Abstract
Mild hypertriglyceridaemia is common in diabetes, but patients with newly presenting or poorly controlled diabetes may have a quantitatively different syndrome of gross hypertriglyceridaemia, which should be treated by optimising glycaemic control before use of lipid lowering drugs
Summary points
Diabetes can be associated with massive hypertriglyceridaemia, with serum triglyceride concentrations ≥100 mmol/l in extreme cases
Severe hypertriglyceridaemia represents an extreme of the classic blood lipid pattern in diabetes. It carries a risk of acute pancreatitis, and will be missed if serum triglycerides are not measured. Lipaemic samples should always prompt measurement of fasting triglyceride by the laboratory
Diabetes and impaired glucose tolerance are common findings in patients with hypertriglyceridaemia. Fasting glucose should be measured, and a glucose tolerance test performed if indicated, in hypertriglyceridaemic patients
Hypertriglyceridaemia due to poor diabetic control does not respond well to lipid lowering agents. Treating the diabetes is the first priority, although many patients will also have an underlying dyslipidaemia
Serious metabolic consequences of poor diabetic control are not reflected in patients' symptoms
Diagnostics lack the robust evidence base available to other interventional medical practices, but considerable consensus guidance obtained from observational and intervention studies is available to guide optimal use of laboratory tests. This includes measurement of serum triglycerides in patients in whom lipid lowering is being considered.
This article considers two cases of hypertriglyceridaemia in diabetes. Such cases are not infrequently referred to lipid clinics or prompt questions about possible laboratory error. In reality they reflect the metabolic consequences of excess glucose substrate in poorly controlled or newly presenting diabetes, usually in association with an underlying defect of triglyceride metabolism. Treatment is of the precipitating factor (that is, hyperglycaemia) rather than with lipid lowering drugs in the first instance, as part of the global management of the diabetes and risk factors for other diabetic complications.
Case 1
An otherwise fit 49 year old man, with a body mass index (weight (kg)/(height (m)2) of 28, attended his general practitioner complaining of tiredness and malaise after a mild flu-like illness. His alcohol consumption was about 16 units a week, his body temperature normal, and his blood pressure 156/98 mm Hg. Clinical examination was otherwise unremarkable, and a full blood count and renal function were checked.
These showed normal full blood count indices, serum sodium concentration 128 mmol/l, and serum creatinine 79 µmol/l. However, his serum was noted in the laboratory to be lipaemic in appearance, and cholesterol and triglyceride tests were added. These showed a total cholesterol concentration of 33.0 mmol/l and triglyceride concentration of 96 mmol/l. A serum glucose assay was added and found the concentration to be 21 mmol/l. Sodium concentration was then measured in serum treated to remove the lipids and was 138 mmol/l. The general practitioner was contacted, and the patient was referred urgently to the local diabetes centre for assessment.
On examination, he was a fit looking man, systematically well, who admitted to polyuria on direct questioning. He was well hydrated with a blood pressure of 152/92 mm Hg, pulse 68 beats/minute. A diagnosis of diabetic hypertriglyceridaemia in newly presenting diabetes was made. The immediate concern was the patient's gross hypertriglyceridaemia, and twice daily treatment with insulin was started, titrated up to 26 units daily.
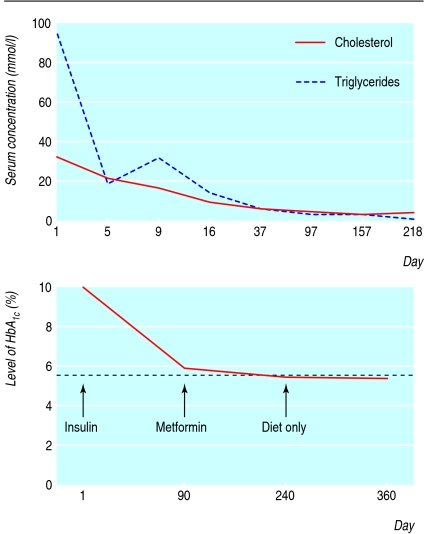
Over the next four weeks his triglycerides fell progressively to 14 mmol/l, and then to 5.6 mmol/l over the subsequent six weeks, at which point his total cholesterol concentration had fallen to 4.7 mmol/l (see fig 1). By 12 weeks after starting insulin, he was asymptomatic, his glycated haemoglobin had fallen from 9.9% to 5.9%, and his treatment was changed to metformin 500 mg twice daily.
