Abstract
There is substantial in-vitro data indicating that curcumin has antioxidant, anti-inflammatory, and anti-amyloid activity. In addition, studies in animal models of Alzheimer’s disease (AD) indicate a direct effect of curcumin in decreasing the amyloid pathology of AD. As the widespread use of curcumin as a food additive and relatively small short-term studies in humans suggest safety, curcumin is a promising agent in the treatment and/or prevention of AD. Nonetheless, important information regarding curcumin bioavailability, safety and tolerability, particularly in an elderly population is lacking. We are therefore performing a study of curcumin in patients with AD to gather this information in addition to data on the effect of curcumin on biomarkers of AD pathology.
INTRODUCTION
Preparations of the plant Curcuma longa Linn have been used to treat various ailments for centuries in Ayurvedic medicine, a traditional Indian system of healing(1). This plant, also known as turmeric, is a member of the Zingiberaceae, or ginger family. Within Ayurveda, turmeric preparations are taken orally to treat dyspepsia, flatulence, liver disease, urinary tract disease and as a “blood purifier.” It is also used externally for pemphigus and other skin diseases, and may be inhaled for the treatment of coryza. Components of turmeric are currently undergoing scientific evaluation for their utility as anti-inflammatory agents(2), value in preventing and treating cancer (3, 4), for the treatment of human immunodeficiency virus (HIV) infection(2) and most recently for the treatment of cystic fibrosis(5). In addition to its medicinal applications, turmeric is used as a dye and food additive. Originally valued because of its ability to maintain the freshness of food, it is a spice commonly used in curry which is currently its most important commercial application.
Curcuma longa Linn is indigenous to South and Southeast Asia where it is grown for commercial use. Turmeric is derived from the rhizome, or root of the plant. Curcumin was isolated in 1815 and the chemical structure was subsequently identified to be diferuloylmethane (Fig. 1). Along with the other curcuminoids (demethoxycurcumin, bisdemethoxycurcumin, and cyclocurcumin), it composes a yellow pigment that is poorly soluble in water and comprises 3–5% of turmeric extract(1). Curcumin has a structure similar to Congo Red (Fig. 1) and, like Congo Red, it binds to and stains amyloid plaques in vivo (unpublished observations). It has been demonstrated in various experimental preparations to have anti-oxidant, anti-inflammatory, and cholesterol-lowering properties (see below), all three of which are felt to be key processes involved in the pathogenesis of Alzheimer’s disease (AD). Epidemiological studies in India, a country where turmeric consumption is widespread, suggest it has one of the lowest prevalence rates of AD in the world(6, 7). Though there are many potential explanations for this observation, a preventative role of curcumin is a possibility. In this paper we summarize the evidence supporting the evaluation of a role for curcumin in the prevention and treatment of human AD.
Fig. (1).
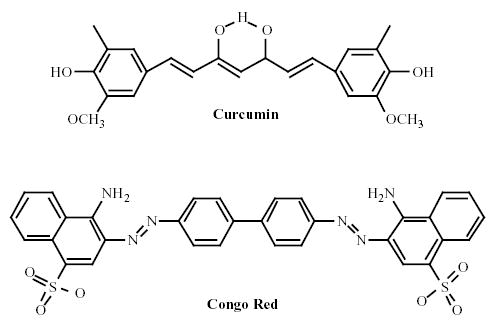
Chemical structures of Curcumin and Congo Red.
IN-VITRO STUDIES
Antioxidant Properties
There is a body of convergent evidence suggesting that oxidative damage plays an important role in the pathogenesis of AD. Elevated levels of the oxidated forms of various organic molecules (lipids, proteins, DNA, and carbohydrates) have been described in brain, cerebrospinal fluid (CSF), blood, and/or urine of AD patients(8, 9). Epidemiological studies have demonstrated an association between dietary antioxidant consumption and a lowered risk for the development of AD(10) although not all have confirmed this observation(11). At least one clinical trial suggested a possible benefit for the anti-oxidant alpha-tocopherol (vitamin E) in slowing the progression of established AD(12). Curcumin exerts potent antioxidant activity, the property that led to its early use as a food preservative.
