variations in antipredator behaviour of male Iberian rock lizards. Anim. Behav. 2005;69:1–9. doi:10.1016/j.anbehav.2004.05.010 [Google Scholar]
- Magnhagen C, Staffan F. Social learning in young-of-the-year perch encountering a novel food type. J. Fish Biol. 2003;63:824–829. doi:10.1046/j.1095-8649.2003.00189.x [Google Scholar]
- Marchetti C, Drent P.J. Individual differences in the use of social information in foraging by captive great tits. Anim. Behav. 2000;60:131–140. doi: 10.1006/anbe.2000.1443. doi:10.1006/anbe.2000.1443 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J, Parker G. The logic of asymmetric contests. Anim. Behav. 1976;24:159–175. doi:10.1016/S0003-3472(76)80110-8 [Google Scholar]
- McGregor P.K, Peake T.M, Lampe H.M. Fighting fish Betta splendens extract relative information from apparent interactions: what happens when what you see isn't what you get. Anim. Behav. 2001;62:1059–1065. doi:10.1006/anbe.2001.1850 [Google Scholar]
- Neff B.D, Sherman P.W. Behavioral syndromes versus darwinian algorithms. Trends Ecol. Evol. 2004;19:621–622. doi:10.1016/j.tree.2004.09.017 [Google Scholar]
- Oliveira R.F, Carneiro M.L.L.A, Canário A.V.M. Watching fights raises fish hormone levels. Nature. 2001;409:475. doi: 10.1038/35054128. doi:10.1038/35054128 [DOI] [PubMed] [Google Scholar]
- Ottoni E.B, de Resende B.D, Izar P. Watching the best nutcrackers: what capuchin monkeys (Cebus apella) know about others' tool-using skills. Anim. Cogn. 2005;8:215–219. doi: 10.1007/s10071-004-0245-8. doi:10.1007/s10071-004-0245-8 [DOI] [PubMed] [Google Scholar]
- Øverli O, Harris C.A, Winberg S. Short term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 1999;54:263–275. doi: 10.1159/000006627. doi:10.1159/000006627 [DOI] [PubMed] [Google Scholar]
- Oyegbile T.O, Marler C.A. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 2005;48:259–267. doi: 10.1016/j.yhbeh.2005.04.007. doi:10.1016/j.yhbeh.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Rutte C, Taborsky M, Brinkhof M.W.G. What sets the odds of winning and losing? Trends Ecol. Evol. 2006;21:16–21. doi: 10.1016/j.tree.2005.10.014. doi:10.1016/j.tree.2005.10.014 [DOI] [PubMed] [Google Scholar]
- Sargeant B.L, Mann J, Berggren P, Krutzen M. Specialization and development of beach hunting, a rare foraging behavior, by wild bottlenose dolphins (Tursiops sp.) Can. J. Zool.—Revue Canadienne De Zoologie. 2005;83:1400–1410. doi:10.1139/z05-136 [Google Scholar]
- Schjolden J, Backstrom T, Pulman K.G.T, Pottinger T.G, Winberg S. Divergence in behavioural responses trout (Oncorhynchus mykiss) with to stress in two strains of rainbow contrasting stress responsiveness. Horm. Behav. 2005;48:537–544. doi: 10.1016/j.yhbeh.2005.04.008. doi:10.1016/j.yhbeh.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Schuett G.W, Harlow H.J, Rose J.D, VanKirk E.A, Murdoch W.J. Levels of plasma corticosterone and testosterone in male copperheads (Agkistrodon contortrix) following staged fights. Horm. Behav. 1996;30:60–68. doi: 10.1006/hbeh.1996.0009. doi:10.1006/hbeh.1996.0009 [DOI] [PubMed] [Google Scholar]
- Schuster S, Wohl S, Griebsch M, Klostermeier I. Animal cognition: how archer fish learn to down rapidly moving targets. Curr. Biol. 2006;16:378–383. doi: 10.1016/j.cub.2005.12.037. doi:10.1016/j.cub.2005.12.037 [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Coe C, Parmigiani S, Koolhaas J. Individual differences in behavior and physiology: causes and consequences. Neurosci. Biobehav. Rev. 2005;29:1–2. doi: 10.1016/j.neubiorev.2004.11.002. doi:10.1016/j.neubiorev.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson J.C. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. doi:10.1016/j.tree.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Sneddon L.U. The bold and the shy: individual differences in rainbow trout. J. Fish Biol. 2003;62:971–975. doi:10.1046/j.1095-8649.2003.00084.x [Google Scholar]