Fig 1 Change in patient's serum total cholesterol and triglyceride concentrations (top) and glycated haemoglobin (bottom) after diagnosis of diabetes and progressive reduction in intensity of treatment
At this point, he started treatment with fenofibrate 267 mg as his triglycerides remained elevated (6.2 mmol/l). These fell to under 3 mmol/l, remaining at this level over the next year. His apolipoprotein E result returned as E2/2, consistent with type III (remnant) hyperlipidaemia.
Six months after presentation, his HbA1c was 5.3%, and metformin was stopped. One year after diagnosis, his glycated haemoglobin had remained within the 5-6% range on diet alone, and his triglycerides remained between 2.0 and 3.0 mmol/l (fig 1).
Case 2
An otherwise fit 52 year old woman was referred by her general practitioner to a lipid clinic because of failure of her mixed hyperlipidaemia to respond to atorvastatin 40 mg once daily and Omacor 2 g twice daily. Her condition had been managed for two years, and until one year previously her lipids had been well controlled, with total cholesterol ranging between 4.5 and 5.2 mmol/l and triglycerides between 2 and 3 mmol/l. Her triglycerides concentration had risen over the previous six months to 8.4 mmol/l despite also taking fenofibrate 267 mg daily.
A fasting blood glucose measure at the time her dyslipidaemia had been discovered had been 5.6 mmol/l. When seen in clinic, the patient was overweight (body mass index 32) with a blood pressure of 148/96 mm Hg but was otherwise physically well. Her serum total cholesterol concentration was 8.3 mmol/l and her triglycerides concentration 9.5 mmol/l. Direct questioning revealed no symptoms of diabetes, although routine blood samples taken to exclude secondary causes for her hyperlipidaemia revealed a fasting blood glucose of 13.7 mmol/l, and a subsequent measurement of her HbA1c was 8.3%. Thyroid and renal function tests were unremarkable, creatine kinase was within the population reference range, but liver tests showed her aspartate transaminase was raised at 73 IU/l (reference range 18-40).
She was reviewed by the clinic dietician and given targeted dietary advice. Six weeks later, she reported home capillary blood glucose measurements mostly between 9 and 11 mmol/l and started taking metformin, titrated up to 1500 mg daily. Ten weeks later, her HbA1c had fallen to 7.3%, at which stage her total cholesterol was 4.3 mmol/l and triglycerides 2.1 mmol/l. Her HbA1c fell to 6.8% over the next three months and her aspartate transaminase level returned to within the reference range. Fenofibrate was subsequently stopped without adverse effect on her triglycerides.
Discussion
These two cases are complementary to the first article in this series1 but describe the specific situation of secondary hyperlipidaemia due to poor diabetes control. Although mild hypertriglyceridaemia with low concentrations of high density lipoprotein (HDL) cholesterol is a classic feature of insulin resistance and characterises the lipid profile in type 2 diabetes, severe hypertriglyceridaemia can occur in poorly controlled disease or at presentation.2
Patients presenting with severe hypertriglyceridaemia not infrequently have an underlying dyslipidaemia, one notable cause being type III (remnant) hyperlipidaemia that is linked to the presence of the apolipoprotein E2 allele.3 It seems that applying a metabolic stress to such people can produce an exaggerated triglyceride response. Similar findings are seen in alcohol induced secondary hyperlipidaemia. High alcohol intake can be associated with severe hypertriglyceridaemia, although this may be limited to predisposed individuals—among patients with normal baseline triglyceride concentrations, heavy drinkers do not necessarily have markedly higher triglycerides than occasional drinkers.4
If triglycerides concentrations are not measured these cases can present as statin resistant hypercholesterolaemia, caused not by an increase in low density lipoprotein (LDL) cholesterol concentrations but by raised serum levels of very low density lipoprotein (VLDL) cholesterol with or without raised chylomicron concentrations.5
Case 1
This case demonstrates several of the features of massive diabetic hypertriglyceridaemia. The patient's lipid results (triglycerides and cholesterol) were out of all proportion to his clinical presentation. The lipid disorder was detected from a lipaemic sample in the laboratory, rather than from a request for lipid measurement. Apart from routine lipid measurement, some cases of massive hypertriglyceridaemia may also present with eruptive xanthomata, abdominal pain, or acute pancreatitis. The case highlights the need to consider lipid measurement early after the diagnosis of diabetes to detect these potentially dangerous abnormalities.