Using a rat liver microsome preparation, Reddy and Lokesh(13) demonstrated that curcumin inhibited lipid peroxidation and Sreejayan and Rao(14) replicated this in a distinct in-vitro model, demonstrating that curcumin, demethoxycurcumin, and desmethoxycurcumin were more potent antioxidants than alpha-tocopherol. Shih and Lin(15) showed that curcumin prevented oxidative damage of DNA in mouse fibroblasts. Kim et al (16) showed that the curcuminoids were more potent antioxidants than alpha-tocopherol using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical trapping assay in which the ability of the compounds to reduce a radical cation is assessed. Multiple experiments therefore provide evidence that curcumin has substantial antioxidant properties.
Anti-Inflammatory Effects
Inflammatory changes (e.g. upregulation of complement, cytokines, acute phase reactants) have been identified in AD brain and inflammation is thought to play a compounding, if not causative role in AD pathogenesis(17). Furthermore, epidemiological studies have consistently demonstrated an association between non-steroidal anti-inflammatory drug (NSAID) use and a subsequent decreased risk for the development of AD(18, 19) though studies of NSAIDs in persons with established AD have been disappointing(20). Curcumin has been shown to have anti-inflammatory effects and may have a role in slowing AD through this mechanism.
Investigators have demonstrated that curcumin inhibits lipoxygenase and cyclooxygenase 2, enzymes that are responsible for the synthesis of the pro-inflammatory leukotrienes, prostaglandins, and thromboxanes(21). It also inhibits AP-1 mediated transcription related to cytokine regulation in vitro(22) and suppresses inducible nitric oxide synthase (iNOS) in activated macrophages(23), processes that promote inflammation. As lipid peroxides also promote inflammation, curcumin’s antioxidant effects also serve to decrease inflammation.
Cholesterol-Lowering Properties
Cholesterol has an influence on β-amyloid (Aβ) deposition and prior use of medications that inhibit the enzyme involved in cholesterol synthesis (3-hydroxy-3-methylglutaryl-coenzyme A, or HMG-CoA reductase inhibitors) have been associated with a decreased risk of AD in epidemiological studies(24). It is proposed that cholesterol interacts with the amyloid cascade in the pathogenesis of AD and studies of drugs affecting cholesterol metabolism for the treatment of AD are being performed. In one study, administration of 500 mg of curcuminoids a day for 7 days reduced levels of serum cholesterol and lipid peroxides in healthy volunteers(25). This suggests another mechanism by which curcumin might exert beneficial effects on AD.
Hemostatic Properties
Curcumin has been demonstrated to exert an inhibitory effect on platelet aggregation that has been attributed to its inhibitory effect on thromboxane A2 production (26, 27). Vascular factors are posited to play a role in AD or in the manifestations of dementia and inhibitors of platelet aggregation may be of benefit.
Anti-Amyloid Properties
There is convergent evidence that toxic effects of Aβ on normal cellular function and viability underlay the pathogenesis of AD. Aβ1–42 has been demonstrated to have adverse effects in a number of cell culture models. Kim et al(16) demonstrated that curcuminoids, but not alpha-tocopherol, protected PC12 rat pheochromocytoma and human umbilical vein endothelial cells from Aβ1–42 injury. These authors speculated that curcumin’s antioxidant properties mediated this effect (see above).
As aggregation of Aβ into fibrils and the subsequent formation of amyloid plaques are posited by many to be important steps in the pathogenesis of AD, these processes are potential targets for therapy. Ono et al(28) performed an in-vitro study in which they measured the effects of curcumin on formation of Aβ fibrils from Aβ1–40 and Aβ1–42 peptides. They found that curcumin inhibited the formation and extension of Aβ fibrils and destabilized preformed Aβ fibrils in a dose-dependent fashion at a range between 0.1 and 1 micro-molar concentration. We have independently confirmed this finding (in press). Additional experiments suggested that curcumin’s effect did not depend on specipic epitopes of Aβ but rather the compound bound to fibrillar Aβ regardless of the specific Aβ sequences of which it was composed.