- Sneddon L.U, Yerbury J. Differences in response to hypoxia in the three-spined stickleback from lotic and lentic localities: dominance and an anaerobic metabolite. J. Fish Biol. 2004;64:799–804. doi:10.1111/j.1095-8649.2004.00361.x [Google Scholar]
- Sneddon L.U, Taylor A.C, Huntingford F.A. Metabolic consequences of agonistic behaviour: crab fights in declining oxygen tensions. Anim. Behav. 1999;57:353–363. doi: 10.1006/anbe.1998.0982. doi:10.1006/anbe.1998.0982 [DOI] [PubMed] [Google Scholar]
- Sneddon L.U, Taylor A.C, Huntingford F.A, Watson D.G. Agonistic behaviour and biogenic amines in shore crabs. J. Exp. Biol. 2000;203:537–545. doi: 10.1242/jeb.203.3.537. [DOI] [PubMed] [Google Scholar]
- Sneddon L.U, Braithwaite V.A, Gentle M.J. Novel object test: examining pain and fear in the rainbow trout. J. Pain. 2003;4:431–440. doi: 10.1067/s1526-5900(03)00717-x. doi:10.1067/S1526-5900(03)00717-X [DOI] [PubMed] [Google Scholar]
- Spoolder H.A.M, Burbidge J.A, Lawrence A.B, Simmins P.H, Edwards S.A. Individual behavioural differences in pigs: intra- and inter-test consistency. Appl. Anim. Behav. Sci. 1996;49:185–198. doi:10.1016/0168-1591(96)01033-7 [Google Scholar]
- Stoinski T.S, Whiten A. Social learning by orangutans (Pongo abelii and Pongo pygmaeus) in a simulated food-processing task. J. Comp. Psychol. 2003;117:272–282. doi: 10.1037/0735-7036.117.3.272. doi:10.1037/0735-7036.117.3.272 [DOI] [PubMed] [Google Scholar]
- Svartberg K, Tapper I, Temrin H, Radesater T, Thorman S. Consistency of personality traits in dogs. Anim. Behav. 2005;69:283–291. doi:10.1016/j.anbehav.2004.04.011 [Google Scholar]
- van Oers K, Klunder M, Drent P.J. Context dependence of personalities: risk-taking behavior in a social and a nonsocial situation. Behav. Ecol. 2005;16:716–723. doi:10.1093/beheco/ari045 [Google Scholar]
- Vilhunen S, Hirvonen H, Laakkonen M.V.M. Less is more: social learning of predator recognition requires a low demonstrator to observer ratio in Arctic charr (Salvelinus alpinus) Behav. Ecol. Sociobiol. 2005;57:275–282. doi:10.1007/s00265-004-0846-x [Google Scholar]
- Whitehouse M.E.A. Experience influences male–male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae) Anim. Behav. 1997;53:913–923. doi:10.1006/anbe.1996.0313 [Google Scholar]
- Wilson D.S. Adaptive individual differences within single populations. Phil. Trans. R. Soc. B. 1998;353:199–205. doi:10.1098/rstb.1998.0202 [Google Scholar]
- Wilson D.S, Coleman K, Clark A.B, Biederman L. Shy–bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J. Comp. Psychol. 1993;107:250–260. doi:10.1037/0735-7036.107.3.250 [Google Scholar]
- Wilson D.S, Clark A.B, Coleman K, Dearstyne T. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 1994;9:442–446. doi: 10.1016/0169-5347(94)90134-1. doi:10.1016/0169-5347(94)90134-1 [DOI] [PubMed] [Google Scholar]
- Winberg S, Øverli O, Lepage O. Suppression of aggression in rainbow trout (Oncorhynchus mykiss) by dietary l-tryptophan. J. Exp. Biol. 2001;204:3867–3876. doi: 10.1242/jeb.204.22.3867. [DOI] [PubMed] [Google Scholar]
- Worden B.D, Papaj D.R. Flower choice copying in bumblebees. Biol. Lett. 2005;1:504–507. doi: 10.1098/rsbl.2005.0368. doi:10.1098/rsbl.2005.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S.R, Fricke R.A, Edwards D.H. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Nagamine M, Uematsu K. Comparison of behavioral responses to a novel environment between three teleosts, bluegill Lepomis macrochirus, crucian carp Carassius langsdorfii, and goldfish Carassius auratus. Fish. Sci. 2005;71:314–319. doi:10.1111/j.1444-2906.2005.00966.x [Google Scholar]