This patient's massive hypertriglyceridaemia responded rapidly to insulin without specific lipid lowering treatment, and subsequently, as his hyperglycaemia was brought under control, he did not require insulin or even oral hypoglycaemic agents to obtain a satisfactory concentration of glycated haemoglobin. High circulating concentrations of triglyceride and free fatty acid are suspected of reducing the effective secretion or action of insulin, with massive hypertriglyceridaemia possibly inducing diabetes.6 It is not possible to say in the present case to what extent the patient's hypertriglyceridaemia may have contributed to his diabetes, but it is interesting that he is now managed on diet alone and maintains a glycated haemoglobin concentration well inside the reference range.
The decision to begin treatment with insulin was a pragmatic one at the time and was based on the severity of the patient's hypertriglyceridaemia, the potential risk of pancreatitis, and a desire to reduce his triglycerides to a safe range as quickly as possible.
Hypertriglyceridaemia affects the measurement of other substances in the blood. Triglycerides can interfere with several assays, notably producing low serum amylase results, which can cause diagnostic difficulty as patients are at risk of pancreatitis, and can produce dilutional effects such as the pseudohyponatraemia seen in this case. Laboratories should identify lipaemic samples (fig 2) and take corrective measures (such as clearing serum with products such as Lipoclear (Statspin, Norwood MA) or using alternative analytical methods). If general practitioners intend to use point of care analyses (near patient testing) in patient management, they should know the specific effects of hypertriglyceridaemia on the test method as the effects vary among methods.
Fig 2 Example of a normal spun blood sample (left) and a lipaemic sample from a diabetic patient with serum triglyceride concentration 150 mmol/l (right). In the latter the serum is densely lipaemic and is topped by a layer of chylomicrons. Courtesy of Dr Andrew Iversen
Case 2
The second case illustrates a common reason for referral to secondary care in patients whose lipid levels do not seem to have responded to a statin but who have not been investigated for secondary causes, or people with known dyslipidaemia who lose lipid control despite increasing intensity of treatment. The large number of patients presenting to clinic with mixed dyslipidaemia who were found to have previously unknown diabetes or impaired glucose tolerance led us locally to change our analytical lipid service to include triglyceride in all lipid profiles.7
In such patients statin treatment may be producing maximal therapeutic effect on LDL, but the serum total cholesterol may still be raised partly as a result of the raised triglycerides. Although some statins are licensed for use in mixed hyperlipidaemia, they remain primarily LDL lowering drugs and have relatively modest effects on triglycerides. They will have limited effect on serum total cholesterol that is raised as a result of high VLDL cholesterol associated with hypertriglyceridaemia. The excess glucose load in patients with uncontrolled diabetes or impaired glucose tolerance is converted into triglycerides by the liver and exported principally in the form of VLDL particles, raising the VLDL (and hence total) cholesterol level. As in the case of alcohol induced hypertriglyceridaemia, drugs which may block the hepatic synthesis or accelerate the metabolism of lipids have limited effect in the face of excess supply of substrate (glucose or alcohol).
Hypertriglyceridaemia secondary to diabetes has been reported to be the commonest presenting cause of pancreatitis attributable to hypertriglyceridaemia,8 and diabetic patients presenting with massive serum triglyceride concentrations should therefore be considered at high risk. The computerised decision support system PRODIGY advises that secondary causes of hyperlipidaemia should be identified and managed before starting lipid lowering treatment,9 and management of hyperglycaemia is described as the key to severe triglyceridaemia in diabetic patients.10 Raised triglycerides is one of the factors included in the Diabetes UK risk assessment for patients11 and should prompt a fasting glucose test in all hypertriglyceridaemic patients.
The choice of lipid lowering drug in patients with an underlying dyslipidaemia once their diabetes is controlled can be difficult. In some cases the lipid profile may reveal a persisting severe hypertriglyceridaemic component, which may prompt use of omega 3 fatty acid compounds or fibrate. In other cases the LDL component may predominate, and the patients should be offered a statin. If both LDL cholesterol and triglyceride concentrations are raised patients will normally require treatment to reduce LDL (statin) and to reduce triglycerides (omega 3 fatty acids or fibrate) usually initiated under specialist guidance. Many of these patients will have elevated liver enzyme activity (γ-glutamyltransferase or γ-glutamyltransaminase) reflecting fatty deposition in the liver, which can be incorrectly attributed to alcohol consumption or seen as a contraindication to lipid lowering drugs. As measures to remove the cause of fatty liver reduce the associated raised transaminases,12 13 appropriate lifestyle advice and, if necessary, drug treatment to reduce triglyceride concentrations would seem appropriate with suitable monitoring.