We have also demonstrated that the in-vitro prevention of Aβ fibril formation by curcumin is visualizable by electron microscopy. Furthermore, curcumin inhibits the formation of antibody-detectable Aβ oligomers(29), which are felt to be intermediates in Aβ aggregation (in press).
Thus curcumin appears to have primary effects on Aβ aggregation in addition to its antioxidant, anti-inflammatory, and platelet aggregation inhibiting properties.
ANIMAL STUDIES
Pharmacokinetic and Pharmacodynamic Studies
In rats, 75% of an orally administered dose (1 gram/kg body weight) was recovered in the feces with only negligible amounts being detected in blood and urine. High doses of curcumin (400 mg or 3.6 mmol/kg body weight) are required to obtain detectable tissue levels in rats(3). This is attributed to extensive metabolism of the compound in the gastrointestinal wall, glucoronidation in the liver, and enterohepatic circulation. There are few published data on central nervous system penetration of curcumin in animals.
Safety Studies
Single doses of 13.6 mmol/kg body weight did not cause any clinical signs in rats(30). Rats treated with up to 3,500 mg/kg body weight per day (9.5 mmol/kg body weight) for 90 days displayed only discolored feces, yellow fur, and hematological changes not considered biologically significant(3). A study in dogs similarly showed lack of toxicity(3). An independent study of rats (1000 mg/kg/day) and monkeys (800 mg/kg/day) for 3 months failed to reveal any evidence of adverse effects on growth, behavioral, biochemical, or histopathological parameters(3). Hepatotoxicity was noted in mice that were fed tumeric and ethanolic tumeric extract (0.05 – 0.25%) for 14 days and rats that were fed a higher dose of tumeric (5%) for 90 days(31).
In some studies curcumin has had ulcerogenic properties, causing gastric ulcerations in rats at doses of 100 mg/kg(32). Furthermore, in a study of rats treated with 50,000 ppm dietary turmeric oleoresin there was an increased incidence of ulcers, inflammation, and hyperplasia of the forestomach, cecum, and colon(3). In other investigations, however, curcumin had antiulcerogenic properties, causing decreased gastric secretion in rats(33) and it has been traditionally used to treat dyspepsia. It is likely that differences in the dose and preparation of the agent account for these disparate observations.
In one study, female mice receiving 50,000 ppm turmeric oleoresin had a significantly increased incidence of thyroid gland follicular cell hyperplasia(34). Animals studies of carcinogenicity, genotoxicity, and teratogenicity have been equivocal or negative(3).
Animal studies therefore indicate that curcumin at high doses is safe though vigilance is suggested for potential hepatic, gastric, and thyroid toxicity.
HUMAN STUDIES
Pharmacokinetic Studies
Human pharmacokinetic studies have been performed in patients with cancer or pre-cancerous lesions. In 15 patients with colon cancer, oral doses of 440–2200 mg were administered daily and curcumin levels measured in the blood, urine, and feces at up to 29 days using high pressure liquid chromatography (HPLC)(35). Though curcumin and its metabolites (curcumin glucuronide, curcumin sulfate, hexahydrocurcumin and hexahydrocurcuminol) were readily measured in feces, none were identifiable in the blood or urine. In another study in patients with precancerous lesions and normal adults, curcumin levels were measured in the blood 2–24 hours after oral ingestion of 500 mg – 8000 mg per day(36). Peak blood levels of 1.77 micromoles were observed 2 hours after the 8000 mg dose with levels decreasing gradually after that. Blood levels in persons receiving 500 – 2000 mg doses were barely detectable. Two patients had repeat pharmacokinetic studies done after taking curcumin regularly for 1 months’ time and no changes were seen in their blood levels. The low blood levels of curcumin seen in this study and the absence of curcumin seen with lower doses are consistent with extensive metabolism of curcumin in the intestinal wall and/or poor absorption. The possibility that curcumin is rapidly metabolized to unmeasured active metabolites would reconcile these findings with the observed biological activity of comparable doses in animal studies. Alternatively, the lack of an internal standard in the HPLC assays used in these studies might render them relatively insensitive to the detection of curcumin(37).
The bioavailability of curcumin may be limited by intestinal and hepatic glucuronidation. Piperine, an inhibitor of glucuronidation, could be administered concomitantly with curcumin to increase bioavailability. Shoba et al demonstrated the feasibility of this approach in both rats and humans(38). When 20 mg of piperine was administered orally with 2 gm of curcumin to volunteers, serum levels were significantly enhanced at 1 hour’s time, increasing total bioavailability by 20-fold. No toxicity was observed in the 10 subjects who participated in this study.
Safety and Tolerability
Turmeric is currently listed as “generally recognized as safe” (GRAS) as a coloring and flavoring agent in food by the United States Food and Drug Administration (FDA)(39). However, curcumin itself is not listed and has been given a temporary acceptable daily intake level of 0.1 mg/kg of body weight(40) pending further study. Its widespread use in food without known adverse effects provides support for its safety. In addition, several relatively short-term studies specifically looking at the safety and tolerability of high doses have been performed.
In short-term studies doses of up to 1,200 mg/day were demonstrated to be well-tolerated in patients with rheumatoid arthritis(41), post-surgical patients(42) and ophthalmology patients(43). In a study of 19 patients with acquired immune deficiency syndrome (AIDS) two patients, one of whom had a history of peptic ulcer disease, developed gastric irritation(44).
In a study of patients with colon cancer treated for 4 months(35), 1 patient on 1,320 mg developed nausea in the first month that resolved despite continued administration of the drug and two patients developed diarrhea (one on 880 mg and one on 2,200 mg/day) and dropped out of the study. In the study of patients with pre-cancerous lesions treated for 3 months, doses of up to 8000 mg/day were tolerated well though higher doses were difficult to tolerate due to their bulky volume(36). In summary, high doses of curcumin appear to be well-tolerated with few adverse effects other than gastric irritation or nausea. To date, however, we are not aware of longer-term safety and tolerability studies in humans nor of studies specifically done in an elderly population as would be encountered in a study of AD.
STUDIES IN ANIMAL MODELS OF AD
In light of the antioxidant, anti-inflammatory, and anti-amyloid effects discussed above, curcumin has become a candidate compound for the prevention or treatment of AD. Several animal models for AD pathology currently exist and investigators have performed studies of curcumin in such animals.
Lim et al(45) studied the effects of curcumin in transgenic mice which carry a human mutation in the amyloid precursor protein (APPsw) that causes AD pathology. APPsw mice display age-related neuritic plaques, inflammation, oxidative damage, and age-related memory deficits but not neurofibrillary tangle pathology. In this study, ten month old APPsw mice were fed diets containing either no curcumin, low-dose (Sigma, 160 ppm), or high-dose curcumin (5000 ppm) for 6 months before being sacrificed. Mice fed low-dose curcumin had decreased total microgial activity, decreased cerebral levels of oxidized proteins, and decreased levels of IL-1β, a cytokine that has been implicated in age-related memory loss(46). Most intriguingly, mice fed low-dose curcumin also had significantly reduced levels of soluble and insoluble Aβ as well as a reduced amyloid plaque burden. An additional observation was that the reduction in microglial activity was localized such that increased activity was observed in the area around amyloid plaques, possibly representing a stimulatory effect of curcumin on phagocytosis of plaques by microglia. APPsw mice fed high-dose curcumin displayed similar decreases in IL-1β and oxidized proteins but no change in Aβ. One possible explanation for the unanticipated results between the low- and high-dose groups is that the multiple mechanisms of action that curcumin has have different dose requirements and time courses that may counterbalance each other at higher doses. More recently we have replicated the effect of low dose curcumin on insoluble Aβ levels in more aged APPsw mice in which AD-like pathology is established (unpublished observations).