Questions and answers: learning points
The series of questions and answers summarised in the box may be found in the second review of best practice in primary care pathology published in the Journal of Clinical Pathology.14 They appeared in the first of this series of primary care laboratory medicine1 and are reproduced here for convenience.
Secondary hyperlipidaemia and hypertriglyceridaemia
When should I screen for secondary hyperlipidaemia and what investigations are required?
In all patients in whom lipid lowering therapy is being considered. The investigations are
Dietary, alcohol, and drug history
Urine dipstick testing for protein
Laboratory or point of care blood glucose
Renal function
Liver enzymes (transaminase)
Thyroid stimulating hormone (if the total cholesterol is >8 mmol/l, unless thyroid disease is suspected clinically)
When should I measure triglycerides at the same time as I measure cholesterol?
In all people being assessed for risk of cardiovascular disease
In all people being considered for lipid lowering treatment
In monitoring, if the first triglyceride level was >2 mmol/l
What triglyceride levels are associated with a risk of pancreatitis and require treatment on this basis?
Serum triglycerides of
5 mmol/l carry a probable increased risk of pancreatitis
10 mmol/l carry a high risk of pancreatitis
20 mmol/l carry a very high risk of pancreatitis
Persistent values over 5 mmol/l justify treatment
I thank Susan Richardson for typing this manuscript; the Clinical Practice Section of the Association of Clinical Biochemists (in particular D B Freedman, A Iversen, T A Gray, N Capps, W G Simpson, D Cassidy, S C Martin, and N A Sattar); the people who kindly reviewed the original work (I S Young, R Gama (Association of Clinical Pathologists), R Neal, and N Campbell (Royal College of General Practitioners)); and S R S Smart, who co-authored the original guidance.
Competing interests: None declared.
This is the seventh article in the series
References
- 1.Smellie WSA. Cases in laboratory medicine Testing pitfalls and guidance in lipid management. BMJ 2006;333:83-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikkila EA. Triglyceride metabolism in diabetes mellitus. Progr Biochem Pharmacol 1973;8:271-99. [PubMed] [Google Scholar]
- 3.Chait A, Brunzell JD, Albers JJ, Hazzard WR. Type III hyperlipoproteinaemia (‘remnant removal disease'). Lancet 1977;ii:1176-8. [DOI] [PubMed]
- 4.Allaway SL, Ritchie CD, Robinson D, Seear T, Reznek R, Fry IK, et al. Detection of alcohol-induced fatty liver by computerized tomography. J R Soc Med 1988;81:149-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durrington PN. Lipoproteins and their metabolism. In: Hyperlipidaemia: diagnosis and management. 2nd ed. Oxford: Butterworth-Heinemann, 1995:25-71.
- 6.Mingrone G, Henriksen FL, Greco AV, Krogh LN, Capristo E, Gastaldelli A, et al. Triglyceride-induced diabetes associated with familial lipoprotein lipase deficiency. Diabetes 1999;48:1258-63. [DOI] [PubMed] [Google Scholar]
- 7.Fortson MR, Freedman SN, Webster PD. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol 1995;90:2134-9. [PubMed] [Google Scholar]
- 8.Smellie WSA, Lowrie R, Wilkinson EL. A laboratory based intervention to improve appropriateness of lipid tests and audit cholesterol lowering in primary care. BMJ 2001;323:1224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHS National Library for Health. PRODIGY hyperlipidaemia guidance. www.prodigy.nhs.uk/hyperlipidaemia/view_whole_guidance (accessed 20 Sep 2006).
- 10.American Diabetes Association 1995 Consensus statement. Detection and management of lipid disorders in diabetes. Diabetes Care 1996;19(suppl 1):S96-102.
- 11.Diabetes UK. Online diabetes risk test. www.diabetes.org.uk/measure-up/accessible-version.aspx
- 12.Davern TJ, Scharschmidt BF. Biochemical liver tests. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. 7th ed. Philadelphia: Saunders, 2002:1227-38.
- 13.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342:1266-71. [DOI] [PubMed] [Google Scholar]
- 14.Smellie WS, Forth JO, McNulty CA, Hirschowitz L, Lilic D, Gosling R, et al, for the Best Practice Working Group. Best practice in primary care pathology. Review 2. J Clin Pathol 2006;59:113-20. [DOI] [PMC free article] [PubMed] [Google Scholar]