Similar effects of curcumin were observed in an animal model of AD in which human Aβ1–40 and Aβ1–42 were infused with a lipoprotein chaperone into the cerebral ventricles of aged female rats(47). Such rats develop Aβ deposits, neurodegeneration, and memory impairment. Reduced levels of 8-EPI-F2 isoprostanes, an oxidative produce of arachidonic acid, and normal levels of synaptophysin, a marker of synaptic integrity were seen in curcumin (2000 ppm), but not ibuprofen-fed rats. Both curcumin and ibuprofen-fed rats had decreased microglial activity. Increased microglial staining was seen in areas surrounding amyloid plaques. We also measured the spatial memory and post-synaptic density 95 (PSD-95) levels, a synaptic protein that anchors N-methyl-D-aspartate (NMDA) receptors, in the brains of younger rats infused with a higher dose of Aβ1–40 and Aβ1–42. Rats fed with curcumin (500 ppm) showed reduced path length and latency in finding the hidden platform in the Morris water maze test, demonstrating superior memory function compared to control-fed rats. Increased PSD-95 and decreased Aβ-stained area also were seen in curcumin-fed rats. These studies suggest that curcumin ameliorates both the pathology and cognitive deficits induced by Aβ infusion in rats.
These findings provide evidence that curcumin can ameliorate the pathology and cognitive deficits in animal models of AD but leaves the mechanism for this activity open to question. We have performed subsequent fluorescence studies that demonstrate that curcumin binds to amyloid plaques in human AD and Tg2576 transgenic mouse brain tissue in-vitro. Furthermore, curcumin was found to bind to amyloid plaques when such mice were either fed curcumin or curcumin injected in the carotid artery (in press). This demonstrates that curcumin crosses the blood-brain barrier in these mice, and direct binding to plaques may be important in its anti-amyloid activity. Animal studies therefore are highly suggestive of efficacy against AD pathology via multiple possible mechanisms. Further study of curcumin in the treatment or prevention of AD in humans is warranted.
A PHASE II, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY OF THE SAFETY AND TOLERABILITY OF TWO DOSES OF CURCUMIN C3 COMPLEX® VERSUS PLACEBO IN PATIENTS WITH MILD-TO-MODERATE ALZHEIMER’S DISEASE
Introduction
We are performing a 24 week, randomized, double-blinded, placebo-controlled study of two doses of curcumin in persons with mild-to-moderate AD. This will be followed by a 24 week extension study in which subjects on curcumin will continue treatment at their assigned dose and those on placebo will receive one of the two doses of curcumin. The purposes of this study are to:
To determine the safety and tolerability of 2000 mg and 4000 mg/day of Curcumin C3 Complex® in patients with AD treated for 6 – 12 months.
To obtain pharmacokinetic data including central nervous system penetration on subjects with AD receiving these doses of orally adminstered Curcumin C3 Complex®.
To gather data on the effects of curcumin on biomarkers thought to be related to the pathology AD and the mechanism of action of curcumin (cholesterol levels, CSF isoprostanes, alpha-1-antichymotrypsin, C-reactive protein, tau, Aβ1–40 and Aβ1–42)
To gather preliminary efficacy data (Alzheimer’s Disease Assessment Scale, cognitive subscale (ADASCog), Neuropsychiatric Inventory (NPI), Activities of Daily Living Scale (ADCS-ADL)) of curcumin in AD.
Design
Thirty-three subjects with mild-to-moderate AD (Mini-Mental Status Examination, or MMSE, scores between 18 and 28, inclusive) defined by National Institute for Neurological and Communicative Disorders and Stroke - Alzheimer’s Disease and Related Disorders Association (NINCDS-ARDA) criteria will be randomized (11 per arm). Subjects may be on stable doses of a cholinesterase inhibitor or memantine but may not be on high doses of antioxidants or anti-inflammatory medications. Subjects will undergo a lumbar puncture at the baseline visit for assessment of biomarker levels and receive either placebo, 2000 mg or 4000 mg Curcumin C3 Complex to be taken in two divided doses each day. These doses were chosen to in an effort to achieve measurable CSF levels of curcumin while maximizing tolerability. Blood draws for toxicological monitoring (CBC, Chemistry Panel, LFTs. PT, PTT, TFTs) will be obtained at screening, 4 week, 12 week, 24 week, 36 week, and 48 week visits. Adverse effects will be assessed with a questionnaire which will be administered at baseline, 4 week, 12 week, 24 week, 36 week, and 48 week visits. Subjects also will be administered the MMSE, ADASCog, NPI, and ADCS-ADL scale at baseline, 24 weeks’ and 48 weeks’ time. At the 24-week visit, subjects will be admitted to the Clinical Research Center and have baseline blood drawn prior to taking their a.m. dose of study medication. They will then undergo serial blood draws for curcumin levels in addition to a second lumbar puncture to again assess biomarker levels and CSF levels of curcumin. Subjects on placebo will then be placed on either of the two doses of curcumin and all subjects will be followed for an additional 24 weeks. The primary analysis will be an intent-to-treat analysis using last-observation-carried-forward data in case of drop-outs. Two tailed T-tests with a significance cut-off of 0.05 will be used
CONCLUSIONS
There is substantial in-vitro data indicating that curcumin has antioxidant, anti-inflammatory, and anti-amyloid activity. In addition, studies in animal models of AD indicate a direct effect of curcumin in decreasing the amyloid pathology of AD. As the widespread use of curcumin as a food additive and relatively small short-term studies in humans suggest safety, curcumin is a promising agent in the treatment and/or prevention of AD. Nonetheless, important information regarding curcumin bioavailability, safety and tolerability, particularly in an elderly population is lacking. We are therefore performing a study of curcumin in patients with AD to gather this information in addition to data on the effect of curcumin on biomarkers of AD pathology.
Acknowledgments
NIA ADRC Grant AG 16570, Institute for the Study on Aging, John Douglas French Foundation, and the Shirley and Jack Goldberg Trust
References
- 1.Majeed M, Badmaev V, Murrray F. Turmeric and the Healing Curcuminoids. New Canaan, CT: Keats Publishing, Inc.; 1996.
- 2.Vlietinck AJ, De Bruyne T, Apers S, Pieters LA. Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med. 1998;64(2):97–109. doi: 10.1055/s-2006-957384. [DOI] [PubMed] [Google Scholar]
- 3.Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, et al. Strategy and planning for chemopreventive drug development: clinical development plans II. J Cell Biochem Suppl. 1996;26:54–71. doi: 10.1002/jcb.240630705. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–98. [PubMed] [Google Scholar]
- 5.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304(5670):600–2. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 6.Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, De-Kosky ST, et al. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57(6):985–9. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 7.Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13(4):439–50. doi: 10.1017/s1041610201007852. [DOI] [PubMed] [Google Scholar]
- 8.Tohgi H, Abe T, Yamazaki K, Murata T, Ishizaki E, Isobe C. Alterations of 3-nitrotyrosine concentration in the cerebrospinal fluid during aging and in patients with Alzheimer’s disease. Neurosci Lett. 1999;269(1):52–4. doi: 10.1016/s0304-3940(99)00406-1. [DOI] [PubMed] [Google Scholar]
- 9.Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, Fitz-Gerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48(5):809–12. [PubMed] [Google Scholar]
- 10.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287(24):3223–9. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 11.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60(2):203–8. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 12.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336(17):1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 13.Reddy AC, Lokesh BR. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol Cell Biochem. 1992;111(1–2):117–24. doi: 10.1007/BF00229582. [DOI] [PubMed] [Google Scholar]
- 14.Sreejayan, Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46(12):1013–6. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 15.Shih CA, Lin JK. Inhibition of 8-hydroxydeoxyguanosine formation by curcumin in mouse fibroblast cells. Carcinogenesis. 1993;14(4):709–12. doi: 10.1093/carcin/14.4.709. [DOI] [PubMed] [Google Scholar]
- 16.Kim DS, Park SY, Kim JK. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1–42) insult. Neurosci Lett. 2001;303(1):57–61. doi: 10.1016/s0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Canadian Study of Health and Aging. risk factors for Alzheimer’s disease in Canada. . Neurology. 1994;44(11):2073–80. doi: 10.1212/wnl.44.11.2073. [DOI] [PubMed] [Google Scholar]
- 19.Andersen K, Launer LJ, Ott A, Hoes AW, Breteler MM, Hofman A. Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer’s disease? The Rotterdam Study. Neurology. 1995;45(8):1441–5. doi: 10.1212/wnl.45.8.1441. [DOI] [PubMed] [Google Scholar]
- 20.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289(21):2819–26. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 21.Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of antiinflammatory actions of curcumine and boswellic acids. J Ethnopharmacol. 1993;38(2–3):113–9. doi: 10.1016/0378-8741(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 22.Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Gautam SC. Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematopathol Mol Hematol. 1997;11(1):49–62. [PubMed] [Google Scholar]
- 23.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60(11):1665–76. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 24.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6(4):345–51. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 25.Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol. 1992;36(4):273–5. [PubMed] [Google Scholar]
- 26.Shah BH, Nawaz Z, Pertani SA, Roomi A, Mahmood H, Saeed SA, et al. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem Pharmacol. 1999;58(7):1167–72. doi: 10.1016/s0006-2952(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava R, Dikshit M, Srimal RC, Dhawan BN. Anti-thrombotic effect of curcumin. Thromb Res. 1985;40(3):413–7. doi: 10.1016/0049-3848(85)90276-2. [DOI] [PubMed] [Google Scholar]
- 28.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75(6):742–50. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 29.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 30.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43(2):86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 31.Deshpande SS, Lalitha VS, Ingle AD, Raste AS, Gadre SG, Maru GB. Subchronic oral toxicity of turmeric and ethanolic turmeric extract in female mice and rats. Toxicol Lett. 1998;95(3):183–93. doi: 10.1016/s0378-4274(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 32.Gupta B, Kulshrestha VK, Srivastava RK, Prasad DN. Mechanisms of curcumin induced gastric ulcer in rats. Indian J Med Res. 1980;71:806–14. [PubMed] [Google Scholar]
- 33.Shakai K. Effects of extracts of Zingiberaceae herbs on gastric section in rabbits. Chem Pharm Bull. 1982;20(37):215. doi: 10.1248/cpb.37.215. [DOI] [PubMed] [Google Scholar]
- 34.NTP Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79%–85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1993;427:1–275. [PubMed] [Google Scholar]
- 35.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7(7):1894–900. [PubMed] [Google Scholar]
- 36.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–900. [PubMed] [Google Scholar]
- 37.Heath DD, Pruitt MA, Brenner DE, Rock CL. Curcumin in plasma and urine: quantitation by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;783(1):287–95. doi: 10.1016/s1570-0232(02)00714-6. [DOI] [PubMed] [Google Scholar]
- 38.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 39.Administration USFaD. Substances generally recognized as safe. Essential oils, oleoresins (solvent-free) and natural extractives (including distillates). 1994.
- 40.Shah RG, Netrawali MS. Evaluation of mutagenic activity of turmeric extract containing curcumin, before and after activation with mammalian cecal microbial extract of liver microsomal fraction, in the Ames Salmonella test. Bull Environ Contam Toxicol. 1988;40(3):350–7. doi: 10.1007/BF01689091. [DOI] [PubMed] [Google Scholar]
- 41.Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–4. [PubMed] [Google Scholar]
- 42.Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24(12):651–4. [PubMed] [Google Scholar]
- 43.Lal B, Kapoor AK, Agrawal PK, Asthana OP, Srimal RC. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res. 2000;14(6):443–7. doi: 10.1002/1099-1573(200009)14:6<443::aid-ptr619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 44.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9(1):161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 45.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21(21):8370–7. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18(8):2974–81. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, et al. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22(6):993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]


